
- Chapter 1 Motions of The Earth
- Chapter 2 Determination Of The Position Of A Place On The Earth’s Surface
- Chapter 3 Air Pressure
- Chapter 4 Landform
- Chapter 5 River

Question 1. What are groups and sub-groups?
Answer:
Groups:
Groups Mendeleev’s periodic table shows that the elements with related chemical properties fall in one below the other forming vertical columns called groups. There are nine group from group I to group VIII and O (zero).
Sub-groups:
Sub-groups Each of the groups from I to VII has been divided into two. Sub-groups A and B.
“WBBSE Class 10 Physical Science Chapter 8 long answer questions, Physical and Chemical Properties of Matter”
Question 2. What are halogen elements? In which do they belong?
Answer:
Halogen elements: The four strongly electro-negative non-metals fluorine (F), chlorine (CI), bromine (Br) and iodine (1) form a family of closely allied elements known as the halogens meaning literally sea self-producer as these elements react with most metals to form compounds similar to sea-salt, sodium chloride.
Position in periodic table: Habzen elements placed in group VII B of the periodic table. The electro-negative character of the elements increases from left to right in a period. So, the strongly electro-negative elements are in the most extreme right group i.e. in group VII B of the period table.

Question 3. What are called alkali metals? In which group do they belong? State the similarity of their chemical properties.
Answer:
Alkali metals: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb) and Caesium (Cs). These five metals are generally known as alkali metals.
Position in the periodic table: The alkali metals are included in the A subgroup of the first group
Similarity:
“Class 10 WBBSE Physical Science Chapter 8 long answer questions, Properties of Matter study material”
Question 4. Discuss the position of hydrogen in the periodic table. Answer: Position of hydrogen in the periodic table :
Reasons for placing in group IA :
Reasons for placing in group VIIB:
Due to such varied tendencies for occupying a seat in the periodic table, hydrogen is often called a naughty element or rogue element.
Question 5. Where are the inert gas elements placed and why?
Answer:
Position of inert gas elements in the periodic table:
Question 6. How is copper purified by the electrolysis method?
Answer:
Purification of copper by electrolysis method :
1. Electrolyte: 15% of CuSO4, solution (aqueous) containing (5-10) % sulphuric acid at 50°C is taken in a voltameter.
2. Electrodes:
3. Electrolysis:
4. Reaction: \(\mathrm{CuSO}_4 \rightleftharpoons \mathrm{Cu}^{2-}+\mathrm{SO}_4^{2-}\)
“WBBSE Class 10 Physical Science Chapter 8, Physical and Chemical Properties of Matter long answer solutions”
Question 7. How is aluminium extracted from the electrolysis method?
Answer:
Extraction of aluminium by electrolysis method :
1. Electrolytes:
2. Electrodes:
Reaction:
At cathode: Al3 + 3e → Al↓
At anode : 3F – 3e→ 3F
⇒ Al2O3 +6F → 2 AIF3+3O
⇒ 6O →3O2
Question 8. Draw the diagram of the covalent radius of hydrogen.
Answer:
The diagram of the covalent radius of hydrogen
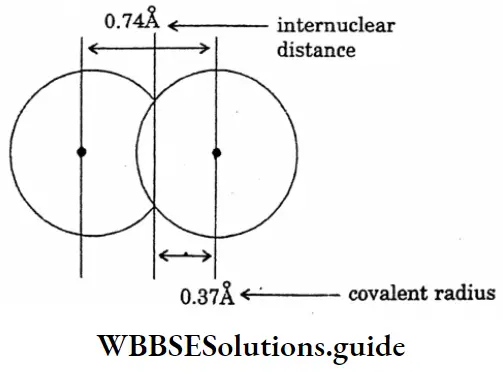
Question 9. Draw the diagram of the showering comparison of Vanderwaal’s radius and covalent radius.
Answer:
The diagram of the showering comparison of Vanderwaal’s radius and covalent radius
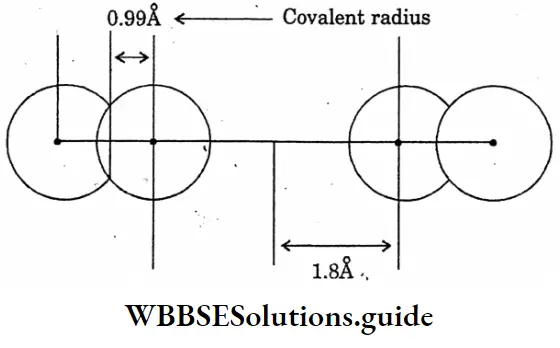
Question 10. State the contact process for the manufacture of sulphuric acid.
Answer:
Contact process for the manufacture of sulphuric acid :
Principle:
1. Formation of SO2: SO2 is prepared by burning sulphur or iron pyrites in excess of air.
Equation: S+ O2 = SO↑ Or,
4 Fes,+ || O2 = 2Fe2O3 + 8SO2 ↑
(Iron pyrities)
2. Formation of sulphur trioxide (SO3): SO3 is prepared by the oxidation of sulphur dioxide with oxygen (from air) in the presence of platinised asbestos or, V2 O5 as catalyst at 450°C.
Equation: \(2 \mathrm{SO}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{SO}_3+192.5 \mathrm{Kj}\)
3. Formation of oleum:
Now sulphur trioxide thus produced is not allowed to react with water directly sulphur trioxide absorbs concentrated sulphuric acid 98% turning it as fuming sulphuric acid or oleum.
Equation: SO3+ H2SO4= H2S2O7 (Oleum)
4. Dilution of oleum to sulphuric acid: Pure and concentrated sulphuric acid is produced by adding slowly a requisite amount of water in fuming sulphuric acid.
Equation:
H2S2O7 >+ H2O = 2 H2SO4
Question 11.
Answer:
The electronic configuration of the element X is: 2, 8, 7,
“WBBSE Class 10 Properties of Matter long answer questions, Physical Science Chapter 8”
Question 12. The atomic number of element A is 20 and that of another element B is 17. Write down their electronic configuration. Will they produce on electrova- lent compound or a covalent compound? What will be their valencies in that case?
Answer:
They will produce an electrovalent compound.
Explanation:
An atom A will give up two (2) valence electrons. Each of the two atoms of B will capture one electron. In the process atoms of A and B attain a stable octet state and atoms of A will be positive ions and those of B will be negative ions.
“Class 10 WBBSE Physical Science Chapter 8, Physical and Chemical Properties of Matter detailed answers”
Question 13. What is the difference between ionic compounds and covalant compounds?
Answer:
Difference between ionic and covalent compounds:
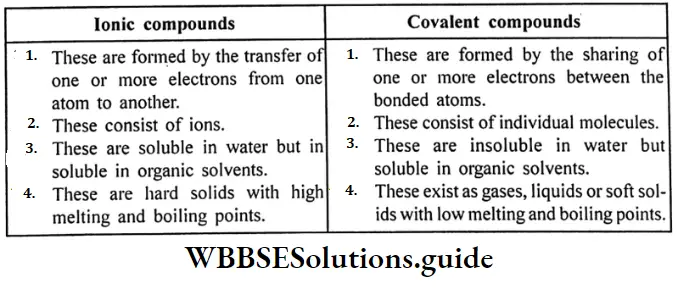
Question 14. How ammonia is prepared in the laboratory?
Answer:
Laboratory preparation of Ammonia:
Ammonia is not collected through the downward displacement of water because it is highly soluble in water.
Equation of the reaction:
2NH4 CI + Ca (OH)2 = 2NH3 ↑ + CaCl2+ 2H2O
↑
2NH4CH+CaO = 2NH3↑ + CaCl2+H2O
Question 15. Prove by experiment that ammonia is dissolved in water and produces an alkaline solution.
Answer:
Fountain experiment:
Arrangement of the experiment: A flask is filled with dry ammonia gas and its mouth is corked. The flask is kept inverted position and is clamped to a stand.
Through a hole in the cork one end of a glass tube is introduced inside the flask. This end of the tube inside the flask is shaped into a jet. The other end of the tube dips in some red litmus solution taken in a beaker.
Explanation:
Conclusion: This experiment proves that ammonia is highly soluble in water and the aqueous solution is alkaline.
“WBBSE Class 10 Physical Science Chapter 8, Physical and Chemical Properties of Matter long answer key”
Question 16. Draw the diagram of the formation of sodium chloride.
Answer:
The diagram of the formation of sodium chloride
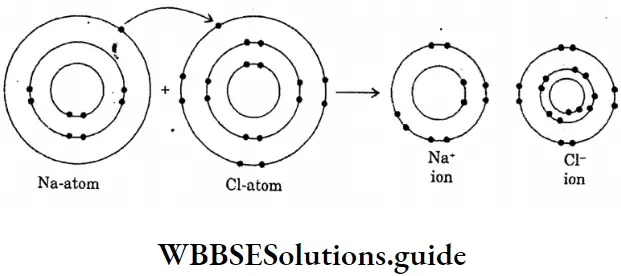
Question 17. Draw the diagram of sodium fluoride :
Answer:
The diagram of sodium fluoride
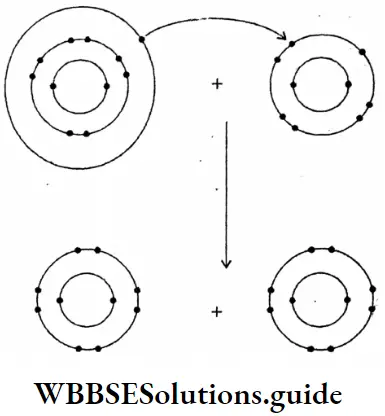
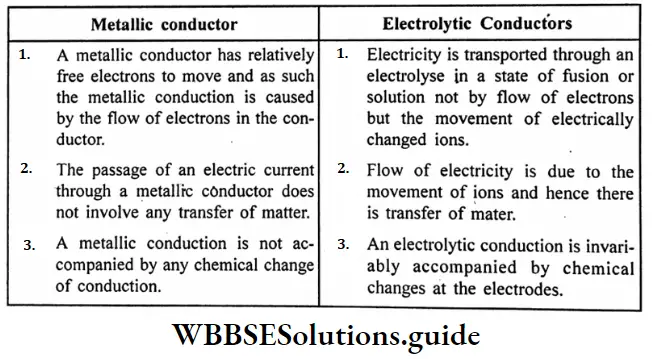
Question 18. What are the main points of difference between metallic conductors on electrolytic conductors?
Answer:
Difference between metallic conductors and electrolytic conductors :
Question 19. What changes are taking place during the electrolysis of an electrolyte?
Answer:
Changes take place during the electrolysis of an electrolyte:
“Class 10 WBBSE Physical Science Chapter 8, Properties of Matter important long answer questions”
Question 20. What are Biodegradable and Non-biodegradable materials?
Answer:
Question 21. Briefly explains thermite welding of iron and steel.
Answer:
Thermite welding of iron and steel
A mixture of 3 parts of ferric oxide and I part of aluminium powder is called a thermite mixture. When the mixture is ignited ferric oxide is reduced to metallic iron by aluminium. Because of the evolution of large amounts of heat, the temperature rises to Magnesium tape
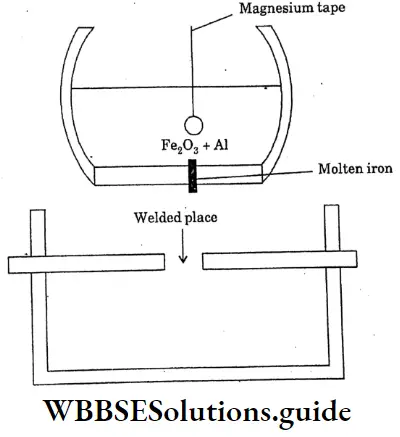
2500c The molten iron thus produced is allowed to fall on the red hot broken rails broken machine parts ect can be welded without removing them from their original sites.
Question Explain the separation of isotopes through the diffusion method.
Answer:
The separation of isotopes through the diffusion method
Though the isotopes have identical chemical properties, some of their physical properties may be quite different because of the difference in their mass numbers.
The most important physical properties of this type are the rate of diffusion, boiling point, and radioactive properties isotopes of elements can be separated by physical methods based on their properties. They may be separated almost completely using a special type of instrument called the mass spectrometer.
Diffusion method:
Since the rate of diffusion of a gas varies inversely to the square root of its molecular mass. Gas molecules with lower atomic mass numbers diffuse more quickly than their heavier sisters.
This principle was very effected actively used by Aston for the separation of isotopes of neon. The more is the difference in the atomic mass numbers, the more effective is the separation.

Question 2. Write the cause of radioactivity.
Answer:
Cause of radioactivity:
The main reason for the radioactivity of an element, as we have noted earlier, is the instability of its nucleus. Such an unstable nucleus tries to attain stability through the expulsion of particles and rays. If there are too many protons in a nucleus they will fall apart and the element will disintegrate. Again, it the nucleus is too heavy with too many neutrons it disintegrates.
“WBBSE Class 10 Physical Science Chapter 7 long answer questions, Atomic Nucleus”
It is observed that in order to attain stability the neutron: Proton ratio must be mear about unity for the elements with low atomic masses while for the heavier elements, it must not exceed 1.5.
Thus we see that most of the radioactive elements have very high atomic masses in 92U234 in comparison to their atomic numbers U has a neutron; proton ratio of 1.537 while radium has 1.56. This is why radium is much more radioactive than uranium.
Question 3. Short Note-Radioactive Equilibrium.
Answer:
Radioactive Equilibrium :
The product element formed by the radioactive disintegration of an element may also be radioactive. The process goes an until the chain is terminated by the birth of a non-radioactive stable element at the end of a series. If we keep a sample of a radioactive element or its salt for a sufficient time, a situation arises when the rate of creation and the rate of disintegration of a product element become equal.
This state for a radioactive series is called a radioactive equilibrium. We see that at the equilibrium.
⇒ \(\frac{\mathrm{dN}_1}{\mathrm{dt}}=\frac{\mathrm{dN}_2}{\mathrm{dt}}=\frac{\mathrm{dN}_3}{\mathrm{dt}}\)
∴ λ1 N1 = λ2 N2 = λ3 N3 = …………
Question 4. Short Note Mass spectrometric method to separate the isotope.
Answer:
Mass spectrometric method:
In this method positive particles of the required gas or vapour formed by bombardment with electrons are first passed through electrical plates with a potential difference (X) of about 1000 volts and two collimating slits. The electrical energy Xe, where e is the charge of the positive particles, is equal to the kinetic energy of the particles.
That is Xe =½ mu2 where m and v are the mass and the charge of the positive particles. The positive particles energing through a slit with almost equal kinetic energy pass a magnetic field (H) between two semicircular.
Question 5. What is binding energy? Short Note-Stability of an atomic nucleus packing fraction.
Answer:
Binding energy
The amount of energy that can be obtained by the amount of mass equal to the mass defect is known as the binding energy.
Packing faction = \(\frac{\text { Actual mass of a nucleus }- \text { Mass number }}{\text { Mass number }} \times 10^4\)
“Class 10 WBBSE Physical Science Chapter 7 long answer questions, Atomic Nucleus study material”
Question 6. What are isotones? Define-nuclear isomers.
Answer:
Isotones
The are elements which differ in both their atomic numbers as well as most numbers but they have the same number of neutrons in their nuclei. They are called isotones.
Nuclear isomers
The two nuclei of an element may have the same atomic number and the same mass number yet differ in their internal nuclear energies. These nuclei are called nuclear isomers.
Question 7. Explain the separation of isotopes by distillation under low pressure.
Answer:
Separation of isotopes by distillation under low pressure
Distillation under low pressure: When a liquid like mercury is distilled under low pressure the lighter isotopes come out more quickly and condense on a cooler surface. The condensate is collected, melted and subjected to the same procedure. B repeating the process the condensate can be made richer in the lighter variety.
Question 8. What do you mean by isobars and isotones?
Answer:
Isobars
Isobars The nuclide of different chemical elements having the same mass number but different atomic numbers are called isobars.
Examples:
Isotones: The nuclides of different chemical elements having the same number of neutrons but different atomic numbers are called isotones.’
Examples: 1H3, 2He4
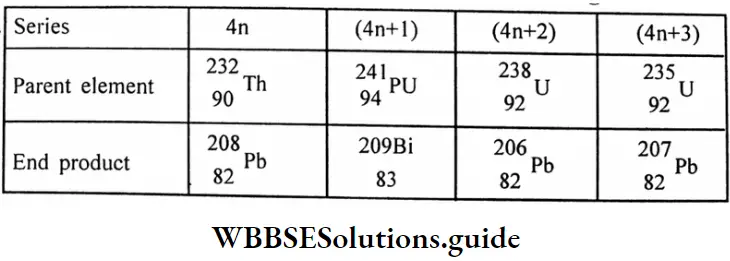
Question 9. What are the parent and end products of different disintegration series?
Answer:
Series
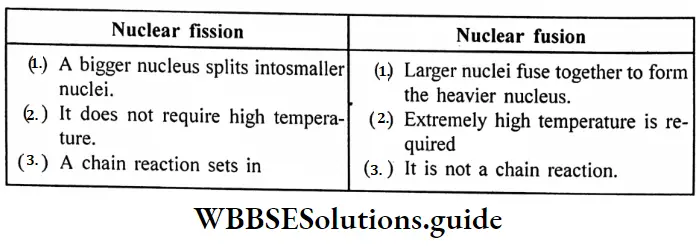
Question 10. What are the differences between nuclear fission and nuclear fusion?
Answer:
Difference between nuclear fission and nuclear fusion :
Question 11. What do you mean by Curie and Becquerel?
Answer:
Curie and Becquerel
“WBBSE Class 10 Physical Science Chapter 7, Atomic Nucleus long answer solutions”
Question 12. A radioactive substance decays at such a rate that after 46 days only 0.25 of its original amount is left to calculate its disintegration constant.
Answer:
Given
A radioactive substance decays at such a rate that after 46 days only 0.25 of its original amount is left
Let, the original amount be No then Amount after 46 days, Nt = 0.25 x No For a nuclear decay,
λ = \(\frac{2.303}{\mathrm{t}} \log \frac{\mathrm{No}}{\mathrm{Nt}}\)
= \(\frac{2.303}{46}\) log4 day-1
= 0.0301day-1
Question 13. A certain nuclide has a half-life of 60 min. If a sample containing 600 atoms is allowed to decay for 90 min, what will be the remaining atoms?
Answer:
Given
A certain nuclide has a half-life of 60 min. If a sample containing 600 atoms is allowed to decay for 90 min
λ = \(\frac{0.693}{60} \mathrm{~min}^{-1}\)
= \(\frac{2.30^3}{90} \log \frac{\mathrm{No}}{\mathrm{N}}\)
⇒ log \(\frac{\mathrm{No}}{\mathrm{N}}\) = 0.4514 Or,
⇒ log \(\frac{\mathrm{No}}{\mathrm{N}}\)= 2.828
∴N = \(\frac{600}{2.823}\)
N= 212
“WBBSE Class 10 Atomic Nucleus long answer questions, Physical Science Chapter 7”
Question 14. The activity of a sample of radioactive element A100 is 6.02 curie. Its decay constant is 3.7 x 10’S’ which calculates the initial mass of the sample.
Answer:
Given
The activity of a sample of radioactive element A100 is 6.02 curie. Its decay constant is 3.7 x 10’S’
Activity = \(\lambda \times \frac{\mathrm{wt}}{\text { at. wt }} \times 6.023 \times 10^{23}\)
\(6.02 \times 3.7 \times 10^{10}=3.7 \times 10^4 \times \frac{w}{100} \times 6.023 \times 10^{23}\)
W= \(\frac{6.02 \times 3.7 \times 10^{10} \times 100}{3.7 \times 1.04 \times 6.023 \times 10^{23}}\)
W= \(10^{-15} \mathrm{~g}\)
Question 15. The half-life period of C is 5760 years. An old piece of wood has a disintegration rate which is 25% of the disintegration rate of an equal weight of a new piece of wood.
Answer:
Given
The half-life period of C is 5760 years. An old piece of wood has a disintegration rate which is 25% of the disintegration rate of an equal weight of a new piece of wood.
Let the rate of disintegration of the new piece = 100
The rate of migration of old pieces = 25
K = \(\frac{0.693}{\frac{t_1}{2}}=\frac{0.693}{5760}\)
t= \(\frac{2.303}{K} \log \frac{a}{a-x}\)
=\(\frac{2.303}{K} \log \left(\frac{\mathrm{lo}}{\mathrm{It}}\right)\)
∴ \(=\frac{2.30 \times 5760}{0.693} \log \frac{100}{25}\)
t= 11523 years.
Question 1. State Joule’s laws of heating effect of current.
Answer:
Joule’s Laws (1841):
1. First Law:
The amount of heat produced in a conductor in a given interval of time of proportion to the square of the current passed. Thus if H be the amount of heat generated in a conductor having resistance R when current 1 passes through it in time t, then
⇒ Hα I2 (When R and t are kept constant)
2. Second law:
The amount of heat produced by a given current in a given time is proportional of the resistance of the conductor.
⇒ H α R (When I and t are kept constant)
3. Third Law:
The amount of heat produced in a given conductor by a given current is proportional to the time for which the current passes.
⇒ Hα t (When I and R are kept constant)
“WBBSE Class 10 Physical Science Chapter 6 long answer questions, Current Electricity”
Combining the three laws, we have:
⇒ H α I2 RT (When 1, R and t vary) or,
H = \(\frac{I^2 R T}{J}\)
IRT (J= mechanical equivalent of heat = 4.2 joule (calorie)
If I am in ampere, R in ohm, t in second, and H in calorie, then
H= \(\frac{I^2 R T}{J}\)
H= 0.241 RT calorie.

Question 2. Short Note:
Answer:
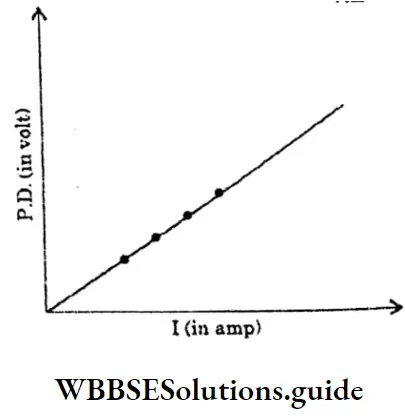
1. Ohmic Resistance :
The resistors, which obey Ohm’s Law are said to have ohmic resistances.
Example: All metal or metallic alloy.
2. Non-ohmic resistance :
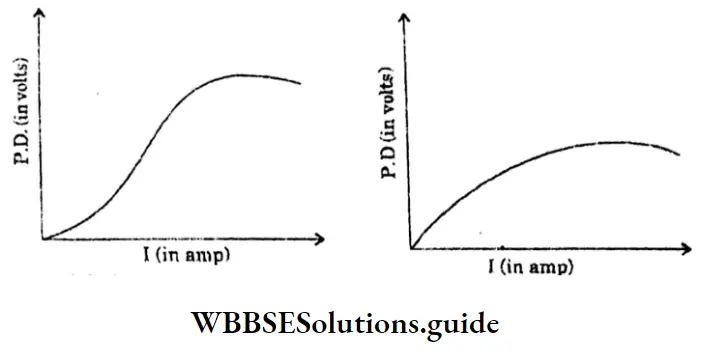
The resistors which do not obey Ohm’s law are said to have non-ohmic resistance.
Example: Electronic value.
“Class 10 WBBSE Physical Science Chapter 6 long answer questions, Current Electricity study material“
Question 3. Two resistors of 30W and 60W are connected in parallel in an electric circuit. How does the current passing through the two resistors compare?
Answer:
Given
Two resistors of 30W and 60W are connected in parallel in an electric circuit
The potential difference across 30W = B potential difference across 60W
i.e I1 R1= I2 R2 Or,
⇒ \(\frac{I_1}{I_2}=\frac{R_2}{R_1}\)
= \(\frac{60 \Omega}{30 \Omega}\)
= 2
Question 4. Short Note-Direct Current.
Answer:
Direct Current:
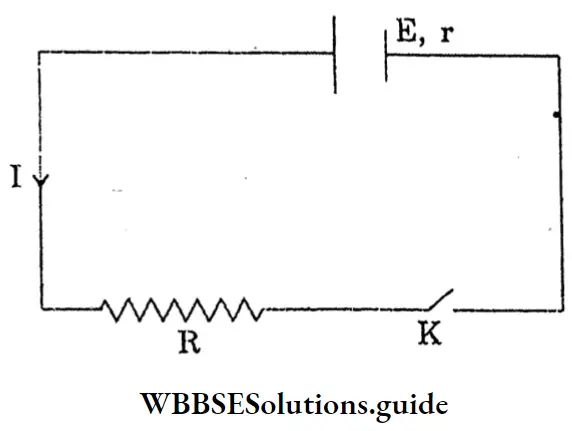
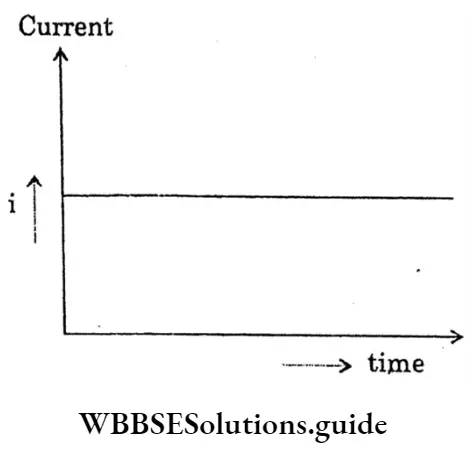
If a resistor connects with the two terminals of an electric cell, then through the resistor a steady current of constant magnitude flows in the same direction (from the positive pole of the cell to the negative pole) and this is called a direct current.
“WBBSE Class 10 Physical Science Chapter 6, Current Electricity long answer solutions”
Question 5. A resistance of 6 Ω is connected with a cell of em. f. 1.5 and negligible internal resistance calculate the current flowing through it.
Answer:
Given
A resistance of 6 Ω is connected with a cell of em. f. 1.5 and negligible internal resistance
V= IR Or, I= \(\frac{V}{R}\)
= \(\frac{1.5}{6}\)
= \(\frac{1}{4}\)
V = 0.25 Amp
The current flowing through it is 0.25 Amp
Question 6. Find the specific resistance of the material of a wire of length 100cm, area of cross-section 0.2 cm,2, and resistance 2 ohms.
Answer:
R = P× \(\frac{1}{\mathrm{~A}}\)
∴ P= \(\frac{\mathrm{RA}}{\mathrm{1}}\)
= \(\frac{20 \times 0.2}{100}\)
= 40 ×10-4 Ohm-cm
So,
R = 20hm
1 = 100 cm
A = 0.2 cm2
P= ?
“WBBSE Class 10 Current Electricity long answer questions, Physical Science Chapter 6”
Question 7. Find effective resistance of the resistors 2 ohm, 4 ohm, 5 ohm connected in
Answer:
We know, for series combination equivalent resistance
R= r1+ r2+r3
= 2+4+5
= 11 ohm.
We also know, for parallel combination equivalent resistance.
⇒ \(\frac{1}{R}=\frac{1}{r_1}+\frac{1}{r_2}+\frac{1}{r_3}\)
= \(\frac{1}{2}+\frac{1}{4}+\frac{1}{5}\)
= \(\frac{19}{20}\)
∴ \(\frac{1}{R}\)= \(\frac{19}{20}\) Or,
= \(\frac{20}{19}\)
R = 1.05 Ohm
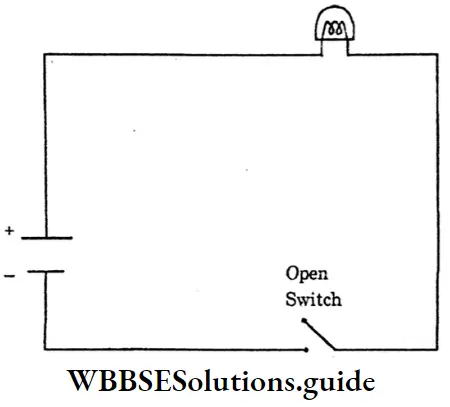
Question 8. Short Note-Electric Circuit.
Answer:
Electric Circuit: A continuous conducting path between the terminals of a source of electricity, is called an electric circuit.
Open electric → An electric circuit in which the flow of current stops, because of an open switch is called an open electric circuit. Closed electric
Open Electric Circuit:
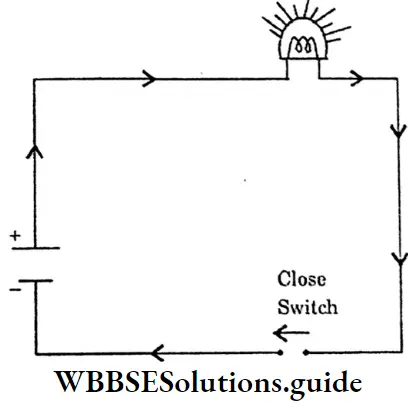
Closed Electric Circuit:
Circuit → An electric circuit in which a current flows continuously, because the switch is closed is called a closed electric circuit.
Question 9. There are two copper wires of equal length. The radius of one is twice the other. Find the ratio of their resistances.
Answer:
Given
There are two copper wires of equal length. The radius of one is twice the other.
We know, R= p \(\frac{1}{A}\)
= \(p \frac{1}{\lambda r^2}\)
(r= radius of the wire)
Since, the length and material are the same.
⇒ \(\mathrm{R} \alpha \frac{1}{\mathrm{r}^2}\)
i.e. \(\frac{R_1}{R_2}=\frac{r_2^2}{r_1^2}=\frac{\left(2 r_1\right)^2}{r_1^2}\)
= 4
∴ The thinner wire has a resistance four times the resistance of the thicker wire.
“Class 10 WBBSE Physical Science Chapter 6, Current Electricity detailed answers”
Question 10. Establish the relation between the emf, terminal voltage and internal resistance.
Answer:
The relation between the emf, terminal voltage and internal resistance
Let, a cell of emf E and internal resistance r is connected to an external resistance R
The total resistance of the circuit = R+r
So, the current drawn from the cells
I= \(\frac{e . m . f \text { of the cell }}{\text { total resistance }}\)
I= \(\frac{E}{R+r}\)
E= I (R+r)
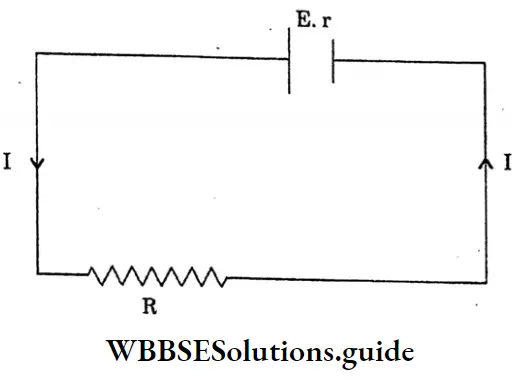
The terminal voltage of the cell, V = IR voltage (v) drops due to internal resistance -I As the work is done carrying a unit positive charge once through a complete circuit,
E = V + v
v = E-V
Internal resistance r = \(\frac{V}{I}\)
= \(\frac{E-V}{I}\)
= \(\frac{E-V}{V / R}\)
= \(R \cdot\left[\frac{E}{V}-1\right]\)
Question 11. A current of 0.5 ampere passes through a wire of resistance 2.5 ohm. for 1 hour. Find the heat produced.
Answer:
Given
A current of 0.5 ampere passes through a wire of resistance 2.5 ohm. for 1 hour.
We know,
H = \(\frac{I^2 R T}{J}\)
= \(\frac{I^2 R T}{4^2}\)
∴ H \(\frac{(0.5)^2 \times 2.5 \times 3600}{4^2}\)
= 535.7 caloric
So,
I = 0.5 ampere
R = 2.5 ohm
t = 1hr.
= 3600 sec.
H =?
“WBBSE Class 10 Physical Science Chapter 6, Current Electricity long answer key”
Question 12. The resistance of a wire of cross-section area 0.01 cm2 is 10 ohm. What is the length of the wire? The specific resistance of the wire is 50 × 10-6 ohm-cm.
Answer:
Given
The resistance of a wire of cross-section area 0.01 cm2 is 10 ohm.
We know,
R = p = \(\frac{\ell}{\mathrm{A}}\) Or,
l= \(\frac{\mathrm{RA}}{p}\) Or,
l= \(\frac{10 \times 0.01}{50 \times 10^{-6}}\)
∴ l= 2000 cm
So,
R= 10 Ohm
A= 0.01 cm2
p= 50 × 10-6
Ohm–cm
1= ?
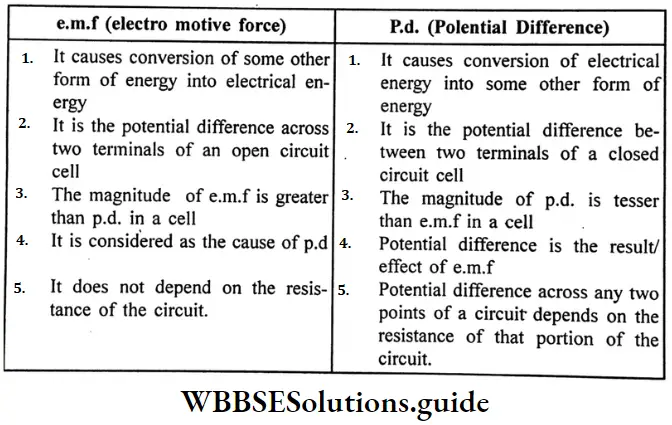
Question 13. What is the difference between e.m.f and p.d?
Answer:
Difference between e.m.f. and p.d. : e.m.f (electromotive force):
Question 14. A resistance of 6Ω is connected with a cell of e.m.f., 1.5 V, and negligible internal resistance, Calculate the current flowing through it.
Answer:
Given
A resistance of 6Ω is connected with a cell of e.m.f., 1.5 V, and negligible internal resistance
V = IR
I = \(\frac{V}{R}\)
I = \(\frac{1.5}{6}\)
I = \(\frac{1}{4}\)
= 0.25 Amp.
“Class 10 WBBSE Physical Science Chapter 6, Current Electricity important long answer questions”
Question 15. A cell of e.m.f. 1.8v is connected to an external resistance of 22 when p.d. recorded at its terminal is 1.6v. Find the internal resistance of cell.
Answer:
Given
A cell of e.m.f. 1.8v is connected to an external resistance of 22 when p.d. recorded at its terminal is 1.6v.
E = 1.8V, V 1.6 volt, R = 2.2
Internal resistance = \(\frac{R(E-V)}{V}\)
= \( \frac{2(1.8-1.6)}{1.6}\)
= 0.25.Ω
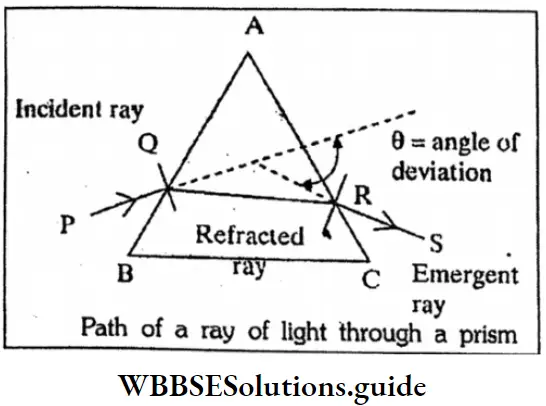
Question 1. Draw a labelled diagram to show refraction of a light ray through a prism.
Or
Path of a ray of light through a prism
Answer:
Path of a ray of light through a prism:
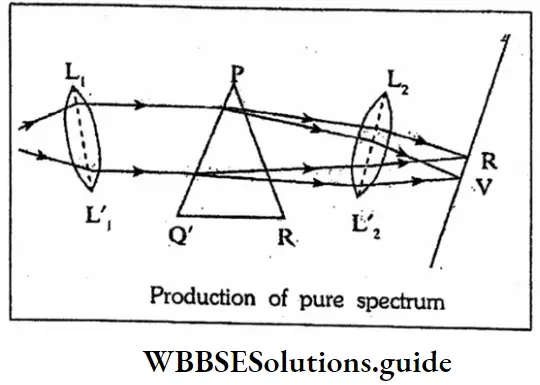
Question 2. What are the conditions of formation of pure spectrum?
Or
Production of pure spectrum
Answer:
Conditions of formation of pure spectrum:
“WBBSE Class 10 Physical Science Chapter 5 long answer questions, Light”
Question 3. How will you explain twinkling of stars?
Answer:
Twinkling of stars:
The light coming from stars is refracted continuously by different layers of the atmosphere before coming to us. Due to there repeated refractions, The apparent position of a star is different from its actual position. The temperature and density of air in the atmosphere continuously changing and so apparent position of the star also changes continuously. Due to this continuous change in the apparent position of a star, it appears to be twinkling.

Question 4. A ray of light shows no disperse emerging from a glass slab. Explain why?
Answer:
A ray of light shows no dispersion on emerging from a glass slab
A rectangular glass slab cut diagonally into two pieces behaves like a pair of prisms placed side by side in reverse order. So the light dispersed by the first prism, recombines by the second prism. Thus there is no dispersion of light in a rectangular glass slab, bulb it is only laterally displaced.
“Class 10 WBBSE Physical Science Chapter 5 long answer questions, Light study material”
Question 5. State the laws of reflection.
Answer:
The laws of reflection:
⇒\(\frac{sini}{sin r}\) = constant
= 1μ2
“WBBSE Class 10 Physical Science Chapter 5 long answer questions, Light”
Physics Class 10 WBBSE Question 6. Deseribe about the action of periscope.
Answer:
The action of periscope
The mirror M, forms a virtual image I1, of the object O and this image 1, acts as virtual object-for the mirror M2, which forms an image I2, which is seen by the eye the final image F2, is also virtual. Since light rays from the object are reflected by two mirrors, the lateral inversion caused by the first mirror is reversed by the second mirror. This males the final image to appear with on lateral invession.
Question 7. State the laws of refraction.
Or
What is snell’s law?
Answer:
Thus, it i and r be the angles of incidence and of refraction respectively when the ray passes from medium 1 to medium 2.
\(\frac{sini}{sinr}\)= Constant
= 1μ2
The second law of refraction was devolped by W, Snell and is know a Snell’s law.
“WBBSE Class 10 Physical Science Chapter 5, Light long answer solutions”
Question 8. Establish the relation between 1μ2 and 2μ1.
Answer:
The relation between 1μ2 and 2μ1
From Snell’s law: sin1/sinr = 2μ1
Where 2 u1, is the R. I. of medium 1 w.r.t. medium combining equations we get
1μ2×2μ1= \(\frac{sin1}{sinr}\)×\(\frac{sinr}{sin1}\) = 1
Or
1μ2X = 1/2μ1
Thus R.I. of medium 2 w.r.t. medium 1 is the reciprocal of R.I of medium 1 w.r.t. medum 2
Question 9. State generalised Snell’s law.
Answer:
Generalisation Of Snell’s law
If i1 and i2 be the angle of incidence and of refraction respectively then we have from Snell’s law
sini1/sini2 =1, μ2
We can write
1, μ2 =μ2 /μ1
⇒μ2/μ1
∴ sini1/sini2= μ2/μ1
Or,μ1 sini1 = μ2 sini2 → Generalised Snell’s Law
Question 10. How far from a concave mirror of radius 2m would you place the object to get an image magnified 3 times?
Answer:
Given m = ± 3 \(\frac{v}{u}\) i.e.u= 7.3u
For real image v = -3u, r = -2m and u is negetive.
So, from relation \(\frac{1}{v}\)+ \(\frac{1}{u}\)= \(\frac{2}{r}\)
Or, – \(\frac{1}{3u}\) – \(\frac{1}{u}\) = – \(\frac{2}{2}\)
For virtual image u =+ 3u, r = -2m and u is negative
So =-1 or, u= = 0.67m
= \(\frac{1}{3u}\) – \(\frac{1}{u}\) = – \(\frac{2}{2}\) Or \(\frac{1-3}{3u}\) = -1
u= \(\frac{2}{3}\)
= 0.67 m
“WBBSE Class 10 Light long answer questions, Physical Science Chapter 5”
Question 11. A concave mirror forms an image of 20 cm high object on a screen placed 5m away from the mirror. The height of the image is 50 cm. Find the focal lenght of the mirror and the distance between mirror and the object.
Answer:
Given
concave mirror forms an image of 20 cm high object on a screen placed 5m away from the mirror. The height of the image is 50 cm.
size of object = 20cm.
Size of the image = 50cm.
u = -5m.

= \(\frac{-10}{7}\)
= −1.43m
And distance between the mirror and the object = 2m.
Question 12. Light falls from glass of refractive index 1.5 to air. Find the angle of incidence for which the angle of deviation is 90°.
Answer:
Light falls from glass of refractive index 1.5 to air.
Refractive index of glass is 1.5. So, the critical angle of glass to air medium is given by
⇒ − q= sin-1(\(\frac{1}{u}\)) sin-1(\(\frac{1}{1.5}\))
= 41.8°
So, reflection in the glas medium takes only when the angle of incidence exceeds 41.8°. Now the angle of deviation. Would be 90° when the angle between the incident ray and the reflected ray becomes 90° i.e. the angle of incidence be equal to 45°.
Question 13. Where should an object be placed from a converging lens of focal length 20cm, so as to obtain a real image of magnification 2 ? u =2, or, v = + 2u, f= + 20cm
Answer:
Given m =\(\frac{u}{u}\)= 2 or , v= +2u, f= +20cm
∴ \(\frac{1}{v}\)– \(\frac{1}{u}\) = \(\frac{1}{f}\) or,
= \(\frac{1}{2u}\) – \(\frac{1}{u}\)
= \(\frac{1}{20}\) or,
= \(\frac{1-2}{2u}\)
∴ u= \(\frac{− 20×1}{2}\)
= – 10 cm
i.e. object to be placed 10 cm in fornt of the lens.
“Class 10 WBBSE Physical Science Chapter 5, Light detailed answers”
Question 14. Two thin lens of focal lengths 15cm. and 30cm. respectively are kept in contact with each other. What is the power of the combined system?
=
Answer: Given
Two thin lens of focal lengths 15cm. and 30cm. respectively are kept in contact with each other.
f1= 15cm, f2 = 30cm.
Then focal length F of the equivalent lens
\(\frac{1}{F}\)= 1/f1+1/f2
\(\frac{1}{15}\) + \(\frac{1}{30}\)
= \(\frac{2+1}{30}\)
= \(\frac{1}{10}\)
Power of the combination = \(\frac{100}{F}\)
= \(\frac{100}{10}\)
= +10
Question 15. The dew drops deposited on hairy leaves appear glittering way?
Answer:
The hairs out the leaf and the surface tension of water create an air gap under the dew drop. Hence some of the rays trying to pass from the drop to the side air gap suffer total internal reflection. Such reflected rays may reach our eyes after refraction through the upper surface of the drop and the drop appears glittering.
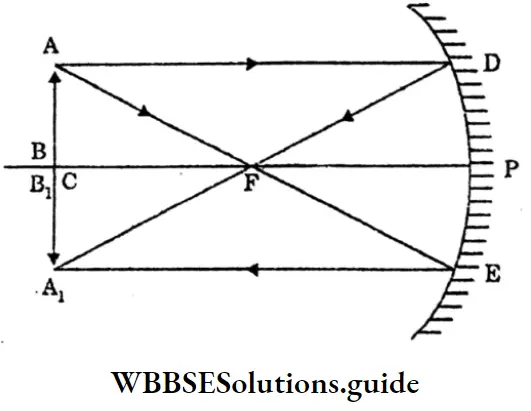
Question 16. Show that, for a spherical mirror the focal length is half of its radius of curvature.
Answer:
P = Pole
F= Focus of the mirror.
PF = f
C = Centre of curvature.
∠BP’C = ∠P’CF (alternate angles.) and
∠BP’C = ∠CP’F (law of reflections i = r)
Hence ∠’P’CF = ∠CP’F
ΔFP’ C is isosceles. Hence P’F = FC
If the aperture of the mirror is small, the point P i very close to the point P, then P’F = PF
∴ PF= FC or, PF + PF = PF + FC
or , 2PF = PC or, PF = ½ PC
or, f = ½ R.
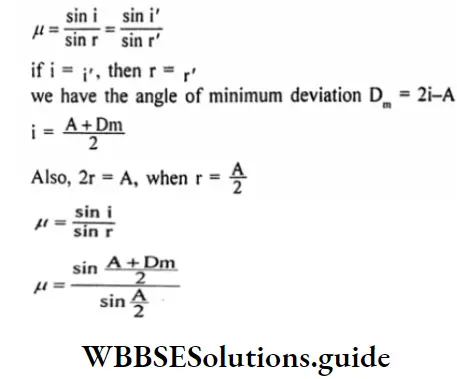
Question 17. Establish relation between the angle of deviation (Dm) and the refractive index of the material of the prism (μ).
Answer:
Let, the angle of deviation be D, the refractive index of the material of the prism be μ.
For minimum deviation, we have ¡=i’
Question 18.
Answer:
1. The Cauchy’s equation: = А+B/ λ2
A, B = Cauchy’s constant
2. The angular dispersion is defined as the difference between the angles of deviation of the extreme colours, red and violet of the spectrum.
“WBBSE Class 10 Physical Science Chapter 5, Light long answer key”
Question 19.
Answer:
1. Condition for dispersion with deviation
⇒(μv-1)A = (μ’y-1)A’
The condition for devotion without dispersion :
⇒ δy -δr (μy-1)A(w-w’)
2.
Question 20. Light of two colours (say X, Y) are sent through a prism. Suppose X bents more than Y. Which colour travels more slowly in the material of the prism?
Answer:
Light of two colours (say X, Y) are sent through a prism. Suppose X bents more than Y.
We know, D, (μx-1)A
D1 = (μy-1)A
⇒ Dy>Dy
⇒ μ x> μy
C/ Vx>C/ Vy
∴ Vy > Vx
The colour X travels more slowly in the material of the prism.
Question 21. Draw a ray diagram for the formation of a concave mirror when the object is at focus F.
Answer:
AB is an object placed at the focus F. The ray AD is incident on the mirror parallel axis and it gets reflected along DF, passing through the focus f. AE appears to be coming from the centre of curvature C, so it gets reflected back along EA. The two reflected rays DF and EA are parallel to each other.
The image is formed at infinity (very far from the mirror) which is real, inverted and highly magnified.
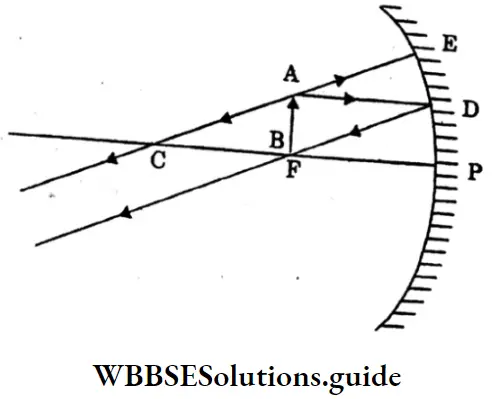
When the object is at focus F.
Thus when the object is at the focus F, the image is at infinity. It is
Question 22. How the angle of minimum deviation of a glass prism changes when it is immersed in a liquid of R.I. greater than I? sin A+D m
Answer:
Minimum deviation of a glass prism:

Hence the angle of minimum deviation decreases.
Question 23. Define-Absorption Spectrum.
Answer:
Absorption Spectrum:
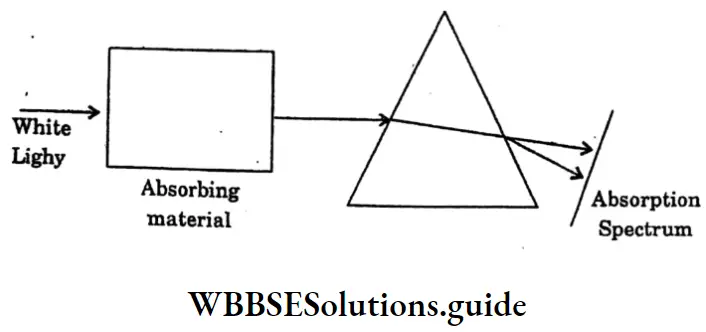
The missing wavelengths provide information about the absorbing material. Such a spectrum is called an absorption spectrum. The absorption spectrum may be of two types depending on the absorbing material and the conditions such as temperature.
Question 24 . An object 4 cm. high is placed at a distance of 10cm from a convex lens of focal length 20cm. Find the position, nature and size of the image.
Answer:
An object 4 cm. high is placed at a distance of 10cm from a convex lens of focal length 20cm.
O= 4cm, u=-10 cm, f= + 20cm.
\(\frac{1}{V}\) = \(\frac{1}{f}\) +\(\frac{1}{u}\)
= \(\frac{1}{20}\) – \(\frac{1}{10}\)
= – \(\frac{1}{20}\)
V= -20 cm
\(\frac{1}{o}\) – \(\frac{V}{u}\)
I = \(\frac{V}{u}\) × 4
= \(\frac{-20}{-10}\) ×4
= 8cm
“Class 10 WBBSE Physical Science Chapter 5, Light important long answer questions”
Question 25. Two lenses one of focal length 20 cm. and another focal length -15cm, are placed in contact. What is the focal length and power of the combination?
Answer:
Two lenses one of focal length 20 cm. and another focal length -15cm, are placed in contact.
f, 20cm=0.2m f15cm – 0.15m
Power of the first lens, P1 = \(\frac{1}{0.2}\) = 5D
Power of the second lens, P2 = \(\frac{-1}{0.2}\)
= 6.67 D
Power of combination, P = P1 + P2 = 5 – 6.67 = -1.67D
Focal length, f= \(\frac{1}{1.67}\) 0.599m
The -ve sign indicates that the combination acts as a concave lens.
Question 26. An object 4 cm. high is placed at a distance of 10cm from a convex lens of focal length 20cm. Find the position nature and size of the image.
Answer:
An object 4 cm. high is placed at a distance of 10cm from a convex lens of focal length 20cm.
0= 4cm, u = -10cm, f= + 20cm,
\(\frac{1}{V}\) =\(\frac{1}{F}\)+ \(\frac{1}{u}\)
= \(\frac{1}{20}\) – \(\frac{1}{10}\)
= – \(\frac{1}{20}\)
V= – 20 cm
\(\frac{I}{O}\)= \(\frac{V}{u}\) or, \(\frac{V}{u}\)×0
= \(\frac{-20}{-10}\)×4
= 8cm
Question 1. What is an anomalous expansion of water?
Answer:
Anomalous expansion of water: Usually liquids expand on heating. But in the case of water, we find a deviation from this general behaviour of the liquids within a certain range of temperature. The volume of water is minimum at 4°C and hence its density is maximum at 4°C. This phenomenon is called the anomalous expansion of water.
Question 2. What is thermo-metric conductivity?
Answer:
Thermometric conductivity: The rate of rise of temperature during the variable state is proportional to \(\frac{K}{P_s}\)(where K = thermal conductivity of the material, PS specific heat.)
This ratio is known as thermometric conductivity or the thermal conductivity per thermal capacity per unit volume.
“WBBSE Class 10 Physical Science Chapter 4 long answer questions, Thermal Phenomena”
Question 3. What is the fundamental principle of calorimetry? What are the conditions?
Answer:
Fundamental principle of calorimetry Heat lost by hot body = Heat gained by cold body.
Conditions:

Question 4. Give a few applications of conductivity.
Answer:
Applications of conductivity:
Ice is packed in sawdust because air which is a bad conductor of heat being trapped in the sawdust prevents the transfer of heat from the surroundings to the ice. So, ice does not melt.
Question 5. Establish the relation: Coefficient of linear \((\Delta \mathrm{l})=\alpha \mathrm{l} \Delta \mathrm{t}\)
Answer:
Let us suppose that a rod has initial l1 at t1° and when it is heated to t2, its length increases to l2.
\(\begin{aligned}(α = constant of proportionality)
This constant is called the coefficient of linear expansion of the solid.
\(\alpha=\frac{\mathrm{l}_2-\mathrm{b}_1 / \mathrm{l}_2}{\left(\mathrm{t}_2-\mathrm{t}_1\right)}\)\(l_2=l_1\left\{1+\alpha\left(t_2-t_1\right)\right\}\)for a small increase in temperature At, if the length I of a rad increases by Al, then,
\(\begin{aligned}“Class 10 WBBSE Physical Science Chapter 4 long answer questions, Thermal Phenomena study material”
Question 6. Establish the relation: ΔS = ΔβSΔt for superficial expansion.
Answer:
If S1 be the initial surface area of a solid substance at t1° and S2, be that at t2 (t2>t1), the coefficient of superficial expansion is denoted by β.
\(\beta=\frac{\left(S_2-S_1\right) / S_1}{\left(t_2-t_1\right)}\) \(s_2=s_1\left\{1+\beta\left(t_2-t_1\right)\right\}\)For a small increase in temperature Δt, if the surface area s of a solid increases by ΔS, then
\(\beta=\frac{\Delta s / s}{\Delta t}\)Δs = βsΔt
Question 7. Establish the relation: ΔV = yVΔt of cubical expansion.
Answer:
If V1 and V2 are the volumes of a solid at t1° and t2°, (t2>t1) respectively, and y is the coefficient of cubical expansion.
\(y=\frac{\left(v_2-v_1\right) / v_1}{\left(t_2-t_1\right)}\)V2 = V1 {1+y (t2-t1)}
For a small rise in temperature Δt, if the volume V of a solid increases by ΔV
\(y=\frac{\Delta V / V}{\Delta t}\)ΔV=YVΔt
Question 8. Establish the relation : \(V_t=V_0\left(1+\frac{t}{273}\right)\) for Charle’s law.
Answer:
Let, Vo denote the volume of a given mass of a gas at 0°C.
Vt = The volume of the same mass of gas at t°C.
The pressure in both cases is the same.
For Charle’s law, the increase in volume of the gas
= \(\frac{\text { volume at } 0^{\circ} \mathrm{C}}{273} \times \text { rise in temp }=v_t-v_0=\frac{v_0}{273} \times t\)
\(v_t=v_0(1+y p t)\)Question 9. Establish the relation Vt = V0 (1+ ypt) for the volume coefficient of a gas.
Answer:
If V0 and V2 are the volume of a given mass of a gas at 0°C and t°C respectively.
\(\mathrm{y}_{\mathrm{p}}=\frac{\text { fractional increase in volume }}{\text { rise of temperature }}=\frac{\left(\mathrm{v}_{\mathrm{t}}-\mathrm{v}_{\mathrm{o}}\right) / \mathrm{v}_{\mathrm{o}}}{\mathrm{t}}\)Vt = V0 (1+ ypt)
Question 10. The thickness of an iron plate is 4mm. and its area is 150 sqm. The temperature of the two sides is 100°C, 30°C and 3940. Caloric heat is conducted in one second from one side to the other. Determine the thermal conductivity of heat.
Answer:
Given
The thickness of an iron plate is 4mm. and its area is 150 sqm. The temperature of the two sides is 100°C, 30°C and 3940. Caloric heat is conducted in one second from one side to the other.
\(\frac{\mathrm{Q}}{\mathrm{t}}=3940 \mathrm{cal} / \mathrm{s}\) , A = 150 cm2 ,
x = 4mm = 0.4 cm2, θ1 = 30°C, θ2 = 100°C
Thermal conductivity of iron.
\(\mathrm{K}=\frac{\mathrm{Qx} / \mathrm{t}}{\mathrm{A}\left(\theta_2-\theta_1\right)}=\frac{3940 \times 0.4}{150(100-30)} \text { c.g.s unit. }=0.15 \text { c.g.s. unit. }\)Question 11. The thermal conductivity of aluminium is 0.50 cal/sec. cm degree. Express the same in units of watt/m degree.
Answer:
Given
The thermal conductivity of aluminium is 0.50 cal/sec. cm degree.
K 0.5 cal/5 cm degree
= 0.5 x 4.2 Joule/s Cm degree
= 0.5 × 4.2 × 100 Joule/s m degree
= 210 watt/m degree.
Question 12. A pond is covered with ice 5cm. thick and the temperature of overlying air is -10°C. Find the rate of conduction of heat by the ice per unit square centimetre area. (Given the Thermal conductivity of ice) = 5 x 10-3 c.g.s unit)
Answer:
Given
A pond is covered with ice 5cm. thick and the temperature of overlying air is -10°C.
k =5 x 10-3 c.g.s unit, A = 1 cm2,
x = 5cm, θ2-θ1, 0-(-10)
or 10°C.
Rate conduction of heat \(\frac{Q}{t}\)
\(=\frac{\mathrm{KA}\left(\theta_2-\theta_1\right)}{\mathrm{X}}=\frac{5 \times 10^{-3} \times 1 \times 10}{5} \mathrm{cal} / \mathrm{s} .\)Physics Class 10 WBBSE Question 13. At what temperature will the volume of a given mass of gas be double of what is at 30°C, if the pressure remains constant?
Answer:
Let, t°C = the required temperature
Applying Charles’s law,
\(\frac{\mathrm{V}}{\mathrm{V}^1}=2=\frac{273+\mathrm{t}}{273+30}\) \(2=\frac{273+t}{303}\)273+ t = 606
t=333°C
“WBBSE Class 10 Physical Science Chapter 4, Thermal Phenomena long answer solutions”
Question 14. The volume of a liquid is 830 m3 at 30°C and it is 850 m3 at 90°C. Find the coefficient of volume expansion of the liquid.
Answer:
Given
The volume of a liquid is 830 m3 at 30°C and it is 850 m3 at 90°C.
If V1 and V2 are the volume of a liquid at temperatures t1°C and t°C respectively.
\(y=\frac{\text { Increase in volume }}{\text { Original volume } \times \text { Rise in temperature }}=\frac{V_2-V_1}{V_1\left(t_2-t_1\right)}\)[V1 = 830m3, t1= 30C, V2 = 850m3, t2 = 90c]
\(y=\frac{850-830}{830(90-30)}=\frac{20}{830 \times 60}=4 \times 10^{-4 /{ }^{\circ} \mathrm{C}}\)Physics Class 10 WBBSE Question 15. The coefficient of cubical expansion of as metal is 7.2×10-5 °C-1. Find the
1. Coefficient linear expansion
\(\alpha=\frac{1}{3} y=\frac{1}{3} \times 7.2 \times 10^{-5}=2.4 \times 10^{-5} /{ }^{\circ} \mathrm{C}\)2. Coefficient of superficial expansion
\(\beta=\frac{2}{3} y=\frac{2}{3} \times 7.2 \times 10^{-5}=4.8 \times 10^{-5} /{ }^{\circ} \mathrm{C}\)Question 16. The base of an iron saucepan has a diameter of 20 cm at 15°C. What will be the increased area of the base of the saucepan when it is filled with boiling water? (Given the coefficient of linear expansion of iron 12 x 10-6 °C)
Answer:
Given
The base of an iron saucepan has a diameter of 20 cm at 15°C.
The radius of the base = 20/2 cm = 10 cm.
S2 – S1 = S1 x B x (t2 – t1), the formula is necessary to define the coefficient of surface expansion.
S1 = π x 102 Cm2, β = 2α = 2 x 12 × 10-6 per°C
(t2 – t1) (100 – 15)°C = 85°C
the required increase in area = (π x 102 x 24 x 10-6 × 85)
= 0.6404 Cm2.
“WBBSE Class 10 Thermal Phenomena long answer questions, Physical Science Chapter 4”
Question 17. The coefficient of apparent expansion of a liquid is 18 x 10-5°C when an iron vessel is used and 14.46 x 10-5°C when an aluminium vessel is used. If the coefficient of iron be 12 x 10-6/0C. Find that of aluminium.
Answer:
Given
The coefficient of apparent expansion of a liquid is 18 x 10-5°C when an iron vessel is used and 14.46 x 10-5°C when an aluminium vessel is used. If the coefficient of iron be 12 x 10-6/0C.
The coefficient of volume expansion of iron = 8 × 12 × 10-6 °C
= 3.6 × 10-5 °C.
The coefficient of real expansion of the liquid is given by
Yr = (18 x 10-5 +3.6 x10-5 )/°C
= 21.6 x 10-5C
If the coefficient of linear expansion of aluminium is a, then
21.6 x 10-5 = 14.46 x 10-5 + 3α
\(\alpha=\frac{7.14}{3} \times 10^{-5 /{ }^{\circ} \mathrm{C}}\)= 2.33 x 10-5/°C
Question 1. What steps should be followed to solve a problem based on the chemical equation by the weight-weight method?
Answer:
The following steps are followed :
“WBBSE Class 10 Physical Science Chapter 3 long answer questions, Chemical Calculations”
Question 2. What is the information available from the formula CaCO3?
Answer:
The formula CaCO3 conveys the following information :

Question 3. What is meant by the balancing of a chemical equation? Why it is necessary?
Answer:
Balancing of a chemical equation: It means making both sides of the equation equal with respect to the kind and number of the atoms of the elements involved.
Balancing of a chemical equation is necessary: Due to the conservation of mass and indestructibility of matter, no atoms can be created or destroyed in a chemical reaction Hence, The same kind of atoms in the same numbers must be present on both sides of the chemical equation.
Question 4. Write the following as balanced equations.
Cu+HNO3 → Cu (N03) + O2
Pb (N03)2 → PbO + NO2 + O2
Al + NaOH + H2O → NaAlO2 + H2
CuO + NH3 → Cu + N2 + H2O
Answer:
Balanced equations are:
3Cu + 8HNO3 = 3Cu (NO3)2 + 2NO – 4H2O
2Pb (NO3)2 = 2pb0+ 4NO2 +O2
2Al + 2NaOH + 2H2O = 2NaAIO2 + 3H2
2NH3 +3CuO = 3Cu + 2n2 + 3H2O
Question 5. What are the limitations of a chemical equation?
Answer:
Limitation of a chemical equation :
Under what conditions a chemical reaction occurs i.e. pressure, temperature, catalyst are not-known.
“Class 10 WBBSE Physical Science Chapter 3 long answer questions, Chemical Calculations study material”
Question 6. Balance the equation KCIO3 → KCI + O2 by trial and error method.
Answer:
KCI03 KCI + O2: It is seen that the number of atoms of oxygen on the left-hand side is 3 whereas in the right-hand side it is 2. To equalize the number of atoms of oxygen on both sides it requires to multiply KCIO3 by 2 and oxygen by 3.
2KCIO3 → KCI +302 SO as to balance this equation if KCI is multiplied by then the equation is proper balance the equation if KCI is multiplied by 2 then the equation is proper. balanced.
2KCIO3 = 2KCI + 302
Question 7. What information is obtained from the chemical equations?
Answer:
A chemical equation gives the following information:
1. Qualitative informations:
2. Quantitative information:
Question 8. Explain the method of balancing a chemical equation by trial and error method.
Answer:
Balancing of a chemical equation by trial and error method :
Question 9. What is the method of writing chemical equations?
Answer:
Method of writing chemical equation :
Initially, the symbols of atoms of elements and formulae of molecules of reactants and products are written.
Question 10. What information is obtained from the equation: 3H2 + N2 = 2NH2
Answer:
The following information is obtained from the equation: 3H2+N2 = 2NH2
Qualitative information: The reactants are hydrogen and nitrogen, and ammonia is the product.
Quantitative information :
“WBBSE Class 10 Physical Science Chapter 3, Chemical Calculations long answer solutions”
Question 11. What information is obtained from the equation? C + O2 = CO2
Answer:
The following information is obtained from an equation: C + O2= CO2
Qualitative informations :
Quantitative information:
Question 12. How many grams of oxygen evolve when 122.5g potassium chlorate is heated? (Given K = 39, CI = 35.5 0 = 16)
Answer:
The balanced equation is : \(\begin{array}{ll}
2 \mathrm{KClO}= & 2 \mathrm{KCl}+3 \mathrm{O}_2 \\
2(39+35.5+3 \times 161 \mathrm{~g} & 3[2 \times 16] \mathrm{g} \\
=245 \mathrm{~g} & =96 \mathrm{~g}
\end{array}\)
By heating 245g KCIO3 96g O2 is obtained
∴ By heating 122.5g KCIO3 \(\frac{96 \times 122.5}{245} \mathrm{~g}\) is obtained.
“WBBSE Class 10 Chemical Calculations long answer questions, Physical Science Chapter 3”
Question 13. 2.6g zinc is treated with excess dil H2SO4 How many grams of oxygen combine with the evolved hydrogen?
Answer:
The balanced equation for the production of hydrogen is:
\(\underset{65 \mathrm{~g}}{\mathrm{Zn}}+\mathrm{H}_2 \mathrm{SO}_4=\mathrm{ZnSO}_4+\underset{(2 \times 1) \mathrm{g}=2 \mathrm{~g}}{\mathrm{H}_2}\)So, 65g of Zinc produces 2g of hydrogen
∴ 2-6 Zinc produces \(\frac{2 \times 2.6}{65} \mathrm{~g}=0.089\) hydrogen
Now, the reaction where hydrogen and oxygen combine is: 2H2 + O2 = 2H2O
So, 4g hydrogen combined with 32g oxygen
0.08g hydrogen combined with \(\frac{32 \times 0.08}{4} \mathrm{~g}=0.64 \mathrm{~g}\)
Question 14. What is the observed loss in weight of 5g? calcium carbonate when it undergoes thermal decomposition?
Answer:
The balanced equation is : \(\begin{aligned}
& \mathrm{CaCO}_3=\mathrm{CaO}+\mathrm{CO}_2 \\
& (40+12+16 \times 3) \mathrm{g}(12+16 \times 2) \mathrm{g} \\
& =100 \mathrm{~g} \quad=44 \mathrm{~g} \\
&
\end{aligned}\)
Loss in weight in the weight of CO2 that escapes 100g CaCO3 produces 44g CO2
∴ 5g CaCO3 produces \(\frac{44 \times 5}{100} \mathrm{~g}=2.2 \mathrm{gCO}_2\)
Question 15. What is the percentage of ammonia in that quantity of ammonium chloride that can produce 5g? ammonia?
Answer:
The balanced equation is :
\(\begin{aligned}So, 34g ammonia is obtained from 107g NH2CI.
Question 16. What weight of potassium chlorate of 96% purity will yield 4.8g oxygen on complete thermal decomposition? (Given, K = 39, CI = 35.5, O = 16)
Answer:
The balance equation is: 2KCIO3 (245g) = 2KCI + 3O2(96g)
∴ 96g oxygen is obtained from 245g KCIO2 of 100% purity.
∴ 4.8g oxygen is obtained from \(\frac{245 \times 4.8}{96} \mathrm{~g}\)
Let x gram of 96% purity contain 12.25g KCIO3 of 100% parity
∴ \(\frac{96}{100} \times 12.56 \text { or, } x=\frac{12.25 \times 100}{96}=12.76 \text { (approx) }\)
So, the required quantity of KCIO3 = 12.76g
“Class 10 WBBSE Physical Science Chapter 3, Chemical Calculations detailed answers”
Question 17. On strong heating, limestone decomposes into quicklime and carbon dioxide. How much quantity of limestone will produced on complete decomposition, 30g of quicklime by the above reaction?
Answer:
The balanced equation is: \(\begin{array}{ll}
\mathrm{CaCO}_3=\mathrm{CaO}+\mathrm{CO}_2 & \\
(40+12+3 \times 16) \mathrm{g} & {[40+16] \mathrm{g}} \\
=100 \mathrm{~g} & =56
\end{array}\)
So 56g, CaO is obtained by the complete decomposition of 100g CaCO3
∴ 30g CaO is obtained by the complete decomposition of \(\frac{100 \times 30}{56} \mathrm{~g} \mathrm{CaCO}_3=53.6 \mathrm{~g} \mathrm{CaCO}_3\)
Thus 53.6g of limestone will have to be decomposed.
Question 18. How many grams of magnesium metal will give 1.2g hydrogen in a complete reaction with dilute H2SO4 (Mg= 24, H = 1) The balanced equation is:
Answer:
So 2g H2 is obtained from 24g Mg
∴ 1.2g H2 is obtained from \(\frac{24 \times 1.2}{2} \mathrm{~g} \mathrm{Mg}=14.4 \mathrm{gMg} .\)
So, 14.4g will be required
Question 19. Calculate the number of moles in 1.5 gm of ammonia.
Answer:
We know, number of mole = \(\frac{\text { Mass given }}{\text { gram molecular mass }}\)
\(\begin{aligned}Question 20. The vapor density of a gas is 40. Calculate the volume of 20 gm of this gas at 27°C and 950 mm pressure.
Answer:
Given
The vapor density of a gas is 40.
The molecular weight of the gas = 2 x vapor density
= 2 × 40 = 80
The volume of 80 gm of gas at S.T.P. is 22.4 Lit
The volume of 10 gm of gas at S.T.P.
\(=\frac{22.4 \times 10}{80}=2.8 \mathrm{Lit}\)Let the volume of this 2.8 liter of gas at 27°C and 950 mm pressure be V Litre
\(\frac{\mathrm{V} \times 950}{273+27}=\frac{2.8 \times 760}{273}\) ⇒V = 7.79 Litre
Hence the volume of the gas at 27°C and 950 pressure is 4.9 Litre.
Question 1. Establish-relation between the pressures and density of a gas at a constant temperature.
Answer:
Relation between the pressures and density of a gas at a constant temperature
Let the volume of the mass m of gas at a constant temperature be V1 when the pressure is P1 and V2 when the pressure is P2.
From Boyle’s law, P1 V1 = P2 V2 →(1)
Let the densities of the gas at the pressure P1 and P2 be P1 and P2 respectively. Substituting in equation (1) we get,
\(\frac{P_1 m}{P_1}=\frac{P_2 m}{P_2}\)\(\frac{P_1}{P_1}=\frac{P_2}{P_2}\) ⇒ Ρ α Ρ
The density of a gas at constant temperature is proportional to pressure.
Question 2. What are the characteristic properties of gases?
Answer:
Characteristic properties of gas:

Question 3. What are the postulates of the kinetic theory of gases?
Answer:
Postulates of Kinetic theory of gases :
“WBBSE Class 10 Physical Science Chapter 2 long answer questions, Behaviour of Gases”
Question 4. A sample of helium has a volume of 520 cm3 at 373 k. Calculates the temperature at which the volume will become 260 Cm3. Assume that the pressure is constant.
Answer:
Given
A sample of helium has a volume of 520 cm3 at 373 k.
According to Charle’s law,
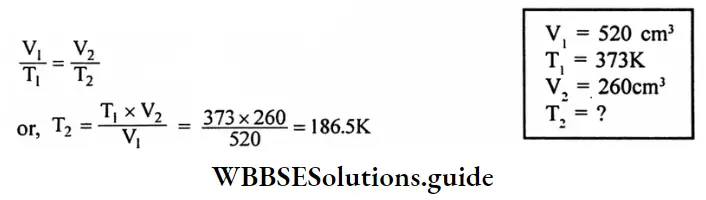
Question 5. Find the volume of N, at 27°C at a pressure of 760 torr, if it occupies 40 mL at STP.
Answer:
Given
at 27°C at a pressure of 760 torr, if it occupies 40 mL at STP.
According to Charle’s law,
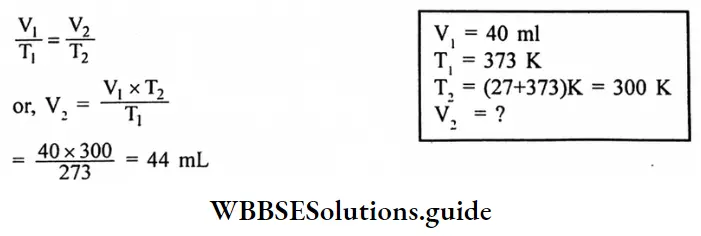
Question 6. A sample of oxygen has a volume of 880 mL and a pressure of 740 torr. What additional pressure is required to reduce the volume to 440mL?
Answer:
Given
A sample of oxygen has a volume of 880 mL and a pressure of 740 torr.
According to Boyle’s law,
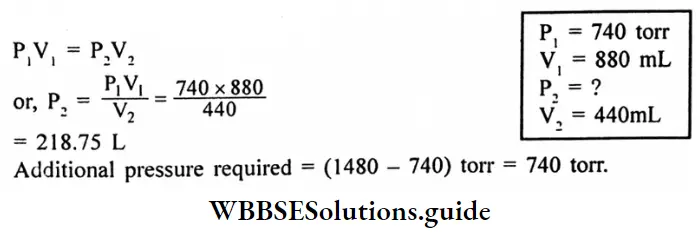
Question 7. 7.0g of a gas at 300K and 1 atmospheric pressure occupies a volume of 4.1 litters. What is the molecular mass of the gas?
Answer:
Given
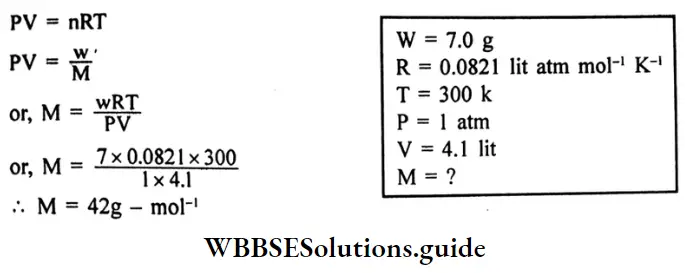
7.0g of a gas at 300K and 1 atmospheric pressure occupies a volume of 4.1 litters.
Applying the ideal gas equation,
Question 8. A steel tank contains carbon dioxide at 27°C and a pressure of 10.0 atm. Calculate the internal gas pressure when the tank and the gas are heated to 100°C.
Answer:
Given
A steel tank contains carbon dioxide at 27°C and a pressure of 10.0 atm.
According to Gay Lussac’s law,
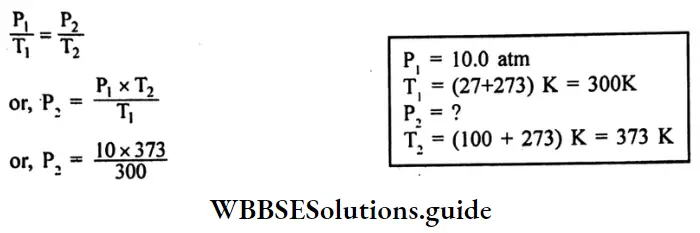
Question 9. A bottle of Volume 1 litre contains gas at 10 atmospheric pressure. How many bottles each of a 1-litre capacity can be filled with the gas at 2 atmospheric pressure at constant temperature?
Answer:
Given
A bottle of Volume 1 litre contains gas at 10 atmospheric pressure
According to Boyle’s law

“Class 10 WBBSE Physical Science Chapter 2 long answer questions, Behaviour of Gases study material”
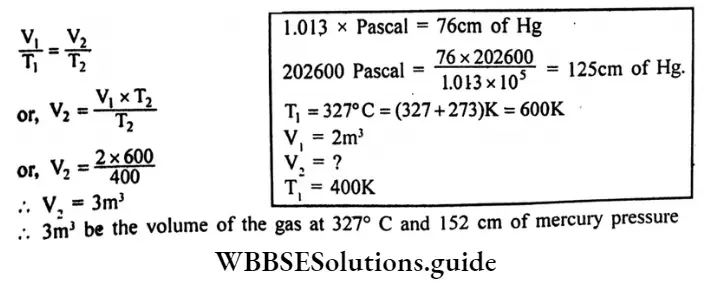
Question 10. The volume of a certain mass of gas at 400k and pressure 202600 pascal is 2 cubic metres. What would be the volume of the gas at 327°C and 152cm of mercury Pressure? [given 1.013 x 105 Pascal 1 atmospheric pressure]
Answer:
Given
The volume of a certain mass of gas at 400k and pressure 202600 pascal is 2 cubic metres.
According to Charle’s law
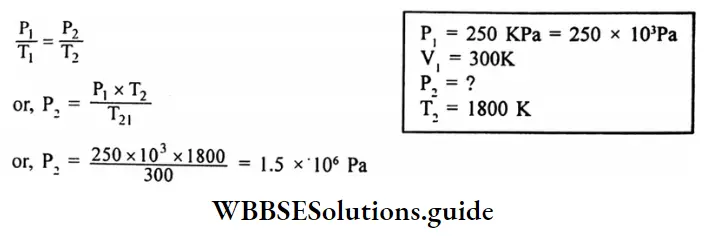
Question 11. An iron cylinder contains helium at a pressure of 250 K pa at 300k. The cylinder can withstand a pressure of 1×106 pa. The room in which the cylinder is placed catches fire. Predict whether the cylinder will blow up before it melts or not. (M.P. of the cylinder 1800K)
Answer:
Given
An iron cylinder contains helium at a pressure of 250 K pa at 300k. The cylinder can withstand a pressure of 1×106 pa. The room in which the cylinder is placed catches fire.
According to Gay-Lussac’s law.
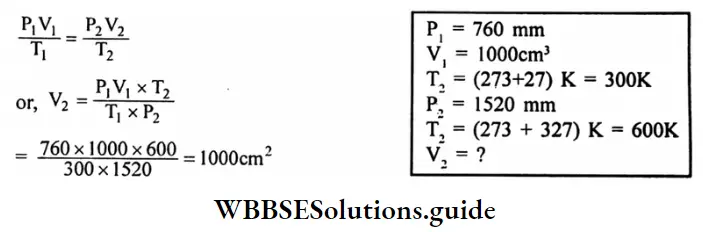
Question 12. A certain quantity of gas occupies a volume of 1000 cm3 at 760 mm and 27°C. Find the volume of the gas if the pressure and temperature are 1520 mm and 327°C.
Answer:
Given
A certain quantity of gas occupies a volume of 1000 cm3 at 760 mm and 27°C.
By combining Boyle’s low and Charle’s low we get.
Question 13. A gas having a temperature 0°C is heated so that its pressure and volume are doubled. What will be the final temperature of the gas?
Answer:
Given
A gas having a temperature 0°C is heated so that its pressure and volume are doubled.
By combining Boyle’s low and Charle’s low we get

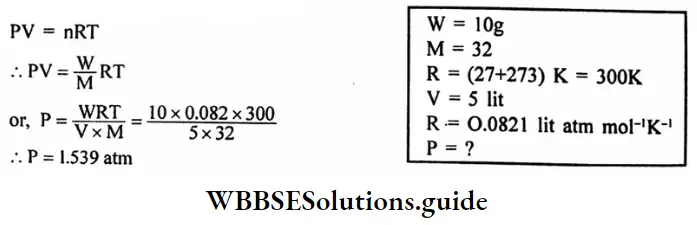
Question 14. 10g of Oxygen are introduced in a vessel of 5 lit capacity at 27°C calculate the pressure of the gas in the atmosphere in the container.
Answer:
Given
10g of Oxygen are introduced in a vessel of 5 lit capacity at 27°C
Applying the ideal gas equation,
Question 15. The volume of 1 gram mole of oxygen gas is 22400 ml at 760 mm. Calculate the temperature of the gas (Given R = 0.082L-atm mol-1 K-1)
Answer:
Given
The volume of 1 gram mole of oxygen gas is 22400 ml at 760 mm.
We know from the ideal gas equation for 1 mole of an ideal gas.
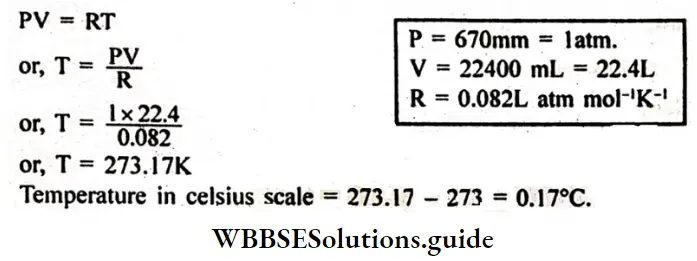
Question 16. Establish the equation:
\(V-t=V_0\left(1-\frac{t}{273}\right)\)
Answer:
Let, Vo denote the volume of a given mass of a gas at 0°C and Vt be the volume of the same mass of the gas at t°C, the pressure in both cases is the same.
From Charle’s law,
The increase in volume of the gas = \(\frac{\text { Volume at } 0^{\circ} \mathrm{C}}{273} \times \text { rise in temperature }\)
∴ \(V_t=V_0+\frac{V_0}{273} \times t\)
\(V_t=V_0\left(1-\frac{t}{273}\right)\)If we consider a temperature lower than 0ºC, say tºC, then the volume of gas is \(v_{-1}=V_0\left(1+\frac{t}{273}\right)\)
“WBBSE Class 10 Physical Science Chapter 2, Behaviour of Gases long answer solutions”
Question 17. Establish: \(\frac{P}{T}=a\)constant for pressure law.
Answer:
If the pressure of a given mass of a gas at temperature t°c and the by P and P’ respectively, then volume remains constant.
We have Pressure law,
\(P=P o\left(1+\frac{t}{273}\right)=\frac{P o T}{273}\) \(p^{\prime}=P_0\left(1+\frac{t^{\prime}}{273}\right)=\frac{P_{o T}}{273}\)Po = pressure of the gas.
TK = absolute temperature corresponding to the temperature t°c.
T1K= absolute temperature to the temperature t1 °C
\(\frac{P}{P^{\prime}}=\frac{T}{T^{\prime}} \) ⇒ \(\frac{P}{T}=\frac{P^{\prime}}{T^{\prime}}\)
⇒ \(\frac{P}{T}\)= Constant when V = constant
∴ P α T
Question 18. Establish the relation PV KT for an ideal gas.
Answer:
Let, P = pressure of a gas
V = Volume of a gas
T= absolute temperature
From Boyle’s law,
\(V \propto \frac{1}{P}\) (T = Constant)
From Charle’s law,
V α T (P= constant)
When P and T both vary
\(V \propto \frac{T}{P}\)⇒ \(V=K \frac{T}{P}\)
\(\frac{P V}{T}=K\)PV = KT
“WBBSE Class 10 Behaviour of Gases long answer questions, Physical Science Chapter 2”
Question 19. What is the general equation is obtained by combining Boyle’s law and Charle’s law. Define-universal gas constant. The general equation is obtained by combining Boyle’s law and Charles’s law.
Answer:
P1 = Pressure of first gas
P2 = pressure of second gas
V1 = Volume of the first gas
V2 = Volume of the second gas
T1 = temperature of the first gas
T2 = temperature of the second gas
Universal gas constant: One gram molecule of all gases occupy the same volume under identical conditions of temperature and pressure the volume of R is the same for all gases R is known as the universal gas constant.
“Class 10 WBBSE Physical Science Chapter 2, Behaviour of Gases detailed answers”
Question 20. Establish a Relation between the pressure, temperature and density of a gas.
Answer:
Relation between the pressure, temperature and density of a gas
Let, P = Pressure of a gas
V = Volume of a gas
T = temperature of a gas
P= density of a gas
m = mass of a gas
M = Molecular weight of the gas
From the ideal gas equation,
\(\frac{P V}{T}=\frac{m}{M} R\) [R= universal gas constant]
\(\frac{\mathrm{PV}}{\mathrm{mT}}=\frac{\mathrm{R}}{\mathrm{M}}\) = Constant
But, \(\mathbf{P}=\frac{\mathbf{M}}{\mathbf{V}}\)
\(\frac{\mathbf{P}}{\mathrm{PT}}\)
Question 21. Why the absolute zero of temperature is called absolute?
Answer:
Question 22. Explain the relation between the pressure-volume of a gas-When the temperature is kept constant.
Answer:
Relation between the pressure-volume of a gas-When the temperature is kept constant
Question 23. Explain the relation between temperature and volume of gas When the pressure is kept constant.
Answer:
Relation between temperature and volume of gas When the pressure is kept constant
Question 24. Write four assumptions of the kinetic theory of gases.
Answer:
Four assumptions of the kinetic theory of gases:
Question 25. Establish: Vr ms = \(\sqrt{\frac{3 \mathrm{PV}}{\mathrm{M}}}\)
Answer:
The square root of mean square speed is called the root mean square speed or rms speed.
\(\mathrm{Vrms}=\sqrt{\Sigma v^2 / N}\)V2 = (Vrms)
We know that, P = \(\frac{1}{3} \mathrm{PV}^2 \mathrm{rms}\)
\(\text { Vrms }=\sqrt{\frac{3 p}{p}}=\sqrt{\frac{3 P V}{M}}\)“WBBSE Class 10 Physical Science Chapter 2, Behaviour of Gases long answer key”
Question 26. Explain briefly the translational kinetic energy of a gas.
Answer:
The total translational kinetic energy of all the molecules of the gas is
\(K=\Sigma \frac{1}{2} m V^2\)= \(\frac{1}{2} m \mathrm{~N} \frac{\Sigma \mathrm{V}^2}{\mathrm{~N}}\)
= \(\frac{1}{2} \mathrm{Mv}^2 \mathrm{rms}\)
The Average kinetic energy of a molecule
\(\frac{K}{N}=\frac{1}{2} \frac{M}{N} V^2 \mathrm{rms}\)We know, \(\mathrm{pV}=\frac{1}{2} \mathrm{MV}^{-2}\) → (1)
From equation → (1)
\(\mathrm{pV}=\frac{2}{3} \frac{1}{2} \mathrm{Mv}^2 \mathrm{rms}\) \(p V=\frac{2}{3} K\) \(K=\frac{3}{2} p V\)
Question 27. State Avogadro’s law
Answer:
Avogadro’s law: At the same temperature and pressure, equal volumes of all gases contain equal numbers of molecules. This is known as Avogadro’s law.
Question 28. Establish Avogadro’s law.
Answer:
Avogadro’s law
Consider equal volumes of two gases kept at the same pressure and temperature.
m1 = mass of a molecule of the first gas
m2 = mass of a molecule of the second gas
N1 = number of molecules of the first gas
N2 = number of molecules of the second gas
P = Common pressure of the two gases
V = Common pressure of the two gases
∴ \(p V=\frac{1}{3} N_1 m_1 V_1^2\)
\(P V=\frac{1}{3} N_2 m_2 V_2\)V1 V2 = rms speeds of the molecules of the first and second gas
\(N_1 m_1 V_1^2=N_2 m_2 V_2^2\) → (1)
As the temperature of the gases is the same, the average kinetic energy of the molecules is the same for the two gases.
\(\frac{1}{2} m_1 v_i==\frac{1}{2} m_2 v_2=\) → (2)
From, (1) and (2)
N1 = N2 Avogadro’s law
Question 29. Establish the relation: Dalton’s law of partial pressure: P=P, +P,+P, +……..
Answer:
In kinetic energy, the pressure exerted by a gas on the walls of a container is due to the collisions of the molecules of the walls.
The total force on the wall is the sum of the forces exerted by the individual molecules.
Suppose there are N1 molecules of gas
1, N2 molecules of gas 2 in the mixture.
The force on a wall of surface area A,
F= force by N1 molecules of gas 1+ force by N2 molecules of gas 2 + ….. = F+F+…
The pressure, \(P=\frac{F_1}{A}+\frac{F_2}{A}+\ldots \ldots\)
If the first gas alone is kept in the container, its N1 molecules will exert a force F1 on the wall.
If the pressure in this case P1 , P1 = \(\frac{F_1}{A}\)
Similar is the case for other gases Thus, P = P1+P2+P3 +…
Question 30. Prive : \(\frac{r_1}{r_2}=\sqrt{\frac{p_2}{p_1}}\) for Graham’s law of diffusion.
Answer:
The rate of diffusion is proportional to the rms speed of the molecules of the gas.
If r1 and r2 be the rates of diffusion of the two gases,
\(\frac{r_1}{r_2}=\frac{V_1, r m s}{V_2, r m s}\) → (1)
\(\text { Vrms }=\sqrt{\frac{3 p}{P}}\)If the pressure of the two gases is the same
\(\frac{\mathrm{V}_1, \mathrm{rms}}{\mathrm{V}_2 \mathrm{rms}}=\sqrt{\frac{\mathrm{p}_2}{\mathrm{p}_1}}\)From equation → (1),
\(\frac{r_1}{r_2}=\sqrt{\frac{p_2}{p_1}}\) ..Graham’s law of diffusion
“Class 10 WBBSE Physical Science Chapter 2, Behaviour of Gases important long answer questions”
Question 31. Prove Ideal gas equation ⇒ PV = nRT
Answer:
Consider a sample of an ideal gas at pressure P, volume V and temperature T.
Let, m = the mass of each molecule
V = rms speed of the molecule
Vtr = rms speed of the gas at the triple point 273. 16K
R = NaK = universal point
= universal gas point
R = 8.314 J K-1 mol-1
This is known as the equation of state of an ideal gas.
Question 32. Write the mathematical form of Max Well’s speed distribution law.
Answer:
The mathematical form of Maxwell’s speed distribution law :
\(d N=r \pi N\left(\frac{m}{2 \pi K T}\right)^{\frac{3}{2}} V^2 e^{-m V^2 / 2 K T}\)Question 33. Write the mathematical expression of Vander Waal’s equation.
Answer:
The mathematical expression of the van der Waals equation.
\(\left(P+\frac{a}{V^2}\right)(V-b)=n R T\)[a, b = constant
a = average force of attraction between the molecules
b = total volume of molecules]
1. A given sample of a substance has a number of parameters which can be physically measured. When these parameters are uniquely specified. So we say that the thermodynamic state of the system is specified.
We know. \(p V=\frac{1}{3} \mathrm{NmV}^2\) →(1)
\(\mathrm{T}=\left(\frac{273.16 \mathrm{k}}{\mathrm{V}^2 \mathrm{tr}}\right) \mathrm{V}^2\) \(V^2=\left(\frac{V_2 {tr}}{273.16 k}\right) T\)Putting this Expression for V2 in qn →(1)
\(\mathrm{pV}=\mathrm{N}\left(\frac{1}{3} \frac{\mathrm{mV}^2 \mathrm{tr}}{273.16 \mathrm{k}}\right) \mathrm{T}\)\(\frac{1}{2} \mathrm{mV}_{\mathrm{tr}}^2\) = average kinetic energy of a molecule at the triple point 273.16K.
As the average kinetic energy of a molecule is the same for all gases at a fixed temperature, mV2 tr is a universal constant. Accordingly. The quantity in the bracket in the equation.
2. above is also a universal constant writing this constant as a k equation.
3. becomes.
pV = NKT → (3)
The Universal constant K = Boltzmann Constant The value of K = 1.38 x 10-23 JK-1
If the gas contains n moles, the number of molecules is N = nXA
(NA 6.02 x 1023mol-1)
NA Avogadro’s Constant
Using eq (3) becomes p = xNA KT
PV = nRT → (4)
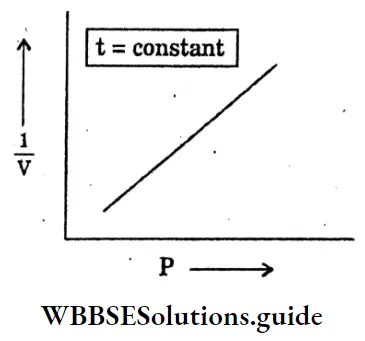
Question 34. Draw the graph Pressure (P) and volume (V) at a constant temperature.
Answer:
Question 1. What is good fuel?
Answer:
Good fuel: Good fuel should have many desirable characteristics. Some of these are as follows:
Question 2. What are the advantages of nuclear energy?
Answer:
The advantages of nuclear energy
The advantages of nuclear energy are as follows: A small amount of radioactive material can generate a huge amount of energy. It does not produce air pollution. A nuclear power plant is more efficient than other power plants.
Question 3. Hydrogen has been used as a rocket fuel would you consider it a cleaner fuel than LPG? Why and why not?
Answer:
Hydrogen is a much cleaner energy source than CNG. CNG is derived from biomass and hence burning the CNG causes air pollution, although on a much smaller scale than coal and petroleum. The use of hydrogen as an energy source does not pollute gases.

Question 4. Can any source of energy be pollution free? Why and why not?
Answer:
Many sources of energy can be pollution free when the burning of biomass is not involved in the production of energy, then there is no chance of pollution. For example, wind energy, solar energy, hydel energy, etc are pollution free.
“WBBSE Class 10 Physical Science Chapter 1 long answer questions, Concerns About Our Environment”
Question 5. What are the limitations of the energy that can be obtained from the oceans?
Answer:
Limitations of energy that can be obtained from the oceans:
These forms of energy can be used only in coastal areas, which would leave a vast portion of human habitation. The technologies for using these energies are still at the experimental stage and hence are very costly and less efficient.
Question 6. Why are looking for an alternate source of energy?
Answer:
Because of the growing population, the energy demand is rising. Fossil fuels are going to be exhausted in the near future and burning them is causing air pollution. Hence, we need to find an alternate source of energy which renewable and environmentally friendly.
Question 7. How has the traditional use of wind and water energy been modified for our convenience?
Answer:
Question 8. What kind of mirror, concave or convex, or plain would be best suited for use in a solar cooker? Why?
Answer:
Explanation: A concave mirror is best suited for use in a solar cooker. The reason for this is the ability of a concave mirror to converge the solar energy at a point. This enables the concave mirror to produce a larger amount of heat compared to other types of mirrors.
Question 9. Short Note-Ozone Layer.
Answer:
-Ozone Layer:
Question 10. What is ultraviolet light? How can the ozone Layer deplete?
Answer:
Ultraviolet light:
Although the concentration of ozone in the ozone layer is very small. It absorbs biologically harmful ultraviolet radiation coming from the sun: Extremely short or vacuum UV is screened out by nitrogen. UV radiation is capable of penetrating nitrogen.
Question 11. Short Note-Exosphere.
Answer:
Exosphere: The exosphere is the outermost layer of Earth’s atmosphere. It extends from the exobase, Which is Located at the top of the Thermosphere at an altitude of about 700 km. above sea level, to about 10,000 km.
The exosphere merges with the emptiness of outer space, where there is no atmosphere. This layer is mainly composed of extreme hydrogen, helium, and several heavier molecules including nitrogen, oxygen, and carbon dioxide closer to the exobase.
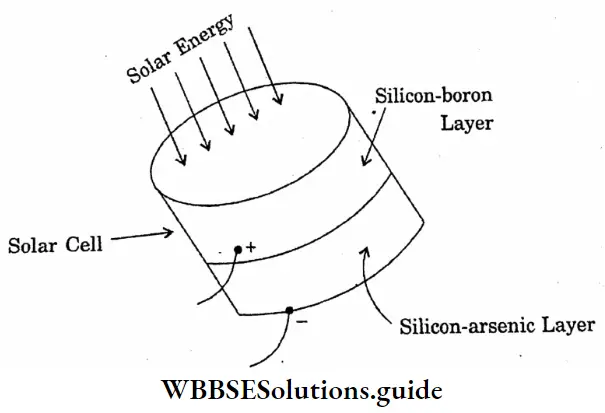
Question 12. Draw a diagram of a solar cell.
Answer:
Diagram of a solar cell
“Class 10 WBBSE Physical Science Chapter 1 long answer questions, Concerns About Our Environment study material”
Question 13. Short Note. Thermosphere.
Answer:
Thermosphere:
The thermosphere is the second highest layer of Earth’s atmosphere. It extends from the Mesopause at an altitude of about 80 km. upto the thermopause at an altitude range of 500-1000km.
Question 14. Short Note-Mesosphere.
Answer:
Mesosphere:
The mesosphere is the third highest layer of Earth’s atmosphere, occupying the region above the stratosphere and below the thermosphere. It extends from the stratopause at an altitude of about 50 km. to the mesopause at 80-85 km above Sea level.
Question 15. Short Note-Stratosphere.
Answer:
Stratosphere :
The Stratosphere is the second lowest layer of Earth’s atmosphere. It lies above the troposphere and is separated from it by the tropopause. This layer extends from the top of the troposphere at 12km about the earth’s surface to the stratopause at an altitude of about 50 to 55 km.
Question 16. Short Note-Green House Effect.
Answer:
Green House Effect: The earth’s atmosphere is transparent to the visible light and infrared radiations of short wavelengths coming from the sun.
“WBBSE Class 10 Physical Science Chapter 1, Concerns About Our Environment long answer solutions”
“WBBSE Class 10 Concerns About Our Environment long answer questions, Physical Science Chapter 1”
Question 17. Briefly describe the thermal power plant.
Answer:
Thermal power plant :
A power plant in which the heat required to make steam to drive turbines is obtained by burning fuels is called a thermal power plant.
“Class 10 WBBSE Physical Science Chapter 1, Concerns About Our Environment detailed answers”
Question 18. What is the cause of global warming? What are the effects of global warming? Write the advantages of generating hydroelectricity.
Answer:
The cause of global warming :
The effect of global warming :
The advantages of Generating Hydroelectricity?