Coordination Compounds And Organometallics
One of the characteristic features of transition elements is their ability to form coordination compounds or complexes. Haemoglobin in blood, chlorophyll in plants, dyes and vitamin B12 are all examples of coordination compounds.
Coordination compounds find manifold applications in chemical analysis, chemical industry, metallurgical processes and medicinal chemistry. The challenging field of bioinorganic chemistry, i.e., the application of inorganic chemistry to living systems, also deals with coordination compounds.
In this chapter, we shall study some aspects of this important class of compounds as well as a new class of compounds—organometallic compounds, which contain metal-carbon bonds.
Coordination Compounds
Coordination compounds are addition compounds and are formed by the addition of stoichiometric amounts of two or more stable compounds. Double salts are also addition compounds but they exist in the crystalline state lose their identity in solution and dissociate into simple ions. In contrast, coordination compounds or complexes retain their identity in solution.
Mohr’s salt [FeSO4.(NH4)2SO4 -6H2O] and potash alum [K2SO4.AI2(SO4)3 -24H2O] are double salts. A solution of Mohr’s salt cannot be distinguished from a solution obtained by mixing ferrous sulphate and ammonium sulphate since both contain Fe2+, NH4 and SO4 ions.
Unlike double salts, coordination compounds do not dissociate in solution. When aqueous ammonia is added to copper(2) sulphate solution, a dark blue colouration is obtained. The addition of alcohol to the solution results in the precipitation of tetraammine copper(2) sulphate as dark blue needle-shaped crystals.
⇒ \(\mathrm{CuSO}_4+4 \mathrm{NH}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CuSO}_4 \cdot 4 \mathrm{NH}_3 \cdot \mathrm{H}_2 \mathrm{O}\)
Tetraammine copper(2) sulphate monohydrate
This is a coordination compound represented as [Cu(NH3 )4]SO4. On dissociation, it forms [Cu(NH3)4]2+ and SO2– ions and thereby retains its identity.
Another example of a coordination compound is potassium hexacyanoferrate(2), also called potassium ferrocyanide, K4[Fe(CN)6]. This on dissolution produces K+ and [Fe(CN)6]4– ions. The complex ion does not dissociate further to give Fe2+ and CN–.
Important terms used In the context of coordination compounds
Coordination entity (complex) A coordination entity or complex comprises a central metal atom or ion attached to and surrounded by a group of anions or neutral molecules. These ions or molecules are called ligands and they can donate an electron pair to the central atom or ion, for example [Cu(NH3)4]2+, [Fe(H2O)6]2+ and [Fe(CN)6]4-.
A coordination entity may be cationic if it has a net positive charge, for example, [CO(NF3)4Cl2]+, and anionic if it bears a negative charge, for example [Ag(CN)2]– or electrically neutral, example [Pt(NH3)2Cl2]. These are also called molecular complexes.
Central atom/ion The metal atom or cation of a coordination entity to which a fixed number of ligands or electron pair donors are attached is called the central atom/ion. For example, the complexes [Cu(NH3)]2+ and [FeCl6]3-, the central ions are Cu2+ and Fe3+ respectively.
Ligands A ligand is a neutral or anionic species capable of donating a pair of electrons to the central metal/ion thus forming a coordinate bond. Ligands may be anions like Cl–, CN–, OH–‘, C2O4– or neutral molecules like NH3, H2O, NH2, CH2 NCH2 NH2 (ethane-1, 2-diamine) all containing lone pair of electrons.
In a coordination entity, the ligand acts as a Lewis base (electron-pair donor) and the metal atom/ion as a Lewis acid (electron-pair acceptor).
Coordination number The total number of sigma bonds formed by ligands surrounding the metal ion is called the coordination number of the central atom/ion. For example, the coordination number of platinum in [Pt (: NH3)4]2+ is 4.
The sigma bonding electrons can be represented as dots preceding the donor atom.
Remember that pi-bonds if any present between the ligand and the central atom/ion are not considered while determining the coordination number.
Coordination polyhedron Ligands in the complex are arranged in a fixed geometric pattern. The spatial arrangement of ligands attached around the central metal atom/ion is called the coordination polyhedron. The common shapes of coordination polyhedra are tetrahedral, square planar and octahedral. Square pyramidal and trigonal bipyramidal entities are also known.
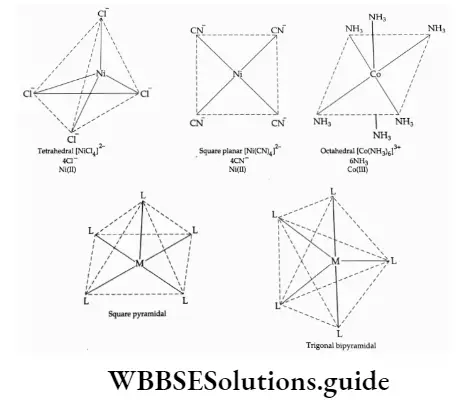
Coordination and ionisation spheres The coordination sphere is made up of the central atom/ion and the ligand. In the formula, it is represented as enclosed in square brackets.
In the complex [Co(NH3)6]Cl3, the coordination sphere is made up of Co3+ and six NH3 molecules (ligand).
A cationic or anionic complex needs anions or cations to preserve its electrical neutrality. Such ions are called counter ions. They are not directly attached to the metal ion and are present in the ionisation sphere, for example in [CO(NH3 )6 ]CI3 the ionisation sphere is made up of three C– ions.
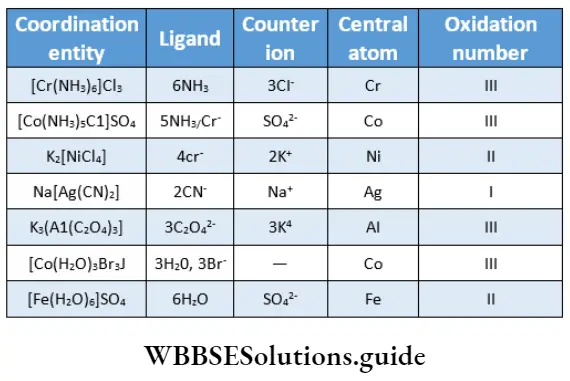
The oxidation number of a central atom The oxidation number of the central metal atom is the charge it would carry if all ligands were parentheses after the name or symbol of the metal.
Some examples of coordination entities, their central atom and the oxidation number are summarised.
Types of ligands Ligands may be classified in two ways: (1) on the basis of the charge they carry, and (2) on the basis of the number of donor atoms they contain.
A ligand may be negative (Cl–, F–, SCN–, etc.), neutral (NH3, H2O) or, very rarely, positive (NH2NH3+).
When a ligand is bound to the central metal ion through one donor atom, it is called a monodentate or unidentate ligand, for example, H2O, NH3, Cl–, CN–, etc. Ligands having two donor atoms are called bidentate or bidentate ligands. Some examples of bidentate ligands are as follows.
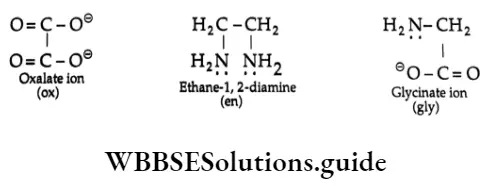
The IUPAC nomenclature has accepted certain short-form notations for some legends. Some of these are ox (oxalate), gly (glycine), EDTA (ethylene diamine tetraacetic acid), en (ethane-1, 2-diamine), etc.
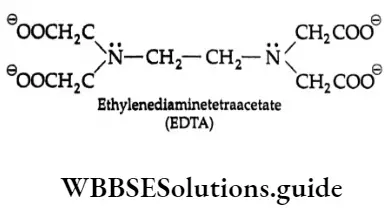
Ligands containing more than two donor atoms are called polydentate. These may be tridentate or tridentate having three donor atoms, tetradentate, having four donor atoms, pentadentate (five) and hexadentate (six). An important hexadentate legend is ethylenediaminetetraacetate (EDTA).
Some ligands may have two donor atoms but the two sites are not used simultaneously. These are called ambidentate ligands, for example, the thiocyanate ion (SCN–) can ligate through either the sulphur or the nitrogen atom.
Similarly, the nitrite ion (NO2–) can coordinate through the nitrogen or the oxygen atom. Chelating ligands and chelates When a bidentate and a polydentate ligand bind a single metal ion using ds two or more (in the case of polydentate) donor atoms, a complex known as a chelate is formed.
Such ligands are mlJed chelating ligands. An example of a chelate is the complex formed between ethylenediamine and Cu2+ it is represented as
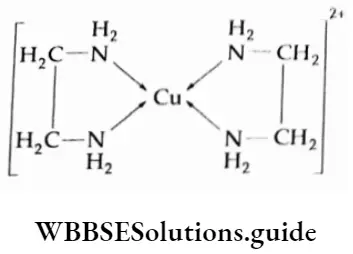
Chelate complexes form a ring structure.
Chelating ligands form more stable complexes than those formed by monodentate ligands. This is called the chelate effect. The five-membered or six-membered rings are the most stable.
A molecule of a bidentate ligand blocks two coordinate sites whereas a tridentate ligand blocks three coordinate sites, and so on.
Therefore, while calculating the coordination number of a metal ion, knowledge of the type of the ligand is important. For example, the coordination number of iron in [Fe(C2O4 )3]3+ is 6 and not 3 as oxalate is a bidentate ligand.
Homoleptic and heteroleptic complexes A homoleptic complex is one in which the metal ion is bound to only one ligand, for example [Co(H2O)6]3+. However, if a metal is bound to more than one ligand, for example, [Co(NH3)4(H2O)6]3+, it is referred to as a heteroleptic complex.

