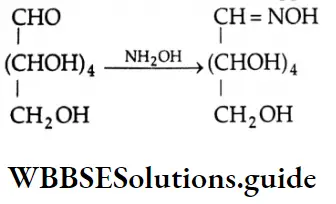
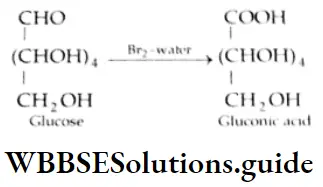
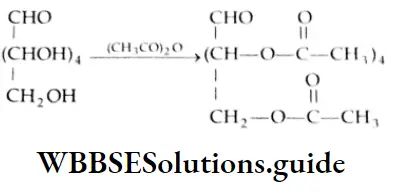
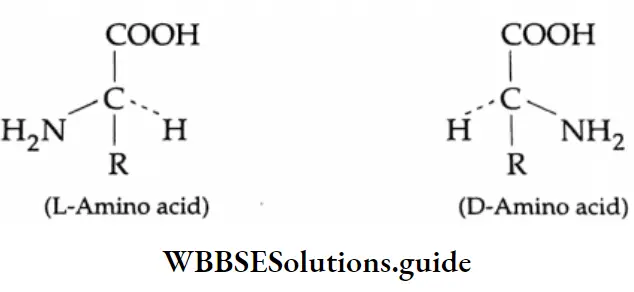
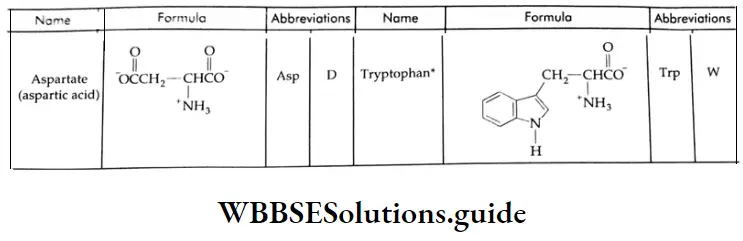
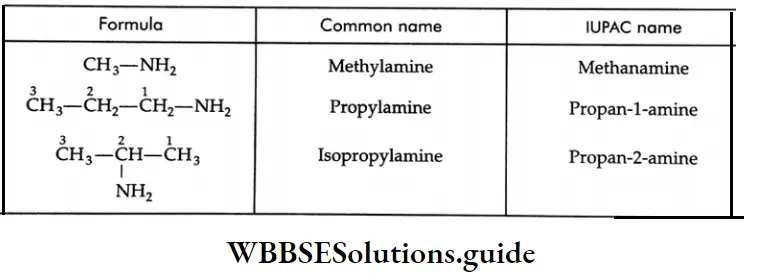
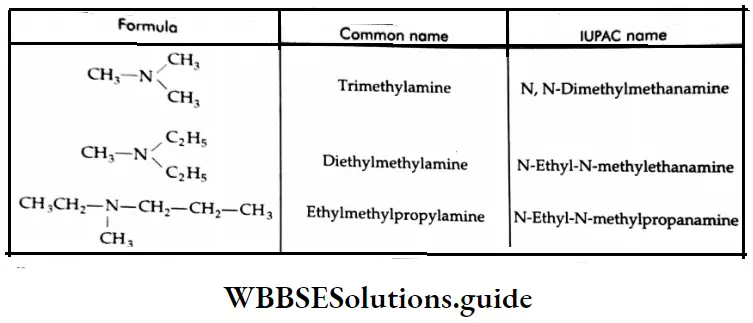
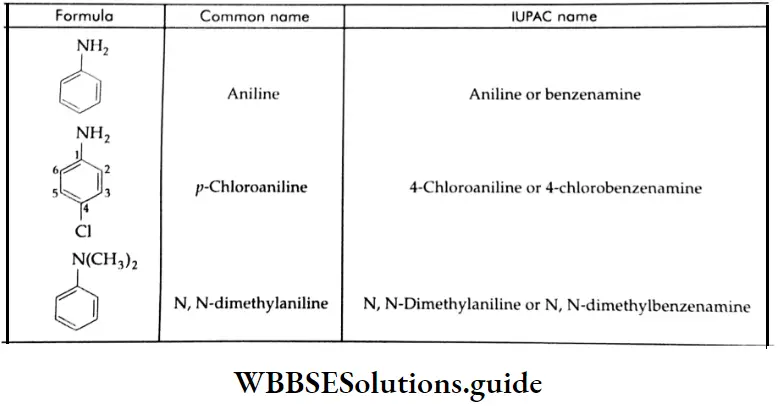
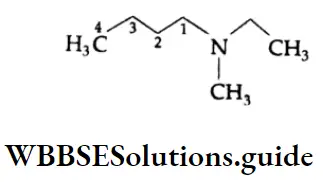
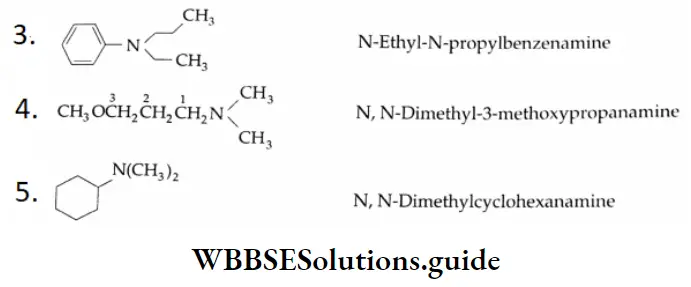
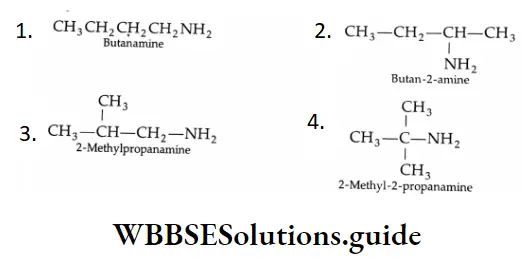
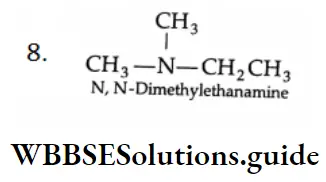
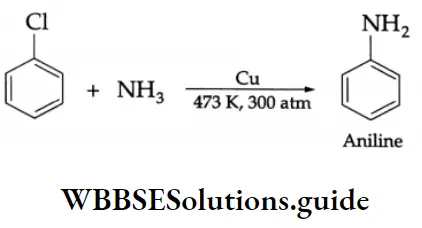
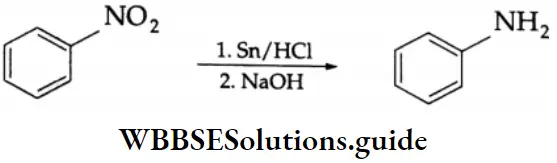
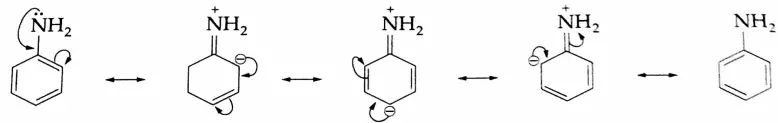
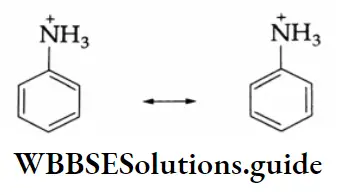
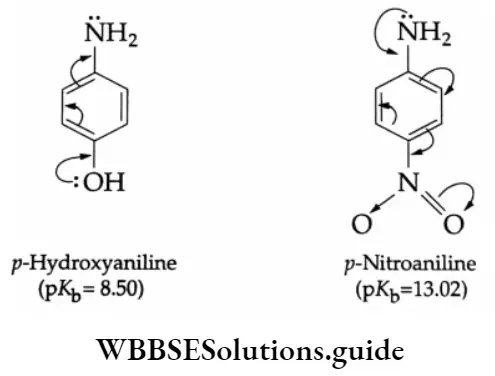
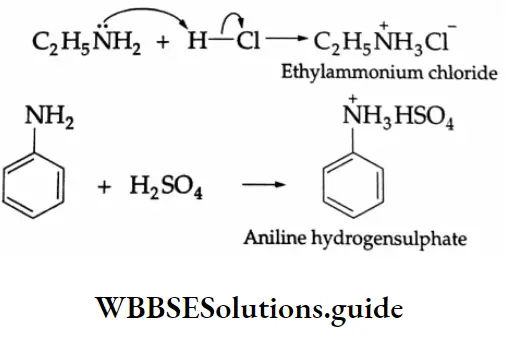
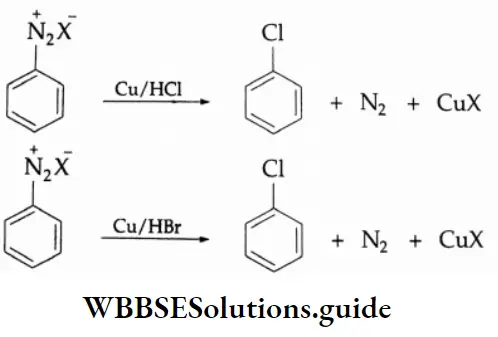
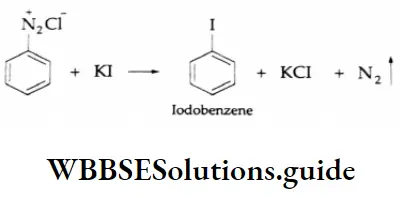
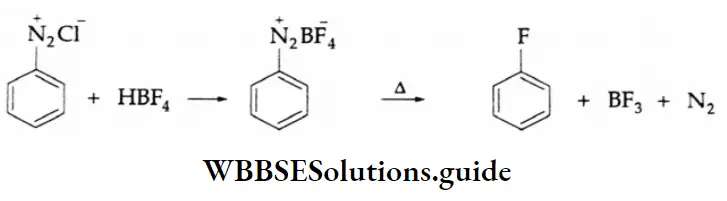
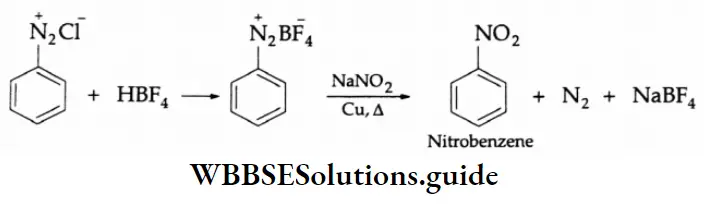
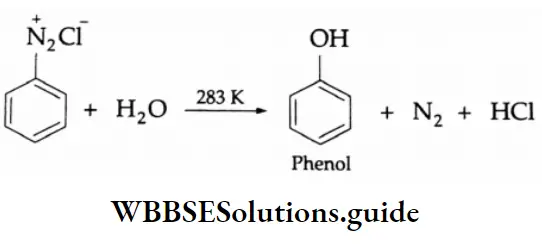
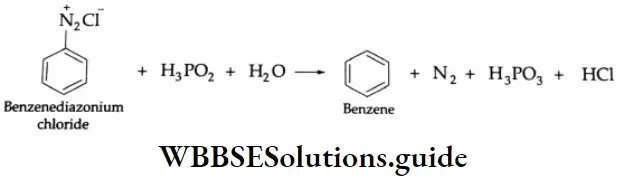
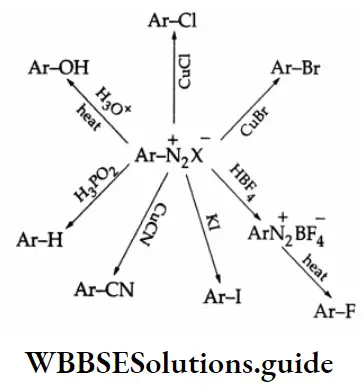
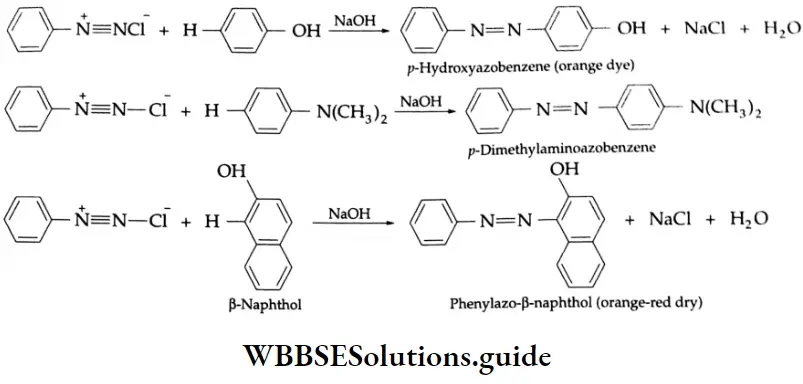
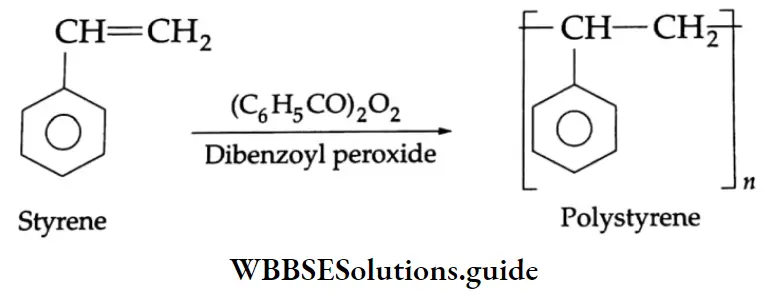
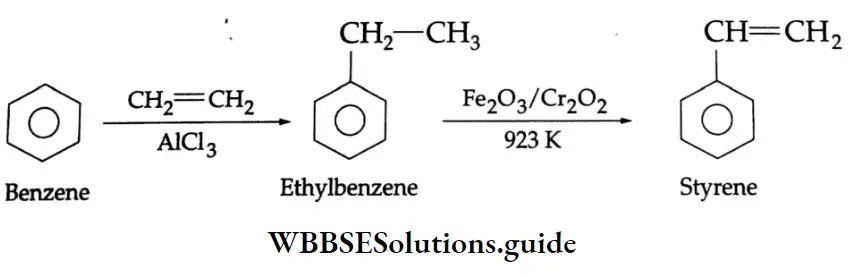
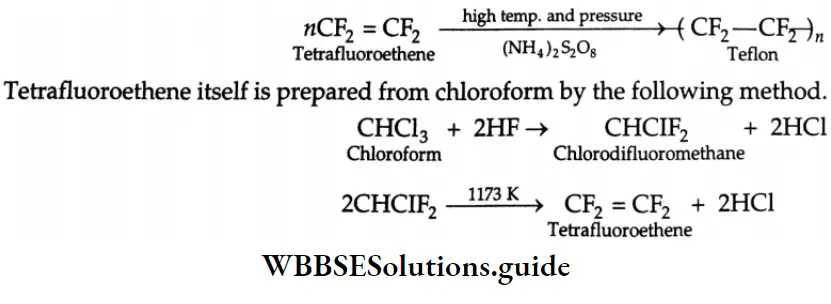
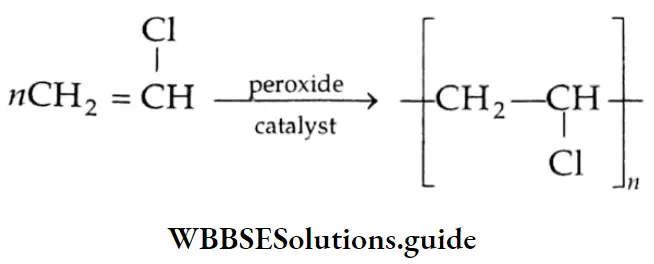
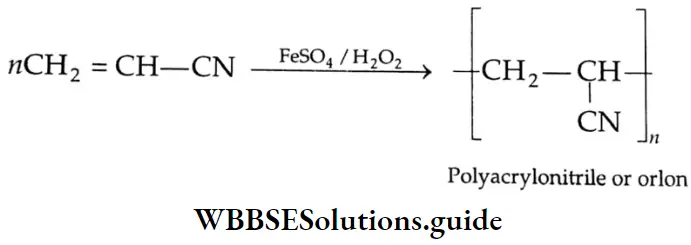
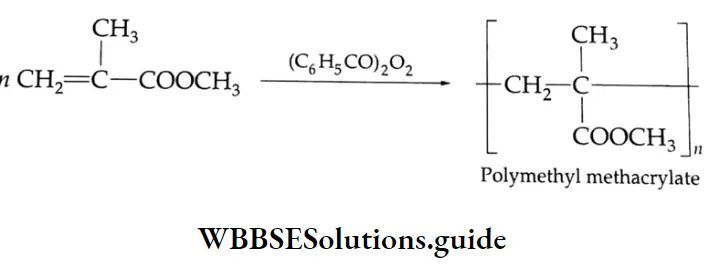
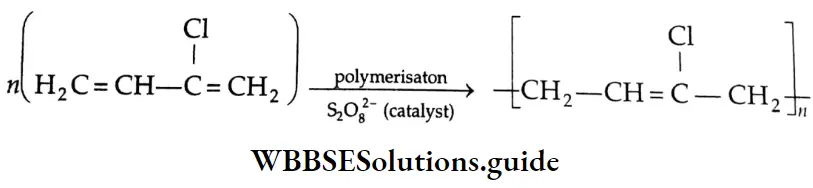
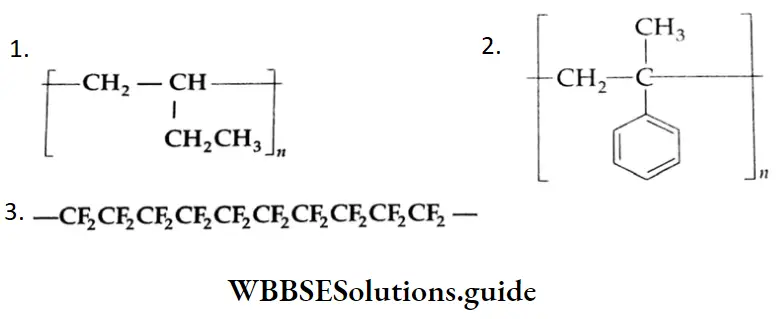
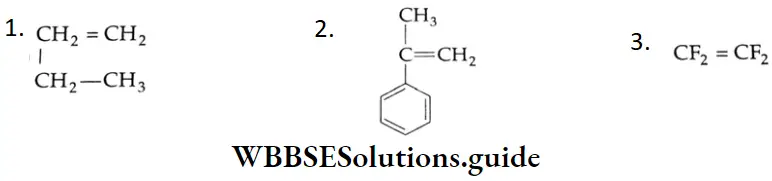
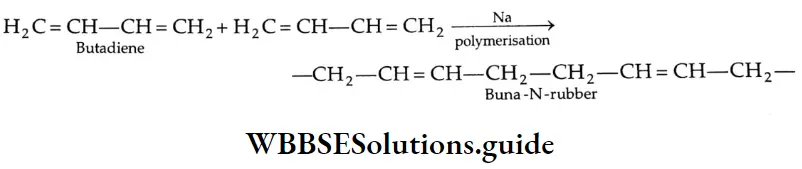
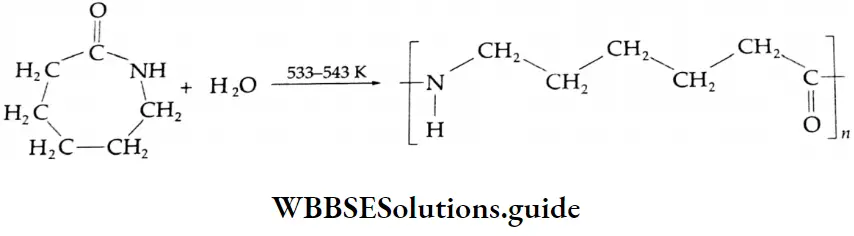
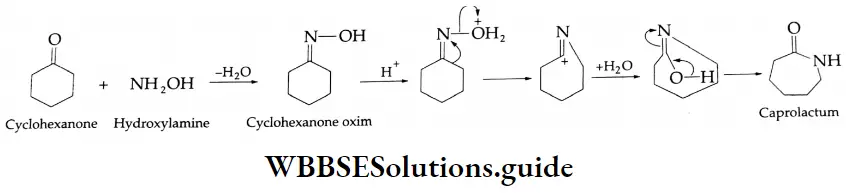
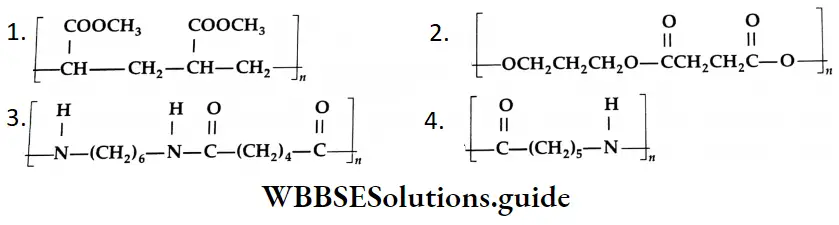
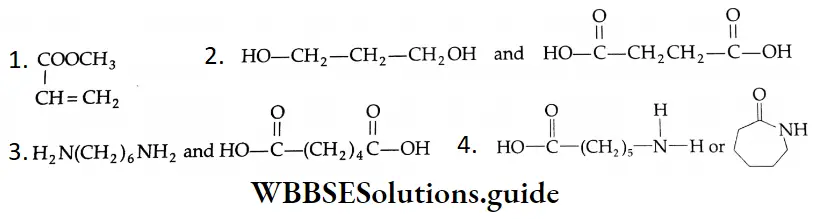
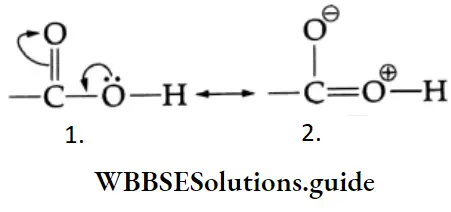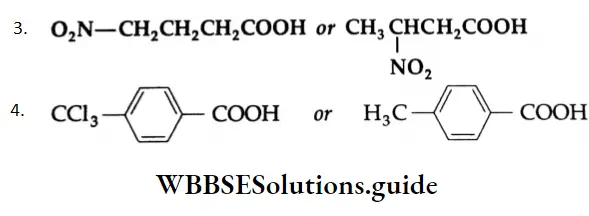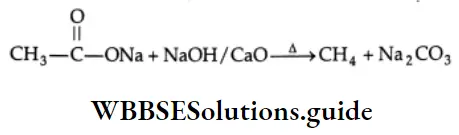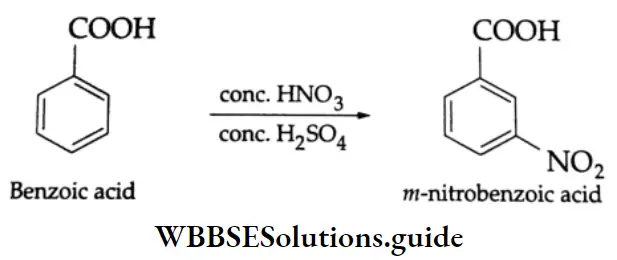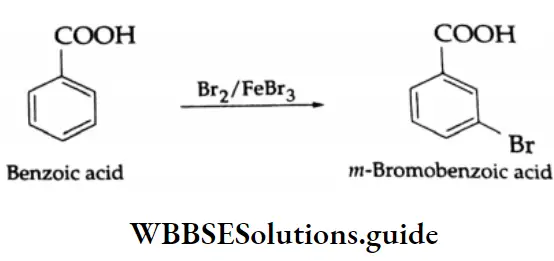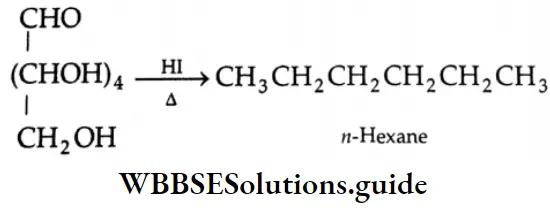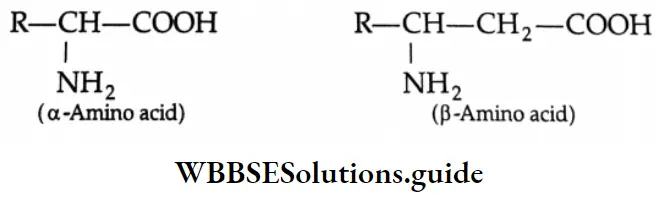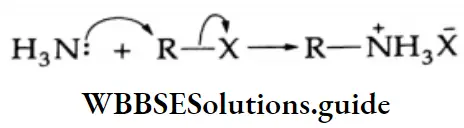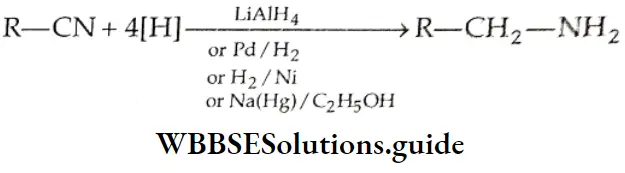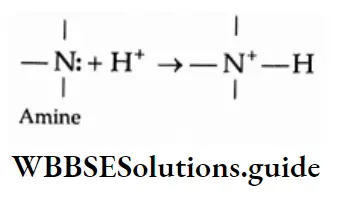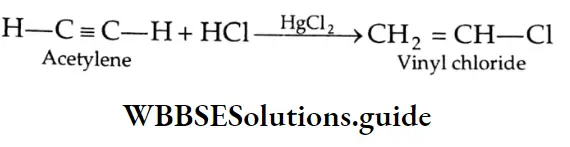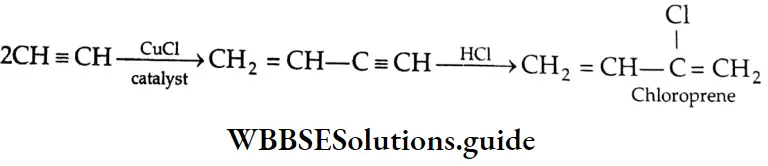Aldehydes, Ketones and Carboxylic Acids
Aldehydes And Ketones
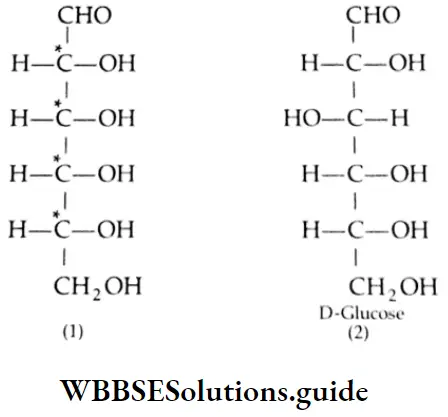
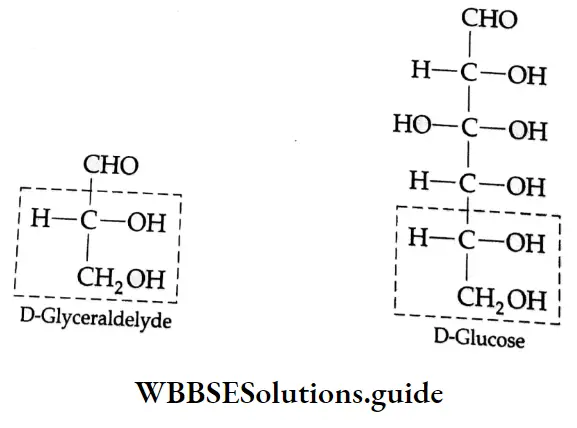
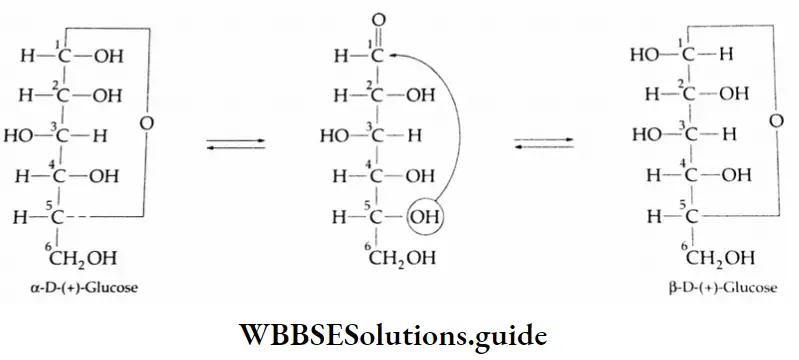
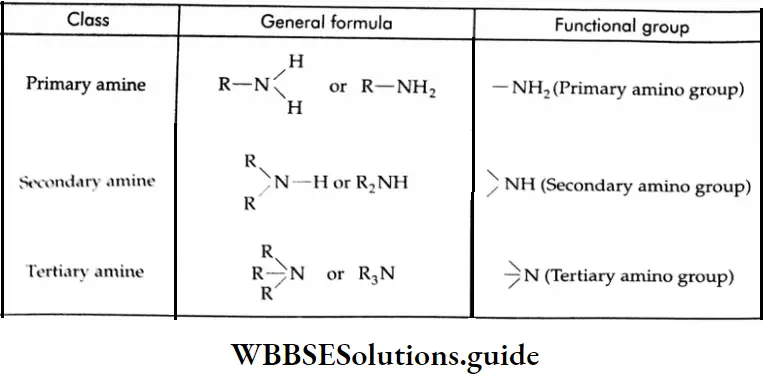
Aldehydes and ketones are compounds containing the carbonyl group (CO). When two alkyl groups are attached to the carbonyl group, the compound is a ketone. When two hydrogens, or one hydrogen and one alkyl group, are attached to the carbonyl group, the compound is an aldehyde.
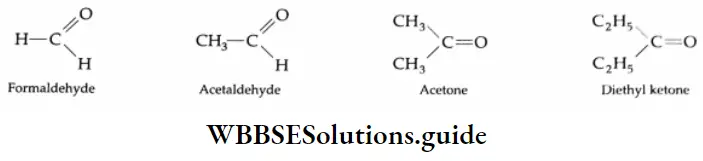
The chemistry of the carbonyl group is extremely important. Aldehydes and ketones are reactive. Many useful products such as dyes, resins, perfumes, plastics and clothes are made from them. Many useful compounds containing these functional groups can be obtained from plants.
Detailed notes on aldehydes, ketones, and carboxylic acids
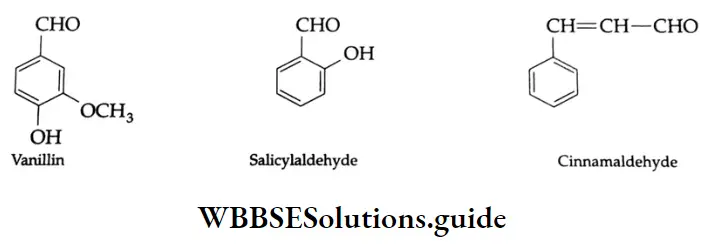
For example, vanillin (from vanilla beans), salicylaldehyde (from meadow sweet) and cinnamaldehyde (from cinnamon). These are flavouring compounds. Vanillin is used for the vanilla flavour in ice creams. Salicylaldehyde has an odour like bitter almonds and is used in perfumery. Cinnamaldehyde is responsible for the flavour of cinnamon.
Nomenclature Of Aldehydes
Aldehydes are named using either of two different systems.
Trivial name
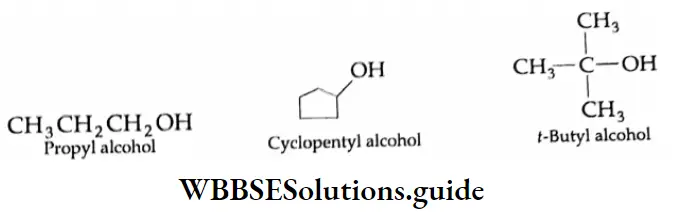
Simple aldehydes are commonly known by their trivial names or common names. According to this system, the name of an aldehyde is derived from the name of the corresponding carboxylic acid by dropping the suffix ic (oic) acid and adding in its place the suffix aldehyde.
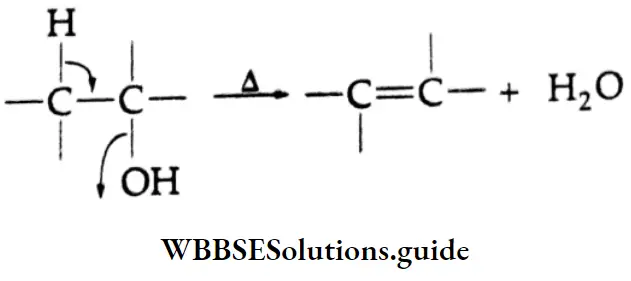
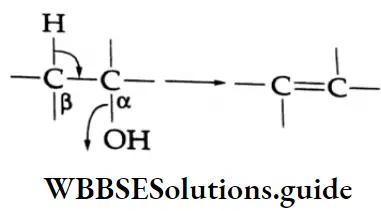
In the common system of nomenclature, the position of an additional substituent is indicated by the Greek letters α, β, γ, etc., a being the carbon attached to the carbonyl group, β being the next carbon, and so on.
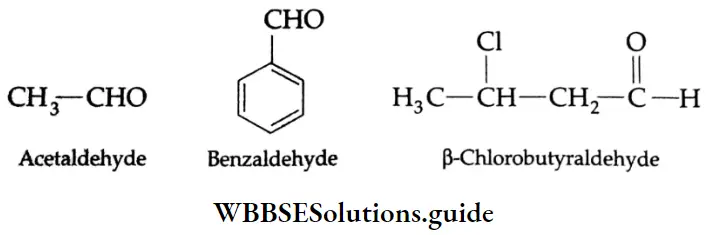
IUPAC name
In the IUPAC system, aldehydes are named by adding the suffix al to the name of the corresponding hydrocarbon, the ‘e’ of the hydrocarbon being omitted.
The substituents on the chain are prefixed in alphabetical order along with the numbers indicating their positions. These numbers are allocated by considering the aldehydic carbon to be the first carbon.
“Functional groups of aldehydes, ketones, and carboxylic acids”
The IUPAC name of a simple aromatic aldehyde in which the aldehydic group is directly attached to the benzene ring is benzenecarbaldehyde. However, the common name benzaldehyde is also retained in IUPAC nomenclature. Other aromatic aldehydes are named as substituted benzaldehydes.
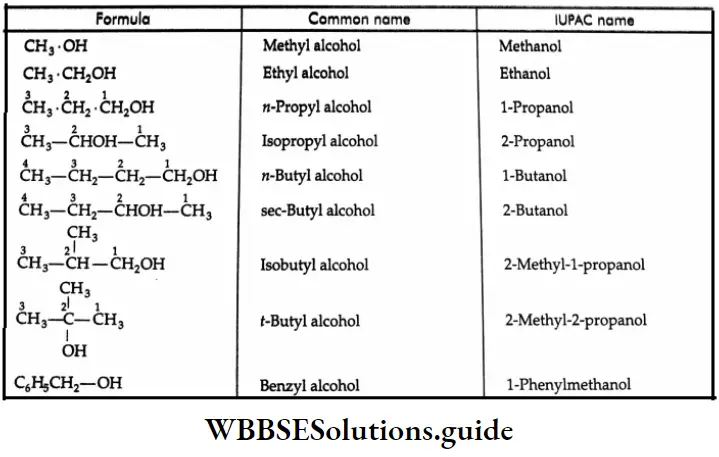
The common and IUPAC names of some aldehydes:
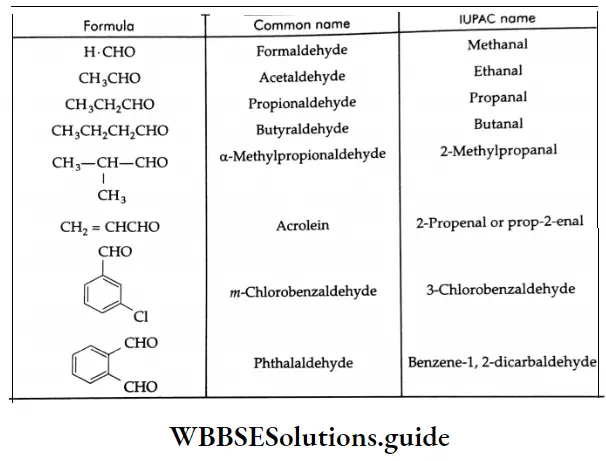
The following are examples of some more aldehydes and their IUPAC names:
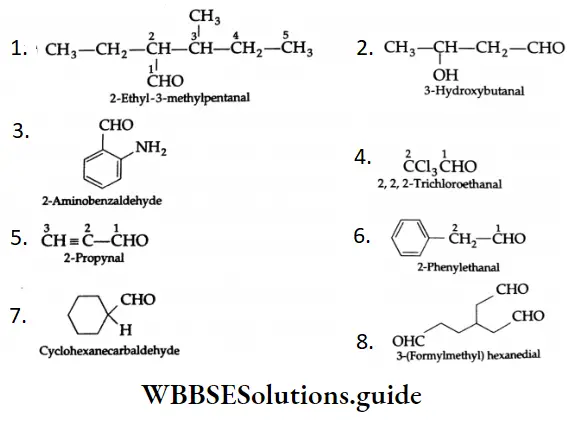
A molecule with a double bond and a triple bond is called an enyne (ene + yne). A molecule with a double bond, triple bond and an aldehyde group is known as a enynal (ene + yne + al). The term is used as a suffix.
The numbering of carbons begins with the carbon of the aldehyde group. The substituents and the functional are located accordingly.
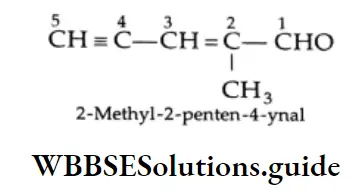
A molecule with two double bonds and an aldehyde group is known as a dienal (diene + al). The term is used as a suffix. Numbering of carbons begins with the carbon of the aldehyde group. The substituents and the functional groups are located accordingly.
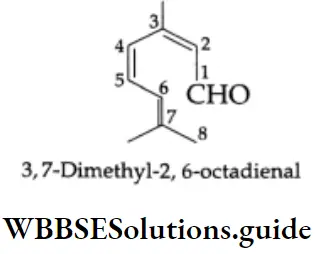
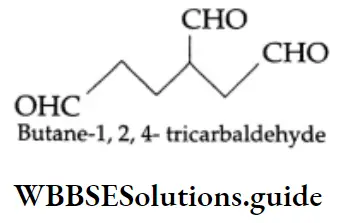
If an unbranched chain is directly linked to more than two aldehydic groups, these aldehydes are named from the parent hydrocarbon by the substitutive use of a suffix, example,tricarbaldehyde. The suffix ‘al’ is not used. Numbering of carbons begins at the end nearest the functional group. The functional groups are located accordingly.
Nomenclature Of Ketones
Ketones too are named using either of two different systems.
Trivial names
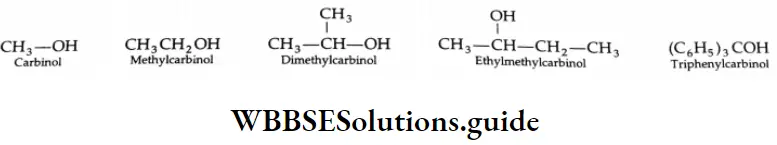
Simple ketones may be named by using the names of the alkyl groups attached to the carbonyl group followed by the word ketone. The names of the alkyl groups are written alphabetically.

IUPAC names
In the IUPAC system ketones are named by adding the suffix ‘one’ to the name of the corresponding hydrocarbon and omitting the final ‘e’ of the hydrocarbon.
“Nomenclature and IUPAC names of aldehydes, ketones, and carboxylic acids”
The position of the carbonyl group is given by a number which can be placed before the parent name as given below or immediately before the suffix (for example, pentane-2-one).
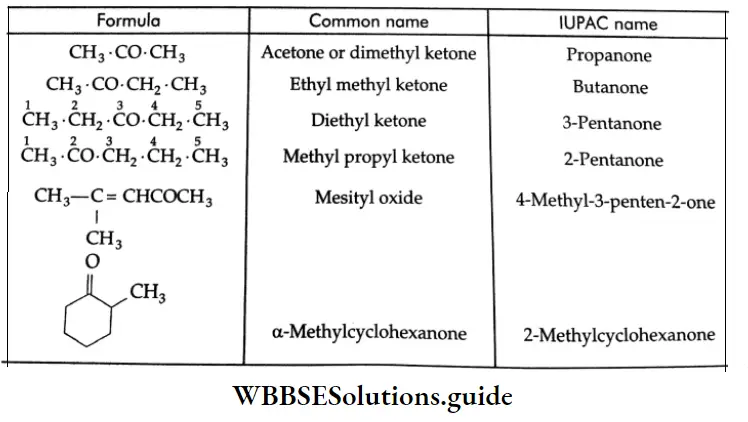
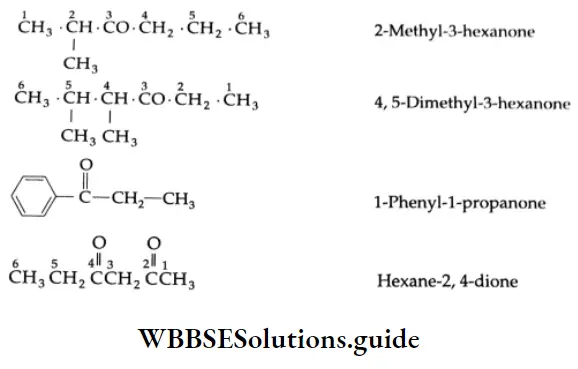
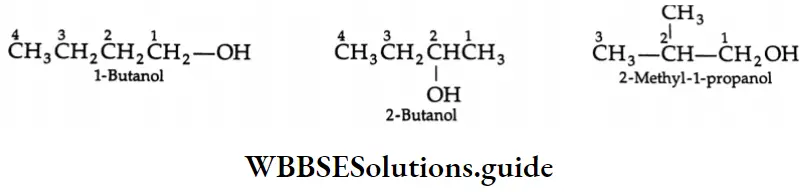
The common and IUPAC names of some ketones:
Ketones Structure
In the IUPAC system, the longest chain containing the carbonyl group is taken as the parent hydrocarbon and the positions of the carbonyl as well as any other substituent present in the molecule are indicated by numbers The longest chain containing the carbonyl group is numbered from the end that gives the carbonyl carbon the lowest number.
A molecule with a double bond and a ketone is called an enone (ene + one) and the term is used as a suffix. The numbering of carbons begins at the end nearest the functional group.
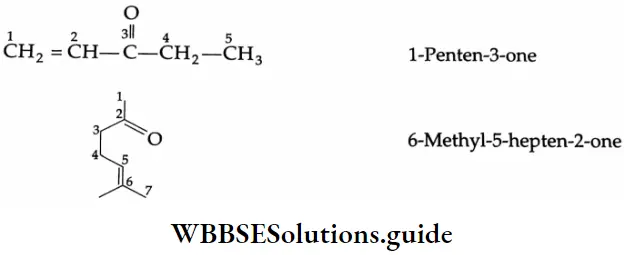
In cyclic ketone, the carbonyl carbon is a
Aldehydes, ketones, and carboxylic acids chapter notes
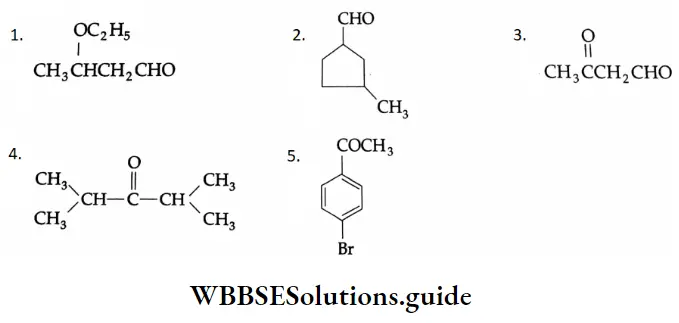
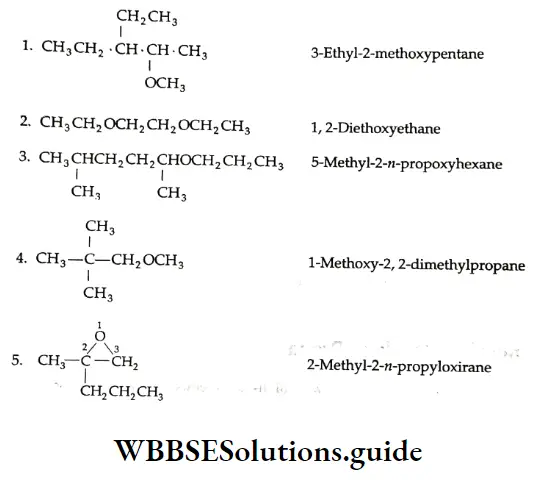
Example 1: Draw the structures of the following compounds.
- β -Ethoxybutyraldehyde
- 3-Methylcyclopentanecarbaldehyde
- 3-Oxob utanal
- Diisopropyl ketone
- 4-Bromoacetophenone
Solution:
The allocation of Greek letters to the carbon atoms in the common system starts from the carbon next to the aldehyde group, whereas the numbers in the IUPAC system always begin with the carbon of the aldehyde group.
⇒ \(\stackrel{\beta}{\mathrm{C}}-\stackrel{\gamma}{\mathrm{C}}-\stackrel{\beta}{\mathrm{C}}-\stackrel{\alpha}{\mathrm{C}}-\mathrm{CHO}\) (Used In Common names)
⇒ \(\stackrel{5}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{1}{\mathrm{C}} \mathrm{HO}\)(Used In IUPAC names
Structures Of The Carbonyl Group Used In IUPAC Names
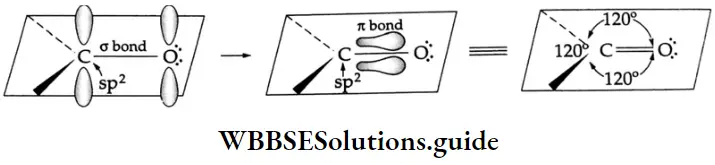
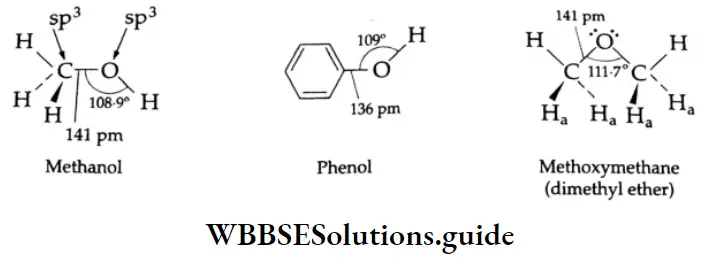
The carbon atom of the carbonyl group is sp2-hybridised and three of its electrons form three o bonds. The molecule is planar and the bond angles are close to 120°. The remaining p orbital of the carbon with one electron overlaps a p orbital of oxygen with one electron to form a bond between these atoms. The oxygen atom also has two lone pairs of electrons that occupy the remaining orbital.
The electrons in the bond of the carbonyl group are not equally shared. They are pulled more towards the more electronegative oxygen atom. As a result, the C-O bond is polarised in the direction C-O. Therefore, the electron-deficient carbonyl carbon is electrophilic (a Lewis acid) in nature and the electron-rich carbonyl oxygen is nucleophilic (Lewis base) in nature.
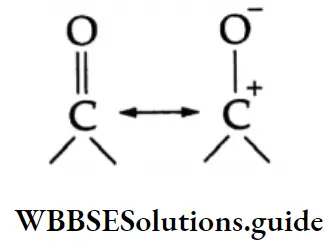
Due to bond polarity, carbonyl compounds have substantial dipole moments.
General Methods Of Preparation Of Aldehydes And Ketones
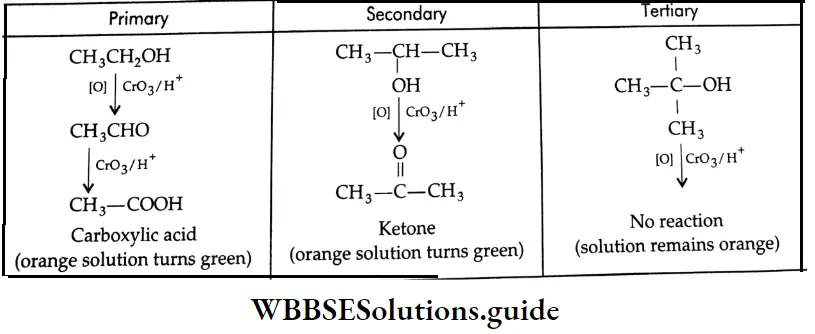
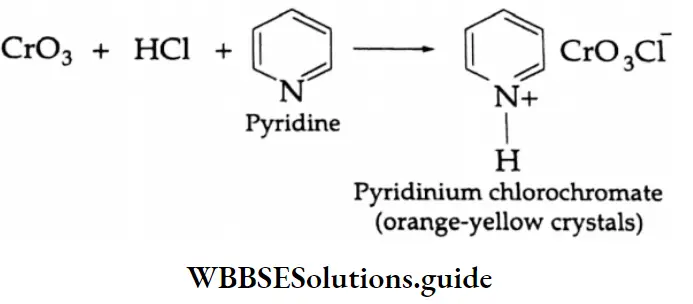
By the oxidation of alcohol
Aldehydes Primary alcohols yield aldehydes upon oxidation with pyridinium chlorochromate (PCC) in a CH2 Cl2 medium. Aqueous methods like Jones oxidation (Na2Cr2O7 and dilute H2SO4 in acetone) are not useful for this purpose since the aldehyde that is formed is further oxidised to a carboxylic acid.
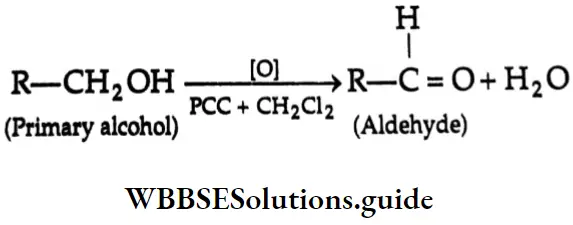
Ketones Secondary alcohols yield ketones upon oxidation with K2Cr2O7/H2SO4 in an acetone medium.
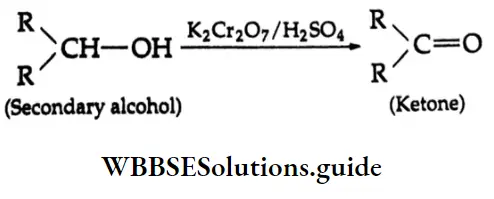
Tertiary alcohols are stable to oxidation under these conditions.
Oppenauer oxidation
Oppenauer oxidation involves the oxidation of primary alcohols and secondary alcohols to aldehydes and ketones respectively in the presence of aluminium isopropoxide, Al[OCH(CH3)2]3

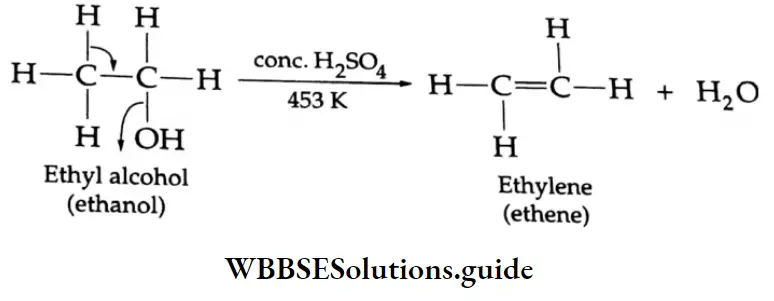
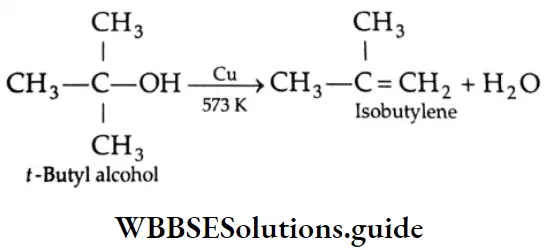
By the dehydrogenation of alcohol
When the vapours of a primary alcohol are passed over hot copper at about 573 K, the alcohol is readily dehydrogenated to yield an aldehyde.
⇒ \(\underset{\text { (Primary alcohol) }}{\mathrm{R}-\mathrm{CH}_2 \mathrm{OH}} \underset{573 \mathrm{~K}}{\stackrel{\mathrm{Cu}}{\longrightarrow}} \underset{\text { (Aldehyde) }}{\mathrm{R}}-\mathrm{CHO}+\mathrm{H}_2\)
Aldehydes, ketones, and carboxylic acids class 12 chemistry notes
On dehydrogenation, secondary alcohols give ketones.
⇒ \(\underset{\text { (Secondary alcohol) }}{\mathrm{R}-\mathrm{CHOH}-\mathrm{R}^{\prime}} \underset{573 \mathrm{~K}}{\stackrel{\mathrm{Cu}}{\longrightarrow}} \underset{\text { (Ketone) }}{\mathrm{R}}-\mathrm{CO}-\mathrm{R}^{\prime}+\mathrm{H}_2\)
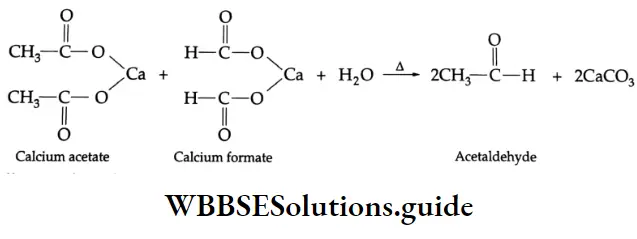
By the pyrolysis of calcium salts of carboxylic acids
When the calcium salts of carboxylic acids are heated to high temperatures, symmetrical ketones are obtained.
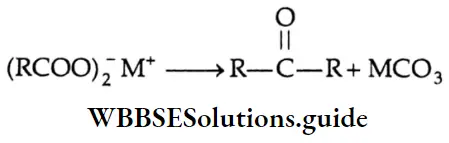
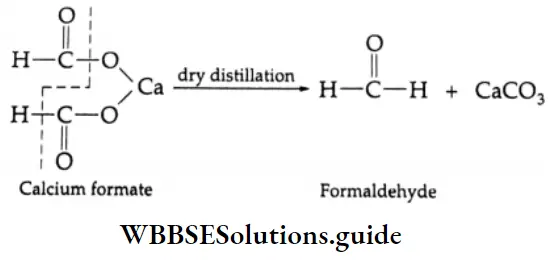
Formation of an aldehyde: On dry distillation, calcium formate gives formaldehyde.
On dry distillation, a mixture of calcium acetate and calcium formate yields acetaldehyde.
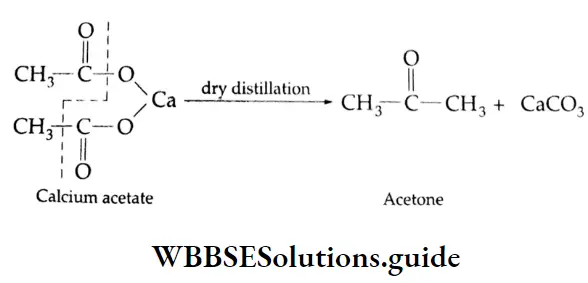
Formation of a ketone: On dry distillation, the calcium salt of a carboxylic acid, other than formic acid, gives a ketone. For example, in dry distillation, calcium acetate gives acetone.
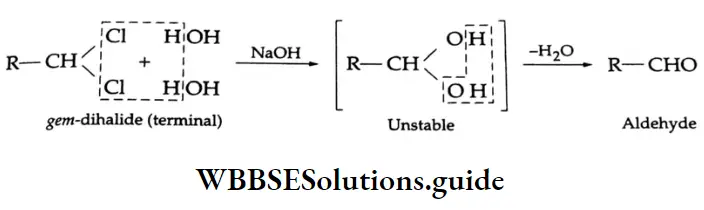
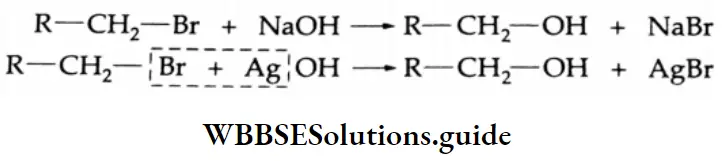
By the hydrolysis of gem-dihalides (1, 1-dihalides)
Formation of an aldehyde Boiling a gem-dihalide with aqueous NaOH gives a dihydroxy compound. Being unstable, this compound loses a water molecule readily to form an aldehyde.
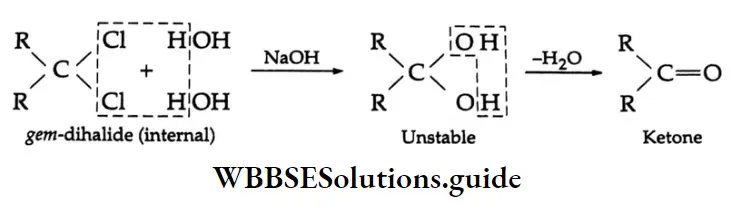
Formation of a ketone If the halo groups are not present on the terminal carbon atom, i.e., the gem-dihalide is an internal dihalide, then a ketone is obtained.
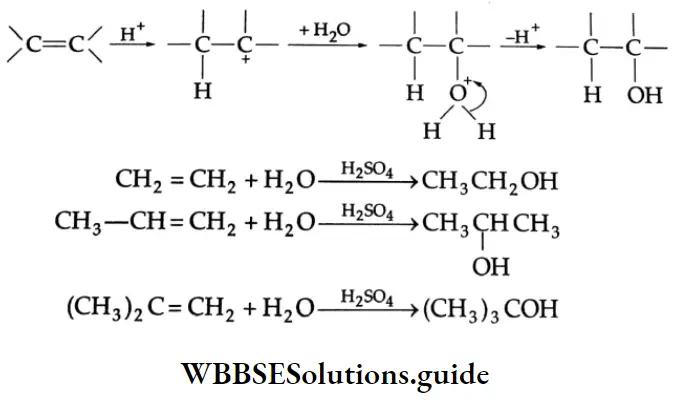
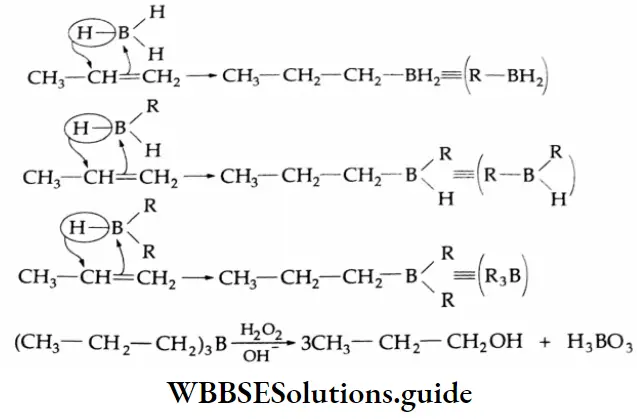
By the hydration of alkynes
The hydration of alkynes with 20% H2SO4 in the presence of a mercuric salt (HgSO4) gives aldehydes or ketones. Formation of an aldehyde On hydration with 20% H2SO4 in the presence of HgSO4 at 353 K, acetylene gives an unstable enol, which soon isomerises to acetaldehyde.
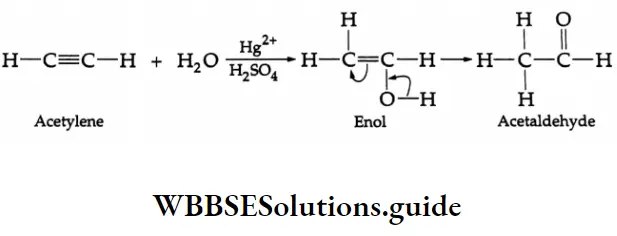
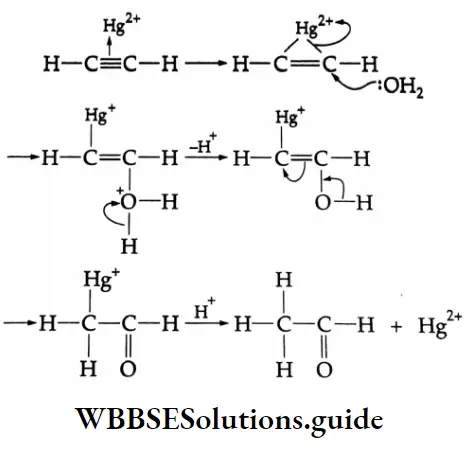
Hydration of alkynes Mechanism:
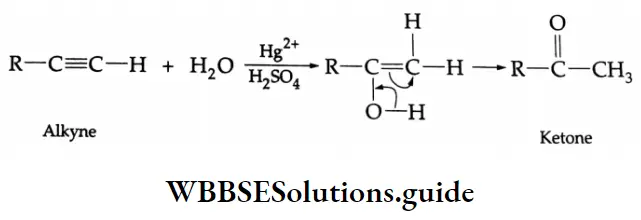
Formation of ketones: On hydration with 20% H2SO4 in the presence of HgSO4 (catalyst) at 353 K, substituted alkynes give ketones.
By ozonolysis
Depending on the structure of an alkene, aldehydes and ketones are obtained by making the alkenes react with ozone and subsequent treatment with zinc dust and water.
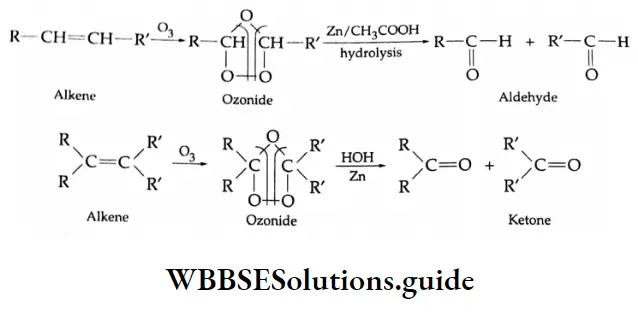
The ozonide may also react with oxidising agents such as H2O2 to give carboxylic acids or with more powerful reducing agents such as NaBH, to give alcohols.
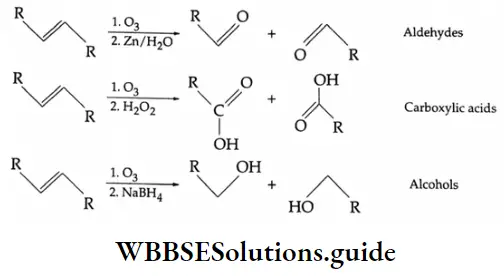
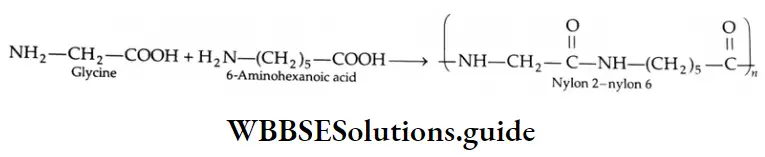
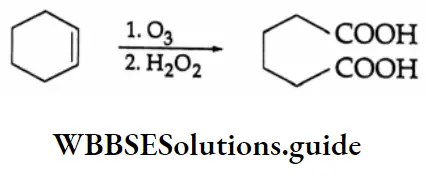
The ozonolysis of cyclohexene is particularly useful as it gives 1, 6-dicarbonyl compounds that are otherwise difficult to make. In the simplest case we get hexane-1, 6-dioic acid (adipic acid), a monomer used in the manufacture of nylon.
Aldehydes And Ketones
By the reduction of acid chlorides
Aldehydes Acid chlorides are reduced with hydrogen gas in the presence of palladised BaSO4 or BaCO3 (Rosenmund reduction) to aldehydes. Ketones are not prepared by this method.
⇒ \(\underset{\text { (Acid chloride) }}{\mathrm{R}-\mathrm{COCl}}+2 \mathrm{H} \stackrel{\mathrm{Pd} / \mathrm{BaSO}_4}{\longrightarrow} \underset{\text { (Aldehyde) }}{\mathrm{R}-\mathrm{CHO}+\mathrm{HCl}}\)
“Physical and chemical properties of aldehydes, ketones, and carboxylic acids”
⇒ \(\underset{\text { Acetyl chloride }}{\mathrm{CH}_3-\mathrm{COCl}}+2 \mathrm{H} \stackrel{\mathrm{Pd} / \mathrm{BaSO}_4}{\longrightarrow} \underset{\text { Acetaldehyde }}{\mathrm{CH}_3 \cdot \mathrm{CHO}}+\mathrm{HCl}\)
The function of BaSO4 is to poison the catalyst. This prevents the reduction of the aldehyde to an alcohol.
Ketones Acid chlorides react with dimethyl cadmium to yield ketones.
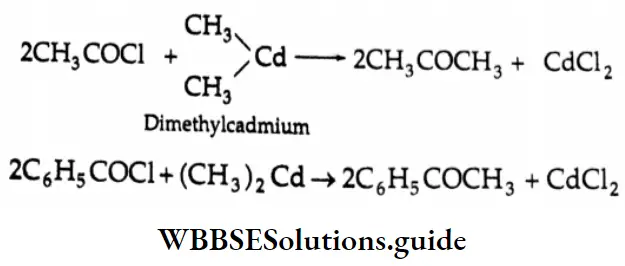
Grignard reagents, being more reactive than dimethyl cadmium, react with ketones to yield 3° alcohols. For this reason, Grignard reagents are not used in such reactions. Dimethyl cadmium does not react with ketones. Dimethyl cadmium can be prepared by making a Grignard reagent react with cadmium chloride.
⇒\(\underset{\text { Methylmagnesium bromide }}{2 \mathrm{CH}_3 \mathrm{MgBr}}+\mathrm{CdCl}_2 \rightarrow \underset{\text { Dimethylcadmium }}{\left(\mathrm{CH}_3\right)_2 \mathrm{Cd}}+2 \mathrm{MgBrCl}]\)
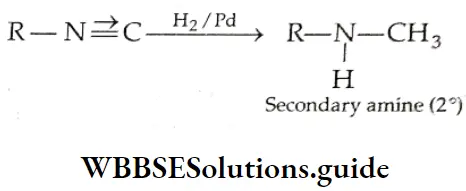
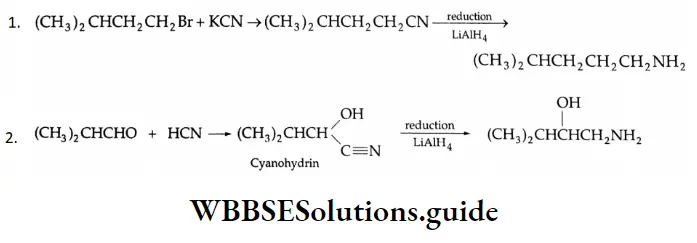
From alkyl cyanides
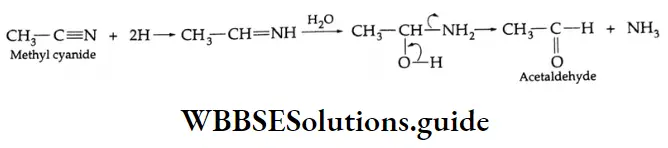
Aldehydes On reduction by stannous chloride and concentrated HCl, an alkyl cyanide (also called an alkyl nitrile) yields an imino chloride, which on hydrolysis with water yields an aldehyde. This reaction is known as the Stephen reaction.
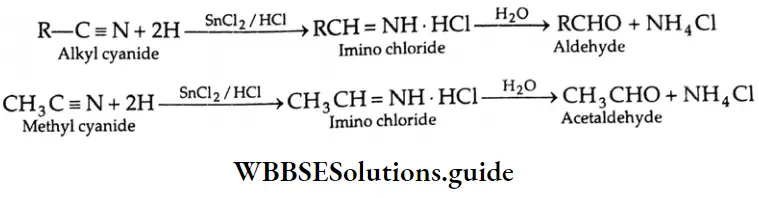
Alkyl cyanides Mechanism:
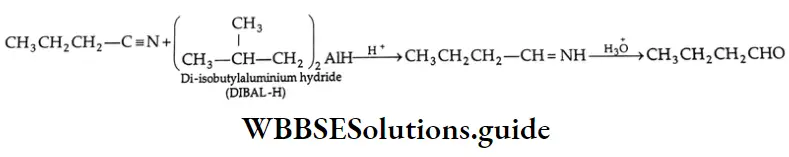
Nitriles may also be selectively reduced by di-isobutylaluminium hydride (DIBAL-H) to imines, which upon hydrolysis give aldehydes.
DIBAL-H is sterically congested and therefore not very reactive. For this reason, it does not reduce the Di isobutyl aluminium hydride (DIBAL-H).
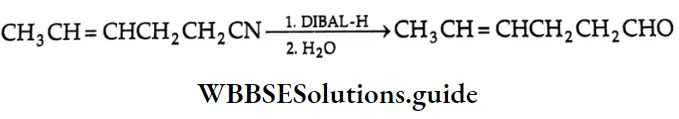
Ethylenic bond of unsaturated nitriles.
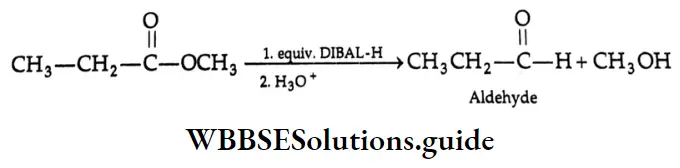
The reaction of an ester with exactly 1 equivalent of DIBAL-H at low temperature gives an aldehyde.
Ketones: On treatment with Grignard reagents, alkyl cyanides give ketones.
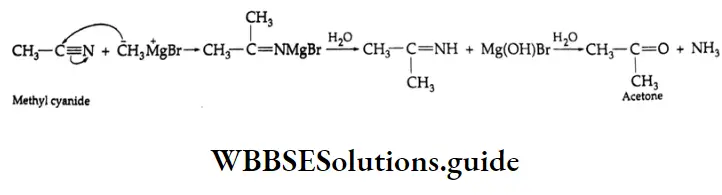
Physical Properties Of Aldehydes And Ketones
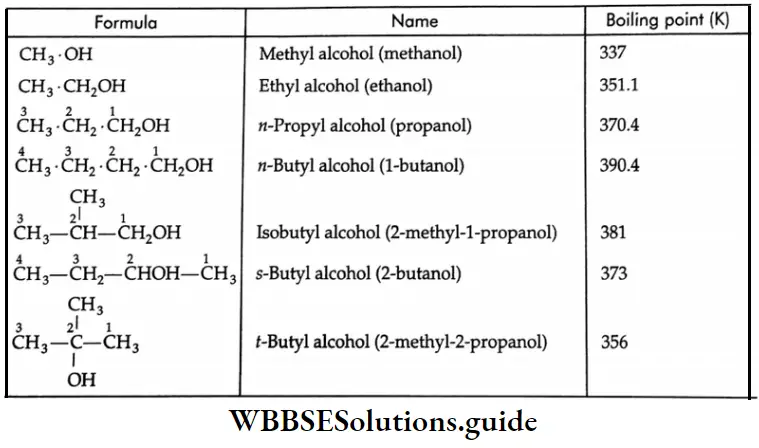
Formaldehyde is a gas while aldehydes or ketones containing up to 11 carbon atoms are colourless liquid Aldehydes and ketones containing more carbon atoms are solids.
Aldehydes and ketones are polar in nature. Their boiling points are higher than those of alkanes of similar molecular masses due to dipole-dipole interaction.
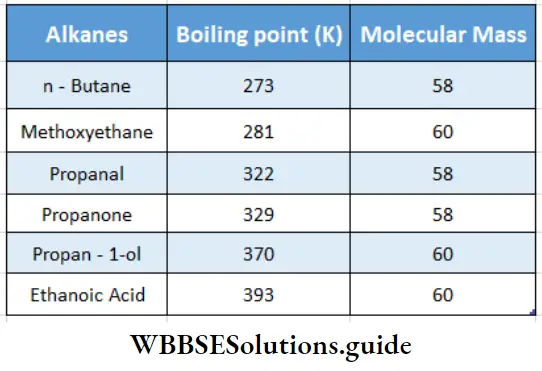
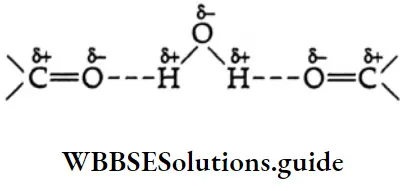
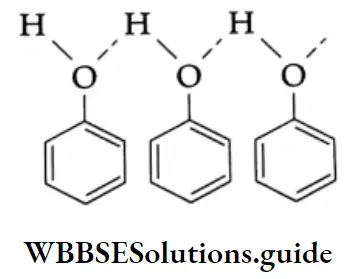
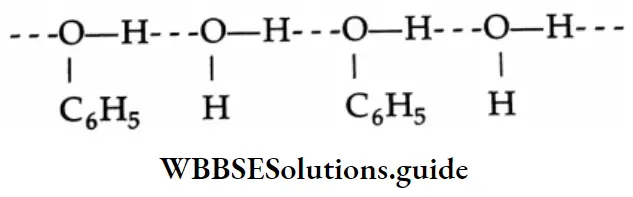
Because aldehydes and ketones are not associated with hydrogen bonds, their boiling points are lower than those of alcohols and carboxylic acids of comparable molecular weights.
The first few members of aliphatic aldehydes and ketones are soluble in water. For example, formaldehyde, acetaldehyde and acetone are soluble in water in all proportions. As the length of the alkyl group chain increases, the solubility of the compound decreases as in alcohols.
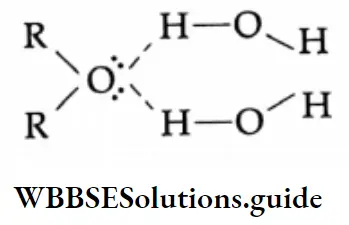
The solubility of lower aldehydes and ketones in water arises from the ability of the oxygen of the carbonyl group to form hydrogen bonds with water molecules.
All aldehydes and ketones are soluble to quite an extent in organic solvents like benzene and methyl alcohol. Formaldehyde and acetaldehyde have an unpleasant odour but higher aldehydes have a fruity smell.
Organic chemistry aldehydes, ketones, and carboxylic acids notes
Some of the ketones have a sweet smell. Many naturally occurring aldehydes and ketones are used as additives in perfumes and as flavouring agents.
Chemical Properties Of Aldehydes And Ketones
Aldehydes and ketones undergo similar chemical reactions because they contain the carbonyl functional group.
Nucleophilic addition reactions
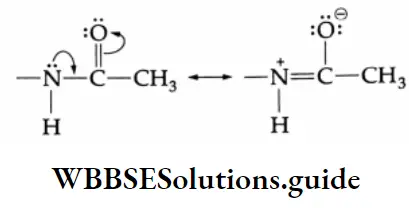
The carbonyl group is represented by the two contributing structures
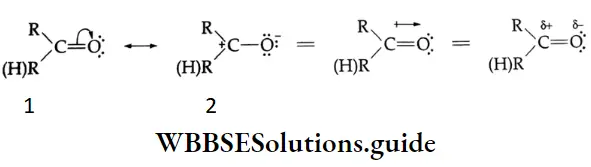
This imparts a substantial dipole moment to the carbonyl group, with the carbon bearing a partial positive harge and the oxygen bearing a partial negative charge.
The polar nature of the carbonyl group and the ability of the oxygen atom to accommodate the extra electron-air facilitates the attack of the nucleophile at the carbonyl carbon. The nucleophile normally adds to the carbony
“Methods of preparation of aldehydes, ketones, and carboxylic acids”
Carbon from a direction approximately perpendicular to the plane of the sp2-hybridised orbitals of the carbonyl carbon. In this process, the hybridisation of the carbonyl carbon changes from sp’ to sp and a tetrahedral alkoxide anion intermediate is formed. The reaction is completed by the abstraction of a proton from the reaction medium to give the addition product.
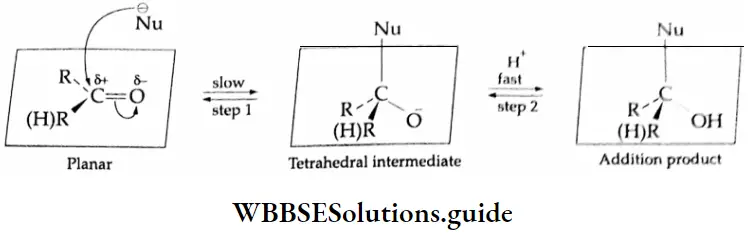
Reactivity of aldehydes and ketones
Aldehydes are more reactive than ketones because of two factors.
Inductive effect The carbonyl carbon atom of a ketone carries two electron-donating groups, whereas that of an aldehyde has only one. Thus the ketone carbonyl carbon atom has less tendency to attract a nucleophile.
Steric effect Bulky groups adjacent to >C=O cause more steric strain in the addition product than in the parent carbonyl and reduce reactivity towards addition.
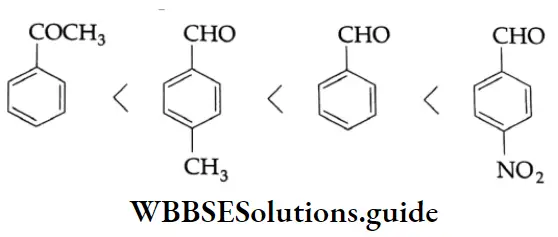
The order of reactivity of different carbonyl compounds towards nucleophilic addition is given below.
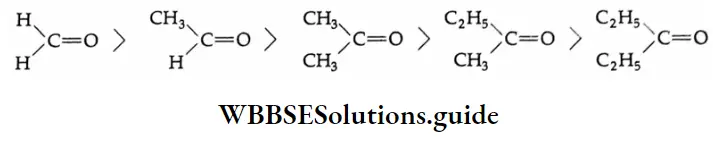
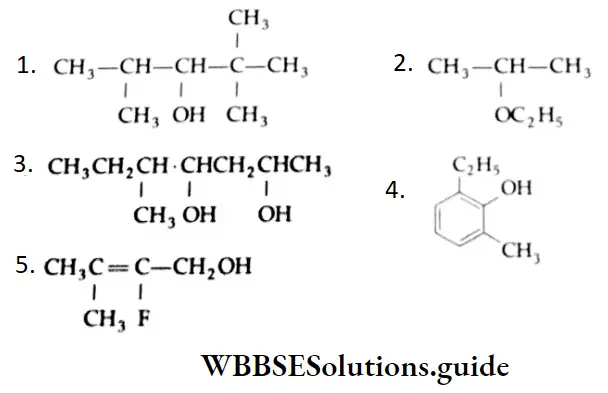
Example 2: Arrange the following carbonyl compounds in increasing order of their reactivity in nucleophilic addition reactions. Give reasons.
1. CH3CHO, CH3CH2CHO, CH3COCH3, CH3COCH2CH3
2.
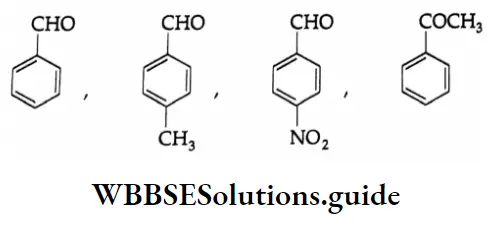
Solution:
1. The reactivity of aldehydes and ketones towards a nucleophile is influenced by the nature of the alkyl group attached to the carbonyl carbon in the following two ways.
- First, an electron-donating alkyl group reduces the positive charge of the carbonyl carbon and makes it less susceptible to nucleophilic attack.
- Secondly, the bulkier alkyl group attached to the carbonyl carbon presents greater steric hindrance than the smaller hydrogen atom to the approaching nucleophile.
From the above considerations, the increasing reactivity of the given compounds towards the nucleophile is as follows.
⇒ CH3COCH2CH3<CH3COCH3<CH3CH2CHO<CH3CHO
2. Similar influences are exerted by an aryl substituent as delocalised orbitals of the ring act as an electron source.
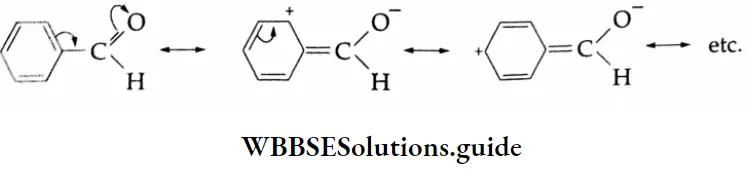
In the case of p-nitrobenzaldehyde, the electron-withdrawing nitro group at the p-position increases the positive character of the carbonyl carbon and thus facilitates the attack of nucleophiles.
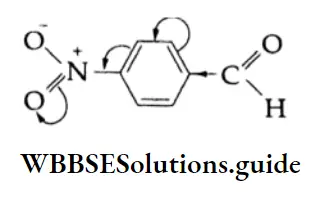
In p-tolualdehyde, the methyl group at the p-position increases the electron density on the carbon of the carbonyl group by hyperconjugation as shown and makes it less reactive than benzaldehyde towards nucleophilic addition.
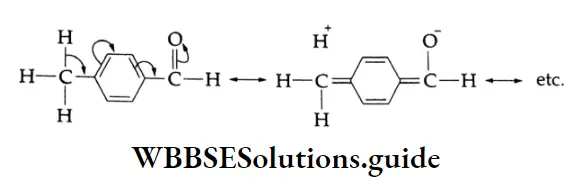
Acetophenone (a ketone) is less reactive than all the given aldehydes. The electron-donating methyl group and л-orbital of the benzene ring, acting as an electron source, make the carbonyl carbon electron-rich and less susceptible to nucleophilic attack. Further, the phenyl group and methyl group attached to the carbonyl carbon present greater steric hindrance to the approaching nucleophile.
Ketone Functional Group
On the basis of the above considerations, the order of increasing reactivity of the given compounds towards nucleophiles is as follows.
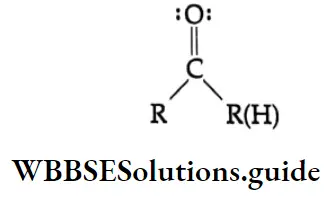
Ketones and aldehydes undergo very similar reactions. In this chapter, unless mentioned otherwise, each reaction discussed is equally applicable to both aldehydes and ketones and a generic structure is used for both.
Examples of nucleophilic addition reactions
Aldehydes and ketones undergo the following nucleophilic addition reactions.
Reaction with hydrocyanic acid (HCN) HCN adds to aldehydes and ketones to produce cyanohydrins.
“Reactivity of aldehydes and ketones in organic reactions”
The reaction is slow with pure HCN. However, in the presence of a base, CN (a stronger nucleophile) is generated, which readily adds to carbonyl compounds to yield the corresponding cyanohydrin.
⇒ \(\mathrm{HCN}+\mathrm{OH}^{-} \rightarrow: \overline{\mathrm{CN}}+\mathrm{H}_2 \mathrm{O}\)
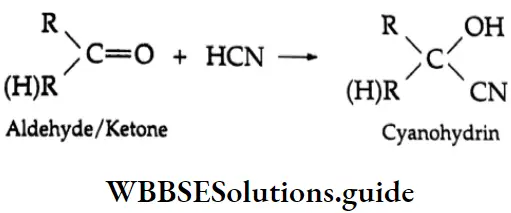
Nucleophilic Mechanism:
The cyanide anion, the nucleophile, adds to the carbonyl carbon to yield an unstable intermediate oxygen anion, which, being a strong base, abstracts a proton from the solvent or HCN to give cyanohydrin.
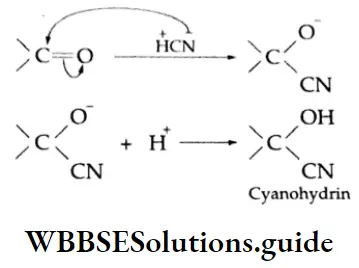
Some plants produce cyanohydrins. The seeds of cherries, plums, peaches and apricots contain cyanohydrins. The odour and flavour of almonds is due to benzaldehyde and the cyanohydrin of benzaldehyde.
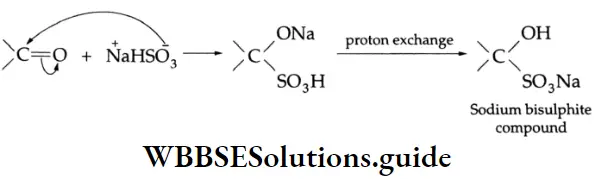
Addition of sodium bisulphite (NaHSO4)
Aldehydes and ketones react with an aqueous, saturated solution of sodium bisulphite to yield a bisulphite product. This product is sparingly soluble in water and can be separated by filtration and decomposed by mineral acids to give back the carbonyl compound. The method is often used for separating a carbonyl compound from a noncarbonyl impurity.
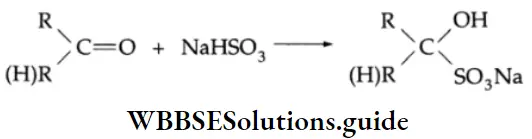
Sodium bisulphite Mechanism:
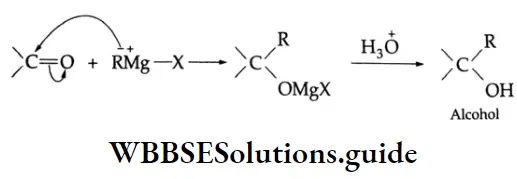
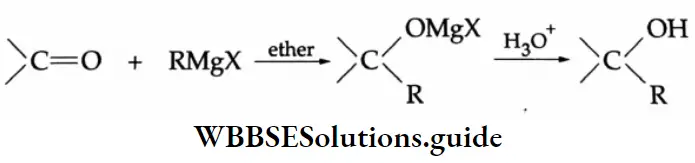
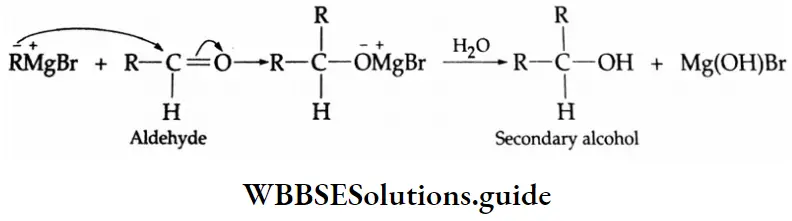
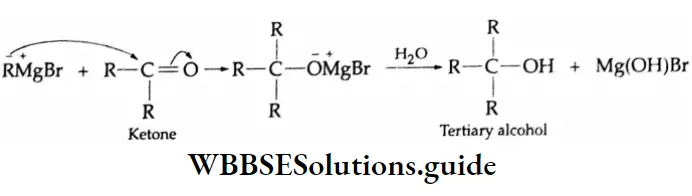
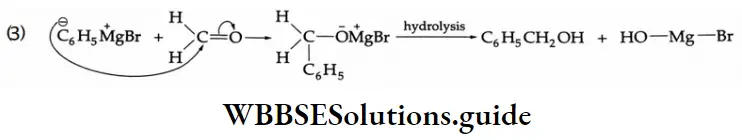
Addition of Grignard reagents
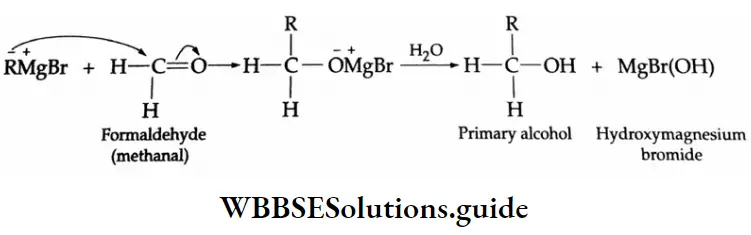
Aldehydes and ketones react with Grignard reagents to produce alcohols. Formaldehyde yields primary (1°) alcohols, other aldehydes give secondary (2°) alcohols and all ketones give tertiary (3°) alcohols.
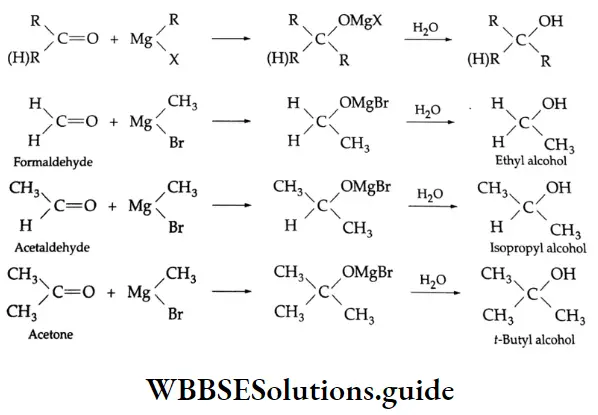
Grignard reagents Mechanism:
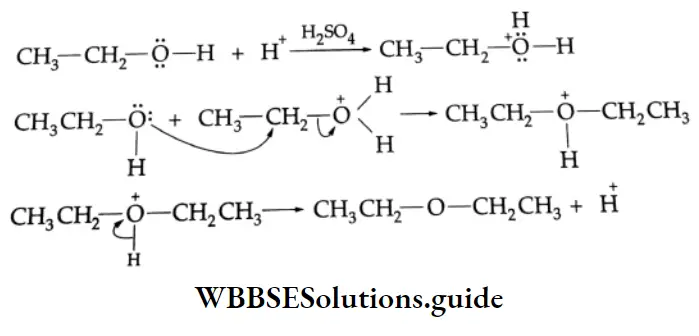
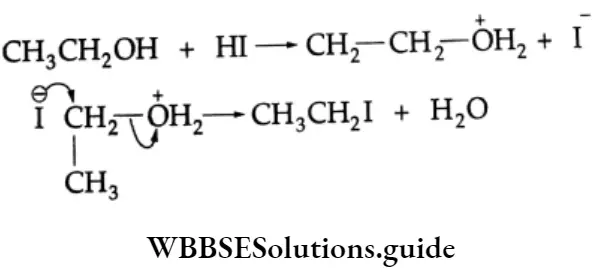
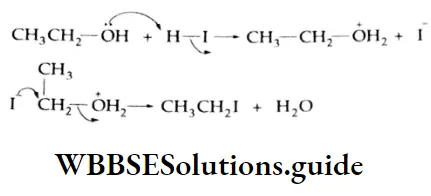
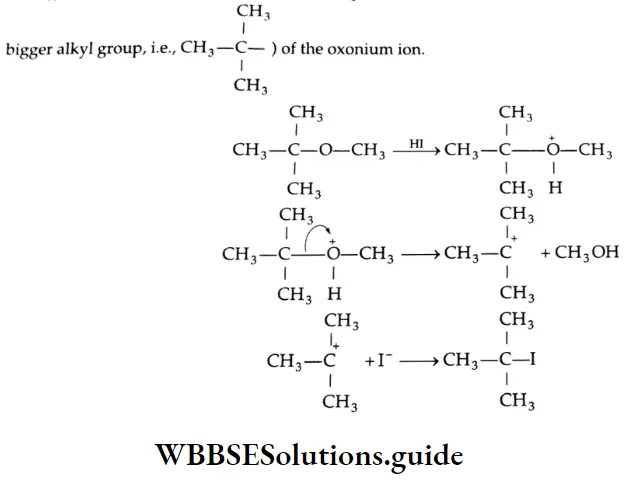
Addition of alcohol
In the presence of dry HCl gas, one mole of an aldehyde reacts with one mole of alcohol to give a hemiacetal (alkoxy alcohol), which again reacts with one mole of the alcohol to yield an acetal (gem-alkoxy compound).
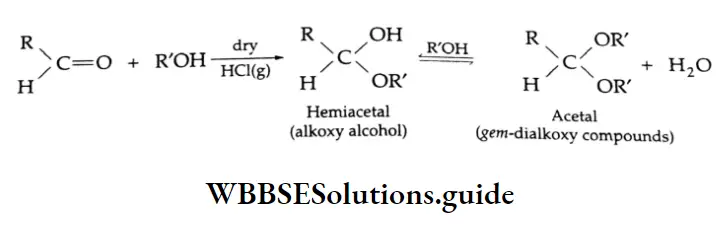
Alcohol Mechanism:
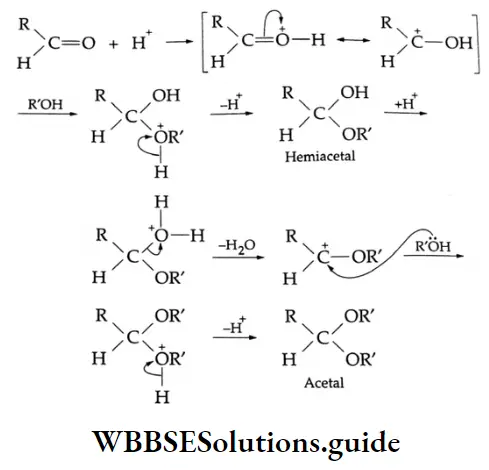
Ketones do not react with monohydric alcohols in the presence of dry HCl but react with ethylene glycol (a dihydric alcohol) to form cyclic ketals.
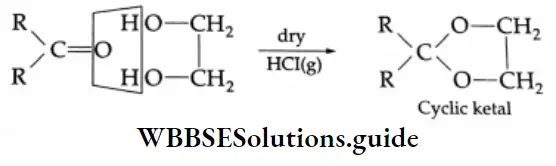
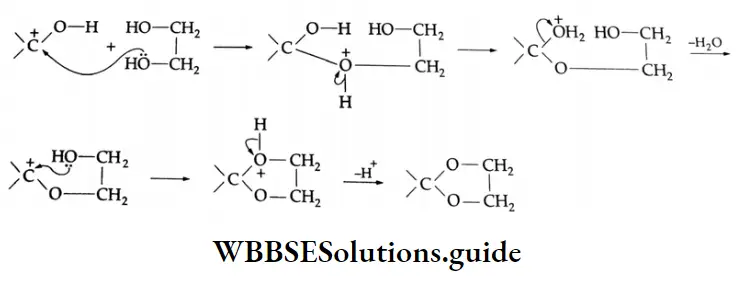
In the presence of dry HCl gas, the oxygen atom of a carbonyl compound is bonded to H*, leaving a stabilised \(\left(>\mathrm{C}=\mathrm{O}+\mathrm{H}^{+} \rightleftharpoons>\mathrm{C}=\stackrel{+}{\mathrm{O}}-\mathrm{H} \leftrightarrow \stackrel{+}{\mathrm{C}}-\mathrm{OH}\right)\) arbonium ion, which can react with ethylene glycol (a nucleophile) to yield a cyclic ketal.
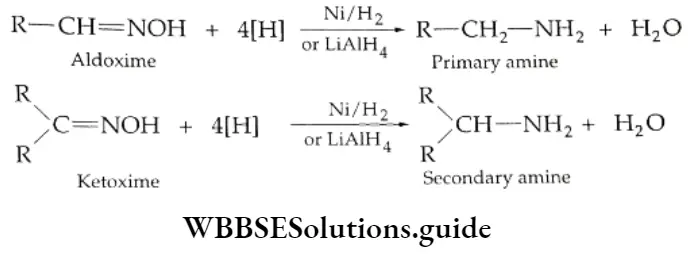
Reaction with ammonia derivative (NH2-Y):
Several derivatives of ammonia such as
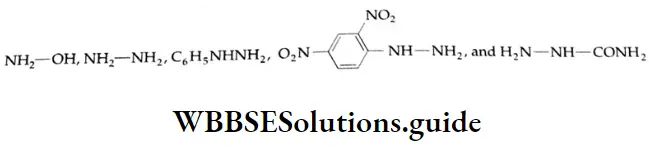
Can take part in addition reactions with aldehydes and ketones. The reaction is catalysed both by acids and alkalis. Under their influence, a molecule of water is eliminated, introducing a double bond between C and N. The products are crystalline, high-melting solids, very useful as derivatives for identification.
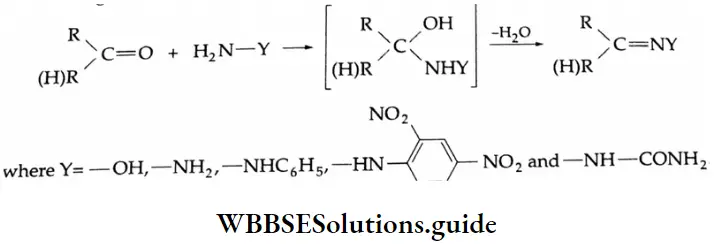
Ammonia derivative Mechanism:
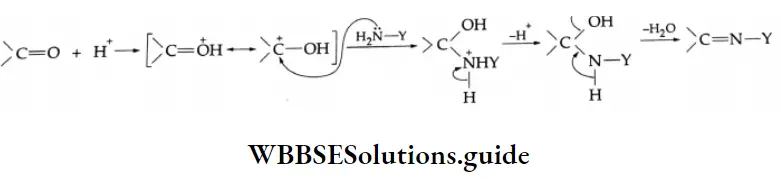
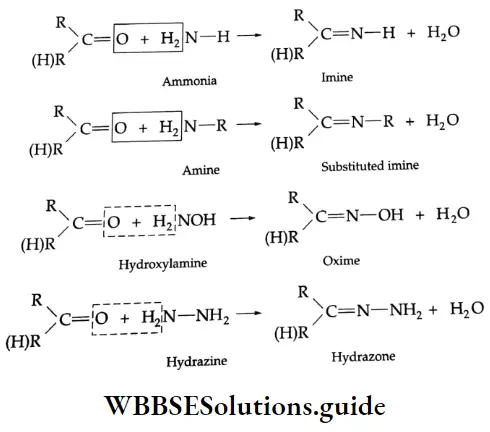
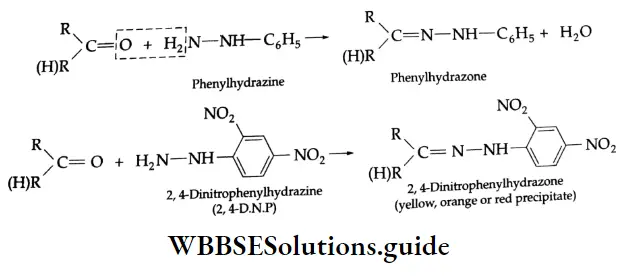
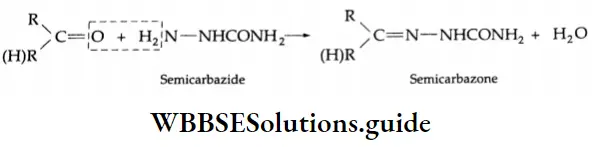
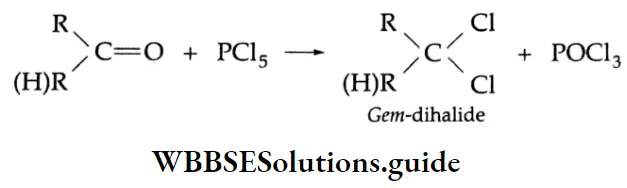
Reaction with PCI5
When aldehydes and ketones are treated with PCl5, the oxygen atom of the carbonyl group is substituted by two chlorine atoms and gem-dihalides are formed.
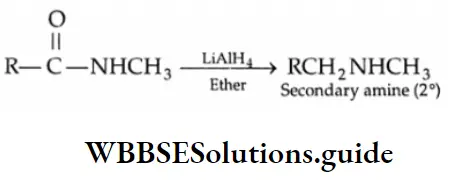
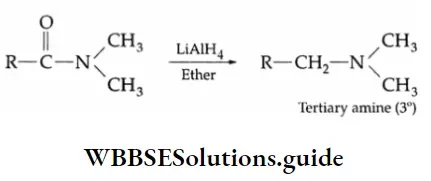
Reduction
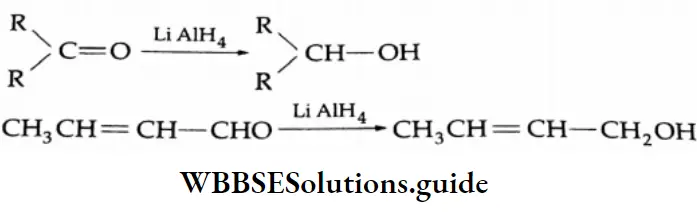
Reducing agents such as LiAlH4, NaBH4 and H2/Ni reduce aldehydes to primary alcohols and ketones to secondary alcohols.
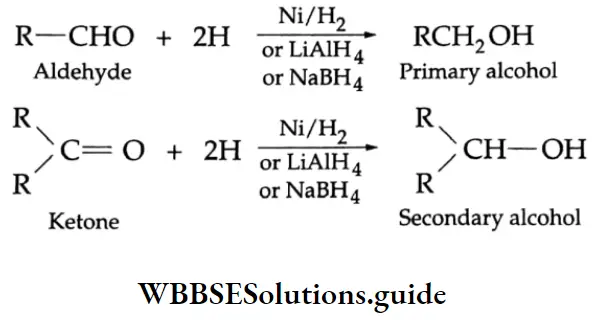
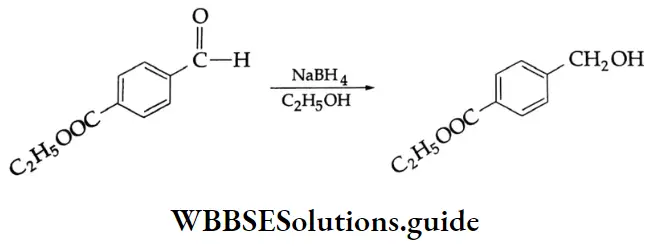
LiAlH4 is a versatile reducing agent. It reduces not only aldehydes and ketones but carboxylic acids, esters and nitriles as well. Sodium borohydride is a weak and selective reducing agent and reduces aldehydes and ketones only, but not carboxylic acids. NaBH4 is also unreactive towards C = C and C=C bonds.
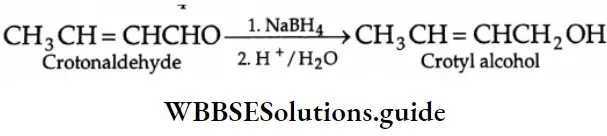
If a molecule contains both an aldehyde and an ester, only the aldehyde will be reduced by NaBH4.
Aldehydes and ketones may be reduced to hydrocarbons on treatment with Zn (Hg) and concentrated HCl. In this reaction, the >C=O group is converted into the >CH2 group. This reaction is known as Clemmensen reduction.
Best revision notes for aldehydes, ketones, and carboxylic acids
⇒ \(>\mathrm{C}=\mathrm{O}+4 \mathrm{H} \frac{\mathrm{Zn}-\mathrm{Hg}}{\mathrm{HCl}}>\mathrm{CH}_2+\mathrm{H}_2 \mathrm{O}\)
Aldehydes and ketones are also easily reduced to hydrocarbons in the presence of excess hydrazine and a strong base on heating. This reaction is known as Wolff-Kishner reduction.
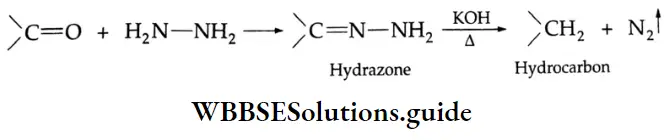
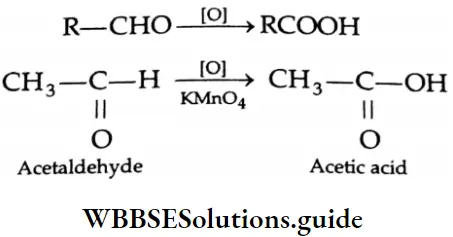
Oxidation of aldehydes
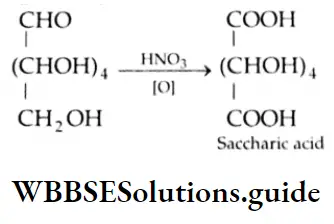
Aldehydes are among the most readily oxidised classes of organic compounds. They are converted to carboxylic acid by numerous oxidising agents such as KMnO4, K2Cr2O7 and HNO3
Aldehydes are also oxidised by relatively weak oxidising agents such as Tollens reagent and Fehling’s solution.
Oxidation by Tollens reagen:
When an aldehyde is warmed with ammoniacal silver nitrate (Tollens reagent), the aldehyde is oxidised to carboxylic acid and the silver ion is reduced to free silver, which deposits in the form of a mirror on the inner wall of the test tube.

Ordinary mirrors are prepared in this way, using formaldehyde.
Tollens reagent is prepared by adding one drop of an aqueous solution of NaOH to a silver nitrate solution (10 mL) and dissolving the resultant precipitate in a minimum quantity of ammonium hydroxide solution.
⇒ \(\mathrm{AgNO}_3+\mathrm{NaOH}+2 \mathrm{NH}_4 \mathrm{OH} \rightarrow \underset{\text { Tollens reagent }}{\mathrm{Ag}\left(\stackrel{+}{\mathrm{NH}_3}\right)_2 \overline{\mathrm{O}} \mathrm{H}}+\mathrm{NaNO}_3+2 \mathrm{H}_2 \mathrm{O}\)
Oxidation by Fehling’s solution:
When heated with Fehling’s solution, an aldehyde is oxidised to a carboxylic acid and the Fehling’s solution is reduced to cuprous oxide (Cu2O) as a brick-red precipitate.
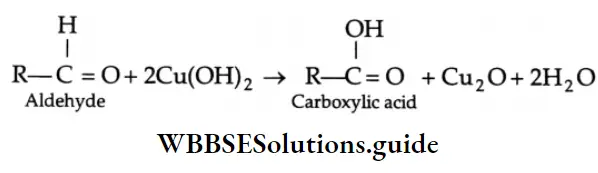
Fehling’s solution is a deep blue solution prepared by mixing equal volumes of Fehling’s solution (1) and Fehling’s solution (2). Fehling’s solution (1) is an aqueous copper sulphate solution. Fehling’s solution (2) is an alkaline solution of sodium potassium tartrate (Rochelle salt).
“Aldehydes and ketones nucleophilic addition reactions explained”
When Fehling’s solution (1) is mixed with Fehling’s solution (2), a deep blue soluble complex is formed, which reacts with an aldehyde to form the sodium salt of a carboxylic acid and a red-brown precipitate of Cu2O.
⇒ \(\mathrm{CuSO}_4+2 \mathrm{NaOH} \longrightarrow \mathrm{Cu}(\mathrm{OH})_2+\mathrm{Na}_2 \mathrm{SO}_4\)
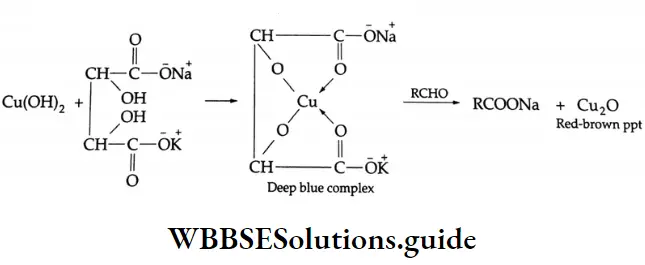
Fehling’s solution can oxidise aliphatic aldehydes only, while Tollens reagent can oxidise aliphatic as well as aromatic aldehydes. These reagents have no effect on ketones.
The aldehyde group undergoes aerial oxidation. For example, crystals of benzoic acid grow inside a bottle filled with benzaldehyde in the presence of sunlight.
Oxidation by Benedict’s reagent:
Benedict’s reagent is an alkaline solution of copper sulphate, sodium carbonate and sodium citrate. On heating with Benedict’s reagent, aliphatic aldehydes yield a red-brown precipitate of Cu2O
Using Benedict’s reagent we can detect the presence of sugar in urine.
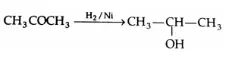
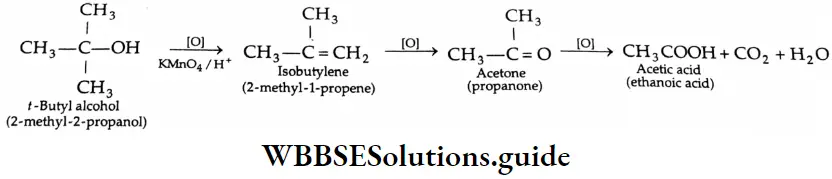
Oxidation of ketones
The oxidation of ketones requires stronger oxidising agents such as permanganate and high temperatures. It involves the cleavage of a carbon-carbon bond adjacent to the carbonyl group, and different carboxylic acids are formed.
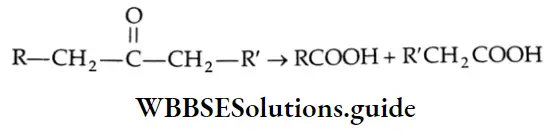
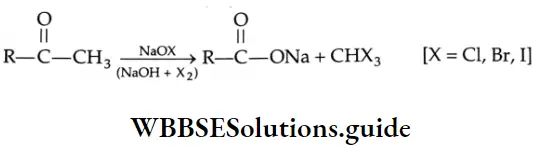
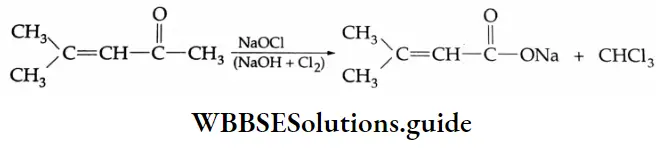
Oxidation of methyl ketones (haloform reaction) Aldehydes and ketones containing a keto methyl group 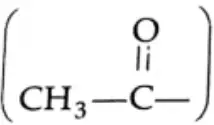 are oxidised by sodium hypohalite (NaOX). Sodium salts of carboxylic acids containing one carbon less than the methyl ketone are formed. The methyl group forms a haloform.
are oxidised by sodium hypohalite (NaOX). Sodium salts of carboxylic acids containing one carbon less than the methyl ketone are formed. The methyl group forms a haloform.
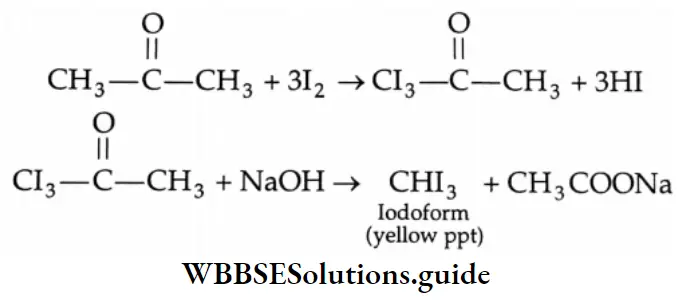
For example, acetone is oxidised by I2/NaOH to give iodoform. The three hydrogens in the methyl group are acidic and are readily displaced by the halogen to yield a triiodo compound which is cleaved under the influence of the alkali to yield iodoform and the corresponding carboxylic acid.
Iodoform is a pale yellow solid and its appearance indicates the presence of a methyl ketone.
Methyl ketones give the corresponding chloroform or bromoform with hypochlorite or hypobromite and so this reaction is known as a haloform reaction.
The hypohalite does not attack a double bond, if present in the molecule.
Compounds containing the methylcarbinol 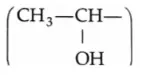 group also respond positively to the iodoform test.
group also respond positively to the iodoform test.
Thus, even ethyl alcohol responds positively to the iodoform test.
Reactions due to α-hydrogen
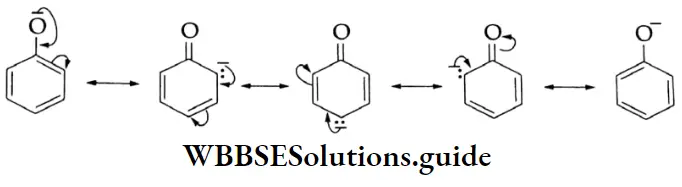
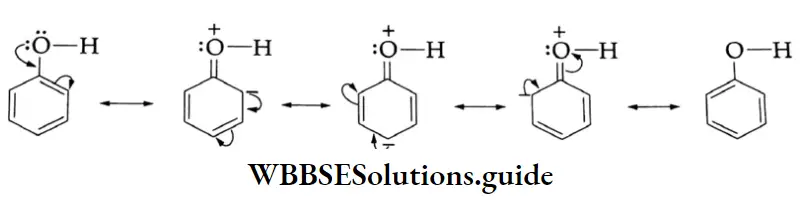
A carbon atom located next to a carbonyl carbon is known as an a-carbon and hydrogens attached to an a-carbon are called a-hydrogens. These hydrogens are acidic due to the electron-withdrawing nature of the carbonyl group and resonance stabilisation of the conjugate base.
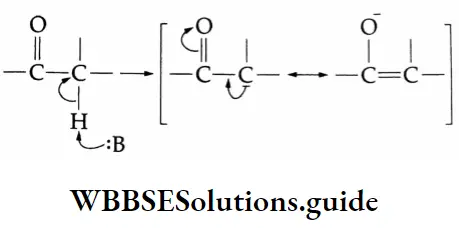
Aldol condensation:
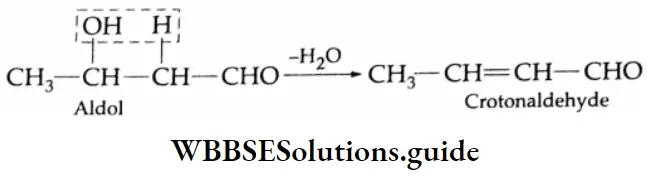
Two molecules of an aldehyde or a ketone containing a-hydrogen undergo condensation in the presence of a dilute alkali to give a β-hydroxy aldehyde (aldol) or a β-hydroxy ketone (ketol). These reactions are called aldol condensation reactions.
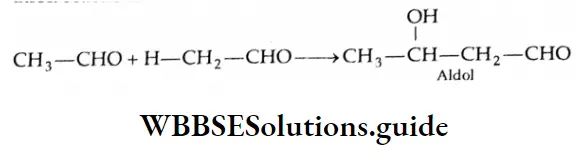
Aldols (aldehyde-alcohols) can be dehydrated easily by heating to yield α, and β unsaturated aldehydes. For example, on heating, β-hydroxybutyraldehyde yields crotonaldehyde.
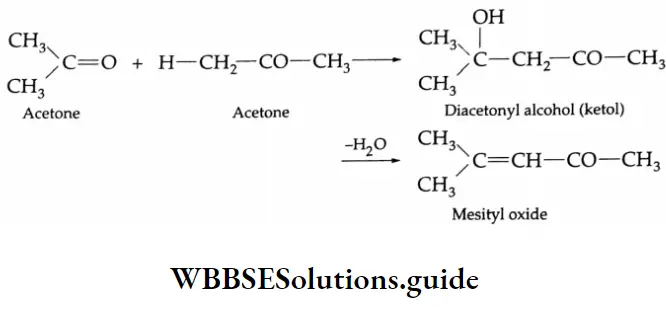
In the presence of Ba(OH)2, two molecules of acetone condense to yield diacetonyl alcohol.
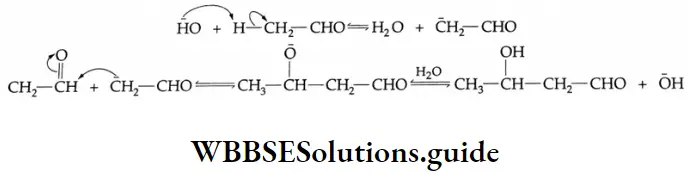
Aldol Condensation Mechanism:
Cross-aldol condensation:
An aldol condensation between two different carbonyl compounds, each containing a-hydrogen, gives a mixture of four products. This reaction is called a cross-aldol condensation.
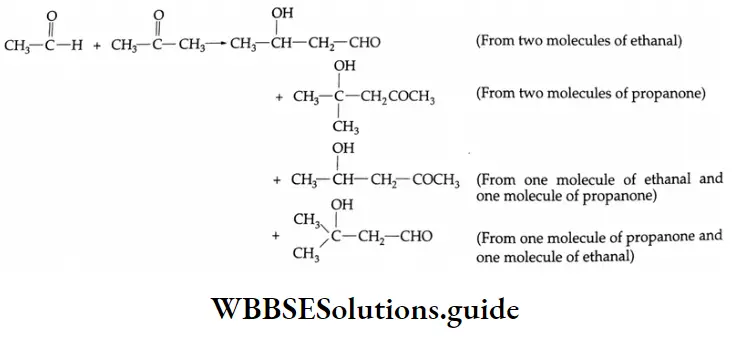
It is generally difficult to separate a mixture of four products. A cross-aldol condensation yielding a pure product can, however, be obtained when one of the reactants has no α -hydrogen (for example, benzaldehyde, or maldehyde).
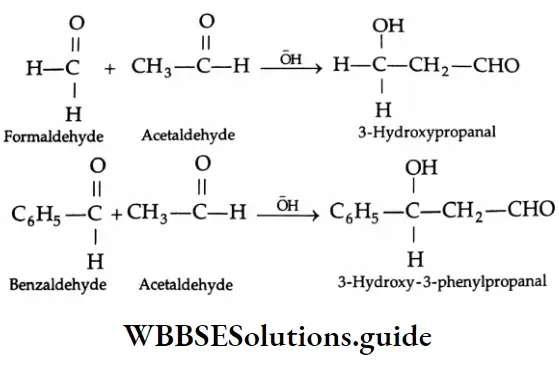
Other reactions
Cannizzaro reaction:
When an aldehyde that has no a-hydrogens is treated with a concentrated aqueous alkali, a disproportionation reaction occurs. One molecule of the aldehyde is reduced to a primary alcohol, and another molecule is oxidised to the corresponding carboxylic acid salt.
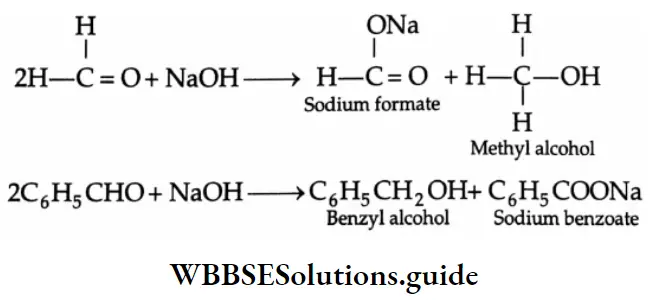
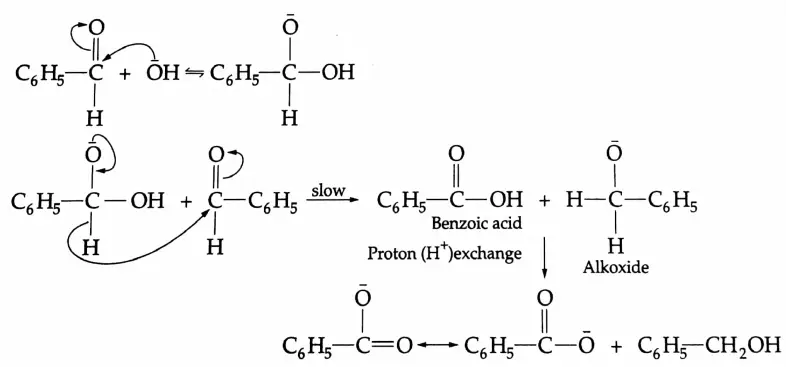
This reaction is known as the Cannizzaro reaction.
Cannizzaro reaction Mechanism:
Crossed Cannizzaro reaction:
When an aldehyde that has no a-hydrogens is treated with formaldehyde and a strong base, it is the formaldehyde rather than the other aldehyde that is oxidised. Such a reaction is known as a crossed Cannizzaro reaction. For example,
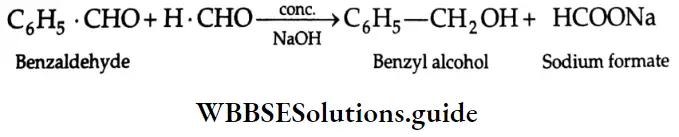
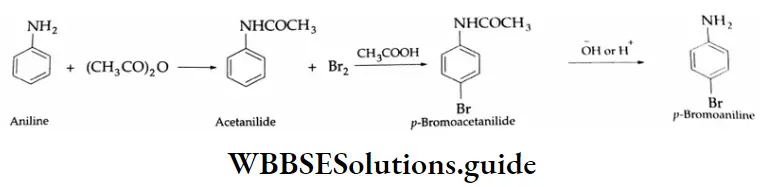
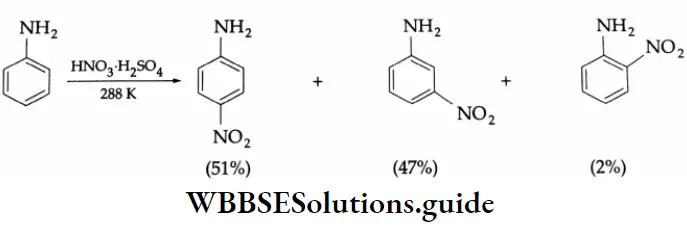
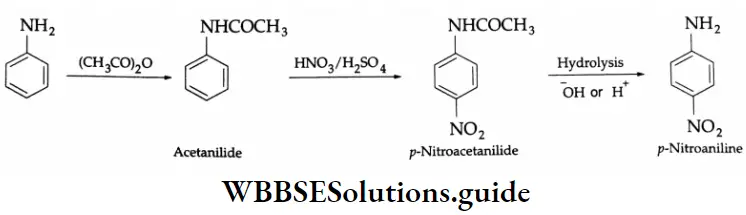
Aromatic Aldehydes
Methods of preparation:
Aromatic aldehydes and ketones are prepared by the following methods.
Aromatic aldehydes
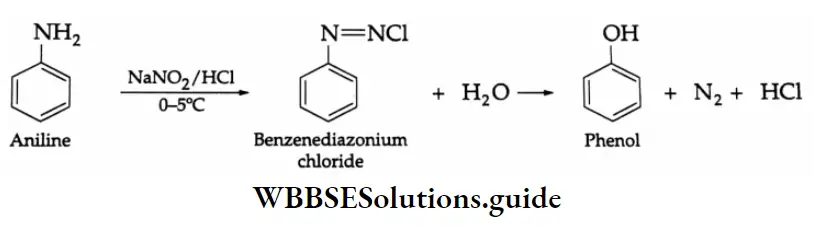
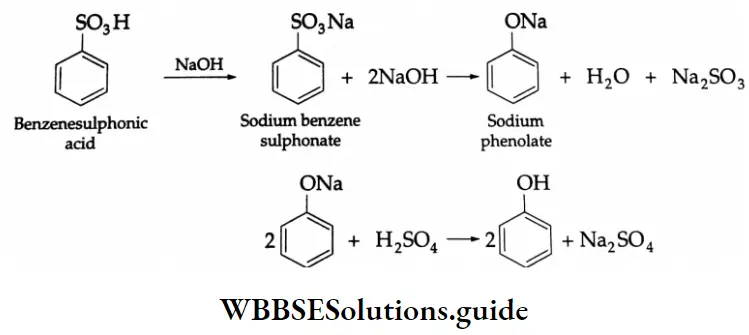
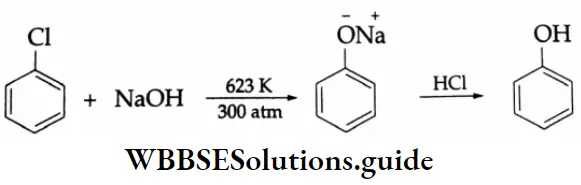
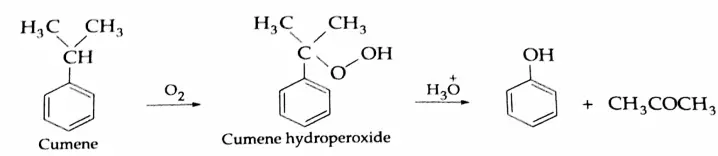
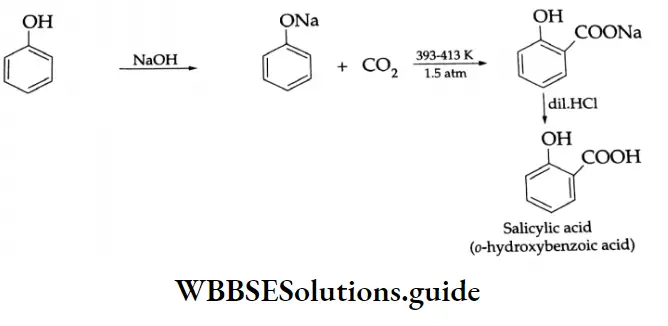
By the oxidation of toluene (Etard reaction), The partial oxidation of toluene by chromyl chloride (CrO2 Cl2) gives a chromium complex, which on hydrolysis yields benzaldehyde.
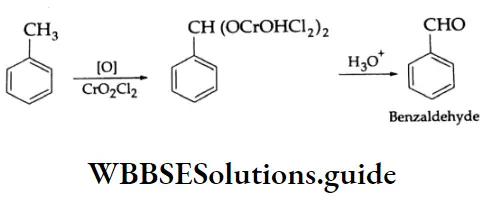
This reaction is known as the Etard reaction. If a strong oxidising agent is used, toluene is oxidised to benzoic acid.
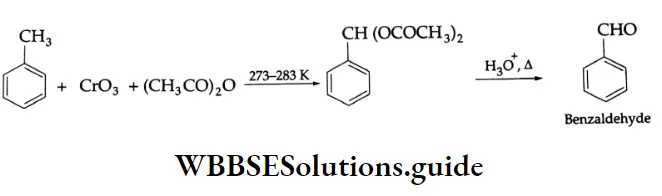
Upon treatment with chromic oxide in acetic anhydride, toluene gives benzylidene diacetate. On hydrolysis with a dilute acid, Benzylidene diacetate yields benzaldehyde.
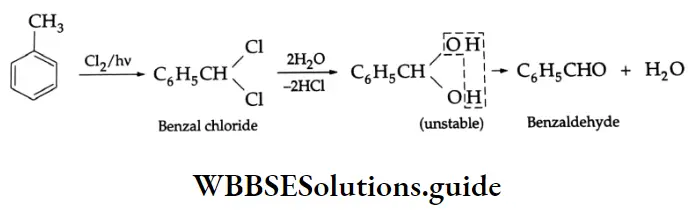
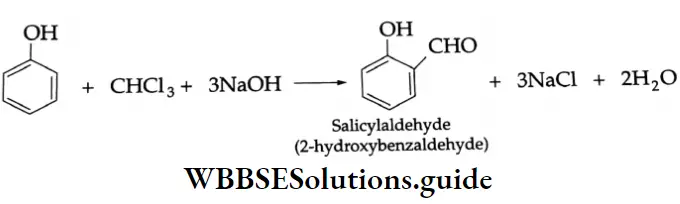
By the hydrolysis of benzal chloride:
On hydrolysis, benzal chloride yields benzaldehyde. Hydrolysis is done by using an aqueous NaOH solution. Benzal chloride is prepared by the chlorination of toluene in the presence of sunlight.
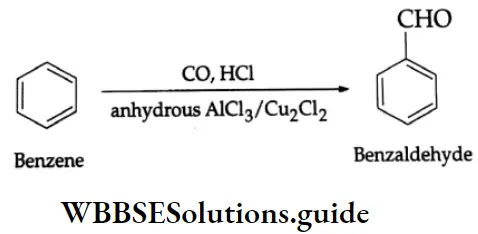
By the Gattermann-Koch synthesis:
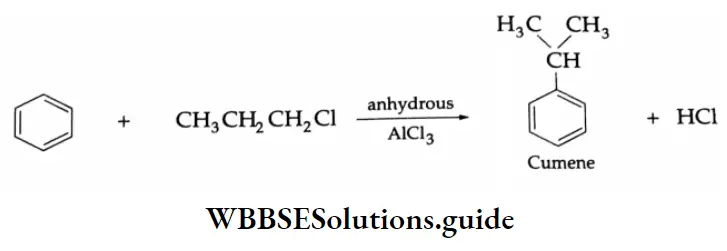
Benzaldehyde is prepared by treating benzene or its derivative with CO+ HCl in the presence of anhydrous AlCl3 and Cu2Cl2
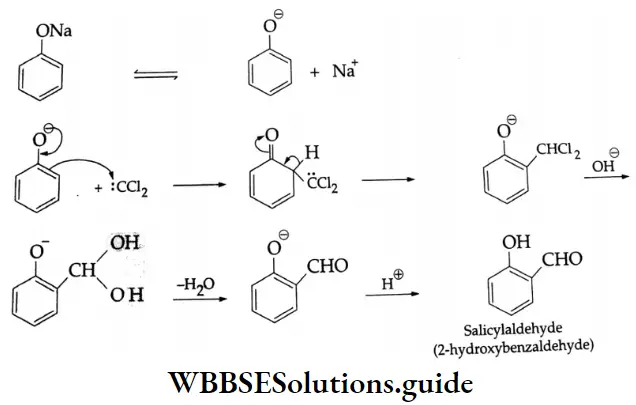
Gattermann-Koch synthesis Mechanism:
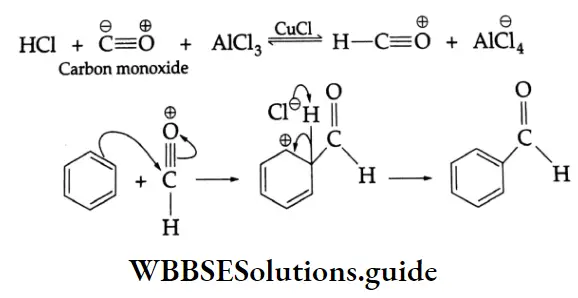
Aromatic ketones
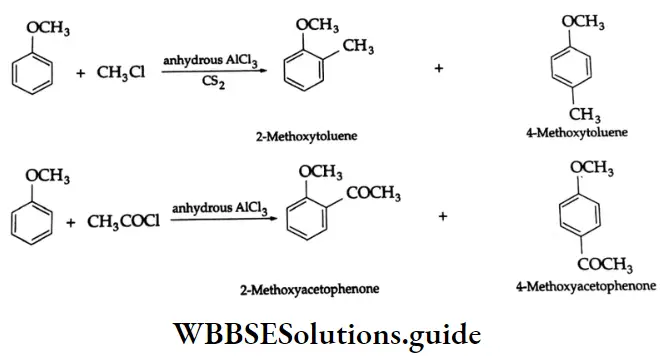
By the acylation of benzene:
Aromatic hydrocarbons react with acyl chloride in the presence of anhydrous AlCl3 to yield aromatic ketones. For example, on treatment with acetyl chloride in the presence of anhydrous AlCl3 benzene or substituted benzene gives acetophenone.
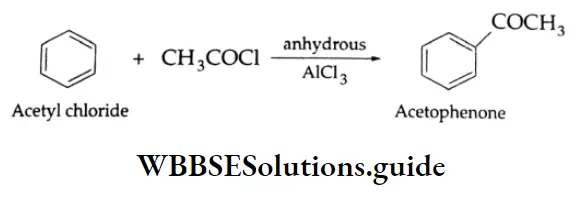
Acylation of benzene Mechanism:
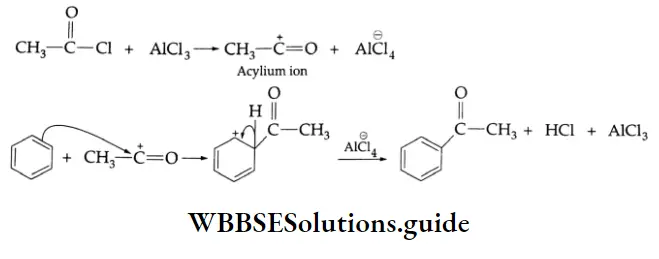
Chemical Reactions
Most of the chemical reactions of benzaldehyde are similar to those of aliphatic aldehydes. We will now discuss some reactions of benzaldehyde which are different from those of aliphatic aldehydes.
Perkin reaction:
On being heated with acetic anhydride in the presence of sodium acetate, benzaldehyde gives cinnamic acid (a, B- unsaturated acid).
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}+\left(\mathrm{CH}_3 \mathrm{CO}\right)_2 \mathrm{O} \stackrel{\mathrm{CH}_3 \mathrm{COONa}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}=\mathrm{CHCOOH}+\mathrm{CH}_3 \mathrm{COOH}\)
This reaction is known as the Perkin reaction.
Perkin reaction Mechanism:
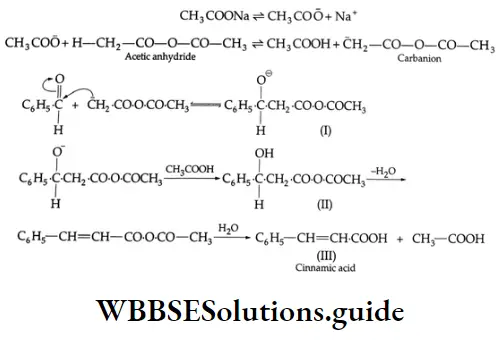
Benzoin condensation
Benzaldehyde and most other aromatic aldehydes undergo a self-condensation, known as benzoin condensation, when treated with potassium cyanide in an alcoholic solution. The product formed from benzaldehyde is called benzoin.
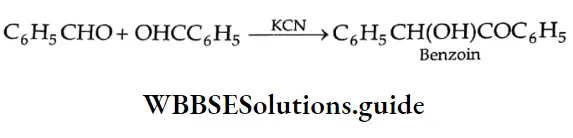
Benzoin condensation Mechanism
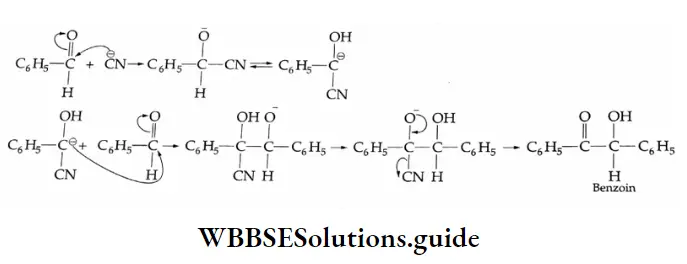
Claisen-Schmidt reaction
Benzaldehyde undergoes condensation with aldehydes and ketones in the presence of a dilute alkali at room temperature to yield unsaturated carbonyl compounds.
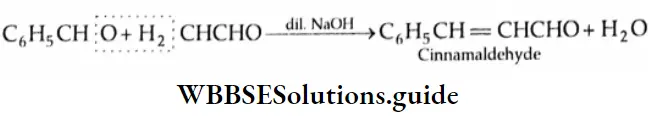
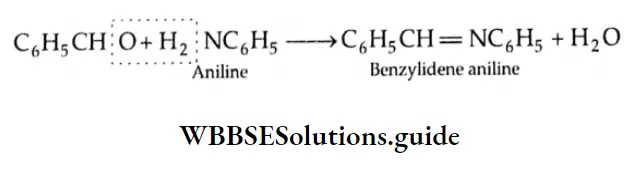
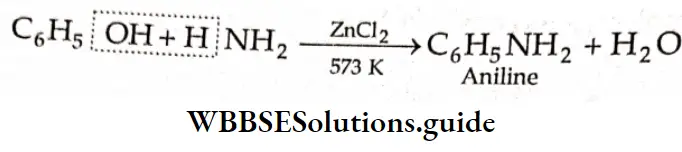
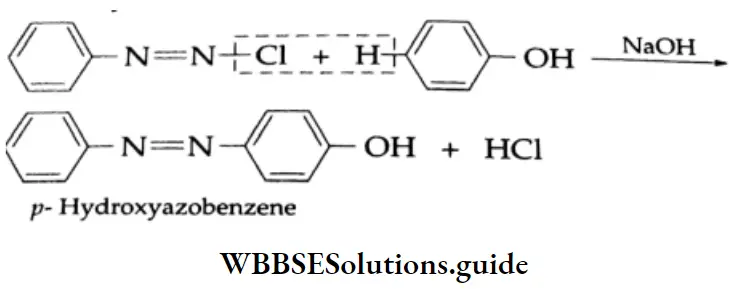
Reaction with aniline
On being heated with aniline, benzaldehyde yields benzylidene aniline (Schiff base).
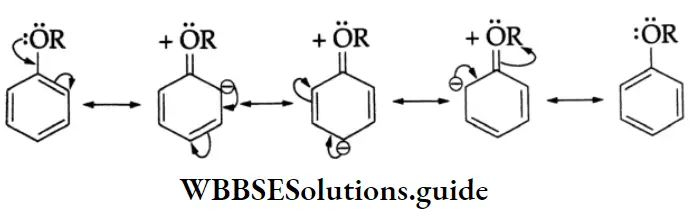
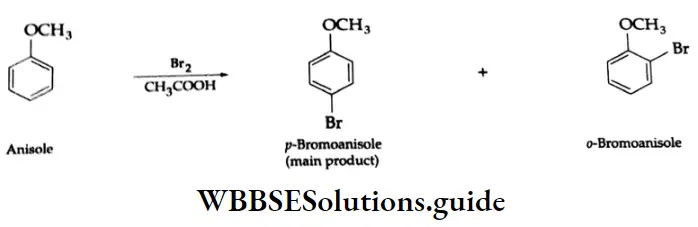
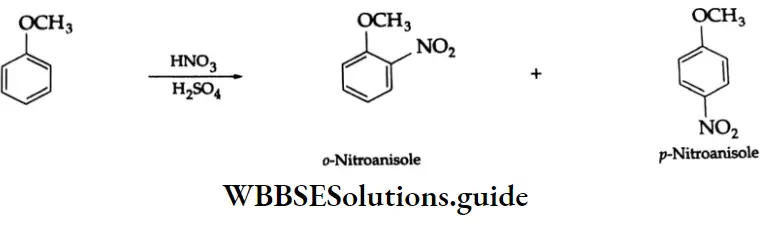
Electrophilic substitution reactions
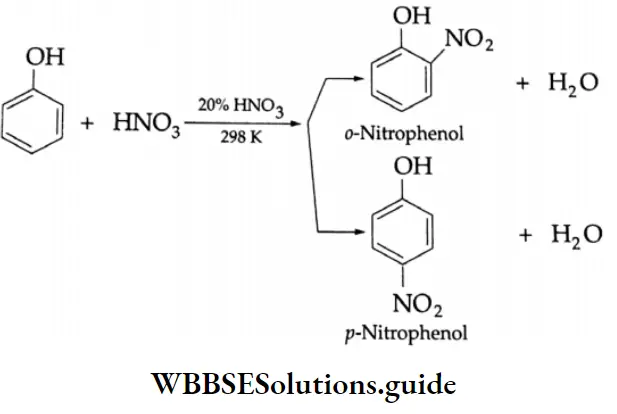
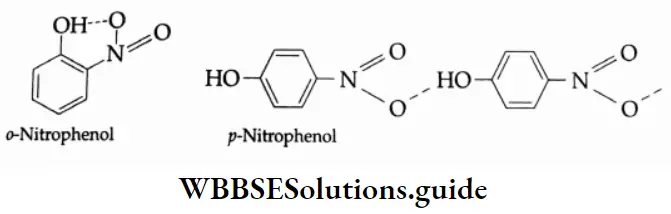
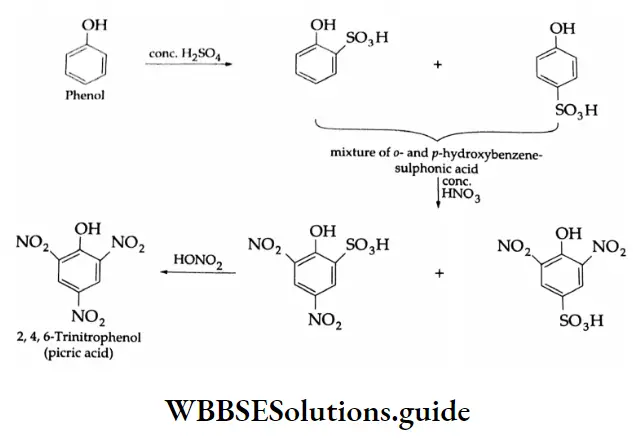
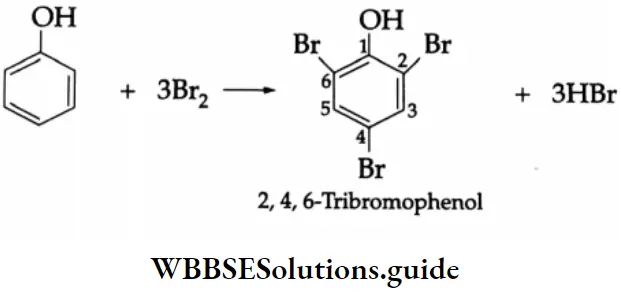
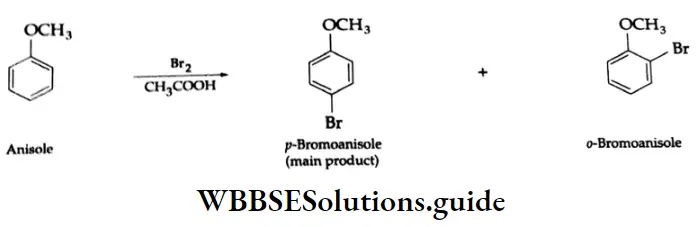
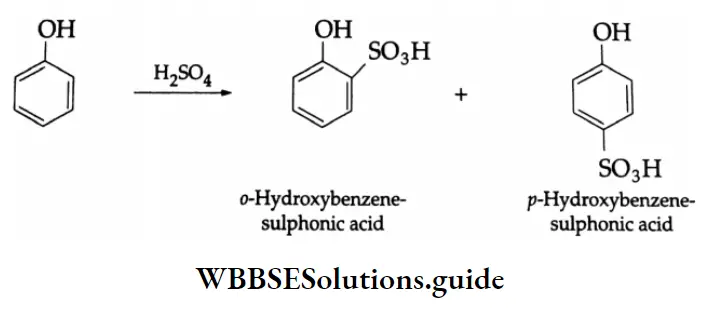
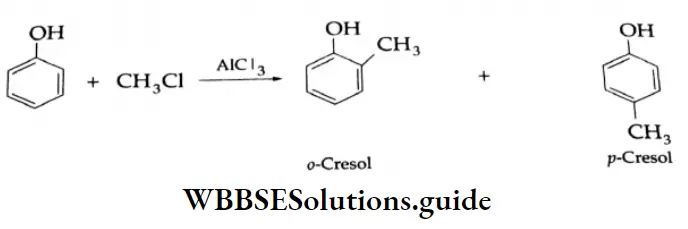
The benzene ring of aromatic aldehydes and ketones undergoes nitration, sulphonation and halogenation to yield the corresponding meta-substituted product.
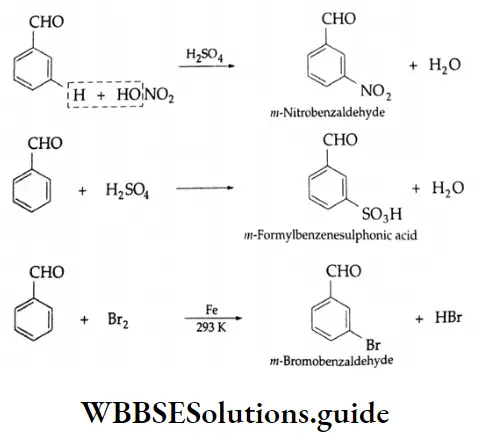
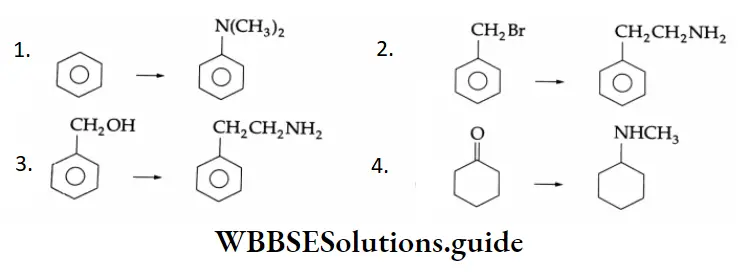
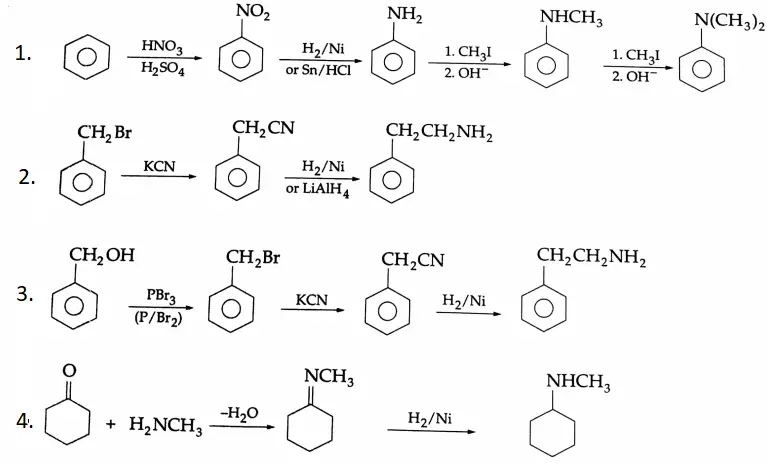
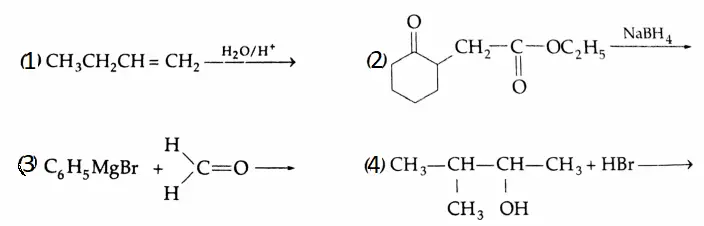
Example 3: Complete each reaction below by supplying the missing starting material, reagent(s) or product.
1. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}\)
2. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CN} \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}\)
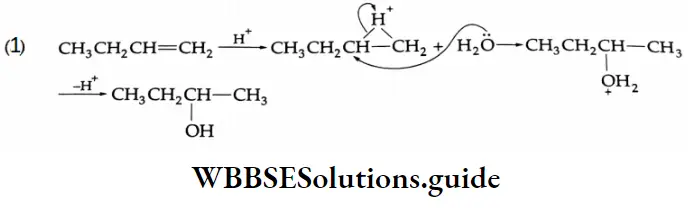
3. \(\mathrm{CH}_3 \mathrm{CH}=\mathrm{CHCH}_3 \rightarrow \mathrm{CH}_3 \mathrm{CHO}\)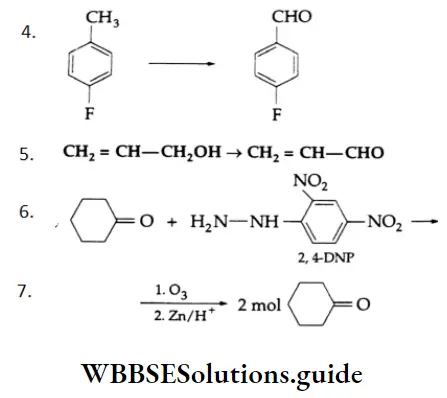
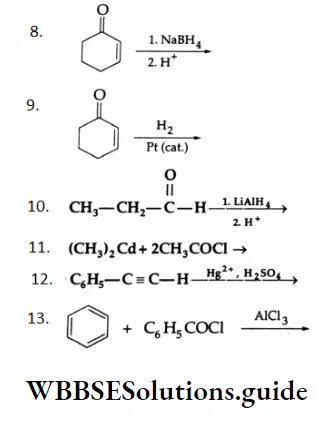
Solution:
1. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH} \stackrel{\mathrm{PCC}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}\)
A primary alcohol is oxidised to an aldehyde by PCC. (i) CH,CH,CN DIBAL-HCH,CH,CHO
2. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CN} \stackrel{\text { DIBAL-H }}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}\)
Aldehydes, ketones, and carboxylic acids important reactions
A nitrile is converted to an aldehyde by DIBAL-H.
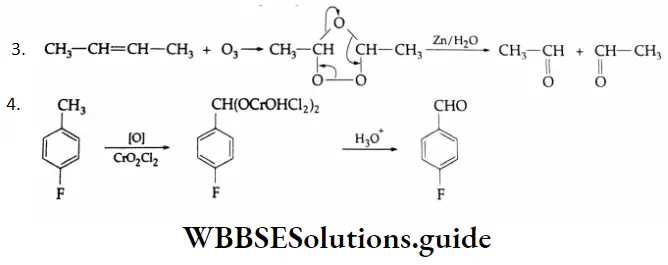
This is an Etard reaction. The partial oxidation of p-fluorotoluene by chromyl chloride (CrO2Cl2) gives a chromium complex, which on hydrolysis yields p-fluorobenzaldehyde.
5. \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2 \mathrm{OH} \stackrel{\mathrm{PCC}}{\longrightarrow} \mathrm{CH}_2=\mathrm{CH}-\mathrm{CHO}\)
A primary alcohol is oxidised to aldehyde by PCC. The double bond is not affected by oxidation with PCC.
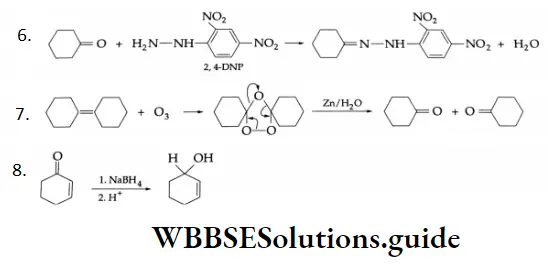
NaBH4 is a weak reducing agent. It only reduces the ketonic group to a secondary alcoholic group. Double bonds are not affected by NaBH4. H2 Pt (cat.)
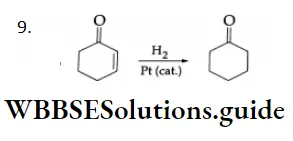
In catalytic hydrogenation, only ethylenic double bonds are reduced. The ketonic group remains unaffected.
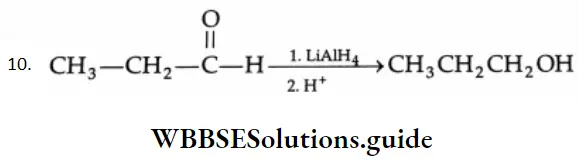
LiAlH is a versatile reducing agent. It reduces the aldehydic group to a primary alcoholic group.
11. \(2 \mathrm{CH}_3 \mathrm{COCl} \stackrel{\left(\mathrm{CH}_3\right)_2 \mathrm{Cd}}{\longrightarrow} 2 \mathrm{CH}_3 \mathrm{COCH}_3+\mathrm{CdCl}_2\)
Acid chlorides react with dimethyl cadmium to give ketones.
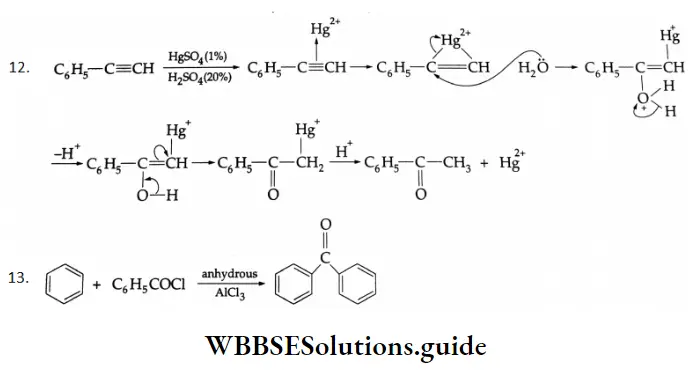
This is a Friedel-Crafts acylation reaction.
Mechanism:
Uses Of Aldehydes And Ketones:
- Formalin (40% aqueous solution of formaldehyde) is used to preserve biological specimens.
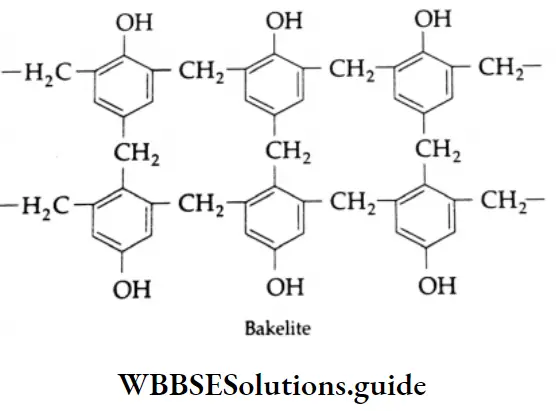
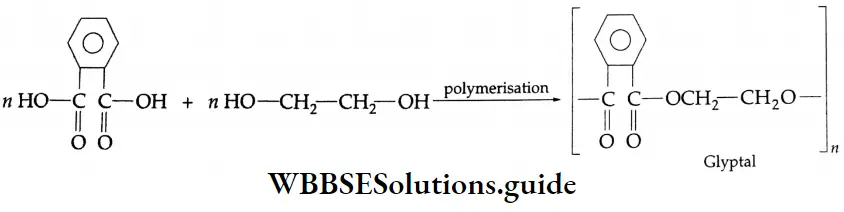
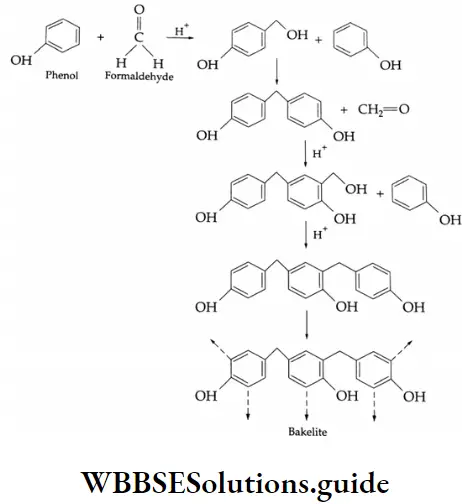
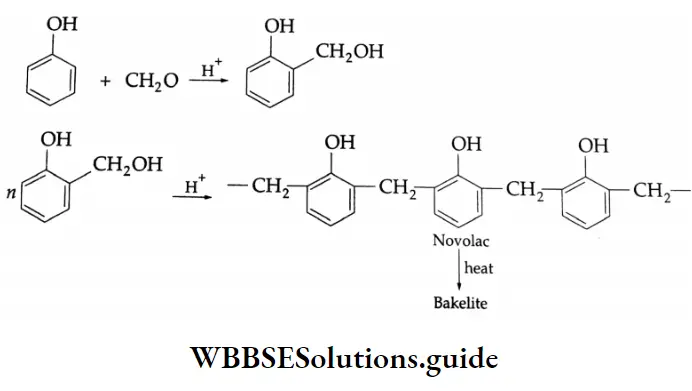
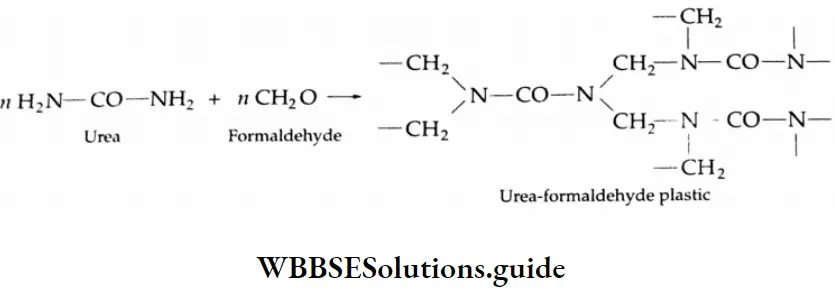
- Formaldehyde is used to prepare the most important plastic bakelite (a phenol-formaldehyde plastic).
- Acetaldehyde is used for manufacturing organic compounds such as acetic acid, ethyl acetate, acetic anhydride and 1-butanol.
- Benzaldehyde is used in perfumery and the dye industry.
- Butyraldehyde, vanillin and camphor are used as flavouring agents in the perfume industry.
Carboxylic Acids
Organic compounds which contain a carboxyl group 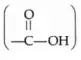 are termed carboxylic acids. The group is so named because it can be considered as a combination of the carbonyl and hydroxyl groups.
are termed carboxylic acids. The group is so named because it can be considered as a combination of the carbonyl and hydroxyl groups.
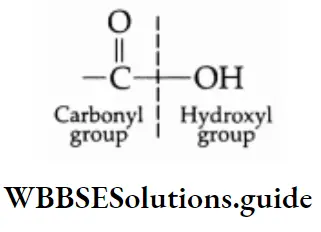
Carboxylic acids may be aliphatic or aromatic. Aliphatic carboxylic acids are compounds in which the carbon of the carboxyl group is attached to an alkyl group and aromatic carboxylic acids are those in which the carbon of the carboxyl group is attached to an aryl group.
The unbranched long-chain monocarboxylic acids (C13 -C18) (monocarboxylic acids are compounds in which only one -COOH group is present) are commonly called fatty acids because many of them are obtained by the hydrolysis of animal fats or vegetable oils.
“Tollens test and Fehling’s test for aldehydes”
Oils and fats are in fact esters of glycerol and fatty acids. Important fatty acids are stearic acid (C17H33COOH), palmitic acid (C15 H31 COOH) and oleic acid (C17H33COOH).
Formic acid is produced by ants and nettles. The sting of ants and nettles irritates the skin. Acetic acid is responsible for the sour taste of vinegar.
Nomenclature
Two systems of nomenclature are currently in use for carboxylic acids.
Trivial name
Simple carboxylic acids are known by their trivial names or common names derived from their natural sources, e.g., formic acid (Latin: formica, ant), acetic acid (Latin: acetum, vinegar), butyric acid (Latin: butyrum, butter). In substituted acids, the positions of the substituents are indicated by the Greek letters a, ẞ, 7, 8 and so on. The carbon atom next to the carboxyl group is a. For example,
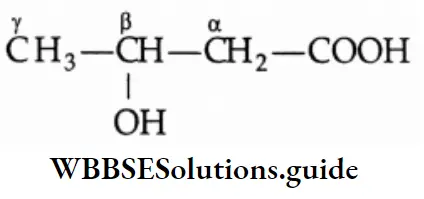
IUPAC name
The IUPAC name of a monocarboxylic acid is derived from the name of the corresponding alkane by dropping the last ‘e’ of the alkane and adding the suffix ‘oic acid’. Positions of substituents on the chain are worked out by considering the carboxylic carbon to be C(1).
Carboxylic acids containing more than one carboxylic acid group are named by retaining the ending’-e’ of the alkane and the number of carboxylic acids are indicated by adding the prefix di, tri, etc., to the term ‘oic acid’. The positions of carboxylic acid groups are located by numbers.
The common and IUPAC names of some carboxylic acids
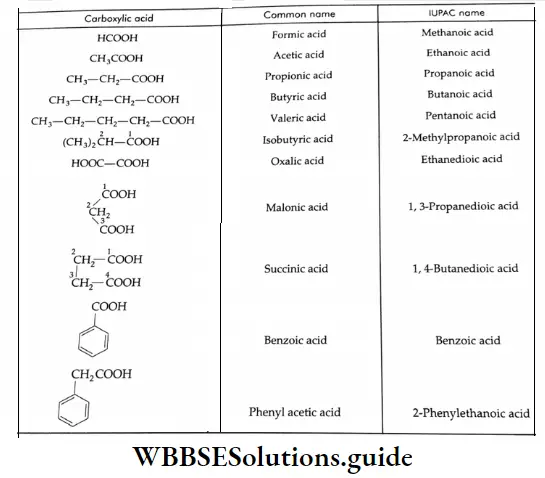
The following are some formulae and IUPAC names of carboxylic acids
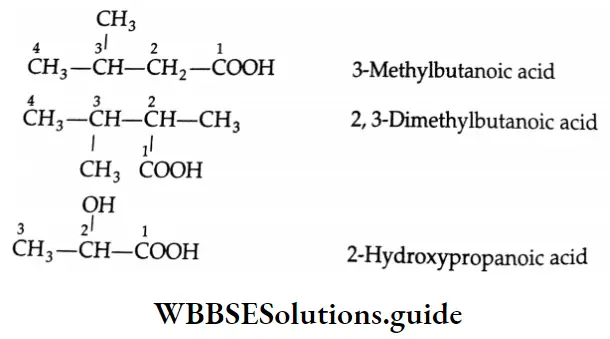
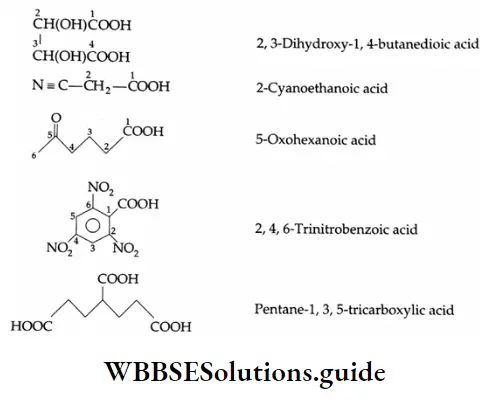
[Note: If an unbranched chain is directly linked to more than two carboxyl groups, these carboxylic acids are named from the parent hydrocarbon by the substitutive used of a suffix such as ‘tricarboxylic acid’, etc., in place of the suffix ‘oic acid’.]
Oxidation and reduction reactions of aldehydes and ketones
Alicyclic carboxylic acids are also named by adding the suffix ‘carboxylic acid’ to the name of a parent hydrocarbon. For example,
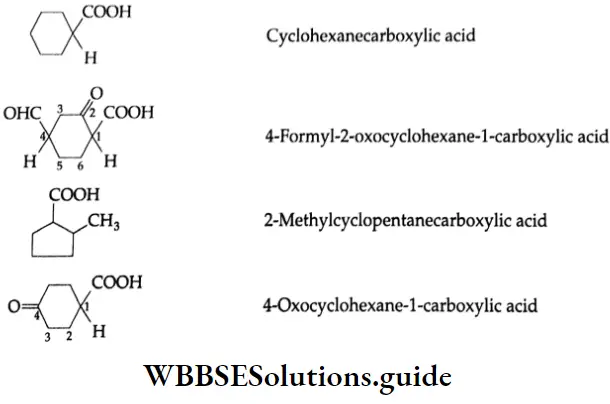
Methods Of Preparation
By the oxidation of primary alcohols or aldehydes
The oxidation of primary alcohols yields a carboxylic acid. The oxidising agent most often used is potassium permanganate in an acidic or alkaline medium, or potassium dichromate or chromium trioxide in an acid medium.
⇒ \(\mathrm{RCH}_2 \mathrm{OH} \underset{\text { 2. } \mathrm{H}_3 \mathrm{O}^{+}}{\stackrel{\text { alkaline } \mathrm{KMnO}_4}{\longrightarrow}} \mathrm{RCOOH}\)
⇒ \(\mathrm{RCH}_2 \mathrm{OH} \stackrel{\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow} \mathrm{RCOOH}\)
The initial product in the oxidation of a primary alcohol is the corresponding aldehyde. However, the aldehyde undergoes oxidation more rapidly than the primary alcohol. So it is normally not shown in the equation. Both aliphatic and aromatic aldehydes are readily oxidised to carboxylic acids even by mild oxidising agents like Tollens reagent. But usually, acid dichromate or permanganate solutions are used for oxidation.
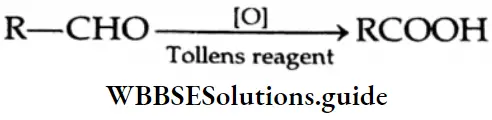
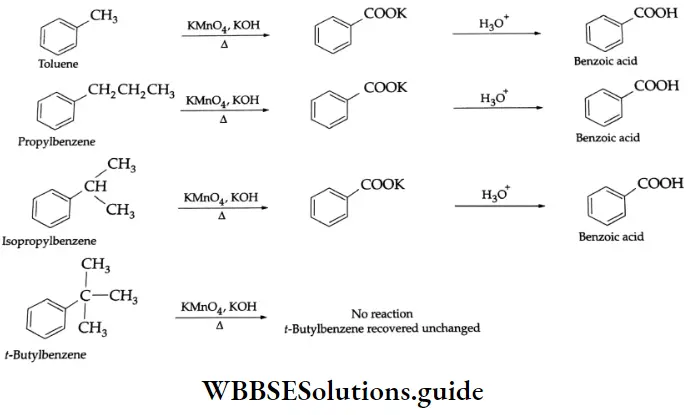
By the oxidation of alkylbenzenes (Side-chain oxidation)
Aromatic compounds with alkyl side chains are oxidised with alkaline KMnO4, acidified K2Cr2O7 or chromic acid to carboxylic acids. For example, toluene, propylbenzene and isopropylbenzene are oxidised to benzoic acid on oxidation with alkaline KMnO4. As t-butylbenzene does not possess hydrogen on the benzyl carbon, it is not oxidised to benzoic acid.
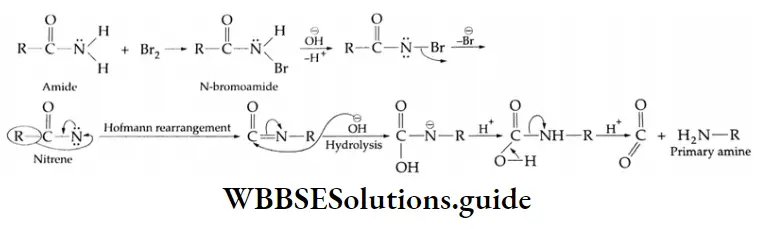
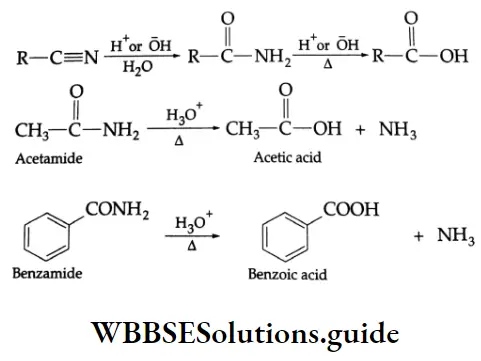
By the hydrolysis of nitriles and amides
Aliphatic and aromatic nitriles give carboxylic acids on hydrolysis upon boiling with acids or alkalis.
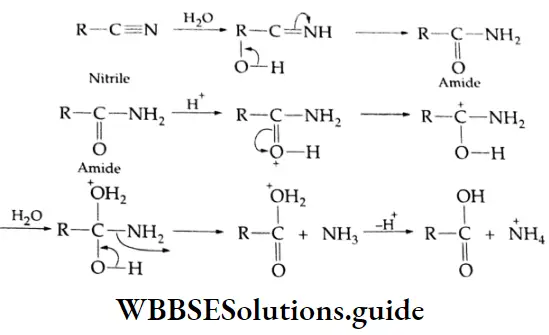
Nitriles and Amides Mechanism:
In order to obtain amides rather than carboxylic acids, mild reaction conditions are used. (Then the reaction stops at the amide stage.)
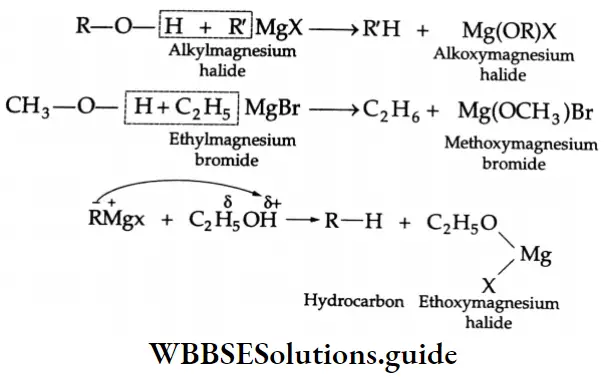
Grignard reagents: Grignard reagents (RMgX) react with carbon dioxide (dry ice is a convenient source of CO2) to yield salts of carboxylic acids. Treatment of the salt with a mineral acid liberates the carboxylic acid.]
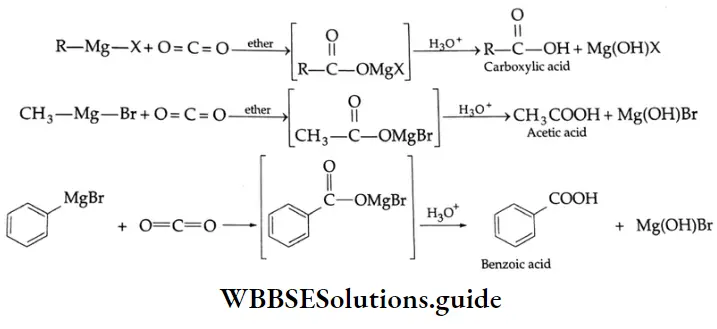
Grignard reagents Mechanism :
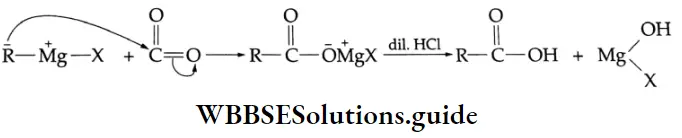
By the hydrolysis of esters, amides, acid halides and acid anhydrides
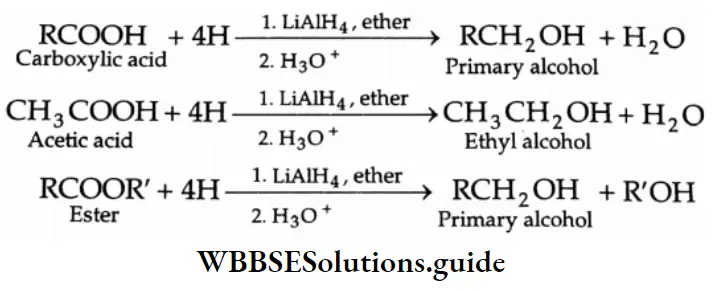
On hydrolysis, esters, amides, acyl halides and acid anhydrides give carboxylic acids.
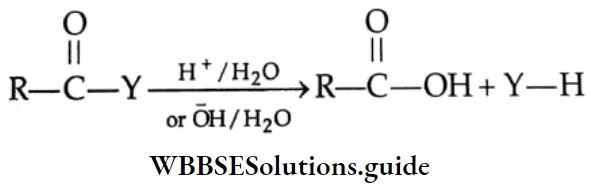
(Y = OR,X,NH2,OCOR)
Hydrolysis of esters Esters undergo hydrolysis by refluxing with dilute HCl or dilute alkali to yield carboxylic acids. Acidic hydrolysis gives carboxylic acid directly but alkaline hydrolysis with dilute NaOH gives the sodium salt of a carboxylic acid, which on acidification yields carboxylic acid.
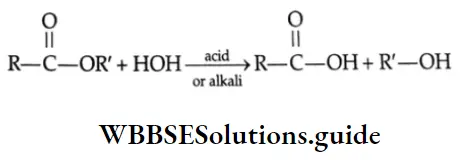
With acid:
⇒ \(\mathrm{CH}_3-\mathrm{COOC}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O} \stackrel{\mathrm{H}^{+}}{\rightleftharpoons} \mathrm{CH}_3 \mathrm{COOH}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\)
With alkali:
⇒ \(\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O} \stackrel{\mathrm{OH}^{-}}{\rightleftharpoons} \mathrm{CH}_3 \mathrm{COOH}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\)
⇒ \(\mathrm{CH}_3 \mathrm{COOH}+\mathrm{OH}^{-} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}_2 \mathrm{O}\)
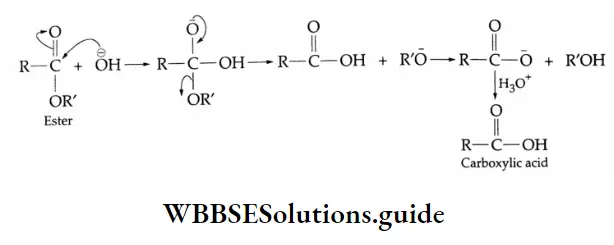
Mechanism of alkaline hydrolysis of esters:
Hydrolysis of amides: On Hydrolosis with acid or alkaline amides yield carboxylic acids
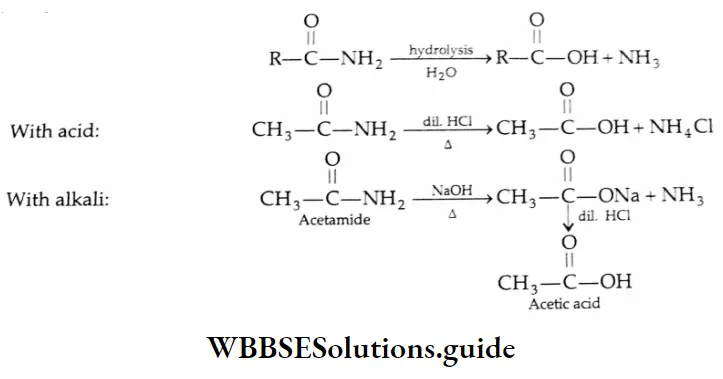
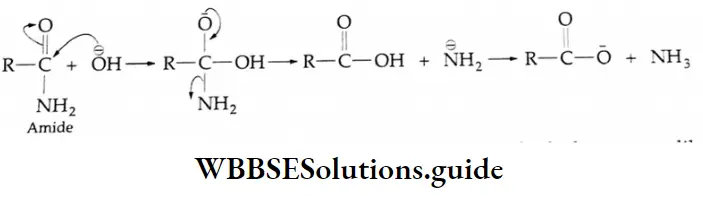
Alkaline hydrolysis of amides Mechanism:
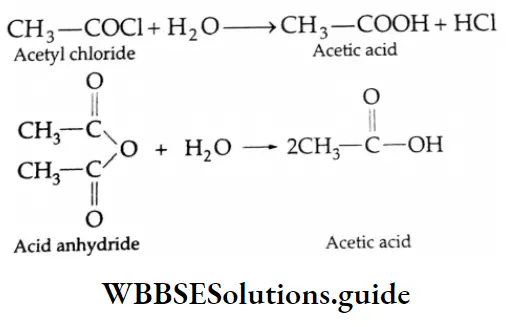
Hydrolysis of acid halides and acid anhydrides:
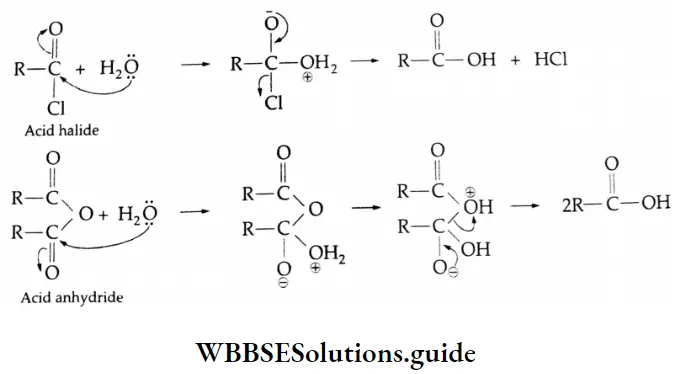
Acid halides and Acid anhydrides Mechanism:
Physical Properties Of Monocarboxylic Acids
Lower carboxylic acids (with up to nine carbon atoms) are liquids with disagreeable odours. Higher members are waxlike solids. They are less volatile and almost odourless. Formic acid and acetic acid are present in traces in the secretions of our skin.
The ability of a dog to differentiate one person from another depends upon its highly developed sense of smell. Since the metabolic processes of different persons are different, the composition of lower carboxylic acids secreted by the skin is different in case of different people and a dog is able to distinguish one person from another.
“Aldol condensation reaction mechanism in ketones”
The boiling points of carboxylic acids are higher than those of other classes of compounds of comparable molecular weight. For example, acetic acid (molecular weight 60) boils at 393 K, but propanol (molecular weight 60) boils at 370 K, and ethyl chloride (molecular weight 64) boils at only 286 K.
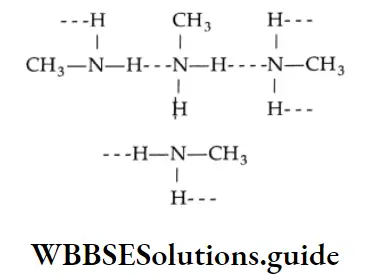
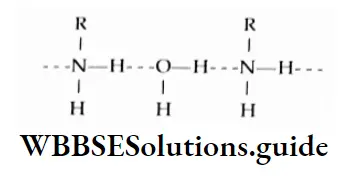
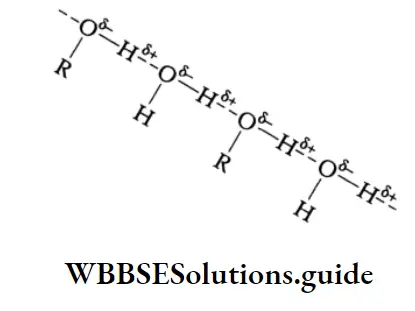
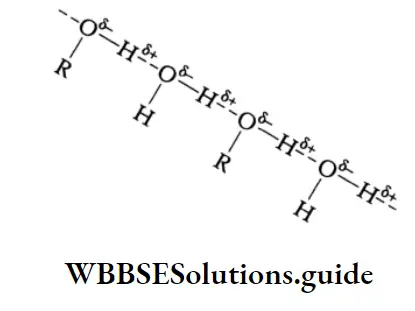
The increase in boiling point is attributed to the hydrogen bonding between the carboxylic acid molecules. More heat is required to break hydrogen bonds in the dimeric structure of carboxylic acids.
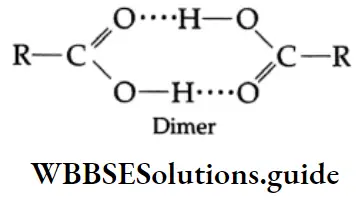
Example 4: Arrange te following compounds in increasing order of their boiling points give reasons
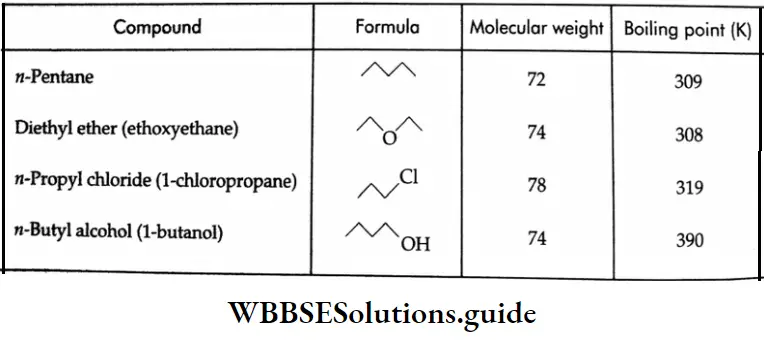
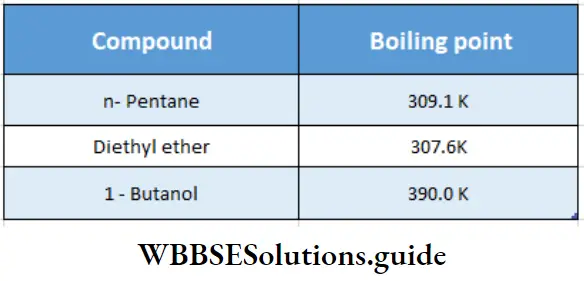
C2H5–O–C2H5, CH3CH2COOH, CH3CH2CH2CH2OH, CH3CH2CH2CHO, CH3CH2CH2 CH2CH3.
Solution:
The boiling points of carboxylic acids are higher than those of other classes of compounds of comparable molecular mass. For example, propanoic acid (molecular mass = 74) boils at 414 K, 1-butanol (molecular mass = 74) boils at 391 K, butanal (molecular mass = 72) boils at 347.7 K, ethoxyethane (molecular mass = 74) boils at 318 K and n-pentane (molecular mass = 72) boils at 319 K. The high boiling point of propanoic (propionic) acid is attributed to the dimeric structure due to hydrogen bonding.
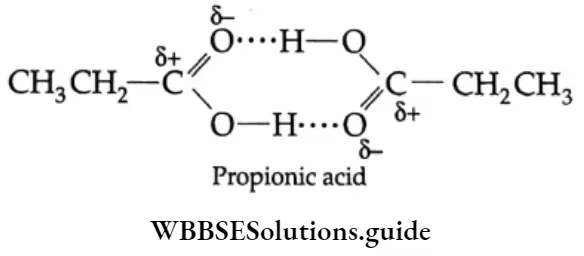
Propionic acid forms even stronger hydrogen bonds than does 1-butanol. The O-H bond of propionic acid is more strongly polarised as O-H due to the adjacent electron-attracting carbonyl group and the hydrogen bridge may be bonded to the more negatively charged carbonyl group rather than to the oxygen of another O-H bond of the carboxylic acid group.
The carbonyl group of butanal 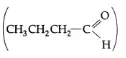 is more polar in nature than the C-O-C group in ethoxyethane (C2H5-O-C2H5). The dipole-dipole attraction of the molecules of butanal is stronger than in ethoxyethane. Therefore, the boiling point of butanal is higher than that of ethoxyethane.
is more polar in nature than the C-O-C group in ethoxyethane (C2H5-O-C2H5). The dipole-dipole attraction of the molecules of butanal is stronger than in ethoxyethane. Therefore, the boiling point of butanal is higher than that of ethoxyethane.
“Cannizzaro reaction step-by-step explanation”
On the other hand, butanal does not form hydrogen bonds, and its boiling point is lower than those of 1-butanol and propanoic acid. n-pentane is nonpolar in nature, the only attractive forces among the molecules of n-pentane being the weak van der Waals attractive forces.
Therefore, the increasing order of boiling points of the given compounds is as follows.
CH3CH2CH2CH2CH3 <CH3CH2OCH2CH3<CH3CH2CH2CHO <CH3CH2CH2CH2OH
<CH3CH2COOH
Lower members of the carboxylic acids are completely soluble in water. This is because of the ability of the carboxyl group to form hydrogen bonds with water molecules. 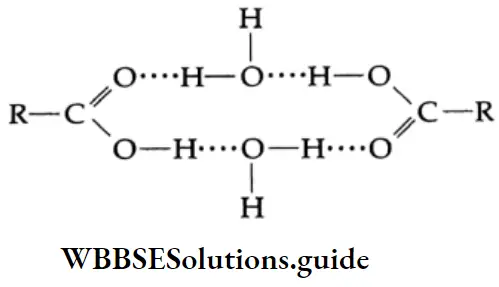
Higher fatty acids are insoluble in water due to the increased hydrophobic nature of long-chain alkyl groups.
Structure Of Carboxylic Acids
A carboxylic acid may be thought of as a resonance hybrid of the following two structures.
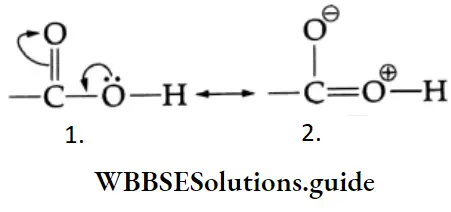
This resonance interaction has two important consequences.
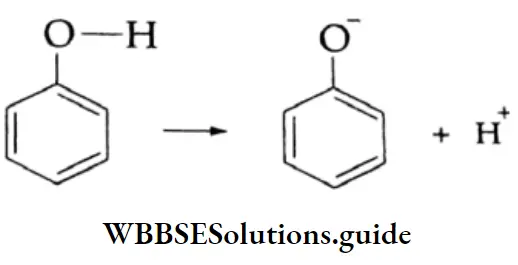
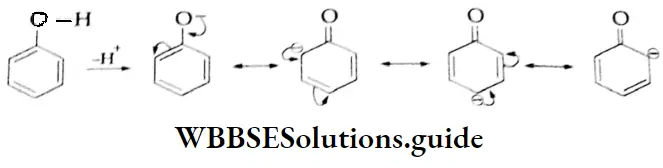
1. The O-H bond is weakened by the electron-withdrawing effect of the carbonyl group so that typical carboxylic acids (pKa =4±1) are much more acidic than alcohol (pKa 17). The conjugate base of a carboxylic acid is also stabilised by resonance. The negative charge is shared equally by both oxygens, making them completely equivalent.
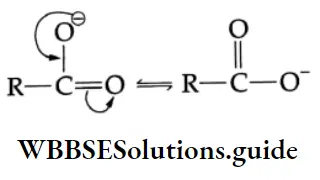
2. The electrophilicity of the carbonyl group is reduced by the electron-donating effect of the O-H group. Therefore, carboxyl carbon is less reactive towards nucleophiles than the carbonyl group of aldehydes and ketones.
Due to the diminished electron-withdrawing capacity of the carbonyl group in carboxylic acids (and their derivatives), α -hydrogens in such compounds are less acidic than those in ketones and aldehydes.
Strength Of Carboxylic Acids
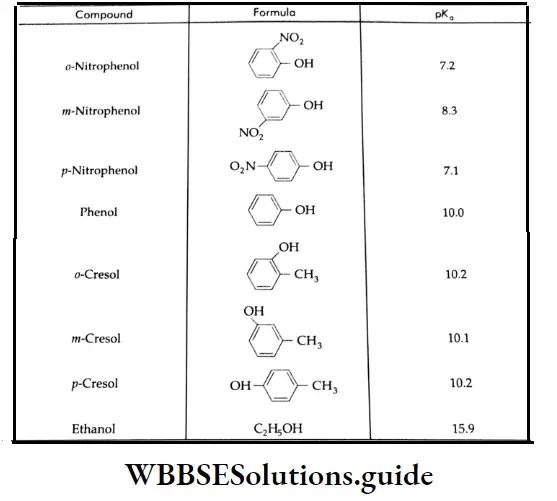
The strength of a Bronsted acid is usually expressed in terms of its pKa value. The lower the pKa value, the stronger is the acid. The pKa values for a large number of carboxylic acids are known.
Table 12.5 pKa values for selected carboxylic acids (R-COOH)
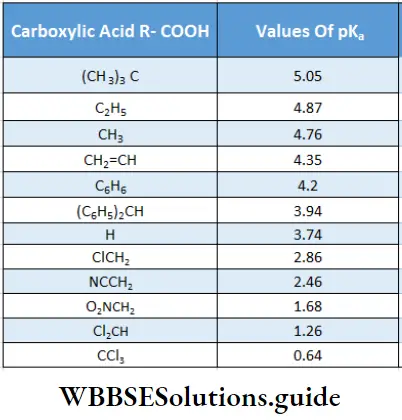
From the table, it is evident that substituted acids (CICH2-COOH, CI2 CH-COOH and CI2 C-COOH) as well as formic acid are stronger than acetic acid.
It is reasonable to expect that electron-donating substituents [Example, CH3-C2H3-,(CH3)2, CH-,(CH3)3C-1 should be acid weakening while electron-withdrawing substituents (-Cl2 -NO2, CN2 etc.) should be acid strengthening. The closer the substituent group is to the carboxyl group, the greater the effect it will have.
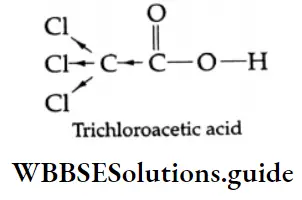
Further, if the number of electronegative halogens on the a-carbon is increased from one to three, the pK value reaches that of a strong mineral acid. The three halogens of trichloroacetic acid exert strong as shown. Due to this, the hydrogen of the -COOH group can readily leave as a proton.
When groups such as C6H5, and vinyl (CH2=CH-) are attached to the carboxyl group then the acid strength should decrease due to resonance, as shown below.
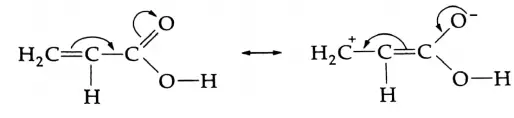
But, in fact, the acid strength increases because an sp2 carbon has greater effective electronegativity than an sp carbon.
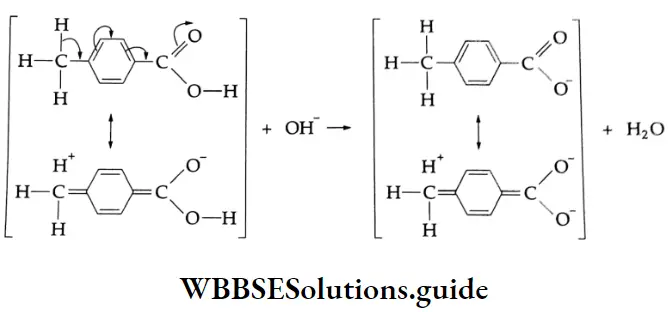
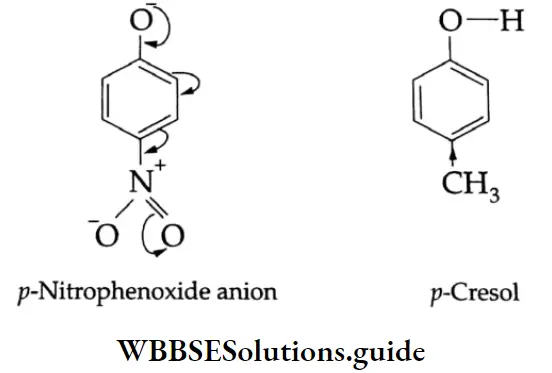
In the presence of electron-attracting groups (NO2, Cl, SO3 H), the acid strength of aromatic carboxylic acids increases and in the presence of electron-donating groups (CH3, OH, OCH3, etc.), the acid strength decreases.
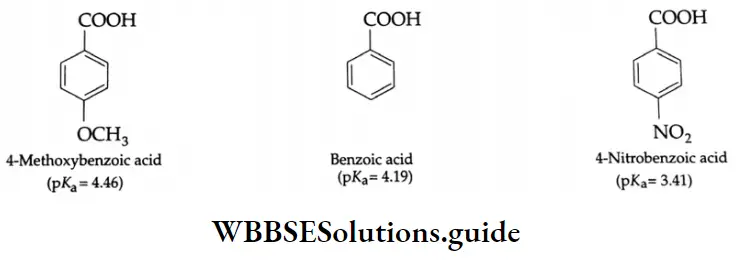
Example: Among the following pairs of acids, which is stronger? Give reasons
1. CH3COOH or CICH2COOH
2. FCH2COOH or CICH2COOH
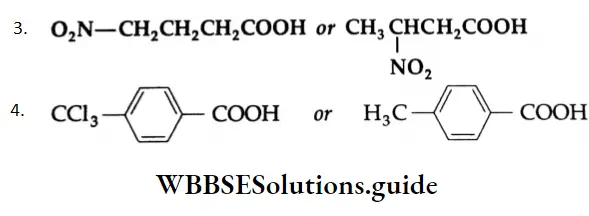
Solution:
Generally, the acidity of a compound is increased by electron-attracting substituents whi electron-donating substituents decrease acid strength. Chloroacetic acid is more acidic tha acetic acid.
The higher acidity of chloroacetic acid is attributed to inductive effect. The chlori atom on chloroacetic acid assists in loosening the O-H bond and making the proton eas removable by pulling the electron towards itself due to its high electronegativity.

The electron-donating methyl group in acetic acid decreases the acid strength.
The larger the electron-withdrawing inductive effect, the greater is the acidity. As a result, fluoroacetic acid is stronger than chloroacetic acid-fluorine is considerably more electronegative than chlorine.
Inductive effect decreases rapidly with distance. The carboxylic acid in which the electron-attracting nitro group is closer to the carboxyl group is stronger because it can pull electrons more effectively. Therefore, 3-nitrobutanoic acid is stronger than 4-nitrobutanoic acid.
The electronegative CCl3– group attracts electrons from the benzene ring, resulting in a positive charge on the carbon atom adjacent to the carboxylic group.
This in turn induces a positive charge on the carboxyl carbon. This results in the release of a proton to yield the carboxylate anion. The carboxylate anion is readily stabilised through resonance and increases the acid strength.
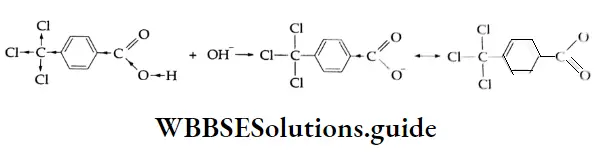
The effect in p-methylbenzoic acid is opposite to that in 4-trichloromethylbenzoic acid. The methyl group is able to donate electrons to the benzene ring through hyperconjugation and built up a negative charge on the carboxyl carbon. This additional charge tends to hold the proton and thus decreases the acid character.
Chemical Properties Carboxylic Acids
The chemical reactions of the carboxylic group involve the following.
- Cleavage of the O-H bond,
- Cleavage of the C-OH bond,
- The COOH group, and
- Substitution reactions in the hydrocarbon part.
Reactions involving cleavage of the O-H bond
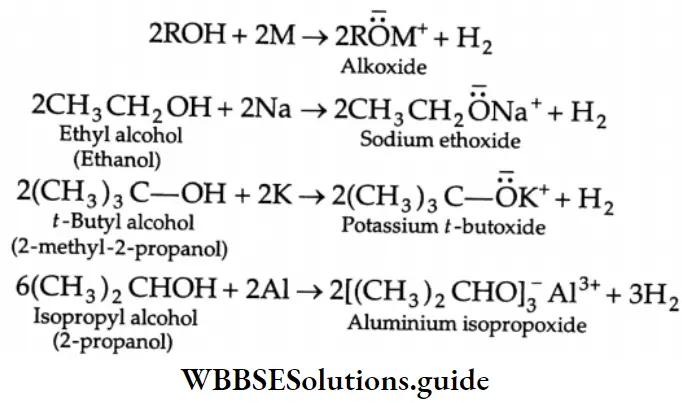
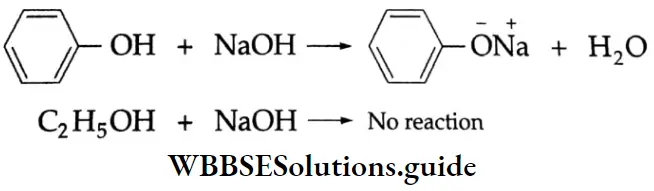
Reactions with metals and alkali Like alcohols, carboxylic acids also evolve hydrogen with sodium metal and like phenols, carboxylic acids react even with weak bases, such as sodium carbonate and sodium bicarbonate, to evolve carbon dioxide.
2CH3COOH + 2Na→2CH3COONa+ H2
CH3COOH + NaOH → CH3COONa +H2O
CH3COOH + NaHCO3-CH3COONa +H2O+CO2↑
Note that the evolution of CO2 is from NaHCO3 and not from the carbxylic acid. This reaction is used to detect the -COOH group in an unknown organic compound. Carboxylic acids evolve CO2 with effervescence.
Carboxylic acids are only partially ionised in an aqueous solution and thus display weak acidic properties. A convenient way to measure the acidity of an acid is in terms of its ionisation constant (Ka) and PKavalues. For acetic acid, the expression for Ka is obtained as follows.
⇒ \(\mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}_3 \stackrel{+}{\mathrm{O}}\)
Preparation and properties of aldehydes, ketones, and carboxylic acids
For the above reaction
⇒ \(K_{\mathrm{a}}=\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]\left[\mathrm{H}_3 \stackrel{+}{\mathrm{O}}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\)
The concentration term for water is neglected since it is not affected to any appreciable extent by the ionisation of the acid. A higher value for Ka implies a strong acid.
The strength of an acid is also indicated by its pK, value.
pKa = -log Ka
Thus, for acetic acid, whose Ka is 1.8 × 10-5, the pKa can be calculated.
PK-log (1.8 × 10-5)=
-0.3+5=4.7.
A smaller value of pKa implies a stronger acid (excellent proton donor). For hydrochloric acid, the pK, value is -7.0. The corresponding values for trifluoroacetic acid, benzoic acid and acetic acid are 0.23, 4.19 and 4.7 respectively.
Stronger acids have pK, values < 1, moderately strong acids have pK, values between 1 and 5, weak acids have pK, values between 5 and 15, and extremely weak acids have pK, values > 15.
Reactions involving cleavage of the C-OH bond
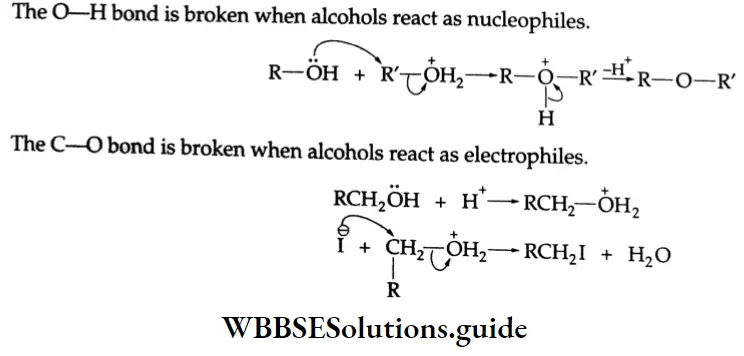
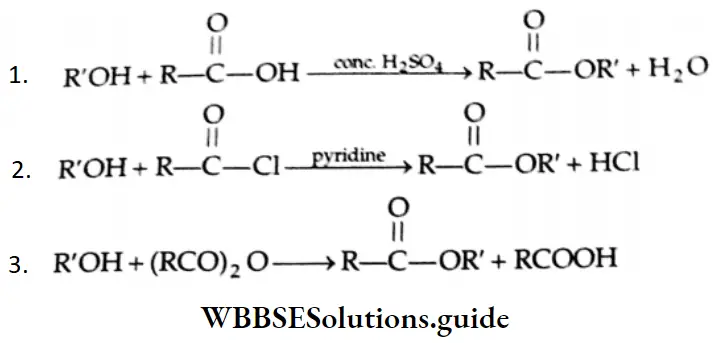
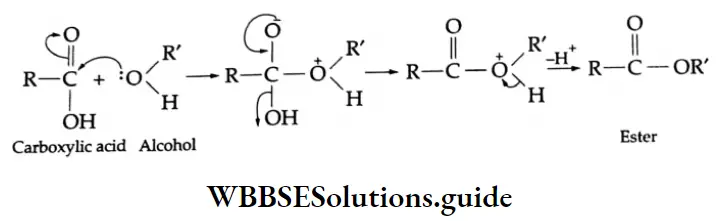
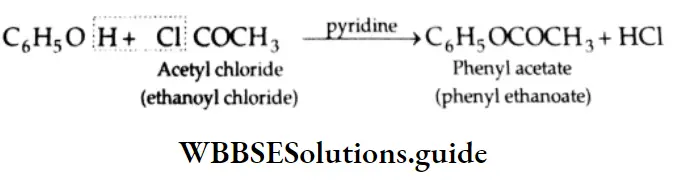
Esterification Carboxylic acids react readily with alcohols or phenol in the presence of catalytic amounts of mineral acids (concentrated H2SO4) to yield esters. The process is called esterification. The conversion of a carboxylic acid into an ester involves nucleophilic substitution at the carbonyl (acyl) carbon.
First of all, the carbonyl oxygen of the -COOH group is protonated, which fascilitates the nucleophilic addition of the alcohol (the alcohol serving as the nucleophile) to the carbonyl group, yielding a tetrahedral intermediate.
Proton transfer of the intermediate converts the acid’s OH group to the protonated form which, being a good leaving group, departs as a water molecule. The protonated ester so formed finally loses a proton to give an ester.
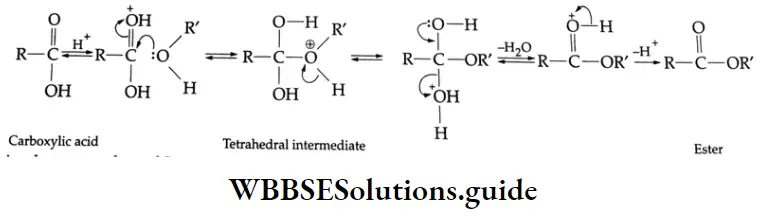
C-OH bond Mechanism:
The reaction has several notable characteristics.
- It is reversible. Completion of the reaction requires either water to be removed by azeotropic distillation o an excess of one of the reagents (usually alcohol) to be used.
- The alcohol’s oxygen is retained in the ester, indicating that the R-O bond does not break during the reaction.
- The reaction requires catalysis by strong acids.
Reactions with PCI5, PCI3 and SOCI2 The reaction of a carboxylic acid with PCl5, PCI3 or
SOCI2 yields an acid chloride.
⇒ RCOOH + PCI5→ RCOC1 + POCl3 + HCl
⇒ 3RCOOH+ PCl3, 3RCOCI+ H3PO3
⇒ RCOOH + SOCI2→ RCOCI+ HCI ↑+ SO2 ↑
With thionyl chloride, the by-products are both gases. With the phosphorus halide, the by-products are either nonvolatile phosphorus acid or the volatile liquid phosphorus oxychloride (boiling point 378 K). The latter may be difficult to separate from the acid chloride if the boiling points of the two are similar. Therefore, thionyl chloride is preferred for the preparation of acid chlorides.
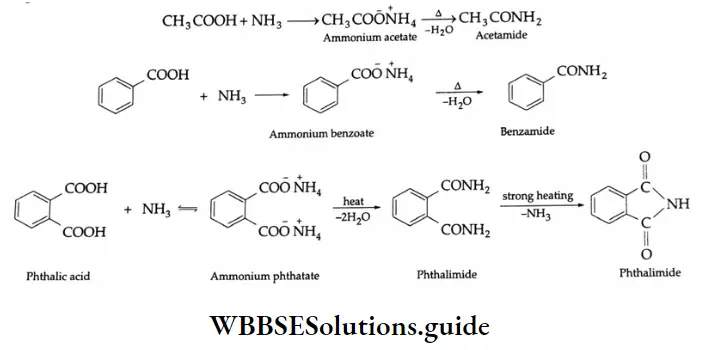
Reaction with ammonia:
Carboxylic acids react with ammonia to yield ammonium salts, which give amides on being heated. For example,
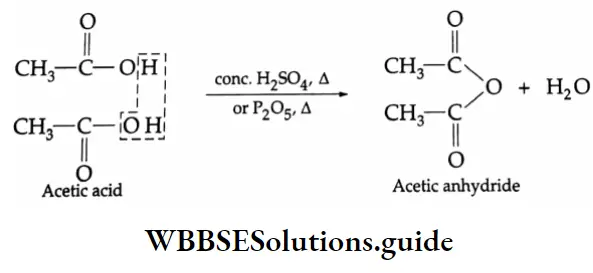
Formation of anhydrides:
On being heated with concentrated H2SO4 or P2O5, a carboxylic acid gives an acid anhydride.
Carboxylic acids may be converted to their anhydrides by making their sodium salts react with the corresponding acid chloride.
⇒ RCOONa + RCOC1→ R–COO–COR+NaCl
Mixed anhydrides can also be prepared by this process.
⇒ RCOONa + R’COC1→ RCO—O—COR’ + NaCl
Reactions involving the carboxyl group
Reduction:
Carboxylic acids are conveniently reduced to primary alcohols upon reaction with lithium aluminium hydride in ether or with diborane, which reduces esters, nitro and halo groups with difficulty. Sodium borohydride is a weak reducing agent and does not reduce the carboxyl group.
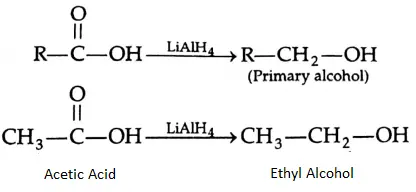
Decarboxylation: on dry distillation with soda lime, the sodium salt of a carboxylic acid gives an alkane.
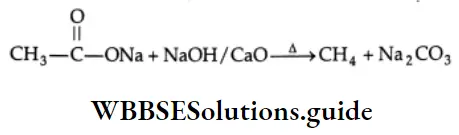
The decarboxylation (removal of CO2) of a carboxylic acid takes place through the carboxylate anion from which the group –CH3 departs along with its bonding electron pair. The carbanion (carbon carrying a negative charge) reacts with a proton to give the alkane.
Decarboxylation Mechanism:
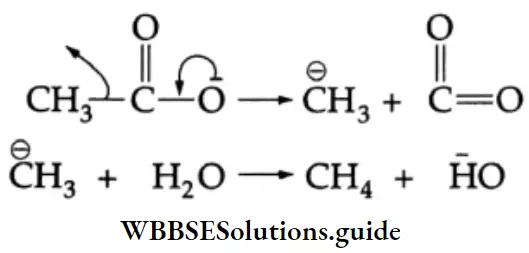
Kolbe’s electrolytic method: In this method, an aqueous solution of a sodium salt of carboxylic acid is subjected to electrolysis to yield an alkane.
⇒ 2CH3COONa+ 2H2O→C2H6 +2CO2 + 2NaOH + H2 at anode at cathode
In another reaction, dry distillation with calcium formate, the calcium salt of a carboxylic acid gives an aldehyde. For example, a mixture of calcium acetate and calcium formate yields acetaldehyde on dry distillation.
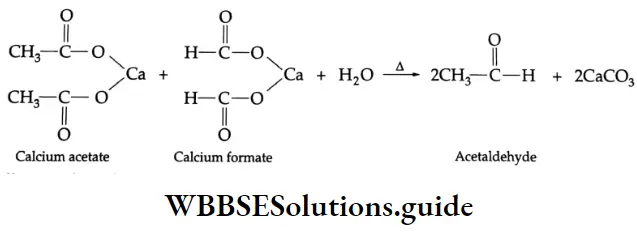
On dry distillation, the calcium salt of a carboxylic acid gives a ketone. For example, calcium acetate, on dry distillation, yields acetone.
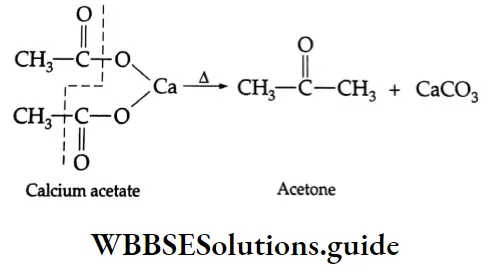
Hunsdiecker reaction:
Carboxylic acids form silver salts when their ammoniacal solutions are treated with silver nitrate. On being refluxed with Br2, the salt forms an alkyl bromide. This is known as the Hunsdiecker reaction.
⇒ \(\underset{\text { Silver carboxylate }}{\mathrm{RCOOAg}}+\mathrm{Br}_2 \underset{\Delta}{\stackrel{\mathrm{CCl}_4}{\longrightarrow}} \underset{\text { Alkyl bromide }}{\mathrm{R}-\mathrm{Br}}+\mathrm{CO}_2+\mathrm{AgBr}\)
Substitution reactions in the hydrocarbon part
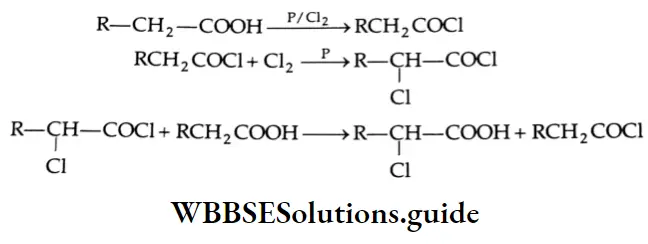
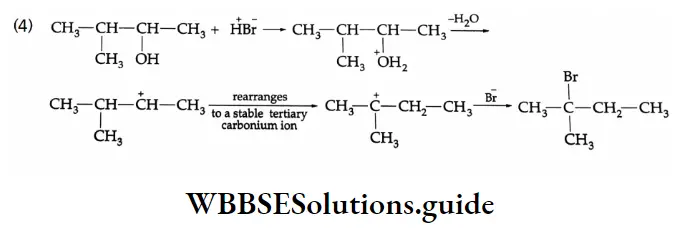
Halogenation: When treated with chlorine or bromine in the presence of red phosphorus, aliphatic carboxylic acids containing a-hydrogen form a-halogen acids.
Similarly,
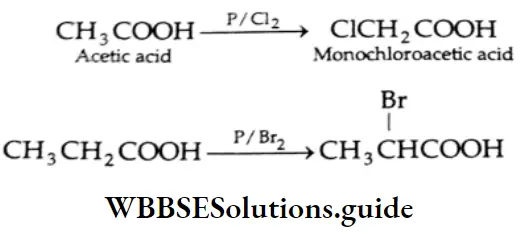
This reaction is called the Hell-Volhard-Zelinsky reaction or HVZ reaction.
Halogenation Mechanism:
Reducing properties of formic acid
Formic acid shows reducing properties in the following reactions.
Reaction with Tollens reagent: On gently warming with Tollens reagent, formic acid gives a grey precipitate of metallic silver.
⇒ HCOOH + 2Ag(NH3)2(Tollens reagent)OH → Ag + CO3 +H3O+4NH3
Reaction with Fehling’s reagent: Upon being heated with Fehling’s reagent, formic acid gives a brick-red precipitate of Cu2O.)
Reaction with mercuric chloride: Formic acid reduces mercuric chloride to mercuric chloride (Hg2Cl2) as a white precipitate. Mercurous chloride is further reduced to mercury as a grey precipitate.
⇒ HCOOH+2HgCl2Hg2Cl2 + CO2 + 2HCl
⇒ HCOOH+Hg2Cl2→ 2Hg(grey ppt.) +CO2+2HCl
Electrophilic substitution reactions of benzoic acid
Nitration:
Aromatic carboxylic acids undergo electrophilic substitution reactions. In benzoic acid, the -COOH group deactivates the benzene ring and is meta-directing. On nitration with concentrated HNO3 and concentrated H2SO4, benzoic acid gives m-nitrobenzoic acid.
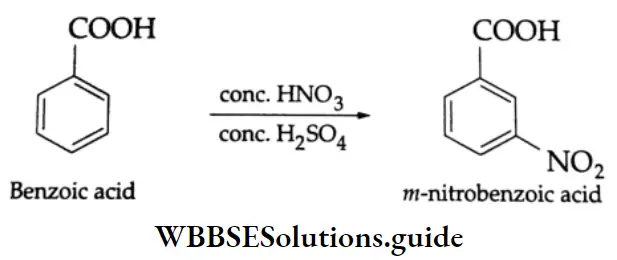
Benzoic acid does not undergo Friedel-Crafts reaction (alkylation or acylation) because this reaction is not possible with a deactivated aromatic nucleus and the catalyst anhydrous AICI3 (Lewis acid) gets bonded with the COOH group as shown below.
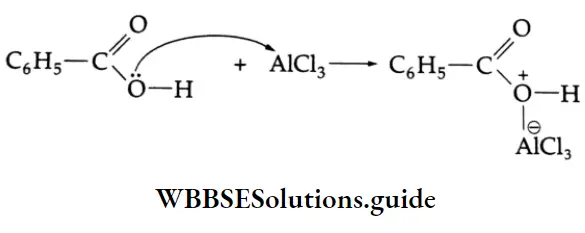
Halogenation:
In the presence of ferric bromide, which acts as a catalyst, benzoic acid reacts with bromine to form m-bromobenzoic acid.
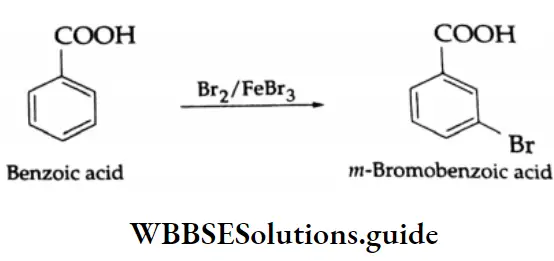
Carboxylic Acids Uses
- Formic acid is used in the rubber, cloth, dye and leather industries.
- Acetic acid is used as a solvent and in the preparation of vinegar.
- .Higher fatty acids are used in the manufacture of soaps and detergents.
Aldehydes, Ketones and Carboxylic Acids Multiple-Choice Questions
Question 1. The functional group of an aldehyde is

Answer: 2.
Question 2. The general formula of aldehydes and ketones is
- Cn H2n-2O
- Cn H2nO
- CnH2n+1O
- CnH2nO2
Answer: 2. Cn H2nO
Question 3. The IUPAC name of acetone is
- Methanal
- Ethanal
- Propanone
- Ethanone
Answer: 3. Propanone
Question 4. Which of the following is used to distinguish between an aldehyde and a ketone?
- Concentrated H2SO4
- Hydrazine
- Tollens reagent
- Nitrous acid
Answer: 3. Tollens reagent
Question 5. On being heated with NaOH solution, formaldehyde gives
- Formic acid
- Acetonw
- Methyl alcohol
- Ethyl formate
Answer: 3. Methyl alcohol
“Differences between aldehydes and ketones based on chemical structure”
Question 6. Which of the following is formed when a mixture of calcium acetate and calcium formate is dry distilled?
- Methanol
- Ethanol
- Ethanal
- Acetic acid
Answer: 4. Acetic acid
Question 7. Which of the following is involved in the Cannizzaro reaction?
- CH3CHO
- HCHO
- HCOOH
- CH3COCH3
Answer: 2. HCHO
Question 8. On dry distillation, calcium acetate gives
- Ethyl acetate
- Calcium formate
- Acetone
- Acetaldehyde
Answer: 3. Acetone
Question 9. Which of the following reagents reacts with aldehydes as well as ketones?
- Tollens reagent
- Fehling’s solution
- Schiff base
- Grignard reagent
Answer: 4. Grignard reagent
Question 10. Which of the following can be used to distinguish between aldehydes and ketones?
- Fehling’s solution
- H2SO4
- NaHSO3
- NH3
Answer: 1. Fehling’s solution
Question 11. Which of the following responds positively to the iodoform test?
- C2H2OH
- CH3OH
- CH3CHO
- C2H4c
Answer: 1 and 3 Or C2H2OH and CH3CHO
“Esterification reaction of carboxylic acids with alcohols”
Question 12. Which of the following is oxidised to acetone?
- CH3 CHO
- C2H5OH
- CH3OH
- CH3-CHOH-CH3
Answer: 3. CH3OH
Question 13. On reacting with chlorine, acetaldehyde gives
- Acetyl chloride
- Chloral
- Dichloroacetic acid
- None of these
Answer: 2. Chloral
Question 14. On being heated with ammoniacal silver nitrate, acetaldehyde gives
- Acetone
- Silver acetate
- A silver mirror
- Formaldehyde
Answer: 3. A silver mirror
Question 15. In the following reaction, what is the appropriate reagent?
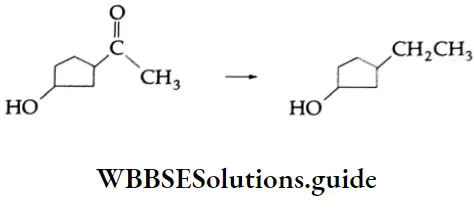
- NH-NH2, KOH
- Zn/Hg,
- H3/Ni
- NaBH4
Answer: 1. NH-NH2, KOH
Question 16. What is the correct order of reactivity of C6H5 MgBr with the following?
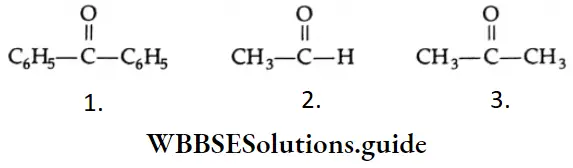
- 1 > 2> 3
- 3 >2 >1
- 2>3>1
- 1 >2 >3
Answer: 3. 2>3>1
Question 17. Which of the following reagents is used to separate acetaldehyde from acetophenone?
- NaHSO3
- C6H5NHNH3
- NH2OH
- NaOH/I2
Answer: 4. NaOH/I2
Question 18. On treatment with 1% HgSO4 and 20% H2SO4, but-1-yne gives
- CH3CH2COCH3
- CH3CH2CH2CHO
- CH3CH2CHO and HCHO
- CH3CH2CHOO and HCOOH
Answer: 1.
“Carboxylic acids acidity and the effect of substituents”
Question 19. The boiling points of the compounds
- CH3CH2CH2CHO
- CH3CH2COOH and HCOOH
- CH3CH2CH2OH
- CH3COOH I
Answer: 3. CH3CH2CH2OH
Question 20. The formation of cyanohydrin from CH3COCH3 is an example of
- Electrophilic substitution
- Nucleophilic substitution
- Nucleophilic addition
- Electrophilic addition
Answer: 3. Nucleophilic addition
Question 21. Which of the following products is obtained when CH3MgBr reacts with formaldehyde?
- C2H2OH
- CH3COOH
- HCHO
- CH3CHO
Answer: 1. C2H2OH
Question 22. In which of the following reactions does an aromatic aldehyde react with acetic anhydride in the presence of sodium acetate to give an unsaturated aromatic acid?
- Friedel-Crafts reaction
- Wurtz reaction
- Perkin reaction
- None of these
Answer: 2. Wurtz reaction
Question 23. Which of the following does not respond positively to the iodoform test?
- 2-pentanone
- 3-pentanone
- Ethanal
- Ethonol
Answer: 2. 3-pentanone
Question 24. Which of the following is Tollens reagent?
- [Ag(NH3)2]+ ion
- Cu(OH)2
- CuO
- Ag2O
Answer: 1. [Ag(NH3)2]+ ion
“Applications and uses of aldehydes, ketones, and carboxylic acids”
Question 25. Which of the following is used to distinguish between aliphatic and aromatic aldehydes?
- Tollens reagent
- Benedict’s reagent
- Schiff base
- Iodoform reaction
Answer: 4. Iodoform reaction
Question 26. Which of the following reactions does benzaldehyde not undergo?
- Aldol condensation
- Benzoin condensation
- Cannizzaro reaction
- Perkin reaction
Answer: 1. Aldol condensation
Question 27. Which of the following will yield acetaldehyde?
- The dry distillation of calcium acetate
- The reduction of acetic acid by LIAIH
- The oxidation of isopropyl alcohol by K2Cr2O7/H2SO4
- The ozonolysis of 2-butene
Answer: 4. The ozonolysis of 2-butene
Question 28. Which of the following reagents is used to distinguish between formic acid and acetic acid?
- Phosphorus pentachloride
- Sodium
- Grignard reagent
- Tollens reagent
Answer: 3. Grignard reagent
“Reduction of aldehydes and ketones to primary and secondary alcohols”
Question 29. Carbon dioxide is evolved when propanoic acid is treated with a NaHCO3 solution. The carbon atom of CO2 results from the
- Methyl group
- Carboxylic acid group
- Methylene group
- Bicarbonate
Answer: 4. Bicarbonate
Question 30. Which of the following does not reduce Fehling’s solution?
- Formic acid
- Acetic acid
- Formaldehyde
- Acetaldehyde
Answer: 2. Acetic acid
Question 31. An organic compound (X), on being heated with K2Cr2O, and H2SO4 gives another compound (Y). The latter on heating with I, and Na2CO3 solution forms an iodoform. Among the following, which one could be the compound (X)?
- CH3COCH3
- CH3OH
- CH3CHO
- CH3CHOHCH3
Answer: 4. CH3CHO
Question 32. Which of the following is used to distinguish between formic acid and acetic acid?
- Sodium
- Mercuric chloride
- Sodium ethoxide
- 2,4-Dinitrophenylhydrazine
Answer: 2. Mercuric chloride
“Oxidation of aldehydes to carboxylic acids using strong oxidants”
Question 33. An ester is hydrolysed by KOH and acidified to get a white precipitate. The ester is
- Methyl acetate
- Ethyl acetate
- Ethyl formate
- Ethyl benzoate
Answer: 4. Ethyl benzoate
Question 34. What are the products formed in the following reaction? \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{COOCH}_3 \stackrel{\mathrm{LiAlH}_4}{\longrightarrow}\)
- C6H5COOCH and CH3OH
- C6H5C3OOH and CH3OH
- C6H5CH2OH and CH3CHO and CH3COOH
- All of these
Answer: 4. C6H5CH2OH and CH3CHO and CH3COOH







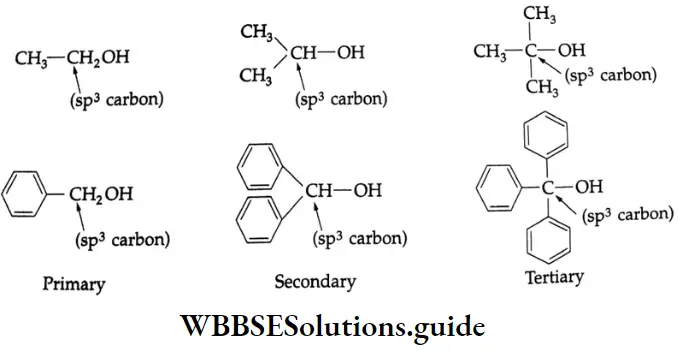
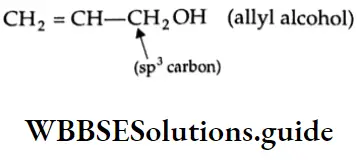
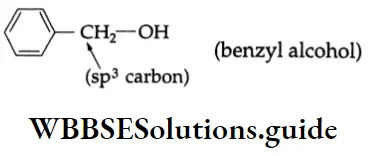
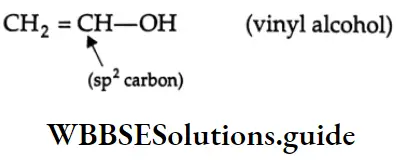

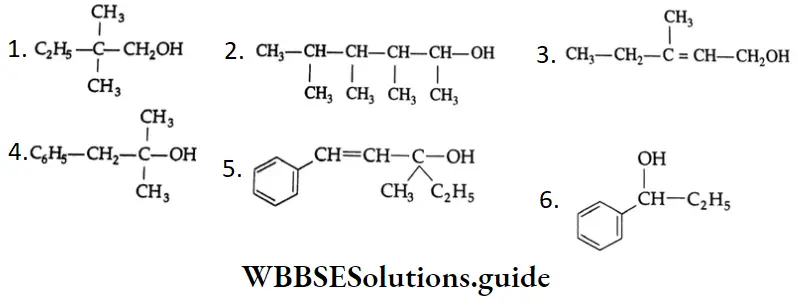
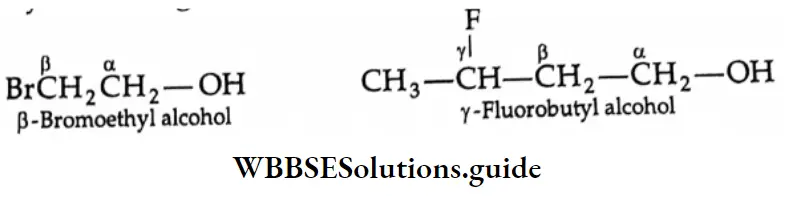

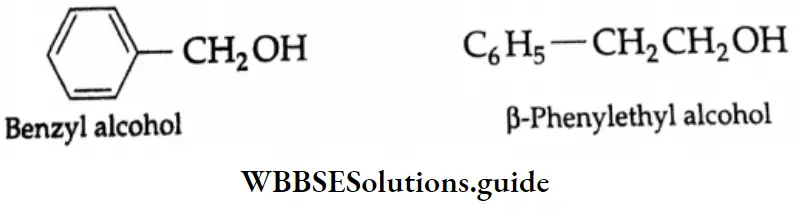

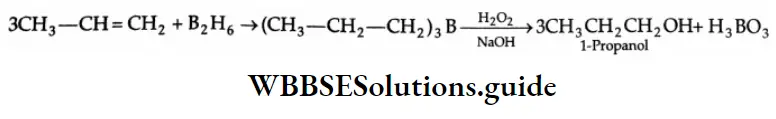
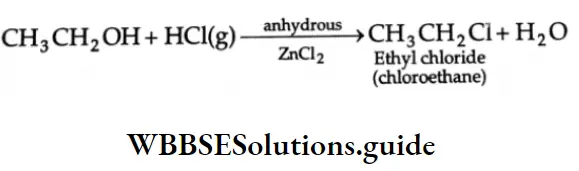
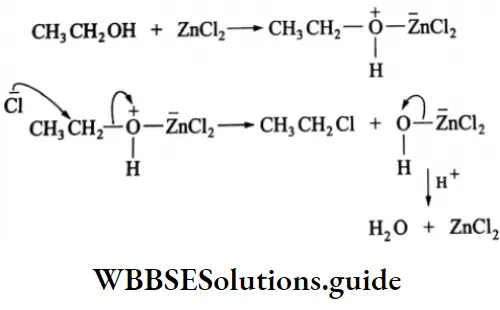
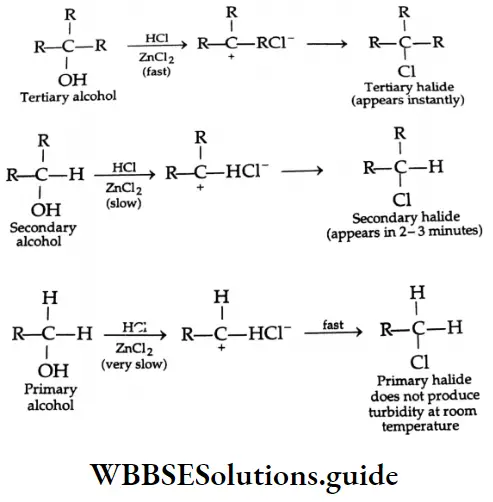
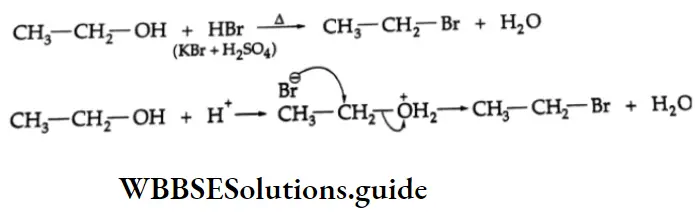
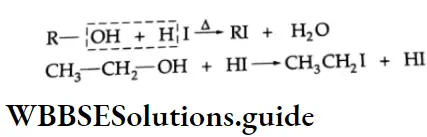
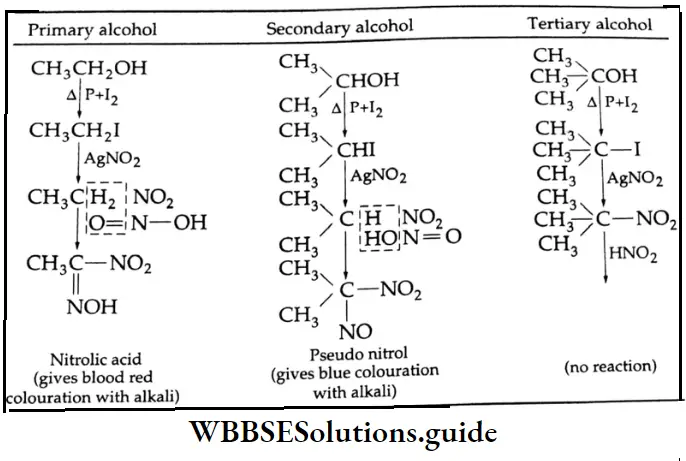
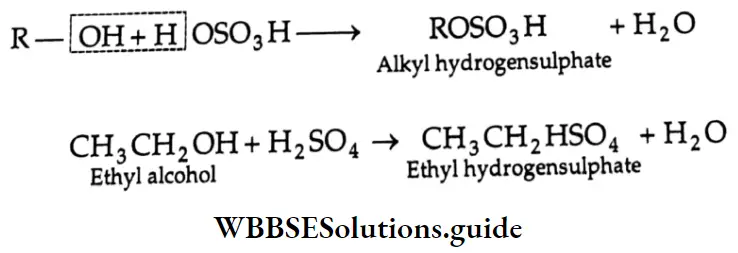
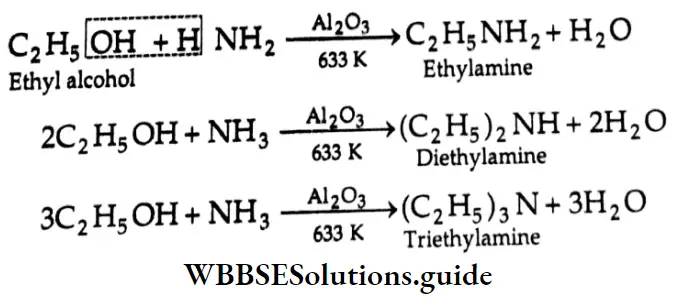
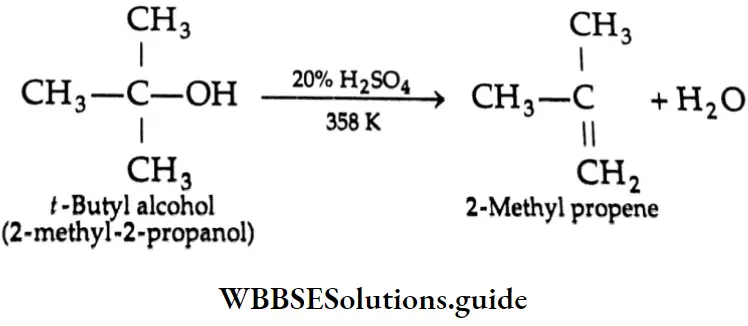
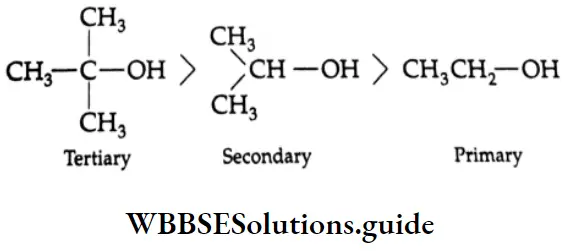
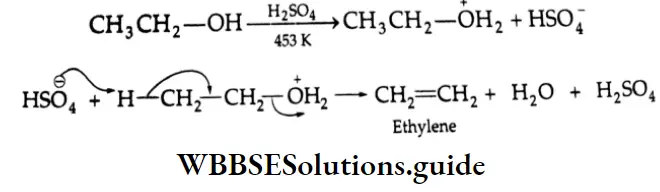
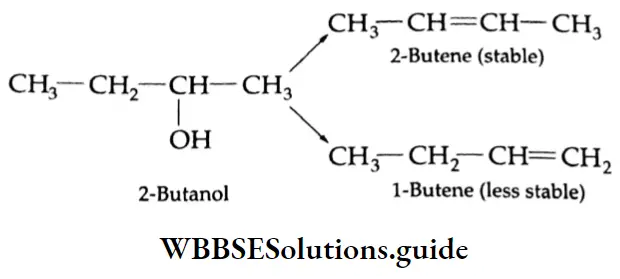
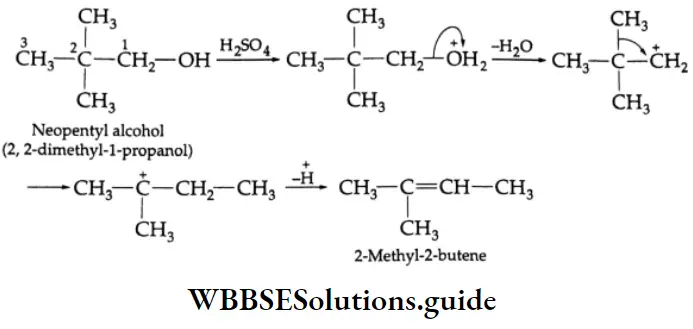
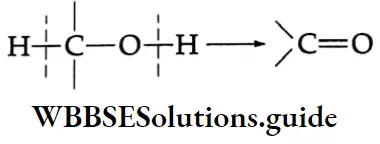
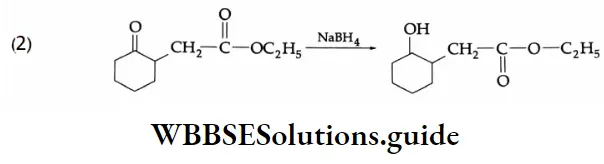

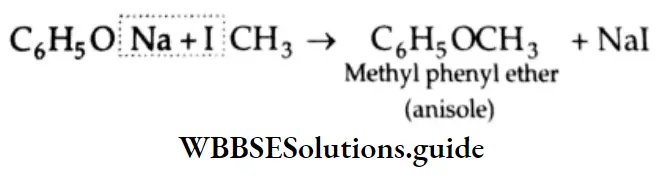
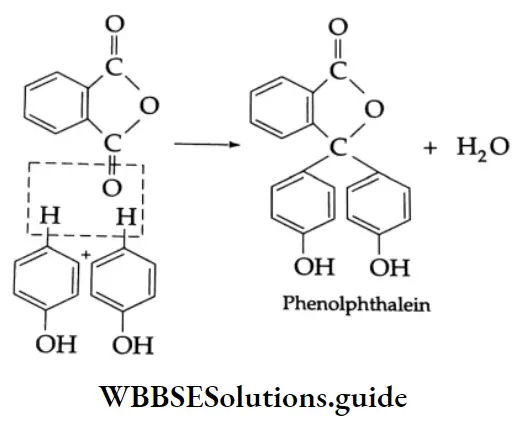
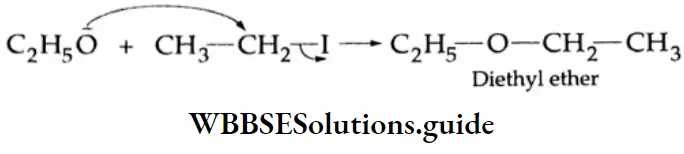
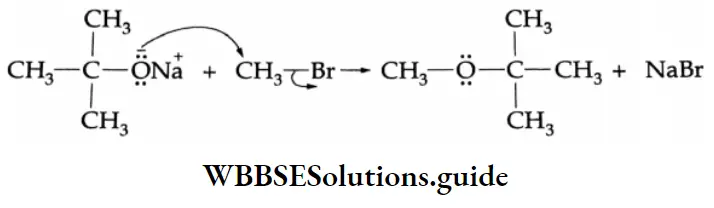
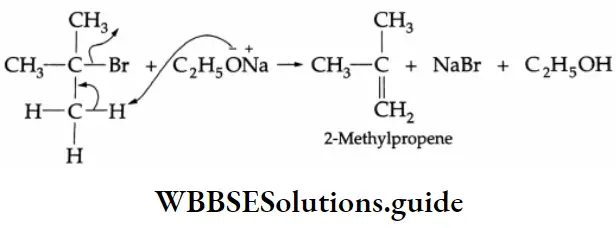
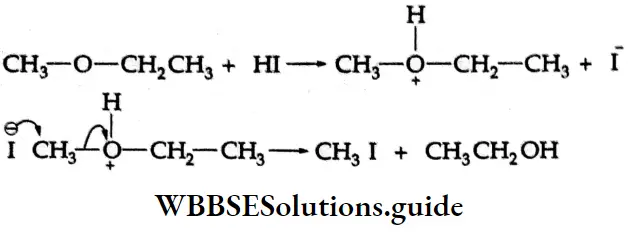
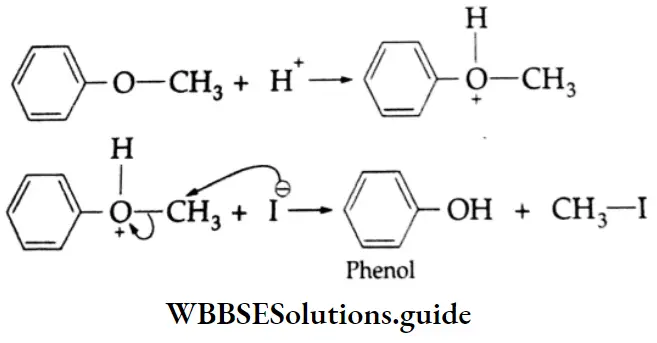
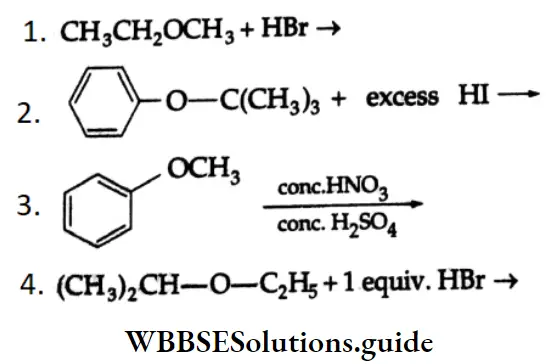
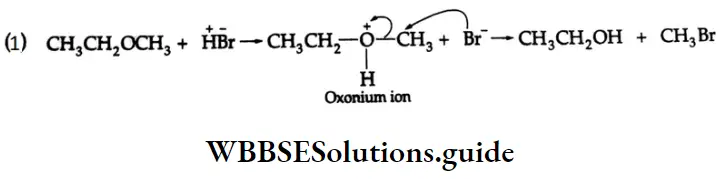
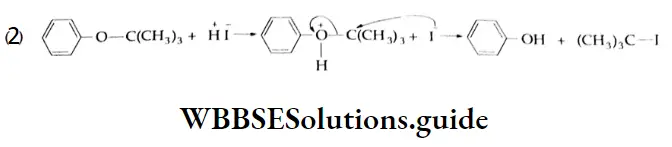
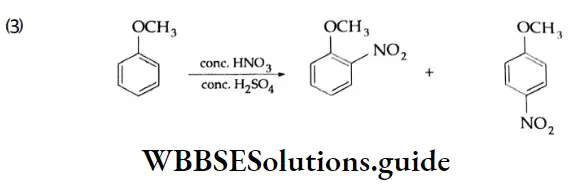
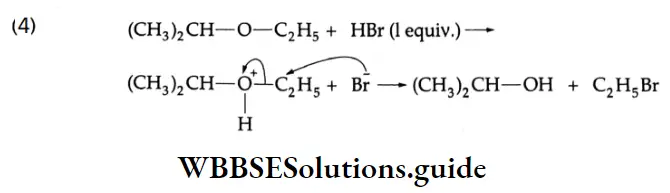






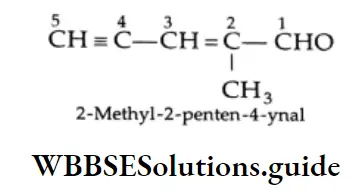





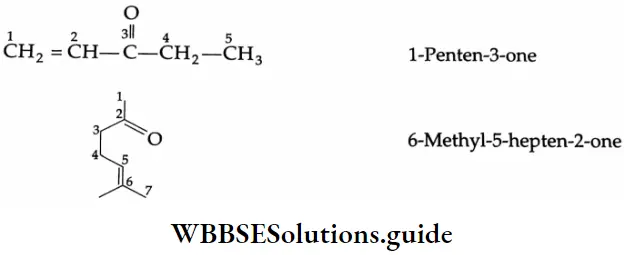


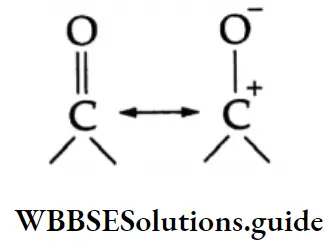

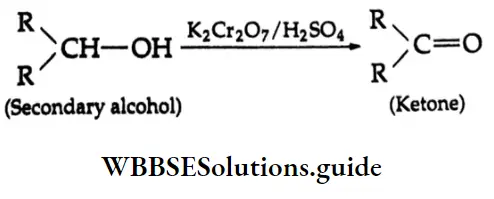

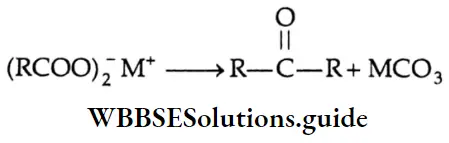

















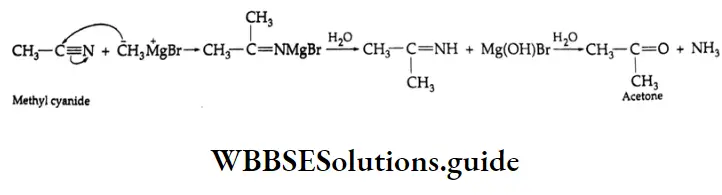











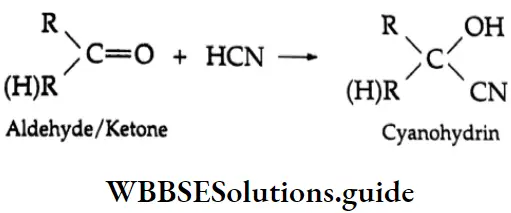







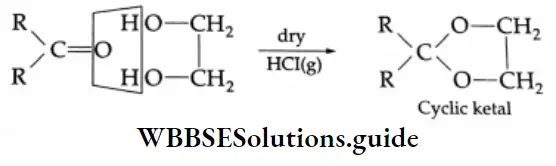









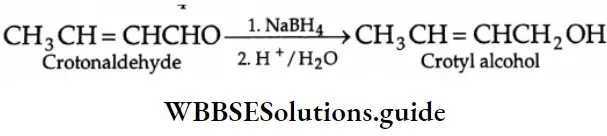




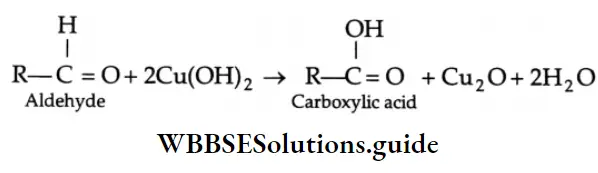

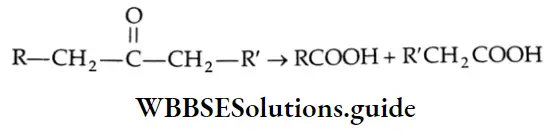
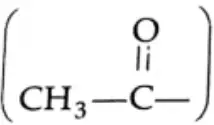 are oxidised by sodium hypohalite (NaOX). Sodium salts of carboxylic acids containing one carbon less than the methyl ketone are formed. The methyl group forms a haloform.
are oxidised by sodium hypohalite (NaOX). Sodium salts of carboxylic acids containing one carbon less than the methyl ketone are formed. The methyl group forms a haloform.


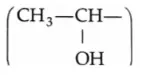 group also respond positively to the iodoform test.
group also respond positively to the iodoform test.








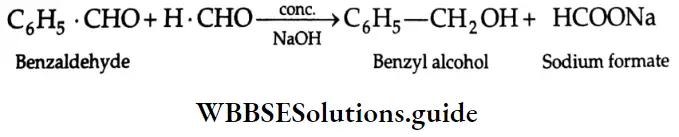










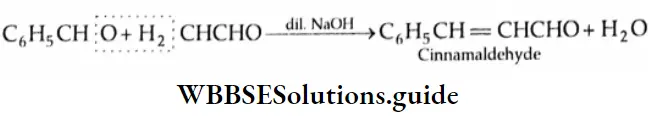







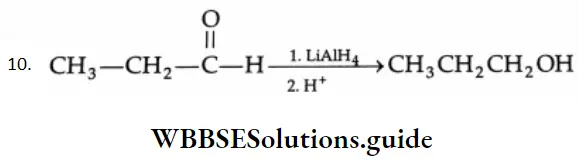

 are termed carboxylic acids. The group is so named because it can be considered as a combination of the carbonyl and hydroxyl groups.
are termed carboxylic acids. The group is so named because it can be considered as a combination of the carbonyl and hydroxyl groups.
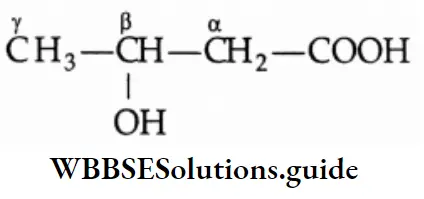




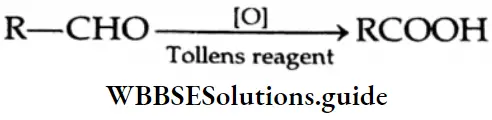




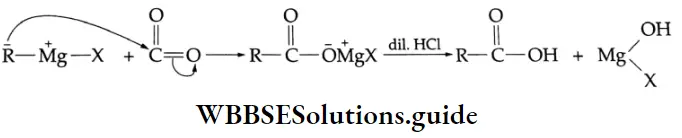
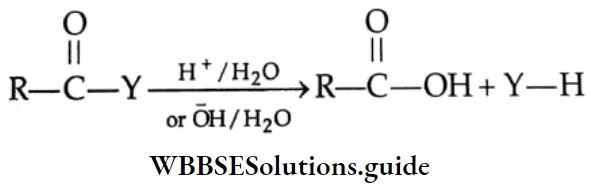








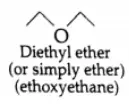
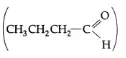 is more polar in nature than the C-O-C group in ethoxyethane (C2H5-O-C2H5). The dipole-dipole attraction of the molecules of butanal is stronger than in ethoxyethane. Therefore, the boiling point of butanal is higher than that of ethoxyethane.
is more polar in nature than the C-O-C group in ethoxyethane (C2H5-O-C2H5). The dipole-dipole attraction of the molecules of butanal is stronger than in ethoxyethane. Therefore, the boiling point of butanal is higher than that of ethoxyethane.