Amines
Amines Definition:
Amines are derivatives of ammonia. Alkylamines are derived from ammonia by replacing one, two, or three of the hydrogen atoms of ammonia with alkyl groups. In arylamines, one, two, or three of the hydrogen atoms of ammonia are replaced by aryl groups.
The amino group (NH2, NH, or N) is present in many important substances. The most significant of them are amino acids, proteins, and alkaloids. Some alkaloids have medicinal properties and some are poisonous.
“amines – compounds containing nitrogen notes”
Morphine, one of the most effective painkillers known, quinine, an important drug for the treatment of malaria, and nicotine, the toxic component in tobacco, are among some of the alkaloids that have been isolated and used by mankind.
Many vitamins, antibiotics, and drugs contain amino groups. Adrenalin, a secondary amine, causes constriction of blood vessels and an increase in blood pressure. Novocaine (a quaternary ammonium salt) is frequently used as a local anesthetic by dentists. A well-known antihistaminic drug diphenylhydramine (Benadryl), is a tertiary amine.
“amines in organic chemistry notes”
Iodofore, a quaternary ammonium salt, is used as an antiseptic. The synthetic fiber nylon is made from two raw materials, one of which is a simple diamine. Benzene diazonium salts are useful intermediates for the preparation of a variety of aromatic compounds. Aniline, an aromatic amine, is used in the synthesis of various types of dyes. It is also used in the synthesis of sulpha drugs.

Amines Structure
In an amine, nitrogen is sp3 hybridized and the molecule is nearly tetrahedral. Of the four sp3-hybridised orbitals, three having one electron each form one sigma bond each with three alkyl groups or two alkyl groups and one hydrogen atom or one alkyl group and two hydrogen atoms.
The fourth hybridized orbital of nitrogen is filled with an electron pair and does not take part in bond formation. Therefore, the geometry of an amine is pyramidal, like that of ammonia.
“classification of amines with examples”
The C-N-C bond angle in trimethylamine is 108°, less than the tetrahedral bond angle of 109.5°. The contraction in bond angle in trimethylamine is possibly due to repulsion between the electrons forming the lone pair and repulsion between the three alkyl groups.
Classification Of Amines With Examples
Amines are classified as primary (1°), secondary (2°), and tertiary (3°), depending on whether one, two, or three hydrogen atoms of ammonia have been replaced by an alkyl or aryl group.
The general formulae and functional groups of primary, secondary and tertiary amines:
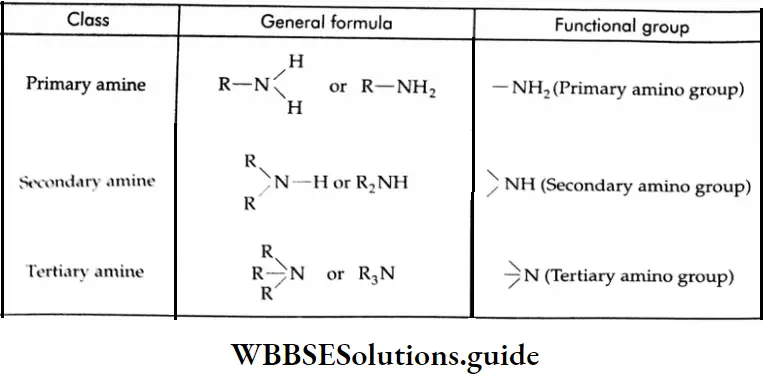
In secondary and tertiary amines, the alkyl or aryl groups may be the same or different. Amines are said to be ‘simple’ when all the alkyl or aryl groups are the same, and ‘mixed’ when they are different.

Nomenclature
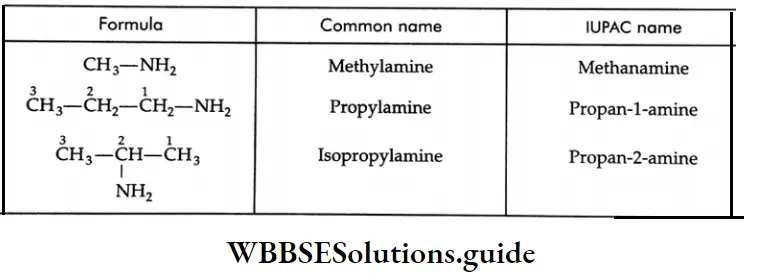
The common names of amines are derived by placing the suffix -amine after the name of the alkyl group. The names are written as single words. In the IUPAC system, primary aliphatic amines are named by adding the suffix -amine to the name of the corresponding alkane, the final ‘e’ of the name of the alkane being omitted. For example, methane-e+amine methanamine.
The common and IUPAC names of some primary amines:
In the case of secondary aliphatic amines, the common name is derived by writing the names of the two alkyl groups in alphabetical order.
When two identical groups are attached to the nitrogen atom, ‘di’ is prefixed to the name of the alkyl group. In the IUPAC system, secondary amines are named as N-substituted derivatives of alkanamines. The longest carbon chain containing an alkyl group attached to nitrogen is taken as the corresponding alkane of the alkanamine.
The common and IUPAC names of some secondary amines:

In the case of tertiary amines, the common name is derived by writing the names of the three alkyl groups in alphabetical order. When three identical groups are attached to the nitrogen atom, tri is prefixed to the name of the alkyl group. In the IUPAC system, tertiary amines are named N, N-disubstituted derivatives of alkanamines. The longest carbon chain containing an alkyl group attached to nitrogen is taken as the corresponding alkane of the alkanamine.
The common and IUPAC names of some tertiary amines:
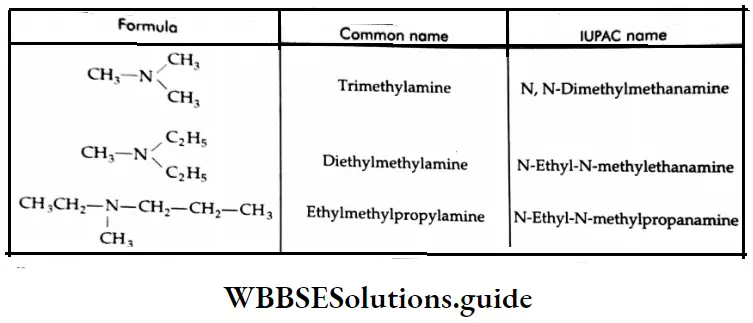
Ethylmethylpropylamine N-Ethyl-N-methyl propanamide
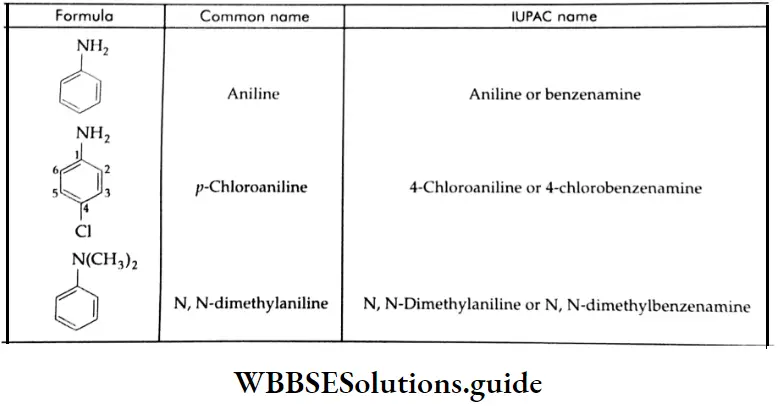
The common and IUPAC name of the simplest aryl amine is aniline. It is also an accepted IUPAC name.
“amines functional group and properties”
Simple derivatives are named substituted anilines. Also, in the IUPAC system, arylamines are named by adding the suffix ‘amine’ to the name of the arene after removing the ‘e’ of the arenes. Thus C6H5 NH2 is named benzenamine.
The common and IUPAC names of some arylamines and substituted arylamines:
Some examples on IUPAC nomenclature:
1. \(\left(\mathrm{CH}_3\right)_3 \stackrel{3}{\mathrm{C}} \stackrel{2}{\mathrm{C}} \mathrm{H}_2 \stackrel{1}{\mathrm{C}} \mathrm{H}_2 \mathrm{NHCH}_3\)
N,3,3 – Trimethyllbutamine
The formula is expended as follows:
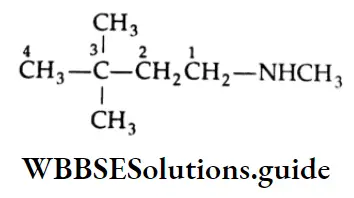
The compound is a secondary amine. Its longest chain has 4 carbons. So, it is an N-substituted butanamine. Here, one methyl group is attached to nitrogen (N) and two methyl groups are attached to C-3.
Therefore, its IUPAC name is N, 3, 3-Trimethylbutanamine.
2.
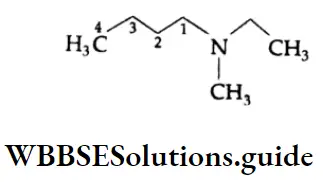
The compound is a tertiary amine. Its longest chain has 4 carbons. So, it is an N, N-disubstituted butanamine. Here nitrogen is attached with one ethyl group and one methyl group. In alphabetical order, ‘ethyl’ would be placed before ‘methyl’. Therefore, the IUPAC name of the compound is N-Ethyl-N-methylbutanamine.
Example 1: What are the eight amines with the formula C4H11N? Classify them as primary secondary and tertiary and write their IUPAC names.
Solution:
Primary amines:
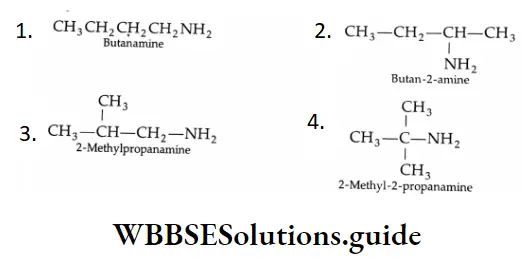
Secondary Amines

Tertiary Amines:
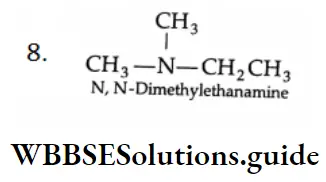
Methods Of Preparation From Alkyl Halides
Aliphatic amines can be prepared by heating alkyl halides with aqueous or alcoholic ammonia solution (excess) in a sealed tube at 373 K.
⇒ \(\mathrm{RX}+\text { conc. } \mathrm{NH}_3 \text { (excess) } \stackrel{\Delta}{\longrightarrow} \mathrm{R}^{+} \mathrm{H}_3 \mathrm{X}^{-}+\mathrm{NH}_3 \stackrel{-\mathrm{NH}_4 \mathrm{X}}{\longrightarrow} \underset{\text { Primary amine }}{\mathrm{RNH}_2}\)
Alkyl halides Mechanism:
This is a nucleophilic substitution reaction.
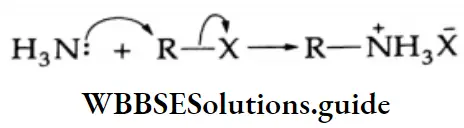
The free amine can also be obtained from the ammonium salt by treatment with NaOH.
⇒ \(\mathrm{R}-\stackrel{+}{\mathrm{NH}_3} \overline{\mathrm{X}} \stackrel{\mathrm{NaOH}}{\longrightarrow} \mathrm{R}-\mathrm{NH}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{NaX}\)
The process of cleavage of the C-X bond by an ammonia molecule called ammonolysis. The following is the order of reactivity of alkyl halides towards nucleophilic reagents.
RI > RBr> RCl
“preparation of amines in organic chemistry”
If a large excess of an alkyl halide is present in the reaction mixture, the primary amine obtained reacts further with the alkyl halide. Then secondary and tertiary amines are formed, and finally a quaternary ammonium salt.
⇒ R-Br+ NH3R-NH2 + HBr
⇒ R-NH2+ R-Br→ R2NH + HBr
⇒ R2 NH+R-Br→ R3N+ HBr
⇒ R3N+R-Br→R4 N+Br–
In this method a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt is obtained. However, the primary amine is obtained as the major product when a large excess of ammonia is used.
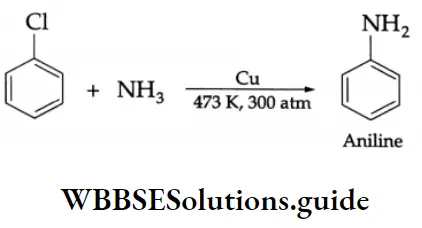
In normal conditions, an aryl halide does not react with ammonia as the halogen is firmly bound to the aryl ring. The reaction occurs under the conditions mentioned in the following chemical equation
By The Reduction Of Nitroalkanes, Cyanides (Nitriles), Amides And Oximes
From nitroalkanes
Nitroalkanes are reduced to primary amines by hydrogen in the presence of finely divided nickel, palladium or platinum as catalyst.
⇒ \(\mathrm{R}-\mathrm{NO}_2+3 \mathrm{H}_2 \stackrel{\mathrm{Ni}}{\longrightarrow} \mathrm{R}-\mathrm{NH}_2+2 \mathrm{H}_2 \mathrm{O}\)
Nitroalkanes are also reduced by LiAlH4 or Fe/HCl.
“chemical reactions of amines with acids”
⇒ \(\mathrm{R}-\mathrm{NO}_2+6[\mathrm{H}] \stackrel{\mathrm{LiAlH}_4}{\longrightarrow} \mathrm{R}-\mathrm{NH}_2+2 \mathrm{H}_2 \mathrm{O}\)
LiAlH4, is expensive and therefore Fe/HCl is preferred.
In the laboratory, nitrobenzene is reduced to aniline by Sn/HCI.
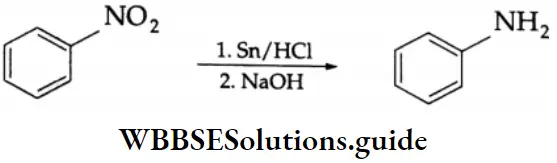
Aromatic nitro compounds are not usually reduced by LiAlH4.
From alkyl cyanides (alkyl nitriles)
Alkyl cyanides are reduced to primary amines by LiAlH4 or Pd/H2
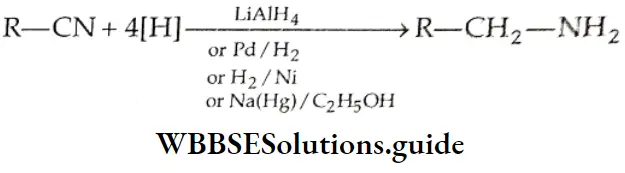
The reduction of an alkyl isocyanide forms a secondary amine.
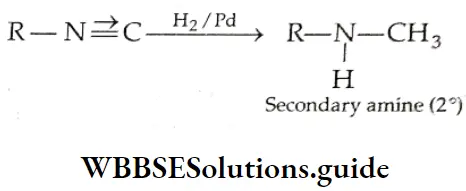
From acid amides
Acid amides are reduced to primary amines by LiAlH4.
⇒ \(\mathrm{R}-\mathrm{CONH}_2+4[\mathrm{H}] \stackrel{\mathrm{LiAlH}_4}{\longrightarrow} \mathrm{R}-\mathrm{CH}_2-\mathrm{NH}_2+\mathrm{H}_2 \mathrm{O}\)
The reduction of N-substituted amides by LiAlH4 gives secondary amines.
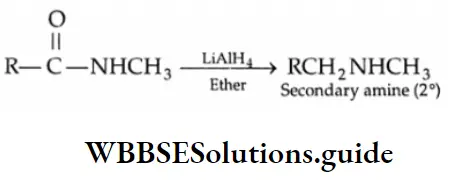
Dialkyl substituted amides, on reduction with LiAIH4 form tertiary amines.
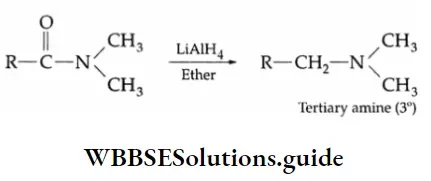
Example 2: Write equations showing how the following conversions may be accomplished
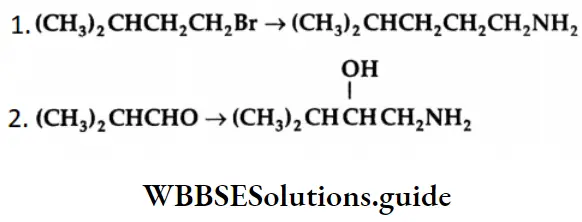
Solution:
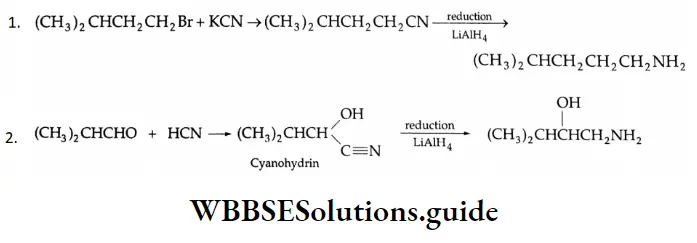
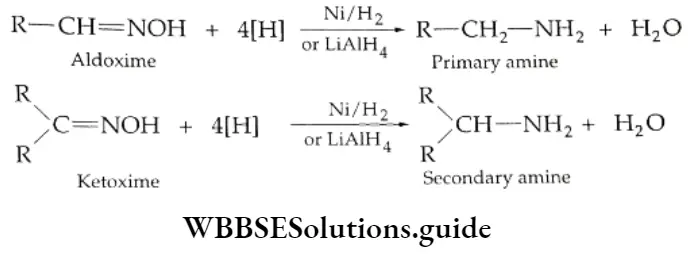
From oximes
The oximes of aldehydes and ketones are reduced to amines by Ni/H2 or LiAlH4.
From The Hofmann Bromoamide Degradation Reaction
Acid amides are converted into primary amines with the loss of one carbon atom by the action of hypobromite (Br2/NaOH).
⇒ \(\underset{\text { Acid amide }}{\mathrm{R}-\mathrm{CONH}_2}+4 \mathrm{NaOH}+\mathrm{Br}_2 \rightarrow \underset{\text { Primary amine }}{\mathrm{R}-\mathrm{NH}_2}+2 \mathrm{NaBr}+\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O}\)
Mechanism Of Hoffmann Bromide Degradation Reaction:
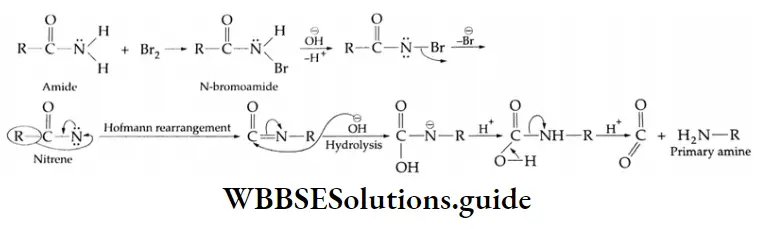
The conversion of the amide to the primary amine involves the migration of the alkyl or aryl group from the carbon atom to the electron-deficient nitrogen atom.
Classification of amines – primary, secondary, and tertiary amines
This is known as Hofmann rearrangement. The entire reaction is also called Hofmann degradation because the amine formed has one carbon atom less than the amide we start with.
On treatment with Br2/NaOH, benzamide gives aniline.
C6H5CONH2+ Br2+ 4NaOH → C6H5NH2+ Na2CO3 + 2NaBr + 2H2O
Example: Illustrate the following processes by means of equations giving reagents and conditions.
- Conversion of an amide to a primary amine with one carbon atom less
- Conversion of an amide to a primary amine with the same number of carbon atoms
Solution:
⇒ \(\mathrm{RCONH}_2+4 \mathrm{NaOH}+\mathrm{Br}_2 \rightarrow \underset{\text { (amine with one } \mathrm{C} \text { atom less) }}{\mathrm{R}-\mathrm{NH}_2}+2 \mathrm{NaBr}+\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{RCONH}_2 \underset{2 . \mathrm{H}_3 \mathrm{O}^{+}}{\stackrel{1 . \mathrm{LiAlH}_4}{\longrightarrow}} \underset{\text { (amine with same number of } \mathrm{C} \text { atoms) }}{\mathrm{RCH}_2 \mathrm{NH}_2}\)
Gabriel phthalimide Synthesis
Gabriel phthalimide synthesis is used to prepare aliphatic primary amines. Phthalimide is acidic and reacts with alcoholic KOH to yield potassium phthalimide, which on being heated with an alkyl halide gives N-alkylphthalimide. On hydrolysis, N-alkyl phthalimide gives an amine and phthalic acid.
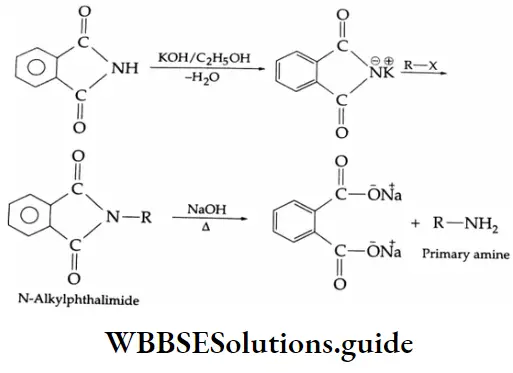
Example 3: Will Gabriel synthesis be useful for the preparation of t-butylamine? Explain.
Solution: No, because we cannot alkylate potassium phthalimide with (CH3)3 C-X. (By dehydrohalogenation, isobutylene will be formed).
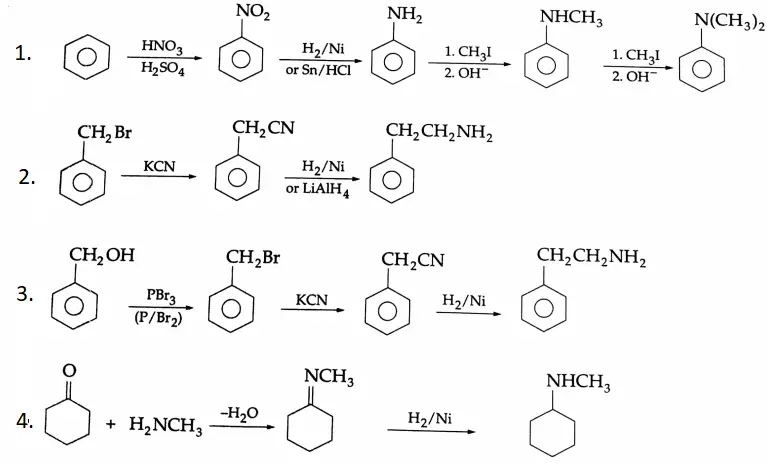
Example 4: In each of the following, how will you obtain the final product from the starting material?
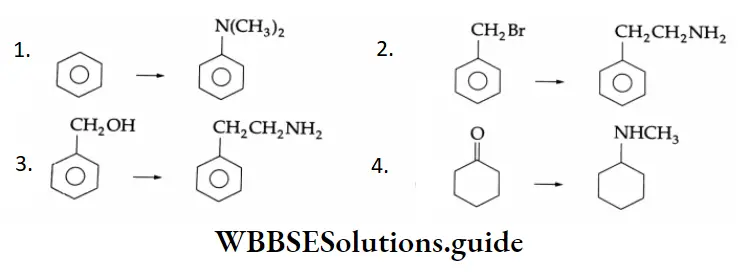
Solution:
Physical Properties
Lower aliphatic amines are gases or low-boiling liquids. Methylamine and ethylamine are gases. n-Propylamine is a liquid at room temperature.
- Pure aniline is a colorless liquid that slowly turns brown due to atmospheric oxidation.
- Diphenylamine (m.p. 327 K) and triphenylamine (m.p. 400 K) are solids.
Boiling point:
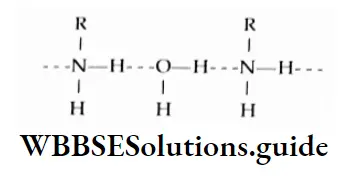
Primary and secondary amines are polar and participate in hydrogen bonding.
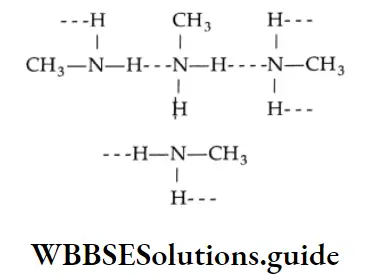
Since nitrogen is not as electronegative as oxygen, the nitrogen-hydrogen bond (N-H—N) is less polar than the oxygen-hydrogen bond (O-H—O). The boiling points of amines are, therefore, lower than those of alcohols of similar molecular weight, but higher than the boiling points of hydrocarbons and other compounds in which no hydrogen bonding is possible, e.g., tertiary amines. We will illustrate the trend in boiling points by considering the examples of n-pentane, butylamine, and n-butyl alcohol.
“basicity of amines explained”
⇒ CH3 CH2CH2CH2CH3-( n-Pentane mol. wt. 72b.p. 309 K)(no hydrogen bonding)
⇒ CH3 CH2CH2CH2NH2 Butylamine mol. wt. 73 b.p. 351 K
⇒ CH3CH2CH2CH2OH n-Butyl alcohol mol. wt. 74 b.p. 391 K
Amine Functional Group
The molecules of primary amines are associated due to intermolecular hydrogen bonding between the nitrogen of one molecule and the hydrogen of another. The molecules of secondary amines are also associated in a similar fashion but to a lesser extent than in primary amines.
A primary amine has two hydrogen atoms available for hydrogen bonding as compared to one hydrogen in a secondary amine. The molecules of tertiary amines are not associated similarly due to the absence of hydrogen atoms available for hydrogen-bond formation.
As the ability to form hydrogen bonds decreases from primary to tertiary amines the boiling points of primary, secondary, and tertiary amines of similar molecular weight also decrease in that order.
Structure, bonding, and properties of amines
⇒ CH3CH2CH2NH2 mol. wt. 59 b.p. 321 K
⇒ CH3NHCH2CH3 b.p. 309 K
Solubility:
Primary, secondary, and tertiary amines with five or fewer carbons are soluble in water as they can form hydrogen bonds with water. The solubility decreases as the hydrophobic alkyl part of the amine increases in size. Amines are soluble in polar solvents like alcohol and ether.
Chemical Properties
The reactivity of amines can be attributed chiefly to the presence of a lone pair of electrons on an electronegative nitrogen. Amines therefore act as nucleophiles.
The basic character of amines
Aliphatic as well as aromatic amines are Lewis bases. The basicity of amines depends upon the readiness with which the lone pair of electrons is available to a proton or to a Lewis acid.
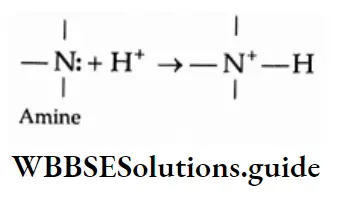
Nitrogen is less electronegative than oxygen, and hence it has a greater tendency to donate its lone pair than does oxygen. Therefore, amines are stronger bases than alcohol, ether or water. However, amines are far weaker bases than hydroxide ions, alkoxide ions, and carbanions.
Relative basicity of amines:
Aliphatic amines (CH3NH2, C2H, NH2, etc.) are more basic than ammonia because the electron-releasing alkyl groups increase the electron density around the nitrogen, thereby increasing the availability of the lone pair of electrons.
We might expect to see an increase in basic strength on going from NH3→RNH2 → R2NH → R3N, due to the increasing inductive effect of successive alkyl groups. The basicity of amines in the gaseous phase follows the expected order
⇒ R3N>R2NH>R-NH2> NH3
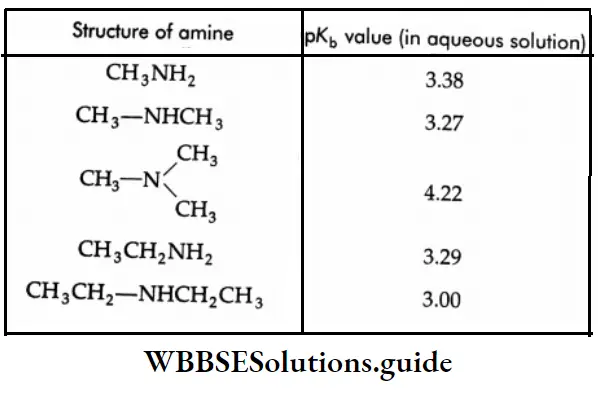
But this order is not observed in the aqueous state as evidenced by their pK, values given in Table.
The pK values of some amines:
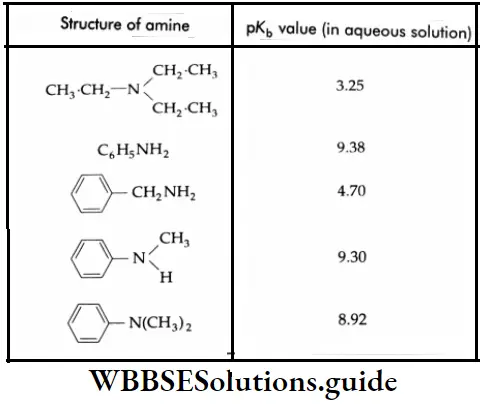
Primary Amine
The introduction of an alkyl group into ammonia increases the basic strength. The introduction of a second alkyl group further increases the basic strength. However, the introduction of a third alkyl group (as in a tertiary amine) decreases the basic strength.
This is because the basic strength of an amine in an aqueous medium is determined not only by electron availability on the nitrogen atom, but also by the extent to which the cation, formed by the uptake of a proton, can undergo solvation, and so become stabilized. The greater the number of hydrogen atoms attached to nitrogen in the cation, the greater is the possibility of solvation via hydrogen bonding between the cation and water.
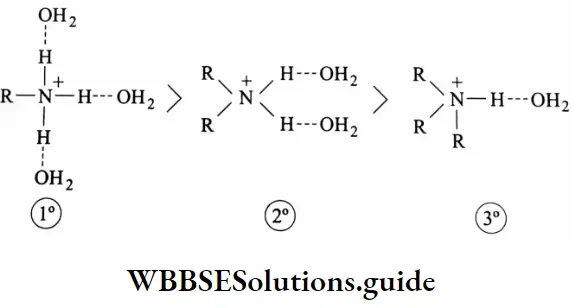
Thus on going along the series, NH3 →R-NH3→ R2NH→R3N, the inductive effect will tend to increase the basicity, but progressively less stabilization of the cation by hydration will occur, which will tend to reduce the basicity.
Therefore, in an aqueous solution, a tertiary amine becomes less basic than a secondary amine. Also, the three alkyl groups around nitrogen in a tertiary amine interfere with protonation, making it less basic. In fact, the basic strength of alkyl amines in an aqueous medium is decided by a combination of the inductive effect, solvation effect, and steric hindrance of the alkyl groups.
“amines vs amides structural difference”
The order of basicity of amines changes if the alkyl group is bigger (for example,C2H5) than the CH3 group due to steric hindrance to hydrogen bonding. The order of basic strength of methyl-substituted amines and ethyl-substituted amines in an aqueous medium is as follows.
⇒ (C2H5)2NH)> (C2H2)3N =C2H5NH2>NH3
⇒ (CH3)2NH >CH3−NH2 > (CH3)3 N > NH3
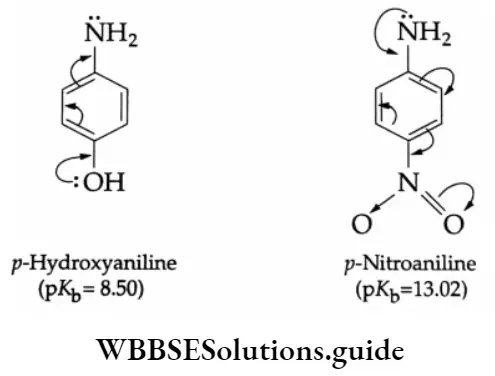
Aniline is less basic than methylamine. The low basicity of aniline is due to the delocalization of the lone pair of electrons on nitrogen with the electrons of the benzene ring, making the lone pair of electrons on nitrogen less available for protonation. The delocalization of the lone pair of electrons with the electrons of the benzene ring is shown in the following resonating structures of aniline.
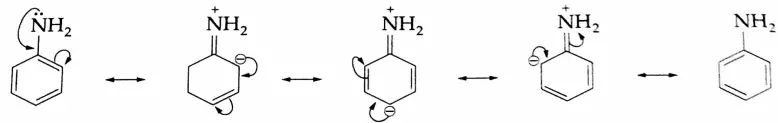
On the other hand, the anilinium ion obtained by the acceptance of a proton is a resonance hybrid of only two structures.
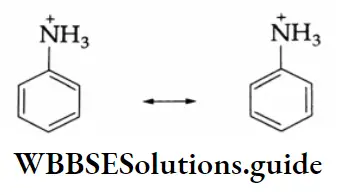
Thus aniline (5 resonating structures) is more stable than the anilinium ion (2 resonating structures) (the greater the number of resonating structures, the greater is the stability). Therefore, the basic nature (or capacity to accept protons) of aniline is less.
Electron-releasing groups like -CH3,-OH and -OMe in the para position increase the basicity of an aromatic amine, while electron-withdrawing groups like -NO2,-CHO, and -COOH reduce the basicity.
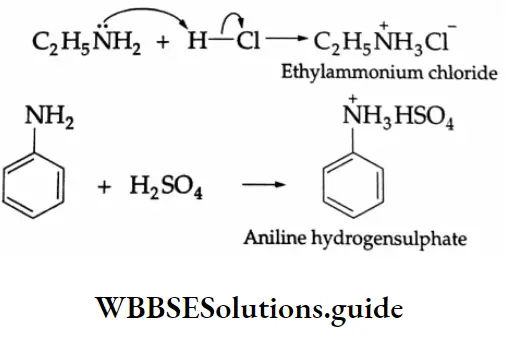
Ammonium Salt Formation
Since amines are basic they react with mineral acids to form ammonium salts.
The free amine can be obtained from the ammonium salt by treatment with NaOH.
⇒ \(\mathrm{R}-\stackrel{+}{\mathrm{NH}_3} \mathrm{X}^{-} \stackrel{\mathrm{NaOH}}{\longrightarrow} \mathrm{RNH}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{NaX}\)
Ammonium salts are soluble in water and are insoluble in organic solvents (example, ether).
On the basis of the above reactions, we can separate amines from nonbasic organic compounds insoluble in water. Suggest how aniline is separated from acetophenone (a ketone) on the basis of the above reactions.
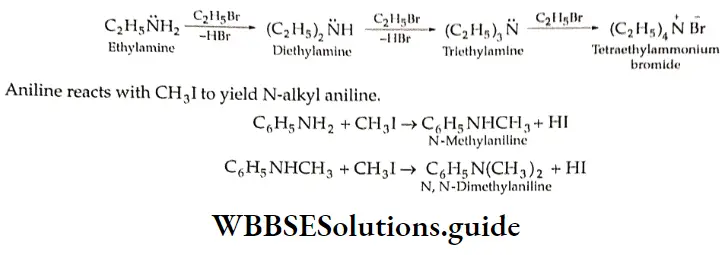
Alkylation
An alkylamine reacts with an alkyl halide to yield a dialkylamine, a trialkylamine, and a tetraalkylammonium halide (quaternary ammonium salt). For example,
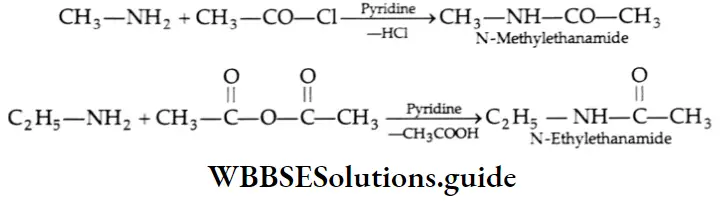
Acylation
Primary and secondary amines react with acyl halides, acid anhydrides, and esters to give substituted amides. This reaction is known as acylation. Tertiary amines do not have a free hydrogen atom and thus do not form amides. The acetylation reaction with acetyl chloride is often carried out in pyridine. Pyridine removes the HCl formed and shifts the equilibrium to the right-hand side.
Mechanism The amine acts as a nucleophile:
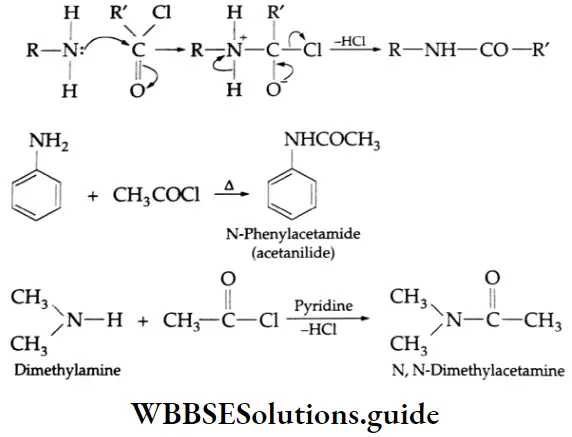
Because of the low basicity of amides, they do not readily undergo further acylation by acid chlorides. Amines react with benzoyl chloride (C6H5COCI) to form substituted benzamides. The reaction is known as benzoylation.
⇒ RNH2+C26H5COCI→ C6H5CONHR (Substituted benzamide)+ HCI
Carbylamine Reaction (Carbylamine Test)
Primary amines (aliphatic or aromatic) react with chloroform and ethanolic potassium hydroxide solution to yield isocyanides.
This reaction is often used as a test for the identification of a primary amine.
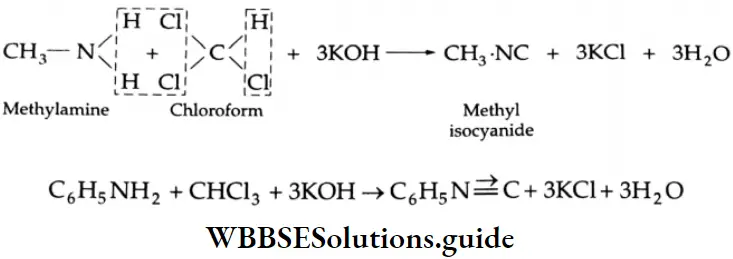
Secondary and tertiary amines do not undergo this reaction.
Reaction With Grignard Reagent
Primary and secondary amines react with a Grignard reagent to yield an alkane containing the same number of carbons as are present in the alkyl group of the Grignard reagent.
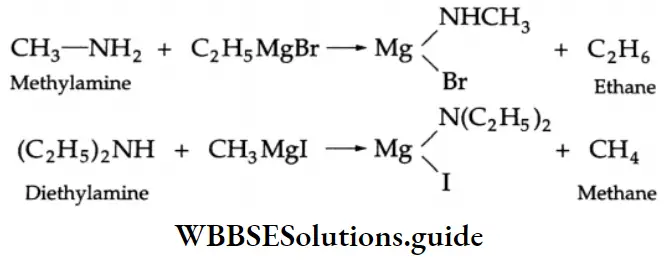
Tertiary amines do not undergo this reaction.
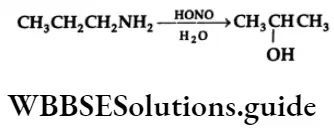
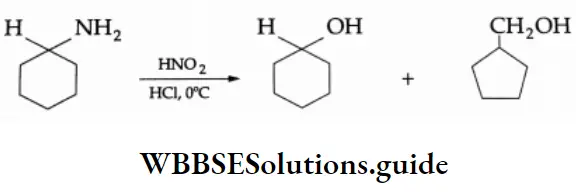
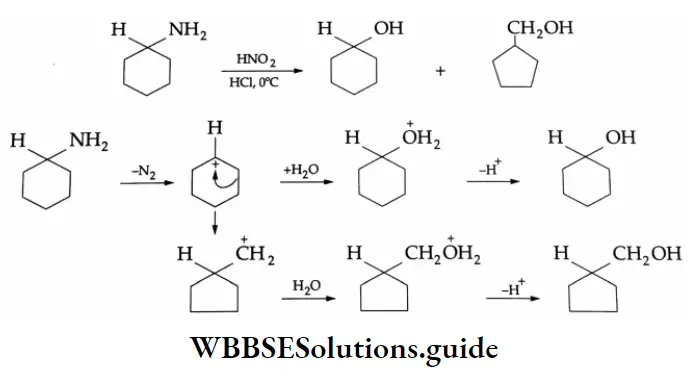
Reaction With Nitrous Acid
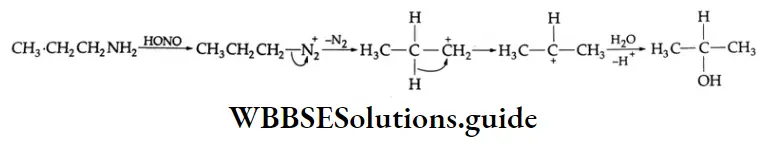
The reaction of aliphatic primary amines with nitrous acid (NaNO2 + HCl) at 273 K yields an unstable alkyl diazonium salt which decomposes to give an alcohol and liberate nitrogen quantitatively. The quantitative evolution of nitrogen is used in the estimation of amino acids and proteins.
⇒ \(\mathrm{R}-\mathrm{NH}_2 \underset{\left(\mathrm{NaNO}_2 / \mathrm{HCl}\right)}{\stackrel{\mathrm{HONO}}{\longrightarrow}}\left(\mathrm{R}-\stackrel{+}{\mathrm{N}_2}\right) \mathrm{Cl}^{-} \stackrel{\mathrm{H}_2 \mathrm{O}}{\longrightarrow} \mathrm{ROH}+\mathrm{N}_2 \uparrow\)
Example 5: Give The Mechanism of the following reaction
Solution:
Aromatic primary amines react with NaNO2/HCl to form stable diazonium salts.
Secondary amines, both aliphatic and aromatic, react with nitrous acid to produce N-nitrosamines, which separate from the reaction mixture as yellow oily liquids.
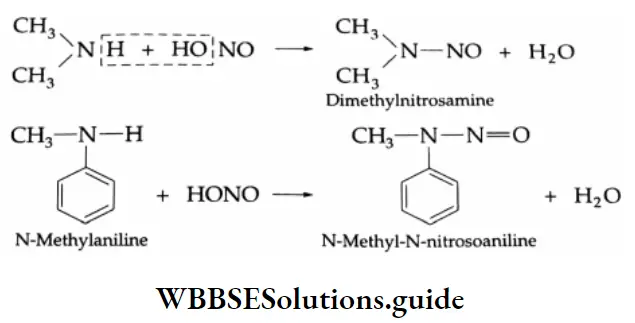
Nitrosamines are poisonous compounds and are carcinogenic.
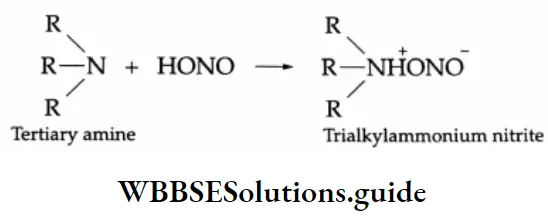
Tertiary amines simply dissolve in nitrous acid, forming a salt.
N, N-dimethylaniline an aromatic tertiary amine, reacts with nitrous acid to yield p-nitroso-N, N-dimethylaniline.
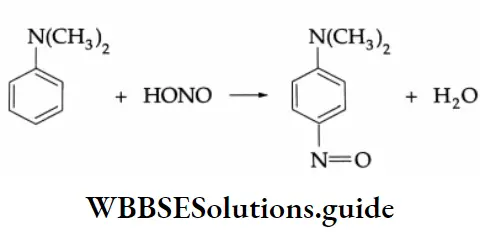
In this reaction, the nitroso group is substituted at the p-position of the benzene ring.
Example 6: Suggest a mechanism for the following reaction.
Solution:
Reaction With Aldehydes And Ketones
Primary and secondary amines react with aldehydes and ketones to form a-hydroxylamines, which usually lose water to give a substituted amine, frequently referred to as a Schiff base.
⇒ CH3CH2NH2 +CH3CHO→ C2H5N (Aldimine (Schiff base) =CHCH3 + H2O
Difference between aliphatic and aromatic amines
Aniline condenses with an aldehyde to form an imine or Schiff base with the loss of water. An aromatic amine forms a more stable Schiff base than an aliphatic amine.
⇒ C6H5NH2+C6H5CHO→C6H5N=CHC6 H5 + H2O
Reaction With Benzene Sulphonyl Chloride
Primary amines react with benzene sulphonyl chloride (Hinsberg reagent) to yield N-alkylbenzene sulphonamide.
⇒ \([\mathrm{R}-\mathrm{NH}_2+\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl} \rightarrow \underset{\text { N-Alkylbenzenesulphonamide }}{\mathrm{R}-\mathrm{NH}-\mathrm{SO}_2 \mathrm{C}_6 \mathrm{H}_5}\)
N-alkylbenzene sulphonamides have an N-H group, the hydrogen of which is acidic due to the powerful electron-withdrawing sulphonyl group. As a result, N-alkylbenzene sulphonamides are soluble in an excess of a cold NaOH solution.
⇒ \(\mathrm{R}-\mathrm{NH}-\mathrm{SO}_2-\mathrm{C}_6 \mathrm{H}_5+\mathrm{NaOH} \rightarrow \mathrm{R}-\stackrel{\ominus}{\mathrm{N}}-\mathrm{SO}_2-\mathrm{C}_6 \mathrm{H}_5 \stackrel{+}{\mathrm{Na}}+\mathrm{H}_2 \mathrm{O}\)
Secondary amines react with benzenesulphonyl chloride to yield N, N dialkylbenzenesulphonamide, which does not dissolve in an NaOH solution.
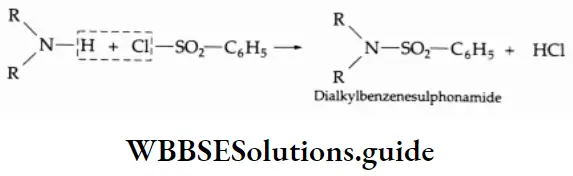
Tertiary amines do not react with benzene sulphonyl chloride.
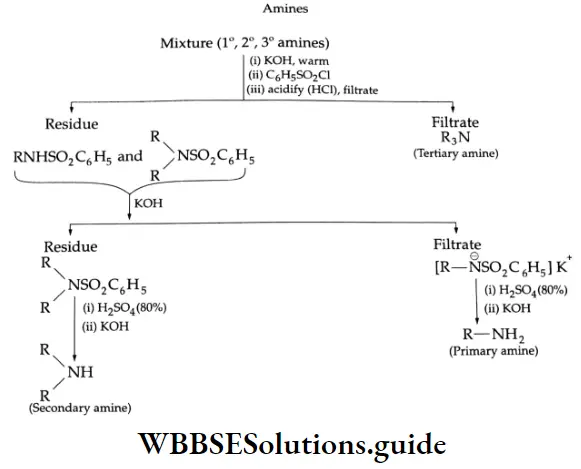
These reactions are used to distinguish between primary, secondary and tertiary amines, and to separate them. The test is known as the Hinsberg test.
Hinsberg test:
- To differentiate primary, secondary, and tertiary amines, the unknown amine is shaken in a test tube with benzene sulphonyl chloride and dilute sodium hydroxide solution. A primary amine is present if a clear solution is obtained.
- If an insoluble layer remains, it can be either due to a secondary or tertiary amine, because a secondary amine forms an insoluble sulphonamide whereas a tertiary amine does not react.
- These two can be distinguished by acidifying the mixture with dilute HCl. If the layer disappears it is a tertiary amine because it will form a water-soluble salt.
- However, the separation of an insoluble compound, a neutral sulphonamide, indicates the presence of a secondary amine.
Separation of primary, secondary, and tertiary amines-Hinsberg method:
- The mixture of amines is treated with aqueous KOH followed by benzene sulphonyl chloride (or p-toluene sulphonyl chloride).
- Primary amines form benzenesulfonamide (soluble in alkalis); the secondary amines also form benzenesulfonamide (but insoluble in alkalis); the tertiary amines remain unaffected.
- The mixture is warmed in a water bath to complete the reaction. The alkaline mixture is acidified with dilute HCI, whereby sulphonamides of primary and secondary amines are precipitated. The solid is filtered and washed with a little cold water. The tertiary amine remains in the filtrate.
- The solid is then boiled with aqueous KOH. The alkali-insoluble sulphonamide of the secondary amine is filtered off. The filtrate is acidified with dilute HCl to precipitate the sulphonamides of the primary amine.
- On boiling the sulphonamides of the primary and secondary amine with 80% H2SO4, and rendering them alkaline with aqueous KOH, the original primary and secondary amines may be recovered.
Electrophilic Substitution Reactions Of An Aromatic Amine (Aniline)
The amino group in aromatic amines is a powerful activating group and is ortho-para directing. This is due to the delocalization of the lone pair of electrons of nitrogen with the electrons of the benzene ring, as shown
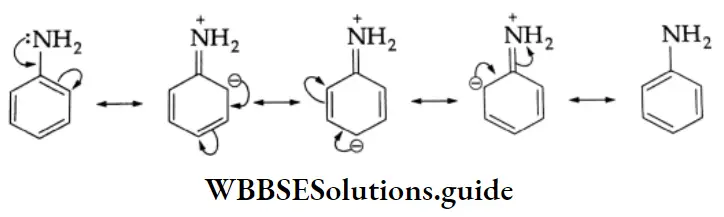
The maximum electron density occurs at the o- and p-positions. Aromatic amines, therefore, undergo typical electrophilic substitution reactions rapidly. In some cases, the rate of the reaction is so great that the poly-substituted product is formed even in mild conditions. In order to prepare a monosubstituted product, the activating influence of the -NH2 group is moderated by introducing an acetyl group.
Halogenation
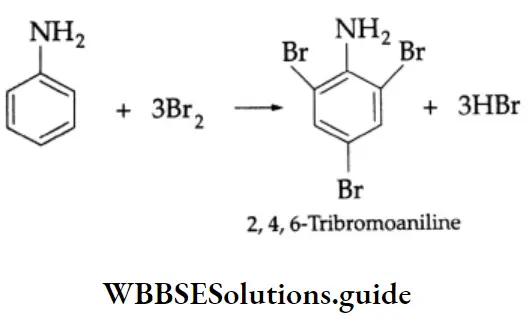
Halogenation of aniline takes place readily at room temperature and does not require the presence of a catalyst. For example, aniline, when treated with bromine water, gives 2, 4, and 6-tribromoaniline.
Nomenclature Of Amines
2, 4, 6-tribromoaniline is formed readily because aniline is extremely susceptible to electrophilic substitution. The substitution tends to occur readily at the ortho and para positions. In order to prepare a monosubstituted aniline, the activating effect of the NH2 group has to be controlled.
IUPAC nomenclature of amines with examples
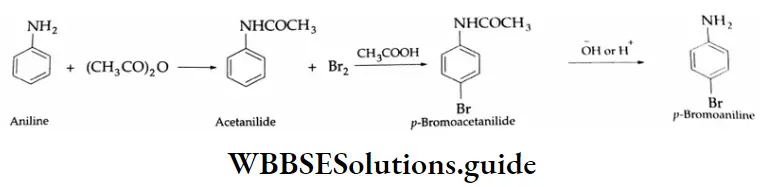
This is usually done by protecting the NH2 group by acetylation with acetic anhydride to get acetanilide. This electrophilic substitution with Br2 in acetic acid gives p-bromoacetanilide. On hydrolysis, this yields p-bromoaniline.
In acetanilide, the lone pair of electrons on nitrogen is attracted toward the strong electron-attracting carbonyl group due to resonance.
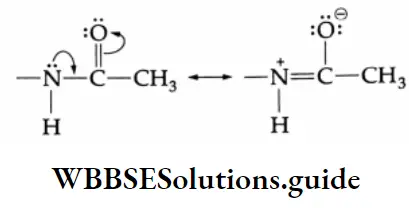
Thus, the lone pair of electrons on nitrogen becomes less available for delocalisation with the electrons of the benzene ring. The reactivity of the NHCOCH, group is thus reduced as compared to that of the -NH2 group.
Nitration
Aniline is susceptible to oxidation and a lot of it is decomposed as tarry material when made to react with concentrated HNO3. On treatment with concentrated HNO3 and concentrated H2SO4, aniline forms the anilinium cation (CH-NH3) by accepting a proton (H*). The anilinium ion is m-directing. Therefore, the product obtained is mainly m-nitroaniline, together with o- and p-nitroaniline.
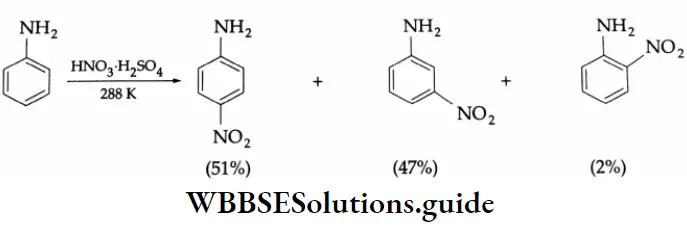
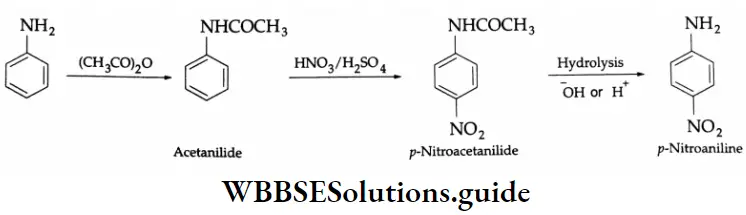
Monosubstituted p-nitroaniline may be obtained according to the following set of reactions. Aniline is first acylated to prepare acetanilide. Acetanilide on nitration (concentrated HNO3 + concentrated H2SO4) gives p-nitroacetanilide, which on hydrolysis yields p-nitroaniline.
Sulphonation
Aniline reacts with concentrated H2SO4 to give anilinium hydrogen sulfate. The salt on heating with concentrated H2SO4, to 453 K forms sulphanilic acid (p-amino benzene sulphonic acid).
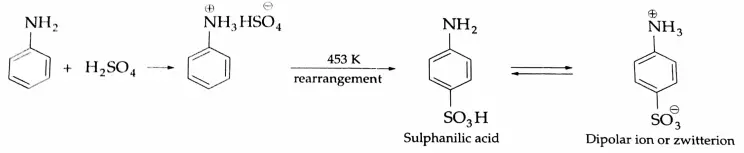
Since sulphanilic acid contains both an acidic group (-SO3H) and a basic group (-NH2) in the same molecule, an ‘inner-salt’ is formed as a zwitterion or dipolar ion. Aniline does not respond to Friedel-Crafts alkylation and acetylation reactions because it forms a salt with AICI3 (Lewis acid) used as a catalyst.
⇒ C6H5NH2 + AlCl3 → C6H5N+H2 AICI–2
Due to this, the nitrogen of aniline becomes positively charged and deactivates the ring. Deactivated compounds usually do not undergo Friedel-Crafts reaction.
Benzenediazonium Salts
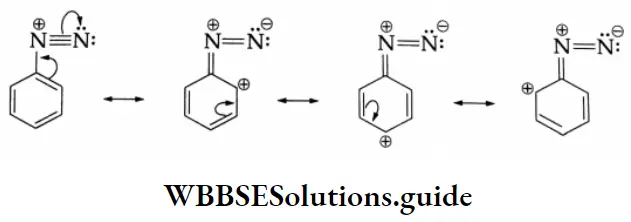
The term diazonium is derived from the Greek word azote (nitrogen). A benzenediazonium salt has the general formula \(\mathrm{C}_6 \mathrm{H}_5-\stackrel{+}{\mathrm{N}} \equiv \mathrm{N} \bar{X}\)where X may be Cl–, Br–, NO3–, or HSO4–. The group N = N- is called the azo group.
The prefix azo is used when both nitrogen atoms (-N-N-) are attached to carbon. When one is attached to carbon and one to some other atom, the suffix diazo is used. While naming these compounds, the suffix diazonium is added to the name of the aromatic hydrocarbon and then the name of the anion is written as a separate word.
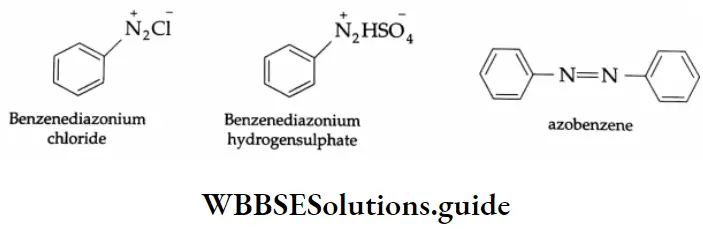
Aromatic amines form aromatic diazonium salts. These are more stable than alkyl diazonium salts. The greater stability of the aromatic diazonium ion is due to resonance.
Preparation
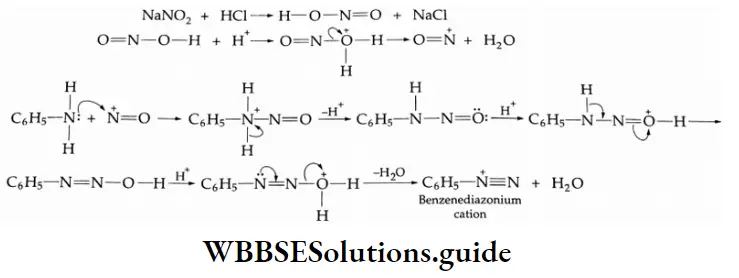
Aniline is dissolved or suspended in a mineral acid (HCI) and cooled to 0°C. Then a cold solution of sodium nitrite is added slowly in slight excess.
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{HCl} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2 \cdot \mathrm{HCl}\)
⇒ \(\mathrm{NaNO}_2+\mathrm{HCl} \rightarrow \mathrm{HNO}_2+\mathrm{NaCl}\)
“importance of amines in pharmaceuticals”
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2 \cdot \mathrm{HCl}+\mathrm{HNO}_2 \rightarrow \underset{\begin{array}{c}
\text { Benzenediazonium } \\
\text { chloride }
\end{array}}{\mathrm{C}_6 \mathrm{H}_5 \mathrm{~N}_2 \mathrm{Cl}}+2 \mathrm{H}_2 \mathrm{O}\)
The process of converting aromatic primary amines to diazonium salts by the action of nitrous acid is known as diazotization.
Physical Properties
Benzenediazonium chloride is a colourless crystalline solid and is readily soluble in water. Aqueous solutions of benzene diazonium chloride are not stable. Nitrogen gas is evolved slowly in cold conditions and rapidly on warming this compound. This compound is relatively stable below 273 K. Bezenediazonium fluoroborate is stable, and can be dried and stored.
Chemical Reactions
Benzene diazonium salts are highly reactive compounds that serve as intermediates in the synthesis of a wide variety of aromatic compounds. The reactions of benzene diazonium salts may be divided into the following two categories.
- Substitution of the diazonium group, and
- Coupling reactions.
1. Substitution of the diazonium group
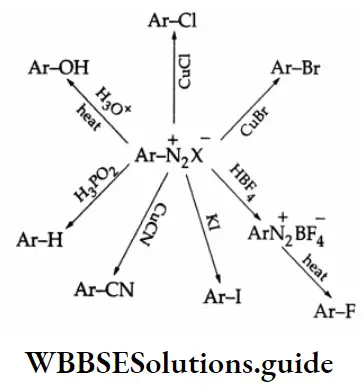
By Cl–, Br– and CN– When a diazonium salt solution is treated with hydrogen chloride, bromide, or cyanide in the presence of the corresponding cuprous halide or the cyanide as catalyst, the diazo group is replaced by the halide group or cyanide group. This is known as the Sandmeyer reaction.
Basicity of amines and factors affecting basicity
In another reaction, when a diazonium salt solution is treated with hydrogen chloride, bromide or cyanide in the presence of copper powder, the diazo group is replaced by the halide group or the cyanide group. This is known as the Gattermann reaction.
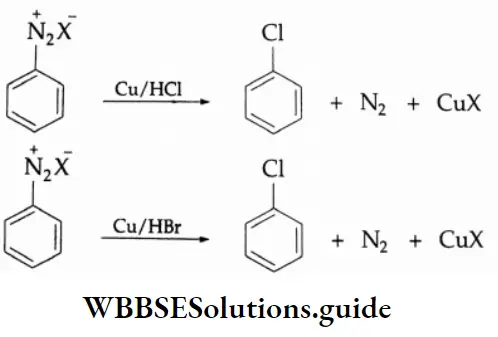
The yield of the halobenzene or cyanobenzene is greater in the Sandmeyer reaction than in the Gattermann reaction.
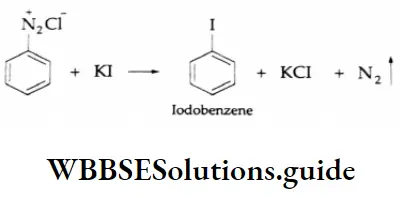
By iodine:
lodine cannot substitute the diazonium group in the benzene ring by halogenation. Therefore, iodobenzene is prepared by making benzene diazonium chloride react with potassium iodide.
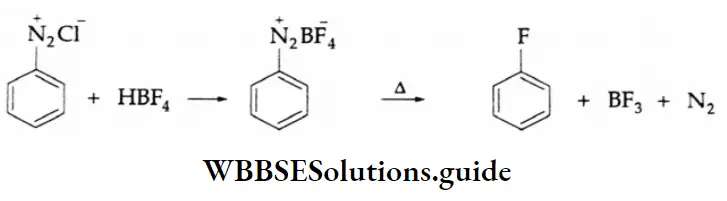
By fluorine:
Aqueous fluoroboric acid, HBF4 reacts with a benzenediazonium salt to yield C6H5N2– BF4–, On gentle heating in the absence of a solvent, the latter decomposes to give fluorobenzene.
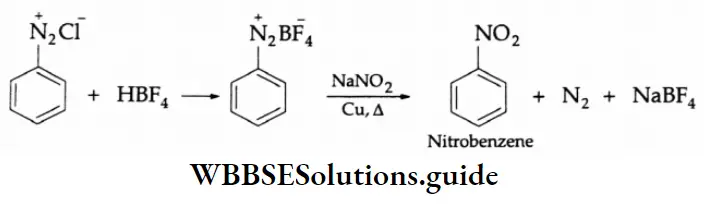
By NO2 group:
On being heated with sodium nitrite in the presence of copper powder, benzene diazonium fluoroborate yields nitrobenzene.
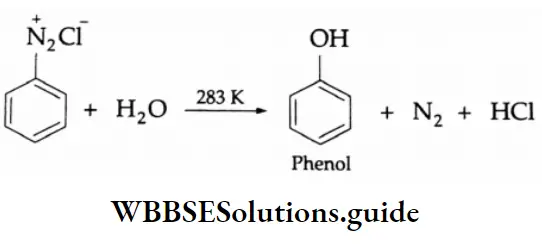
By OH group:
At 283 K, an aqueous solution of benzenediazonium chloride yields phenol.
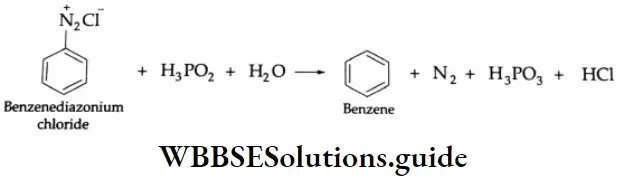
By hydrogen:
Benzene diazonium chloride is reduced by hypophosphorous acid (H3 PO2) to benzene.
In all the above reactions, nitrogen is displaced from the aromatic ring and escapes as a gas.
Summary of the syntheses using diazonium salts:
2. Coupling reactions
When diazonium salts are treated with phenols or aromatic amines in a basic medium, azo compounds (azo compounds contain the group-N-N-) are formed. These are known as coupling reactions.
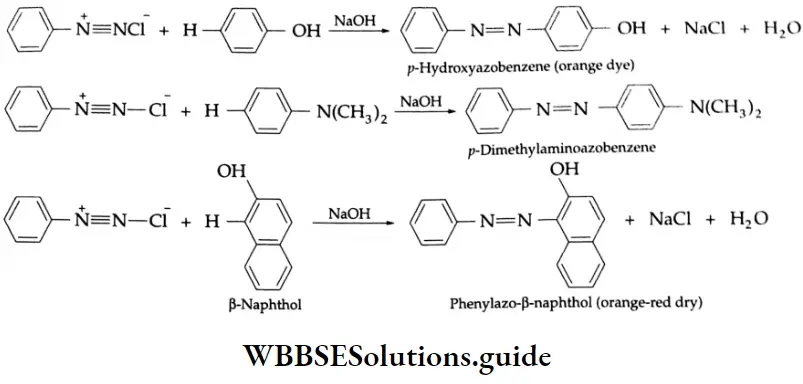
These reactions are a kind of electrophilic aromatic substitution in which aromatic diazonium ions act as poor electrophiles. They attack a benzene ring that has an activating group on the ring.
The coupling takes place para to the OH or -NR2 group unless this position is blocked. The azo compounds are usually colored (orange, red, or yellow). The double-bonded nitrogen atoms are largely responsible for giving the azobenzenes their colors. A group like -NN-is called a chromophore.
“applications of amines in industry”
The last coupling reaction described above is used in the detection of primary aromatic amines. Aromatic amines on diazotization give diazonium salts which on treatment with β-naphthol form a red/orange dye. The formation of a red/orange dye confirms the presence of an aromatic amino group.
When a benzene diazonium ion reacts with aniline, a somewhat different reaction takes place. Instead of attacking the ring, the ion bonds to the nitrogen atom of aniline, and a yellow solid is obtained.
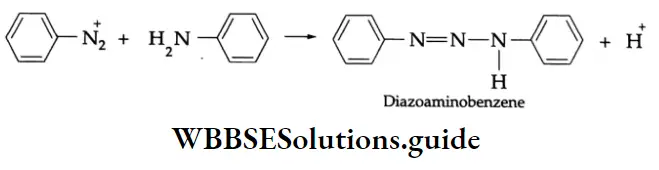
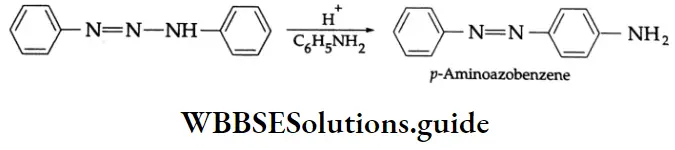
Diazoaminobenzene reacts with excess aniline and acid to give the aromatic substitution product, p-aminoazobenzene.
Amines Multiple-Choice Questions
Question 1. Which of the following is the functional group of a primary amine?
- —NH–
- -NH2
- +NH3
- NH3
Answer: 2. -NH2
Question 2. Amines are derivatives of
- CO2
- Urea
- NH3
- None of these
Answer: 3. NH3
Question 3. Which of the following is formed when ethylamine reacts with nitrous acid?
- C2H2OH
- CH3COOH
- C2H2NO2
- None of these
Answer: 1. C2H2OH
Question 4. Which of the following is formed when methylamine is heated with chloroform and alcoholic KOH?
- CH3NC
- CH2CN
- CH3CHO
- CH3OH
Answer: 1. CH3NC
Question 5. A Grignard reagent reacts with a primary amine to produce
- An alkane
- Tertiary amine
- None of these
- A secondary amine
Answer: 1. An alkane
Question 6. The reduction of nitroalkane gives a
- Secondary amine
- Primary amine
- A higher amine
- Quaternary salt
Answer: 3. A higher amine
“naming of amines according to IUPAC”
Question 7. Which of the following is formed when acetamide reacts with Br2/NaOH?
- Acetone
- Methylamine
- Ammonia
- Acetaldehyde
Answer: 2. Methylamine
Functional groups in nitrogen-containing organic compounds
Question 8. Which of the following is formed when aniline reacts with fuming sulphuric acid?
- Sulphonic acid
- Sulphanilic acid
- Benzene sulphonic acid
- Benzoic acid.
Answer: 2. Sulphanilic acid
Question 9. Which of the following is formed when aniline is heated with chloroform and alcoholic KOH?
- Chlorobenzene
- p-Hydroxyaniline
- Carbylamine
- Acetanilide
Answer: 3. Carbylamine
Question 10. What is the hybridization state of N in an amine?
- Sp3
- Sp2
- Sp
- Sp3d
Answer: 1. Sp3
Question 11. Among the following, which is the correct order of basic strength?
- NH>CH3NH2> NF3
- CH3NH2>NH3>NF3
- NF3> CH3NH2> NH3
- NH3> NF3> CH3NH2
Answer: 2. CH3NH2>NH3>NF3
Question 12. Which of the following is the most basic?
- C6H5NH2
- (CH3)2> NH
- (CH3)3N
- NH3
Answer: 2. (CH3)2> NH
Question 13. Which of the following is the order of basic strength of amines in a benzene solution?
- CH3NH2> (CH3)3N> (CH3)2NH
- (CH3)3N> (CH3)2NH> CH3NH2
- CH3NH2> (CH3)2NH> (CH3)3N
- (CH3)3N> CH3NH2>(CH3)2NH
Answer: 2. (CH3)3N> (CH3)2NH> CH3NH2
Question 14. Which of the following is the most reactive towards electrophilic substitution?
- Nitrobenzene
- Aniline
- Aniline hydrochloride
- N-Acetylaniline
Answer: 2. Aniline
Question 15. Which of the following is the most basic?
- C6H5NH2
- p-NO2-C6H4NH2
- m-NO2-C6H4NH2
- C6H5CH2NH2
Answer: 4. m-NO-CH4NH2
Question 16. What are the products formed when aniline is heated with concentrated HNO, and concentrated H2SO4?
- o- and p-Nitroaniline
- p-Nitroaniline
- Tarry material
- There is no reaction.
Answer: 3. Tarry material
Question 17. The reaction of a primary amine with chloroform and ethanolic KOH is called the
- Carbylamine reaction
- Kolbe reaction
- Reimer-Tiemann reaction
- None of these
Answer: 1. Carbylamine reaction
Question 18. Benzene diazonium chloride reacts with phenol in a weak basic medium to give
- Diphenyl ether
- p-hydroxy azobenzene
- Chlorobenzene
- Benzene
Answer: 2. p-hydroxy azobenzene
Question 19. Which of the following does not react with acetyl chloride?
- Methylamine
- Ethylamine
- Dimethylamine
- Trimethylamine
Answer: 3. Dimethylamine
Question 20. Hinsberg reagent is
- Benzene sulphonic acid
- Benzenesulphonamide
- p-Toluenesulphonyl chloride
- None of these
Answer: 3. p-Toluenesulphonyl chloride
Question 21. How many primary amines have the molecular formula C4H11N?
- 1
- 2
- 3
- 4
Answer: 4. 4
Question 22. In reaction with aqueous HNO2 at low temperatures, a compound produces an oily nitrosamine. The compound is likely to be
- Methylamine
- Ethylamine
- Diethylamine
- Triethylamine
Answer: 3. Diethylamine
“reaction of amines with nitrous acid”
Question 23. An amine reacts with benzenesulphonyl chloride and the product is insoluble in NaOH. The amine is likely to be
- A primary amine
- A secondary amine
- A tertiary amine
- All of these
Answer: 2. A secondary amine
Question 24. Which of the following compounds is the least basic? a secondary amine

Answer: 3
Comparative basicity of amines, ammonia, and substituted amines
Question 25. What is the correct order of basicity of the following compounds?

- (2)>(1)>(3) > (4)
- (3)> (1)>(2) > (4)
- (1)>(3)>(2)>(4)
- (1)>(2)>(3)>(4)
Answer: 3. (1)>(3)>(2)>(4)
