NEET Physics Atoms Notes
Atoms
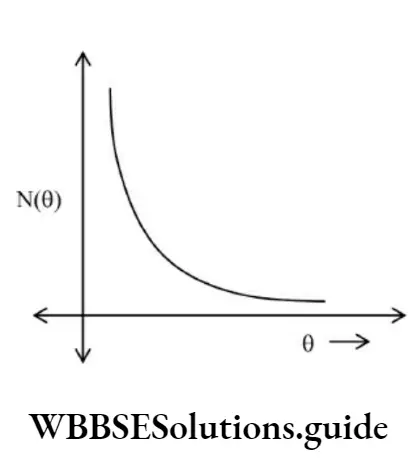
Important facts about Rutherford’s α–α-scattering experiment:
- Most of the α–particles do not suffer collisions with the gold foil.
- Only about 0.14% of the incident α–particles scatter by more than 10.
- About 1 in 8000 α–particles deflect by more than 900.
Graph of the number of alpha particles scattered versus scattering angle
Read And Learn More: NEET Physics Notes
NEET Physics Atoms Important Questions
Rutherford argued that, for large angle deflection there must be a massive positively charged body in every atom where the total positive charges of the atom are concentrated.
Hence Rutherford is credited with the discovery of the nucleus.
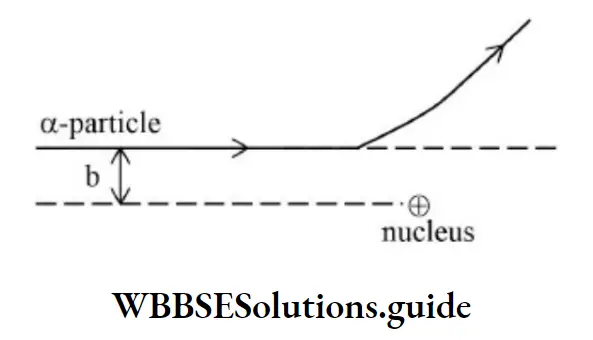
Impact Parameter
It is the perpendicular distance of the initial velocity vector of the α–particle from the center of the nucleus.
Best tricks to solve Atoms problems for NEET
The impact parameter is given by,
\(\mathrm{b}=\frac{\mathrm{Ze}^2 \cot \left(\frac{\theta}{2}\right)}{4 \pi \mathrm{E}_0\left(\frac{1}{2} m v^2\right)} \Rightarrow \mathrm{b} \propto \cot \left(\frac{\theta}{2}\right)\)The trajectory of α–particle depends on the impact parameter.
In case of a head-on collision, the impact parameter is minimum and the α–particle rebounds back.
For a large impact parameter, the α–particle goes nearly undefeated.
Distance of closest approach: At the distance of closest approach entire K.E. of the alpha particle gets converted into electrostatic P.E.
i,e., \(\begin{aligned}
\frac{1}{2} \mathrm{mv}^2 & =\left(\frac{1}{4 \pi \varepsilon_0}\right) \frac{(\mathrm{Ze})(2 \mathrm{e})}{\mathrm{r}_0} \\
\mathrm{r}_0 & =\frac{9 \times 10^9 \times 4 \mathrm{Ze}^2}{m v^2}
\end{aligned}\)
Bohr’s Model of Atom NEET Questions and Solutions
Bohr’s Quantum Condition
The angular momentum of an electron in the nth stationary orbit is given by,
\(\mathrm{mvr}=\mathrm{n} \frac{\mathrm{h}}{2 \pi} \quad \mathrm{n}=1,2,3, \ldots \ldots \ldots\)When an electron makes a transition from a higher energy state to a lower energy state, the difference in energy is emitted in the form of electromagnetic radiation.
\(\mathrm{E}_2-\mathrm{E}_1=\mathrm{hv}\)The orbital radius of electrons in the ‘nth’ orbit is given by:
\(\begin{aligned}& \mathrm{r}_{\mathrm{n}}=\frac{\mathrm{n}^2 \mathrm{~h}^2 \varepsilon_0}{\pi \mathrm{mZ} \mathrm{e}^2} \\
& \mathrm{r}_{\mathrm{n}} \propto \frac{\mathrm{n}^2}{\mathrm{Z}}
\end{aligned}\)
For hydrogen atom, \(r_n \propto n^2\)
The radius of nth orbit in a hydrogen atom is,
\(\mathrm{r}_{\mathrm{n}}=0.53 \mathrm{n}^2\) A

The orbital velocity of electrons is given by:
\(\begin{aligned}& v_{\mathrm{n}}=\frac{\mathrm{Ze}^2}{2 \varepsilon_0 \mathrm{nh}} \\
& \mathrm{v}_{\mathrm{n}} \propto \frac{\mathrm{Z}}{\mathrm{n}} \\
& \mathrm{v}_{\mathrm{n}}=\frac{\mathrm{Z}}{\mathrm{n}} \frac{\mathrm{c}}{137}
\end{aligned}\)
Where ‘c’ is the speed of light.
In the case of the hydrogen atom,
\(\mathrm{v}_{\mathrm{n}}=\frac{1}{\mathrm{n}} \frac{\mathrm{c}}{137}\) \(\text { If } \mathrm{n}=1, \mathrm{v}_{\mathrm{n}}=\frac{\mathrm{c}}{137} \simeq 2.2 \times 10^6 \mathrm{~ms}^{-1}\)NEET Atoms Previous Year Questions with Solutions
Expression for total energy
\(\begin{aligned}& E_n=-\frac{1}{n^2} \frac{m Z^2 e^4}{8 \varepsilon_0^2 h^2} \\
& E_n \propto \frac{Z^2}{n^2} \\
& E_n=(-13.6) \frac{Z^2}{n^2} e V
\end{aligned}\)
For hydrogen atoms,
\(\mathrm{E}_{\mathrm{n}}=\frac{-13.6}{\mathrm{n}^2} \mathrm{eV}\)If n = 1, then,
E = –13.6 eV
∴ The ionization energy of the hydrogen atom in the ground state is 13.6 eV & its ionization potential is 13.6 V.
\(\begin{aligned}& \text { K.E. }=+\frac{1}{\mathrm{n}^2} \frac{\mathrm{mZ^{2 }} \mathrm{e}^4}{8 \varepsilon_0^2 \mathrm{~h}^2} \\
& \text { P.E. }=-\frac{1}{\mathrm{n}^2} \frac{\mathrm{mZ}^2 \mathrm{e}^4}{4 \varepsilon_0^2 \mathrm{~h}^2} \\
& |\mathrm{E}|=\text { K.E.; P.E. }=2 \mathrm{E}
\end{aligned}\) \(\text { wave number, } \quad \frac{1}{\lambda}=R Z^2\left[\frac{1}{\mathrm{n}_1^2}-\frac{1}{\mathrm{n}_2^2}\right]\)
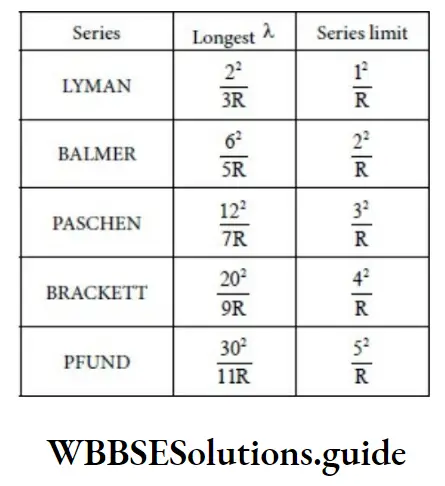
Energy Level Diagram For Hydrogen

No. of spectral lines emitted is given by, \(\frac{\mathrm{n}(\mathrm{n}-1)}{2}\)
Hydrogen Spectrum and Energy Levels NEET
The time period of revolution of an electron is given by,
\(\begin{aligned}& T=\frac{2 \pi r}{v} \\
& T \propto \frac{r}{v} \Rightarrow T \propto \frac{n^2}{\frac{1}{n}} \Rightarrow T \propto n^3 \\
& f \propto \frac{1}{T} \Rightarrow f \propto \frac{v}{r} \Rightarrow f \propto \frac{1}{n^3}
\end{aligned}\)
The wavelength of light emitted by hydrogen atoms is,
\(\lambda=\frac{12,420}{\Delta \mathrm{E}(\mathrm{eV})}^{\circ} \mathrm{A}\)The atom stays in the excited state for about 10 nanoseconds.
Rydberg’s constant is not the same for all elements.
It is the same for elements having the same number of electrons.
The permitted value of ‘n’ ranges up to. However, only values up to 7 have so far been observed.
Balmer series was the first observed spectral series.
The B.E. of the electron in the ground state of hydrogen is called rydberg.
⇒ 1 rydberg = 13.6 eV
