Chapter 7 Atomic Nucleus Broad Answer Type Questions
Question Explain the separation of isotopes through the diffusion method.
Answer:
The separation of isotopes through the diffusion method
Though the isotopes have identical chemical properties, some of their physical properties may be quite different because of the difference in their mass numbers.
The most important physical properties of this type are the rate of diffusion, boiling point, and radioactive properties isotopes of elements can be separated by physical methods based on their properties. They may be separated almost completely using a special type of instrument called the mass spectrometer.
Diffusion method:
Since the rate of diffusion of a gas varies inversely to the square root of its molecular mass. Gas molecules with lower atomic mass numbers diffuse more quickly than their heavier sisters.
This principle was very effected actively used by Aston for the separation of isotopes of neon. The more is the difference in the atomic mass numbers, the more effective is the separation.

Question 2. Write the cause of radioactivity.
Answer:
Cause of radioactivity:
The main reason for the radioactivity of an element, as we have noted earlier, is the instability of its nucleus. Such an unstable nucleus tries to attain stability through the expulsion of particles and rays. If there are too many protons in a nucleus they will fall apart and the element will disintegrate. Again, it the nucleus is too heavy with too many neutrons it disintegrates.
“WBBSE Class 10 Physical Science Chapter 7 long answer questions, Atomic Nucleus”
It is observed that in order to attain stability the neutron: Proton ratio must be mear about unity for the elements with low atomic masses while for the heavier elements, it must not exceed 1.5.
Thus we see that most of the radioactive elements have very high atomic masses in 92U234 in comparison to their atomic numbers U has a neutron; proton ratio of 1.537 while radium has 1.56. This is why radium is much more radioactive than uranium.
Question 3. Short Note-Radioactive Equilibrium.
Answer:
Radioactive Equilibrium :
The product element formed by the radioactive disintegration of an element may also be radioactive. The process goes an until the chain is terminated by the birth of a non-radioactive stable element at the end of a series. If we keep a sample of a radioactive element or its salt for a sufficient time, a situation arises when the rate of creation and the rate of disintegration of a product element become equal.
This state for a radioactive series is called a radioactive equilibrium. We see that at the equilibrium.
⇒ \(\frac{\mathrm{dN}_1}{\mathrm{dt}}=\frac{\mathrm{dN}_2}{\mathrm{dt}}=\frac{\mathrm{dN}_3}{\mathrm{dt}}\)
∴ λ1 N1 = λ2 N2 = λ3 N3 = …………
Question 4. Short Note Mass spectrometric method to separate the isotope.
Answer:
Mass spectrometric method:
In this method positive particles of the required gas or vapour formed by bombardment with electrons are first passed through electrical plates with a potential difference (X) of about 1000 volts and two collimating slits. The electrical energy Xe, where e is the charge of the positive particles, is equal to the kinetic energy of the particles.
That is Xe =½ mu2 where m and v are the mass and the charge of the positive particles. The positive particles energing through a slit with almost equal kinetic energy pass a magnetic field (H) between two semicircular.
Question 5. What is binding energy? Short Note-Stability of an atomic nucleus packing fraction.
Answer:
Binding energy
The amount of energy that can be obtained by the amount of mass equal to the mass defect is known as the binding energy.
- The atomic mass of an isotope of an element is expected to be equal to the Sun of the fundamental particles present in it. In AMU the mass of a proton or a neutron is nearly equal to unity while that of an electron is negligible.
- Atomic masses of isotopes of elements are expected to be approximately whole numbers. Called atomic mass numbers.
Packing faction = \(\frac{\text { Actual mass of a nucleus }- \text { Mass number }}{\text { Mass number }} \times 10^4\)
“Class 10 WBBSE Physical Science Chapter 7 long answer questions, Atomic Nucleus study material”
Question 6. What are isotones? Define-nuclear isomers.
Answer:
Isotones
The are elements which differ in both their atomic numbers as well as most numbers but they have the same number of neutrons in their nuclei. They are called isotones.
Nuclear isomers
The two nuclei of an element may have the same atomic number and the same mass number yet differ in their internal nuclear energies. These nuclei are called nuclear isomers.
Question 7. Explain the separation of isotopes by distillation under low pressure.
Answer:
Separation of isotopes by distillation under low pressure
Distillation under low pressure: When a liquid like mercury is distilled under low pressure the lighter isotopes come out more quickly and condense on a cooler surface. The condensate is collected, melted and subjected to the same procedure. B repeating the process the condensate can be made richer in the lighter variety.
Question 8. What do you mean by isobars and isotones?
Answer:
Isobars
Isobars The nuclide of different chemical elements having the same mass number but different atomic numbers are called isobars.
Examples:
- 18 Ar40
- 19K40
- 20Ca40
Isotones: The nuclides of different chemical elements having the same number of neutrons but different atomic numbers are called isotones.’
Examples: 1H3, 2He4
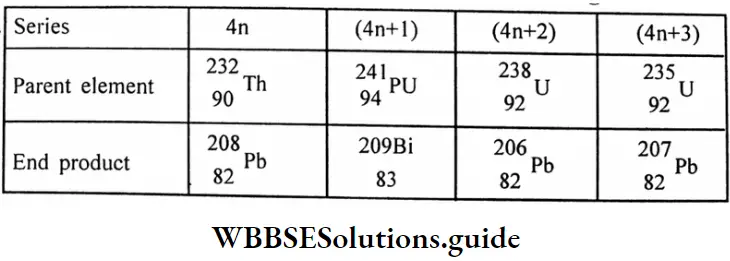
Question 9. What are the parent and end products of different disintegration series?
Answer:
Series
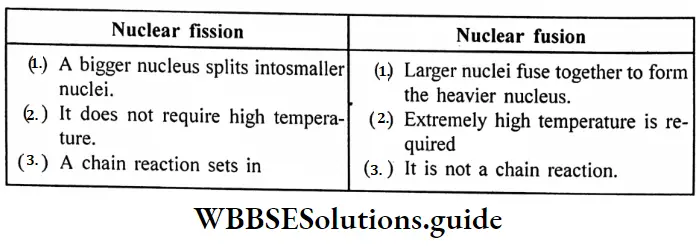
Question 10. What are the differences between nuclear fission and nuclear fusion?
Answer:
Difference between nuclear fission and nuclear fusion :
Question 11. What do you mean by Curie and Becquerel?
Answer:
Curie and Becquerel
- Curie If a radioactive substance disintegrates at the rate of 3.7 × 1010 disintegrations per second, its activity is said to be 1 curie.
- Becquerel (SI unit): If a radioactive substance has 1 disintegration per second, it is said to have an activity of one Be equal.
“WBBSE Class 10 Physical Science Chapter 7, Atomic Nucleus long answer solutions”
Question 12. A radioactive substance decays at such a rate that after 46 days only 0.25 of its original amount is left to calculate its disintegration constant.
Answer:
Given
A radioactive substance decays at such a rate that after 46 days only 0.25 of its original amount is left
Let, the original amount be No then Amount after 46 days, Nt = 0.25 x No For a nuclear decay,
λ = \(\frac{2.303}{\mathrm{t}} \log \frac{\mathrm{No}}{\mathrm{Nt}}\)
= \(\frac{2.303}{46}\) log4 day-1
= 0.0301day-1
Question 13. A certain nuclide has a half-life of 60 min. If a sample containing 600 atoms is allowed to decay for 90 min, what will be the remaining atoms?
Answer:
Given
A certain nuclide has a half-life of 60 min. If a sample containing 600 atoms is allowed to decay for 90 min
λ = \(\frac{0.693}{60} \mathrm{~min}^{-1}\)
= \(\frac{2.30^3}{90} \log \frac{\mathrm{No}}{\mathrm{N}}\)
⇒ log \(\frac{\mathrm{No}}{\mathrm{N}}\) = 0.4514 Or,
⇒ log \(\frac{\mathrm{No}}{\mathrm{N}}\)= 2.828
∴N = \(\frac{600}{2.823}\)
N= 212
“WBBSE Class 10 Atomic Nucleus long answer questions, Physical Science Chapter 7”
Question 14. The activity of a sample of radioactive element A100 is 6.02 curie. Its decay constant is 3.7 x 10’S’ which calculates the initial mass of the sample.
Answer:
Given
The activity of a sample of radioactive element A100 is 6.02 curie. Its decay constant is 3.7 x 10’S’
Activity = \(\lambda \times \frac{\mathrm{wt}}{\text { at. wt }} \times 6.023 \times 10^{23}\)
\(6.02 \times 3.7 \times 10^{10}=3.7 \times 10^4 \times \frac{w}{100} \times 6.023 \times 10^{23}\)
W= \(\frac{6.02 \times 3.7 \times 10^{10} \times 100}{3.7 \times 1.04 \times 6.023 \times 10^{23}}\)
W= \(10^{-15} \mathrm{~g}\)
Question 15. The half-life period of C is 5760 years. An old piece of wood has a disintegration rate which is 25% of the disintegration rate of an equal weight of a new piece of wood.
Answer:
Given
The half-life period of C is 5760 years. An old piece of wood has a disintegration rate which is 25% of the disintegration rate of an equal weight of a new piece of wood.
Let the rate of disintegration of the new piece = 100
The rate of migration of old pieces = 25
K = \(\frac{0.693}{\frac{t_1}{2}}=\frac{0.693}{5760}\)
t= \(\frac{2.303}{K} \log \frac{a}{a-x}\)
=\(\frac{2.303}{K} \log \left(\frac{\mathrm{lo}}{\mathrm{It}}\right)\)
∴ \(=\frac{2.30 \times 5760}{0.693} \log \frac{100}{25}\)
t= 11523 years.
