Respiration In Plants Introduction
You have learned in your previous chapter that plants take in carbon dioxide during photosynthesis. But do you know, that plants also take in oxygen They do so during respiration. All living organisms need a continuous supply of food for growth. They also need energy to carry out various life processes. Food is the source of this energy.
During photosynthesis, chlorophyllous cells trap solar energy and convert it to chemical energy (ATP). The chemical energy is stored as bond energy in food (glucose).
However, the energy in the food is to be made available to the cells in a utilizable form. Respiration is the biochemical process that breaks down the food with a subsequent release of energy and CO2.
Respiration In Plants Definition: Respiration is a catabolic, biochemical process, that involves the stepwise, complete, or incomplete oxidation of complex organic molecules (glucose) either in the presence or in the absence of oxygen with the release of energy as ATP required for various cellular metabolic activities.
“respiration in plants notes for class 11 biology”
Cellular Metabolic Reactions
Metabolism (derived from the Greek word metabole means ‘change’) or metabolic reaction is the set of life-sustaining chemical transformations within the cells of living organisms. The word metabolism also H refers to the sum of all chemical reactions that occur in living organisms.
The chemical reactions of metabolism are organized in metabolic pathways. In these pathways, one chemical is transformed through!; a series of steps into another chemical by a sequence of enzymes.
Metabolic reactions are usually divided into two categories
- Anabolic reactions are the building up of components of cells from smaller units with the help of energy; for example synthesis of protein and nucleic acids.
- Catabolic reactions are the breaking down of organic matter into simpler forms to release energy; for example cellular respiration.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
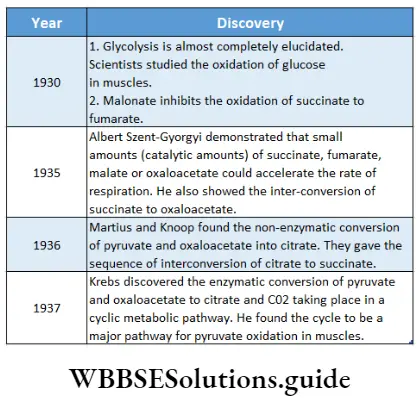
Historical perspective: Several important discoveries have led us toward the modern concept of cellular respiration. Some significant discoveries are mentioned in the table below.
” plants and respiration”
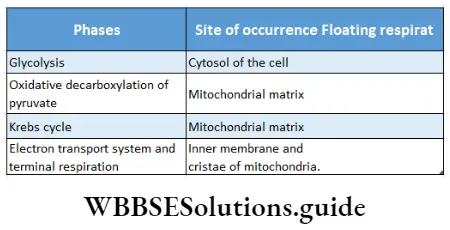
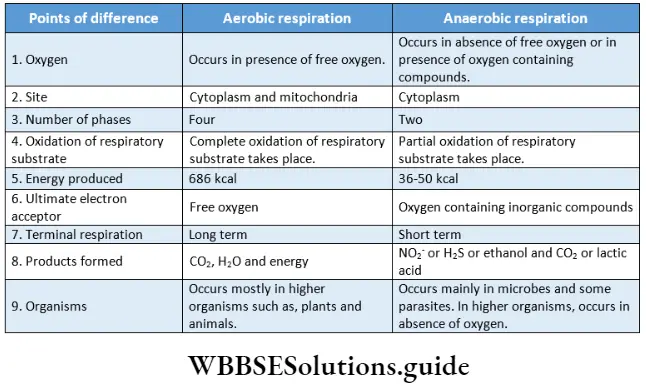
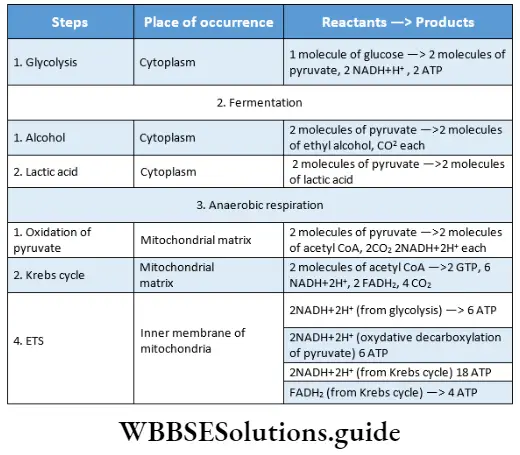
Site of respiration: Respiration or oxidation of food occurs in all living cells. This is also known as cellular respiration. Respiration involves various steps that occur in specific parts of a cell.
Site of respiration in prokaryotic cells: Prokaryotic cells do not contain mitochondria. In this type of cell, respiration occurs in the cytoplasm. In prokaryotes, respiration occurs in a specialized structure made up of a cell membrane called a mesosome.

Site of respiration in eukaryotic cells: In eukaryotic cells, respiration is complete in four different phases at different sites.
These are listed in a tabular manner as follows:
Time of respiration: Respiration occurs all the time, whether it is night or day. If respiration stops, even for a few seconds, organisms die.
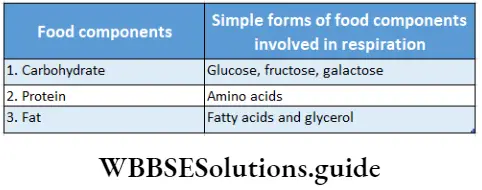
Respiratory substrate: The substances that undergo oxidation during respiration to release energy are known as respiratory substrates. These include carbohydrates, protein, fat, and organic acids.
These are present in the protoplasm of the cells. Among these, glucose (a simple carbohydrate) is the main respiratory substrate and is known as the starting point of respiration.
Types of respiration depend on the types of respiratory substrates:
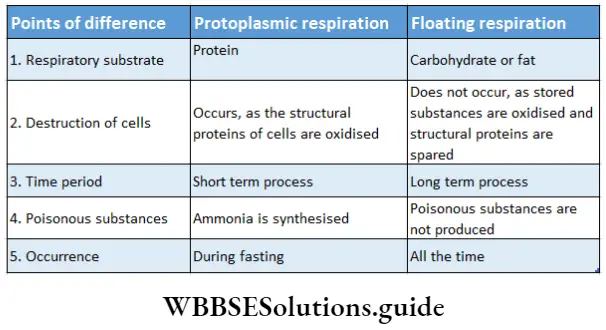
Depending on respiratory substrates, respiration is of two types—floating respiration and protoplasmic respiration.
Floating respiration: In this type of respiration, carbohydrate or fat is utilized as respiratory substrate. Generally, this type of respiration occurs in cells under normal conditions.
“detailed notes on respiration in plants with diagram”
Protoplasmic respiration: In this type of respiration, protein is utilized as a respiratory substrate. This type of respiration generally occurs in the cells during fasting, when stored carbohydrates and fats are used up completely.
This type of respiration uses structural proteins of cells because stored protein is rarely present in the cells. In this process, ammonia is synthesized by the oxidation of amino acids, which is harmful to the cells.
Respiration is an exothermic process: Organic substances present in the cell, undergo oxidation (fully or partially) during respiration. This releases the energy present in the food as ATP and heat. Hence, respiration is known as an exothermic and exergonic process.
Respiration is a catabolic process: During respiration, the complex organic components of the cell break down to form simple organic substances (in the case of aerobic respiration CO2 and H2O). This releases the energy from food. As a result, the amount of organic substances and the dry weight of the cell gradually decrease Hence, respiration is known as a catabolic process.
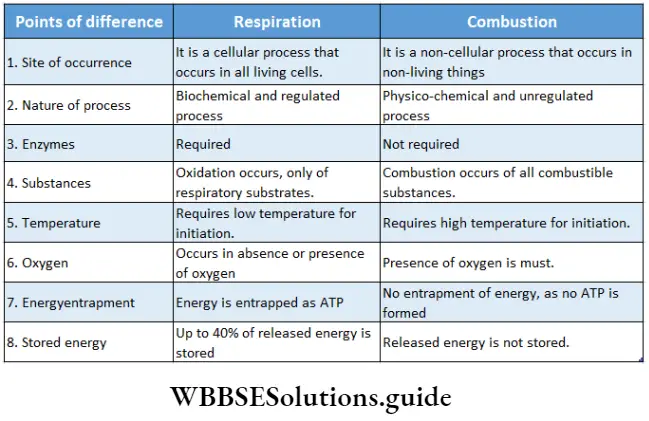
Respiration and combustion: In 1789 Lavoisier, first suggested that respiration is a type of controlled combustion. He also said that respiration and combustion have similarities as well as dissimilarities.
Similarities between respiration and combustion:
- O2 is involved in both processes. However, free O2 is used mainly during aerobic respiration.
- Both processes release stored energy.
- Both the processes involve breaking down of chemical bonds within the organic compounds.
- Both processes convert complex organic substances into their ample forms.
- CO2 is produced during both respiration and combustion.
- H2O is produced as the by-product in both processes.
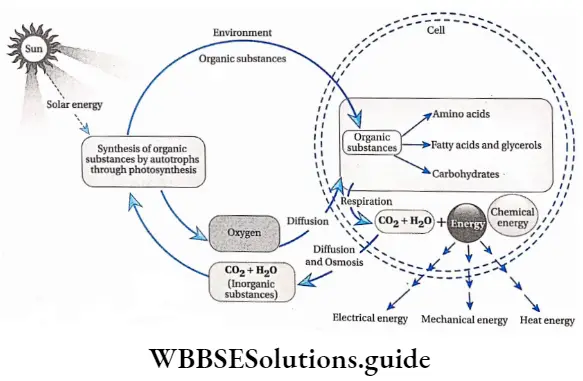
Importance of respiration: Respiration has immense importance in living organisms, such as
Release energy: The stored energy of the food is released by respiration in the form of heat. Organisms utilize this energy to carry out various life processes. The excess energy remains stored in the form of ATP (chemical energy).
Utilization and transformation of energy: The energy released during respiration is used for various physical activities, such as locomotion, movement, reproduction, growth, etc. The energy is also transformed into different forms such as heat, kinetic energy, etc.
“types of respiration in plants with examples”
Maintenances of O-CO balance: Oxygen (which is evolved during photosynthesis) from the environment is utilized during aerobic respiration. CO2 required in photosynthesis enters the environment through respiration. Hence, respiration and photosynthesis, maintain O2-CO2 balance in the environment
Bioluminescence
The emission of light, by certain living organisms, is known as bioluminescence. The light is produced due to the oxidation of luciferin protein. Enzyme luciferase oxidizes luciferin.

Some marine fish and fireflies emit light by this process. This property is also found in certain bacteria, fungi, and also in suckers of some octopus species. example Vibrio fishcheri, black dragon fish, etc.
Do plants Breathe?
The plants do not have any specific respiratory organ. The exchange of gases (that occurs with the help of the diffusion process) takes place after the entry of oxygen into the numerous air spaces present in the cells of the leaf, stem, and root.
The parts of the plant that are involved in the gaseous exchange are—the general body surface of the plant (stems, roots, fruits, and seeds), lenticels (openings in the bark of the tree trunk), stomata (present in the leaves and young stems), pneumatophores (roots that grow upward from the soil or water).
Why do plants not have special respiratory organs?
- Plant tissues are formed of loosely bound cells, so, the exchange of gases takes place by diffusion.
- Each plant part takes care of its own need for gaseous exchange. The transportation of gases from one part to another in a plant is almost negligible.
- Plants require less amount of O2 to carry out their life processes. During photosynthesis, the availability of O2 in the cells is so abundant that the exchange of gases with the environment is not needed at all.
- Each living cell in a plant is located quite close to the surface of the plant.
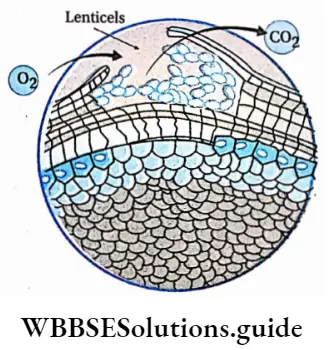
Lenticels: Numerous small pores are found in the bark of woody stems and roots of certain trees and shrubs. These pores help in a gaseous exchange known as lenticels.
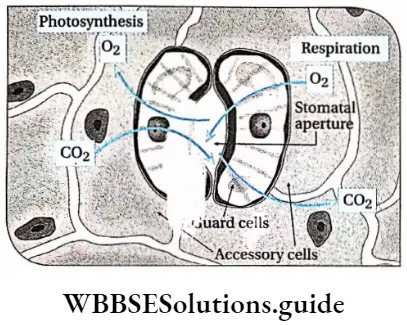
Stomata: Numerous tiny pores found in the epidermis of the leaves and young stems, are known as stomata. A stoma contains an opening called a stomatal aperture. This opening is guarded by two bean-shaped guard cells.
The guard cells are surrounded by other epidermal cells known as accessory cells. A gaseous exchange takes place through the stomatal aperture between the leaf and the atmosphere.
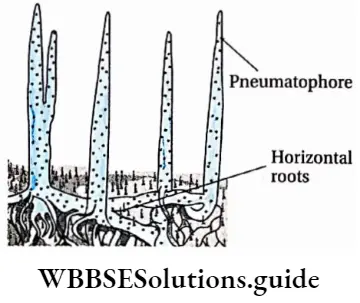
Pneumatophore: Mangrove plants like Rhizophora, Ceriops, etc., mostly grow in muddy and saline soil. This type of soil contains very low levels of O2 which hampers the gaseous exchange in the roots of these plants.
So, their roots grow erect branches that remain above the soil or water. These roots contain tiny pores (called pneumatophores) through which gaseous exchange takes place. Such roots are called pneumatophores.
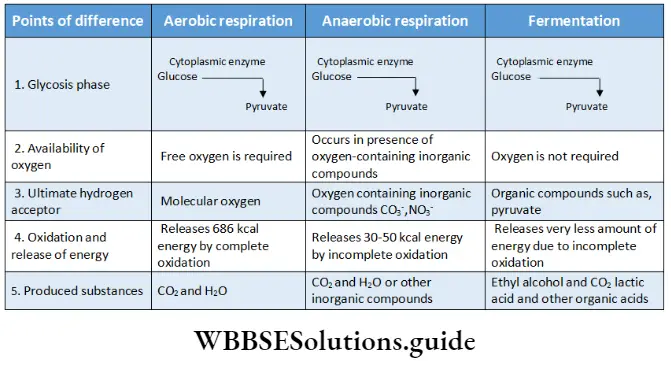
Respiration In Plants Types Of Cellular Respiration
Respiration involves the oxidation of respiratory substrates in cells and also the reduction of an electron acceptor. Based on the utilization of oxygen and the ultimate electron acceptor,
Respiration is of two types
- Aerobic
- Anaerobic.
“aerobic and anaerobic respiration in plants explained”
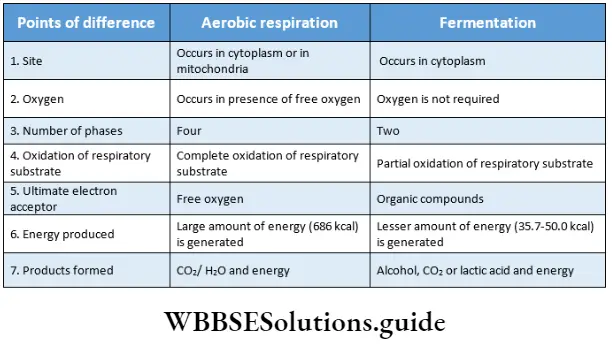
Respiration In Plants Aerobic Respiration
The word aerobic is derived from the Greek words, aer—’air’ and bios—’life’. The organisms performing aerobic respiration are called aerobes.
Aerobic Respiration Definition: The process that involves the complete oxidation of respiratory substrates (glucose) in the presence of O2 with the release of water and free CO2, with complete conversion of static energy into chemical (as ATP) and heat energy, is known as aerobic respiration.
Aerobic Respiration Site: Aerobic respiration is found in all living cells of higher plants and animals. Phases of aerobic respiration take place in the cytoplasm and mitochondria of the cell.
Overall reaction

Aerobic Respiration Summary:
Aerobic respiration includes four phases:
- Glycolysis,
- Oxidative decarboxylation of pyruvate,
- Krebs cycle,
- Electron transport system and terminal respiration.
Experiment to demonstrate aerobic respiration
Aerobic respiration can be demonstrated with the help of a simple experiment using germinating gram seeds.
Materials required: Two conical flasks, two single-holed corks, two delivery tubes bent twice at right angles with small and long arms, two beakers, two sample tubes, thread, colored water, freshly prepared 20% potassium hydroxide solution, germinating and dry non-germinating gram seeds, petroleum jelly, cotton.
Aerobic Respiration Procedure: Seed coats of the germinating seeds are removed carefully. A cotton bed is prepared at the base of the conical flask and is made wet by sprinkling water. The wet cotton protects the germinating seeds from getting dry. Now, decorated seeds are placed on the wet cotton bed.
- Freshly prepared 20% KOH solution is poured into a sample tube. The sample tube is then suspended inside the conical flask with the help of thread with proper care to avoid the spilling of solution in the conical flask. The mouth of the conical flask is covered with a single-holed cork.
- The small arm of the delivery tube is fitted with the cork and the long arm is dipped in the beaker containing coloured water. The cork connections are made airtight with petroleum jelly.
- Another setup is prepared with dry non-germinating seeds which are kept directly in the conical flask. This setup will act as a control setup. Both the set up are kept undisturbed for one hour and then the result is observed.
Aerobic Respiration Observation: The level of the colored water in the delivery tube is raised in the setup having germinating seeds. No such rising of water level is observed in the second set up having non-germinating seeds.
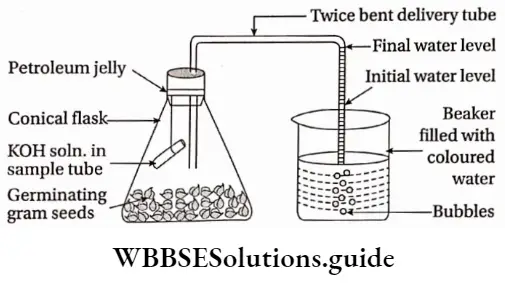
Inference and explanation: The rise of the level of colored water in the delivery tube shows a partial vacuum of water in the system. the component of air present in the system has been consumed by the germinating seed component of air (mainly O2 and not N2 or CO2 because higher organisms can not absorb atmospheric N2 or CO2) and releases CO2.
- This released CO2 is absorbed by KOH present in the sample tube. Thus, a partial vacuum is produced which causes the rise of the colored water level. In the control setup, the dry seeds are not respiring at all, so, they do not use the oxygen.
- Thus, no vacuum is created. So, the colored water level remains stable at its initial position. Therefore, it can be concluded that germinating seeds perform aerobic respiration.
Aerobic Respiration Precautions:
- Seeds should be well germinated.
- Removal of seed coats should be done carefully.
- All the connections should be made airtight.
- The free ends of the delivery tubes should be dipped in colored water.
- Levels of the water should be marked appropriately.
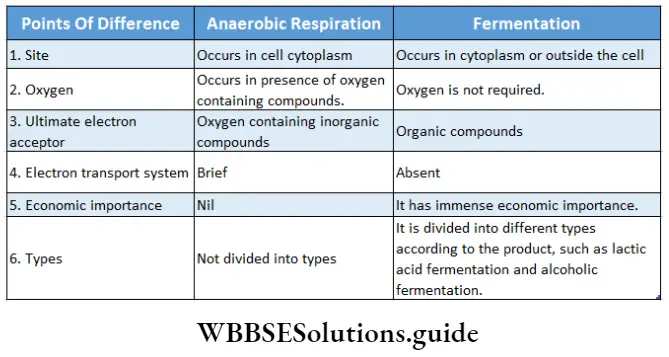
Respiration In Plants Anaerobic Respiration
The word Anaerobic word is derived from Greek words, viz., art—’ without’, aer—’air’, and bios—’life’. The anaerobically respiring organisms are called anaerobes.
Anaerobic Respiration Definition: The respiration that involves incomplete oxidation of respiratory substrate (glucose) present in the cells of anaerobic organisms, in the presence of oxygen-containing inorganic substances (NO3–, CO32– etc.,) resulting in the release of carbon dioxide and less amount of energy (ATP) is known as anaerobic respiration.
“glycolysis, Krebs cycle, and electron transport chain notes”
Anaerobic Respiration Site:
- Anaerobic respiration is mainly found in microorganisms such as anaerobic bacteria, Monocystis, yeast, etc. This type of respiration is also found in certain cells of higher plants such as, in potato tuber.
- It mainly takes place in the cytoplasm and mesosome (a cellular structure made up of cell membranes).
- In higher groups of organisms, aerobic respiration occurs when a comparatively very small amount of oxygen is made available to the cell or when oxidation of substrates does not occur due to the absence of mitochondria.
Overall Reaction

Anaerobic Respiration Summary:
Anaerobic respiration is completed in two phases:
- Glycolysis, and
- Incomplete oxidation of pyruvic acid.
Experiment to demonstrate anaerobic respiration
Anaerobic respiration can be demonstrated with the help of a simple experiment using germinating gram seeds.
Materials required: Deep Petri dish, test tube, bent forceps, mercury, KOH pellets, germinating gram seeds
Anaerobic Respiration Procedure:
- Seed coats of the germinating seeds are removed carefully. The deep Petri dish is half-filled with mercury. Then the test tube is also filled with mercury up to 5/6th part and the remaining portion is filled with peeled germinating seeds.
- Now, the mouth of the test tube is closed with the thumb and then inverted vertically over the mercury-filled Petri dish. As the seeds are lighter than mercury they float over mercury tov/ards the closed end of the inverted test tube.
- The thumb is then removed carefully. The whole setup is then kept undisturbed for half an hour.
- Observations are recorded after half an hour and then KOH pellets are introduced into the test tube with a pair of bent forceps. KOH pellets rise upwards to the closed end of the tube. A final observation is made after 5-10 minutes.
“respiration in plants NEET notes with key points”
Anaerobic Respiration Observations:
- An initial observation is made after half an hour. It is seen that the level of the mercury has displaced downwards due to the collection of some gas towards the upper end of the test tube.
- But, after the introduction of KOH pellets in the test tube, the level of mercury is raised again and the tube is filled with mercury.
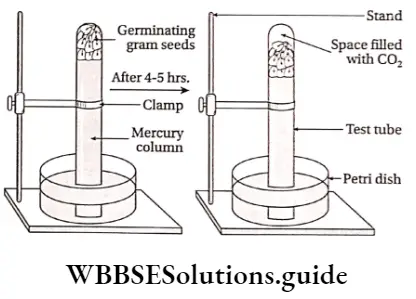
Inference and explanation: The test tube is completely filled with mercury and peeled germinating seeds at the beginning of the experiment. So, there is no air left in the tube. The germinating seeds thus are allowed to respire anaerobically.
- During anaerobic respiration, seeds release a gas which is collected at the top of the test tube by downward displacement of mercury.
- This gas is obviously CO2 as it is absorbed by KOH, which again results in the rise of the mercury column in the test tube.
- Thus it is inferred that anaerobic respiration takes place in germinating gram seeds and CO2 gas is evolved during this process.
Anaerobic Respiration Precaution:
- The seed coats should be peeled carefully.
- Mercury should be handled carefully.
- The mouth of the test tube should be smooth.
- The amount of mercury in the Petri dish should be sufficient enough so that no air enters the test tube while it is inverted.
- KOH pellets should not be touched with hands. They should be always handled with forceps.
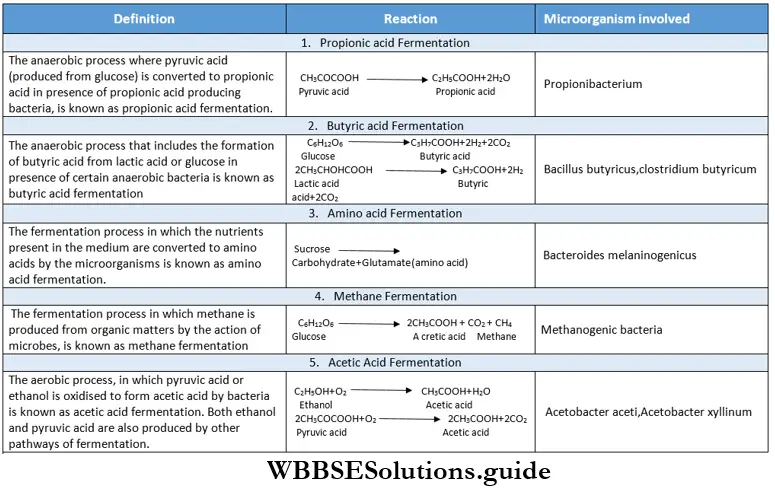
Fermentation
Fermentation is a type of anaerobic respiration that occurs in anaerobes. The term fermentation originated from the Latin word fermentum which means ‘to boil’.
Fermentation Definition: The process in which incomplete oxidation of respiratory substrate occurs in the absence of oxygen and in which certain organic compounds are produced resulting in the release of a low amount of energy, is known as fermentation.
It is a process in which the organic compounds are chemically changed, in the absence of oxygen, by microorganisms. Louis Pasteur first described the concept of fermentation in yeasts.
Fermentation Site: Fermentation occurs in microorganisms such as bacteria, yeast, etc. It takes place in specific body cells of higher organisms.
Characteristics of fermentation:
- Fermentation is an intracellular process.
- This is an enzyme-dependent process. For example, Zymase is required for ethyl alcohol fermentation, and lactate dehydrogenase is required in lactic acid fermentation.
- The process of fermentation produces certain organic compounds, such as ethyl alcohol, lactic acid, butyric acid, propanoic acid, etc.
- This process does not involve the electron transport system.
- In this process, organic compounds act as electron donors as well as the ultimate electron acceptor.
Classification of fermentation:
Depending on the end products, fermentation can be divided into the following types—
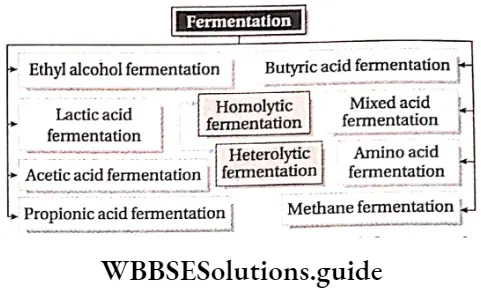
Among all these types, ethyl alcohol fermentation and lactic acid fermentation are the most important.
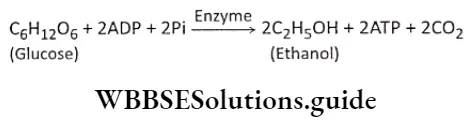
Ethyl alcohol fermentation or alcoholic fermentation: The fermentation process where glucose is partially oxidized by the action of cytosolic enzymes in the absence of oxygen to produce CO2, ethyl alcohol, and a low amount of heat energy, is known as ethyl alcohol fermentation or alcoholic fermentation. This process is observed in Saccharomyces cerevisiae (yeast).
The reaction of ethyl alcohol fermentation is:
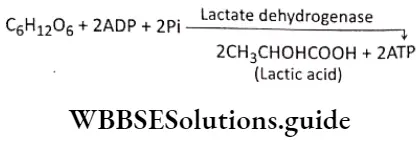
Lactive acid fermentation: Lactic acid fermentation is a metabolic process in which glucose and other six-carbon sugars are partially oxidized by the enzyme present in the cytosol, in the absence of oxygen, to form lactic acid or lactate and to generate ATP. It occurs in some bacteria (Lactobacillus sp., Lactococcus sp., etc.), and muscle cells of animals. The reaction of lactic acid fermentation is-
Fermentation Summary:
Fermentation is completed in two phases:
- Glycolysis, and
- Anaerobic oxidation of pyruvic acid.
Advantages and disadvantages of fermentation
The process of fermentation has both advantages and disadvantages.
Fermentation Advantages:
- In microorganisms, the fermentation process is the only way to get energy from food. Their cellular metabolism depends on fermentation.
- The process of fermentation is used for preparing bread, cakes, wine, and other alcoholic drinks.
- Fermentation is also used to produce vinegar.
- Fermentation plays an important role in the tanning and curing of leather.
- It is also used to induce fragrance in tea and tobacco.
Fermentation Disadvantages:
- A small amount of energy is released by this process (net gain is 2 molecules of ATP per glucose molecule). This small amount of energy is sufficient for small organisms such as bacteria, yeasts, etc. However, this small amount of energy is not sufficient for the larger organisms.
- After excessive exercise, a large amount of lactic acid is produced in the muscles by fermentation. Muscles become fatigued due to the accumulation of lactic acid in them.
- As a result of incomplete oxidation, the end products of fermentation also contain some amount of static energy which is not used for the metabolic activities of the cell.
- Certain toxic compounds are often formed in our food due to fermentation.
” respiration in plants class 11 handwritten notes”
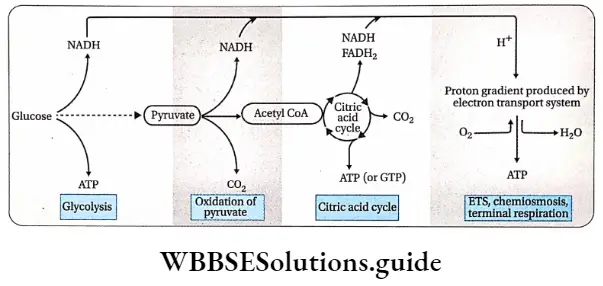
Respiration In Plants Mechanism Of Cellular Respiration
Glucose is the primary respiratory substrate for any living cell. Complete oxidation of glucose occurs by several biochemical reactions, during respiration (aerobic). Many intermediate substances are formed during this process.
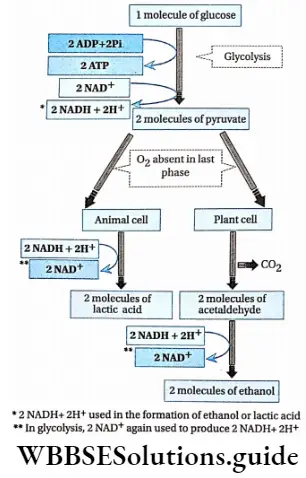
Oxidation of glucose is completed in four steps:
- Glycolysis,
- Fate of pyruvic acid (anaerobic and oxidative decarboxylation of pyruvate),
- Krebs’ cycle,
- Terminal respiration and electron transport system.
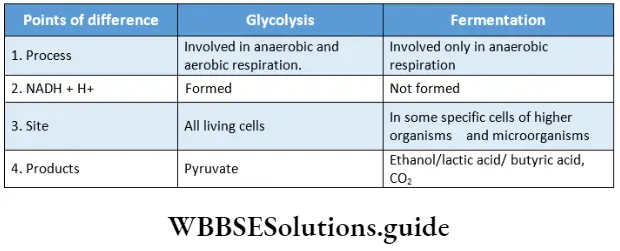
Respiration In Plants Glycolysis
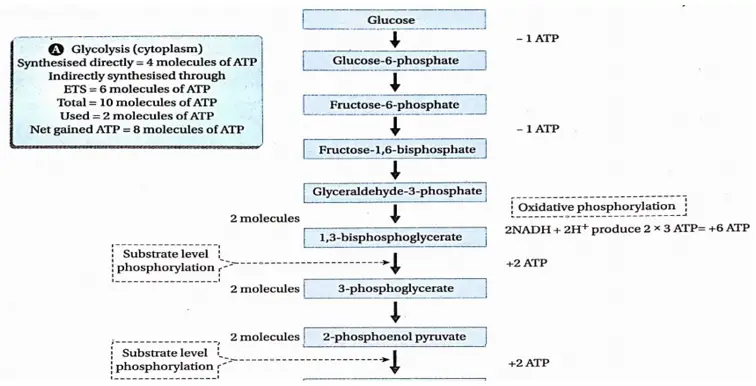
The term ‘glycolysis’ (glycols = sugar, lysis = breakdown) is generally used to refer to the dissolution of sugar. It is nearly, a universal pathway in all biological systems. This pathway was discovered by Gustav Embden, Otto Meyerhof, and J. Parnas in 1930. So, this pathway is also known as Embden-Meyerhof-Parnas (EMP) pathway.
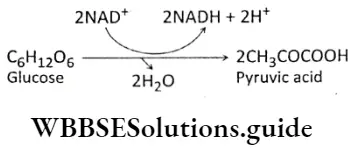
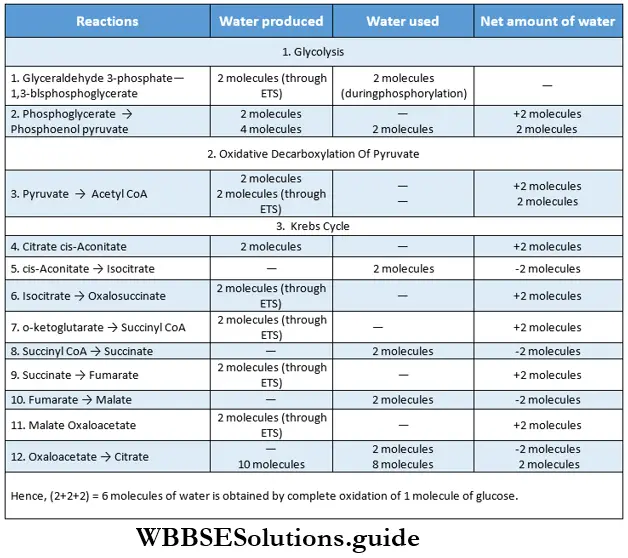
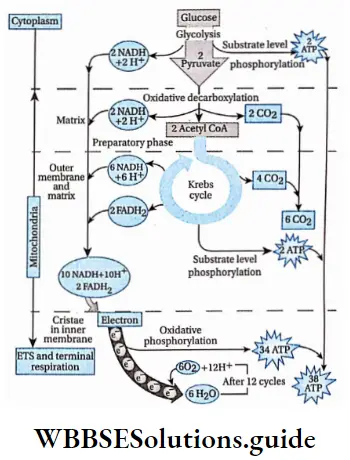
Glycolysis Definition: Glycolysis is the biochemical process, in which glucose is oxidized to pyruvic acid without the presence of free oxygen within the cell cytoplasm and produces 2 molecules of ATP, 2 molecules of NADH+H+, and 2 molecules of water.
This is the first phase of cellular respiration. Glycolysis includes a sequence of reactions that converts glucose into pyruvate along with the production of ATP. In aerobes, glycolysis is the initiating phase of the citric acid cycle and the electron transport chain. In the case of anaerobes, it is the only energy-yielding phase.
Glycolysis Site: Glycolysis occurs in the cytoplasm of an aerobe or anaerobe. This is because the required for this process are present in the cytoplasm.
Overall reaction
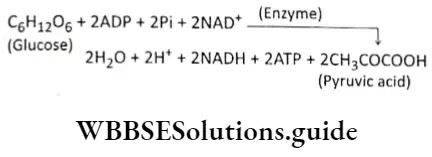
Glycolysis Components: Glucose, hexokinase, isomerase, phosphofructokinase, aldolase, dehydrogenase, kinase, mutase, etc., and cofactors such as NAD+, Mg2+, ADP, ATP
Glycolysis Characteristics:
- The glycolytic pathway does not require oxygen (anaerobic condition).
- Pyruvate is formed as the end product. In anaerobic conditions, pyruvate moves to mitochondria to participate in the citric acid cycle. Under anaerobic conditions, pyruvate is converted to other organic compounds in the cytoplasm only.
- Generally, this pathway is an emergency energy-yielding pathway for cells in the absence of oxygen.
- Here,1 molecule of glucose converts into 2 molecules of pyruvic acid (pyruvate). CO2 is not produced at all.
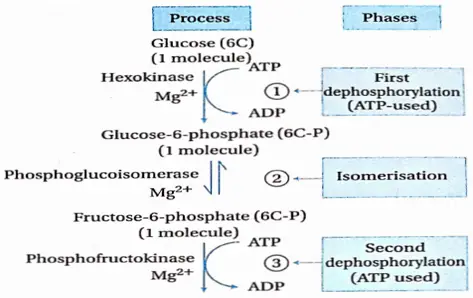
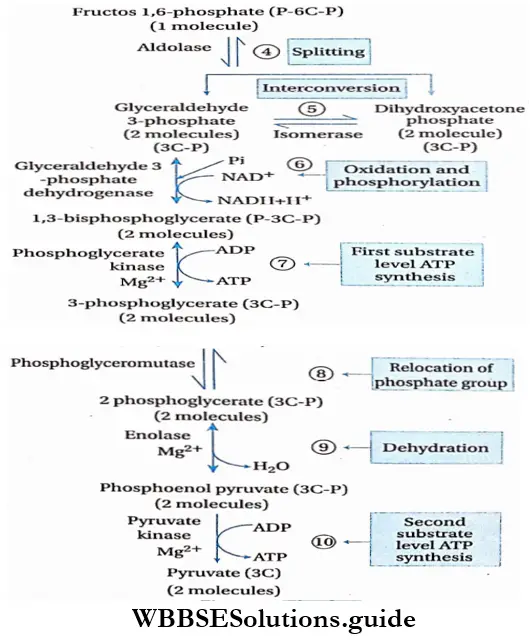
Glycolysis Process: Glycolysis takes place in two phases. In the first phase, glucose is enzymatically phosphorylated by ATP and ultimately cleaved to yield 2 molecules of glyceraldehyde 3-phosphate (GAP).
This is an energy investment phase. In the second phase of glycolysis, the GAP is oxidized to form 1, 3-bisphosphoglycerate. it is then converted into 3-PGA and ATP. 3-phosphoglyceric acid (PGA) is then converted to 2-PGA which after dehydration yields phosphoenol pyruvate (PEP).
chapter 14 biology class 11 notes
It donates its phosphate group to ADP to form ATP and produces free pyruvic acid as the final product. Both processes are energy-producing phases.
Energy investment phase: 2 ATP molecules are required in five steps.
The steps are discussed below:
First phosphorylation: This step requires enzyme hexokinase, Mg2+, and ATP. This step includes the phosphorylation of glucose at the sixth position by ATP to yield glucose 6-phosphate. This is an irreversible reaction and the first phosphorylation of glycolysis.
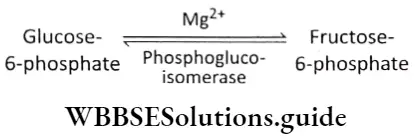
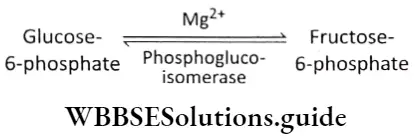
Isomerization: The next step in glycolysis is the isomerization of glucose-6-phosphate to fructose-6-phosphate. The reaction is catalyzed by phosphoglucoisomerase. It is a reversible reaction.
Second phosphorylation: In this reaction, a second molecule of ATP is required to phosphorylate fructose-6-phosphate in its 1-position to yield fructose-1, 6-bisphosphate. This reaction is catalyzed by 6-phosphofructokinase. It requires Mg2+. It is also an irreversible step.

Interconversion of triose phosphates: The two 3-carbon compounds, i.e., DHAP and GAP, are isomers. DHAP is a ketotriose, whereas GAP is an aldotriose. DHAP is converted to GAP by isomerization and it is catalyzed by triosephosphate isomerase. This reaction is very fast and reversible.

Energy-producing phase: This phase includes the oxidoreduction steps as well as the phosphorylation step. In this step, ATP is generated from ADP.
Oxidation and phosphorylation of glyceraldehyde 3-phosphate convert to 1,3-bisphosphoglycerate (BPG) reacting with inorganic phosphate(pi) and NAD. The enzyme catalyzing this reaction is glyceraldehyde 3-phosphate dehydrogenase. NAD+ is also reduced to form NADH+H+. This is a reversible reaction.
⇒ \(\mathrm{GAP}+\mathrm{NAD}^{+}+\mathrm{Pi} \stackrel{{\mathrm{GAP} \\ \text { dehydrogenase }}}{\rightleftharpoons} 1,3-\mathrm{BPG}+\mathrm{NADH}+\mathrm{H}^{+}\)
Glycolysis in RBC of mammals
The following phases of reaction occur in RBCs of mammals instead of first at the use of ATP synthesis in glycolysis
1. 1,3-bisphosphoglycerate is first converted to 2,3-bisphosphoglycerate.
⇒ \(\text { 1,3-bisphosphoglycerate } \stackrel{\text { Enzyme }}{\longrightarrow} \text { 2,3-bisphosphoglycerate }\)
2. 2,3-bisphosphoglycerate is again converted to 3-phosphoglycerate.
⇒ \(\text { 2,3-bisphosphoglycerate } \stackrel{\text { Enzyme }}{-} \underset{\mathrm{Pi}}{\longrightarrow} \text { 3-phosphoglycerate }\)
First substrate level ATP synthesis and formation of 3-phosphoglycerate: 1,3-bisphos phoglycerate converts into 3-phosphoglycerate by the action of phosphoglycerate kinase and Mg2+. In this stage, ATP is formed from ADP and Pi. ATP is formed by first substrate-level phosphorylation. It is also an irreversible process.
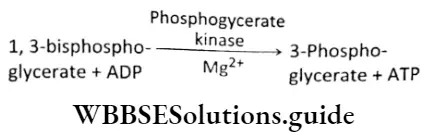
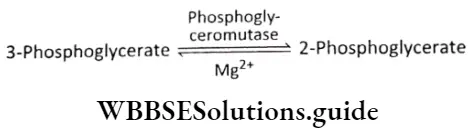
Isomerization or rearrangement: In this reaction, an intramolecular rearrangement or isomerization takes place. The phosphate molecule present at the third carbon atom of the 3-phosphoglycerate shifts to the second carbon atom of the molecule. This change forms 2-phosphoglycerate (2-PGA). This reaction is catalyzed by the enzyme phosphogly- ceromutase.
Dehydration: In this reaction, 2-phosphoglycerate is j dehydrated to form phosphoenol pyruvate (PEP), Enolase catalyzes this reaction. This dehydration reaction markedly elevates the transfer potential of the phosphoryl group. This is also a reversible reaction. One molecule of water is released in this reaction.
⇒ \(\text { 2-Phosphoglycerate } \underset{\mathrm{Mg}^{2+}}{\stackrel{\text { Enolase }}{\rightleftharpoons}} \begin{gathered} \text { Phosphoenol } \\ \text { pyruvate } \end{gathered}+\mathrm{H}_2 \mathrm{O}\)
Second substrate level ATP synthesis and formation of pyruvate: This last reaction involves the formation of pyruvate along with the simultaneous generation of ATP. The transfer of the phosphoryl group from PEP to ADP is catalyzed by pyruvate kinase. This phosphorylation reaction is known as second substrate-level phosphorylation.

The reaction has been found to be irreversible under intracellular conditions. The enzyme requires either Mg2+ or Mn2+ with which it must form a complex before binding the substrate.
Significance of glycolysis:
- This is the common and important glucose metabolism pathway for all the organisms.
- It is the obligatory pathway for carbohydrate breakdown to generate intracellular energy.
- The end product (pyruvate) of this pathway is considered the main substrate for both aerobic and anaerobic respiration. Pyruvate is also converted to acetyl CoA, which is considered the main substrate for the TCA cycle.
- Two molecules of ATP are obtained in this phase of fermentation and 8 molecules of ATP are obtained in this phase of aerobic respiration.
- The NADH+H+ produced during glycolysis is used for different metabolic activities.
- The intermediate products of glycolysis are used for other metabolic activities, such as
- Intermediate product phosphoenolpyruvate (PEP) helps in the synthesis of auxin, tryptophan, phenylalanine, anthocyanin, etc.
- In glycolysis, glycerol produced from fat metabolism, gets converted into dihydroxyacetone phosphate and participates in aerobic respiration, and also produces energy.
- Dihydroxyacetone phosphate produces glycerol during fatty acid metabolism.
Where has the hydrogen gone?
- Glucose contains 12 hydrogen atoms. The pyruvate molecule produced from it contains only 8 hydrogen atoms. What will be the fate of the remaining 4 hydrogen atoms?
- NADH + H+ is produced during the formation of glyceraldehyde 3-phosphate from 1,3-bisphosphate. This phase occurs twice so, 2x(NADH + H+) is produced.
- It is known that 1 hydrogen atom of glucose reduces NAD+ to NADH. The other H+ comes from glyceraldehyde 3-phosphate. Hence, a total of 2 hydrogen atoms from glucose are required in this phase.
- In another reaction of glycolysis, phosphoenolpyruvate is produced from 2-phosphoglycerate. This reaction gives out one molecule of water (dehydration). Hence, one H+ is released.
- This also occurs twice so two H+ are released. Among 12 hydrogen atoms of glucose, 4 hydrogen atoms are used in the above-mentioned reactions. Therefore, a total of 8 hydrogen atoms are found in 2 molecules of pyruvate.
Respiration In Plants Fate Of Pyruvate
The fate of pyruvate differs according to the absence or presence of O2.
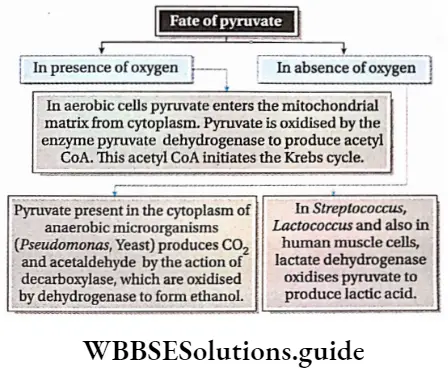
Anaerobic decarboxylation of pyruvate
The mechanism of fermentation resembles that of aerobic respiration up to glycolysis. Pyruvate undergoes decarboxy location without oxygen to yield ethyl alcohol or lactic acid, depending upon the organism and type of tissue.
Alcoholic fermentation: Yeasts can respire both aerobically and anaerobically. If the fungi are not in contact with the atmosphere they respire anaerobically and the pyruvate is oxidized without the presence of oxygen.
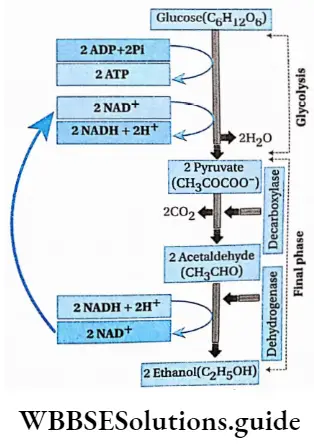
Pyruvate Process:
Pyruvate produced by glycolysis produces acetaldehyde through decarboxylation (removal of CO2). The enzyme involved in this process is pyruvate decarboxylase. It requires Mg2+ and a tightly bound coenzyme thiamine pyrophosphate (TPP) to complete the reaction.
In this step of alcoholic fermentation, the aldehyde is reduced to ethanol by the enzyme alcohol dehydrogenase, which contains Zn2+ ions at its active site. Ethanol and CO2 are thus the end products of alcoholic fermentation.
Why do microorganisms carry out fermentation for a longer period of time?
The intermediate products of fermentation are waste products. The cell excretes these waste products so that they cannot stop the fermentation process. As the wastes are not stored and catabolised within the cells, waste of energy is minimal. The cell density decreases and the fermentation process continues.
The organisms which respire by the process of fermentation, can survive with less amount of ATP how?
Higher organisms require a large amount of energy to carry out their metabolic processes. They get this energy through aerobic respiration. However, the organisms, that carry out fermentation, require less amount of energy to continue their life process. As a result, their respiration process is short and yields less ATP, which is sufficient for their survival.
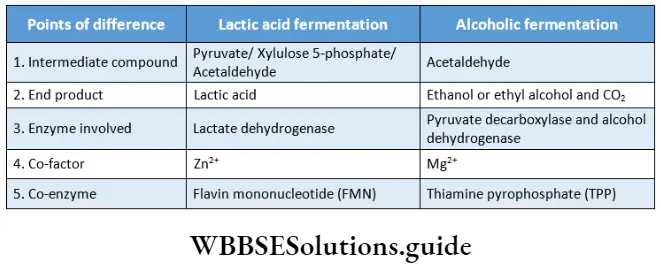
Lactic acid fermentation: The lactic acid bacteria and muscle cells (in the absence of oxygen) respire anaerobically. The pyruvate undergoes decarboxylation to form lactic acid.
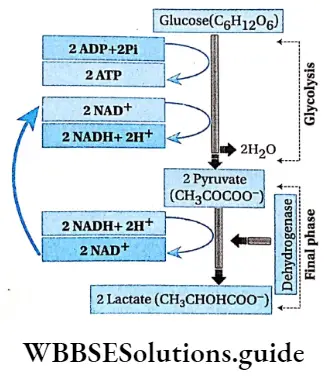
Pyruvate Process:
- One molecule of glucose yields 2 molecules of pyruvate, 2 ATP molecules, 2 NADH+ 2H+, and 2 molecules of water in glycolysis.
- Pyruvate acts as an electron acceptor. Lactic acid is directly produced in the final phase by the reduction of pyruvate, catalyzed by lactate dehydrogenase.
- This reaction requires flavin mononucleotide (FMN) as a coenzyme of the enzyme lactate dehydrogenase and Zn2+ ions. CO2 has not evolved.
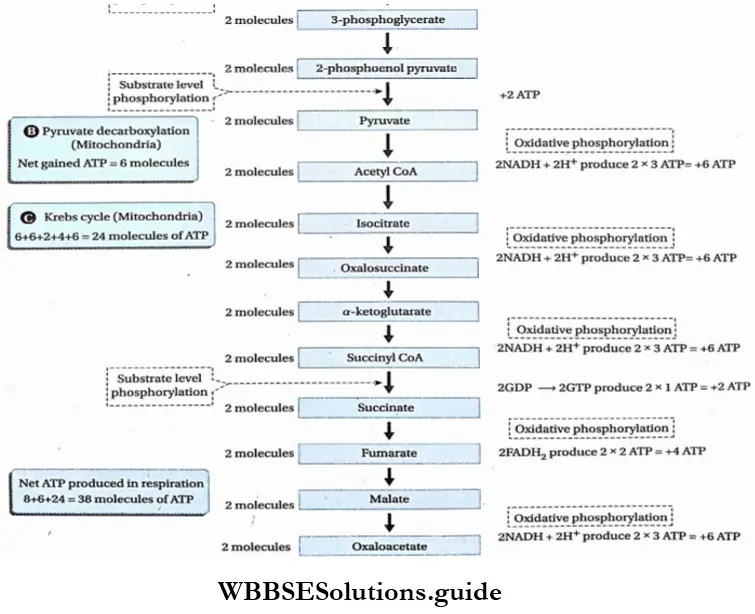
Oxidative decarboxylation of pyruvate
This is the second phase of aerobic respiration. Scientist Lynen (1951) provided a reaction for this oxidative process.
Oxidative decarboxylation of pyruvate Definition: conversion Oxidativeof pyruvate decarboxylation into acetyl-CoAofbypyruvatethe action of the pyruvate dehydrogenase and co-enzyme A, resulting in the formation of NADH++ H+ and release of CO2.
Site: All the reactions of this process occur in the mitochondrial matrix.
Overall reaction:
The overall reaction of oxidative decarboxylation of pyruvate is:
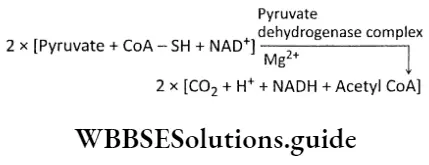
Pyruvate Components: Pyruvate, thiamine pyrophosphate (TPP), lipoic acid, NAD+, FAD, co-enzyme A (CoA-SH), Mg2+, and pyruvate dehydrogenase.
In eukaryotes, the pyruvate dehydrogenase complex is composed of 30 molecules of pyruvate dehydrogenase, 12 molecules of dihydrolipoyl dehydrogenase, and 60 molecules of dihydrolipoyl transacetylase.
Pyruvate Process:
The process of oxidative decarboxylation is described as follows:
1. This step is catalyzed by pyruvate dehydrogenase whose prosthetic group is thiamine pyrophosphate (TPP). Pyruvate undergoes decarboxylation to yield CO2 and the hydroxyethyl derivative of TPP. TPP remains attached to the acetaldehyde, so it is known as active acetaldehyde
⇒ \(\text { Pyruvate }+\mathrm{TPP} \underset{\text { dehydrogenase }}{\stackrel{\text { Pyruvate }}{\longrightarrow}} \text { Active acetaldehyde }+\mathrm{CO}_2\)
2. Active acetaldehyde produces acetyl lipoic acid. The hydroxyethyl group of TPP is oxidized to form an acetyl group. The acetyl group is then transferred to the sulfur atom of lipoic acid and releases TPP by reacting with lipoic acid.
⇒ \(\begin{array}{ll} \text { Active acetaldehyde }+ \text { Lipoic acid } \longrightarrow & \text { TPP+ } \\ \text { (dihydrolipoyl transacetylase) } & \text { Acetyl lipoic acid } \end{array}\)
“difference between respiration in plants and animals”
3. Acetyl lipoic acid reacts with coenzyme A to produce dihydrolipoic acid and acetyl CoA. Acetyl CoA thus formed then leaves the enzyme complex and enters the TCA cycle.
4. The oxidized form of lipoic acid is regenerated by the enzyme dihydrolipoyl dehydrogenase whose reducible prosthetic group is tightly bound to FAD.
5. The resulting FADH2 which remains bound to the enzyme is re-oxidised in the final step by NAD+ with the formation of NADH+ H+.

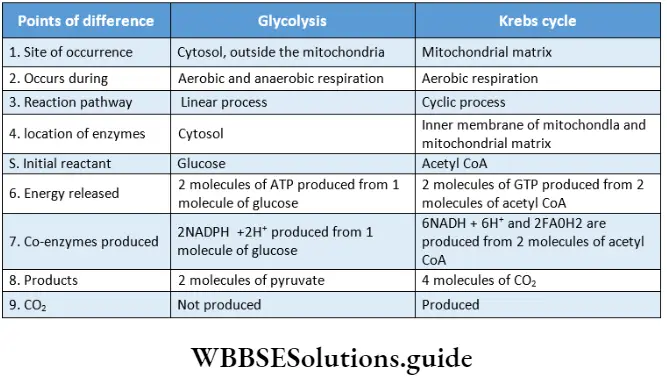
Respiration In Plants Krebs Cycle
This is the third phase of aerobic respiration. This cycle was first described by Sir Hans Adolf Krebs, in 1937. In 1953, he received the Nobel Prize for his work. This cycle is named after him as the Krebs cycle. This cycle is also known as the citric acid cycle as the first product of this cycle is citric acid or citrate.
Krebs Cycle Definition: The Krebs cycle is the cyclic process, occurring in the mitochondrial matrix of living cells, where acetyl CoA is completely oxidized to produce different organic acids, carbon dioxide, water, and ATP with the help of enzymes.
Krebs Cycle Site: This cycle occurs in the mitochondrial matrix of any living cell. The essential enzymes for this cycle are present in the mitochondrial matrix.
Only succinate dehydrogenase is present on the inner side of the inner mitochondrial membrane. Due to this, the step of conversion of succinate to fumarate occurs in the inner mitochondrial membrane.
Overall reaction
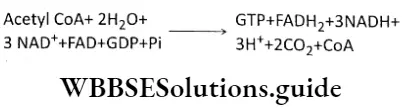
Krebs Cycle Components: Acetyl CoA, GDP, FAD, NAD+, H2O, enzymes.
Characteristics of Krebs cycle:
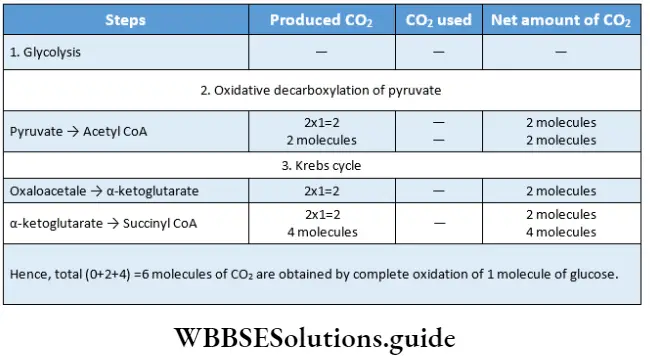
- This cycle includes 10 steps for the complete oxidation of 1 molecule of acetyl CoA.
- Oxidation takes place in four steps. Hydrogen, present in the organic compounds, takes part in the oxidation process. Among all the oxidation steps, CO2 is released in one step. The step is known as oxidative decarboxylation.
- In two steps of this cycle, 1 molecule of CO2 is released.
- This cycle takes 3 molecules of water and releases 1 molecule. So, this cycle requires 2 molecules of water in total.
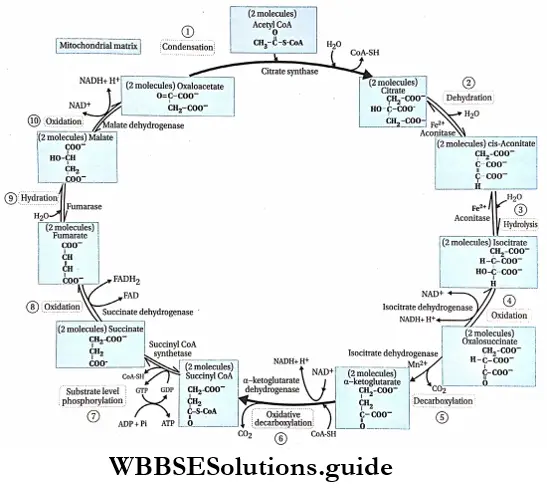
Steps of Krebs cycle
The complete oxidation of acetyl CoA, (which is formed in glycolysis) occurs in the mitochondrial matrix through this cycle.
The steps are given below:
Step 1 Condensation: This initial reaction of the citric acid cycle is catalyzed by citrate synthase. Here, the 4-carbon compound oxaloacetate binds with an acetyl group of 2-carbon compound acetyl CoA to yield the 6-carbon compound citrate, the first tricarboxylic acid intermediate of the cycle. For this reason, the cycle is also known as the tricarboxylic acid cycle or TCA cycle.

Step 2 Dehydration: Citrate must be isomerized to isocitrate to enable the 6-carbon unit to undergo oxidative decarboxylation. The isomerization of the citrate is accomplished by dehydration followed by hydration.
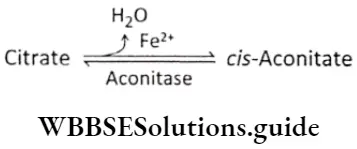
After releasing one molecule of water, citrate is converted to cis-aconitate by the action of the enzyme aconitase. Fe2+ acts as the cofactor. is converted to cis-aconitate by the action of the enzyme aconitase. Fe2+ acts as the cofactor.
Step 3-Hydration:
Now, cis-aconitate undergoes hydration and gets converted to isocitrate. This reaction is also catalyzed by aconitase.
⇒ \(\text { cis-Aconitate }+\mathrm{H}_2 \mathrm{O} \underset{\mathrm{Fe}^{2+}}{\stackrel{\text { Aconitase }}{\longrightarrow}} \text { Isocitrate }\)
Step 4 Oxidation: This is one of the four oxidation-reduction reactions in the TCA cycle. In this step, isocitrate is oxidized to form oxalosuccinate. The oxidation of isocitrate is catalyzed by isocitrate dehydrogenase in the presence of divalent cations J Mg2+/Mn2+ and NAD+.
⇒ \(\begin{aligned} & \text { Isocitrate }+\mathrm{NAD}^{+} \stackrel{\begin{array}{c} \text { Isocitrate } \\ \text { dehydrogenase } \end{array}}{\longrightarrow} \mathrm{NADH}+\mathrm{H}^{+} \\ & + \text {Oxalosuccinate } \\ & \end{aligned}\)
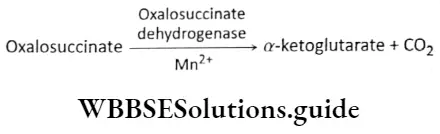
Step 5 Decarboxylation: The decarboxylation of oxalosuccinate produces a 5-carbon compound ar-ketoglutarate and 1 molecule of CO2. This reaction is catalyzed by oxalosuccinate dehydrogenase.
The formation of ar-ketoglutarate involves both oxidation and carboxylation. Hence, both steps 4 and 5 together are known as oxidative decarboxylation.
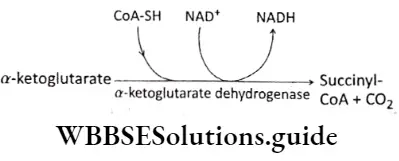
Step 6 Oxidative decarboxylation: The conversion of isocitrate to or-ketoglutarate is followed by a second oxidative decarboxylation reaction, with the formation of 4-carbon compound succinyl CoA from or-ketoglutarate.
The reaction produces another molecule of CO2 and NADH in the citric acid cycle. The reaction is catalyzed by the enzyme orketoglutarate dehydrogenase. This stage is the second decarboxylation stage of the citric acid cycle.
Step 7 Substrate level phosphorylation: Succinyl CoA is converted to succinate by the enzyme succinyl CoA synthetase. One molecule of H2O is H2O required in this reaction. The energy released by this reaction is stored in GDP to form GTP. Next, 1 phosphate from GTP combines with ADP to form ATP. This is the only substrate-level phosphorylation of the TCA cycle.
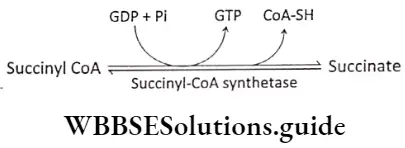
In fact, this is the only reaction in the TCA cycle, that directly yields a high-energy phosphate bond.
Step 8 Oxidation of succinate: Succinate is oxidized to fumarate by the enzyme succinate dehydrogenase, which contains covalently-bound prosthetic group FAD. This enzyme is tightly bound to the inner mitochondrial membrane. FAD accepts hydrogen and is converted to FADH2.
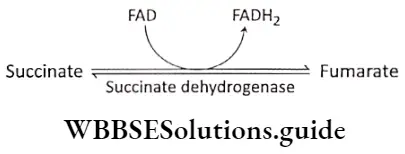
Step 9 Hydration: The next step in the cycle is the hydration of fumarate to malate. Fumarase or fumarate hydratase catalyses this reaction. One molecule of water combines with fumarate in this reaction.
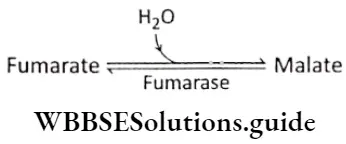
Step 10 Oxidation of malate: In this last reaction of the cycle, the malate dehydrogenase catalyzes the conversion of malate to oxaloacetate. The hydrogen produced in the reaction is accepted by the NAD+ to form NADH+ H+.
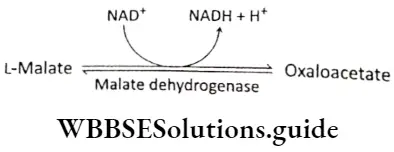
Succinate, fumarate, malate, and oxaloacetate are all 4 carbon compounds.
Different names of the Krebs cycle
- TCA cycle: The first product of the Krebs cycle is citrate and some other products (like aconitate, isocirate, and oxalosuccinate) contain 3 carboxylic groups (-COOH). Hence, this cycle is also known as the tricarboxylic acid cycle (TCA cycle).
- Citric acid cycle: Citrate (citric acid) is the first product of the Krebs cycle, so it is also known as the citric acid cycle.
Significance of Krebs cycle
Krebs cycle plays many important roles in an organism.
Catabolic and energy-generating role:
- The citric acid cycle is a very effective metabolic pathway for the complete conversion of carbohydrates.
- The main purpose of the cycle is to convert potential chemical energy into metabolic energy in the form of ATP.
- This energy is used for various activities- of the living cells,
- Three classes of organic fuels, viz., carbohydrate, lipid, and protein are degraded through the TCA cycle.
- During the process, 2 ATP along with 6 NADH and 2 FADH2 are produced. The energy carriers NADH and FADH2 produced from this cycle help to yield ATP during oxidative phosphorylation.
“mechanism of respiration in plants step by step”
Biosynthetic role:
- The TCA cycle also fuels a variety of biosynthetic processes.
- Several nucleic acids, organic acids, amino acids, keto acids, and other compounds are formed from some intermediate products of this cycle.
- Or-ketoglutarate, succinyl-CoA, fumarate, and oxaloacetate are the precursors of the above-mentioned organic substances.
- A transamination reaction converts a-ketoglutarate directly to glutamate, which can then serve as a common precursor of proline, arginine, and glutamine.
- Oxaloacetate can be transaminated to produce aspartate.
- Aspartic acid itself is a precursor of the pyrimidine nucleotides. It is a key precursor for the synthesis of asparagine, methionine, lysine, threonine, and isoleucine.
- Oxaloacetate can also be decarboxylated to yield PEP, which is a key element for the synthesis of various organic compounds such as— aromatic amino acids phenylalanine, tyrosine, and tryptophan.
- Oxaloacetate also plays an important role in the formation of 3-phosphoglycerate and its conversion to amino acids serine, glycine, and cysteine.
- Succinyl-CoA provides most of the carbon atoms of the porphyrins.
- Succinyl CoA is the precursor for phytochrome, chlorophyll, and cytochrome.
- Acetyl CoA is the precursor for anthocyanin, phenyl yS-cyanin, and fatty acid.
Krebs cycle: a brief overview
- Complete oxidation of 1 molecule of acetyl CoA occurs in one Krebs cycle. So, two Krebs cycles are required for complete oxidation of 2 molecules of acetyl CoA.
- Four oxidative phases (4th, 6th, 8th, and 10th phases) are present in the Krebs cycle.
- The only exothermic reaction occurs in the 7th phase of the Krebs cycle.
- The only exothermic reaction occurs in the 7th phase of the Krebs cycle.
- All the phases of the Krebs cycle, except the 4th phase, occur in the mitochondrial matrix.
- The enzyme succinate dehydrogenase is present in the inner mitochondrial membrane; so, the 4th phase occurs in the inner mitochondrial membrane.
- During the cycle, the citrate molecule loses 2 carbon molecules as carbon dioxide, to become oxaloacetate, which will help to repeat the cycle again.
- The Krebs cycle is responsible for producing about 24 ATP molecules.
- Three molecules of water are used up in the Krebs cycle (1st, 3rd, and 4th places) whereas, only 1 molecule is produced.
Respiration In Plants Phosphorylation
The addition of a high-energy phosphate group to an organic compound or a protein is known as phosphorylation.
Some examples of phosphorylation are:
⇒ \(\mathrm{AMP}+\mathrm{Pi}+\text { Energy } \rightleftharpoons \mathrm{ADP}\)
⇒ \(\mathrm{ADP}+\mathrm{Pi}+\text { Energy } \rightleftharpoons \mathrm{ATP}\)
⇒ \(\text { Glucose + ATP } \rightleftharpoons \text { Glucose 6-Phosphate + ADP }\)
Phosphorylation Types: Three types of phosphorylation are found in respiration.
They are—
Substrate-level phosphorylation: Substrate-level phosphorylation is a type of metabolic reaction that results in the formation of ATP or GTP by the direct transfer and donation of a phosphoryl group (PO32-) to ADP or GDP respectively from a high-energy containing phosphorylate intermediate, in presence of the enzyme.
For example, the excess energy of phosphoenolpyruvate combines with ADP in the form of inorganic phosphate to form ATP.
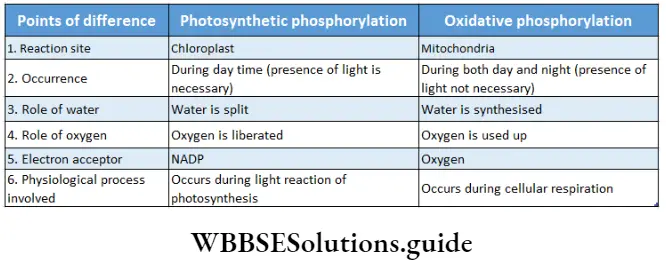
Oxidative phosphorylation: Oxidative phosphorylation is the metabolic pathway in which cells use enzymes to oxidize nutrients to release energy through different carriers of the electron transport system to form ATP in the mitochondria.
The formation of ATP by the energy released during the transportation of electrons through different carriers in the electron transport system is known as oxidative phosphorylation.
Photophosphorylation: In plants during the light reaction, ATP is synthesized from ADP and Pi using the energy of light. This process is called photophosphorylation. This process is described elaborately in chapter 13.
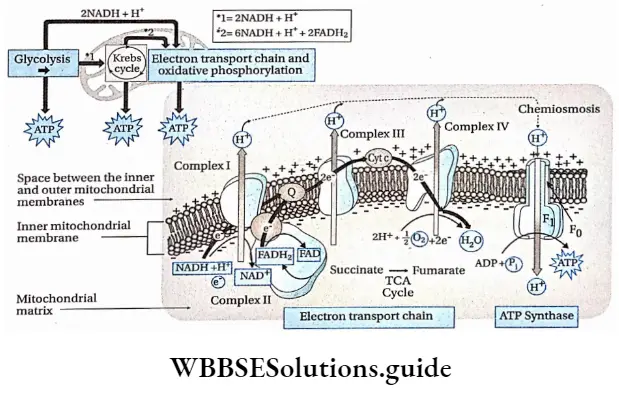
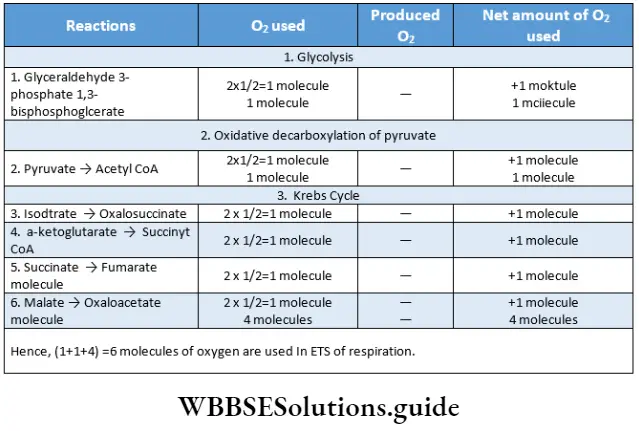
Electron Transport System Or ETS Terminal Respiration
This is the last phase of aerobic respiration. In this phase, NADH+ H+ and FADH2, produced during Krebs cycle and glycolysis, are oxidized.
Electron Transport System Or ETS Terminal Respiration Definition: An electron transport system is a system, that involves a series of electron carriers of coenzymes and cytochromes that help in the transportation of electrons from a reduced substance to its ultimate electron acceptor along with increasing redox potential with loss of energy at each step.
Electron Transport System Or ETS Terminal Respiration Site: In prokaryotic cells, ETS occurs in the cell membrane, and in eukaryotes, it takes place in the inner mitochondrial membrane.
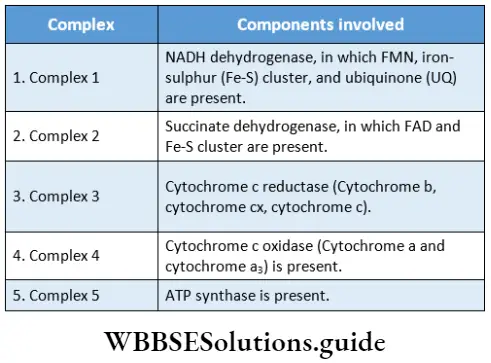
Components: There are several electron carriers, that take part in the electron transport system, present in the inner mitochondrial membrane.
The carriers are:
- NAD+ (nicotinamide adenine dinucleotide),
- FMN (flavin mononucleotide) or FAD (flavin adenine dinucleotide),
- Co-enzyme Q or ubiquinone,
- Cytochrome-b,
- Cytochrome-c,
- Cytochrome-a
- Cytochrome-a3.
According to the modem view of the electron transport system, there are five multi-protein complexes present in the inner mitochondrial membrane.
The overall reaction of ETS and terminal respiration:
ETS and terminal respiration can be represented by the following reactions:
⇒ \(\mathrm{NADH}+\mathrm{H}^{+}+3 \mathrm{ADP}+3 \mathrm{Pi}+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{NAD}^{+}+\mathrm{H}_2 \mathrm{O}+3 \mathrm{ATP}\)
⇒ \(\mathrm{FADH}_2+2 \mathrm{ADP}+2 \mathrm{Pi}+\frac{1}{2} \mathrm{O}_2 \longrightarrow \mathrm{FAD}+\mathrm{H}_2 \mathrm{O}+2 \mathrm{ATP}\)
Characteristics of ETS:
- The complexes are sequentially present in the inner mitochondrial membrane.
- The electron carriers or the hydrogen of the complexes are arranged in the form of a chain.
- Transportation of electrons through the chain of electron carriers occurs like a downhill journey to its more electronegative neighbor (i.e., a gradual decrease in free energy).
- In the end, this electron is accepted by molecular oxygen. Then it combines with hydrogen to form water.
- During electron transport, the electron donor is oxidized and the electron acceptor is reduced. This is known as the redox reaction and it is catalyzed by enzyme reductase. Here, the electron donor and the acceptor are together known as redox pairs.
- In some steps of the electron transport system, high-energy ATP is produced by the phosphorylation of ADP in the presence of ATP synthase.
Process Of Ets
The process of electron transport in the inner mitochondrial membrane involves
The sequential occurrence of the following events:
1. Complex-1 oxidizes the NADH which is generated in the cytosol and mitochondrial matrix by the citric acid cycle. NADH donates 2 protons and 2 electrons to the flavin mononucleotide (FMN) to form FMNH2 Now, FMN releases the protons out of- the mitochondria and transfers electrons to ubiquinone via Fe-S molecules.
⇒ \(\mathrm{NADH} \longrightarrow \mathrm{NAD}+\mathrm{H}^{+}+\mathrm{e}^{-}\)
2. During the oxidation of succinate to fumarate, FAD reduces to form FADH2 in complex II. In the next step electrons donated by FADH2, are also transferred to ubiquinone directly through the Fe-S cluster.
⇒ \(\mathrm{FADH}_2 \longrightarrow \mathrm{FAD}+2 \mathrm{H}^{+}+2 \mathrm{e}^{-}\)
3. Ubiquinone gets reduced by accepting electrons from complex I and complex 2. Reduced ubiquinone then transfers electrons to complex 3.
4. In complex 3, cytochrome b accepts the electron from reduced ubiquinone and transfers the electron to cytochrome c via cytochrome cx. Complex III also releases the protons to the outer chamber of the mitochondria.
5. In complex 4, cytochrome a and a3 accept electrons from complex 3. The electrons are passed to oxygen through cytochrome a3. Now, O2, two electrons, and two protons combine to form one molecule of metabolic water.
⇒ \(\mathrm{H}_2+\frac{1}{2} \mathrm{O}_2+2 \mathrm{e}^{-} \longrightarrow \mathrm{H}_2 \mathrm{O}\)
Molecular O2 is required in this last phase and the reaction is known as terminal respiration.
Some important facts about ETS
Some important and interesting facts about ETS are:
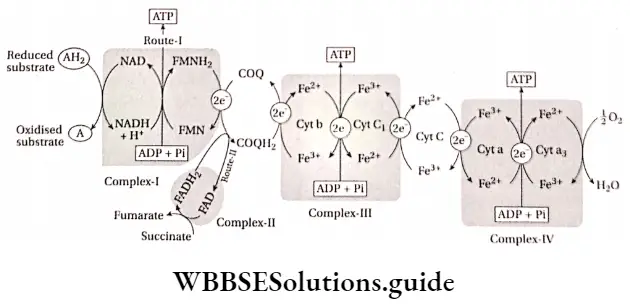
Two routes of ETS: Hydrogen released by the intermediate compounds during respiration is accepted by NAD+ or FAD to form NADH + H+ and FADH2. Electrons from these compounds move to ETS by two routes.
Route 1: Electrons from NADH + H+ move to ubiquinone via FMN
Route 2: Electrons from FADH2 directly move to ubiquinone.
Due to the above-mentioned routes of the electron transport system, FMN is known as the first electron carrier of ETS, and FAD is known as the second electron carrier of ETS. Now, these electrons are transported to cytochrome. In the end, these electrons combine with oxygen in the environment.
ATP production in different phases of ETS
Route 1: Three molecules of ATP are produced in this route.
The steps are:
⇒ \(\mathrm{NAD}^{+} \rightarrow \mathrm{FMN}\)
⇒ \(\text { Cytochrome b } \longrightarrow \text { Cytochrome } c_1\)
⇒ \(\text { Cytochrome a } \longrightarrow \text { Cytochrome } \mathrm{a}_3\)
Route 2: Two molecules of ATP are produced in this route. The steps are—
⇒ \(\text { Cytochrome b } \rightarrow \text { Cytochrome } c_1\)
⇒ \(\text { Cytochrome a } \longrightarrow \text { Cytochrome } \mathrm{a}_3\)
Energy is released in different steps:
- 9.3 kcal energy is released during the transfer of electrons from NAD+ to FMN.
- 8.3 kcal energy is released during the transfer of electrons from cytochrome b to cytochrome c.
- 24.4 kcal energy is released during the transfer of electrons from cytochrome a to cytochrome a3.
Relation between ETS and cellular respiration:
- NADH+H+ and FADH2 are produced in glycolysis and the Krebs cycle undergoes oxidation in ETS. These oxidized forms, again act as oxidizing agents in glycolysis and Krebs cycle.
- Three molecules of ATP are formed from one molecule of NADH+H+ and two molecules of ATP are produced from 1 molecule of FADH2 in ETS during the oxidation phase.
- The molecular oxygen produces water by accepting protons and electrons.
“short notes on respiration in plants for quick revision”
Production of 3 ATP from NADH+H+ and 2 ATP from FADH2:
- Electrons released from NADH+Hf activate the first proton pump, Complex 1 (NADH dehydrogenase). Later, Complex 3 and Complex 4 are also activated by electrons.
- In each of the steps, 1 molecule of ATP is produced. Hence, electrons released from NADH+ produce 3 ATP by activating 3 proton pumps.
- The electrons released from FADH2 activate Complex 3 via ubiquinone. The electrons also activate the Complex 4. So, here 2 ATP are produced by activating two proton pumps.
Oxidative phosphorylation:
In cellular respiration, energy is conserved by NADH + H+ and FADH2 produced in glycolysis and Krebs cycle. Electrons, released during ETS are transferred to molecular oxygen through NADH+H+ and FADH2. Molecular oxygen produces water by accepting the electron. As a result, NADH+H+ and FADH2 are oxidized.
The free energy, produced during this reaction, helps in the synthesis of ATP, with the help of ATP synthase present in the FI portion of the oxysome. This coupling of ATP synthesis to NADH/FADH2 oxidation is known as oxidative phosphorylation. So, ETS is also known as oxidative phosphorylation.
Relation between ETS and proton pump
After releasing the single electron, the nucleus of the hydrogen atom contains only one proton. This proton acts as a proton pump. In ETS, the proton moves in the opposite direction of the proton pump due to the effect of ATP synthase which produces ATP.
⇒ \(\mathrm{H}-\mathrm{e}^{-} \longrightarrow \mathrm{H}^{+} \text {(proton) }\)
Two protons (2Hf) are required for the formation of molecules of ATP.
Oxidative phosphorylation in ETS
The proton flow and the enzyme ATP synthase of the inner mitochondrial membrane play an important role in oxidative phosphorylation.
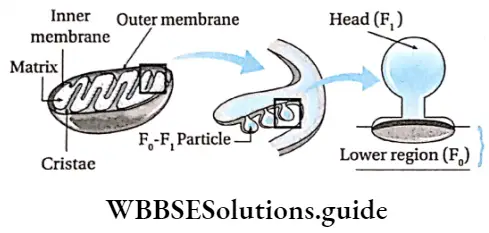
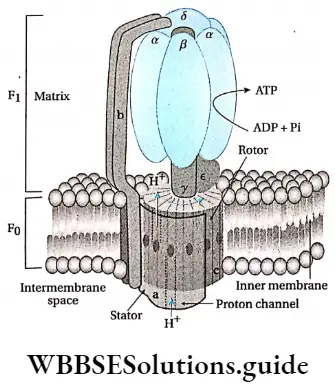
Structure F0-F1 and ATP synthesis:
- There are several folds, known as cristae found in the inner mitochondrial membrane.
- These are present in the mitochondrial matrix. The cristae bear many tennis-racket-like structures known as F0-F1 particles Fernandes-Moran subunits or oxysomes.
- The lower portion of the F0-F1 particle remains embedded in the inner mitochondrial membrane.
- The Fx particle, (head) remains attached to the F0 particle through a stalk and projects inside the matrix.
- The electron carriers of ETS are present in the lower portion of the F0-F1 particle. The enzyme ATP synthase is present in this particle. So, this article is also known as F0-F1 ATP synthase.
- The F portion of this article is composed of five subunits, their ratio is 3α: 3β: lγ: 1δ: l∈. α and β subunits form a cylindrical structure and the y subunit acts as the rotor in the middle of the cylinder, The y and e subunits are present at the stalk region of the complex. F0 is composed of three subunits, viz., a, b, and c. Their ratio is la: 2b: 12c. Subunit ‘c’ contains the proton channel.
Proton flow: During the transportation of electrons through the electron carriers present in the F0 region of the F0-F1 particle, protons move from the mitochondrial matrix to the outer chamber.
A pH gradient is generated, due to this condition, in the inner mitochondrial membrane with a greater concentration of protons in the outer chamber than in the matrix. The difference in concentration of protons across the inner mitochondrial membrane is called proton gradient. This proton gradient generates the proton motive force.
Chemiosmotic Theory
Peter D. Mitchell proposed this chemiosmotic theory in 1961. The theory suggests, that most ATP synthesis in respiring cells takes place due to the electrochemical (proton) gradient across the inner mitochondrial membranes. This process uses the energy of the proton motive force formed by the oxidation of energy-rich molecules.
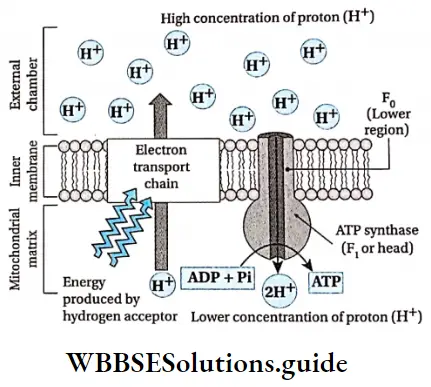
Explanation:
- In respiration, besides the transportation of electrons, protons are also pumped across the membrane (i.e., between the inner and outer membrane at the region of cristae). This causes a change in pH and electrochemical gradient in the matrix.
- To balance this condition, protons are again sent back to the matrix by the proton motive force generated in the outer chamber. The protons move into the matrix through the F1 region of the ATP synthase. This process is known as chemiosmosis. As the protons move through the F0-F1 particle, so it is also known as a proton channel.
- This process stimulates the synthesis of ATP from ADP and Pi. Here, ATP is synthesized by the effect of the proton gradient and the process of chemiosmosis.
Its Inhibitors
The electron transport system can be inhibited by various chemicals.
Cyanide: Prevents transportation of electrons from cytochrome a3 to oxygen.
Carbon monoxide: Prevents transportation of electrons from cytochrome a3 to oxygen by showing affinity to cytochrome oxidase.
Dinitrophenol: Prevents synthesis of ATP
Antimycin A: Prevents transportation of electrons from cytochrome b to cytochrome c1.
Significance of terminal respiration
The significances of terminal respiration are:
- It is an exergonic process.
- The energy released by electrons, flowing through the ETS, is used to transport protons across the inner mitochondrial membrane, by a process called chemiosmosis.
- This generates potential energy in the form of a pH gradient and an electrical potential across the inner mitochondrial membrane. Protons are allowed to flow back across the membrane and down this gradient, through the enzyme called ATP synthase. It is present in the inner mitochondrial membrane.
- This enzyme uses this energy to generate ATP from ADP and inorganic phosphate, by a phosphorylation reaction.
- In this process, oxygen terminally accepts the electrical and is reduced to water. If this step does not occur, the entire flow of the reaction will stop and the respiratory process will no longer take place.
Respiration In Plants Energy Relation In Respiration
- Adenosine triphosphate (ATP) is a highly energized molecule. Energy remains stored in the form of ATP in the living cells which supplies energy for its physical activities.
- An inorganic phosphate combines with adenosine monophosphate (AMP) to form adenosine diphosphate (ADP). Again, another inorganic phosphate combines with ADP to form adenosine triphosphate (ATP).
- An important part of ATP is the three phosphate groups. The last phosphate group remains attached to the ADP through the energy-rich bond.
- This gets hydrolyzed in the presence of water and releases 8.15 kcal energy. This reaction is catalyzed by the ATPase enzyme.
⇒ \(\mathrm{ATP}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{ADP}+\mathrm{Pi}+8.15 \mathrm{kcal}\)
Respiration In Plants Energy Currency
Commonly, currency is a form of money, used actually as a medium of exchange. It is used as a basis of trade and is circulated within an economy. Similarly, ATP plays an important role as a currency that circulates energy in the ce||. |t also acts as a medium of energy exchange.
The role of ATP as energy currency is as follows:
- Adenosine triphosphate (ATP), a nucleotide composed of adenine, ribose, and three phosphate groups, is perhaps the most important energy-rich compound in a cell. It acts as an energy pool in the cells.
- Energy is released by the breakdown of the ATP molecules and they are able to supply energy for biochemical processes that require energy. So, ATP is best named as energy currency.
- The main function of ATP is to form a connection between cellular respiration and the amount of energy required by the cell.
- ATP supplies energy for various physiological processes such as muscle contraction, respiration, nerve function, protein contraction, respiration, nerve function, protein synthesis, active absorption of cells, etc
- Hydrolysis of ATP releases energy and produces ADP and inorganic phosphate. This released energy is used up by living organisms. In turn, ATP is again produced during respiration to restore the energy currency of cells.
Respiration In Plants Respiration And Energy
Calculations of energy generated during different types of respiration have been discussed below.
Aerobic respiration: In aerobic respiration, the complete oxidation of 1 molecule of glucose produces 686 kcal or 2870 kJ energy (1 kcal=4.18 kJ). Some amount of this energy is stored in the ATP as chemical energy. The rest of the energy is released as heat energy.
- The energy, released due to the breakdown of ATP, is used for various biological processes in the cell. In addition to the typical life processes, free energy is also utilized in muscle relaxation and contraction, emission of light in fire-fly, electric current in electric-ray fish, etc.
- The complete oxidation of one molecule of glucose produces 38 ATP molecules. For the synthesis of 1 molecule of ATP, 8.15 kcal or 34 kJ is required.
- Hence, 38×8.15 kcal = 309.7 kcal energy is required for the synthesis of 38 molecules of ATP. So, it can be assumed that, out of the 686 kcal energy of 1 molecule of glucose, 309.7 kcal energy is used for the synthesis of 38 ATP molecules.
- Rest 686-309.7 = 376.3 kcal or 1569 kJ energy is released as heat energy. So, 45% of the total energy is used up for the synthesis of ATP and 55% is released as heat energy. So, the efficiency of aerobic respiration is 45% only.
Anaerobic respiration: In anaerobic respiration, 50 kcal or 210 kJ energy is produced from 1 molecule of glucose. Two molecules of ATP are synthesized from 16.3 kcal, which is 33% of this energy. The rest of the energy (67%) is released as heat. Thus, the efficiency of anaerobic respiration is 33%.
Fermentation: Two molecules of ATP are produced in the fermentation process. So, 2×8.15kcal = 16.3 kcal or 68 kJ energy remains bound with 2 ATP molecules. The efficiency of fermentation is equal to that of anaerobic respiration.
Respiration In Plants Respiratory Balance Sheet
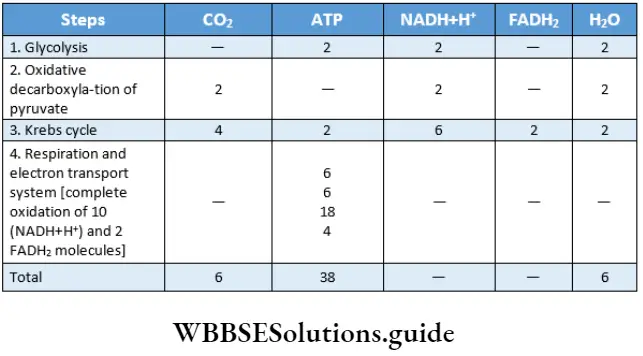
The components formed by complete oxidation of 1 molecule of glucose in aerobic respiration, are 38 molecules of ATP, 6 molecules of CO2, and 6 molecules of water. The balance sheet of ATP produced and consumed in the process of respiration is given below.
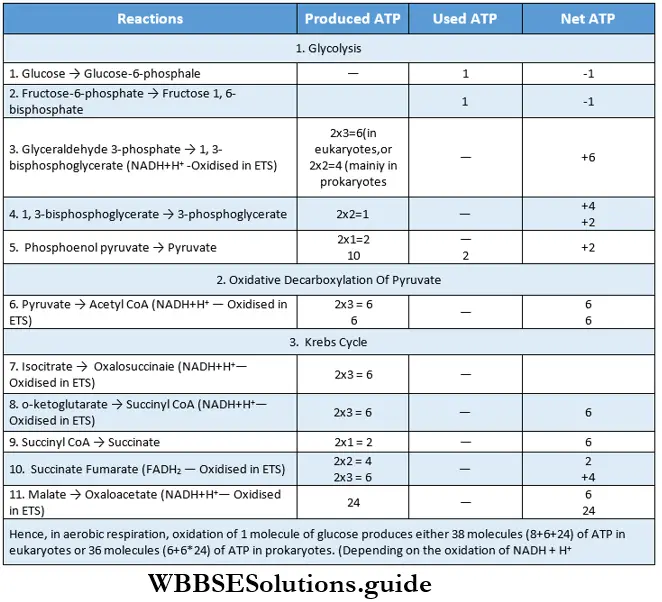
Respiration In Plants Aps Production In Glycolytic Phase Of Anaerobic And Aerobic Respiration
The number of ATP varies in the glycolytic phase of anaerobic and aerobic respiration. The aerobic glycolysis produces more ATP than the anaerobic process.
Two molecules of ATP are produced in the glycolysis phase of anaerobic respiration or fermentation: During fermentation, an aerobic process, NADH+H+ is produced in glycolysis. NADH+H+ oxidizes pyruvate to form lactic acid or ethyl alcohol. As a result, oxidative phosphorylation or terminal respiration does not take place in the process of fermentation.
So, in fermentation, ATP is not produced through oxidative phosphorylation. Four molecules of ATP are formed in the process of glycolysis and among them, two molecules are utilized during the same process. So, total ATP produced during fermentation is 4-2 = 2 ATP
Eight molecules of ATP are produced in the glycolysis phase of aerobic respiration: During aferobic respiration, in the glycolysis phase, NADH+H+ is produced by oxidative phosphorylation. During terminal respiration, three ATP are produced by the oxidative photophosphorylation.
This phase occurs twice, so, 3×2 = six molecules of ATP are produced. Four molecules of ATP are produced during glycolysis and two molecules are used by the process. So, the total number of ATPs produced by the glycolysis stage of aerobic respiration is 6+(4-2)=6+2=8.
Respiration In Plants Modern Concept Of ATP Production
Previously it was thought that 38 molecules of ATP are generated during aerobic respiration by oxidation of one molecule of glucose. However, the modern concept of ATP production differs from the earlier knowledge.
- Some protons, through the inner mitochondrial membrane, move to the mitochondrial matrix. These protons are pumped by the ATP synthase present in the inner membrane of mitochondria.
- The proton gradient between cytoplasm and mitochondria is also used for the transport of pyruvate in the mitochondrial matrix. As a result, NADH+H+ is converted to NAD+ providing 2.5 ATP. This number was previously thought of as three ATP, via electron transport system (ETS). On the other hand, FADH2 converted to FAD provides 1.5 ATP. This number was previously thought of as two ATP.
- In aerobic respiration, 5 steps (each step occurs twice) are involved in the production of NAD++e–+e– +H++H+ NADH+H+ So, ATP is produced in 5 steps. = (5×2.5)x2=25 ATP.
- Conversion of FAD —FADH2 occurs only in one step So, ATP produced = (1×1.5)x2=32 ATP.
- Total number of ATP found in substrate level phosphorylation = 6. Among these, 2 molecules of ATP are used during glycolysis. So, net production of ATP according to the modern concept = (25+3+6)-2= 32 ATP.
Respiration In Plants Amphibolic Pathway
The term amphibolic is derived from the Greek word amphi meaning ‘both sides’. This term was proposed by B. Davis (1961). It is used to describe a biochemical pathway that involves both catabolism and anabolism.
Definition: The pathway that includes both an anabolic reaction (to generate metabolic intermediates for biosynthesis) and a catabolic reaction (to generate energy) is known as an amphibolic pathway.
Respiration In Plants’ Anabolic And Catabolic Functions
Reactions, involved in glycolysis and the Krebs cycle, are mainly catabolic in nature. But most of the reactions are reversible in these two processes. As a result, many intermediate compounds are formed which help to produce various intermediate organic compounds necessary for growth through anabolic reactions.
- A given molecule at a given moment may go for a catabolic or anabolic pathway as per a cell’s requirement. However, both pathways cannot occur simultaneously.
- Thus, the respiratory pathways not only disseminate organic compounds and provide energy, but they also provide precursors for the biosynthesis of macromolecules that constitute living systems.
- So, truly, the respiratory pathways are called amphibolic pathways. According to the cell’s need, an enzyme (or enzymes) regulates and determines whether a pathway will function as an anabolic or catabolic pathway.
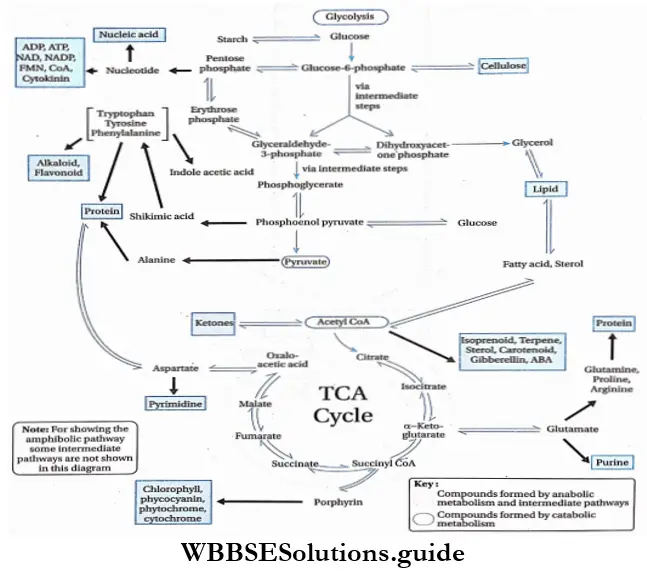
Anabolic functions of the respiratory pathway
The anabolic functions of the respiratory pathway are as follows—
- Acetyl CoA is required for the formation of fatty acids, steroids, and carotenoids.
- Porphyrins are formed from succinyl CoA, which are the major components of chlorophyll and phytochrome.
- Glutamate, which helps in the formation of purines, is formed from a-ketoglutarate.
- Aspartate, which helps in the formation of pyrimidine, is formed from oxaloacetate.
- Alanine produced from pyruvate, helps in protein synthesis.
- Glucose is synthesized from oxaloacetate through gluconeogenesis.
- Starch is produced as a result of reversible reactions in glycolysis. Starch is the stored food, found in plants.
- In glycolysis, cellulose is formed from glucose-6-phosphate. Cellulose is the main structural component of the cell wall of plant cells.
- Nucleotides are formed from glyceraldehyde -3-phosphate through the pentose phosphate pathway. These are required for the formation of nucleic acid.
Catabolic functions of the respiratory pathway
The catabolic functions of the respiratory pathway are as follows
- Glucose is converted to pyruvate in glycolysis and ATP is produced.
- Acetyl CoA and oxaloacetate combine to form citrate. This citrate undergoes various processes and produces oxaloacetate and CO2.
- This pathway acts as an oxidation pathway for sugar, amino acids, and fat.
- Reduced coenzymes are produced in four steps of the Krebs cycle. Another coenzyme is produced during the formation of acetyl CoA from pyruvate.
- This reduced acetyl CoA is produced in the matrix, near the inner mitochondrial membrane. This coenzyme helps in the transfer of electrons and protons in the electron transport system and produces ATP and H2O.
Respiration In Plants Respiratory Quotient Or Nutrients
Respiratory Quotient Or Nutrients Definition: RQ is defined as the ratio of the volume of carbon dioxide (CO2) given off by the organism during respiration, to the volume of oxygen (O2) absorbed at the same time.
Expression of RQ or respiratory quotient: RQ or respiratory quotient is calculated by the following formula
⇒ \(\mathrm{RQ} \text { or Rate of respiration }=\frac{\text { Volume of } \mathrm{CO}_2 \text { released }}{\text { Volume of } \mathrm{O}_2 \text { absorbed }}\)
RQ in cell or tissue differs, according to the chemical nature of respiration and respiratory substrate.
“respiration in plants notes PDF download”
Respiration In Plants Different Types Of Respiratory Substrates And Their QR
Depending on respiratory substrates, RQ is of different values.
RQ of carbohydrates
Carbohydrate is the main respiratory substrate in aerobic respiration and fermentation. In aerobic respiration, carbohydrates (glucose, fructose) are completely oxidized, and partial oxidation of carbohydrates occurs in fermentation.
In aerobic respiration: In this process, the amount of O2 required for complete oxidation of carbohydrates is equal to the amount of CO2 evolved. So, in this case, the value of RQ is 1.
Oxidation reaction and RQ of aerobic respiration is given below
⇒ \(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6+6 \mathrm{O}_2 \rightarrow 6 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}+\text { Energy }\)
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{6 \mathrm{CO}_2}{6 \mathrm{O}_2}=1\)
In fermentation: In this process, O2 is not used for partial oxidation of carbohydrates, but some amount of CO2 is produced. So, in the case of fermentation, the value of RQ is infinite.
The oxidation reaction and RQ of fermentation are given below
⇒ \(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6 \stackrel{\text { Enzyme }}{\longrightarrow} 2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+2 \mathrm{CO}_2+\text { Energy }\)
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{2 \mathrm{CO}_2}{0}=\infty \text { (infinity) }\)
RQ of fats
- Fats also can be used as the respiratory substrate. At first, fat breaks to form fatty acids and glycerol. It mainly occurs during the germination of oilseeds such as mustard seeds, groundnuts, etc.
- Complete oxidation of glycerol will show RQ = 0.7. Fats contain considerably more hydrogen and carbon atoms than oxygen atoms.
- So fatty acids require extra O2 than that is required for carbohydrate metabolism, for complete oxidation. In this process, a low amount of CO2 is produced compared to the amount of oxygen used. In this case, the value of RQ is less than 1.
RQ of glycerol: The reaction of tripalmitin, a triglyceride, also known as glycerol tripalmitate, and RQ is given below:
⇒ \(\begin{aligned} & 2 \mathrm{C}_{51} \mathrm{H}_{98} \mathrm{O}_6+145 \mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} 102 \mathrm{CO}_2+98 \mathrm{H}_2 \mathrm{O}+\text { Energy } \\ & \text { Tripalmitin } \\ & \qquad R Q=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{102 \mathrm{CO}_2}{145 \mathrm{O}_2}=0.7 \end{aligned}\)
RQ of fatty acid: Oxidation reaction and RQ of palmitic acid, a fatty acid is given below—
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{4 \mathrm{CO}_2}{11 \mathrm{O}_2}=0.36\)
RQ of Proteins
Amino acids are produced in those cases, where proteins are used as substrate. Amino acids also have fewer oxygen atoms than hydrogen and carbon atoms. Hence, amino acids need more oxygen for oxidation. The respiratory quotient is hence less than one.
The oxidation reaction and RQ of alanine, an amino acid are given below:
⇒ \( 2 \mathrm{C}_3 \mathrm{H}_7 \mathrm{O}_2 \mathrm{~N}+6 \mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} \mathrm{CO}\left(\mathrm{NH}_2\right)_2+5 \mathrm{CO}_2+5 \mathrm{H}_2 \mathrm{O} Alanine\)
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{5 \mathrm{CO}_2}{6 \mathrm{O}_2}=0.83\)
RQ of organic acids
Organic acids released by succulent plants are rich in oxygen and hence, require a low amount of oxygen for oxidation. More CO2 is produced in this case. Therefore, the respiratory quotient of organic acids is always greater than 1.
Oxygen is not required in aerobic respiration. So, the theoretical respiratory quotient is infinity (∞).
RQ of oxalic acid:
Oxidation reaction and RQ of citric acid is given below
⇒ \( 2(\mathrm{COOH})_2+\mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} 4 \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}+\text { Energy } Oxalic acid\)
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{4 \mathrm{CO}_2}{\mathrm{O}_2}=4\)
RQ of citric Acid: Oxidation reaction and RQ of citric acid is given below—
⇒ \( 2 \mathrm{C}_6 \mathrm{H}_8 \mathrm{O}_7+9 \mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} 12 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}+\text { Energy } Citric acid\)
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{12 \mathrm{CO}_2}{9 \mathrm{O}_2}=1.33\)
RQ of malic acid:
Oxidation reaction and RQ of malic acid is given below:
⇒ \(\mathrm{C}_4 \mathrm{H}_6 \mathrm{O}_5+3 \mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} 4 \mathrm{CO}_2+3 \mathrm{H}_2 \mathrm{O}+\text { Energy }\) Malic acid
RQ of tartaric acid: Oxidation reaction and RQ of tartaric acid is given below—
⇒ \( 2 \mathrm{C}_4 \mathrm{H}_6 \mathrm{O}_6+5 \mathrm{O}_2 \stackrel{\text { Enzyme }}{\longrightarrow} 8 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}+\text { Energy }\) Tartaric acid
⇒ \(\mathrm{RQ}=\frac{\text { Produced } \mathrm{CO}_2}{\text { Consumed } \mathrm{O}_2}=\frac{8 \mathrm{CO}_2}{5 \mathrm{O}_2}=1.6\)
Respiration In Plants Significance of RQ
The significance of RQ is as follows:
- RQ value determines the type of respiration.
- It provides information regarding the respiratory substrate. The chemical nature of the substrate can be determined by the RQ value.
- The RQ value helps to know the type of changes or abnormalities occurring in the body. In the case of acidosis, the rate of respiration increases. So, more amount of CO2 is given out. As a result, the value of the RQ rises. In case of alkalosis, the opposite happens, hence the value of RQ becomes less.
- During exercise RQ rises. This is because lactic acid and CO2 are produced in the body.
- After a long period of fasting RQ will be less than 1. This is because proteins stored in the body are used as a respiratory substrate during fasting.
- In germinating seeds, initially, anaerobic respiration takes place followed by aerobic respiration. Here, the RQ changes, from less than 1 to more than 1.
- In CAM plants, during the night, O2 consumption takes place without the associated CO2 evolution and the RQ value becomes zero or even negative.
Compensation point
- It is the point at which no gaseous exchange is observed between the photosynthetic organ and environment as the rate of photosynthesis and rate of respiration is equal. Compensation point depends on two factors—CO2 and light.
- A compensation point can be reached by a plant at a specific CO2 concentration prevailing in the environment when the plant is illuminated with non-limiting light intensity. The CO2 compensation point of C3 plants is 25-100 ppm and of C4 plants is less than 5 ppm.
- Similarly, at the light-compensation point, the photosynthetic tissue does not show any gaseous exchange at a specific value of light intensity through it receiving non-limiting CO2. Heliophytic plants show light-compensation points at 100-400 ft candles.
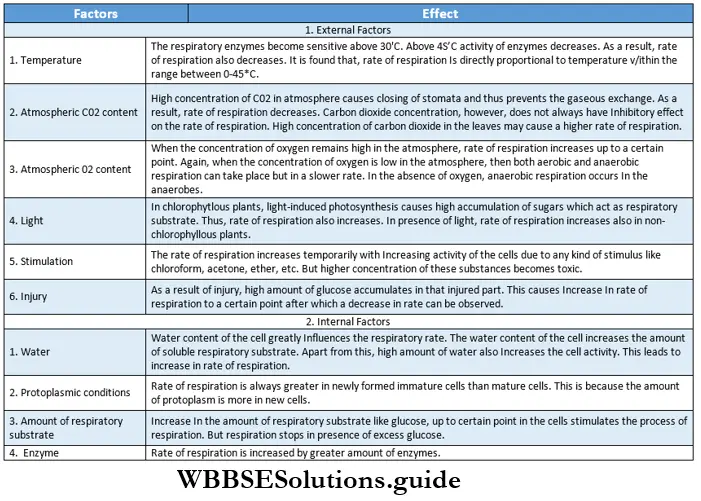
Respiration In Plants Factors Affecting Respiration
Respiration is affected by certain factors. Mainly, factors are of two types:
- External factors—oxygen, light, temperature, carbon dioxide, etc.
- Internal factors— water content of the cell, enzymes, protoplasmic conditions, etc. All the factors are briefly discussed below.
Respiration In Plants Notes
Cytochromes: Iron-containing (heme) proteins that serve as electron carriers in respiration, photosynthesis, and other oxidative reactions.
Electron carriers: Molecules capable of accepting one or two electrons from one molecule and donating them to another in the process of electron transport.
Flavin mononucleotide (FMN): Riboflavin phosphate, a co-enzyme of oxidation-reduction enzyme. Ft-candle or
Foot-candle: A unit of measure of the intensity of light falling on a surface equal to 1 lumen per square foot. Originally it was defined with reference to how bright a standardised candle is burning at one foot away from a given surface.
Heliophyte: A plant that thrives under bright sunlight. They are also called sun-stroke plants. Example Sempervivum tectorum.
Mesosome: An organelle of bacteria that appears as an invagination of the plasma membrane and performs functions like cellular respiration, DNA replication, etc.
Proton motive force: The force that facilitates the transfer of Ft-candle or foot-candle: A unit of measure of the intensity protons or electrons across a membrane downhill the electrochemical potential.
Tripafmitin: A triglyceride derived from the fatty acid called palmitic acid.
Points To Remember
- Respiration is a catabolic process, that occurs in all living cells, where energy is released by oxidation of complex organic substances present in the cell.
- Cellular respiration is the stepwise oxidation of complex organic substances, where O2 is used, and H2O and CO2 are produced along with the release of energy.
- During respiration, energy from the organic substances is released as heat energy. This chemical energy is stored in the ATP as chemical energy and is released in the form of heat.
- Fat is used as fuel in some parts of the plants. For example, fat is used by oil seeds during germination.
- About 40% of the total energy produced by cellular respiration is used for metabolic activities and the rest is released as heat.
- Six molecules of O2 are required for oxidation of 1 gram molecule of glucose. This means, 1 molecule of oxygen is required to generate 114.3 kcal energy.
- Carbohydrate is the respiratory substrate in floating respiration. Protein is the respiratory substrate for cytoplasmic respiration.
- Excess energy released during respiration is later used for various activities such as active transport, cell division, bioluminescence, etc.
- Glycolysis is the common initial step for both aerobic and anaerobic respiration.
- Four phases of aerobic respiration are—glycolysis, oxidative decarboxylation of pyruvate, Krebs cycle, and electron transport chain.
- Glycolysis is an anaerobic process, that occurs in cytoplasm. This process breaks 6-C glucose molecules to produce 2 molecules of pyruvate via 7 intermediate steps. Two ATP and 2 NADH+2H+ are also produced in this process.
- Oxidative decarboxylation of pyruvate occurs in the mitochondrial matrix. Pyruvate produces acetyl CoA by releasing a molecule of CO2. Molecules of NADH + H+ are also produced here.
- Krebs cycle occurs in mitochondria. GTP, CO2, NADH+H+, FADH2 are produced in this phase.
- ATP is known as cellular energy currency. 30.6 kJ energy is by hydrolysis of one molecule of ATP.
- Two high-energy bonds are present in one molecule of ATP—one as a terminal and the other as a carbon phosphate bond.
- Four ATP molecules are produced during glycolysis and 2 ATP molecules are produced during the Krebs cycle by substrate-level phosphorylation.
- Substrate-level phosphorylation occurs in skeletal muscles and the brain. Phosphocreatine donates a phosphate group to ADP and converts it to ATP. Chemical energy is released by ATP as heat energy.
- Thirty-four molecules of ATP are produced in aerobic respiration by oxidative phosphorylation.
- Anaerobic respiration occurs only in the cell cytoplasm
- In aerobic respiration, 38 molecules of ATP are produced by 1 molecule of glucose. The efficiency of aerobic respiration is 45%.
- Mainly there are two types of fermentation—lactic acid fermentation (lactic acid is the end product) and alcoholic fermentation (ethyl alcohol is the end product).
- Lactic acid fermentation is mainly of two types— homolactic and heterolactic.
- In fermentation, 33% of energy is stored in 2 molecules of ATP.
- The ratio of CO2 produced in aerobic and anaerobic respiration is 3:1.
- Only the glycolytic cycle occurs in RBCs due to a lack of mitochondria.
- Through the electron transport chain, 32 or 34 molecules of ATP are produced.
- Cytochrome is an iron-containing electron transport protein. Cytochrome a3 contains both iron and sulfur clusters.
- Co-enzyme Q is known as ubiquinone. It acts as an electron receiver in the electron transport chain (ETS).
- Only highly energized GTP is produced in the Krebs cycle. It is produced by substrate-level phosphorylation.
- Oxidative decarboxylation of pyruvate is known as the gateway of aerobic respiration. It is the connector between glycolysis and the Krebs cycle.
- 80-90% of glucose is metabolized in the Krebs cycle.
- Fat is used during respiration, in the form of fatty acid and glycerol. A fatty acid is converted to acetyl CoA, which takes part in the Krebs cycle. Glycerol is converted to dihydroxyacetone phosphate, which takes part in glycolysis.
- Protein is used during respiration, in the form of amino acids. Unnecessary amino acids release their amino groups by the process of deamination. The rest of the amino acids are converted to acetyl CoA or pyruvate. About 5% of the total energy required for the metabolic activities of the body, is released during glycolysis.
- NADP is known as co-enzyme II and NAD is also known as DPN or co-enzyme I.
- The high rate of respiration, in ripe fruits, is known as climacteric respiration. Some ETS inhibitors are— 2,4-dinitrophenol, cyanide, and antimycin-A.
- Ganong’s respirometer is used to measure the rate of respiration and the value of RQ.
- Irreversible reactions are found in 3 steps of glycolysis—
- Glucose Glucose 6-phosphate,
- Fructose 6-phosphate Fructose 1,6- bisphosphate,
- 2-phosphoenol pyruvate —> Pyruvate.
- In yeast, bacteria, and lower plants, a different pathway is found instead of the Krebs cycle. This pathway is known as the glyoxylic acid cycle and is considered as the bypass of the Krebs cycle.
- Respiration does not occur in the tracheid, trachea, and schlerenchyma, because they do not contain cytoplasm.
- In bacteria, respiration occurs in mesosomes instead of mitochondria. Krebs cycle does not occur in RBCs due to lack of mitochondria.
