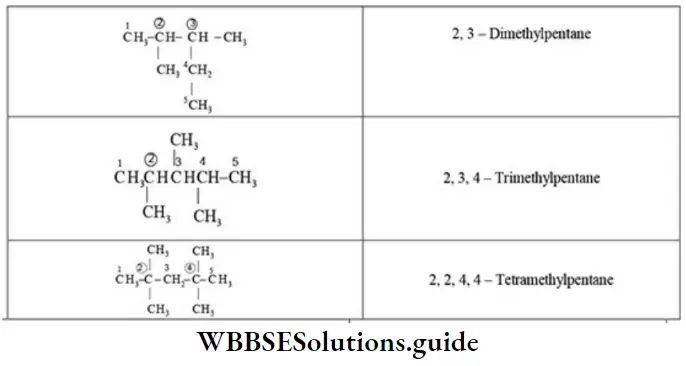
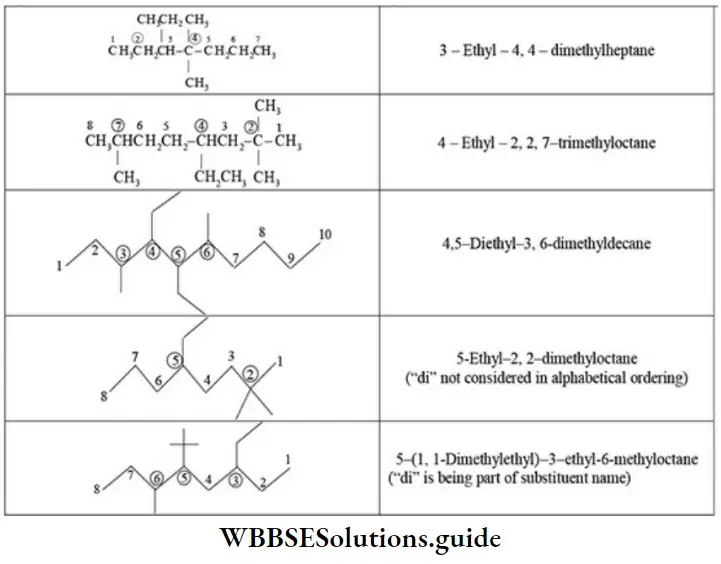
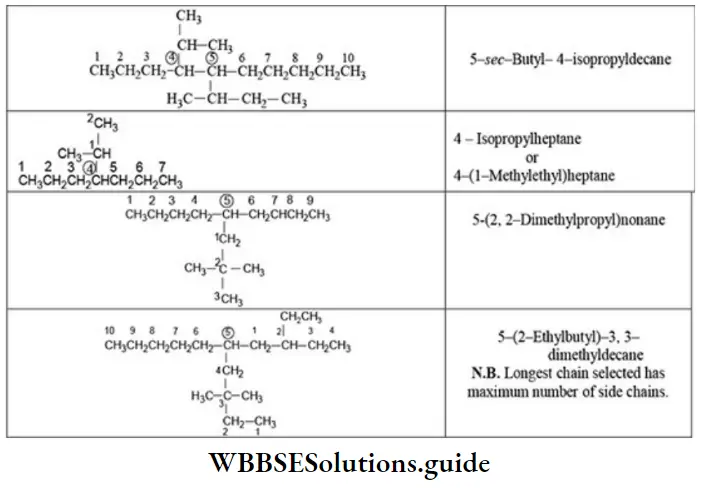
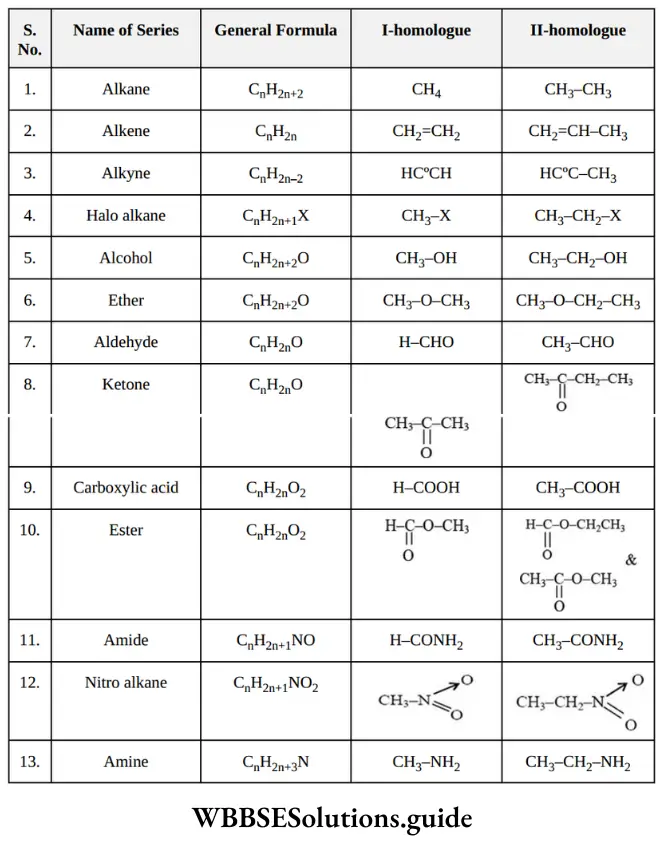
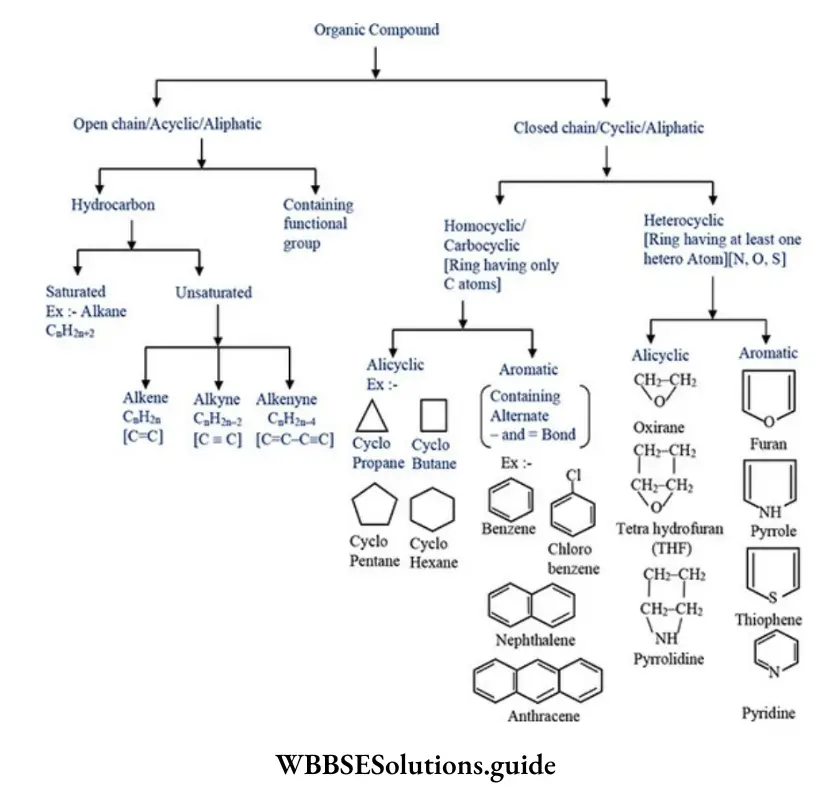
Quantitative Analysis
Estimation of carbon and hydrogen by Liebig’s combustion method
The quantitative analysis deals with the determination of percentage of various elements. The following methods are employed for the determination of the percentage composition of elements present in the organic compounds.
Read And Learn More: NEET General Organic Chemistry Notes, Question And Answers
Percentage of hydrogen in the compound = \(\frac{2}{18} \times \frac{\text { mass of water } \times 100}{\text { mass of organic compound }}\)
Percentage of carbon in the compound = \(\frac{12 \times \text { mass of carbon dioxide } \times 100}{44 \times \text { mass of organic compound }}\)
Depending upon the composition of organic compounds, the following modifications are made in the estimation of carbon and hydrogen by Liebigs method.
Estimation of nitrogen by Kjeldahl’s method
Nitrogen By Kjeldahl’s Method Principle is based on the quantitative conversion of nitrogen of the organic compound to ammonium sulphate by sulphuric acid. The reaction product is treated with an alkali and the ammonia released is determined. From this the amount of nitrogen in the organic compound is calculated.
⇒ \(\underset{\text { organic compound }}{[\mathrm{C}, \mathrm{H}, \mathrm{N}, \mathrm{S}]} \underset{\text { heat }}{\stackrel{\text { cooc. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2+\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4\)
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4+2 \mathrm{NaOH} \stackrel{\text { heat }}{\longrightarrow} \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{NH}_3\)
Quantitative Techniques Definition
This method is simple, convenient, and largely used for the estimation of nitrogen in foodstuffs, drugs, fertilizers, and many other organic compounds. However, this method cannot be employed for the estimation of nitrogen in the following types of organic compounds.
Organic compounds containing nitrogen in aromatic rings such as pyridine, quinoline, etc.,
NEET organic chemistry quantitative analysis notes
Organic compounds containing nitro (-NO2) and diazo (- N = N -) groups.
% of nitrogen in the compound = \(\frac{\text { volume of the acid }\left(\mathrm{cm}^3\right) \times \text { normality of the acid } \times 1.4}{\text { mass of organic compound in } \mathrm{g}}\)
Estimation of Nitrogen by Duma’s method
Nitrogen By Duma’s method Principle: The organic compound containing nitrogen when heated with excess of copper oxide in the atmosphere of carbon dioxide, yields nitrogen in addition to carbon dioxide and water.
⇒ \(\underset{\text { Organic compound }}{\left[\mathrm{C}_{\mathrm{x}} \mathrm{H}_y \mathrm{~N}_z\right]}+\left(2 \mathrm{x}+\frac{\mathrm{y}}{2}\right) \mathrm{CuO} \rightarrow\left(2 \mathrm{x}+\frac{\mathrm{y}}{2}\right) \mathrm{Cu}+\mathrm{xCO}_2+\frac{\mathrm{y}}{2} \mathrm{H}_2 \mathrm{O}+\frac{\mathrm{z}}{2} \mathrm{~N}_2\)
Traces of nitrogen oxides formed during combustion of organic compound are reduced to nitrogen by passing the gaseous mixture over a heated copper gauze. The percentage of nitrogen present in a given organic compound is calculated from the volume of nitrogen collected over potassium hydroxide solution from a known mass of organic compound.
Percentage of nitrogen = \(\frac{28 \times V_0 \times 100}{22,400 \times m}\)
where V0 = volume of nitrogen at STP; m = mass of organic compound in grams.

Estimation of Halogen by Carius method
Halogen By Carius Method Principle: A known mass of an organic compound is heated with fuming nitric acid and a few crystals of silver nitrate in a sealed tube called the Carius tube. The silver halide is formed.
⇒ \(\underset{\text { Organic compouad }}{[\mathrm{C} \mathrm{H} \mathrm{X}]}+[\mathrm{O}] \underset{\text { beat }}{\stackrel{\text { fuming } \mathrm{HNO}_3}{\longrightarrow}} \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{HX}\)
⇒ \(\mathrm{HX}+\mathrm{AgNO}_3 \rightarrow \underset{\text { Preceipitate }}{\mathrm{AgX}}+\mathrm{HNO}_3\)
Quantitative analysis of organic compounds NEET study material
Percentage of halogen = \(\frac{\text { atomic mass of } \mathrm{X}}{\text { molecular mass of } \mathrm{AgX}} \times \frac{\text { mass of silver halide in grams }}{\text { mass of organic compound in grams }} \times 100\)
Percentage of chlorine = \(\frac{35.5}{(108+35.5)} \times \frac{\text { mass of silver chloride in grams }}{\text { mass of organic compound in grams }} \times 100\)
Percentage of bromine = \(\frac{80.0}{(108+80)} \times \frac{\text { mass of silver bromide in grams }}{\text { mass of organic compound in grams }} \times 100\)
Percentage of iodine = \(\frac{127}{(108+127)} \times \frac{\text { mass of silver iodide in grams }}{\text { mass of organic compound in grams }} \times 100\)
- This method does not give satisfactory results in the estimation of iodine as Agl is slightly soluble in nitric acid and iodide may oxidize to iodine to some extent.
- The results of this method is not very accurate in case of polyhalogenated aromatic compounds
- Estimation of fluorine can not be carried out as AgF is soluble in water.
Estimation Of Sulfur by Carius Method
Sulfur By Carius Method Principle: An organic compound is digested with fuming nitric acid in a sealed tube. The sulfur present in the compound is quantitatively oxidised into sulphuric acid. Sulphuric acid so formed is precipitated as barium sulfate by adding excess of barium chloride.
Percentage of sulphur = \(=\frac{32}{233} \times \frac{\text { mass of barium sulphate in grams }}{\text { mass of organic compounds in grams }} \times 100\)
Estimation of phosphorous by Carius method
Phosphorous By Carius Method Principle: The phosphorus present in the organic compound is oxidised to orthophosphoric acid by heating with fuming nitric acid. The phosphoric acid so obtained is precipitated as MgNH4PO4 which on ignition is converted into Mg2P2O7.
⇒ \(\underset{\text { Organic component }}{[\mathrm{C}, \mathrm{H}, \mathrm{P}]}+[\mathrm{O}] \underset{\text { nitric acid }}{\stackrel{\text { filming }}{\longrightarrow}} \mathrm{H}_3 \mathrm{PO}_4+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
⇒ \(\mathrm{H}_3 \mathrm{PO}_4+\left[\mathrm{NH}_4 \mathrm{Cl}+\underset{\text { magnetic mixture }}{\mathrm{NH}_4 \mathrm{OH}}+\mathrm{MgCl}_2\right] \rightarrow \underset{\text { white precipitate }}{\mathrm{MgNH}_4 \mathrm{PO}_4}\)
NEET chemistry quantitative analysis solved examples
⇒ \(2 \mathrm{MgNH}_4 \mathrm{PO}_4 \stackrel{\text { ignite }}{\longrightarrow} \underset{\text { magnesium pyophloctuse }}{\mathrm{Mg}_2 \mathrm{P}_2 \mathrm{O}_7}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{NH}_3\)
⇒ \(\text { Percentage of phosphorus }=\frac{62}{222} \times \frac{\text { mass of } \mathrm{Mg}_2 \mathrm{P}_2 \mathrm{O}_7 \text { in grams }}{\text { mass of organic compound in grams }} \times 100\)
Estimation of oxygen: There is no direct method for the estimation of oxygen present in the organic compound. The percentage of oxygen in the compound is generally estimated by difference.
Percentage of oxygen = 100 – [sum of percentage of all other elements present in it] The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements. However, oxygen can also be estimated directly as follows:
A definite mass of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red-hot coke when all the oxygen is converted to carbon monoxide. This mixture is passed through warm Iodine pentoxide (I2O5) when carbon monoxide is oxidised to carbon dioxide producing iodine.
⇒ \(Compound \stackrel{\text { heat }}{\longrightarrow} \mathrm{O}_2\)+ other gaseous products
⇒ \(\left.2 \mathrm{C}+\mathrm{O}_2 \stackrel{1373 \mathrm{~K}}{\longrightarrow} 2 \mathrm{CO}\right]\) x 5 ……..(1)
⇒ \(\left.\mathrm{I}_2 \mathrm{O}_5+5 \mathrm{CO} \longrightarrow \mathrm{I}_2+5 \mathrm{CO}_2\right]\) x 2…..(2)
Quantitative Analysis Chemistry
On making the amount of CO produced in equation (1) equal to the amount of CO used in equation (2) by multiplying equations (1) and (2) by 5 and 2 respectively; we find that each mole of oxygen liberated from the compound will produce two moles of carbon dioxide.
Thus 88 g carbon dioxide is obtained if 32 g oxygen is liberated.
Let the mass of organic compound taken be mg
NEET important questions on quantitative analysis with solutions
Mass of carbon dioxide produced be m1g
∴ \(\mathrm{m}_1 \mathrm{~g}\) carbon dioxide is obtained from \(\frac{32 \times \mathrm{m}_1}{88} \mathrm{~g} \mathrm{O}_2\)
∴ Percentage of oxygen = \(\frac{32 \times \mathrm{m}_1 \times 100}{88 \times \mathrm{m}} \%\)
The percentage of oxygen can be derived from the amount of iodine produced also.
Presently, the estimation of elements in an organic compound is carried out by using microquantities of substances and automatic experimental techniques. The elements, carbon, hydrogen and nitrogen present in a compound are determined by an apparatus known as CHN elemental analyser. The analyzer requires only a very small amount of the substance (1-3 mg) and displays the values on a screen within a short time.
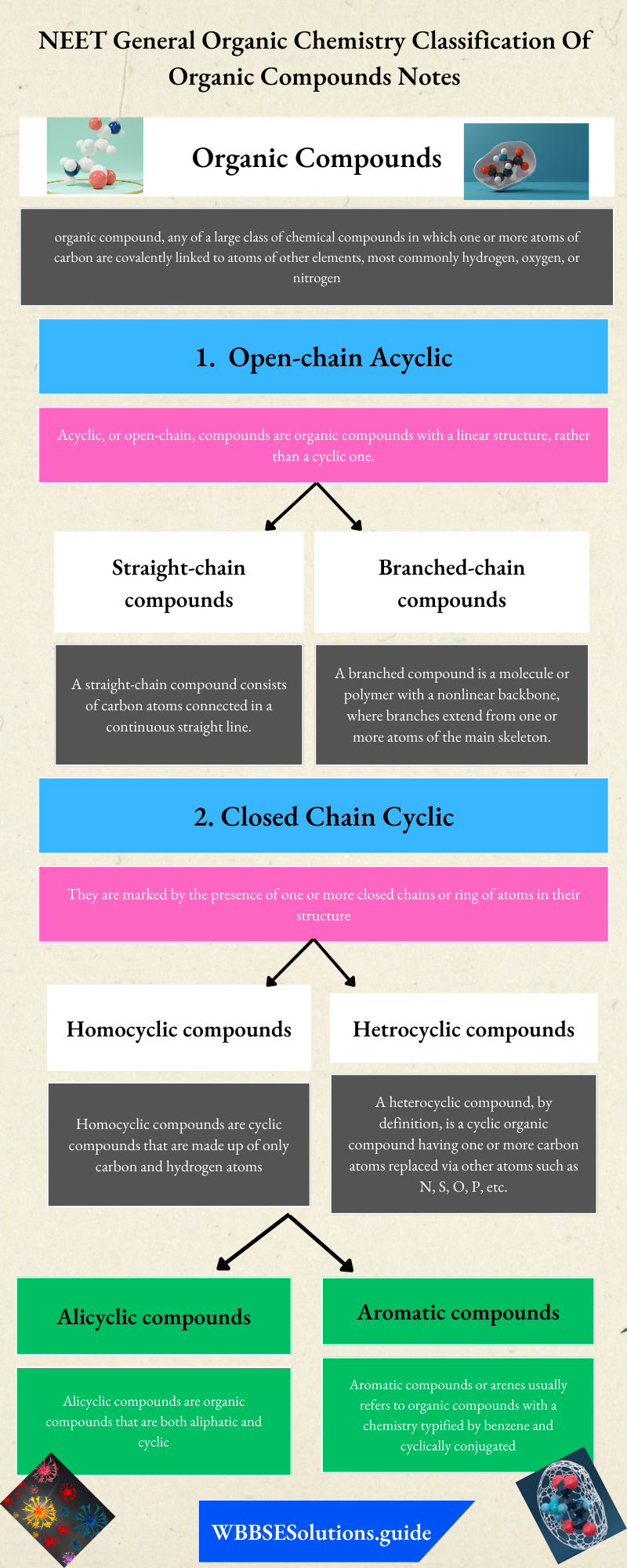
Empirical and Molecular Formulae: We know that a chemical formula gives the representation of a molecule of a substance in terms of symbols of various elements present in it. The determination of a formula of the substance involves the chemical analysis to determine
- The constituent elements present.
- The relative number of elements of each type present.
The chemical formula may be of two types:
- Empirical formula and
- Molecular formula
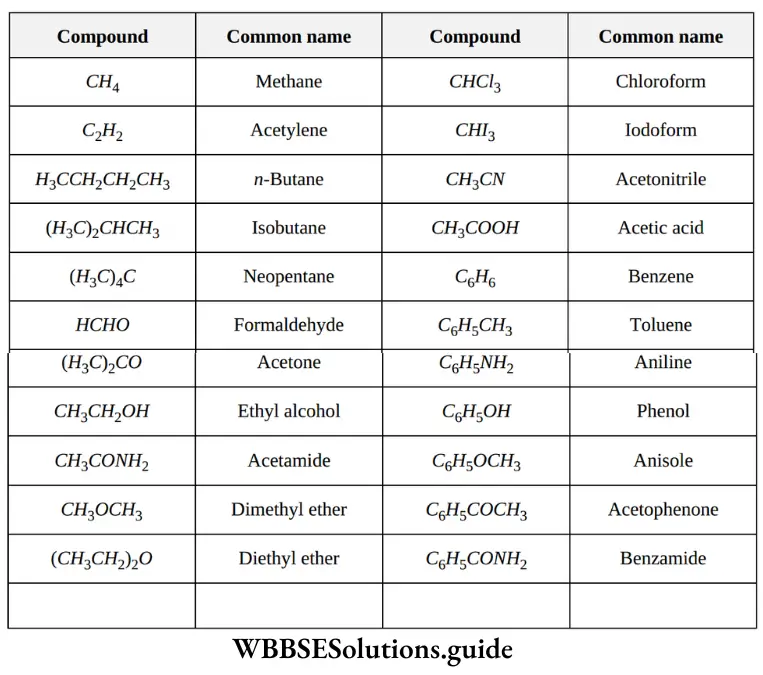
1. Empirical formula: The formula that gives the simple whole number ratio of the atoms of various elements present in one molecule of the compound is called empirical formula. For example, empirical formula of benzene is CH which indicates that atomic ratio of C: H in benzene is 1:1.
2. Molecular formula: The formula which gives the actual number of atoms of various elements present in one molecule of the compound is called molecular formula.
For example, molecular formula of benzene is C6H6 which tell that one molecule of benzene contains 6 atoms of carbon and 6 atoms of hydrogen.
Relation between empirical and molecular formulae; Molecular formula and empirical formula are related as molecular formula = n(empirical formula)
where n is a simple whole number and may have values 1, 2, 3 … It is equal to n = \(\frac{\text { molecular mass }}{\text { empirical formula mass }}\)
Methods of quantitative analysis in organic chemistry NEET
For example, the molecular mass of benzene is 78. The empirical formula of benzene is CH and therefore, its empirical formula mass is 13.
Thus, n = \(\frac{\text { molecular mass }}{\text { empirical formula mass }}=\frac{78}{13}=6\)
Therefore, the molecular formula of benzene = (CH)6 = C6H6.
It may be noted that in many cases, the value of ‘n’ comes out to be one and, therefore, empirical formula and molecular formula are same in these cases. For example, empirical and molecular formulae are same for methane (CH4), propane (C3H8), sucrose (C12H22O11) etc.
Determination of the empirical formula of a compound: Once the percentage composition is known, the ratio of the numbers of atoms of each element present in the compound can be calculated to get the empirical formula. The method is to divide the percentage composition of each element by its atomic mass and to factorise the resulting numbers to obtain simple whole numbers. The steps involved in determining the empirical formula are:
Step 1: The relative number of atoms (also called atomic ratio) of various elements in the molecule of the compound is given by the following relation.
Relative number of atoms = \(\frac{\text { percentage of an element }}{\text { atomic mass of the element }}\)
Step 2: The simplest ratio of the atoms of the elements is obtained by dividing the relative numbers of atoms by the least value.
Step 3: Round off the value to the nearest whole number and multiply, if necessary, by a suitable integer to make them whole numbers. This gives the simplest whole-number ratio.
Step 4: Write the chemical formula with the simplest ratio of the atoms. The formula, thus, obtained represents the empirical formula of the compound.
Determination of the molecular formula of a compound
Step 1: Determine the empirical formula.
Step 2: Calculate the empirical formula mass by adding the atomic masses of the atoms in the empirical formula.
Step 3: Determine the molecular mass by a suitable method.
Step 4: Determine the value of n, as ‘n’ = \(\frac{\text { molecular mass }}{\text { empirical formula mass }}\)
Round off ‘n’ to the nearest whole number.
Estimation of carbon and hydrogen in organic compounds NEET
Step 5: Multiply the empirical formula by ‘n’ to get the molecular formula.
For example, if a compound has 23.3% carbon, 4.85% hydrogen, 40.78% nitrogen, and the remainder is oxygen, the empirical formula is calculated as follows:
C = \(\frac{23 \cdot 3}{12}\), H = \(\frac{4 \cdot 85}{1}\), N = \(\frac{40 \cdot 78}{14}\) , O = \(\frac{31 \cdot 07}{16}\)
C = 194, H = 45, N =2.95, O = 1.94
Dividing by the smallest number, we get
C = \(\frac{1 \cdot 94}{1 \cdot 94}\) = 1; H = \(=\frac{4.85}{1.94}\) = 2.5: N = \(\frac{2.95}{1.94}\) = 1.5 and O =\(\frac{1.94}{1.94}\) = 1
The simplest whole number ratio is C2H5N3O2.
From the empirical formula, the molecular formula is arrived at by dividing the molecular mass by the empirical formula mass and the resulting whole number is multiplied with the empirical formula to get the molecular formula.
For example, if the empirical formula is CH2 and the molecular mass is 56, the molecular formula is 56/14 = 4, 4 x CH2 = C4H




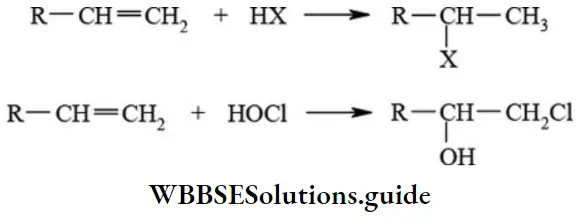
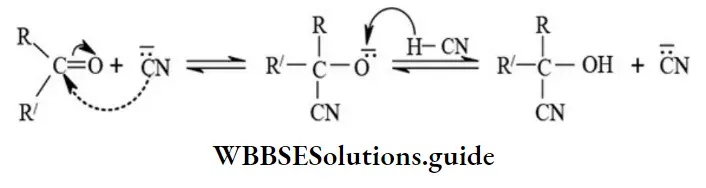
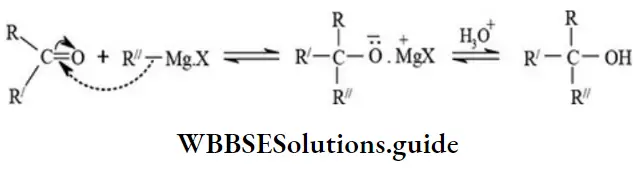
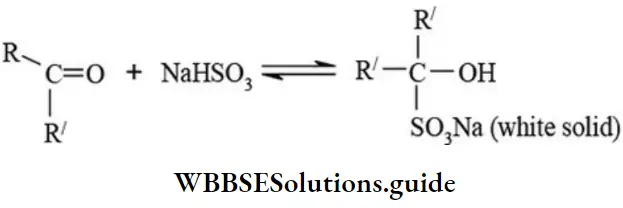
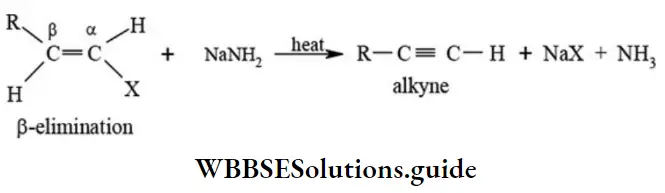
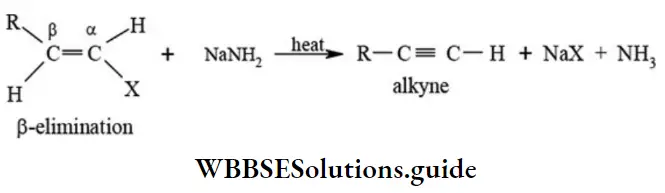
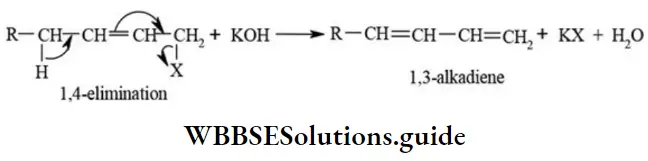
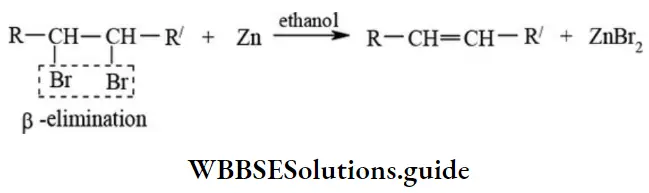
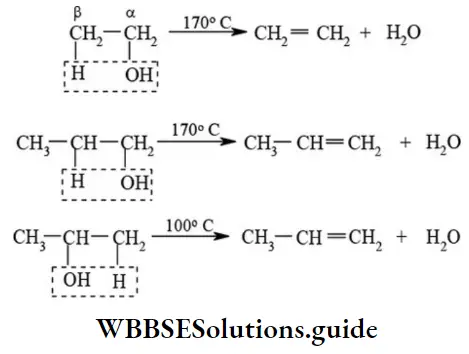
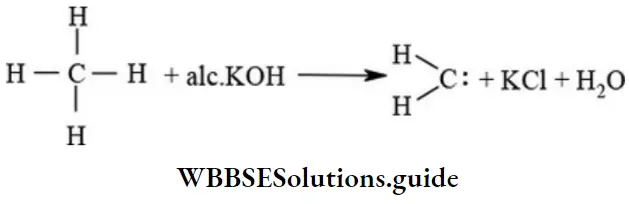
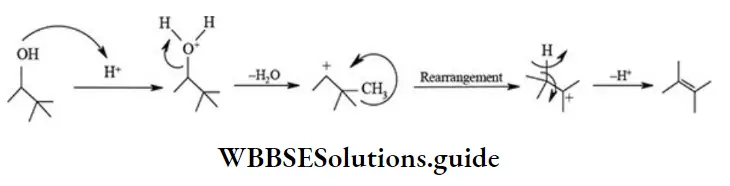

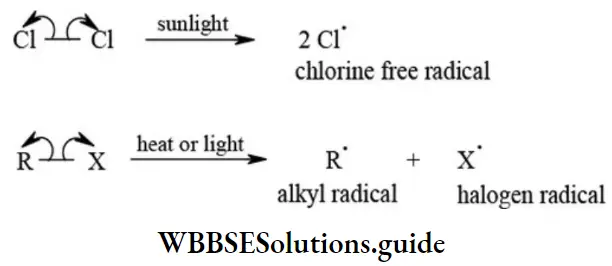

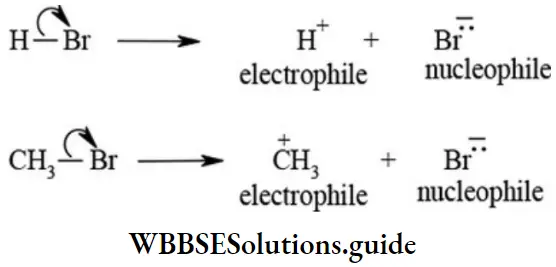
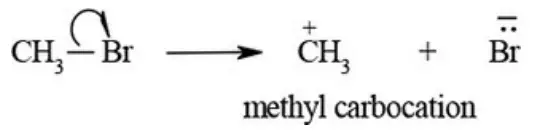
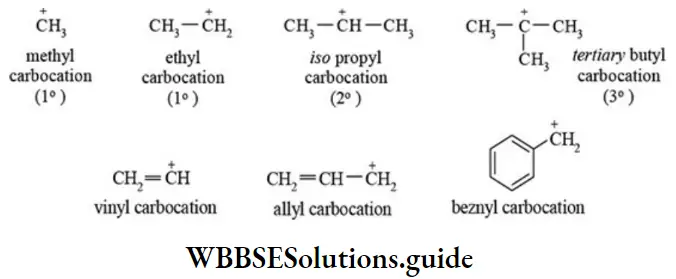
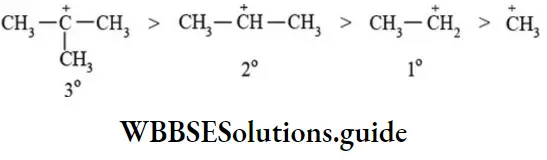
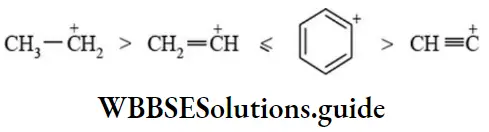
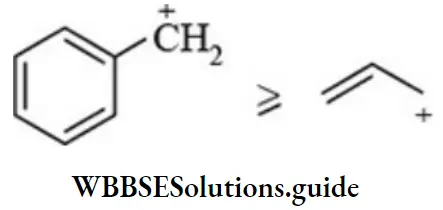
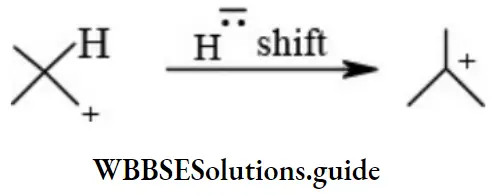
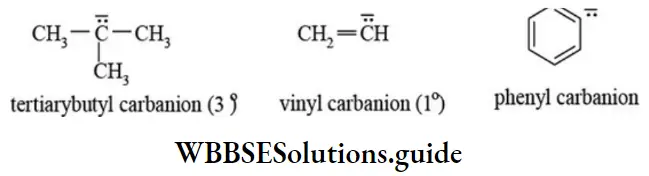
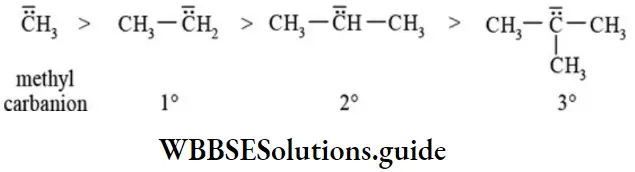
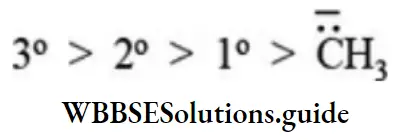
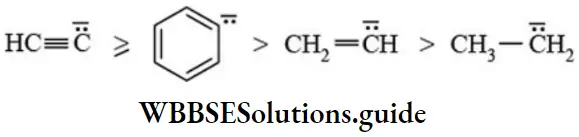
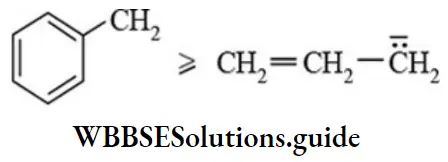
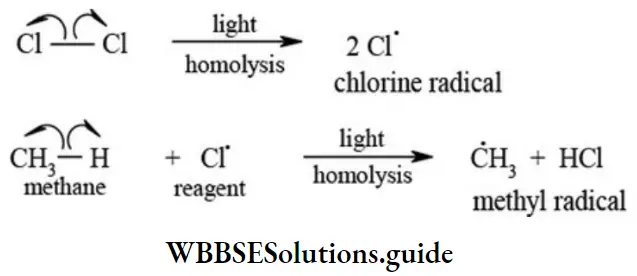
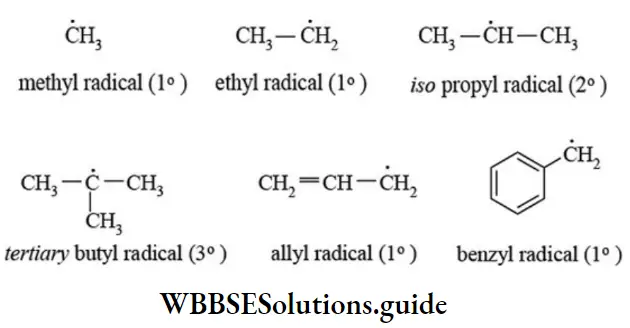
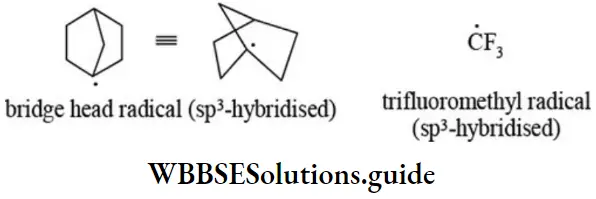

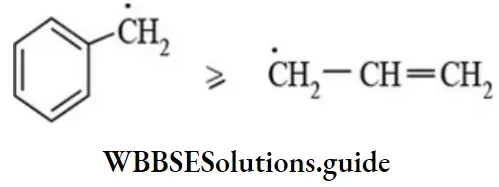
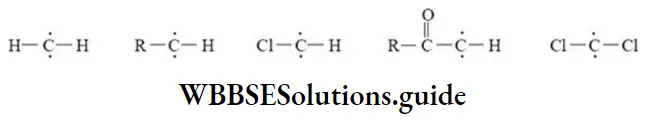
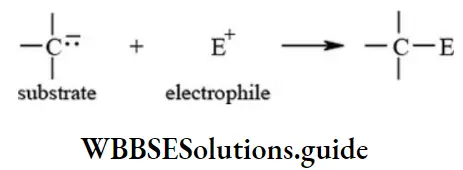

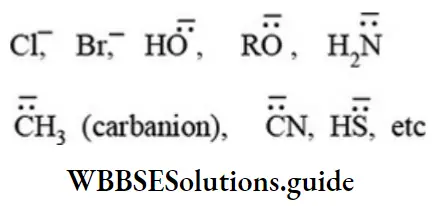

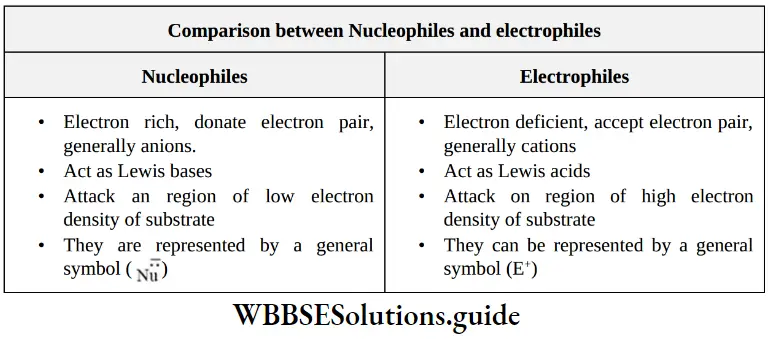

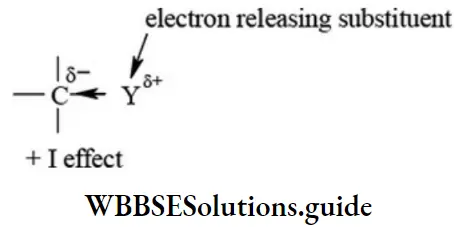
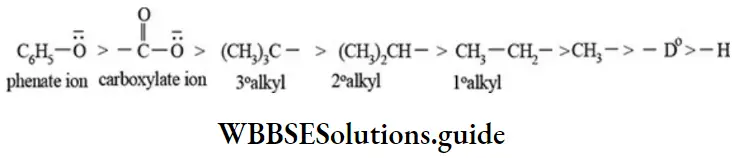
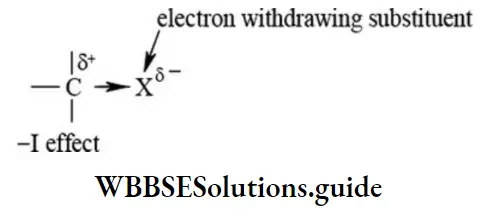
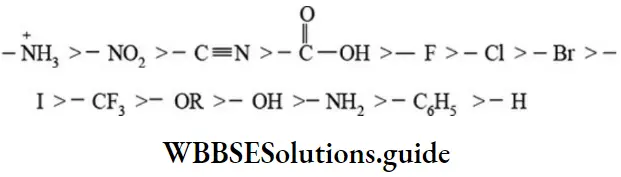
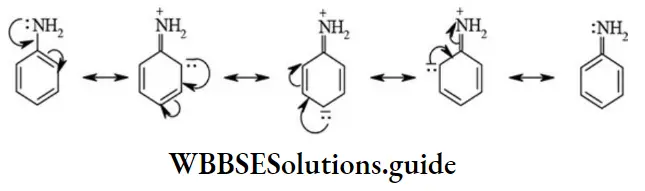

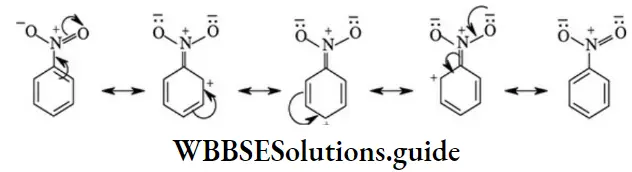
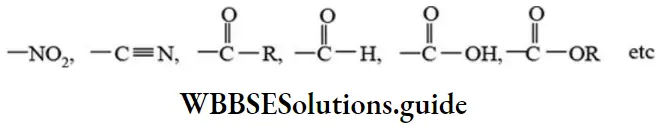
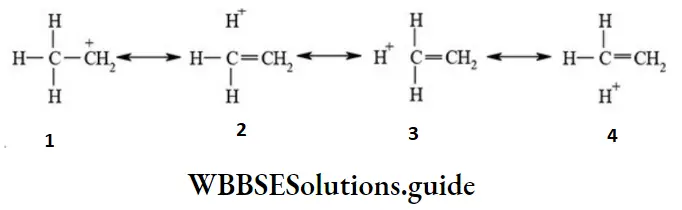
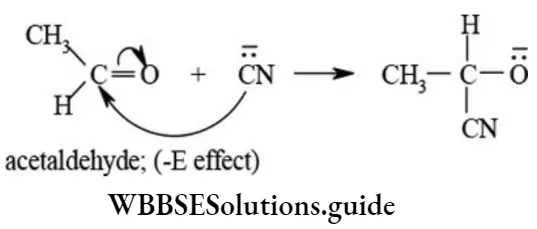
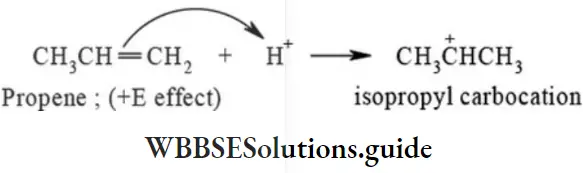
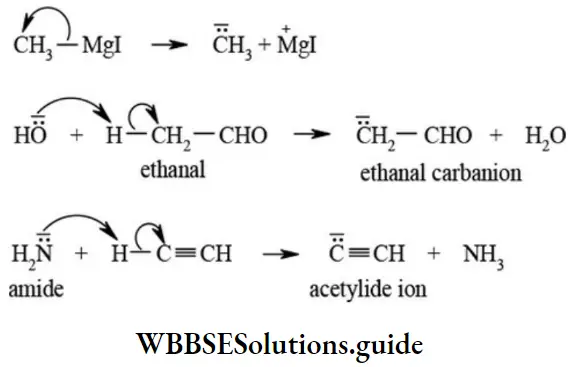
 etc. exert both +R and -R effects.
etc. exert both +R and -R effects.