Nomenclature Of Organic Compounds
The nomenclature deals with the naming of millions of organic compounds. The following systems are employed.
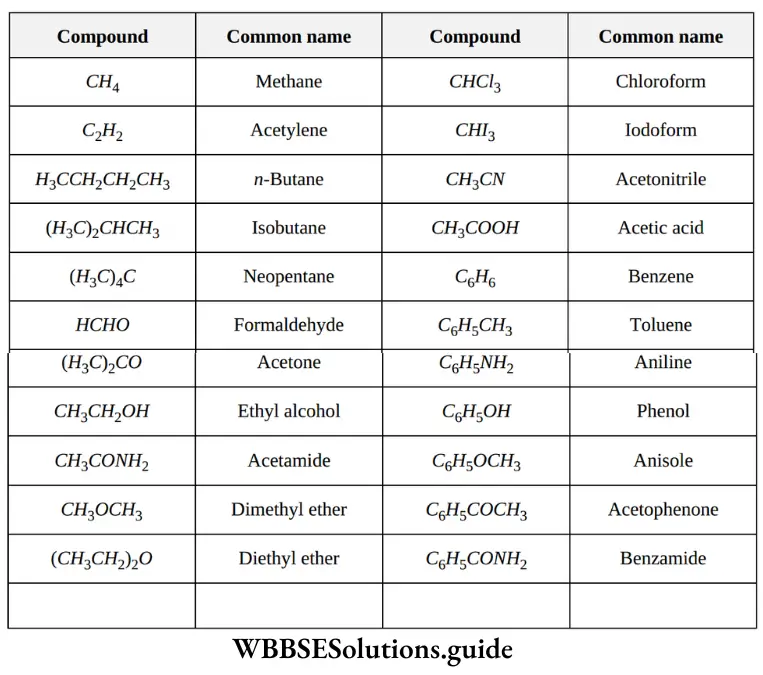
1. Trivial system (common system): It is the oldest system of naming organic compounds. In the early stages of the development of organic chemistry, organic compounds were named after the source from which they were first isolated. Generally, the names chosen had Latin or Greek roots. The following illustrations justify the statement.
Read And Learn More: NEET General Organic Chemistry Notes, Question And Answers
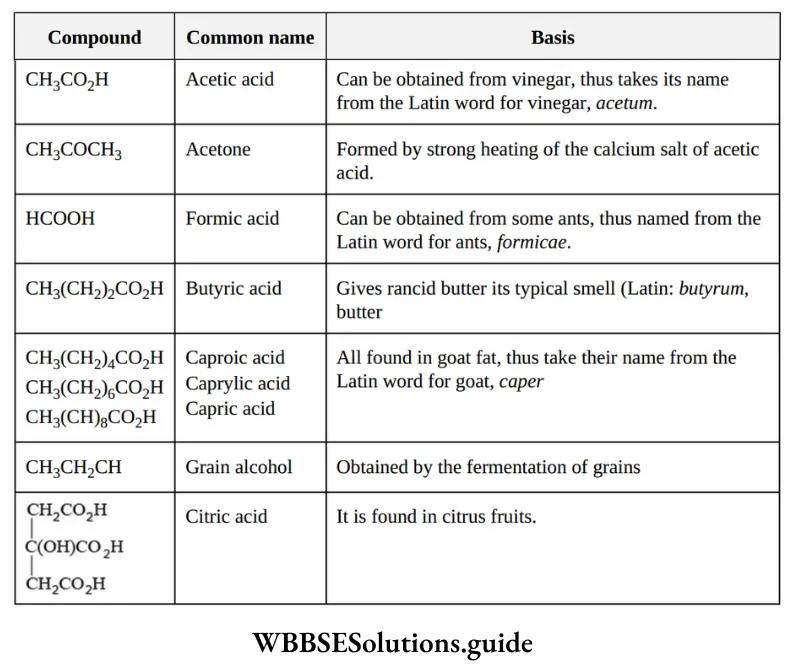
NEET organic chemistry nomenclature notes
Iupac Nomenclature
IUPAC nomenclature of organic compounds for NEET
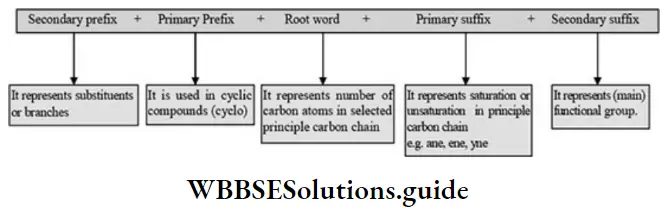
2. IUPAC System – Saturated hydrocarbons are the parent compounds and other organic compounds as their derivatives obtained by substituting one or more hydrogen atoms with functional groups. IUPAC name of any organic compound may consist of three parts. “prefix-root word-suffix”.

NEET chemistry nomenclature rules and examples
Root word- is assigned to organic molecule based on the number of carbon atoms present in the main parent chain
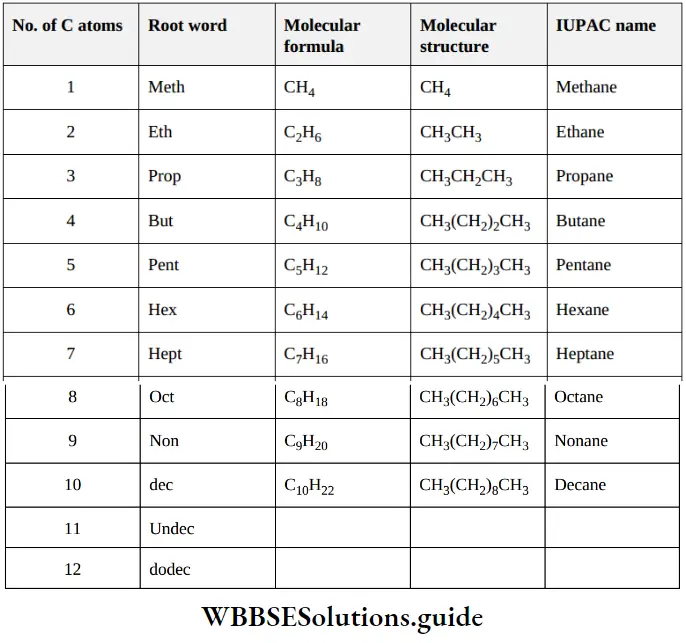
IUPAC naming of organic compounds chart for NEET
Suffix – is based on the nature of bonds and functional group present in the molecules. It is of 2 types,
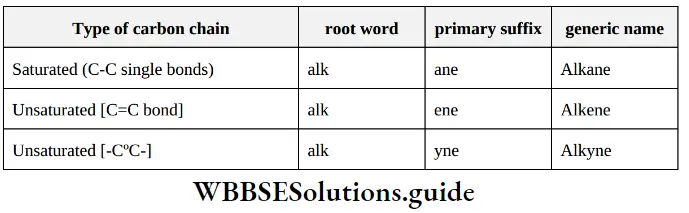
(1) Primary suffix: A primary suffix is added next to the root word to indicate whether the parent chain is saturated or unsaturated.
Note: A carbon-carbon double bond or triple bond must be included in parent chain even if it contains less number of carbon atoms.
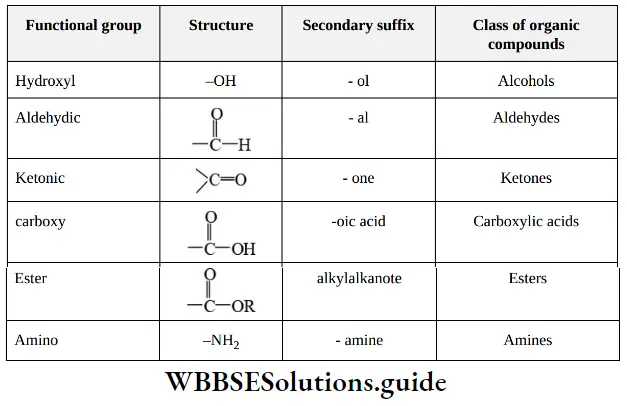
(2) The secondary suffix added next to the primary suffix to indicate the presence of a functional group in organic compounds which determines the class of organic compounds.
NEET important nomenclature questions with answers
Secondary suffixes of a few functional groups are given.
Note – While adding the secondary suffix, the letter ‘e’ of the primary suffix (i.e., ane, ene, and yne) is dropped if the secondary suffix begins with a vowel (a, e, i, o, or u). It is retained if a secondary suffix begins with a consonant.
3. Prefix (substituent): All the groups which are not names in parent chain and functional groups are called as substituents. Its name placed before the root word. Example: – Alkyl group, halo atoms, nitro group
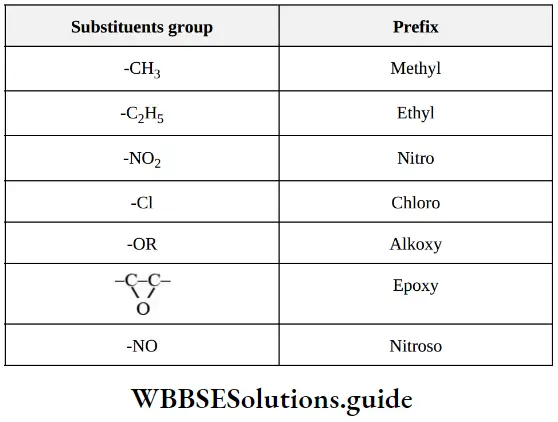
Shortcut tricks to learn IUPAC nomenclature for NEET
Alkyl Groups
Alkyl groups derived from an alkane, by removing a hydrogen atom bonded to carbon. These groups are named simply by dropping -ane from the name of the corresponding alkane and replacing it by -yl. R is a general symbol, general formula for an alkyl group is CnH2n+1, because it contains one less hydrogen atom than the parent alkane, CnH2n+2.
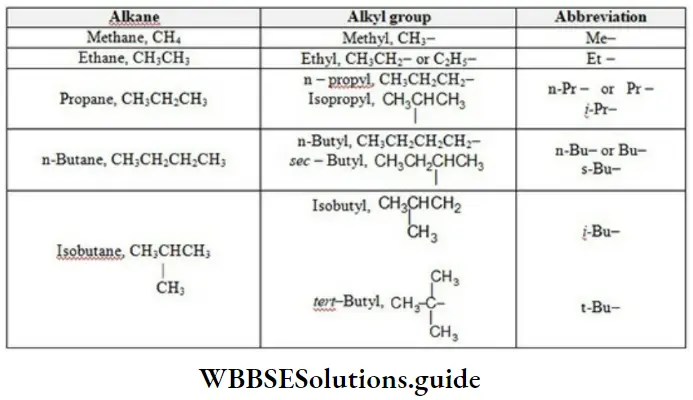
NEET chemistry nomenclature solved examples
Among the alkyl groups we encounter the problem of isomerism.
- While only one alkyl group can be derived from methane (the methyl, CH3-)
- Ethane (the ethyl, CH3CH2-)
- Two or more alkyl groups can be derived from higher alkanes.
Alkyl groups Examples
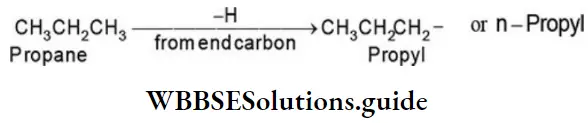
1. From propane (C3H8), two alkyl groups can be derived. Removal of one of the hydrogens from one of the end carbon atoms gives an alkyl group that is called the propyl group or n-propyl group.
Removal of one of the hydrogens from the middle carbon atom gives an alkyl group that is called the isopropyl group.
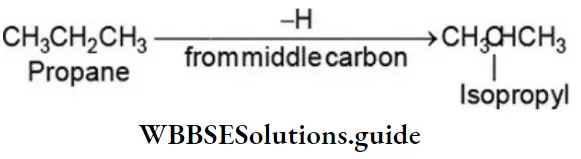
Both these alkyl groups contain the propane chain but differ in the point of attachment of the group to the rest of the molecule.
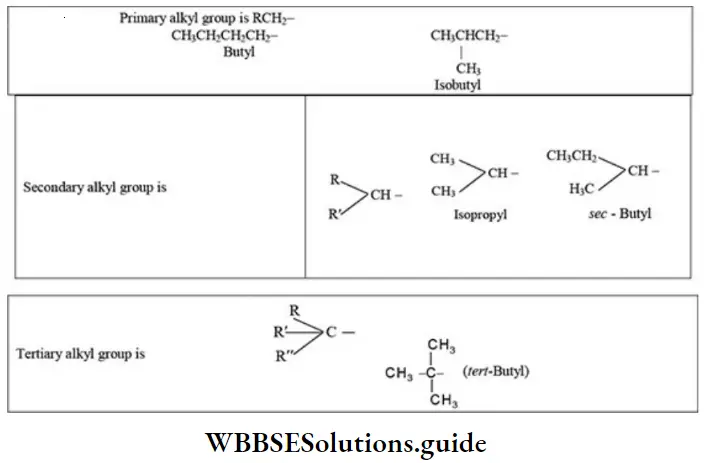
2. There are four butyl groups, two derived from the straight-chain n-butane, and two derived from the branched-chain isobutane. These are given the designations: n- (normal), sec¬secondary), iso-, and tert- (tertiary) as shown below:
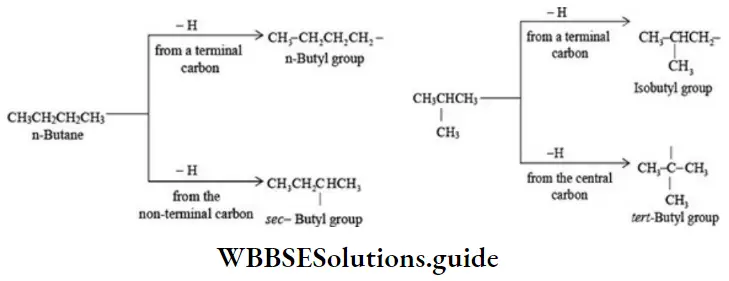
An alkyl group is described as,
- Primary if the carbon at the point of attachment is bonded to only one other carbon,
- As secondaryif bonded to two other carbons,
- Tertiary if bonded to three other carbons. Thus, if r is any hydrocarbon radical, the different kinds of alkyl groups are
RCH2 -Primary
R2CH– Secondary
R2C- Tertiary
Alkyl Group Problems
Problem 1: Classify each of the following alkyl groups as primary, secondary, or tertiary: Butyl, Isopropyl, Isobutyl, sec-butyl, and tert-Butyl.
Solution:
Best notes for organic chemistry nomenclature NEET
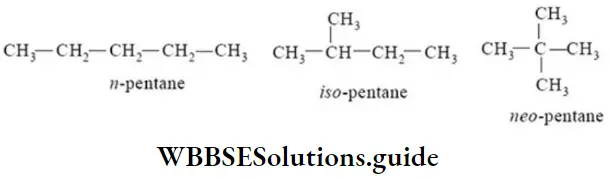
EPrefix n-(normal) is used for those alkanes in which all the carbon atoms form a continuous chain with no branching.
CH3CH2CH2CH3 (n-butene)
CH3CH2CH2CH2CH3 (n-pentane)
EPrefix iso is used for those alkanes in which one methyl group is attached to the next-to-end carbon atom (second last) of the continuous chain.
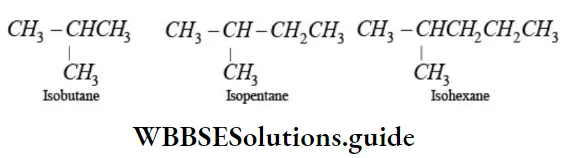
EPrefix neo is used for those alkanes which have two methyl groups attached to the second last carbon atom of the continuous chain.

Rules for naming of open chain Organic compound.
Iupac Nomenclature Rules
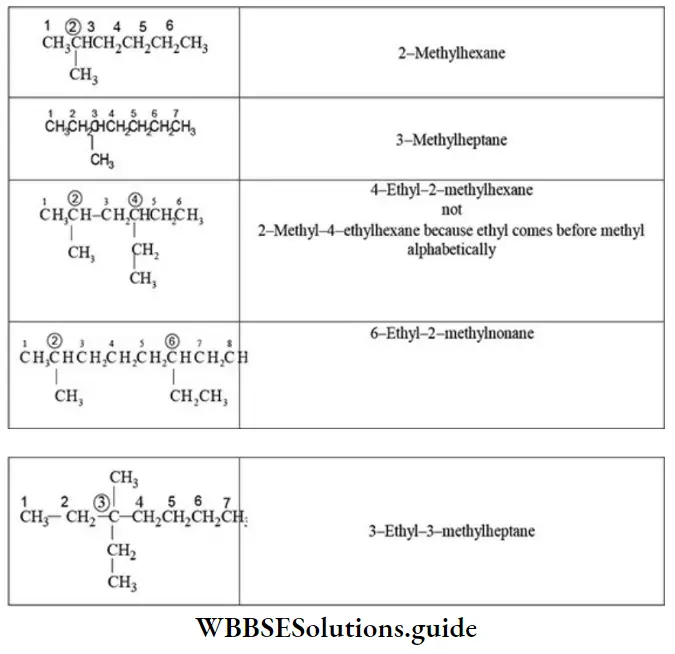
Rule 1 – Longest chain rule (root word rule): The continuous carbon chain containing maximum carbon atoms including the function group is selected. It is called the parent chain. For one to four carbon atoms of the parent chain, special root words are used but for chains of five or more carbon atoms Greek number roots are employed.
The generic root word for any parent chain is ‘alk’.
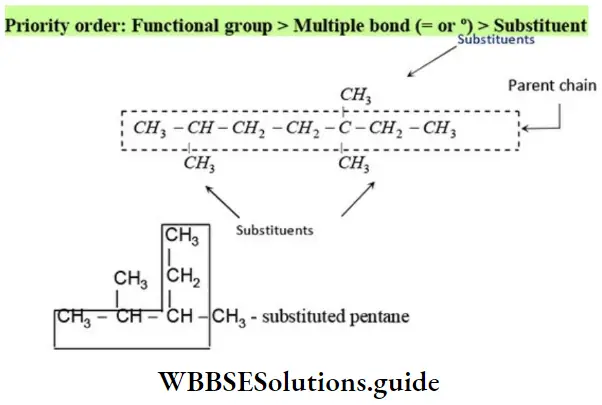
Similarly, the following hydrocarbon will be regarded as a substituted hexane because the longest continuous carbon chain (parent chain/ root chain) contains six carbon atoms.
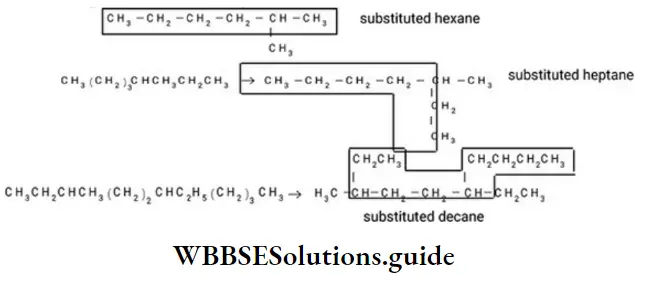
Note – If two different chains of equal length are possible, the chain with maximum number of side chains or alkyl groups is selected. The number used to specify the position of the substituents is called locant.
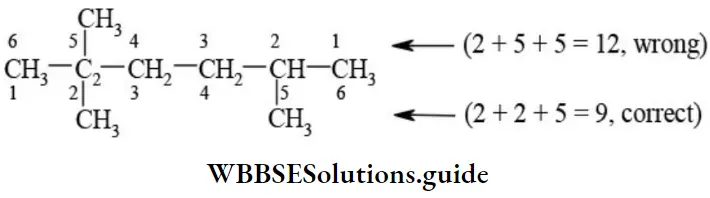
Rule 2 – Lowest sum rule (rule of locant): Parent carbon chain is numbered using Arabic numerals 1, 2, 3, 4, and 5 in such a way that functional groups or substituents containing carbon receive least number.
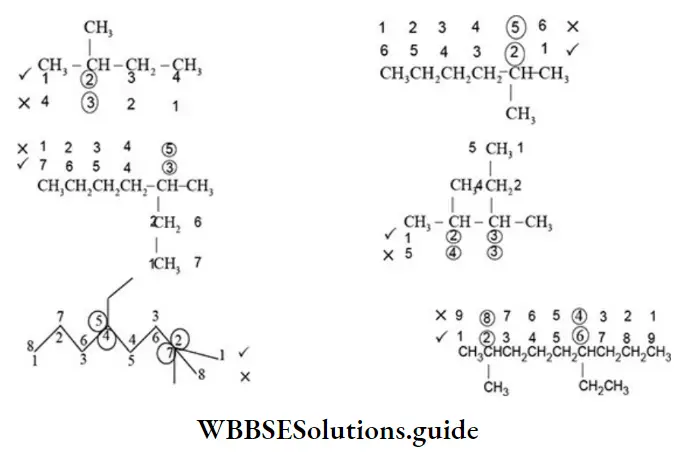
E When the parent chain contains two or more substituents, the numbering is done from the end where the sum of the locants is least.
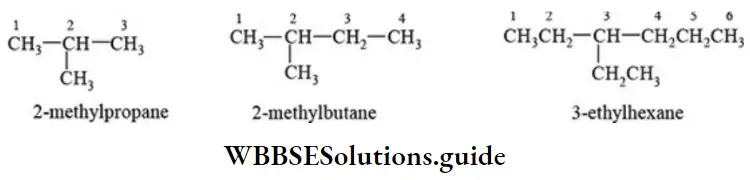
E Parent chain with one substituent: Prefix the name of the substituent to the root word of parent chain and indicate its position. The name of the substituent is separated from its locant by a hyphen (-). The name of the organic compound is written as one word.
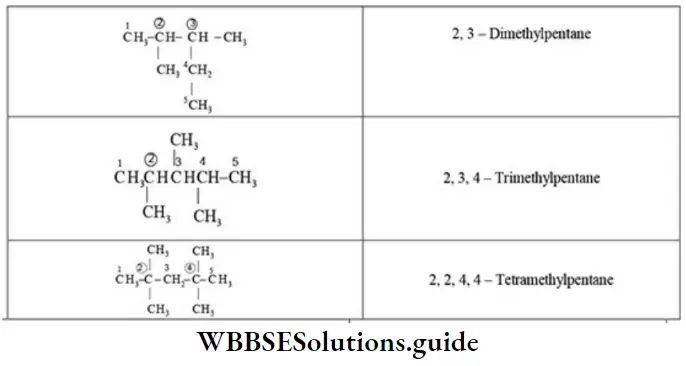
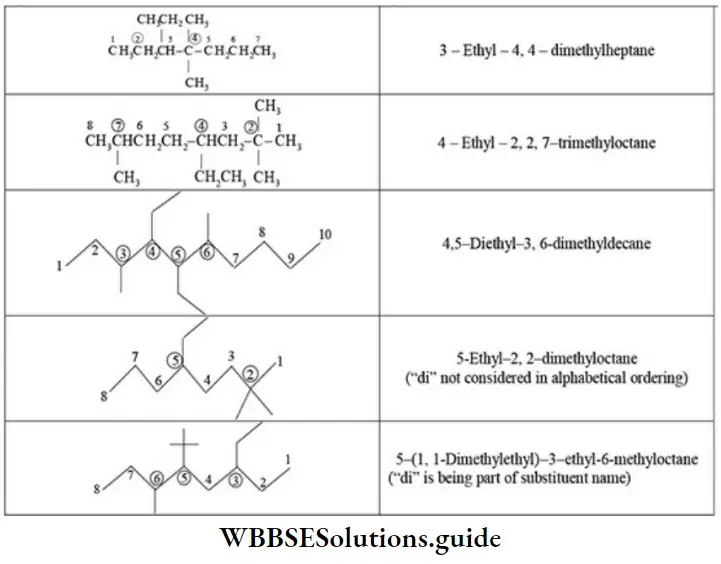
E Naming of different substituents: When two or more different substituents are present on the parent chain, they are named in alphabetical order along with their appropriate locants.
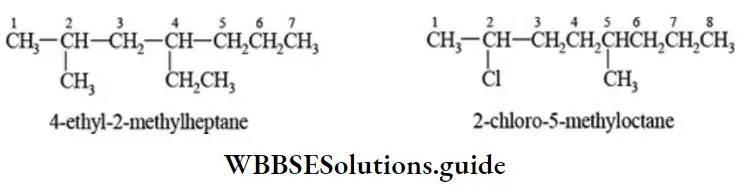
E Numbering of different substituents at equivalent positions: If two different alkyl or halo groups are present at equivalent positions, the numbering of the parent chain is done in such a way that the substituent which comes first in the alphabetical order gets the lower number.
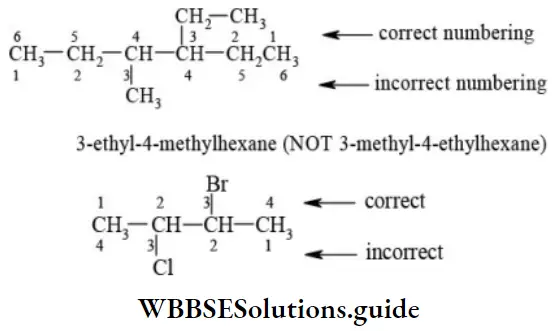
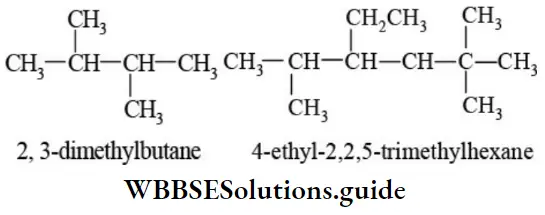
E Presence of the same substituent more than once: If the same substituent occurs more than once, the prefixes di, tri, tetra are prefixed to the name of the substituent. It may be noted that the position and name of the substituent are separated by a hyphen (-) whereas the numerals representing the positions of the substituents are separated by commas.
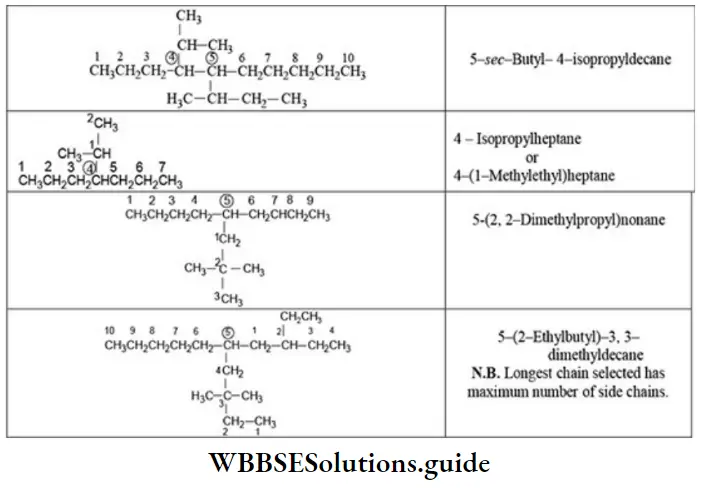
E Naming a complex substituent: In case the substituent on the parent chain is complex (i.e., it has a branched chain), it is named as a substituted alkyl group by numbering the carbon atom of this group attached to the parent chain as 1. The name of complex substituent is enclosed in the bracket to avoid confusion with the numbers of parent chain.
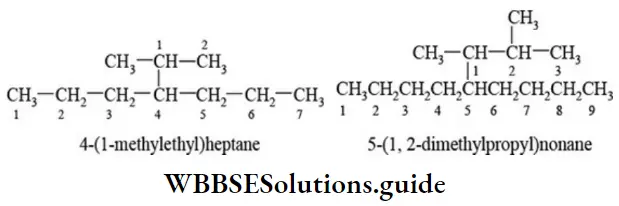
If same complex substituent occurs more than once on the parent chain, prefixes bis, tris, tetrakis, etc., are used before the name of the complex substituent. The application of IUPAC rules to the structure of a molecule to arrive at IUPAC name is illustrated as follows.
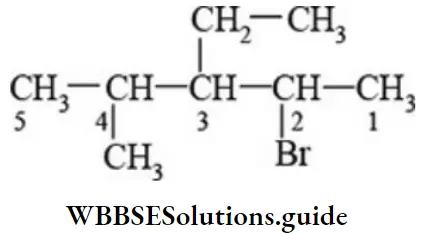
Functional group priority in IUPAC nomenclature NEET
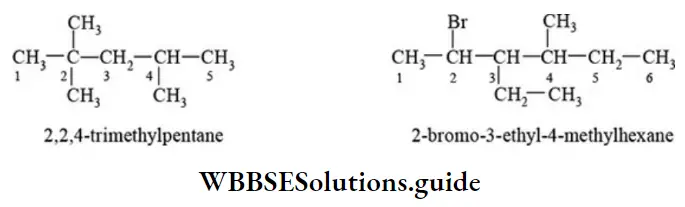
- Parent chain has 5 carbon atoms, and it has more substituents. The root word is ‘pent’.
- The primary suffix is ‘ane’
- Sum of locants is 9, the substituent that comes first in alphabetical order gets the lower number.
- Prefixes to the rootword are bromo, ethyl, and methyl. Hence, IUPAC name is “2-bromo-3-ethyl-4-methyl pentane”
A few examples are given below: