WBBSE Class 10 Physical Science Question Answer In English
Multiple Choice Questions Physical Science And Environment
Question 1. Which among the following gases absorb long wavelength infrared radiation emitted from the earth’s surface
- N2
- O2
- CH3
- He
Answer: 3. CH3
Question 2. At STP, 2-24 L is occupied by
- 4.4 g CO2
- 0.64g SO
- 28 g CO
- 16g O. [C= 12. O -16. S = 32]
Answer: 1. 4.4 g CO2
Read And Learn More: WBBSE Solutions For Class 10 Physical Science And Environment
Question 3. How many molecules of CO2 will be produced when I mole C reacts completely with 1 mole O2?
- 6.022 x 1023
- 1.806 x 1024
- 6.022 x 1022
- 6.022 x 1024
Answer: 3. 6.022 x 1023
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 2”
Question 4. For a solid, how many types of thermal expansion coefficients are there?
- One
- Two
- Three
- Four
Answer: 3. Three

Question 5. Which one of the following has the highest wavelength?
- x-ray
- y-ray
- Infrared ray
- Ultraviolet ray
Answer: 3. Infrared ray
Question 6. In the case of refraction if the angle of incidence and the angle refraction are 45° and 30° respectively, then the angle of deviation is
- 75°
- 15°
- 7-5°
- 37.50
Answer: 2. 15°
Question 7. Temperature remains unchanged if the potential difference between the two ends of a conductor is V and the current through the conductor is I, which of the following is true?
- V∞ I
- V∞ I2
- V ∞ I-1
- V ∞ r-2
Answer: 1. V∞ I
Question 8 . The relation among electromotive force (V), work (W) and charge (Q) is
- Q=WV
- Q=V/W
- Q=V/W2
- Q = W/V
Answer: 4. Q = W/V
Question 9. For the atom produced by ẞ-particle emission from a radioactive atom
- Mass number increases
- Atomic number increases
- Mass number decreases
- Atomic number decreases
Answer: 2. Atomic number increases
Question 10. To which group of the long
- Group 1
- Group 16 (e)
- Group 17
- Group 2
Answer: 3. Group 17
Question 11. Solid state of which of the following compounds is composed of ions?
- Sodium chloride
- Hydrogen chloride
- Naphthalene glucose
Answer: 1. Sodium chloride
Question 12. Which of the following has the highest ability to conduct electricity?
- Pure water
- An aqueous solution of Sugar cane
- Liquid hydrogen chloride
- Aqueous solution of acetic acid
Answer: 4. Aqueous solution of acetic acid
“Class 10 WBBSE Model Question Paper 2023, Physical Science and Environment Set 2 study material”
Question 13. In the first step of fixation of nitrogen which of the following compounds as a result of lightning
- NO
- NO2
- N2O5
- HNO3
Answer: 1. NO
Question 14. Which of the following is the formula of bauxite, ore of aluminum?
- Al2O3
- Al2O3H2O
- Al20O3 2H2O
- A1F3 . 3NaF
Answer: 3. Al20O3 2H2O
Question 15. Which of the following is the alkyl group containing two carbon atoms
- Methyl
- Ethyl
- Propyl
- Isopropyl
Answer: 2. Ethyl
Class 10 Physical Science WBBSE Physical Science And Environment Answer The Following Questions
Question 1. Write down the unit of calorific value of the fuel.
Or
Does the temperature increase of decrease with an increase in altitude in the strato- sphere?
Answer:
Kilo Joule/Kg OR The temperature increases.
Question 2. Which radiation, coming from the sun is prevented by the ozone layer from falling on the earth’s surface?
Answer: Ultra Violet Ray (UV ray).
Question 3. State whether the following. the statement is true or false: The volume of gas molecules is taken into consideration in Avogradro’s raw.
Answer: False.
Question 4. The product of volume and pressure of how many grams of Ni gas is 244 liter atmosphere at STP? [N= 14].
Answer: 280g.
Question 5. State whether the following statement is True or False: The constituent particles of a material change position during the conduction of heat through it.
Or, The width and the cross-section of a conductor remain unchanged, what is the relation between the thermal resistance and thermal conductivity of that conductor?
Answer:
False or \(R_T \alpha \frac{1}{K}\) [d and A constant]
Question 6. What will lie the angle of incidence when a ray of light passes through the centre of curvature of a concave mirror?
Answer: 0°.
Question 7. How many rectangular surfaces are there in a prism?
Answer: 3.
Question 8. Give an example of a semiconductor.
Answer: Silicon (Si).
Question 9. A thin wire and a thick wire of the same conducting material have the same length. Which one of them will carry more current connected to the same potential difference?
Answer: Thick wire.
Question 10. Mention one misuse of the nuclear fission reaction.
Or
Which law explains the release of huge amounts of energy in nuclear fusion?
Answer:
Atom Bomb Or E = mc [m = Decreasing mass, C = Vacuum, E = Produced energy).
Question 11. Match the right column with the left column:
Left column- Right column
Oxide layers protect from attack by water vapor- AI
Group 1 cement of the logn periodic table having the least reducing property accelerates the rusting of iron – LI
When the metal remains exposed to air metal slowly develops green patches on its surface – Cu
Group 2 cement of the long periodic table having the least atomic radius – Bc
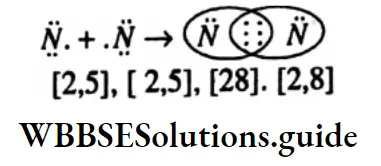
Question 12. Draw the Lewis dot structure of N? molecules, (atomic number of N is 7)
Answer:
Question 13. Which kind of electricity is used in electrolysis?
Answer: Direct Current (dc)
Or
Write down the cathode reaction in the electrolysis of acidulated water using platinum electrodes.
Answer: H + e → H, H+H → H2↑
Question 14. In electroplating gold on brass, what is the electrolyte used?
Answer: Solution of Potassium Aurocyanide [K[Au(CN)2].
Question 15.What colour is formed in the reaction of ammonia with Nessler’s reagent?
Answer: Copper-brown color.
“WBBSE 2023 Madhyamika Physical Science and Environment, Set 2 Model Question Paper download”
Question 16. Write down the formula of the precipitate formed when H’S gas is passed through an aqueous solution of silver nitrate.
Or
Write the name of the compound which is formed by the reaction of nitrogen with magnesium metal nl a high temperature.
Answer: Ag2S or Magnesium Nitride (Mg2 N2).
Class 10 Physical Science WBBSE
Question 17. What is the value of the H-C-H bond angle in methane? 1 Or, Write the IUPAC name of CH CH2COOH- 1.
Answer: 109° 28′ OR Propanoic Acid.
Question 18. What is the industrial source of CGN7?
Answer: The gaseous substances contained in the petroleum mine or coal mine.
WB Class 10 Physical Science Question Answer Physical Science And Environment Answer The Following Questions
Question 1. What is the concept of sustainable development?
Answer:
The concept of sustainable development
Sustainable development means obtaining and utilizing natural resources discriminately so that they do not get exhausted completely, keeping the future generation in mind.
Question 2 . 1 g of a gas at 7°C and 2-atmosphere pressure occupies a volume of 410 mL. Determine the molar mass of the gas. (R=0-082 litre atmosphere mole’ 11C1}
Or
A fixed mass of gas occupies a volume of 273 cm3 at STP. At what pressure the above gas will occupy a volume of 300 cm3 at 27″C?2
Answer:
Given
1 g of a gas at 7°C and 2-atmosphere pressure occupies a volume of 410 mL.
Mass of gas (W) = 1 g.
Temperature (T) = 7° c=(273+7) k=280k.
Pressure(P0 = 2 atm and volume (v) = 410 ml =0.41L
Let the molar mass of gas = M
From PV = (\(\left(\frac{W}{M}\right)\))
M= \(\frac{W R T}{P V}\) Or,
M= \(\frac{1 \times 0.082 \times 280}{2 \times 0.41}\)
= 28
∴ The molar mass of gas = 28 g. mol-1
Or
Answer: Primary Pressure of the gas (P1) = 76 cm mercury pressure, Temperature (T1)=273k and volume (V1)=273 cm and final temperature (T2)=(27+273) K = 300k, volume (V2)= 300 cm. Let, final pressure = P2.
From the combined law of Charles and Boyle’s
⇒ \(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\) Or
⇒ \(P_2=\frac{P_1 V_1 T_2}{T_1 V_2}\)
⇒ \(P_2=\frac{76 \times 273 \times 300}{273 \times 300}\)
P2= 76
∴ The final pressure of the gas = 76 cm mercury pressure
Question 3. What is the refractive index of a medium?
Or
Which type of detection of vision is rectified a convex lens?
Answer:
The refractive index of a medium is \(\frac{\sin i}{\sin r}\) = 1μ2
That is the refraction of the second medium in respect of the first medium
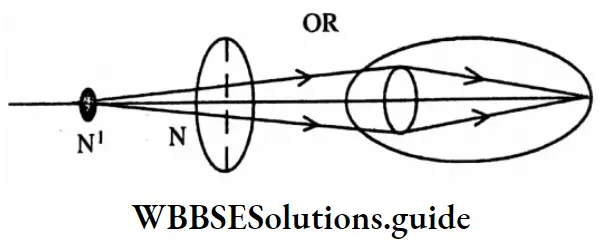
Suppose, near a person with a long type of defect of vision, there is point N and near his eyes, there is point N. The vocal length of the convex lens
will be such that after refraction through the lens the irregular image of point N1 is formed at point N1. So, the person sees point N1 at point N1
“WBBSE Madhyamika 2023 Physical Science and Environment, Set 2 Model Question Paper with solutions”
Question 4. Two resistances r1, and r2, when connected separately to the same potential difference, it was seen that the current flowing through r1, was six times the current flowing through r1. Determine the ratio of and r2
Answer:
Given
Two resistances r1, and r2, when connected separately to the same potential difference, it was seen that the current flowing through r1, was six times the current flowing through r1.
Let, Potential difference = V and through resistances r1 , and r2, the current following is I1 and I2 respectively.
I = 6I2
In case of r1 V= I ______________(1)
In case of r2V = r____________ (2)
By comparing 1 and 2 we get
I1 r1 = I2r2
Or \(\frac{r_1}{r_2}=\frac{I_1}{I_2}=\frac{I_2}{6 I_1}=\frac{1}{6}\)
∴ r1 : r2= 1:6
Question 5. How did Kossel explain the formation of ionic bonds?
Or
Liquid hydrogen chloride cannot conduct electricity, but molten sodium chloride can conduct electricity. Explain.
Answer:
Koss el’s explanation –
- At the time of the chemical bond between two different elements, an electro-positive atom of the element gives out one or more electrons from its orbit and an other electro-negative atom of another element receives the refused electron in its outermost orbit.
- Thus, the two atoms, like their nearest neutral gas, gain a symmetrical electron pattern.
- Then, the Cation and the Anion with the coulombian attraction force, form an ionic bond.
Or
Hydrogen chloride is a compound with the same valency; so, there is no ion even when it is liquid. So, liquid hydrogen chloride is not able to conduct electricity.
Again NaCl is a ionic compound even in the solid state, it has Na* and Cl- ions in it.
Question 6 . Distinguish between Sodium chloride and naphthalene by two physical properties.
Answer:
Difference between Sodium chloride and naphthalene by two physical properties

Question 7. Between two aqueous solutions, one is Ferric chloride and the other is Aluminium
chloride. How would you identify the Ferric chloride solution using an aqueous solution of ammonia? Answer with a balanced chemical equation.
Answer:
In two solutions, by adding the solution of ammonia separately, brown residual is formed. That solution can be identified as Ferric Chloride. Moreover, the other solution with white gum-like residual is the solution of aluminum chloride.
Equation:
FeCI3 +3NH4OH → Fe(OH)3↓+3NH4 CI
AICI3 +3NH4OH → Al(OH)3 +3NH4 CI
Question 8. Why zinc blend can be called both mineral and ore of zinc?
Or
Mention two ways of preventing rusting of iron.
Answer: Zinc blend (ZnS) is naturally hard and metallic are found in mines. Hence it is called the ore of zinc.
Or
- Galvanization or the layer of melted zinc on iron prevents rusting.
- Tar Clour etc. prevent rusting.
Question 9. Write with a balanced chemical equation what happens when methane is burnt in oxygen.
Or
Mention one use of each acetic acid and ethyl alcohol.
Answer: CH4 (methane) +2O2 – CO2+H2O
Or
- Acetic Acid – Vinegar, preserves fish and flush, to cook pickle.
- Ethyl alcohol – Resin, scent artificial rubber and fiber, medicine when produced takes ethyl alcohol as a soluble agent.
WBBSE Class 10 Physical Science Question Answer Physical Science And Environment Answer The Following Questions
Question .1 What is meant by the moler volume of a gas? Mention two reasons for the deviation of real gases from the behavior of ideal gases.
Answer:
Moler volume of a gas:
In a certain temperature and pressure the volume of 1-mole quantity of a gaseous element is called the molar volume of the gas.
Ideal gas molecules are similar to mass point, but real gas molecules, no matter how small. They are, and cannot be ignored. Molecules of ideal gases have no force of attraction and distraction among them. Molecules of real gases show attractive force.
Question 2. How many grams of ammonium sulfate is required to prepare 558 g of by the reduction of Fe2O3 with Al at high temperature? How many moles of Fe2O3required in the reaction? [Fe=55 8. Al = 27.0 = 16]
Or
By heating 32.1 g ammonium chloride with calcium hydroxide 10.2g NH3 33.3 g CaCI, and 10.8 g H2O are obtained. How many grams of calcium hydroxide take part in the reaction? How many mole of NH3, and how many liters of NH3, at STP are formed in the reaction? (N = 14. H=1)
Answer
Fe2O3+2 Al→ 2Fe + Al2O3
2×278→ 2×55.8g
2×55.8g Fe needs 2x27g Al.
558g Fe needs = \(\frac{2 \times 27 \times 558}{2 \times 55.8}\)
= 270g Al.
Again,
2x27g Al reacts with 1 mol Fe2O3.
270g Al reacts with mol Fe2O3
Or
Answer: As per the law of the regularity of mass, the mass of NH4 Cl+ mass of Ca(OH)2=
NH3 mass + CaCl2 mass + H2O mass
32.1g+ mass of Ca(OH)2
= 10.2g+33.3g + 10.8g
Or
Mass of Ca(OH)2= (10.2 +33.3 + 10.8)g-32.1g = 54.3g-32.1g = 22.2g
We know, 17g NH3 = 1 mol NH3
10.2g NH3 =\(=\frac{1}{17} \times 10.2 \mathrm{~mol}\)x 10.2 mol
= 0.6mol
In STP, 1 mol. NH, volume = 22.4litre
∴ 0.6 mol. NH3 volume = 22.4×0.6 litre = 13.44 litre.
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 2 PDF”
Question 3. Which quantities remain fixed in the definition of the volume expansion coefficient of a gas? Name a non-metal which is a good conductor of heat.
Or
What is meant by ‘the linear expansion coefficient of copper is 17 x10-6 °C? Why does the value remain the same even in Kelvin scale?
Answer:
- The quantities remaining fixed are
- The pressure of the gas, and
- Mass of the gas.
Diamond is a nonmetal which is a good conductor of heat.
Or
The linear expansion coefficient of copper is 17×10-6 means if 1°C temperature is increased of any copper rod, the length of the rod will increase by 17×10-6 portion of the primary length of the rod.
Since the change of 1°C and the change of 1 K are the same, the value remains the same in Kelvin Scale also.
Question 4. What type of mirror is used by dentists? Why a ray of light does not deviate as a result of refraction through a glass slab?
Answer:
The dentists use a concave mirror
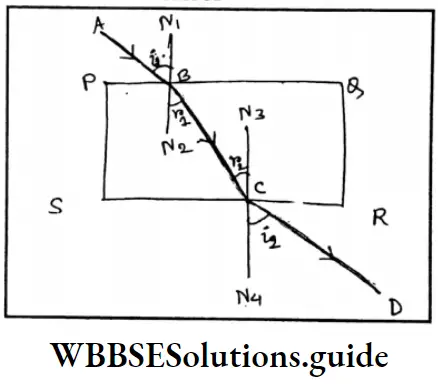
Here PQRS is a rectangular glass slab. ABCD is the direction of a ray of light. N1N2 and N3 N4 are two perpendiculars on B and C points where the reflection takes place. Al point B, because of refraction of light, ABN1 = (angle of incidence) and CBN2 =r, (refraction angle). At point C, because of refraction, BCN3 =r2 (angle of incidence) DCN4 = i2 (refraction angle).
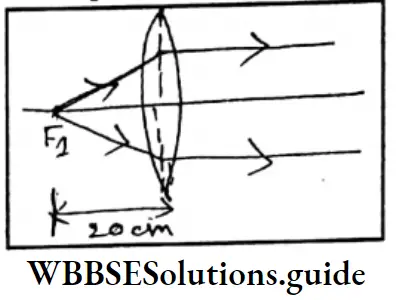
Question 5. When an object is placed 20 cm away from a convex lens, no image is obtained on either side of the lens. What is the focal length of the lens? If the refractive index of glass with respect to air is 1.5, what is the refractive index of air with respect to glass?
Or
The length of an object is 5 cm. An image of length 10 cm is obtained when it is placed at.a distance of 2 cm in front of a convex lens. What is the linear magnification and image distance?
Answer:
Given
The length of an object is 5 cm. An image of length 10 cm is obtained when it is placed at.a distance of 2 cm in front of a convex lens.
The slab of glass is rectangular. The two opposite sides are parallel. Hence,
⇒ N1 N2 II, N3 N4
Again N1 N2 II, N3 N4and transversal is BC.
<N2 C= <BCN3 (Alternative Angle)
r1 = r2 , Again, refraction angle i2 = i1 AB || CD i1 = i2.
∴ The ray does not deviate
The focal length of the lens = 20cm
At a distance of 20 cm from the convex lens, an object is placed. Then the refracted rays of light will be parallel.
∴ In respect of air, the refraction index of glass will be
⇒ \(g^\mu \text { air }=\frac{1}{\text { air } \mu g}=\frac{1}{1.5}\)
= 0.67
Air μg = 1.5
Or
Answer:
In respect of glass, a refractive index of air will be –
- Length of the object (h1) = 5cm
- Length of the image (h2 ) = 10cm
∴ Linear magnification = m
= \(\frac{h_2}{h_1}\)
= \(\frac{10}{5}\)
∴ m = 2
∴ Image distance (V) = mu = (2×2) m = 4cm.
“Class 10 WBBSE Madhyamika Model Question Paper 2023, Physical Science Set 2 practice questions”
Question 6. Write in brief the basic principle of hydroelectric power generation.
Answer:
The basic principle of hydroelectric power generation
The principle of hydropower is that the potential energy of water that is stored at great heights in the dam is changed to kinetic energy by allowing the water to flow at high speed. The kinetic energy of flowing water is utilized to produce electricity.
Question 7. A current of 1A flow when an electric bulb is connected to 220 V mains. What would be the current when the same bulb is connected to 110V mains?
Or
Find the ratio of resistances for two bulbs of 220V-60W and 220V-60W.
Answer:
A current of 1A flow when an electric bulb is connected to 220 V mains.
Potential difference (V2) = 220V and current flow in the bulb (I1) = 1A.
Again Potential difference (V2) = 110 V
Let, the current flow in the bulb for the second time = I,
Resistance of the bulb = R
∴ V1 =I1R ___________(1)
V2 =I2R______________(2)
∴ \(\frac{V_1}{V_2}=\frac{I_1}{I_2}\) Or,
⇒ \(I_2\frac{V_2 I_2}{V_1}\)
⇒ \(I_2\frac{110 \times 1}{220}\)
I2= 0.5A
Or
Answer:
R= \(\frac{V_2}{P}\)______________(1)
\(=\frac{220 \times 220}{60} \Omega\)____________(2)
\(\frac{110 \times 110}{60} \Omega\)____________(3)
⇒ \(2 \div 3=\frac{R_1}{R_2}=\frac{220 \times 220}{60} \times \frac{60}{110 \times 110}\)
= \(\frac{R_1}{R_2}\)
∴ R1 : R2
Question 8. Explain why a new element is formed by a particle emission but no new element is formed by y-ray emission from a radioactive element.
Answer:
The nature of a particle is like He2+
- From the nucleus of a radioactive element, when a particle is emitted, the mass number of the new nucleus is 4 units less.
- The atomic number is less by 2 units So, so a new element is formed a radioactive element.
- Again, the Y-ray has a small wavelength. It is electromagnetic. In the case of y-rays, the atomic number is unchanged So, no new element is formed.
Question 9. Write down Dobereiner’s law of triads. Arrange Cl, Br, I, F in increasing ‘order of their oxidizing power.
Or
What is the important conclusion of Moscley’s experiment? What is me impor- tance of this conclusion in regard to the periodic table?
Answer:
Dobereiner’s law of triads.
Dobereiner’s law of triads states that the atomic mass of the middle element of a triad is he arithmetic mean of the atomic masses of the two other elements. Increasing order of oxidizing power-1<Br<el<F.
Or
Answer: Mosley concluded that there were three unknown elements between aluminum and gold. There were only 92 elements up to and including uranium and 14 rare-earth elements.
The importance of this conclusion in the case of the periodic table:
- Mendeleev’s periodic law is rectified.
- The elements are arranged in the periodic table by atomic number instead of atomic masses.
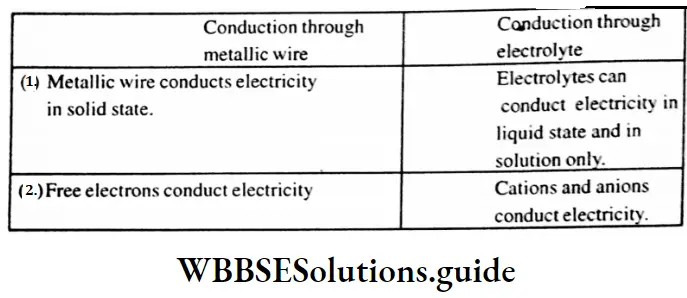
Question 10. Write two differences between the conduction of electricity through a metallic wire and an electrolyte during electrolysis. In the electrolytic refining of copper metal, impure copper rod is used as which electrode?’
Answer:
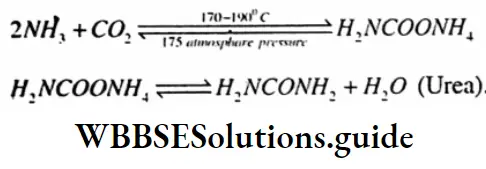
Question 11. Write the names of the chemicals used and the balanced chemical equation in the industrial production of urea.
Answer:
The chemicals are :
- Liquid ammonia
- Liquid CO2
- Balanced chemical equation
“WBBSE Class 10 Model Question Paper 2023, Physical Science and Environment Set 2 exam pattern”
Question 12. (A) and (B) are two unsaturated hydrocarbons, each containing 2 carbon atoms. On reaction with bromine per molecule and (B) adds two molecules of bromine per molecule. Write the structural formula of (A) and (B). Write a balanced chemical equation of the reaction of (B) with bromine.
Answer:
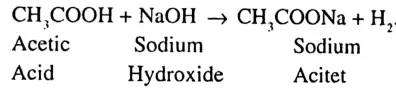
Or
Write a balanced chemical equation of the reaction of sodium hydroxide with acid. Which one between just and polyethylene is environment-friendly for packaging and why?
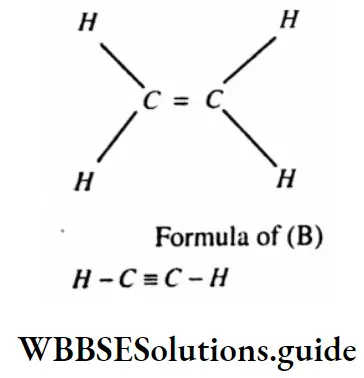
(A) and (B) are two unsaturated hydrocarbons, each containing 2 carbon atoms. On reaction with bromine per molecule and (B) adds two molecules of bromine per molecule.
The balanced chemical equation of the reaction of (B) with bromine
HC ≡ CH+2Br2—CHBr2–CHBr2( 1, 1, 2, 2-Tetrabromithane)
Or
Answer:
The equation is –
Jute is environment-friendly. It is formed with cellulose organic polymer. So, it is decomposed by bacteria and fungi, etc. easily. Hence, it is environmentally friendly.
