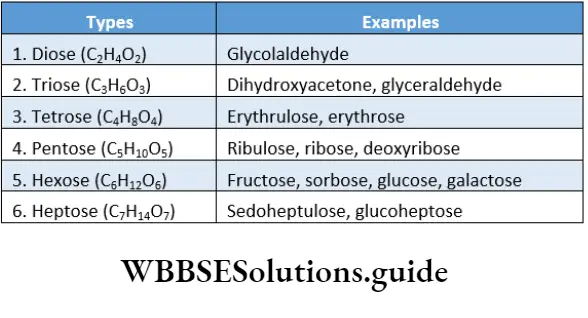
Monosaccharides
Monosaccharides Definition: The simplest form of carbohydrates that do not hydrolyse further into smaller units are called monosaccharides.
Monosaccharides Types: Monosaccharides are classified on the basis of—
- The number of carbon atoms and
- Reducing group presence.

Properties of monosaccharides: The different properties of monosaccharides are discussed below.
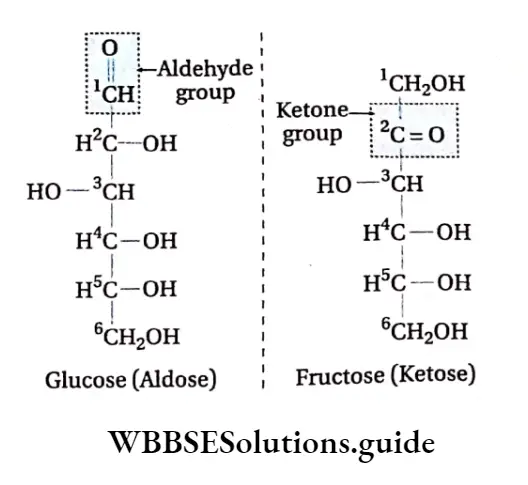
Presence of aldehyde or ketone group: They essentially contain an aldehyde or ketone group in their structure.
Monosaccharides glucose and fructose notes
Examples of monosaccharides are glucose, fructose, ribose etc.

The simpler carbohydrates are known as sugars. The sugars are named according to the presence of the aldehyde or ketone group. Those that contain –CHO (aldehyde) are called aldoses and those that contain C = 0 (ketone) are ketoses.
Isomerism: The empirical Formula of monosaccharide is Cn(H2O)n. The value of ‘n’ ranges from three to eight.
Pentose and hexoses have an open chain or ring structure. Except for dihydroxyacetone, all other monosaccharides have an asymmetric carbon atom (chiral carbon) i.e., four different atoms or groups of atoms (substituents) bonded to its four valencies.
The presence of asymmetric carbon atoms allows the formation of isomers. The compounds which have the same structural formula but differ only in spatial configuration are called stereoisomers or geometric isomers.
Glucose (aldohexose) with 4 asymmetric carbon atoms has 2n = 24 = 16 stereoisomers (n = The number of asymmetric carbon atoms) while fructose (ketohexose) has 23 = 8 stereoisomers.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
Depending on the orientation of the H and OH groups around the asymmetric carbon atom, a sugar may exist as D and L stereoisomers that are mirror images of each other.
When the OH group around the carbon atom adjacent to the terminal primary alcohol carbon is on the right, the sugar is a member of the D series and when it is on the left, it is a member of the L series.
The majority of the monosaccharides occurring in mammals are of the D configuration.
Molecular structure: The monosaccharides have mainly two types of molecular structures—
- Free Chain Structure And
- Ring-Like Structure.
Aldose sugars generally show a free chain structure. This structure can also be explained by Fischer’s projection. On the other hand, monosaccharides with 5-6 C atoms have a ring-like structure.
The carbonyl group(-C=0) is attached covalently to the O-atom of the OH group, to form the ring structure.
“monosaccharide example “
The ring structure is of two types— or -ring and -ring. They are stereoisomers of each other.
Due to optical rotation, they can be converted to each other by mutarotation. The carbon atom1 (C-l) is called anomeric carbon.
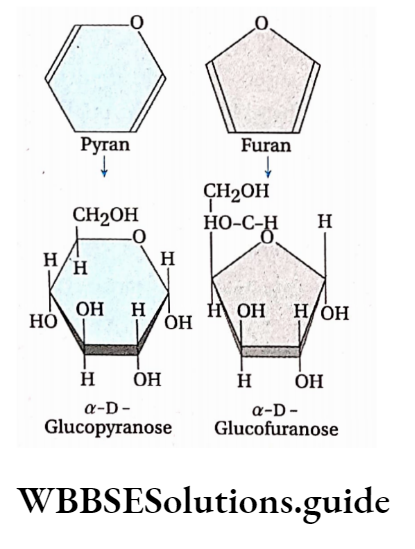
Pyranose and furanose formation: Ring forms are of two types—
- Pyranose and
- Furanose.
The Pyranose ring form is hexagonal with five carbon atoms and one oxygen atom.
It is formed when the aldehyde group attached to the C-l atom of sugar is attached to its hydroxyl group at carbon atom 5, forming a six-member ring.
The Furanose ring form is pentagonal with four carbon atoms and one oxygen atom. It is formed when the keto group at 2nd carbon reacts with the hydroxyl group at 5th carbon, forming a five-member ring.
Difference between glucose and fructose monosaccharides
Ester formation: Due to the presence of an alcoholic hydroxyl (-0H) group in the structure, it reacts with inorganic acids to form its esters. Example: Glucose-l-phosphate is the phosphate ester of Glucose.
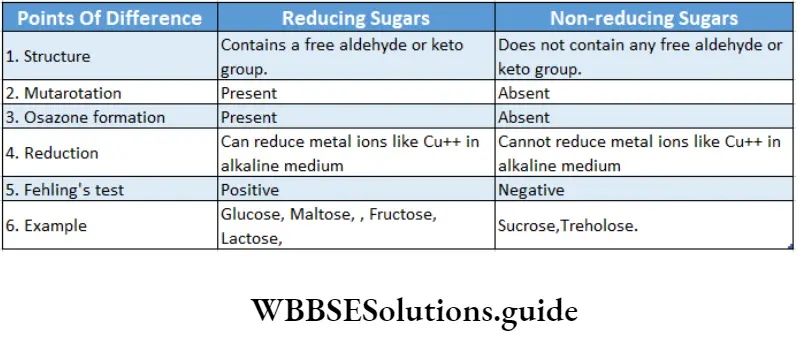
Reducing properties: Due to the presence of free aldehyde or keto group, all monosaccharides can reduce metal ions like cupric ions (Cu++) etc.
Oxidising properties: Monosaccharides get oxidised to several sugar acids containing the -COOH group.
In the case of hexoses, number 1 or number 6 carbon atoms may get oxidised to -COOH groups.
Structure and function of glucose and fructose
When a number of carbon gets oxidised, exonic acid is produced, for example, gluconic acid. When C6 gets oxidised, uronic acid is produced, for example, glucuronic acid.
Optical Isomerism
It is the isomerism based on the optical activity of a sugar. The optical activity occurs due to the presence of asymmetric carbon atoms in its molecule.
The optical activity of a sugar refers to the rotation of the plane of polarised light (light in which the waves vibrate in a single plane) passing through a solution of that sugar. If the light is rotated in a clockwise manner or to the right, then the sugar is referred to as dextrorotatory or d-sugar or (+)sugar.
Monosaccharides definition types and examples
On the other hand, if the light is rotated in an anti-clockwise manner or to the left, then the sugar is referred to as levorotatory or l-sugar or (-)sugar. These are the optical isomers of each other.
Mutarotation
It is the change in the optical activity of a freshly prepared aqueous solution of sugar until its optical rotation attains a stable equilibrium.
Epimers
Several isomers of glucose are formed due to the exchange of H+ and OH- ions, within C-2, C-3 and C-4 of the molecule. These are known as epimers. Example Mannose, galactose etc.
Effect of concentrated acid: The reaction of sugar with a strong mineral acid produces a furfural compound.
Effect of mild alkali: Both aldoses and ketoses react with mild alkali solution, to form enediols which are powerful reducing agents.
Osazone formation: Osazones are a class of carbohydrate derivatives found when reducing sugars react with phenylhydrazine.
Glucose vs fructose metabolism in the body
By studying the crystalline structure of the osazone formed, the carbohydrate can be identified. For example, glucose osazone is needle-shaped and long, while maltose appears as a bunch of grapes.
Hexosamine formation: The Hydroxyl group of hexose sugar when replaced by an amino group forms a structure called hexosamine. It is also called amino sugar.
Glucosamine is a type of sugar, formed from glucose.
Glycoside formation: Glycosides are molecules in which a sugar is bound to the hydroxyl group of a non-sugar moiety.
The replaceable hydrogen atom is replaced by alcohol, phenol or sterol group. Example digitonin, and fluorine.
They have a bitter medicinal taste, The leaves and roots have large amounts of glycosides.
Condensation: Several monosaccharides form larger molecules through chemical bonds by the condensation process.
Compound Carbohydrate
Two or more monosaccharide units when joined together by glycosidic bonds form compound carbohydrates.
The formula of compound carbohydrate is (C6H12O6)n- (H2O)n-1.
Compound carbohydrates are of two types—Oligosaccharides and Polysaccharides.
Oligosaccharides-simple Compound Carbohydrates
Oligosaccharides Definition: Simple carbohydrates which are composed of two to ten molecules of monosaccharides joined by glycosidic bonds are known as oligosaccharides.
Oligosaccharides Structure: When two or more monosaccharide units, either similar or dissimilar, link to each other by bonds, an oligosaccharide is formed.
” structure of glucose and fructose”
Two monosaccharide molecules join each other by a glycosidic bond between 1st carbon atom of one monosaccharide molecule and 2nd or 4th or 6th carbon atom of another monosaccharide molecule.
Each glycosidic bond formation involves the removal of one molecule of water.
Oligosaccharides Types: On the basis of a number of monosaccharide units or monomers, these are disaccharides, trisaccharides, tetrasaccharides, pentasaccharides etc.
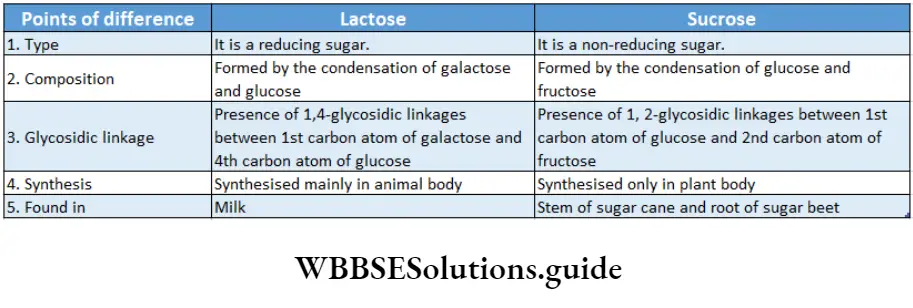
Oligosaccharides Disaccharides: Disaccharides are composed of two molecules of monosaccharides (C6H1206)2-(H20).
The biologically important disaccharides present in plants are sucrose (glucose + fructose) and maltose (glucose + glucose).
In animals disaccharide, lactose (galactose glucose) is present.
Sucrose or cane sugar is abundantly found in sugarcane, beets, carrots and fruits.
It is formed by the condensation of one molecule of D-glucose and one molecule of D-fructose.
Here, the 1, 2-glycosidic bond is formed between the aldehyde group of glucose and the keto group of fructose.
Due to the absence of a free aldehyde or keto group, sucrose is a non-reducing sugar.
Maltose or malt sugar, a reducing aldose, is found in cereals, like oat, barley, wheat, etc.
A maltose molecule is formed by the bonding of two D-glucose molecules through a 1, 4-glycosidic bond.
It is a reducing sugar as the aldehyde group of one of the glucose molecules is free. It is used in making beer.
Lactose or milk sugar is found in milk in the form of gritty crystals of the milk whey.
It is formed by the condensation of one molecule of D-galactose and one molecule of D-glucose through a 1, 4-glycosidic bond between the 1st carbon aldehyde group of galactose and the 4th carbon of glucose.
It is also a reducing sugar as the aldehyde group of the glucose molecule is free.
Trisaccharides: Trisaccharides are composed of 3 molecules of monosaccharides, for Example, raffinose (glucose + fructose + galactose) found in cottonseed and sugar beet.
Tetrasaccharides: Tetrasaccharides yield 4 monosaccharides on hydrolysis, for Example, stachyose (glucose + fructose + galactose + galactose), the only tetrasaccharide known to exist in plants.
Pentasaccharides: Pentasaccharides yield five monosaccharide units, Example verbascose (fructose + glucose + galactose + galactose + galactose).
Derived monosaccharide
Any substance derived from monosaccharides by reduction of the carboxyl group by oxidation or by replacement of one or more hydroxyl groups and forms a modified, different complex structure with different properties is called derived monosaccharide.
Examples are saline, glucosamine, glucuronic acid, phosphate j sugar, amino sugar and vitamin C (ascorbic add).
Different types of derived monosaccharides are essential for different physiological functions of the body.
For example—
- Vitamin C is required for the functioning of the different enzymes and also to treat the disease, scurvy,
- Amino sugar prevents protein synthesis in several bacteria.
- Glucuronic acid is an important constituent of saliva,
- Phosphate sugar plays an important role in forming nucleic acid and the release of energy.
Properties:
- They are water-soluble.
- They generally have a sweet taste.
- They are stored as storage carbohydrates. For example, sucrose is stored in sugarcane and sugar beet as reserve food.

Polysaccharides—Complex Compound Carbohydrates
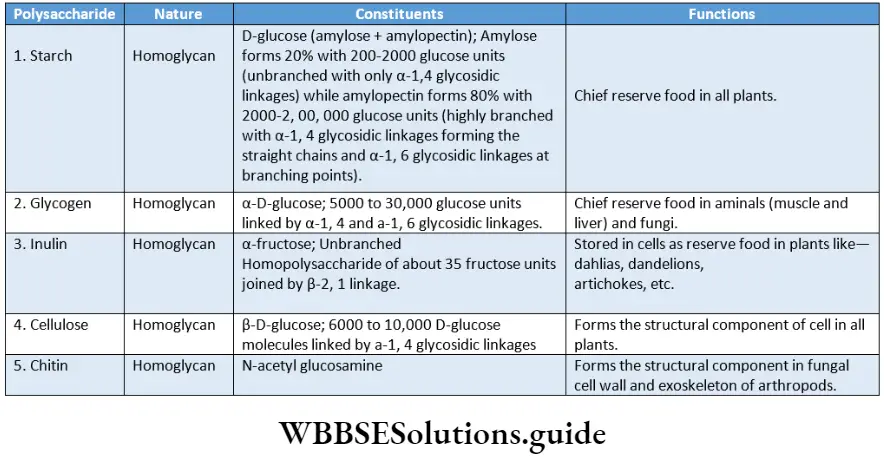
Polysaccharides Definition: The polymers consisting of 20 to 107 monosaccharide units, joined by glycosidic linkages, are called polysaccharides.
Classification of polysaccharides: Depending upon the chemical structure, nature and functions, polysaccharides are of the following types.
According to the chemical structure
Homoglycans or homopolysaccharides: The polysaccharides formed by condensation of a single type of monosaccharides are called homoglycans or homopolysaccharides; for Example starch (glucose units), inulin (fructose units), agar (galactose units), etc.
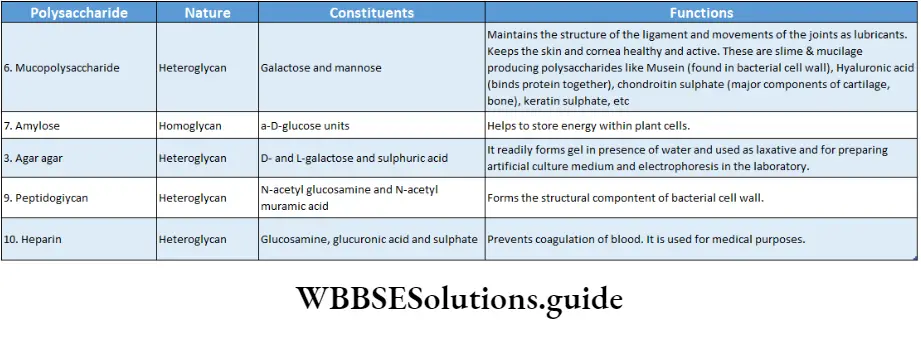
Heteroglycans or heteropolysaccharides: The polysaccharides formed by the condensation of two or more kinds of monosaccharide units are called heteroglycans or heteropolysaccharides; for Example chitin, glycoproteins, peptidoglycans, hyaluronic acid, heparin, mucopolysaccharides, etc.
According to the nature of the components
- Pentosan: The polysaccharides formed by units of pentoses are called pentosans. Example Xylan.
- Hexosan: The polysaccharides formed by units of hexoses are called hexosans. Example Galactan.
According to the nature of the components
Pentosan: The polysaccharides formed by units of pentoses are called pentosans. Example Xylan.
Hexosan: The polysaccharides formed by units of hexoses are called hexosans. Example Galactan.
Fructosan: Homopolysaccharides of fructose units are known as fructosans, for Example, inulin etc.
Galactosan: The homopolysaccharides of galactose units are known as galactosans, Example D-galactosan.
Within cells, there are many oligosaccharides formed by three or more units.
These are not present as free molecules but remain linked to lipids or proteins, to form glycoconjugates.
The carbohydrates attached to proteins are called glycoproteins and to lipids are called glycolipids.
According to functions
Storage or nutrient polysaccharides: They serve as reserve food and provide nourishment, for example, starch, glycogen, inulin, etc.
Structural polysaccharides: They take part in the structural framework of the cell. The cell walls in bacteria, plants and exoskeletons in animals are formed with polysaccharides.
Examplecellulose (in the plant cell wall), chitin (in the fungal cell wall, the exoskeleton of arthropods), peptidoglycan (in the bacterial cell wall), etc.
Complex polysaccharides: They are colloidal materials of high molecular weight. They are formed of polysaccharides and non-sugar components.
They are capable of forming gels or have adhesive properties, for example, glycoproteins, peptidoglycans, agar agar, heparin, hyaluronic acid, keratin sulphate, etc.
They bind proteins in cell walls and connective tissue and water in interstitial spaces.
Biological importance of carbohydrates
Carbohydrates have immense importance in living organisms. They also have various commercial uses in human life.
Storage food: Starch and glycogen are major storage or nutrient polysaccharides and serve as reserve food providing nourishment.
Energy source: Glucose is the primary source of energy as it is the ultimate substrate in cellular respiration. The calorific value of glucose is 4.1 kcal/g.
Structural compound: Cellulose, chitin and peptidoglycan form structural compounds of living systems like cell walls and exoskeleton.
Protection: Mucilage forms a gelatinous protective coat in many aquatic algae and bacteria.
Glycoproteins form a protective layer, glycocalyx, on the inner lining of the intestine.
Anticoagulant: Heparin prevents intravascular blood coagulation.
Medicinal value: Mucopolysaccharides like husk of isabgol (Plantago ovate), mucilage of Aloe, agar agar, algin etc., obtained from brown and red algae are of medicinal value.
Roughage: Cellulose keeps the digestive tract in functional fitness by acting as roughage.
Functions of glucose and fructose in human body
Synthesis of vitamins: Lactose helps in the growth of certain bacteria within the intestine, which in turn synthesises vitamin B-complex.
Protein-sparing action: Carbohydrates can act as supplementary food mainly for protein as well as lipid synthesis.
Mucopolysaccharides: Algin obtained from brown algae is used as a stabiliser in ice cream, toothpaste, shaving creams, face creams etc. Algin is also used in the manufacture of surgical fibres, capsule covers, flameproof plastics and security glass. Pectin is used as jelly.
Source of timber, paper, and fibres: Cellulose: fibres of cotton and jute are used for making textiles, ropes, bags etc. Cellulose-rich wood is used in making furniture, tools and sports goods.
