Atomic Mass
Atomic Mass:
After Dalton said that the atoms of a substance had a particular mass, the problem that assailed scientists was how to find this mass. An atom is too small to be seen or isolated, so it was not possible to determine its mass by weighing.
Theoretically, though the mass of an atom could be determined by weighing a large sample of an element and then dividing it by the number of atoms it contained. But no one could come up with a method to count the number of atoms in a sample of an element.
- Avogadro’s hypothesis at least provided a way to find the relative masses of elements. Since equal volumes of different gases under similar conditions of temperature and pressure contain equal numbers of molecules it stands to reason that if one weighs equal volumes of hydrogen and oxygen under the same temperature and pressure one would be able to know the weights of equal numbers of hydrogen and oxygen molecules.
- Comparing the two weights, one would be able to determine how much heavier (or lighter) an oxygen molecule is compared to a hydrogen molecule. This is exactly what scientists did and they found that a molecule of oxygen is 16 times heavier than a molecule of hydrogen. They knew by then that one molecule of hydrogen contains two atoms of hydrogen and that one molecule of oxygen contains two atoms of oxygen, so they concluded that an atom of oxygen is 16 times heavier than an atom of hydrogen.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
Similarly, the relative (compared to hydrogen) atomic masses of other gases were determined. Berzelius and the Italian chemist Stanislao Cannizzaro (separately) did some pioneering work in determining the relative atomic masses of various elements. The atomic mass of hydrogen was taken as one, and the relative atomic masses of other elements were determined with the hydrogen atom as the reference. The choice of this reference was made by Dalton because hydrogen is the lightest element.
Atomic Mass Of Elements
- The relative atomic mass of oxygen was actually found to be 15.88 on this scale. Later, the oxygen atom was chosen as the reference and it was assigned a mass of 16. This was done for convenience because with the oxygen atom as the reference the relative atomic masses of the other elements worked out to whole numbers or close to whole numbers. Also, oxygen was considered more reactive, forming a large number of compounds. On this scale, the mass of a hydrogen atom works out to 1.008.
- Much later, the exact mass of the hydrogen atom was determined by an indirect method and turned out to be L66 x 10-24 g. However, to get back to the determination of relative atomic masses, in 1961, the International Union of Chemists selected a new reference for expressing them. They accepted 12C with the mass number 12 (the stable isotope of carbon) as the standard for comparing the atomic and molecular masses of elements and compounds. Today this scale depends upon measurements of atomic mass by mass speptrqmeters. A. mass spectroipeter is an instrument used for making accurate measurements of mass by comparing the mass of art atom with the mass of a particular atom chosen as the standard.
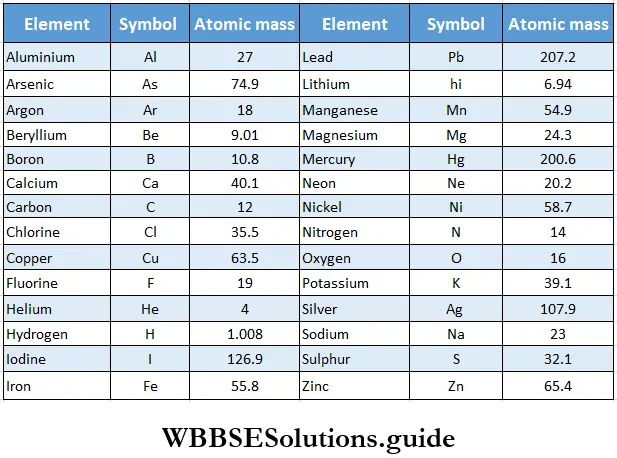
- Now, when we refer to the atomic mass of an element, we mean its relative atomic mass compared to 1/12 of the mass of an atom of 12C, which is taken as 12. In other words, 1/12 of the mass of a 12C atom is taken as the standard and is referred to as 1 atomic mass unit (amu). However, IUPAC has recommended a symbol (u) (unified mass) in place of amu. The average atomic masses of hydrogen, oxygen, nitrogen, and sulphur on this scale work out to 1.008,16,14 and 32 u respectively. The atomic masses of some common elements with the carbon-12 isotope as the reference are given in Table.
Average atomic mass: A large number of elements occur in nature as a mixture of several isotopes. The abundance of an isotope is defined ns the percentage of atoms of that isotope ih a sample of the element. The relative abundance of an element is fixed, thus independent of its state. For example, chlorine exists as two isotopes of masses Cl-35 and Cl-37. The relative abundance (per cent occurrence) of Cl-35 is 75.770 and that of Cl-i7 is 24.229. Also the precise f atomic mass of both the isotopes Cl-35 and Cl-37 are 34.9698 u and 36.9659 u respectively. The average atomic mass of chlorine can be calculated as follows.
Atomic Mass Of Common Elements
Average atomic mass of chlorine = \(=\frac{(34.9698 \times 75.770)+(36.9659 \times 24.229)}{100}=35.45 \mathrm{u}\)
The gram-atomic mass of an element is that quantity of it whose mass in grams is numerically equal to its atomic mass. In other words, it is the atomic mass of art element expressed in grams. It is also known as one gram-atom of the element. Atomic mass of oxygen = 16 u.
Gram-atomic mass of oxygen (or one gram-atom of oxygen) = 16 g.
Molecular mass: The average relative mass of a molecule compared to 1/12 of the mass of an atom of carbon (12C) is called its molecular mass. In other words, the molecular mass of a substance indicates how many times a molecule of it is heavier than l/12th the mass of an atom of carbon. The molecular mass of carbon dioxide, for example, is 44.0 u, which means a carbon dioxide molecule is 44 times heavier then 1/12th of a carbon atom.
The molecular mass of a compound or an element can be calculated by adding the atomic masses of all the atoms in one molecule of the compound or element. For example, the molecular formula of water is H2O.
Atomic Weights Of Elements
Therefore, the molecular mass of water (H2O) = (2 x 1008 u) for 2 hydrogen atoms + (1 x 16.0 u) for one oxygen atom = 18.02 u.
In case of ionic substances, we find out the relative formula mass instead of the molecular mass. The formula mass of a substance is the sum of the atomic masses of all the atoms in a formula unit of the compound. For example, the formula mass of NaCl is 58.5 u. The atomic masses of sodium and chlorine are 23.0 u and 35.5 u respectively.
The molecular mass of a substance (element or compound) expressed in grams is called its gram-molecular mass. The amount of the substance is referred to as one gram-molecule of the substance.
∴ Molecular mass of CO2 = 44.0 u.
Gram-molecular mass of CO2 (or one gram-molecule of CO2) = 44.0 g.
Note: Units like the gram-atom, gram-molecule, and gram-equivalent are no longer used by scientists. The mole has replaced these units.
