WBBSE Class 10 Physical Science Question Answer In English
Multiple Choice Question And Answers Physical Science And Environment
Question 1. Which among the following gases does not help in the depletion of ozone in the ozone layer?
- NO
- NO2
- CFC
- CO2
Answer: 4. CO2
Question 2. What is the value of PV for 11.2 litres of an ideal gas at STP?
- 2RT
- RT
- 0.5RT
- 11.2 RT
Answer: 3. 0.5RT
Read And Learn More: WBBSE Solutions For Class 10 Physical Science And Environment
Question 3. According to the following chemical equation CH4+2O2→ CO2 +2H2O What volume of 0, will be required to burn 10 moles of CH4 at STP?
- 448L
- 224L
- 44.8L
- 22.4L
Answer: 1. 448L
Question 4. Which among the following substances has the highest heat conductivity?
- Silver
- Diamond
- Copper
- Aluminium
Answer: 2. Diamond

Question 5. If a beam of red light and a beam of violet light are incident at the same angle on the inclined surface of a prism from an air medium and produce angles of refraction r and v respectively, which of the following is correct?
- r = v
- r= \(\frac{1}{V}\)
- r> v
- r < v
Answer: 4. r<v
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 1”
Question 6. A point source of light is placed at the centre of curvature of a concave mirror. The angle of deviation of the rays incident on the mirror from this source and reflected from it is
- 0°
- 180°
- 90°
- 360°
Answer: 2. 180°
Question 7. Coulomb’s law related to electric charges is applicable when the two charges
- One is the point, One is spherical
- Both are spherical
- One is the point, One is extended
- Both are points
Answer: 4. Both are points.
Question 8. The characteristics of a fuse wire are
- High resistance high melting point
- Low resistance, low melting point
- Low resistance high melting point
- High resistance low
Answer: 2. High resistance low melting point.
Question 9. Present in a particle
- One proton, one neutron
- One proton
- Two protons two neutrons
- One electron
Answer: 3. Two protons, two neutrons.
“Class 10 WBBSE Model Question Paper 2023, Physical Science and Environment Set 1 study material”
Question 10. Which of the following is not a periodic property of elements?
- Density
- Melting point
- Boiling point
- Radioactivity
Answer: 4. Radioactivity
Question 11. In which of the following compounds there is no existence of molecules?
- Hydrogen chloride
- Calcium oxide
- Methane
- Ammonia
Answer: 4. Ammonia.
Question 12. Which of the following statements is correct in the case of electrolysis of CuSO4 solution using Cu electrodes?
- The mass of the cathode decreases
- The mass of the anode increases
- The concentration of CuSO4 in the solution decreases
- The concentration of CuSO4 in the solution remains unchanged
Answer: 4. The concentration of CuSO4 in the solution remains unchanged.
Question 13. What colour is produced when H2S gas is passed through an alkaline aqueous solution of sodium nitroprusside?
- Violet
- Orange
- Deep blue
- Green
Answer: 1. Violet.
Question 14. The formula of red haematite, an area of iron, is
- FeO
- Fe2O3
- Fe3O
- FeCO3
Answer: 3. Fe2O3
Question 15. By the reaction of aqueous NaHCO3 with which of the following compounds CO2 is produced?
- CH3 CH2 OH
- CH3CHO
- CH3 COCH3
- CH3 COOH
Answer: 4. CH3 COOH
Class 10 Physical Science WBBSE Physical Science And Environment Answer The Following Questions
Question 1. Which fuel gas is harvested from the coal bed?
Answer: Methane
Or
Name a gas present in the air, the increase in the amount of which causes global warming.
Answer: CO2
Question 2. Name an energy source which can be used for sustainable development.
Answer: Solar energy.
Question 3. Write whether the following statement is true or false. The speed of the gas molecules contained in a closed vessel at fixed temperature and pressure is the same.
Answer: True.
Question 4. What is the nature of the V versus T graph according to Charles’s law?
Answer: If the straight line increase backwards, it will go through the main point.
Question 5. Write whether the following statement is true or false. Among copper, invar and iron the linear expansion coefficient of iron is the lowest.
Answer: True.
Or
What is the unit of volume expansion coefficient?
Answer: K^(-1).
“WBBSE 2023 Madhyamika Physical Science and Environment, Set 1 Model Question Paper download”
Question 6. What is meant by the pole of a spherical mirror?
Answer: It refers to the middle point of the spherical mirror.
Question 7. Write down one use of X-Ray.
Answer: Medical science.
Question 8. Name a machine where electrical energy is converted to mechanical energy.
Answer: Electric motor.
Question 9. Apart from the live wire, what are the two other wires in the household circuit?
Answer: Neutral wire and earth wire.
Question 10. Which kind of nuclear reaction produces energy in a nuclear reactor?
Answer: Nuclear division.
Or
Give an example of a natural radioactive element.
Answer: Uranium / Thorium.
Question 11. Match the Right column with the left column.
Answer:
Left column – Right column
Krypton – A noble element
Neptunium – Transuranic element
Copper – Prepared by carbon reduction of the oxide of the metal
Zinc – In the alloy brass, the metal whose percentage amount is higher than that of the other metal.
Question 12. Between chloroform and sodium chloride which is not soluble in water?
Answer: Chloroform.
Question 13. Name e metal which is extracted by the process of electrolysis.
Answer: Aluminium (Al)
Or
Which is the anode in the electroplating of silver or brass spoon?
Answer: Silver.
Class 10 Physical Science WBBSE
Question 14. Which energy causes chemical reactions during electrolysis?
Answer: From electrical energy to chemical energy.
Question 15. Show with the help of an appropriate litmus paper that the aqueous solution of ammonia is alkaline in nature.
Answer: Red litmus paper will become blue.
Question 16. NaOH+H2S →______+H2O
Answer: Na2S.
Question 17. Write one use of Urea.
Answer: Fertiliser.
Question 18. Write the structural formula of propanone.
Answer: CH3-CO-CH3
Or
Wohler first prepared an organic compound from an inorganic compound in the laboratory. What is the organic compound?
Answer: Urea.
Question 19. Give an example of a biodegradable natural polymer.
Answer: Cellulose of Protein.
Class 10 Physical Science WBBSE Physical Science And Environment Answer The Following Questions
Question 1. Write with reason in which layer among the layers of the atmosphere the pressure is the highest.
Answer:
The pressure is the highest in Troposphere, because among all the layers of the atmosphere, the temperature is the lowest ture Moreover, when temperature decreases, pressure increases.
Question 2. Find out the ratio of the Volumes occupied by 32g O2 and 44g CO2 gases at 27°C temperature and 700 mm Hg pressure (C= 12, O= 16).
Answer:
Molar mass of O2 = (16+16) 32 g = 1 mol
Molar mass of CO2 = (12+32) 44 g = 1 mol
So, Volume of both is = 22.4 litre.
Ratio of volume = 1:1
Or
A fixed mass of a gas occupies a volume of 520 cm3 at -13°C temperature. Keeping the pressure unchanged, when the gas is heated the volume of the gas increases to 700 cm. What is the final temperature of the gas in degrees Celsius?
Answer:
Given
A fixed mass of a gas occupies a volume of 520 cm3 at -13°C temperature. Keeping the pressure unchanged, when the gas is heated the volume of the gas increases to 700 cm.
Mass of the gas const. (m)
According to the problem
T1 =-13°C= 273° + (-13°)K
= 260K.
V1= 520 cm3
P1 = 76 cm [Mercury pressure]
T2 = ?
V2 = 700 cm3
P2 = 76cm.
⇒ \(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)
⇒ \(\frac{76 \times 520}{260}\)
∴ \(=\frac{76 \times 700}{T_2}\)
T2 = 350
= (350-273)
= 77°C.
Final temperature = 77° C./350K.
“WBBSE Madhyamika 2023 Physical Science and Environment, Set 1 Model Question Paper with solutions”
Question 3. Mention two features of the image formed by a simple camera.
Answer:
- The image is real.
- The image is inverted.
Or
Where in front of a concave mirror image of an extended object placed at infinity will be formed by the mirror? Mention one feature of the image.
Answer: The object should be placed within the focus of the mirror. The image will be real and inverted.
Question 4. Mention one similarity and one dissimilarity between electromotive force and potential difference?
Answer:
Similarity – Both are related to energy.
Dissimilarity:
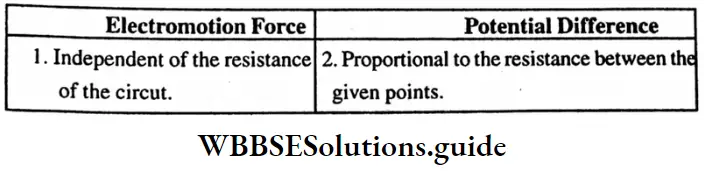
Question 5. By giving an example of an iconic compound show that its ions do not obey the octet rule.
Answer:
PCI2 (The electron number in the outermost orbit is 10. P is the central atom.
Or
Explain why the melting point of sodium chloride is much greater than that of glucose.
Answer: Salts have high intermolecular forces. Hence, they have a higher melting point than covalent compounds like glucose.
Question 6. Show that F forms an ionic bond with Na but it forms a covalent bond with H (The atomic number of H F and Na are 1, 9 and 11 respectively).
Answer:
H and F both are non-metals; so, they share their electrons and make covalent bonds. But as Na is Metal. It gives e to F and Na+, and F– are formed and they form ionic bonds.
Question 7. Write with the balanced chemical equation, what happens when Nitrogen gas is passed over calcium carbide heated at 1100°C.
Answer:
⇒ \(\mathrm{CaC}_2+\mathrm{N}_2 \stackrel{1100^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{CaCN}_2+\mathrm{C}\)
Question 8. Write the balanced chemical equation of the reaction for the formation of metallic iron from ferric oxide by thermit process. Write an application of the process.
Answer:
⇒ \(\mathrm{Fe}_2 \mathrm{O}_3+2 \mathrm{Al} \stackrel{\text { Heat }}{\longrightarrow} 2 \mathrm{Fe}+\mathrm{Al}_2 \mathrm{O}_3\)
Or
Write the balanced chemical equation of the reaction that occurs when a piece of metallic CuSO What information is obtained from this reaction about the relative position of Cu and Fe in the activity series of metals?
Answer:
Fe + CuSO4– FeSO4 + Cu↓
On the submerged portion of Fe, the red metallic copper layer is formed
Question 9. Select the members of a homologous series from the following compound and arrange them in increasing order of their molecular weights: CH3 COOH, CH3CH2OH, CH3OCH3, CH3OH, C2 H4, C2H6, CH3CH2OH, C3H4
Answer:
The members are CH3CH2OH, CH3OH. Arranged in increasing order – CH3OH, CH3C H2OH.
Or
Write with an example what is meant by functional group.
Answer:
Function groups are specific substituents within melecules that are respon- sible for the characteristic chemical reaction of those melecules. Example-
⇒ CH3 – CH2– OH (Ethyl alcohol)
⇒ CH3– O – CH3 (Diethyl ether)
Class 10 Physical Science Solution WBBSE Physical Science And Environment Answer The Following Questions
Question 1. State Avogadero’s law.
At a certain fixed temperature and pressure, the molar volumes (V/n) of the real gases are nearly equal and at STP the limit is 22.4 L met. How Avogadro’s law can be arrived at from this information obtained from experiments?
Answer:
Avogadero’s law
Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.
Given
At a certain fixed temperature and pressure, the molar volumes (V/n) of the real gases are nearly equal and at STP the limit is 22.4 L met.
1 mol of gas contains 6.022×1023 numbers of atoms.
6.022×1023 numbers of atomic volumes are very merged in respect of the volume of gas.
Question 2. A and B react to produce C according to the following chemical equation. 2A+ B →2C A, B and C are the formulas for three gaseous substances. The vapour densities of A and B are 32 and 16 respectively. Find out the vapour density of C.
Answer:
Given
A and B react to produce C according to the following chemical equation. 2A+ B →2C A, B and C are the formulas for three gaseous substances. The vapour densities of A and B are 32 and 16 respectively.
2A+B-2C
Vapour density of A = 32
Vapour density of B = 16
We know,
Vapour density = 1/2 x molecular mass.
∴ Molecular mass of A = (2 x 32) = 64 g
∴ Total Molecular mass reactant (64×2) + 32 = 160 g
B(2×16)= 32 g
∴ Mass of 2C = 160g
Mass of C= \(\frac{160}{2}\)g
C= 80g
∴ Vapour density of C = 1/2 x molecular mass
= \(\frac{1}{2}\) x 80=40 g
Or
According to the following chemical equation:
- 2ZnS +3O2 → 2ZnO +2SO2 from 100 mole of ZnS
- How many grams of ZnO, and
- How many mol of SO, will be produced? (Zn65.5, S = 32, O= 16)
Answer:
2ZnS +3O2 → 2ZnO +2SO2
⇒ 2 x (65.5 + 32) → 2 x (65.5+ 16)
⇒ 195→ 163
1= 163/195
ZnO = 8,150gm
195→ (32+32) = 128
195→128
1 → 128/195
9750→ \(\frac{128 \times 9750}{195}\)
= 6400gm
= \(\frac{6400}{64}\)
= 100 mole SO2
- 8,150 gm of ZnO, and
- 100 mole of SO, will be produced.
Or
Give an example of the volume expansion of a liquid on heating. The area of a solid substance at a temperature of T1 K is A sqm and that at a temperature of T1K is A, sqm. Write down the mathematical expression of or the coefficient of area expansion with unit of that solid substance.
Answer:
Given
The area of a solid substance at a temperature of T1 K is A sqm and that at a temperature of T1K is A, sqm.
The volume of alcohol in an alcohol thermometer increases at the time of increase in temperature.
At temperature T1 the area is A1 of the solid
At T2 the area is A2
∴ The mathematical expression of the coefficient of area expansion is \(\beta=\frac{A_2-A_1}{T_2-T_1}\)
Unit cm/°c in CGS
mr-1 in SI.
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 1 PDF”
Question 3. Write down the three factors on which the conduction of heat through a solid substance depends.
Answer:
The factors are:
- Area of the width intersection
- Change of temperature, and
- Length of the solid substance.
Question 4. What is the dispersion of light? Will there be a dispersion of white light within a glass slab after retraction when white light is incident on the glass slab at an angle of 45°?
Answer: The phenomenon of splitting white light into its constituent colours is known as the dispersion of light.
Answer: Yes.
Question 5. The principal section of the prism is an equilateral triangle If a ray of light is incident at an angle of 30° on one of the refracting surfaces and emerges at an angle of 45° from the other refracting surface, what is the angle of deviation?
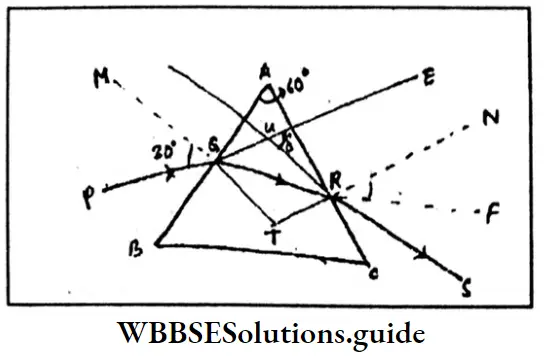
δ =i1+i2– A
= 30°-45-60° (As it is an equilateral triangle)
= 15°
Question 6. Two metallic conductors A and B of the same length have resistivities of 1.6×108 Ω and 3.2×10-8 m respectively. These two conductors are separately connected to the same potential difference what would be the ratio of their cross sections in order to have the same current flowing through each of them?
Answer:
Given
Two metallic conductors A and B of the same length have resistivities of 1.6×108 Ω and 3.2×10-8 m respectively. These two conductors are separately connected to the same potential difference
R= \(\frac{P l}{A}\) and
V=IR
R= \(\frac{V}{l}\)
R = \(\frac{V}{l}\)
= \(\frac{P l}{A}\)
As potential difference(v), current (I)and Length(l) are
∴ \(\frac{P}{A}\) Constant
∴ \(\frac{P_1}{A_1}=\frac{P_2}{A_2}\)
⇒ \(\frac{1.6 \times 10^{-8}}{A_1}=\frac{3.2 \times 10^{-8}}{A_2}\)
⇒ \(\frac{A_2}{A_1}=\frac{2}{1}\)
⇒ A1 : A2 = 1:2
Or
The series combination of two 10Ω-ohm resistances is connected in parallel combination with a 20-ohm resistance. Determine the equivalent resistance of the final combination.
Answer:
Given
The series combination of two 10Ω-ohm resistances is connected in parallel combination with a 20-ohm resistance.
Series combination:
∴ Equivalent resistance = Rp.
∴ Rp = R1+R2
= 10Ω+10Ω = 20 Ω
∴ Rp =20 Ω
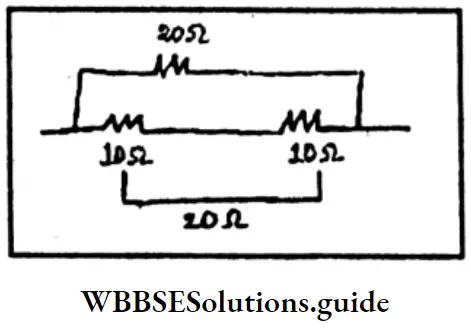
Parallel combination
Equivalent resistance = \(\frac{1}{R p}\)
⇒ \(\frac{1}{R p}=\frac{1}{R_1}+\frac{1}{R_2}\)
= \(\frac{1}{20}+\frac{1}{20}\)
= \(\frac{1+1}{20}\)
= \(\frac{2}{20}\)
= \(\frac{1}{10} \Omega\)
Rp=10 Ω
“Class 10 WBBSE Madhyamika Model Question Paper 2023, Physical Science Set 1 practice questions”
Question 7. What is meant by electrical power? The dating of a bulb is written as 220V- 100W. What is its meaning?
Answer:
Electrical power
Electrical power is the rate of consumption of electrical energy in respect of the time of some electric machine. We mean that the bulb is to be used at 220 volts potential difference to glow fully and the electrical energy will be spent at the rate of 100 joule/second or a power of 100w will be required.
WB Class 10 Physical Science Question Answer Question 8. From which part of the atom is the radioactive rays emitted? Which of the radioactive rays has the highest penetrating power and which has the highest ionising power?
Answer:
From the nucleus:
- γ Ray has the highest penetrating power.
- α Ray has the highest ionising power.
Question 9. Mention the dissimilarity of properties of hydrogen with one property of group 1 elements and two properties of group 17 elements.
Answer:
Dissimilarity with group 1 elements Hydrogen is non-metal and diatomic gas. Group I elements or bases are monoatomic hard substances.
- Hydrogen is an electropositive element; halogens are electronegative.
- Hydrogen is a reductant, but halogens are oxidants.
Arrange as directed:
Or
- Na(11), K (19), Li (3), and Rb (37) belong to Group 1 of the long periodic table according to decreasing order of atomic radius.
- S (16) O (8), Te (52), and Se (34) belong to Group 16 of the long periodic table according to increasing order to electronegativity.
- Ca(20), Be (4), Sr (38), and Mg (12) belong to group 2 of the long periodic table according to decreasing order of reducing power.
(The atomic numbers have been given within the first brackets after the symbols of the elements):
- Rb> K > Na > Li
- Te< Se <S<0
- Sr>Ca> Mg> Be [Reducing powers increases from up to down]
Question 10. On what basis electrolytes have been classified as strong and weak system slectrolytes? Give an example of a strong electrolyte.
Answer:
The electrolytes are classified as strong and weak on the basis of how much they are ionised. Strong electrons are ionised almost completely the weak electro- lytes are ionised a little.
NaOH is a strong electrolyte.
“WBBSE Class 10 Model Question Paper 2023, Physical Science and Environment Set 1 exam pattern”
Question 11. Write mentioning the name of the catalyst and condition, how nitric oxide is manufactured by oxidising ammonia with the help of aerial oxygen. Write also the balanced chemical equation of the reaction.
Answer: Catalyst- Pt or Pt Rh. condition 5-7 atm, 700° – 800° c, 0.0014 second.
⇒ \(4 \mathrm{NH}_3+5 \mathrm{O}_2 \frac{\text { Pt or Pt Rh (Catalyst })}{5-7 \mathrm{~atm}, 700-800^{\circ} \mathrm{C}, 0.0014 \mathrm{sec}}=4 \mathrm{NO}+6 \mathrm{H}_2 \mathrm{O}\)
Question 12. Two different organic compounds A and B have the same molecular formula of C2H6O, A reacts with metallic sodium to produce hydrogen gas but B does not react with metallic sodium. Write structural formulas of the compounds A and B. Write the balanced chemical equation of the reaction of A with metallic sodium. A = CH3CH2OH B = CH3OCH3
Two different organic compounds A and B have the same molecular formula of C2H6O, A reacts with metallic sodium to produce hydrogen gas but B does not react with metallic sodium.
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH} \mathrm{Na} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{ONA}+\frac{1}{2} \mathrm{H}_2\)
Or
Write the condition for the reaction of the addition of hydrogen to ethylene. Write the balanced chemical equation of the reaction. Mention one use of CNG.
Answer: In the presence of Pt, Pd or raney nickel, the reaction of the addition of hydrogen is dove
⇒ \(\mathrm{CH}_2=\mathrm{CH}_2+\mathrm{H}_2 \frac{\mathrm{Pt}, \mathrm{pd}}{\text { or, RaneyNi}} \mathrm{CH}_3–\mathrm{CH}_3\)
CNG is used as fuel.
