Haloalkanes And Haloarenes
Haloalkanes:
An alkyl halide (haloalkane) or aryl halide (haloarene) is formed when one of the hydrogens in an alkane or benzene molecule is replaced by a halogen atom (fluorine, chlorine, bromine or iodine). For Example,
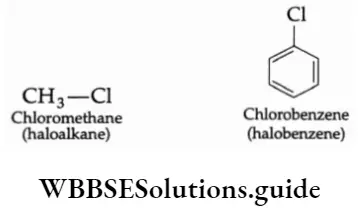
Chemists often use R-X as a general formula for alkyl halides; R stands for any alkyl group and X for any halogen. The aryl group in a halobenzene is denoted by the symbol Ar to distinguish it from the alkyl group R.
These classes of compounds find wide applications. For Example, chlorine-containing antibiotics, Chloramphenicol and Aureomycin are very effective in the treatment of bacterial infections. Chloroquine is used as a drug in the treatment of malaria.
Haloalkanes and haloarenes class 12 chemistry notes
Our body produces the iodine-containing hormone, thyroxine, the deficiency of which causes a disease called goitre. These are all aryl halides. A majority of alkyl halides are employed as insecticides and there exists virtually no soil microorganism which can degrade them into nontoxic metabolic products
Classification On The Basis Of The Number Of Halogen Atoms
Haloalkanes or haloarenes are classified as mono-, di- or tri-halogen compounds depending on whether they contain one, two or three halogen atoms. For Example,
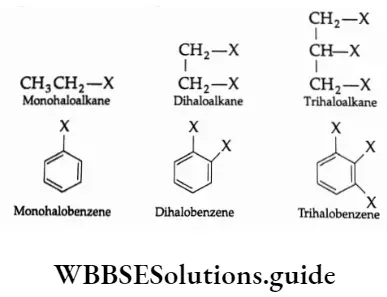
Monohalo compounds are further classified on the basis of the hybridisation of the carbon atom to which the halogen atom is attached.
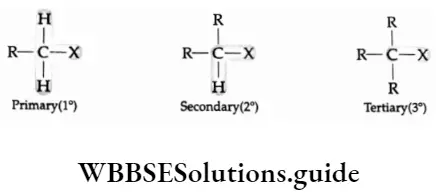
Compounds contalnting an sp3 C—X bond (X = F, Cl, Br, I)
1. Alkyl halides or haloalkanes (R—X) Alkyl halides other than methyl halides are classified as primary (1°), secondary (2°) or tertiary (3°), depending upon the number of carbons attached to the halogen-bearing carbon.
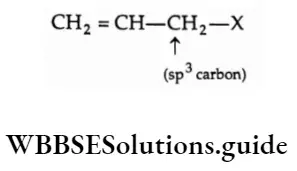
2. Allyl halides In an allyl halide, the halogen atom is attached to that sp -sp-hybridised carbon which is bonded to the carbon forming a carbon-carbon double bond.
3. Benzylic halide In a benzylic halide, the halogen atom is attached to that sp3 -hybridised carbon which is bonded to a benzene ring.
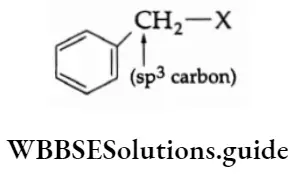
Compounds containing an sp2 C—X bond
1. Vinylic halides In a vinylic halide, the halogen atom is bonded to the sp2-hybridised carbon atom that forms a carbon-carbon double bond.
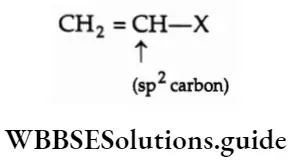
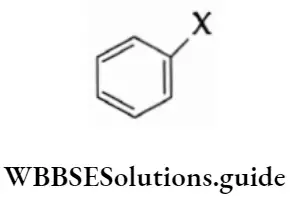
2. Aryl halides In an aryl halide, the halogen atom is attached to the sp2-hybridised carbon atom of an aromatic ring.
Dihalo compounds (dihalides) are classified as vicinal (or vie) dihalides and geminal (or gem) dihalides. Molecules with two halogen atoms connected to the same carbon are called geminal (or gem) dihalides. Molecules with two halogen atoms connected to neighbouring carbons, as in the case of 1, 2-dibromoethane, are called vicinal (or vie) dihalides.
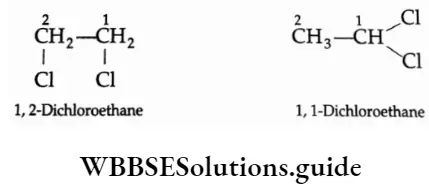
Nomenclature
The common names of alkyl halides are simply the alkyl derivatives of the corresponding hydrogen halides. he /UPAC names are halo derivatives of the corresponding hydrocarbons. In the common names, the prefixes n-, •c- (s-) and text- (/-) indicate normal, secondary and tertiary respectively.
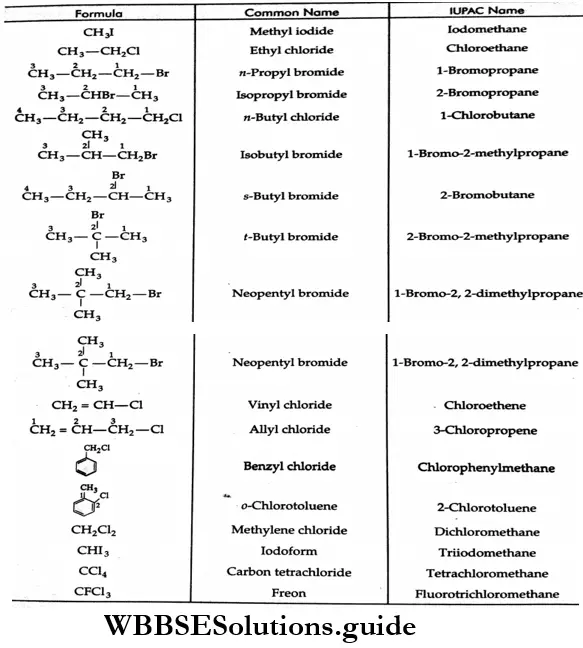
Example Write the IUPAC names of the following.
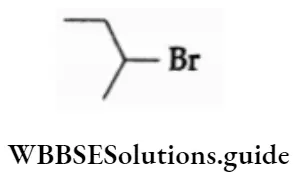
2-Bromobutane
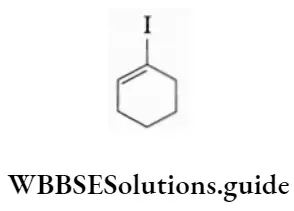
1-Iodocyclohexene
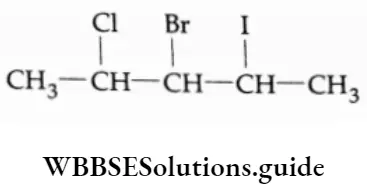
3-Bromo-2-chloro-4-iodopentane
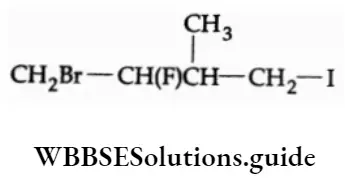
1-Bromo-2-fluoro-4-iodo-3-methyl butane
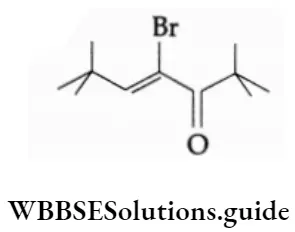
4-Bromo-2, 2, 6, 6-tetramethyl-4-heptene-3-one
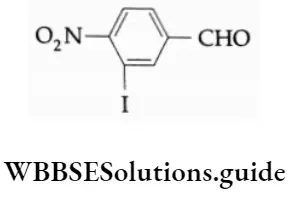
3-Iodo-4-nitrobenzaldehyde
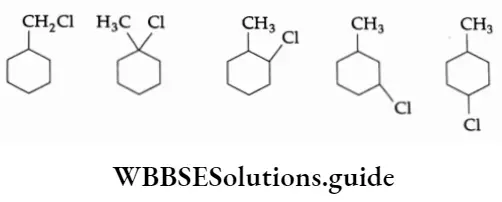
Example Write the structures of all the possible isomers of the monochloro derivative of methylcyclohexane (excluding stereo structures).
Solution
Methods Of Preparation From alkanes
Cyclic and acyclic hydrocarbons can be chlorinated or brominated in the presence of visible or ultraviolet light. For Example, methane on chlorination yields a mixture of methyl chloride, methylene chloride, chloroform and carbon tetrachloride.
⇒ \(\mathrm{CH}_4+\mathrm{Cl}_2 \stackrel{\text { hv }}{\longrightarrow} \underset{\text { Methyl chloride }}{\mathrm{CH}_3 \mathrm{Cl}}+\mathrm{HCl}\)
Haloalkanes and haloarenes chapter important questions and answers
⇒ \(\mathrm{CH}_3 \mathrm{Cl}+\mathrm{Cl}_2 \stackrel{\text { hv }}{\longrightarrow} \underset{\text { Methylene chloride }}{\mathrm{CH}_2 \mathrm{Cl}_2}+\mathrm{HCl}\)
⇒ \(\mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{Cl}_2 \longrightarrow \underset{\begin{array}{c}
\text { Trichloromethane } \\\text { (chloroform) }\end{array}}{\mathrm{CHCl}_3}+\mathrm{HCl}\)
⇒ \(\mathrm{CHCl}_3+\mathrm{Cl}_2 \longrightarrow \underset{\begin{array}{c}\text { Tetrachloromethane } \\\text { (carbon tetrachloride) }\end{array}}{\mathrm{CCl}_4}+\mathrm{HCl}\)
Haloalkanes And Haloarenes Class 12 Notes
Mechanism
⇒ \(\mathrm{Cl}: \mathrm{Cl} \stackrel{\text { heat }}{\longrightarrow} \mathrm{Cl}^{\circ}+\mathrm{Cl}^{\cdot}\)
Chlorine-free radical
⇒ \(\mathrm{Cl}^{\cdot}+\mathrm{CH}_4 \longrightarrow \mathrm{HCl}+\underset{\begin{array}{c} \text { Methyl free } \\ \text { radical } \end{array}}{\mathrm{CH}_3}\)
⇒ \(\cdot \mathrm{CH}_3+\mathrm{Cl}: \mathrm{Cl} \longrightarrow \underset{\text { Methyl chloride }}{\mathrm{CH}_3 \mathrm{Cl}}+{ }^{\circ} \mathrm{Cl}\)
⇒ \(\mathrm{CH}_3 \mathrm{Cl}+\cdot \mathrm{Cl} \longrightarrow \underset{{\text { Chloromethyl } \\ \text { free radical }}}{\cdot \mathrm{CH}_2 \mathrm{Cl}+\mathrm{HCl}}\)
⇒ \(\cdot \mathrm{CH}_2 \mathrm{Cl}+\mathrm{Cl}: \mathrm{Cl} \longrightarrow \underset{\begin{array}{c} \text { Methylene } \\ \text { chloride } \end{array}}{\mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{Cl}}\)
⇒ \(\mathrm{CH}_2 \mathrm{Cl}_2+\cdot \mathrm{Cl} \longrightarrow \underset{{\text { Dichloromethyl } \\ \text { free radical }}}{\cdot \mathrm{CHCl}_2}+\mathrm{HCl}\)
⇒ \(\cdot \mathrm{CHCl}_2+\mathrm{Cl}: \mathrm{Cl} \longrightarrow \underset{\text { Chloroform }}{\mathrm{CHCl}_3+{ }^{\circ} \mathrm{Cl}}\)
⇒ \(\mathrm{CHCl}_3+\mathrm{Cl} \longrightarrow \underset{{\text { Trichloromethyl } \\ \text { free radical }}}{\cdot \mathrm{CCl}_3}+\mathrm{HCl}\)
⇒ \(\cdot \mathrm{CCl}_3+\mathrm{Cl}: \mathrm{Cl} \longrightarrow \underset{{\text { Carbon } \\ \text { tetrachloride }}}{\mathrm{CCl}_4}+\cdot \mathrm{Cl}\)
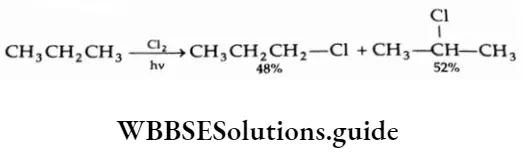
The ease of substitution at various carbon atoms is tertiary > secondary > primary, which is the same as the stability of the various alkyl radicals. For Example, the chlorination of propane yields a mixture of 1-chloropropane and 2-chloropropane.
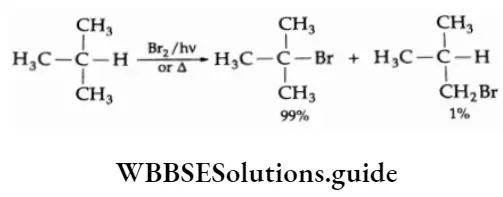
The bromination of an alkane is similar to chlorination except that the rate of the reaction is slow. The bromination of isobutane gives tert-butyl bromide as the major product.
Fluorohydrocarbons cannot be prepared by the direct fluorination of alkanes, because the reaction is explosive. Fluorine can, however, be introduced by the replacement of chlorine from an alkyl chloride using mercurous fluoride.
2CH3CI+ Hg2F2 →2CH3F+ Hg2Cl2
The iodination of an alkane is reversible and leads to the regeneration of the alkane.
Haloalkanes And Haloarenes Class 12 Notes
⇒ \(\mathrm{R}-\mathrm{H}+\mathrm{I}_2 \rightleftharpoons \mathrm{R}-\mathrm{I}+\mathrm{HI}\)
The reaction is, therefore, not synthetically useful and direct iodination is usually achieved by using a strong oxidising agent (HIO3 or HNO3 ) to destroy the HI formed in the process.
HIO3 + 5HI→3I2 + 3H2O
From Alkenes
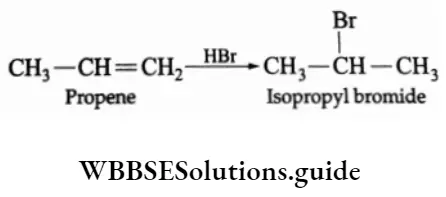
1. Addition of HX
The addition of hydrogen halides to alkenes gives a variety of alkyl halides. HF, HBr and HI can be added at room temperature, while HCI is added at a higher temperature.
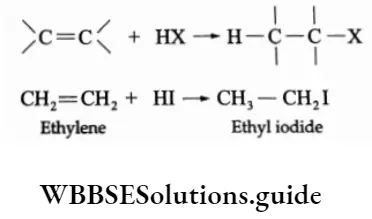
In the case of unsymmetrical olefins, the reaction proceeds under the Markovnikov rule.
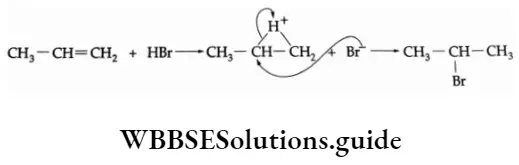
Mechanism The hydrogen halides add on to the olefins by an ionic mechanism.
In the presence of light or peroxide, the addition of hydrogen bromide follows a free-radical mechanism the product is formed according to the anti-Markovnikov rule.
Mechanism
⇒ \(\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2+\mathrm{HBr} \stackrel{\text { peroxide }}{\longrightarrow} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br}\)
⇒ \(\mathrm{HBr} \stackrel{\text { peroxide }}{\longrightarrow} \dot{\mathrm{H}}+\dot{\mathrm{Br}}\)
⇒ \(\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2+\dot{\mathrm{Br}} \longrightarrow \mathrm{CH}_3-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_2-\mathrm{Br}\)
⇒ \(\mathrm{CH}_3-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_2-\mathrm{Br}+\mathrm{HBr} \longrightarrow \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br}+\dot{\mathrm{Br}}\)
In contrast to the addition of HBr, the free radical addition of HI and HC1 to olefins normally does not take place.
⇒ \(\dot{\mathrm{X}}+\mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2 \longrightarrow \mathrm{XCH}_2 \dot{\mathrm{C}} \mathrm{H}_2 \quad\left[\begin{array}{ll}
\mathrm{X}=\mathrm{Cl} ; & \Delta H=-26 \mathrm{kcal} \mathrm{mol}^{-1} \\
\mathrm{X}=\mathrm{Br} ; & \Delta H=-5 \mathrm{kcal} \mathrm{mol}^{-1} \\
\mathrm{X}=\mathrm{I} ; & \Delta H=+7 \mathrm{kcal} \mathrm{mol}^{-1}
\end{array}\right]\)
⇒ \(\mathrm{XCH}_2 \dot{\mathrm{C}} \mathrm{H}_2+\mathrm{HX} \longrightarrow \mathrm{XCH}_2 \mathrm{CH}_3+\dot{\mathrm{X}}\left[\begin{array}{ll}
\mathrm{X}=\mathrm{Cl} ; & \Delta H=+5 \mathrm{kcal} \mathrm{mol}^{-1} \\
\mathrm{X}=\mathrm{Br} ; & \Delta H=-11 \mathrm{kcal} \mathrm{mol}^{-1} \\
\mathrm{X}=\mathrm{I} ; & \Delta H=-27 \mathrm{kcal} \mathrm{mol}^{-1}
\end{array}\right]\)
For the addition of HCl, the first step is exothermic and so the addition of the chlorine atom takes place readily but the second step is endothermic so this step is not possible. The initially formed radical is unable to decompose HCI.
For the addition of HBr, both the steps are exothermic and so the addition takes place readily. For the free radical addition of HI, the first step is endothermic. Therefore, no HI will add on to an olefin.
Haloalkanes And Haloarenes Class 12 Notes
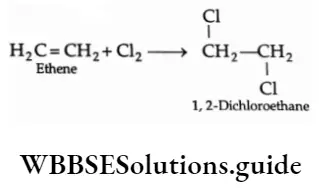
2. Addition of halogens
Dihalides are formed by the addition of halogens to alkenes.
From Alcohols
Alcohols are important starting materials for the preparation of alkyl halides. Various reagents such as PCI5, PBr3, PI3 and SOCl2 readily displace the alcoholic group to yield the corresponding halides.
⇒ \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH}+\mathrm{PCl}_5 \longrightarrow \underset{\text { Ethyl chloride }}{\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Cl}+\mathrm{POCl}_3+\mathrm{HCl}}\)
⇒ \(3 \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH}+\mathrm{PI}_3 \longrightarrow \underset{\text { Ethyl iodide }}{3 \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{I}+\mathrm{H}_3 \mathrm{PO}_3}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{SOCl}_2 \longrightarrow \underset{\text { Ethyl chloride }}{\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}}+\mathrm{SO}_2+\mathrm{HCl}\)
The three hydrohalogen acids HI, HBr and HC1 also react with alcohols but at different rates i.e., HI > HBr > HCI
⇒ \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH}+\mathrm{HI} \stackrel{\Delta}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_2-\mathrm{I}+\mathrm{H}_2 \mathrm{O}\)
With primary alcohols, hydroiodic acid reacts most readily while hydrochloric acid requires zinc chloride as a catalyst and hydrobromic acid displays an intermediate reactivity
⇒ \(\underset{\text { Ethyl alcohol }}{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}+\mathrm{HCl} \stackrel{\text { anhydrous } \mathrm{ZnCl}_2}{\longrightarrow} \underset{\text { Ethyl chloride }}{\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}}+\mathrm{H}_2 \mathrm{O}\)
Mechanism
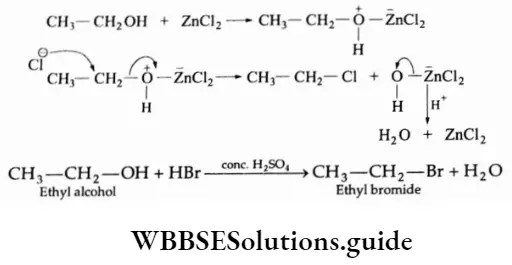
Furthermore, a tertiary alcohol reacts more rapidly than a secondary alcohol, which in turn reacts faster than a primary alcohol.
The Halogen-Exchange Method
This method is used to prepare alkyl iodides which are otherwise not easily obtained. It involves treating an alkyl chloride or bromide with a solution of sodium iodide.
⇒ \(\underset{(\mathrm{X}=\mathrm{Cl}, \mathrm{Br})}{\mathrm{R})}+\mathrm{X}+\mathrm{NaI} \stackrel{\text { acetone }}{\longrightarrow} \underset{\text { Alkyl iodide }}{\mathrm{R} \mathrm{I}}+\mathrm{NaX}\)
This reaction is called the Finkelstein reaction.
An alkyl fluoride is best prepared by heating an alkyl chloride or alkyl bromide in the presence of mercurous fluoride.
2CH3 —C1+ Hg2F2→2CH3—F+Hg2Cl2
This reaction is known as Swart’s reaction.
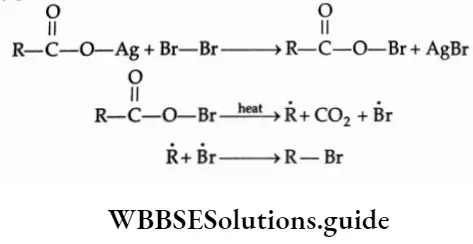
By The Hunsdiecker Reaction
Alkyl halides and aryl halides can be prepared by decomposing the silver salt of carboxylic acid using chlorine or bromine in the presence of CC14.
⇒ \(\underset{\text { iilver carboxylate }}{\mathrm{RCOOAg}}+\mathrm{Br}_2 \underset{\Delta}{\stackrel{\mathrm{CCl}_4}{\longrightarrow}} \mathrm{R}-\mathrm{Br}+\mathrm{CO}_2+\mathrm{AgBr}\)
The reaction probably proceeds through a free-radical mechanism as shown below.
Physical Properties
Melting and boiling points
The melting and boiling points of alkyl halides are higher than those of alkanes with the same number of carbon atoms. This is because of their greater dipole-dipole and van der Waals attractions.
In alkyl halides having the same alkyl groups, the boiling and melting points increase with the increase in the atomic weight of the halogen. Thus, the boiling point of methyl fluoride is the lowest while that of methyl iodide is the highest.
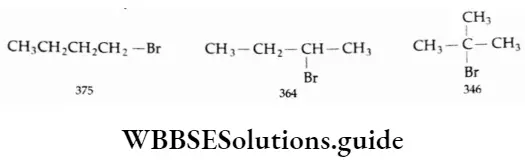
In the case of isomeric alkyl halides, the boiling point depends upon surface area. The boiling point of the primary halide is the highest and that of the tertiary halide is the lowest.
Specific gravity
Most halides are liquids. The bromides, iodides and polyhalides, in general, have a specific gravity greater than and are, therefore, heavier than water.
Solubility
Alkyl halides are insoluble in water because of their inability to form hydrogen bonds with water.
Haloalkanes And Haloarenes Class 12 Notes
Chemical Reactions
Alkyl halides mainly undergo the following two types of chemical reactions.
- Nucleophilic substitution reactions
- Elimination reactions
In addition, alkyl halides undergo some other characteristic reactions which we will discuss subsequently.
Nucleophilic Substitution Reactions
All the halogens are significantly more electronegative than carbon. As a result, a carbon-halogen bond is polar. The majority of reactions that alkyl halides undergo involve heterolytic cleavage of the carbon-halogen bond, with the halogen departing as X-.

In such a case, the halogen is referred to as a departing nucleophile. The order of reactivity of alkyl halides is
R—I>R—Br>R—Cl>>R—F
The strengths of the C—X bonds and dipole moments have been measured and are listed.
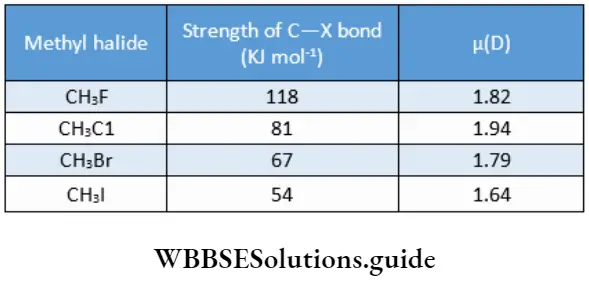
It is clearly the easiest to break a C—I bond and the most difficult to break a C—F bond. Iodine is the leaving group and fluorine is a very bad one with the other halogens in between.
In this chapter, we will confine our attention to reactions in which the halogen is attached to a saturated (i.e., sp3 -hybridised) carbon.
The main nucleophilic substitution reactions that alkyl halides (in particular ethyl bromide) undergo are as follows.
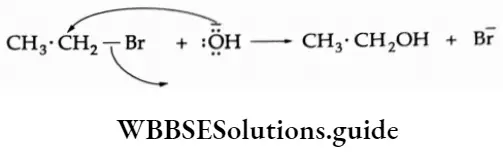
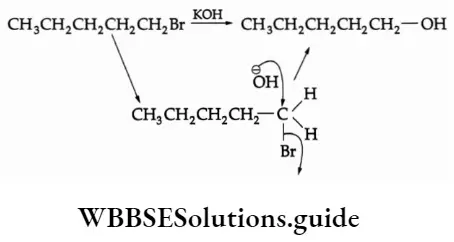
1. Hydrolysis The reaction of ethyl bromide with aqueous NaOH gives ethyl alcohol.
C2H5Br + NaOH -> C2H5OH + NaBr
Mechanism
Solvolysis If the attacking nucleophile is the solvent, the reaction is called a solvolysis reaction.
2. Reaction with potassium hydrosulphide Ethyl bromide on treatment with aqueous alcoholic KSH yields alcohol.
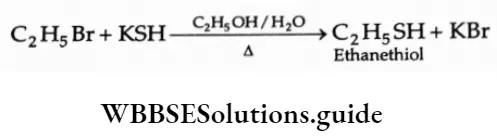
3. Reaction with sodium alkoxide On treatment with sodium ethoxide, ethyl bromide gives diethyl ether.
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{C}_2 \mathrm{H}_5 \text { ŌNa } \rightarrow \mathrm{C}_2 \mathrm{H}_5-\mathrm{O}-\mathrm{C}_2 \mathrm{H}_5+\mathrm{NaBr}Diethyl ether\)
4. Reaction with ammonia On being heated with concentrated ammonia in a sealed tube, ethyl bromide gives a mixture of amines.
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{Br}+\mathrm{NH}_3 \rightarrow \underset{\text { Ethylamine }}{\mathrm{C}_2 \mathrm{H}_5-\mathrm{NH}_2}+\mathrm{HBr}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{Br}+\mathrm{C}_2 \mathrm{H}_5-\mathrm{NH}_2 \rightarrow \underset{\text { Diethylamine }}{\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{NH}}+\mathrm{HBr}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{NH} \rightarrow \underset{\text { Triethylamine }}{\left(\mathrm{C}_2 \mathrm{H}_5\right)_3 \mathrm{~N}}+\mathrm{HBr}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{Br}+\left(\mathrm{C}_2 \mathrm{H}_5\right)_3 \mathrm{~N} \rightarrow \underset{{\text { Tetraethylammonium } \\ \text { bromide }}}{\left(\mathrm{C}_2 \mathrm{H}_5\right)_4 \mathrm{~N}^{+} \mathrm{Br}^{-}}\)
Ammonia is a nucleophilic reagent.
Haloalkanes And Haloarenes Class 12 Notes
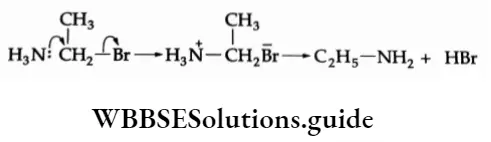
Similarly ethylamine, diethylamine and triethylamine act as nucleophilic reagents.
5. Reaction with silver acetate On being heated with the silver salt of carboxylic acid, ethyl bromide yields an ester.
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{Br}+\underset{\text { Silver acetate }}{\mathrm{CH}_3-\mathrm{CO} A \stackrel{+}{\rightarrow}} \rightarrow \underset{\text { Ethyl acetate }}{\mathrm{C}_2 \mathrm{H}_5-\mathrm{O}-\mathrm{CO}}-\mathrm{CH}_3+\mathrm{AgBr}\)
Important reactions of haloalkanes and haloarenes
In this reaction, the acetate anion is the nucleophilic reagent.
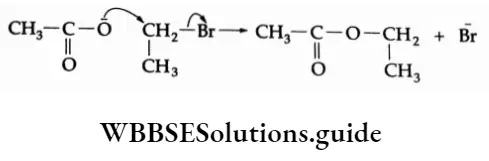
6. Substitution by the cyanide (nitrile) anion On being heated with an alcoholic solution of KCN, ethyl bromide gives ethyl cyanide (ethyl nitrile).
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{AgCN} \longrightarrow \underset{\text { Ethyl isocyanide }}{\mathrm{C}_2 \mathrm{H}_5-\stackrel{+}{\mathrm{N}} \equiv \mathrm{C}+\mathrm{AgBr}}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{Br}+\mathrm{KCN} \longrightarrow \underset{\text { Ethyl cyanide }}{\mathrm{C}_2 \mathrm{H}_5-\mathrm{CN}+\mathrm{KBr}}\)
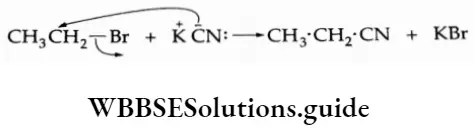
KCN is ionic and provides predominantly cyanide ions in solution. The carbon and nitrogen atoms of the CN: ion are in a position to donate electron pairs. However, the attack takes place mainly through carbon and not through nitrogen because the resulting C—C bond in alkyl cyanide (C—CN) is stronger than the C N bond.
Upon reacting with silver cyanide (AgCN), ethyl bromide gives ethyl isocyanide.
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{AgCN} \longrightarrow \underset{\text { Ethyl isocyanide }}{\mathrm{C}_2 \mathrm{H}_5-\stackrel{+}{\mathrm{N}} \equiv \mathrm{C}+\mathrm{AgBr}}\)
Silver cyanide, AgCN, is essentially covalent. Therefore, the lone pair on the nitrogen is mainly available for covalent bond formation, resulting predominantly in the formation of isocyanides.
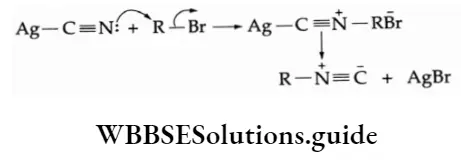
7. Reaction with silver nitrite On being heated with silver nitrite, an alcoholic solution of alkyl halide gives a mixture of nitroalkane and alkyl nitrite. These are separated by fractional distillation.
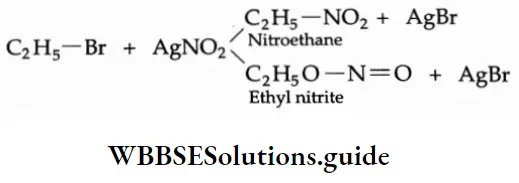
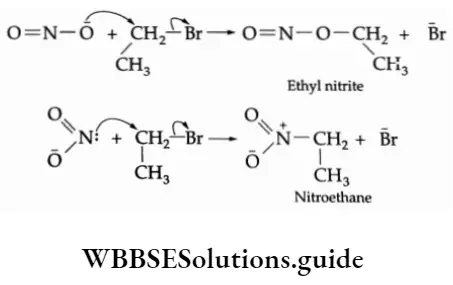
Mechanism The nitrite anion \((\mathrm{O}=\ddot{\mathrm{N}}-\overline{\mathrm{O}})\) is an ambident nucleophile with two different points of linkage. The linkage through oxygen results in the formation of alkyl nitrites while that through the nitrogen atom leads to the formation of nitroalkanes.
8. Reaction with sodium acetylide On treatment with sodium acetylide, ethyl bromide yields a higher alkyne.
For Example,
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{Na}-\mathrm{C} \equiv \mathrm{CH} \rightarrow \mathrm{C}_2 \mathrm{H}_5-\mathrm{C} \equiv \mathrm{CH}+\mathrm{NaBr}1-Butyne\)
Sodamide (NaNH2 ) reacts with acetylene to give sodium acetylide.
⇒ \(\mathrm{H}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}+\stackrel{+}{\mathrm{N}} \mathrm{a}-\mathrm{NH}_2 \rightarrow \mathrm{H}-\mathrm{C} \equiv \overline{\mathrm{C}}-\stackrel{+}{\mathrm{N}} \mathrm{a}+\mathrm{NH}_3\)
Mechanisms of nucleophilic substitution reactions
A nucleophilic substitution reaction may be described as one in which a substituent is replaced by a nucleophile
The following general equation represents nucleophilic substitution
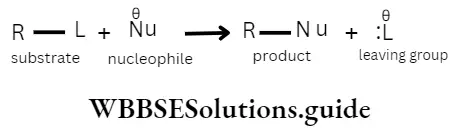
The above type of traction can take place in the following two ways.
Possibility 1 The leaving group is ejected first and then the nucleophile comes in.
Possibility 2 The leaving group is ejected at the same time as the nucleophile is attached.
Let us now examine each of the possibilities separately.
According to possibility 1, the leaving group goes out with the bonded pair of electrons, leaving a carbonium ion in the first step. In the second or final step the nucleophile, with its lone pair of electrons, interacts with the carbonium ion to form the product.
1. First step:
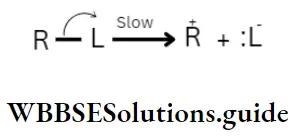
2. Second step:
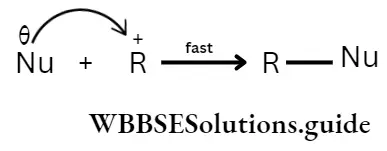
The rate of the overall reaction in a multistep process is dependent on the slow step. In the example cited above, the slow step (rate-determining step) is the first step
- Requiring more energy than the second step.
- Which creates a charged species out of a neutral molecule.
Rate of overall reaction α rate of slow step reaction (1)
α[substrate]
The rate of the reaction is dependent on the concentration of the substrate molecule only and is independent of the concentration of the nucleophile because the nucleophile becomes involved only after the rate-determining step. Thus the reaction is unimolecular and is termed SN1—S stands for substitution, N for nucleophilic and 1 indicates that the reaction is unimolecular.
Example s of SN1 reactions Tertiary halides usually undergo SN1 displacements. For Example, f-butyl bromide is hydrolysed to f-butyl alcohol through an unimolecular process.
⇒ \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}-\mathrm{Br} \stackrel{\mathrm{O}}{\longrightarrow}\left(\mathrm{CH}_3\right)_3 \mathrm{C}-\mathrm{OH}+\stackrel{\ominus}{\mathrm{Br}}\)
The rate of the reaction depends only on the concentration of the alkyl halide and is independent of the concentration of the added nucleophile. The reaction occurs according to the following rate equation:
Rate=K1[(CH3)3C—Br]
The mechanism of the reaction involves two steps. In the first step, the halide ionises to form an intermediate carbonium ion, which then readily combines with the nucleophile to form the product.
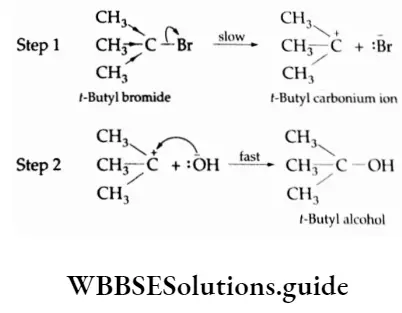
It might seem that the addition of a nucleophile, i.e., an \(\mathrm{O} \stackrel{\ominus}{\mathrm{H}}\) ion, would affect the rate of the reaction in Step 2. But this step is so much faster than Step 1 that the effective rate measured is that of Step 1, i.e., the ionisation step.
Tertiary alkyl halides undergo SN1 reactions very fast because of the high stability of tertiary carbonium ions. The order of reactivity of alkyl halides in the case of SN1 reactions is tertiary halide > secondary halide > primary halide.
Allylic and benzylic halides undergo SN1 reactions in which the intermediate carbonium ion is stabilised by resonance.
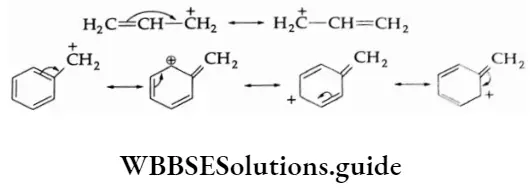
There are some nucleophilic substitution reactions in which the substrate (R L) as well as the nucleophile \(: \stackrel{\ominus}{N u}\) take part in the rate-determining step. In such a case, the nucleophile attacks the carbon atoms from the side opposite to that of the leaving group and forms a pentavalent transition state which finally collapses to give the product (R—Nu) and the leaving group \(\text { (: } \stackrel{\ominus}{\mathrm{L}})\)
⇒ \(: \stackrel{\ominus}{N u}+R-L \frac{\text { slow }}{\text { (a) }}[\mathrm{Nu}–\mathrm{R}–\mathrm{L}] \underset{\text { (b) }}{\stackrel{\text { fast }}{\rightarrow}} \mathrm{Nu}-\mathrm{R}-: \stackrel{\ominus}{\mathrm{L}}\)
Definition and classification of haloalkanes with examples
The rate of the reaction depends upon the concentration of the substrate (R—L) as well as that of the nucleophile \(\text { (: } \stackrel{\ominus}{\mathrm{Nu}})\) both the reactants being involved in the rate-determining step (a).
Rate of reaction α rate of slow step (a)
⇒ \(\propto[R-L][\stackrel{\ominus}{N u}]\)
Since the rate of the reaction is dependent on the concentration of the substrate and that of the nucleophile, the reaction is bimolecular and is termed an SN2 reaction, 2 indicating that the reaction is bimolecular.
Example s of SN2 reactions Primary halides usually undergo SN2 reactions. For Example, methyl bromide is hydrolysed to methyl alcohol upon reacting with an aqueous solution of sodium hydroxide. The hydroxide ion displaces the bromide ion.
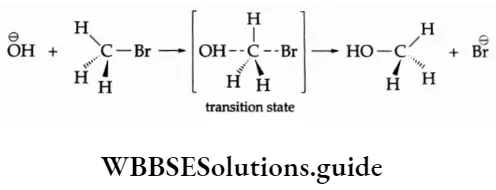
[The solid wedge represents the bond above the plane of the paper, the dashed line below the plane of the paper and a straight line represents a bond on the plane of the paper.]
The hydroxide ion attacks the carbon atom bearing the leaving group from behind and the bromide ion
departs from the opposite side. In the transition state, the \(\mathrm{O} \stackrel{\ominus}{\mathrm{H}}\) ion and the \(\stackrel{\ominus}{\mathrm{Br}}\) ion is only partially bonded to the central carbon atom. Finally, a new covalent bond is formed between the hydroxide ion and the carbon atom with the loss of the bromide ion.
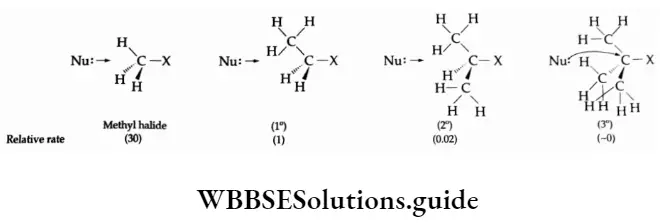
Methyl halides undergo SN2 reactions most rapidly because there are only three small hydrogen atoms Tertiary halides are the least reactive because bulky alkyl groups slow down the approaching nucleophiles. The order of reactivity of alkyl halides in the case of SN2 reactions is primary halide > secondary halide > tertiary halide.
Stereochemical aspect of nucleophilic substitution reactions
Before discussing the stereochemical aspect of nucleophilic substitution reactions in detail, it is useful to discuss some terms used in optical isomerism such as optical activity, chirality, retention of configuration, inversion of configuration and racemisation.
Plane polarised light and optical activity An ordinary beam of light vibrates in all directions. A plane of polarised light is one which vibrates entirely in one direction. An ordinary beam of light can be converted to a plane polarised light by passing it through a specially constructed prism, called Nicol prism. Certain compounds may rotate the plane polarised light either to the left (anticlockwise) or to the right (clockwise).
Compounds which rotate plane polarised light to the left are said to be laevorotatory or of the 1-form. The degree of rotation of light in such cases is prefixed by a – (minus) sign. If the light is rotated to the right, the compound is said to be dextrorotatory or of the d-form. The degree of rotation of light in such cases is prefixed by a + (plus) sign.
The el¬ and /- isomers of a compound are known as optical isomers and the phenomenon is termed optical isomerism. The optical activity of a compound is identified and estimated by an instrument known as a polarimeter.
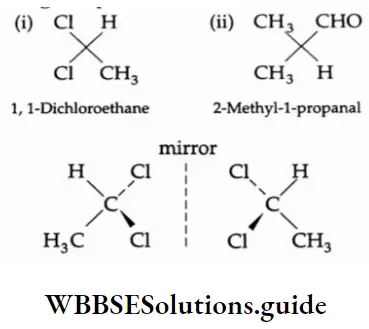
Enantiomers, diastereomers and chirality Enantiomers are structures that are not identical but are mirror images of each other.
Stereoisomers that are not mirror images of one another are called diastereoisomers. The term diastereoisomer is sometimes shortened to diastereomer.
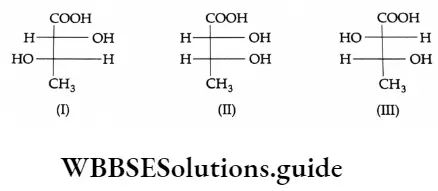
1 and 3 form a pair of enantiomers. I and 2, and II and IE, form a pair of diastereomers. Structures are said to be chiral if they cannot be superimposed on their mirror images. A hand cannot be superimposed on its mirror image.
If you hold your left hand up to a mirror, the image looks like a right hand. When we try to superimpose our hands on each other, we simply cannot do so. A hand and a foot are chiral objects whereas a ball and a glass are achiral objects. Achiral structures are superimposable in their mirror images.

A carbon atom carrying four different groups is a chiral carbon. Such a carbon is sometimes also referred to as an asymmetric carbon atom. For Example,
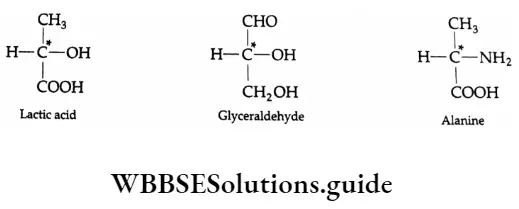
The asymmetric carbon atoms are indicated by a star (*).
Examples of achiral carbon-containing compounds:
Inversion, retention and racemisation To understand these terms consider the following general reaction.
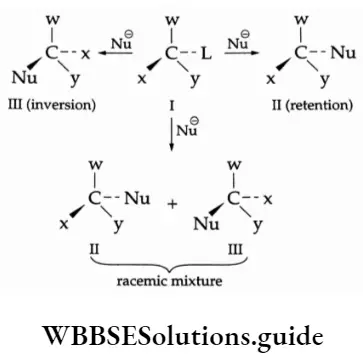
If (2) is obtained the process is called retention of configuration because (1) and (2) have a similar configuration. If (3) is obtained the process is known as inversion of configuration because the configuration of 3 is inverted with respect to (1).
If a 50: 50 mixture of (2) and (3) is obtained then the process is called racemisation. The resulting racemic product is optically inactive as one isomer will rotate plane polarised light in the direction opposite to that in which the other isomer will.
Now let us consider the stereochemical aspect of SN1 and SN2 reactions taking the Example of optically active alkyl halides.
In the case of optically active alkyl halides, an SN1 reaction involves racemisation, i.e., an equimolecular mixture of the d- and l- isomers is produced. This is possible because the carbonium ion formed as an intermediate in
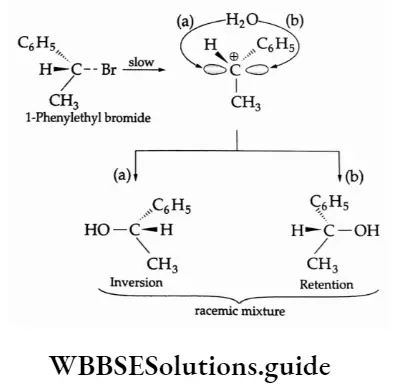
The rate-determining step has an equal chance of being attacked from both sides, giving one isomer having the same configuration and the other having the opposite configuration. This process may be illustrated by citing the Example of the hydrolysis of I-phenyl ethyl bromide.
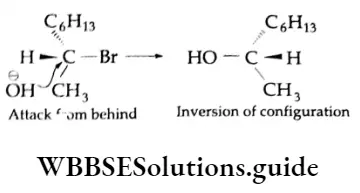
However, the SN2 reaction of an optically active compound proceeds with a complete inversion of configuration. This is because the nucleophile will be linked to the opposite side with respect to the leaving group (halide ion) in an optically active alkyl halide.
This process may be illustrated by citing the Example of the hydrolysis of 2-bromooctane. The hydrolysis of the optically active 2-bromooctane with sodium hydroxide gives the optically active 2-octanol with an inverted configuration.
Such reactions are generally accompanied by a change in optical rotation and its direction as well. It is termed as Walden inversion or flipping. The reaction may be likened to an umbrella flipping in a high wind, the attacking nucleophile being analogous to a high wind.
SN2 vs SN1
The two mechanisms termed SN1 and SN2 are the extremes.
The solvent, substrate structure, nucleophile and, to a lesser extent, the leaving group and reaction temperature, nil contribute to determining the mechanism. We shall briefly examine the effect of some of these parameters.
Solvent A reaction is favoured by an ionising solvent whose dielectric constant is high. Ionising solvents accelerate the rate of an SN1 reaction while that of an SN2 reaction is relatively solvent-independent. Substrate structure In SN2 reactions the order of reactivity of various alkyl halides follows the sequence
CH3 —X > primary > secondary > tertiary
In contrast to SN2 reactions, the sequence of reactivity of alkyl halides follows the following order in SN1 reactions.
Tertiary > secondary > primary > CH3 —X.
Nucleophile While the nature of the nucleophile does not alter the rate of an SN1 reaction, that of an SN2 reaction is altered by the nucleophile. The high concentration of a good nucleophile will favour an SN2 reaction.
Elimination Reactions
A reaction involving the loss of two atoms or groups from a molecule, without there being any substitution b) other atoms or groups, is known as an elimination reaction. This is the reverse of an addition reaction.
Most commonly a proton is lost from one carbon whereas a nucleophile is lost from the adjacent carbon; these tw< carbon atoms are usually referred to as |i- and a-carbons respectively.
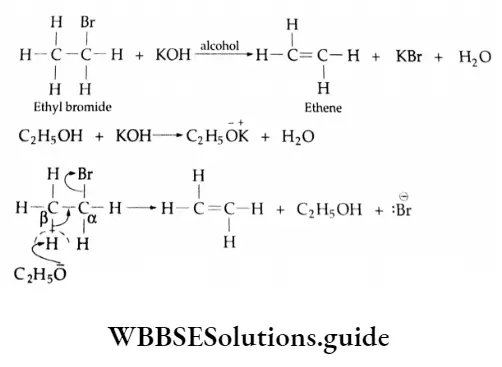
The reaction is known as a reaction or 1, 2-elimination reaction.
Orientation in an elimination reaction
Sometimes elimination reactions lead to mixtures of alkene products; usually, one alkene is formed as a major product and the other as a minor product. There are two empirical rules governing orientation in those reactions.
1. Saytzev rule (Zaitsev rule) states that an alkyl halide capable of forming a double bond in either direction of the chain preferably yields that alkene in which there are a greater number of alkyl groups attached to the double bond. That is, the more substituted olefin is formed as the major product. For Example, 2-bromobutane gives 2-butene as the major product.
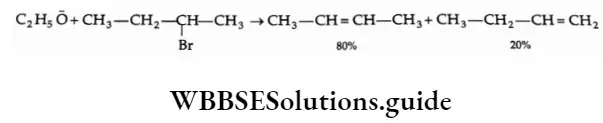
The stability of alkenes follows the order
R2C=CR2>R2C=CHR>RCH=CHR>RCH=CH2>CH2=CH2
The greater stability of the more-substituted alkene is explained on the basis of hyperconjugation.
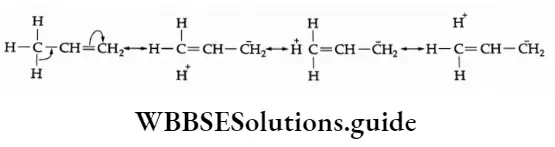
The number of resonating structures increases in more-substituted alkenes and consequently increases stability.
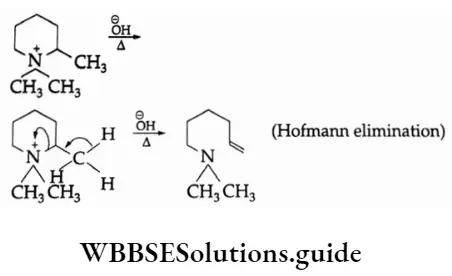
2. The Hofmann rule states that quaternary ammonium salts yield predominantly the least-substituted alkene.
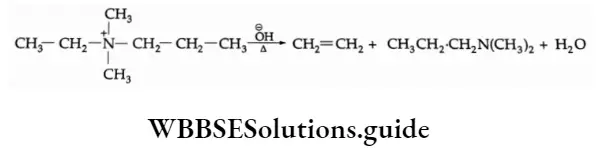
In this case, the hydroxide ion abstracts a proton from the β-carbon, resulting in the formation of a double bond and expulsion of a tertiary amine.
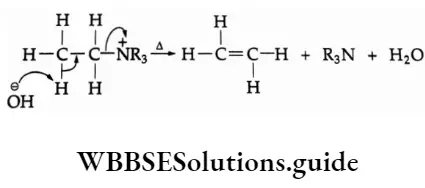
If a quaternary ammonium salt has ethyl as well as propyl groups attached to the positively charged nitrogen the propyl group shows relatively little tendency to lose a β-hydrogen.

Let us now see how the nature of the nucleophile determines whether a reaction will be a substitution read or an elimination reaction.
Basicity
The more basic the nucleophile the more likely is that elimination will replace substitution as the main reaction of an alkyl halide.
For Example,
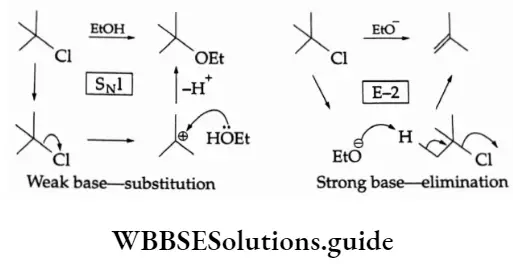
Size
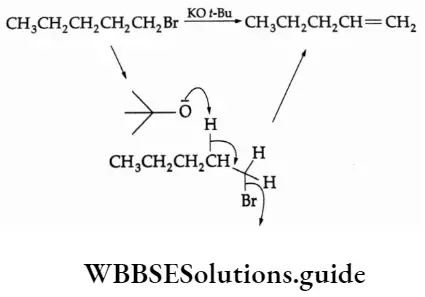
One of the best bulkier bases for promoting elimination and avoiding substitution is potassium f-butoxide. The large alkyl substituent makes it hard for the negatively charged oxygen to attack carbon in a substitution reaction but it has no problem attacking hydrogen.
Small nucleophile—substitution
Large nucleophile—elimination
Example Among the following, which alkyl halide would you expect to react more rapidly by the SN2 mechanist Explain.
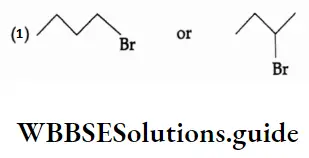



Example Which of the following SN1 reactions would you expect to take place more rapidly? Explain.
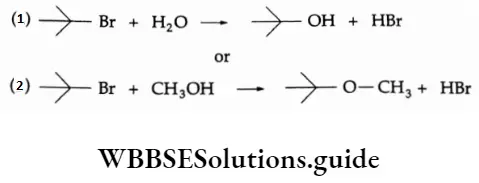
Solution (1), is because water is more polar than CH3OH.
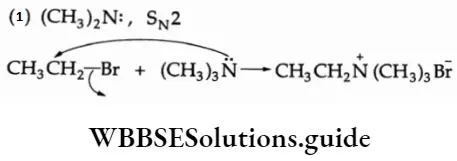
Example Suggest a reagent (or reagents), and state the type of reaction and mechanism involved for each of the following transformations.
1. CH3CH2— Br -> CH3CH2N(CH3)3
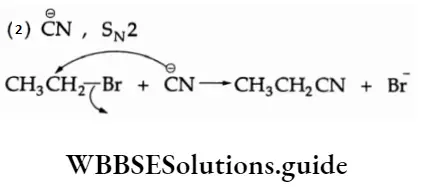
2. CH3CH2Br -> CH3CH2CN
Example Write the structure of the expected product. Indicate the type of reaction and the mechanism involved.
Solution
Temperature
Temperature has an important role to play in deciding whether a reaction is an elimination or a substitution reaction. Elimination is favoured at high temperatures.
Reaction With Metals
1. Alkyl halides react with metallic sodium in dry ether to form alkanes containing an even number of carbon atoms.
⇒ \(2 \mathrm{C}_2 \mathrm{H}_5-\mathrm{I}+2 \mathrm{Na} \longrightarrow \underset{\text { Butane }}{\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_3+2 \mathrm{NaI}}\)
This reaction is known as the Wurtz reaction.
Mechanism The reaction is believed to proceed through a nucleophilic attack by the alkyl ion on the alkyl halide.
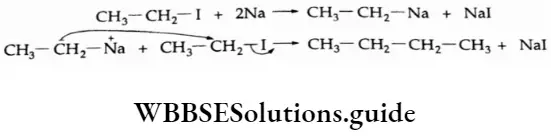
2. Alkyl halides react with metallic zinc in dry ether to form an alkane containing an even number of carbon atoms.
Nomenclature of haloalkanes – IUPAC rules and common names
⇒ \(2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{I}+\mathrm{Zn} \longrightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_3+\mathrm{ZnI}_2\)
This is called the Frankland reaction.
Mechanism
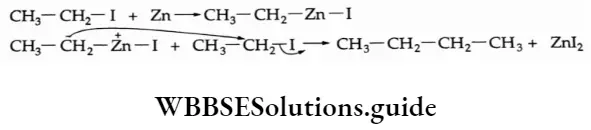
3. When alkyl or aryl halides are refluxed with magnesium in dry ether, alkyl or aryl magnesium halides (R—Mg—X) are formed. They are known as Grignard reagents. They are organometallic compounds. (The metal magnesium is attached to the carbon.)
⇒ \(\mathrm{R}-\mathrm{X}+\mathrm{Mg} \stackrel{\text { dry ether }}{\longrightarrow} \underset{(\mathrm{R}=\text { alkyl, aryl) }}{\mathrm{R}-\mathrm{Mg}-\mathrm{X}}\)
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{Mg} \underset{\text { ether }}{\stackrel{\text { dry }}{\longrightarrow}} \mathrm{C}_2 \mathrm{H}_5 \mathrm{MgBr}\)
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{Br}+\mathrm{Mg} \underset{\text { ether }}{\stackrel{\text { dry }}{\longrightarrow}} \mathrm{C}_6 \mathrm{H}_5 \mathrm{MgBr}\)
Grignard reagents form loose covalent bonds with the solvent ether molecules and form solutions in ether.
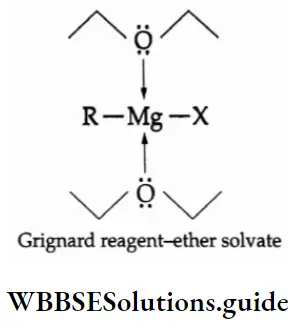
Since the electronegativities of magnesium and carbon are very different, the Grignard reagents may 1 regarded as polar compounds which are sources of nucleophilic carbanions R.
⇒ \(\stackrel{\delta-}{\mathrm{R}}-\stackrel{\delta_{+}^{+}}{\mathrm{Mg}}-\mathrm{X} \longrightarrow \stackrel{\ominus}{\mathrm{R}}+\stackrel{+}{\mathrm{M} g X}\)
Example Is (CH3)3 CO K+ an organometallic compound? If not, why?
Solution
No, the metal is bonded to an oxygen, not a carbon. This is an example of an alkoxide salt.
Haloarenes
Aryl halides (haloarenes) are those compounds in which the halogen atom (F, Cl, Br, I) is attached directly benzene ring. Unlike alkyl halides, these compounds are characterised by their relative inertness towards r common nucleophiles.
Nomenclature
Simple aryl halides are named by prefixing the halo derivative to the word benzene, for example, iodobenzene. The three disubstituted isomers are differentiated by the use of ortho-, meta- and para-.
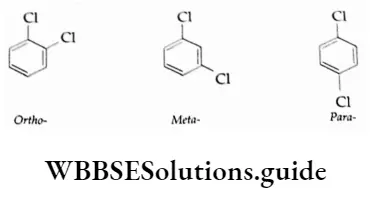
Numbering, however, is required when more than two halo atoms or other substituents are attached to the ring.
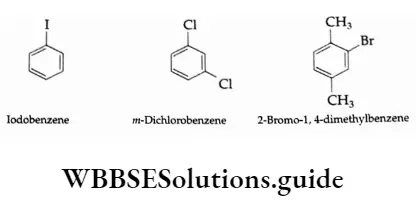
Iodobenzene m-Dichlorobenzene 2-Bromol, 4-dimethylbenzene
Methods Of Preparation
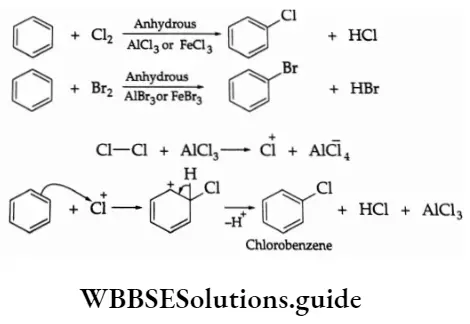
Halogenation
The chlorination or bromination of benzene is carried out at an ordinary temperature in the presence of iron or a Lewis acid (AICI3 or FeCI3 ).
From diazonium salts
This is the most general method used to prepare all types of aryl halides. An aromatic amine is first diazotised. The diazonium salt can then be reacted with a metal halide to obtain the aryl halide.
Aryl chlorides and bromides are conveniently prepared by the treatment of a freshly prepared solution of diazonium salt with cuprous chloride or cuprous bromide. This is known as the Sandmeyer reaction.
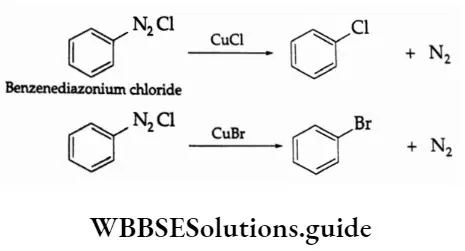
Aryl iodides are, however, obtained by treating the diazonium salt with aqueous potassium iodide.
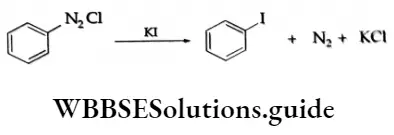
For the preparation of aryl fluorides, the solution of benediazonium chloride is treated with fluoroboric acid, HBF4. The solid benzene diazonium fluoroborate, ArN2+BF4–, which precipitates out, is filtered, washed and dried. When heated, the fluoroborate decomposes to give the aryl fluoride.
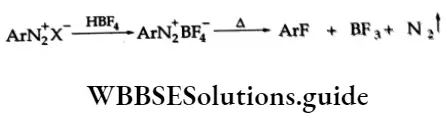
Hunsdlockor reaction
Aryl bromides are obtained by heating the silver salts of aromatic acids with bromine.
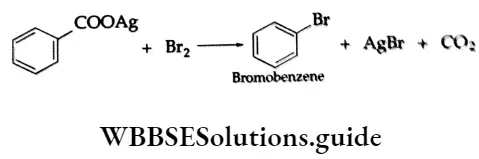
Physical Properties
Aryl halides are polar. They are insoluble in water. For aryl halides having the same aryl group but different halogen atoms, the melting and boiling points increase with the increase in molecular weight.
For Example, the melting point of iodobenzene is the highest and that of fluorobenzene is the lowest, p-isomers, being symmetrical, have high melting points. They are better packed in the crystal lattice.
Chemical Reactions
Nucleophilic substitution
Aryl halides do not generally undergo nucleophilic substitution reactions due to the following reasons.
1. Resonance effect In an aryl halide, the electron pair on the halogen atom is in conjugation with the benzene ring.
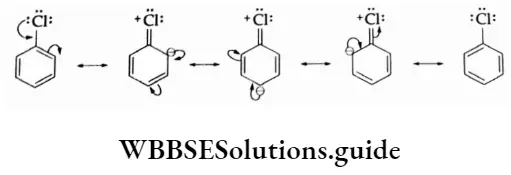
This gives the C—Cl bond a double bond character and thus the bond length decreases. The shorter the bond length, the greater the bond strength and the lower the reactivity. Thus, under ordinary conditions, a nucleophilic attack does not take place.
2. In an alkyl halide, the carbon attached to the halogen is sp3 -sp3-hybridised. However, in an aryl halide, the carbon attached to the halogen is sp2 -sp2 -hybridised. The sp2 -sp2 -hybridised carbon with greater s-character is electronegative. Thus, the breaking of the C—X bond becomes difficult.
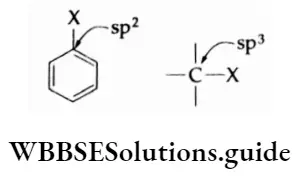
However, nucleophilic substitution reactions of aryl halides are possible in extreme conditions
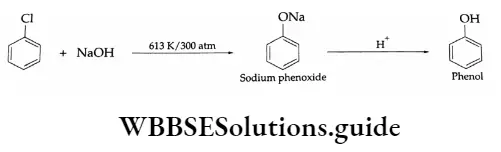
1. Substitution by hydroxyl group On being heated with aqueous sodium hydroxide at 613 K and 300 atm, chlorobenzene gives phenol.
2. Substitution by the amino group The reaction of chlorobenzene with ammonia at high temperature (573 K) and high pressure yields aniline.

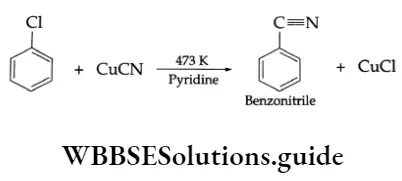
3. Substitution by the —CN group On being heated in the presence of cuprous cyanide at 473 K, chlorobenzene gives benzonitrile (phenyl cyanide).
The above reactions 1-3 take place easily when electron-attracting groups such as —NO2, — C = N and —SO3 H are present at the ortho- and para-positions. Note the condition of the reactions.
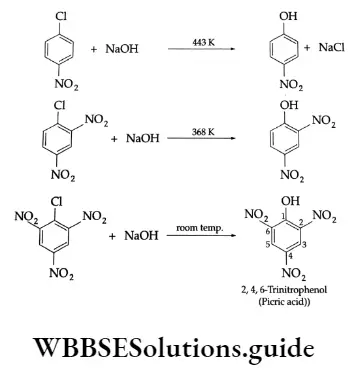
The presence of an electron-withdrawing group at the 0- and p- positions increases the rate. The presence of an electron-attracting group on any position of the ring does not increase the rate equally. No effect on the reactivity of an aryl halide is observed by the presence of an electron-withdrawing group at the m- position.
The mechanism is as follows.
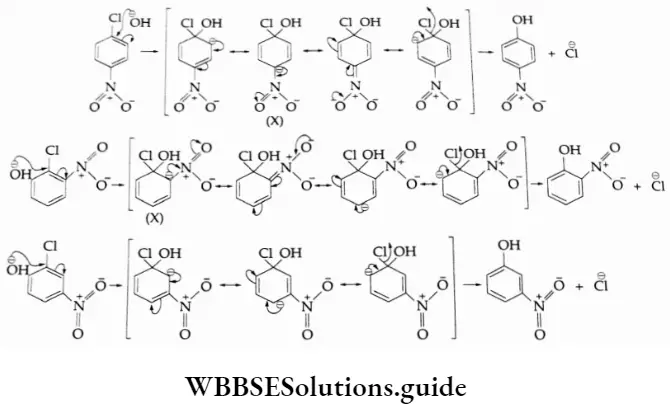
The presence of a nitro group at the 0- and p- positions withdraws the electron cloud from the benzene ring. As a result, the attack of the nucleophile on a halobenzene becomes easier. The resulting carbanion thus formed gets stabilised through resonance.
Preparation of haloalkanes from alcohols and hydrocarbons
The negative charge appearing at the 0- and p- positions (see structure X) with respect to the halogen substituent is also stabilised by the nitro group through resonance. In the case of m-nitrobenzene, none of the resonating structures bears a negative charge on the carbon atom bearing the nitro group.
The presence of the nitro group at the m- position therefore does not stabilise the negative charge and hence they do not undergo nucleophilic substitution easily.
Electrophilic substitution
The benzene ring of an aryl halide undergoes the usual electrophilic substitution reactions such as halogenation, nitration, sulphonation and Friedel-Crafts reactions. In an aryl halide, the halogen atom deactivates the ring due to its more electronegative character but at the same time, it is 0- and p- directing as is evident from the resonating structures of aryl halides.
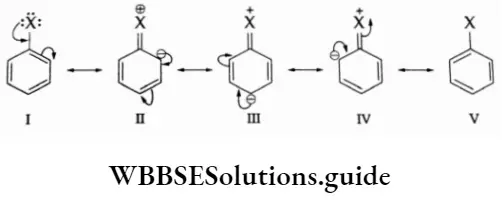
Due to resonance, the electron density increases more at the 0- and p- positions than at the m- position. Tt electrophilic substitution reactions in aryl halides require slightly more extreme conditions as compared to those in benzene.
1. Halogenation
2. Nitration
3. Sulphonation
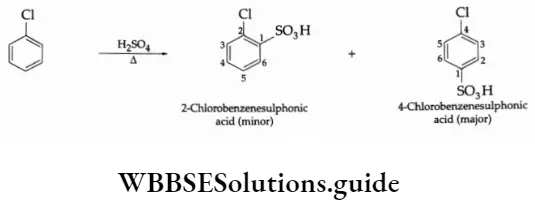
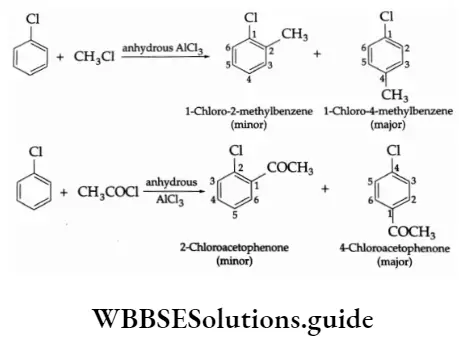
4. Friedel-Crafts reaction
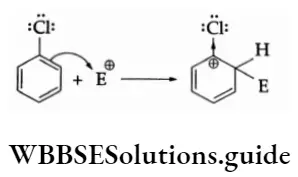
Example Chlorine is an electron-withdrawing group. Yet it is 0- and p- directing in electrophilic substitution reactions. Why?
Solution:
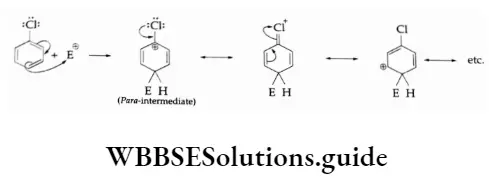
Chlorine withdraws electrons through the -I-effect and releases electrons through resonance. In electrophilic reactions, chlorine cannot stabilise the following carbocation through the -I-effect.
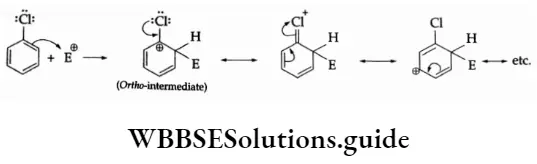
Through resonance, chlorine in chlorobenzenes makes the ortho- and para- para–intermediates more stable.
Reactions with metals
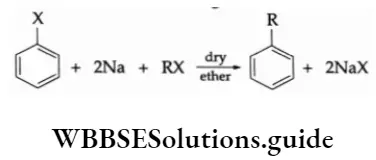
1. Wurtz-Fittig reaction On being heated with an alkyl halide and metallic sodium in dry ether, an aryl halide yields alkyl benzene. This reaction is called the Wurtz-Fittig reaction.
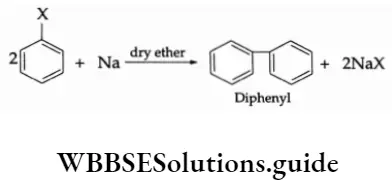
2. Fittig reaction Two molecules of an aryl halide, on being heated with sodium in the presence of dry ether, give diphenyl. This reaction is called the Fittig reaction.
Polyhalogen Compounds
Dichloromethane (methylene chloride)
Dichloromethane is widely used as a
- Solvent.
- Paint Remover.
- Propellent In Aerosols.
A high concentration of methylene chloride in the air may cause dizziness, nausea and numbness of fingers and toes.
Methylene chloride has to be handled with care. Direct contact with the eyes can bum the cornea and direct contact with the skin may cause intense burning and mild redness of the skin. Methylene chloride also affects our central nervous system.
Trichloromethane (chloroform)
Chloroform is used as a solvent for fats, waxes, resins, etc. Fortified by alcohol, it finds use as a general anaesthetic, but its use is discouraged because of its harmful side effects such as nausea and liver toxicity.
The major use of chloroform is in the production of the refrigerant freon. Inhaling chloroform causes dizziness and headache. Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is, therefore, stored in closed, dark bottles.
⇒ \(2 \mathrm{CHCl}_3+\mathrm{O}_2 \stackrel{\text { light }}{\longrightarrow} \underset{\text { Phosgene }}{2 \mathrm{COCl}_2}+2 \mathrm{HCl}\)
Triiodomethane (iodoform)
It is used as an antiseptic. Due to its bad smell, it has been replaced by other formulations containing iodine, such as tincture iodine.
Tetrachloromethane (carbon tetrachloride)
Carbon tetrachloride is used
- As A Fire Extinguisher Under The Name Pyrene.
- As A Refrigerant.
- In The Synthesis Of Chlorofluorocarbons.
- As A Dry-Cleaning Agent.
Exposure to CCI4 causes liver cancer. It also causes dizziness, nausea and vomiting. 1’his chemical may irritate the eyes on contact.
Freons
The chlorofluorocarbon compounds of methane and ethane are collectively known as freons. They are used extensively as noncorrosive coolants, lubricants and nonpoisonous fire extinguishing liquids. They are also used as aerosol propellants, and for refrigeration and air-conditioning purposes.’
Freons and ozone depletion The ozone (O3) layer in the stratosphere shields the earth from ultraviolet (UV) radiation that is harmful to living organisms.
Freons often make their way into the troposphere and diffuse unchanged into the stratosphere. They initiate a free-radical chain reaction that can upset the natural ozone balance. The reactions are as follows:
⇒ \(\mathrm{CFCl}_3+h v \rightarrow \dot{\mathrm{C} F C l}{ }_2+\dot{\mathrm{C}} \mathrm{l}\)
⇒ \(\dot{\mathrm{Cl}}+\mathrm{O}_3 \rightarrow \mathrm{ClO}+\mathrm{O}_2\)
⇒ \(\mathrm{ClO}+\mathrm{O} \rightarrow \mathrm{O}_2+\dot{\mathrm{Cl}}\)
In the chain-initiating step, UV light causes the homolytic cleavage of one C—Cl bond of the freon. The chlorine atom destroys thousands of molecules of O3 before it diffuses out of the stratosphere or reacts with other substances. This reaction, therefore, is responsible for the depletion of the ozone layer.
DDT (p, p’-dichlorodiphenyltrichloroethane)
DDT is used as an insecticide. It is effective against mosquitoes that spread malaria. The use of DDT is banned in many countries because it is harmful and non-biodegradable.
Haloalkanes And Haloarenes Multiple-Choice Questions
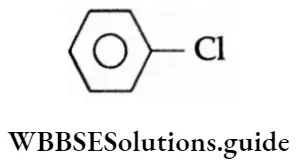
Question 1. Which of the following is an aryl halide?
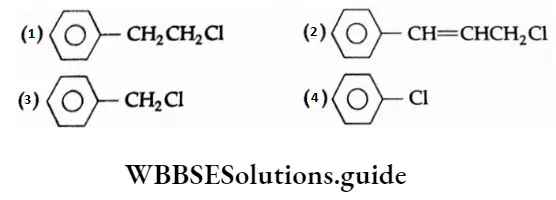
Answer: 4.
Question 2. Which of the following is an allyl halide?
- CH2 = CH—Cl
- HC = C—CH2CI
- CH2 = CH—CHCICH3
- CH2 = CH—CH2CH2CI
Answer: 3. HC = C—CH2CI
Question 3. Which of the following is a vinyl halide?
- CH2 = CH—CHB1CH3
- CH3—C(Br) = CH2
- CH2 = CH—CH2—CH2—Cl
- HC H C—Br
Answer: 2. CH3—C(Br) = CH2
Question 4. How many isomers (including stereoisomers) are possible for C4H9Br?
- 3
- 2
- 4
- 5
Answer: 4. 5
Question 5. How many isomers does C5H11CI have?
- 3
- 4
- 6
- 8
Answer: 4. 8
Question 6. The reaction of ethyl bromide with metallic sodium in dry ether gives
- Ethane
- Butane
- Propane
- Ethylene
Answer: 2. Butane
Question 7. Which of the following is used as an anaesthetic agent?
- Chloroform
- Iodoform
- Acetylene
- Methane
Answer: 1. Chloroform
Question 8. On being boiled with alcoholic KOH, ethyl bromide gives
- Ethyl Alcohol
- Ethylene
- Acetylene
- Methane
Answer: 2. Ethylene
Question 9. The IUPAC name of secondary butyl chloride is
- 3-chloroquine
- 1-chloro-2-methylpropane
- 2-chloroquine
- 2-chloro-2-methylpropnne
Answer: 2.1-chloro-2-methylpropane
Question 10. The reaction CH3CH2Br + OH–→CH3CH2OH + Br– is
- SN1
- SN2
- Elimination Reaction
- None Of These
Answer: 2. SN2
Question 11. The conversion of ethyl bromide to alcohol using aqueous KOH is an Example of
- An Addition Reaction
- A Substitution Reaction
- Dehydrohalogenation
- An Elimination Reaction
Answer: 2. A Substitution Reaction
Question 12. The products of the reaction (CH3)3C— Br + CH3ONa→ are
- (CH3)3C—ONa + CH3Br
- (CH3)2C = CH2 + CH3OH + NaBr
- (CH3)3C—OCH3 + NaBr
- (CH3)3C—CH2OH + NaBr
Answer: 3. (CH3)3C—OCH3 + NaBr
Question 13. The reaction of (CH3)3CMgCl with D2O gives
- (CH3)3CD
- (CH3)3COD
- (CD3)3CD
- (CD3)3OD
Answer: 1. (CH3)3CD
Question 14. Which of the following is a refrigerant?
- COCl2
- CCl4
- CF4
- CF2Cl2
Answer: 4. CF2Cl2
Question 15. Which of the following has a high dipole moment?
- CH3CI
- CH2CI2
- CHCI3
- CCI4
Answer: 1. CH3CI
Physical and chemical properties of haloalkanes
Question 16. On being heated with aqueous NaOH, chloroform gives
- CH3COONa
- HCOONa
- CH3OH
- HCOOH
Answer: 2. HCOONa
Question 17. The main product of
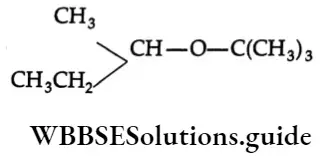
- CH3CH=CHCH3
- CH2=CH—CH2—CH3152.00 ne of these
- No Golden Passport to Hindi for Class 11
Answer: 2. CH3CH=CHCH3
Question 18. DDT is
- An Insecticide
- Biodegradable
- Nonbiodegradable
- None Of These
Answer: 1. An Insecticide
