Chapter 8 Physical And Chemical Properties Of Matter Broad Answer Type Questions
Question 1. What are groups and sub-groups?
Answer:
Groups:
Groups Mendeleev’s periodic table shows that the elements with related chemical properties fall in one below the other forming vertical columns called groups. There are nine group from group I to group VIII and O (zero).
Sub-groups:
Sub-groups Each of the groups from I to VII has been divided into two. Sub-groups A and B.
Question 2. What are halogen elements? In which do they belong?
Answer:
Halogen elements: The four strongly electro-negative non-metals fluorine (F), chlorine (CI), bromine (Br) and iodine (1) form a family of closely allied elements known as the halogens meaning literally sea self-producer as these elements react with most metals to form compounds similar to sea-salt, sodium chloride.
Position in periodic table: Habzen elements placed in group VII B of the periodic table. The electro-negative character of the elements increases from left to right in a period. So, the strongly electro-negative elements are in the most extreme right group i.e. in group VII B of the period table.
Question 3. What are called alkali metals? In which group do they belong? State the similarity of their chemical properties.
Answer:
Alkali metals: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb) and Caesium (Cs). These five metals are generally known as alkali metals.
Position in the periodic table: The alkali metals are included in the A subgroup of the first group
Similarity:
- Alkali metals are strong electropositive.
- The oxides of these metals are strongly basic.
- Each has valency 1.
WBBSE Class 10 Physical Science Solutions
Question 4. Discuss the position of hydrogen in the periodic table. Answer: Position of hydrogen in the periodic table :
Reasons for placing in group IA :
- Like the alkali metals, hydrogen is electropositive.
- The valence of hydrogen is I, alkali metals are also non-valent.
- During the electrolysis of a fused chloride of an alkali metal, deposits at the cathode; in the electrolysis of a dilute aqueous solution of hydrogen chloride, hydrogen collects at the cathode.
- Like alkali metals, hydrogen has a reducing property and forms stable oxides like water (H2O).
- Like hydrogen, each of the alkali metals. Li, Na, K, Rb Cs, Fr possesses one valence-lectron.
Reasons for placing in group VIIB:
- Like halogens, hydrogen is a non-metatallic element.
- The valency of hydrogen and that of each halogen is 1.
- Halogen molecules are diatomic, a hydrogen molecule is also diatomic.
- The halogens from metallic halides like, NaCl, KBr, Kl etc. Hydrogen also produces salt-like hydrides like L, H, NaH, CaH, etc with the strongly electropositive element and hydrogen here acts as an electro-negative element.
- Atoms of halogen elements may transform to negative halide ions by taking one electron each.
- For example, x+eX; X = F, Cl, Br, I and hydrogen atom also may transform to a negative hydride ion by capturing one electron: H+e→ H.
- Like the halogens hydrogen also can produce covalent compounds reacting with electro-negative elements like S, O etc.
Due to such varied tendencies for occupying a seat in the periodic table, hydrogen is often called a naughty element or rogue element.
Question 5. Where are the inert gas elements placed and why?
Answer:
Position of inert gas elements in the periodic table:
- He, Ne, Ar, Kr, Xe, and Rn. These are inert elements. They are each gaseous and monoatomic. These are chemically non-reactive. As these elements do not react, they have zero valency.
- So they are included in the O (zero) group of the periodic table. Besides Helium, all the inert gases have eight (8) electrons in their outermost orbit. There are only 2 electrons in the outermost orbit of Helium.
Question 6. How is copper purified by the electrolysis method?
Answer:
Purification of copper by electrolysis method :
1. Electrolyte: 15% of CuSO4, solution (aqueous) containing (5-10) % sulphuric acid at 50°C is taken in a voltameter.
2. Electrodes:
- Cathode: Pure thin copper plate.
- Anode: Thick impure copper plate.
3. Electrolysis:
- On electrolysis, copper dissolves from the anode and deposits on the cathode. Thus gradually the anode plate wears out and the cathode plate thickens.
- The copper obtained in the way 99.99% purity.
4. Reaction: \(\mathrm{CuSO}_4 \rightleftharpoons \mathrm{Cu}^{2-}+\mathrm{SO}_4^{2-}\)
- At cathode: Cathode : Cu2-+2e →Cu↓
- At anode: Cu – 2e → Cu2-
WBBSE Class 10 Physical Science Solutions
Question 7. How is aluminium extracted from the electrolysis method?
Answer:
Extraction of aluminium by electrolysis method :
1. Electrolytes:
- Alumina (Al2O3) 20%
- Fused Cryolite (AIF3, 2NaF 60%)
- CaF2, 20%.
2. Electrodes:
- Cathode: Inner lining of carbon of the steel tank.
- Anode: Thick carbon rod suspended into a fused electrolyte
Reaction:
At cathode: Al3 + 3e → Al↓
At anode : 3F – 3e→ 3F
⇒ Al2O3 +6F → 2 AIF3+3O
⇒ 6O →3O2
Question 8. Draw the diagram of the covalent radius of hydrogen.
Answer:
The diagram of the covalent radius of hydrogen
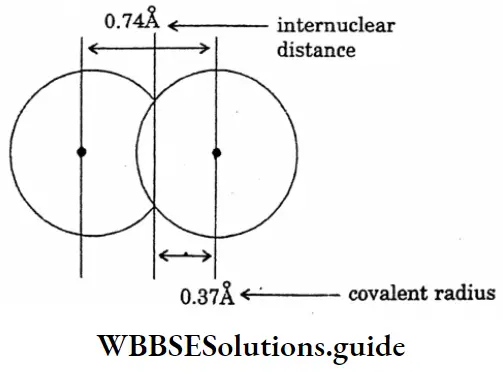
Question 9. Draw the diagram of the showering comparison of Vanderwaal’s radius and covalent radius.
Answer:
The diagram of the showering comparison of Vanderwaal’s radius and covalent radius
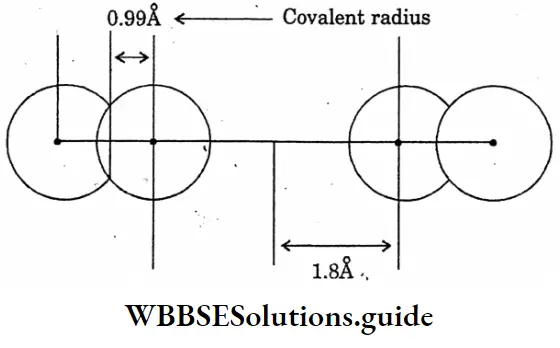
Question 10. State the contact process for the manufacture of sulphuric acid.
Answer:
Contact process for the manufacture of sulphuric acid :
Principle:
1. Formation of SO2: SO2 is prepared by burning sulphur or iron pyrites in excess of air.
Equation: S+ O2 = SO↑ Or,
4 Fes,+ || O2 = 2Fe2O3 + 8SO2 ↑
(Iron pyrities)
2. Formation of sulphur trioxide (SO3): SO3 is prepared by the oxidation of sulphur dioxide with oxygen (from air) in the presence of platinised asbestos or, V2 O5 as catalyst at 450°C.
Equation: \(2 \mathrm{SO}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{SO}_3+192.5 \mathrm{Kj}\)
3. Formation of oleum:
Now sulphur trioxide thus produced is not allowed to react with water directly sulphur trioxide absorbs concentrated sulphuric acid 98% turning it as fuming sulphuric acid or oleum.
Equation: SO3+ H2SO4= H2S2O7 (Oleum)
4. Dilution of oleum to sulphuric acid: Pure and concentrated sulphuric acid is produced by adding slowly a requisite amount of water in fuming sulphuric acid.
Equation:
H2S2O7 >+ H2O = 2 H2SO4
Question 11.
- Write down the electronic arrangement of the element 17X 35 What is the valency of the element?
- Will the element form an onion or cation?
- What type of valency will be exhibited when the element combines with sodium?
Answer:
The electronic configuration of the element X is: 2, 8, 7,
- As an atom of the element tends to capture or share one electron to attain stable of the octet, its valency is 1.
- It will form an anion by capturing electrons.
- The element will exhibit electro-valency when it combines with sodium since sodium is a metal, an atom which tends to release one electron.
Question 12. The atomic number of element A is 20 and that of another element B is 17. Write down their electronic configuration. Will they produce on electrova- lent compound or a covalent compound? What will be their valencies in that case?
Answer:
- The electronic configuration of A is: 2, 8, 8, 2.
- The electronic configuration of B is: 2, 8, 7.
They will produce an electrovalent compound.
Explanation:
An atom A will give up two (2) valence electrons. Each of the two atoms of B will capture one electron. In the process atoms of A and B attain a stable octet state and atoms of A will be positive ions and those of B will be negative ions.
- A and B thus form an electrovalent compound.
- As an atom of A loses two electrons.
- So A has valency 2 (two) and since each atom of B captures one electron, The valency of B will be one (1).
WBBSE Class 10 Physical Science Solutions
Question 13. What is the difference between ionic compounds and covalant compounds?
Answer:
Difference between ionic and covalent compounds:
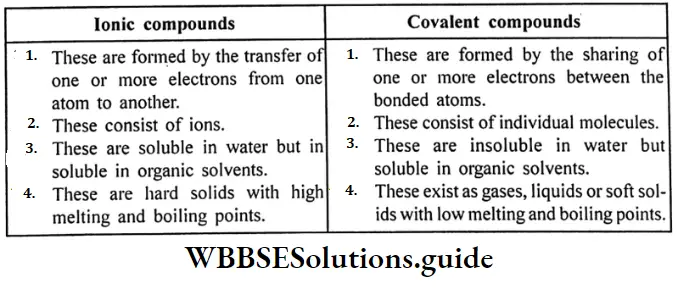
Question 14. How ammonia is prepared in the laboratory?
Answer:
Laboratory preparation of Ammonia:
- Chemicals required: Ammonium chloride (NH4, CI) and quick lime (CaO) or dry slaked lime (Ca (OH2)–
- Condition: Normally ammonia gas is obtained in the laboratory by heating a mixture of ammonium chloride with slaked lime. Instead of using slaked lime quick lime may also be used.
- Collection: Ammonia is lighter than air, it may be collected by the downward displacement of air.
Ammonia is not collected through the downward displacement of water because it is highly soluble in water.
Equation of the reaction:
2NH4 CI + Ca (OH)2 = 2NH3 ↑ + CaCl2+ 2H2O
↑
2NH4CH+CaO = 2NH3↑ + CaCl2+H2O
- Precautions: The ingredients, the test tube, the delivery pipes and the gas jar should be absolutely dry. All the connections in the apparatus should be leak-proof.
- Drying of ammonia: As ammonia is a basic substance, it cannot be dried by acidic drying agents like cones. H2SO, or P2O5,
- The gas is absorbed by fused CaCl2, with the formation of an addition compound CaCl2, 8NH3. SO, fused CaCl2, cannot be used to dry ammonia. It is best dried with the basic drying agent, quick lime (CaO).
Question 15. Prove by experiment that ammonia is dissolved in water and produces an alkaline solution.
Answer:
Fountain experiment:
Arrangement of the experiment: A flask is filled with dry ammonia gas and its mouth is corked. The flask is kept inverted position and is clamped to a stand.
Through a hole in the cork one end of a glass tube is introduced inside the flask. This end of the tube inside the flask is shaped into a jet. The other end of the tube dips in some red litmus solution taken in a beaker.
- Operation: Now ice or ether is poured upon the flask.
- Observation: A blue fountain produces inside the flask.
Explanation:
- Due to the evaporation of ether, the flask is cooled. Ammonia gas inside the flask contracts, as a result, there is a partial vacuum inside the flask.
- At this stage, if the stop cock is opened the red litmus solution rushes inside the flask and ammonia is dissolved in it.
- Due to vacuum created inside there is a formation of the fountain and the solution becomes blue.
Conclusion: This experiment proves that ammonia is highly soluble in water and the aqueous solution is alkaline.
WBBSE Class 10 Physical Science Solutions
Question 16. Draw the diagram of the formation of sodium chloride.
Answer:
The diagram of the formation of sodium chloride
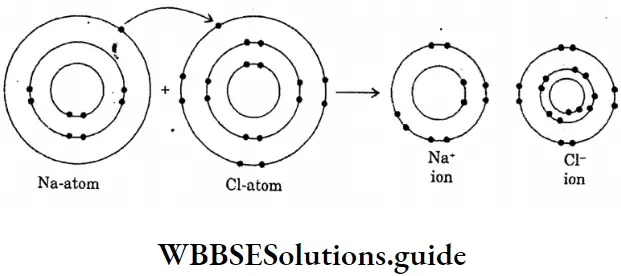
Question 17. Draw the diagram of sodium fluoride :
Answer:
The diagram of sodium fluoride
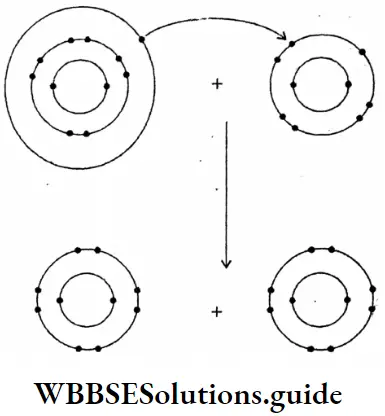
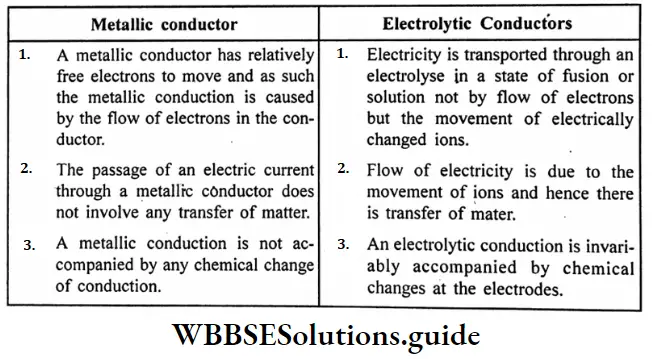
Question 18. What are the main points of difference between metallic conductors on electrolytic conductors?
Answer:
Difference between metallic conductors and electrolytic conductors :
Question 19. What changes are taking place during the electrolysis of an electrolyte?
Answer:
Changes take place during the electrolysis of an electrolyte:
- When fused or dissolved in water, electrolyte splits up into oppositely charged particles called ions.
- On passing an electric current, the cations migrate towards the cathode while the anions migrate towards the anode.
- The cations on reaching the cathode gain electrons from it and from neutral atoms which get deposited on the cathode.
- The anions on reaching the anode lose electrons and get converted into neutral atoms which may be collected as such or they may undergo some secondary change to from some other products.
Question 20. What are Biodegradable and Non-biodegradable materials?
Answer:
- Biodegradable materials: Materials of vegetable and animal origin invariable decay and decompose into CO2, H2O, N2 or NH3 by the combined action of sometimes natural agencies like air, water, subshene etc. These are called biodegradable materials.
- Non-biodegradable materials: Synthetic materials like plastics, polythene, Teflon, PVC etc. are not decomposed by these natural agencies even for a long time. These are called non-biodegradable materials.
Question 21. Briefly explains thermite welding of iron and steel.
Answer:
Thermite welding of iron and steel
A mixture of 3 parts of ferric oxide and I part of aluminium powder is called a thermite mixture. When the mixture is ignited ferric oxide is reduced to metallic iron by aluminium. Because of the evolution of large amounts of heat, the temperature rises to Magnesium tape
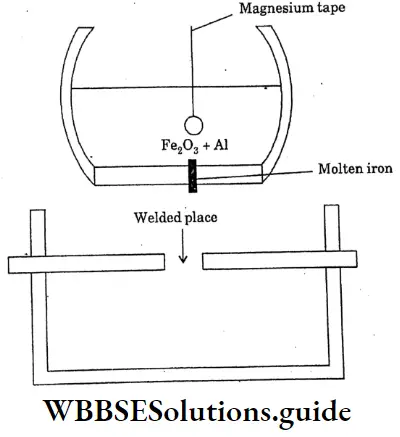
2500c The molten iron thus produced is allowed to fall on the red hot broken rails broken machine parts ect can be welded without removing them from their original sites.
