WBBSE Class 10 Physical Science Question Answer In English
Multiple Choice Questions Physical Science And Environment
Question 1. Which of the following greenhouse gases has the maximum contribution towards global warming?
- N2O
- CH4
- CO2
- H2O
Answer: 3. CO2
Question 2. According to Boyle’s law what is the PV-P graph?
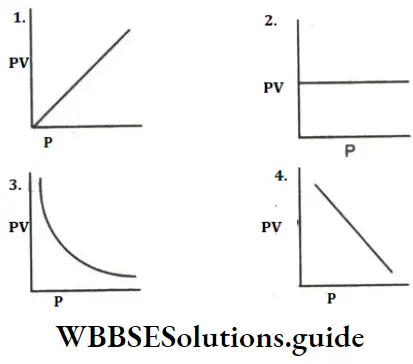
Answer: 2 PV-P Graph
Read And Learn More: WBBSE Solutions For Class 10 Physical Science And Environment
Question 3. If the vapour density of a carbon-containing gaseous substance.is 13. Which of the following can be its molecular formula?
- CO2
- C2H4
- C2H
- C2H2
Answer: 4. C2H2
Question 4. The unit of coefficient of linear expansion of a solid is
- m
- nr!
- °C-1
- °C
Answer: 3. °C-1
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 3”

Question 5. An object is placed in between the optical centre and the focus of a thin convex lens. What is the nature of the image of the object?
- Real and inverted
- Virtual and inverted
- Rea and erect
- Virtual and erect
Answer: 4. Virtual and erect
Question 6. When a ray of light is incident perpendicularly on a transparent glass slab, what will be its angle of deviation?
- 0°
- 180°
- 30°
- 90°
Answer: 1. 0°
Question 7. Which of the units given below is the SI unit of resistance?
- Volt
- Ampere
- Coulomb
- Ohm
Answer: 4. Ohm
“Class 10 WBBSE Model Question Paper 2023, Physical Science and Environment Set 3 study material”
Question 8. In a domestic electric circuit, the fuse wire is connected to which of the following? live line
- Earth line
- Live line
- Neutral line
- Both live and neutral line
Answer: 2. Live line
Question 9. β-ray emitted from a radioactive clement is an electromagnetic wave
- A stream of electrons
- A stream of neutrons
- A stream of protons
- Electromagnetic wave
Answer: 1. A stream of electrons
Question 10. How many groups are there in the long periodic table?
- 7
- 8
- 9
- 18
Answer: 4.18
Question 11. Information of which of the following compounds’ octet rule is not obeyed?
- NaCl
- LiH
- KCl
- CaO
Answer: 2. LiH
Question 12. Which of the following can conduct electricity?
- Molten NaCl
- Liquid HCI
- Solid NaCl
- Aqueous solution of glucose
Answer: 1. Molten NaCl
Question 13. What will be the colour of (the resulting solution when excess aqueous ammonia is added to an aqueous solution of copper sulphate?
- Yellow
- Green
- Deep blue
- Brown
Answer: 3. Deep blue
Question 14. In which of the following alloys zinc is present?
- Bell metal
- Brass
- Bronze
- Duralumin.
Answer: 2. Brass
Question 15. Which of the following is a saturated hydrocarbon?
- C3H6
- C2H
- C2H2
- C2H6
Answer: 4. C2H6
WBBSE Class 10 Physical Science Question Answer Physical Science And Environment Answer The Following Questions
Question 1. Mention one use of biogas.
Or
What is the role of NO in the decomposition of ozone in the ozone layer?
Answer:
Use of biogas
Biogas-1st is used as a fuel.
Or
Role of NO – No reacts with ozone(O3) and decomposes NO2 and O2.
Question 2. Among charcoal, petrol and ethanol which one is a fossil fuel?
Answer: Petrol is a fossil fuel.
“WBBSE 2023 Madhyamika Physical Science and Environment, Set 3 Model Question Paper download”
Question 3. Under constant pressure, at what temperature in degrees Celsius, the volume of an ideal gas will be zero according to Charles’ law?
Answer: The volume of an ideal gas will be zero at a temp. of -273° C.
Question 4. What is the unit of M in the equation PV –RT? (Symbols have usual meaning)
Answer: Unit of M-g/mol.
Question 5. Whether the following statement is ‘true’ or ‘false’? The real expansion of any liquid depends on the expansion of the vessel in which it is kept.
Or
Among iron, invar and copper which one has the least coefficient of linear ex- pansion?
Answer: True.
Or
Answer: Invar has the least coefficient of linear expansion.
Question 6. Between the angle of incidence and the angle of refraction which one is greater when light travels from a rarer to a denser medium?
Answer: The angle of incidence is greater than the angle of refraction.
Question 7. What type of mirror is used in the viewfinder of a motor car?
Answer: Concave mirror.
Question 8. How does the resistance of a semiconductor change with the increase of temperature?
Answer: The resistance of a semiconductor decreases with an increase in temperature.
WBBSE Class 10 Physical Science Question Answer
Question 9. Which type of energy is transformed into electrical energy in a dynamo?
Answer: Mechanical energy.
Question 10. Arrange [5 and 7-rays in ascending order of their penetrating power.
Or
Which kind of nuclear reaction is the source of the sun’s energy?
Answer: α<β<γ.
Or
Nuclear fusion.
Question 11. Match the right Column with the left Column:
Left Column – Right Column
An alkali metal K – The most electronegative element F
An element whose anion Fe accelerates the rusting of iron – Extracted from haematite – Fe
Extracted from haematite K – An alkali metal – K
Most electronegative element Cl – An element whose anion accelerates the rusting of iron Cl
Question 12. What type of chemical bond is present in CaO?
Answer: CaO has an ironic bond between them.
Question 13. What is used as a cathode to electroplate silver over a copper spoon?
Or
Give an example of a compound whose aqueous solution is a weak electrolyte.
Answer:
A copper spoon is used as a cathode.
Or
Weak electrolyte → NH3
Question 14. During electrolysis which electrode is called a cathode?
Answer: The electrode connected to the negative terminal of a battery is called the cathode.
Question 15. State one use of liquid ammonia.
Or
Write the formula of the precipitate formed when an aqueous ammonia solution is added to an aqueous solution of aluminium chloride.
Answer:
Use of liquid Ammonia-used as a refrigerant in ice making.
Or
NH4Cl precipitate is formed.
Question 16. In the laboratory preparation of nitrogen, an aqueous solution of which compound is mixed will aqueous solution of ammonium chloride and healed?
Answer: A concentrated solution of aqueous sodium nitrate is used.
Question 17. Write the IUPAC name of CH3, CH2CHO.
Or
Wine the structural formula of positional CH3 CH2 CH2 OH.
Answer: Propanal.
Or
Question 18. Mention one use of polytetrafluoroethylene.
Answer: Use coating articles and cookware to make them non-sticky.
WB Class 10 Physical Science Question Answer Physical Science And Environment Answer The Following Questions
Question 1. What is methane hydrate?
Answer:
Methane hydrate
Methane hydrate is a crystalline solid consisting of a methane molecule surrounded by a cage of interlocking water molecules.
Question 2. The pressure of a fixed mass of a gas at a temperature of 0°C. is doubled while the volume is halved. What will be the final temperature of the gas?
Or
Under constant pressure fixed mass of a gas is heated from 0°C to 546°C. What is the ratio of the final volume of the gas to its initial volume?
Answer:
Given
The pressure of a fixed mass of a gas at a temperature of 0°C. is doubled while the volume is halved.
No, the final temperature will not change As, PV = K according to Boyle’s law.
⇒ 2P × V/2 = PV = K
Or
According to Charle’s law,
Vt = V0 (1+_t/273)
Or, Vt = V0 (1+2)
Or, Vt= 3V0
Therefore, volume changes 3times the initial volume.
“WBBSE Madhyamika 2023 Physical Science and Environment, Set 3 Model Question Paper with solutions“
Question 3. What is meant by the optical centre of a convex lens?
Or
Why does the earth’s sky appear blue during daytime?
Answer:
Optical centre of a convex lens
If a ray of light strikes one surface of a lens that emergent ray from the other surface is parallel to it, and the corresponding refracted ray passes through a definite point on the principal axis. The point is the optical centre of the lens.
Or
According to Rayleigh’s law,
Scattering a 1/ γ4 [y wavelength of light]
The lesser the wavelength of light, more will be the scattering of light, since we know, the wavelength of blue is much less and is sensitive to our eyes. So sky appears blue during day time.
Question 4. State Lenz’s law related to electromagnetic induction.
Answer:
Lenz’s law related to electromagnetic induction
According to Lenz’s law, the induced current will be such that it will oppose the cause producing it.
Question 5. Write with an example how according to Lewis’s concept a covalent bond is formed.
Or
Why the bond in sodium chloride cannot he expressed as Na-CI?
Answer:
Each atom achieves a Lewis octet by forming a double bond. A covalent bond takes two electrons it occurs between two atoms and the electrons are shared euqally. Example water (H2O).
Or
The bond in Sodium chloride cannot be expressed as Na-Cl because the solution contains only Na+ and Cl- ions and water, but not the metal Na(S).
Question 6. Give one example each of a liquid and a solid covalent compound.
Answer:
Liquid covalent bond → H2O.
Solid covalent bond → CH4
Question 7. Write with a balanced chemical equation what happens when H2S gas is passed through an aqueous copper sulphate solution.
Answer: CuSO4 +H2S→ CUS+H2SO4
Question 8. Write down the cathode reaction when an aqueous solution of MSO2 (M = metal) is electrolysed. Write with reason whether the reaction is oxidation or reduction.
Or
Give one use of each copper and aluminium.
Answer:
Cathode reaction –
M2++ 2e → M.
Reductions placed at the cathode.
Or
Coppered used to make household utensils.
Aluminium is used to make the external parts of aeroplanes.
Question 9. What is the condition of the substitution reaction of methane with chlorine? Write tile balanced chemical equation of the first step of the reaction.
Or
Write with a balanced chemical equation what happens when ethanol reacts with metallic sodium.
Answer:
Condition → displacement of one atom occurs.
→ Occurs in unsaturated hydrocarbons.
Or
2C2H5 OH+2Na →2C2H5ONa+H2
Class 10 Physical Science WBBSE Answer The Following Questions
Question 1. Establish an ideal gas equation on the basis of Boyle’s law, Charles’s law and Avogadro’s law.
Answer: Equation according to Boyle’s law.
⇒ PV=K (P = Pressure of gas, V= its volume, K= proportionality constant.) Charles law – VIT K = Constant.
⇒ Avogadro’s law – PV=nRT [ R= molar gas constant]
Question 2. SO2 required for the industrial production of sulphuric acid is produced by burning iron pyrites in excess air current.
The chemical equation of the reaction is given below:
4FeS2+11O2→ 2Fe2O3 +8SO2. How many grams of FeS2 is required for the production of 512g of SO2 Fe= 56, S= 32, O=16).
Or
By heating 200g of a metal carbonate 112g of metal oxide and a gaseous compound are produced. Vapour density of the gaseous compound is 22. How many moles of the gaseous compound is produced in the reaction?
Answer:
Given
By heating 200g of a metal carbonate 112g of metal oxide and a gaseous compound are produced. Vapour density of the gaseous compound is 22.
SO2 512g.
Molecular mass of SO2 →32+32 = 64
Moles of SO2 = 512/64 = 8mol.
For 8moles of SO2
4moles of FeS2 is required
Molecular mass of FeS2 → 56 +64 = 120g.
Therefore, 4moles of FeS2 → 120 x 4 = 480g.
480g of FeS2 is required.
Or
200g → 112g
MCO3 MO
Vapour dencity = 22. Mass of the gaseous component = 200-112= 8g.
Molecular weight = 2 x V.D
= 2 x 2.2
= 4.4
No of moles= 8/4.4
= 1.81 moles.
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 3 PDF”
Question 3. What is thermal conductivity? What is its SI unit?
Or
Define the coefficient of surface expansion, Write its SI unit.
Answer:
Thermal conductivity
The coefficient of thermal conductivity at the material of a substance is numerically equal to the quantity of heat that conducts in one second normally through a slab of unit length and unit area, that difference of temperature between its end faces, being one degree. Its SI Unit is Jm’ Or, Wm’ K-‘.
Or
It is defined as the fractional change in the surface area of the solid per degree rise in temperature.
β = \(\frac{S_2-S_1}{S_1\left(t_2-t_1\right)}\) S1 and S2 are the surface areas of a solid at t1 and t2 respectively. Y= coefficient of surface expansion unit
α = K-1 or °C-1. γ = K-1 or, °F-1
β = K-1 Or °C-1 Or °F γ = K-1 Or °C-1 Or °F-1
Question 4. How can an erect and magnified image be formed with the help of a convex lens? With the help of which type of lens long-sightedness can be rectified?
Answer: An erect and magnified image can be formed by keeping the object between the focus and the optical centre of a convex lens.
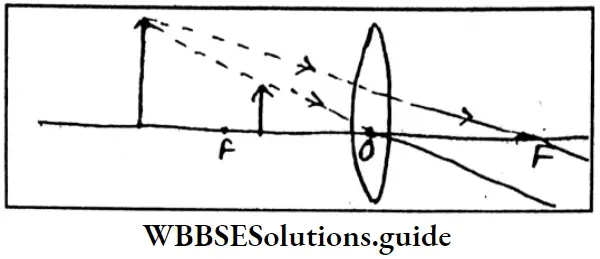
Long-sightedness can be rectified using a concave lens.
Question 5. If the velocity of light in a medium is 2x10s m/s, what will be the refractive index of that medium?
Or
The refractive index of a medium with respect to air is V2. If the angle of incidence of a ray of light in air is 45° determine the angle of deviation for that ray in case of refraction.
Answer:
If the velocity of light in a medium is 2x10s m/s
We know,
⇒ \(\mu\frac{v}{c}=\mu=\frac{2 \times 10^8}{3 \times 10^8}\)
= \(\frac{2}{3}\)
= 0.67
Or
Given,
⇒ μ = \(\sqrt{2}\). We know
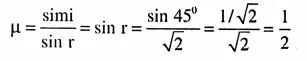
= r= \(\sin ^{-1}\left(\frac{1}{2}\right)\)
= r= 30°
∴ i-r
= S= 45 – 30 = 15°
∴ Angle of Direction is 15°
Question 6. Write ionic’s laws related to the heating effect of current.
Answer:
Joule’s Laws:
- The amount of heat produced in a conductor in a given interval of time is proportional to the square of the current passed. H∞l (when R and t are constant).
- The amount of heat produced by a given current in a given time is proportional to the resistance of the conductor. H∞ R (when I and t are constant).
- The amount of heat produced in a given conductor by a given current is proportional to the time for which the current passes. H∞t (when I and R are constant).
“WBBSE Madhyamika Model Question Paper 2023, Physical Science and Environment Set 3 sample paper
Question 7. Calculate the equivalent resistance when a wire of resistance 10 ohm is divided in two equal parts and connected in parallel combination.
Or
There are two 60-watt lamps and two 80-watt fans in a house. The lamps and fans run 5 hours daily. Find out the expense in a month if a unit of electricity costs Rs. 4/-. (assume one month 30 days).
Answer:

Equivalent resistance
→ 1/Rl= \(\frac{1}{R}\)+ \(\frac{1}{R}\) = \(\frac{2}{R}\)
∴Rl = \(\frac{2}{R}\)
Or
Given, 2 60W bulb & 2 80W bulb.
∴ Total Power
= 2 (60 + 80)
= 2 x 140
= 280w
Question 8. Compare the charge and ionising power of the a and y rays. Mention one use of radioactivity.
Answer:
Pow in BOT unit
⇒ \(\frac{280 \times 5}{1000}\)
= 1. 4 B.O.T
Total expense = 1.4 x 4 x 30 = Rs. 168

One use of radioactivity: To estimate age: Dating technique.
Question 9. What is meant by the ionisation energy of an atom of an element? Arrange Li, Rb, K and Na in the increasing order of their ionisation energy.
Or
Mention the similarity of properties of hydrogen with one property of Group 1 elements and two properties of Group 17 elements.
Answer:
Ionisation energy of an atom of an elemen
It is the minimum energy required to remove a valence from a neutral gaseous atom Rb < K < Na < Li.
Or
Like all other Group 1 elements it has one electron in its outermost shell. Like all other group 17 elements it acquires noble gas configuration on gaining one electron.
Question 10. What is present along with pure alumina in the molten mixture which is electrolysed for the extraction of aluminium by electrolysis? What are used as cathode and anode in this electrolysis?
Answer:
Fluospar and Cryolite is used in the molten mixture.
Cathode – Graphite
Anode – Graphite rods.
“WBBSE Class 10 Model Question Paper 2023, Physical Science and Environment Set 3 exam pattern”
Question 11. Write the conditions and balanced chemical equation for the industrial production of ammonia by Haber’s process.
Answer:
Hober’s Process.
\(\mathrm{N}_2+3 \mathrm{H}_2 \underset{550^{\circ} \mathrm{C}}{\stackrel{200 \mathrm{atn}}{\longrightarrow}} 2 \mathrm{NH}_3\)Condition.
A pressure of 200 atm is required. Higher than this pressure may damage the plant.
A temperature of 550° C is maintained below this temp the process will be very slow.
A catalyst like finely divided iron is used, so that the maximum surface area of the catalyst can be used & molybdenum is used to increase its efficiency.
“Class 10 WBBSE Madhyamika Model Question Paper 2023, Physical Science Set 3 practice questions”
Question 12. The molecular formula of an organic compound is C2H4O2. The compound is soluble in water and on the addition of NaHCO3. to the aqueous solution of the compound CO2 evolved. Identify the organic compound. Write, with conditions and a balanced chemical equation, the reaction of the compound with ethanol.
Or
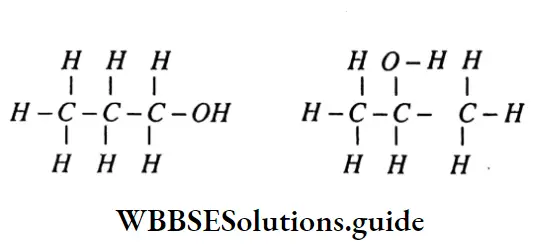
Compare three properties of organic and inorganic compounds..
Answer:
The molecular formula of an organic compound is C2H4O2. The compound is soluble in water and on the addition of NaHCO3. to the aqueous solution of the compound CO2 evolved.
Organic Compound is – CH3COOH
CH3COOH + C2H5OH H2SO4 → CH3 -C-O-C2H5 +H2O
Or