Photophosphorylation
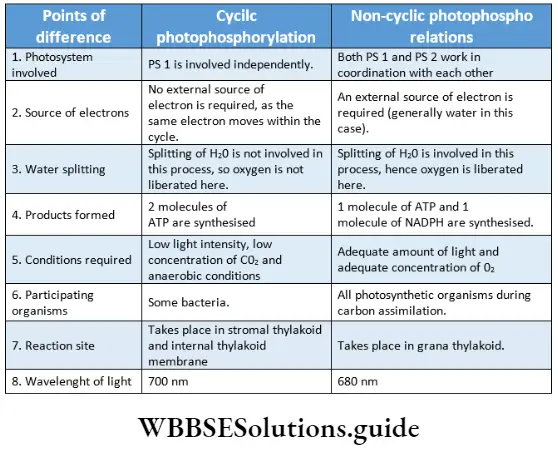
Cyclic And Noncyclic Photophosphorylation
Photophosphorylation Definition: The synthesis of ATP from ADP and Pi using the energy of light is called photophosphorylation.
It can be of two types
- Cyclic and
- Non-cyclic photophosphorylation.
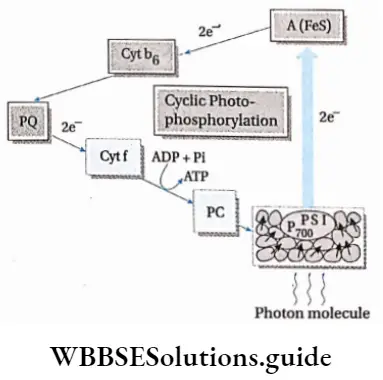
Cyclic Photophosphorylation:
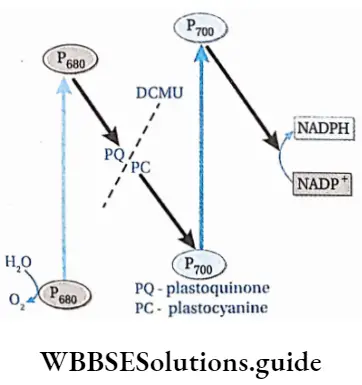
The photophosphorylation process which occurs along with cyclic electron transport is known as cyclic photophosphorylation.
Cyclic Photophosphorylation
Photophosphorylation Process:
- Only PS 1 or P is involved in the process.
- Chlorophyll dimers of the reaction centre absorb light rays of wavelength more than 680 nm.
- After absorbing light in the form of a photon, an electron is released, leading to the oxidation of the reaction centre.
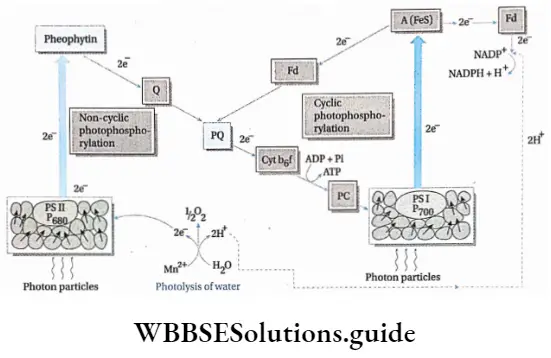
- The reaction centre becomes positively charged and the electron released now forms ferredoxin reducing substance (FRS).
- The electron is carried by the electron carriers like quinone (Q), plastoquinone (PQ), FeS protein and finally ferredoxin (Fd). Now the electron, from Fd, returns to P700, via Cyt b6f and plastocyanin.
- The electron creates a proton gradient which leads to the formation of 2 ATP molecules from 2ADP and Pi.
- Due to the absence of photolysis of water, O2 and NADPH are not produced.
Limitations of cyclic photophosphorylation
- It is an incomplete mechanism, that occurs only when non-cyclic photophosphorylation is prevented.
- It is active only within PS I.
- As the non-cyclic photophosphorylation stops, carbon assimilation no longer takes place. This reduces the rate of photosynthesis.
- O2 is not produced, hence NADPH is also not formed.
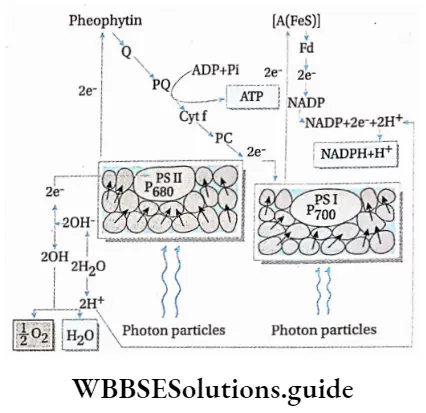
Non-cyclic photophosphorylation: The photophosphorylation process which occurs along with non-cyclic electron transport is known as non-cyclic photophosphorylation.
Non-cyclic photophosphorylation Process:
- Photosystem 1, photosystem 2 and cytochrome b6f complex are involved in the transport of protons and electrons.
- PS 2 absorbs light of 680 nm wavelength (in the red part of the spectrum) and its reaction centre, P680, gets excited. It releases a pair of electrons and becomes P680+. This reaction centre can, later on, absorb electrons released by the splitting of water.
- The electrons released by P680 are accepted by the primary electron acceptor Pheo,
- The electrons are transferred from Pheo to PCT. Simultaneously, PQ- also accepts 2H+ from stroma to form PQH2.
- PQH2 is oxidised and the two protons are released into the lumen and form semireduced plastosemiquinone (PQH). One electron is given to cytochrome f, in cyt b6f complex, via a Fe-S protein.
- PQH is now further oxidised to form PQ. This electron is now transferred to cytochrome b6, in cyt b6f complex.
- The electron of cytochrome f is transferred to a Cu-containing electron carrier, plastocyanin (PC).
- This electron is now transferred to PS 1.
- Simultaneously, PS 1 absorbs light having a wavelength of 700 nm (in the far-red part of the spectrum) and its reaction centre P700 gets excited. It expels the electrons, which are accepted by a Fe-S protein. P700 becomes P700+.
- These electrons are accepted by ferredoxin (Fd), which is also a Fe-S protein.
- P700+ pair in PS 1 accepts two electrons from reduced plastocyanin and becomes P700–
- By the catalytic activity of Fd-NADP oxidoreductase, electrons are transferred to NADP+, forming NADPH+ H+, thereby completing non-cyclic electron transport.
- A continuous supply of water is essential for this process. O2 is released by this process.
Experiments by different scientists to prove— ‘Water releases O2 during photosynthesis’
In 1931, Van Niel demonstrated that H2S is required for stabilising CO2 in photosynthetically active bacteria. In these bacteria, sulphur is formed instead of O2.
⇒ \(6 \mathrm{CO}_2+12 \mathrm{H}_2 \mathrm{~S} \longrightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+12 \mathrm{~S}+6 \mathrm{H}_2 \mathrm{O}\)
In 1937, Robert Hill observed that isolated chloroplasts can evolve oxygen in the absence of CO2. His finding was one of the first indications that the source of the electrons in the light reactions was in fact water. In his experiment, he used an artificial electron acceptor.
The artificial electron acceptor intercepts the electrons before they cascade down to P700 (the reaction centre of PS 1), but after they have gone down the electron transport chain. Thus, the Hill reaction is formally defined as the photo-reduction of an electron acceptor by the electrons of water, with the evolution of oxygen. Various dyes can be used as artificial electron acceptors (A). The general equation, known as the Hill Reaction can be written as follows—
Non-Cyclic Photophosphorylation
⇒ \(2 \mathrm{~A}+2 \mathrm{H}_2 \mathrm{O} \underset{\text { chlorophyll }}{\stackrel{\text { sunlight }}{\longrightarrow}} 2 \mathrm{AH}_2+\mathrm{O}_2\)
In vivo, or in the organism, the final electron acceptor is NADP+. However, during the experiment, a dye is used as an artificial electron acceptor. It changes colour as it is reduced. DCIP (2,6-dichlorophenolindophenol) is a dye which is blue in its oxidized form and colourless in its reduced form. It is called the Hill Reagent.
In 1941, Samuel Ruben and Martin Kamen used radioactive oxygen (O18)-containing water, to prove that O2 is released from H20 (H2O18)
⇒ \(12 \mathrm{H}_2 \mathrm{O}^{18}+6 \mathrm{CO}_2 \underset{\text { chlorophyll }}{\stackrel{\text { sunlight }}{\longrightarrow}} \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+6 \mathrm{O}_2^{18}+6 \mathrm{H}_2 \mathrm{O}\)
Later, in 1952, Arnon and Emerson proved that the hydrogen acceptor in photosynthesis is NADP+. So, NADP+ is the natural Hill reagent.
The growth of weeds can be reduced by blocking photophosphorylation
Herbicides such as chlorophenyl dimethyl urea (DCMU) and chlorophenyl dimethyl urea (CMU) can prevent electron transport during non-cyclic photophosphorylation. As a result, on the application of these chemicals, the light-independent phase is blocked, and no more glucose is synthesised by photosynthesis. This reduces the growth of the weeds.
Significance of photophosphorylation
Liberation of oxygen: Splitting of water releases oxygen, during photophosphorylation.
ATP production: Cyclic photophosphorylation produces 2 molecules of ATP, while non-cyclic photophosphorylation produces 1 molecule of ATP.
Reduction of NADP: Non-cyclic photophosphorylation reduces NADP+ to NADPH and H+.
Cyclic Electron Transport Chain
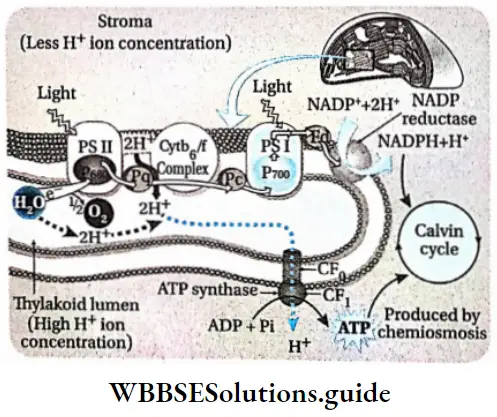
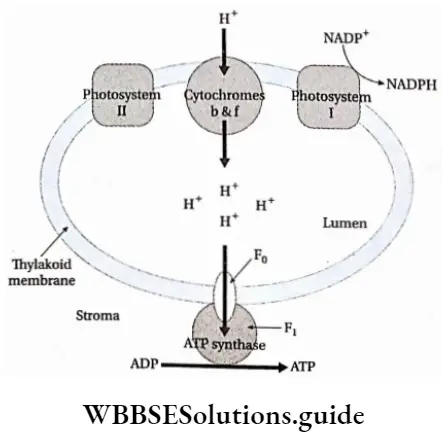
The chemiosmotic hypothesis of synthesis of ATP
The chemiosmotic mechanism of ATP formation was first proposed by Peter Mitchell (1966). He received the Nobel Prize in 1978 for proposing the above hypothesis. According to this theory, a proton concentration gradient is established across the thylakoid membrane due to photosynthetic electron transport.
The chemiosmotic mechanism of ATP formation is as follows—
- H+ ions, released through the splitting of water (photolysis), accumulate within the thylakoid lumen.
- Primary electron acceptors or carriers are present outside the thylakoid membrane.
- These primary electron carriers carry the electrons to the H-acceptor.
- At the same time, these primary electron carriers also carry H+ ions from the stroma into the thylakoid lumen.
- As the H+ ion from the carrier is released into the thylakoid lumen, the electron bound to the carrier is transferred to the next carrier.
- NADP reductase is present outside the thylakoid membrane. It is the last of the series of electron carriers that carries electrons from PS 1 to NADPH. It accepts the electron from the previous carrier and a proton from the stroma to reduce NADP+ into NADPH+H+.
- Due to this reaction, the concentration of H+ ions decreases in the stroma but increases in the thylakoid lumen. This causes the pH to decrease within the thylakoid lumen.
- A proton gradient is created due to the difference in the concentration of protons across the thylakoid membrane.
- The potential energy stored in the form of a proton gradient is electrical as well as chemical in nature.
- As the concentration of hydrogen ions in the lumen increases further, the ions move through the ATP synthase enzyme to the stroma. This movement of the proton generates a kind of energy called proton motive force, which is used to phosphorylate ADP to ATP.
- Towards the end of the electron transport chain, specific enzyme molecules synthesise ATP by combining ADP and Pi, using this proton motive force. These are known as CF0-CF1 particles (ATP synthase or ATPase).
- These CF0-CF-1 particles are structurally similar to the F0-F1 particles of mitochondria.
- CF0 part is rod-like and is strongly attached to the thylakoid membrane. The CF-L part is a spherical structure placed above the CF0 part.
- Since the H+ ions are transported through the CF0 part, it is known as the proton channel. The H+ ions are transported through this transmembrane channel by facilitated diffusion.
- Energy is released for every 3H+ ions transported through the CF0 to the stroma. This energy is utilised to cause a conformational change in the CF0 particle. This enables it to catalyse the synthesis of 1 molecule of ATP from ADP and Pi.
- Hence, it is observed that a membrane, proton pump, proton gradient and CF0-CF1 particles are required for the chemiosmotic mechanism.