Chemistry In Every Day Life
Chemistry influences every aspect of life. Food, clothing, furniture, medicines, and so on, are all associated with chemistry. Sugar and rubber (from plants); oils, fats, and proteins (from animals); insecticides, dyes, perfumes, explosives, lubricants, solvents, refrigerants, and fuels (petroleum) are products of organic compounds.
In this chapter, we shall discuss some important aspects of the chemistry of drugs, food, and cleansing agents.
Drugs
- A drug is a chemical that has a low molecular mass (~100 to 500 u). It interacts with a macromolecular target(s) and brings about a biological response. If this biological response helps prevent, manage or cure a disease, the chemical is called a medicine. Medicines are also used to alleviate pain.
- However, medicines should not be taken in dosages higher than required. Doing so would cause adverse effects. Remember that contraceptives and nutrients are not considered to be medicines/drugs.
- WHO (1966) has given a more comprehensive definition.
- “A drug is any substance or product that is used or is intended to be used to modify or explore physiological systems or pathological states for the benefit of the recipient.”
- Ehrlich defined the term chemotherapy as treatment with chemicals that are toxic to infectious microorganisms but harmless to humans.
“class 12 chemistry chapter 16 notes”
Classification Of Drugs
Drugs are classified in four different ways.
- One classification is based on the pharmacological effect of the drugs available for the treatment of a particular type of symptom/disease. For example, analgesics are painkillers, antipyretics reduce fever, and antiseptics destroy or arrest the growth of microorganisms (bacteria).
- Some drugs have a similar action on any biochemical process. For example, antihistamines inhibit the action of histamines, which cause allergic reactions.
- Yet another classification is based on chemical structure. Drugs with common structural features do have similar pharmacological activity. For example, sulphonamides have the following common structural features.
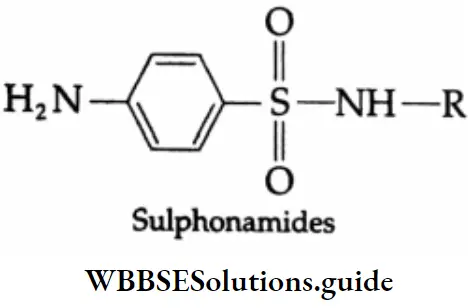
Drugs may also be classified based on molecular targets. They usually interact with macromolecules such as carbohydrates, lipids, proteins, and nucleic acids. These macromolecules are called target molecules or drug targets. Drugs with common structural features may have the same mechanism of action on targets.
Class 12 chemistry chapter 16 chemistry in everyday life notes
Catalytic Activity Of Enzymes
Almost all biological reactions in our body are carried out under the catalytic influence of enzymes. Hence, enzymes are a very important target of drug action. Drugs can either increase or decrease the rate of enzymatically mediated reactions.
Enzymes perform two major functions in their catalytic activity.
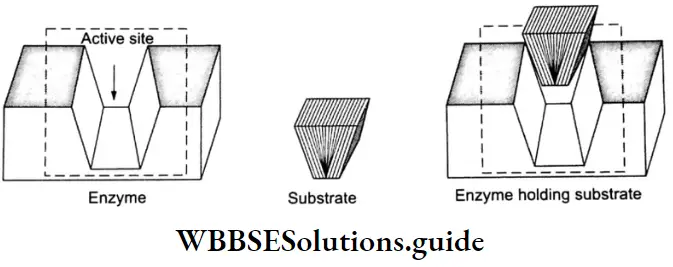
1. The catalytic activity of an enzyme is due to the presence of a specific site, on its surface, called the active site.
- This site is characterized by the presence of functional groups that form weak bonds, such as ionic bonds, hydrogen bonds, or van der Waals bonds, with the substrate molecule.
- The enzyme can also be attached to the substrate through dipole-dipole attraction.
- An enzyme has a distinct cavity in which the substrate is bound. The cavity contains an active center in which the amino acids are grouped in such a way as to enable them to combine with the substrate. The substrate induces a conformational change in the enzyme.
- This aligns amino acid residues or the other groups on the enzyme in the correct spatial orientation for substrate binding.

2. After attaining the abovementioned appropriate steric orientation, the reactants (enzyme and substrate) react to form the products. As the enzyme surface has no affinity for the product molecules, the latter leaves the enzyme surface quickly to make room for fresh molecules of substrates to be bound at the active site.
Inhibition Of Enzymes
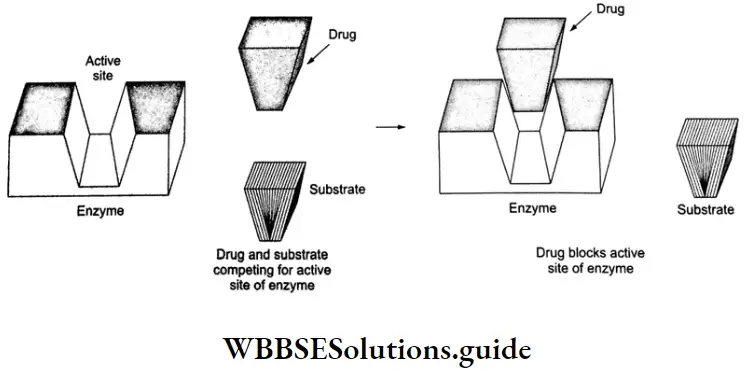
Inhibition of enzymes is a common mode of drug action. Drugs can block the binding site of an enzyme and thus prevent the binding of the substrate. Such drugs are called enzyme inhibitors.
Drugs inhibit the attachment of the substrate to the active site in two different ways.
1. Some drugs compete with the normal substrate for attachment to the active site of the enzyme. Such drugs are called competitive inhibitors.
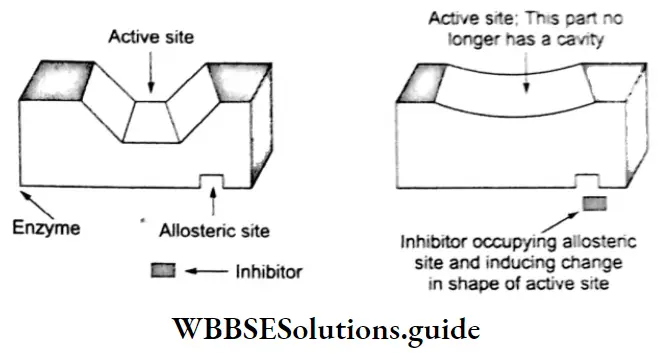
2. Some drugs react with an adjacent site (called an allosteric site) and not with the active site of the enzyme. They alter the enzyme in such a way that it loses its catalytic property—the attachment of inhibitors at the allosteric site changes the shape of the active site in such a way that the substrate is unable to recognize the active site.
“chemistry in everyday life class 12 notes”
The enzyme is made ineffective permanently if the bond formed between the inhibitor and the enzyme is a strong covalent bond. In such a case, the enzyme-inhibitor complex is degraded by the body, and a new enzyme is synthesized.
Receptors As Drug Targets
Many drugs exert their physiological effects by binding to a specific cellular binding site called a receptor. A drug (agonist) interacts with its receptor by the same kinds of bonding interactions-hydrogen bonding, electrostatic attractions, and van der Waals interactions.
The most important factor in bringing together a drug and a receptor is a close fit: the greater the affinity of a drug for its binding site, the higher is the drug’s potential biological activity.
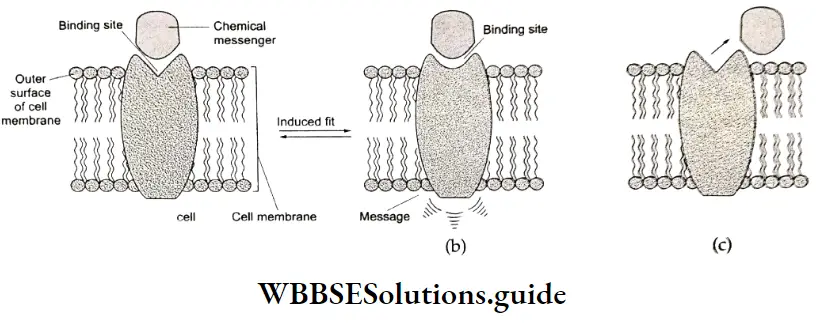
Agonists: These are drugs that mimic a natural messenger and activate a receptor to produce an effect. These come into play when the natural messenger is not available.
Antagonists: Antagonists attach themselves to receptors and prevent them from functioning. They are useful when a message is required to be blocked.
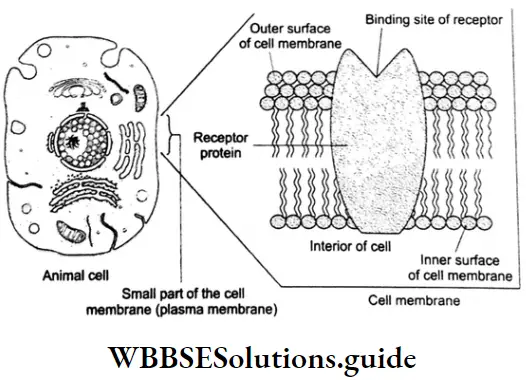
Transfer Of Message Into The Cell By The Receptor
Our body has a large number of different receptors which interact with different chemical messengers. (Chemical messengers are in fact chemicals.) These receptors bind with specific chemical messengers because their binding sites (active sites) have different shapes, structures, and amino acid compositions.
To accommodate a chemical messenger, the shape of the receptor site changes. This causes the transfer of a message into the cell. The chemical messenger gives a message to the cell without in fact entering the cell.
Types Of Drugs
Antacids
Under normal conditions, the stomach contains a certain amount of hydrochloric acid. The H+ ion of HCl participates in the process of digestion. An excess of this acid causes indigestion.
Excess HCl can be produced due to various reasons-overeating, the ingestion of certain kinds of spicy food, and increased stress.
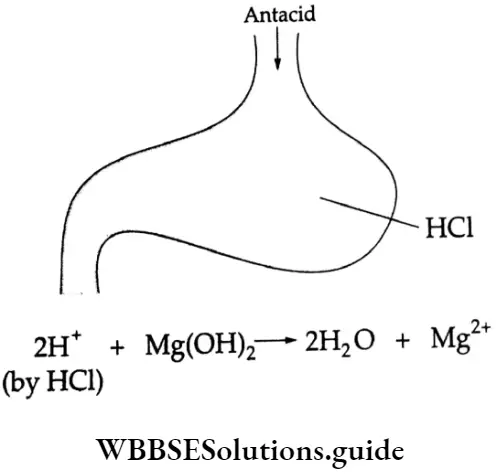
The function of an antacid is to relieve indigestion by reducing the amount of stomach acid (gastric acid) to a normal level by neutralisation. Various compounds such as magnesium hydroxide, aluminium hydroxide and sodium bicarbonate, which have basic properties, can reduce acidity.
⇒ \(\mathrm{NaHCO}_3+\mathrm{H}^{+} \rightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2+\mathrm{Na}^{+}\)
⇒ \(\mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{H}^{+} \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{Mg}^{2+}\)
⇒ \(\mathrm{Al}(\mathrm{OH})_3+3 \mathrm{H}^{+} \rightarrow 3 \mathrm{H}_2 \mathrm{O}+\mathrm{Al}^{3+}\)
“important topics in chemistry in everyday life”
However, excessive use of bicarbonate can cause hypersecretion of hydrochloric acid by the cells of the stomach lining, causing ulcers. Such ulcers can be life-threatening in advanced stages and the only treatment is to remove the ulcerated part of the stomach by surgery.
Metal hydroxides such as magnesium hydroxide and aluminum hydroxide are better options because they are insoluble and do not increase the pH value above 7. Although antacids do relieve acidity, they do not cure the cause of hyperacidity. Unless the cause of the hyperacidity is removed, antacids bring only temporary relief.
Chemistry in everyday life class 12 short notes
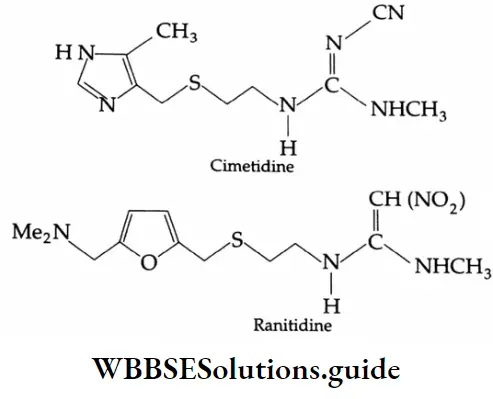
Histamine, a chemical, also stimulates the secretion of pepsin and hydrochloric acid in the stomach. A major breakthrough in the treatment of hyperacidity came when cimetidine, an antihistamine, was discovered to prevent the interaction of histamine with the receptors present in the stomach wall.
When cimetidine is administered, less acid is released in the stomach. Later, another drug, ranitidine (Zantac) was discovered and widely used in the treatment of hyperacidity.
Antihistamines
- Histamine has various functions. It causes dilation of blood capillaries. In addition, it makes the capillaries more permeable to blood fluids.
- Thus these fluids can readily leak out of the capillaries and cause swelling of the tissues. The compound causes contraction and spasms of the smooth muscles in the bronchial tubes.
- It can produce skin swellings and stimulate the glands that secrete watery nasal fluids, mucus, tears, saliva, and so on.
- Histamine is released in the body due to an allergic reaction caused by dust, pollen, or certain kinds of food.
- The net effect of its actions includes runny nose, congestion, and sneezing. Antihistamine compounds work against the action of histamine.
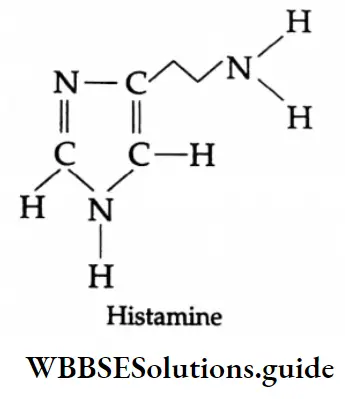
Antihistamines and histamines have certain structural features in common, as shown below.
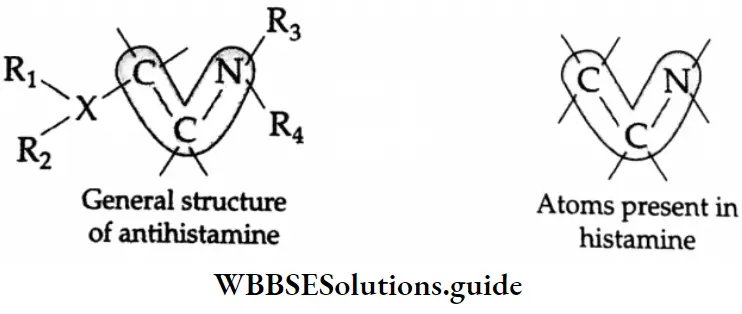
Due to the similarity in their structural features, antihistamines mimic histamines in their chemical reactions and can take the place of histamines. This, in effect, blocks the action of histamines and the symptoms caused by histamines begin to disappear.
“applications of chemistry in daily life”
Some commonly used antihistamines are Diphenylhydramine (Benadryl), Brompheniramine (Dimetapp), Promethazine (Phenergan), and Terfenadine (Trexyl, Seldane).
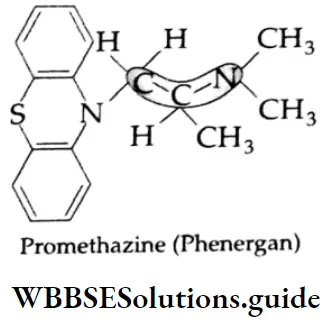
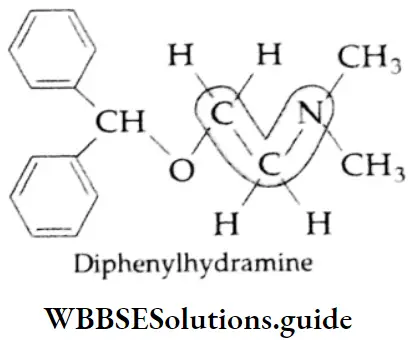
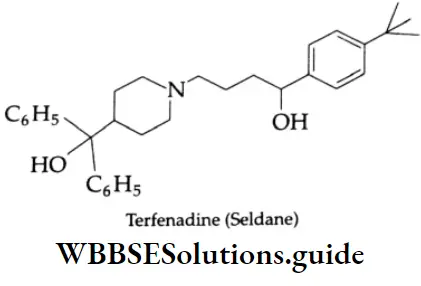
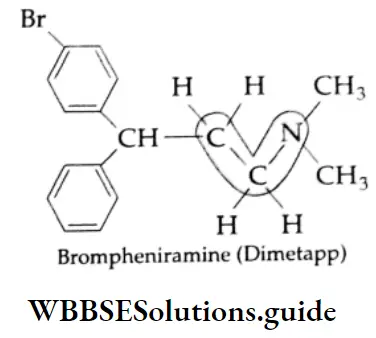
Antihistaminic agents are used primarily in the management of certain allergic disorders.
Many antihistamines such as diphenylhydramine and brompheniramine produce variable degrees of central nervous system depression and as such they all have sedative action. Terfenadine is a nonsedative antiallergic.
“drugs and medicines in chemistry class 12”
Some antihistaminic drugs and their brand names:
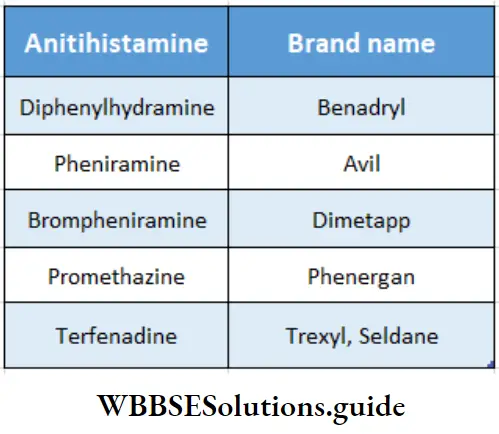
Excess histamine production by the body also causes the hypersecretion of hydrochloric acid by the cells of the stomach lining, leading to the development of ulcers.
The antihistamines that block the histamine receptors, thereby preventing the allergic responses associated with excess histamine production, do not affect HCl production.
“role of chemistry in food and nutrition”
The reason is that a second kind of histamine receptor triggers the release of acid into the stomach.
