WBCHSE Class 11 Chemistry Notes On Atomic Structure And Subatomic Particles
The atom as pictured by Dalton, Berzelius, Cannizzaro, and Avogadro was an indestructible particle, which could neither be created nor destroyed. It was thought to be indivisible, and the smallest building block of matter.
“Subatomic particles, their discovery, characteristics, and significance, WBCHSE Class 11 notes”“
This concept of the atom, as you have read in the previous chapter, could not explain how or why atoms combine, or how exactly atoms of different elements differ from each other.
- The explanation had to wait until the end of the nineteenth century when some path-breaking experiments proved that the atom is made of still smaller particles—electrons, protons, and neutrons. These discoveries led to a clearer understanding of the chemical behavior of atoms.
- They also led to the formulation of quantum mechanics, since Newtonian mechanics could not describe the motion of the electron. The laws of quantum mechanics helped to explain the stability of the atom in terms of the arrangement of electrons in the atom and the characteristic radiation emitted by atoms.
“WBCHSE Class 11 Chemistry, atomic structure, discovery of subatomic particles, notes”
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
Faraday’s Contribution
The earliest indication that scientists had regarding the existence of a relation between matter and electricity came from the observation that static electricity is generated when substances like glass (or ebonite) are rubbed against silk (or fur). Michael Faraday, an English physicist, performed some experiments in the 1830s, which made this connection more evident.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
Discovery Of Subatomic Particles
Faraday showed that when electricity is passed through an electrolyte, the electrolyte undergoes chemical changes. The phenomenon is called electrolysis and the quantitative results obtained by Faraday are expressed in Faraday’s laws of electrolysis.
“WBCHSE Class 11, chemistry notes, on atomic structure, fundamental particles, and their properties”
According to these laws,
- The weights of the elementary substances produced (liberated) during electrolysis are proportional to the quantity of charge (current x time) passed through the electrolyte and
- The number of moles of various substances liberated by a fixed quantity of charge bears a simple, whole-number ratio with each other.
- These results showed clearly that there is a relation between atoms and electricity. They were also very similar to the laws of chemical combination. Only years later, a scientist called Stoney claimed that Faraday’s laws on the interaction between matter and electricity were like the laws of chemical combination, which dealt with the interaction between different materials.
- He claimed further that since the laws of chemical combination led to the formulation of the atomic theory of matter, Faraday’s laws should imply the discrete nature of electricity. In other words, that electricity must be made of discrete particles and he called these particles of electricity electrons.
Discovery Of The Electron
The electron was discovered during experiments involving the passage of electric current through a gas. Under ordinary conditions, gases do not conduct electricity.
Discovery Of Subatomic Particles
However, if a gas is filled in a sealed tube fitted with electrodes and the pressure inside the lube is lowered to about 10-2 atm, the gas starts conducting electricity when a high voltage (5000-10,000 V) is applied across the electrodes. Such a tube is called a gas-discharge tube. The neon signs we are so familiar with are gas-discharge tubes filled with neon.
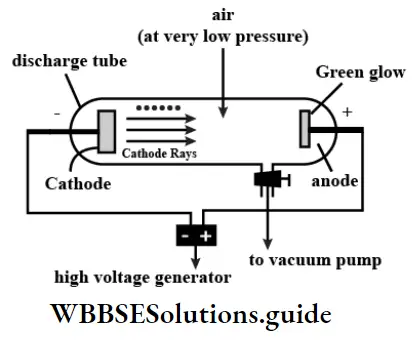
Discovery Of The Electron Cathode rays: When the pressure inside the discharge tube is 1 atm, no electric current flows through the tube even on applying high voltage. The pressure of the gas inside the tube can be decreased by pumping out the gas with the help of a vacuum pump. As the pressure is reduced to about 10-2 atm, a purple light is emitted if the gas being used is air. The whole tube appears purple.
Discovery Of Subatomic Particles Class 11 Notes
- The color of the light depends on the gas being used. If the pressure is reduced much further to about 10-4 atm, the purple light disappears and the part of the glass tube directly opposite to the cathode begins to glow with a greenish light.
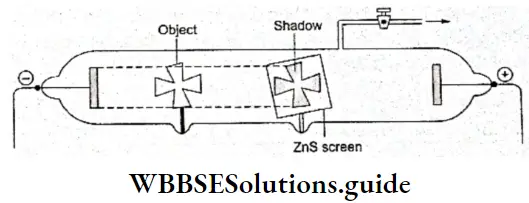
- A British physicist, Sir William Crookes, who conducted this experiment in 1879, showed that the green glow (fluorescence) is caused by the bombardment of invisible rays emitted by the cathode. An object placed inside the tube, between the cathode and the opposite end of the tube, casts a sharp shadow on the wall of the tube.
- Besides, the glow can then be seen only around the shadow. This proved that the glow was due to the emission of rays from the cathode and that the rays moved in straight lines. These rays are called cathode rays.
“Discovery of subatomic particles, WBCHSE Class 11, chemistry notes, and key concepts”
Discovery Of The Electron Properties of cathode rays: A series of experiments conducted by several scientists ultimately proved that cathode rays are a stream of electrons. Becquerel’s discovery of radioactivity and subsequent experiments conducted by other scientists to study the phenomenon of radioactivity acted as secondary proof to establish that the particles emitted by the cathode are electrons.
Class 11 WBCHSE Atomic Structure Notes And Subatomic Particles
Discovery Of Subatomic Particles Class 11 Notes
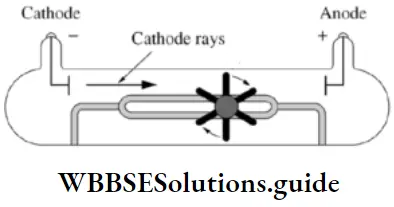
- But first, it had to be proved that the emission from the cathode consisted of a stream of material particles. This was done by placing a light paddle wheel in the path of the cathode rays. It was seen that the rays rotate the paddle wheel, which could happen only if they comprised particles with mass and energy.
- A metal foil placed in the path of the cathode rays gets heated if the rays are focused on it. This, too, shows that emission from the cathode consists of fast-moving particles. Of course, light rays could also cause the foil to get heated. Later experiments did show that cathode rays consist of electrons and light rays, but we will not concern ourselves with that at the moment.
Discovery Of Subatomic Particles Class 11 Notes
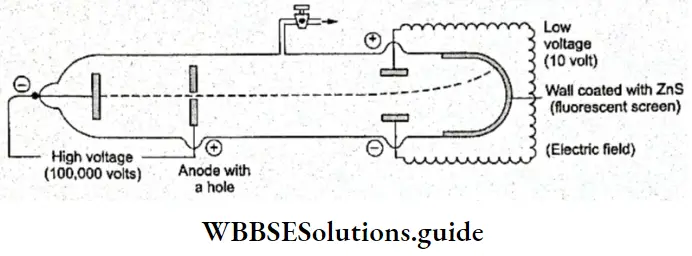
- The next thing that needed to be investigated was whether the particles emitted by the cathode were charged. An electric field was applied to the beam of cathode rays as shown in Figure and it was seen that the rays were deflected towards the positive plate. This proved that the particles of the cathode rays are negatively charged.
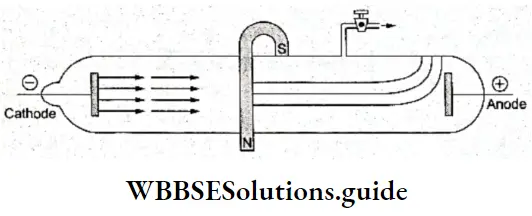
- When a magnetic field was applied to the beam of cathode rays, the rays were deflected towards the south pole, as negatively charged particles should.
Other experiments conducted to investigate the properties of cathode rays showed that they ionize the gas through which they pass (as charged particles should) and that they produce X-rays when they are made to fall on metals, such as tungsten.
“WBCHSE Class 11, chemistry notes, on atomic structure, protons, neutrons, and electrons”
Discovery Of The Electron Charge To Mass Ratio (e/m): Sir J J Thomson, a British physicist, observed the deflection of cathode rays under the influence of an electric field and a magnetic field applied simultaneously and at right angles to the electric field in a specially designed discharge tube.
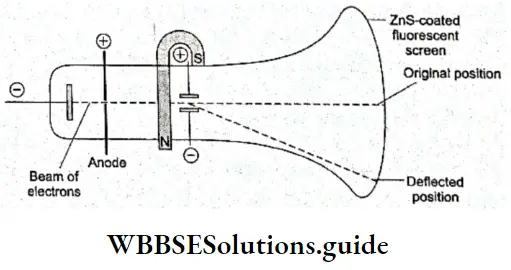
- He then adjusted the two fields so that the cathode rays would strike a fluorescent screen placed at the end of the tube opposite the cathode at the original position as did before the two fields were applied. From his observations, he calculated the charge (e) to mass (m) ratio of the electron.
- It turned out to be the same (1.76 x 108 C g-1) irrespective of the nature of the cathode or the gas taken in the discharge tube. This proved that electrons, the negatively charged subatomic particles, are universal constituents of matter.
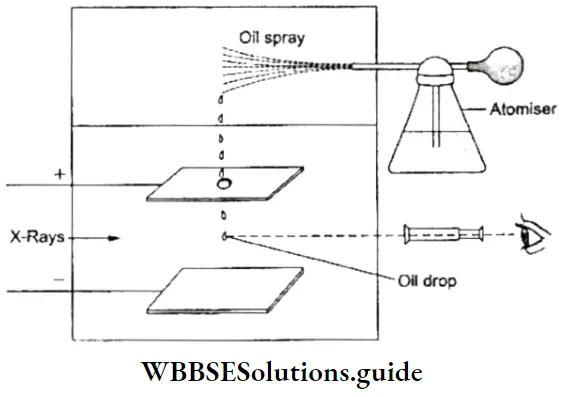
Discovery Of The Electron Charge: In 1909, Robert Millikan, an American physicist, measured the charge carried by an electron by doing an experiment that has come to be known as the Millikan oil-drop experiment. He allowed a spray of oil droplets (produced by an atomizer) to enter the apparatus through a small hole and fall between two charged plates.
- He observed the motion of the droplets of oil as they fell under the influence of the force of gravity through a telescope. He then passed X-rays into the chamber to ionize the air inside. The electrons thus produced imparted a negative charge to the oil droplets which then came under the influence of the electric field between the charged plates.
- He adjusted the electric field until it balanced the gravitational field. (In a balanced field a droplet would either be stationary or move with constant velocity per the first law of motion.) The magnitude of the electric field required to balance the two fields would be a measure of the charge on the droplet of oil. The charge on a droplet, according to Millikan’s observations, was always a whole-number multiple of 16 x 10-19 C. He, therefore, concluded that the charge on an electron is 16 x 10-19 C.
Discovery Of Mass Of The Electron: The mass of an electron can be calculated quite simply from the results of Thomson’s and Millikan’s experiments.
According to Thomson,
⇒ \(\frac{e}{m}=1.76 \times 10^8 \mathrm{Cg}^{-1}\)
According to Millikan, e =1.6 x 10-19 C.
∴ mass of electron (m) = \(\frac{e}{e / m}\)
= \(\frac{16 \times 10^{-19}}{1.76 \times 10^8}=9.1 \times 10^{-28} \mathrm{~g}\)
= \(9.1 \times 10^{-31} \mathrm{~kg}\)
“Atomic structure, discovery of electrons, protons, and neutrons, WBCHSE Class 11”
Anode Rays Or Canal Rays
After the discovery of the electron began the search for its positive counterpart. Since the atom as a whole is electrically neutral, scientists began to look for positively charged subatomic particles. In 1886, Eugen Goldstein, a German physicist, performed an experiment that led to the discovery of the proton.
- He used a perforated cathode instead of the normal one used in the gas discharge tube.
- When he passed an electric discharge through the tube, he observed a glow on the wall of the tube behind the perforated cathode. This meant that a stream of particles (or light) was moving towards the cathode, passing through it and causing the tube to glow.
- The rays were named anode rays or canal rays. These rays traveled in a direction opposite to that of the cathode rays.
Investigations followed, as in the case of cathode rays, and the following conclusions were reached.
- Anode rays travel in straight lines.
- They consist of material particles.
- They are positively charged since they are deflected towards the negatively charged plate in an electric field.
- They are deflected by a magnetic field.
- Their charge-to-mass (e/m) ratio depends upon the gas used in the discharge tube.
- The mass of the positively charged particles in the anode rays is equal to the atomic mass of the gas used.
Origin Of Cathode And Anode Rays: When a high voltage is applied across the gas-discharge tube, the atoms or molecules of the gas get ionized, resulting in the formation of positively charged ions and negatively charged particles. The negatively charged particles, we know, are electrons.
⇒ \(\underset{substack{\text { a neutral } \\ \text { gaseous atom }}}{\mathrm{A}} \stackrel{\text { ionised }}{\longrightarrow} \mathrm{A}^{+}+\mathrm{e}^{-}\)
Under the influence of the high voltage, the electrons move at a high velocity towards the anode. In the process, they ionize more neutral gas atoms or produce more electrons and positive ions, The negatively charged particles or electrons constitute cathode rays, while the positively charged ions constitute anode rays.
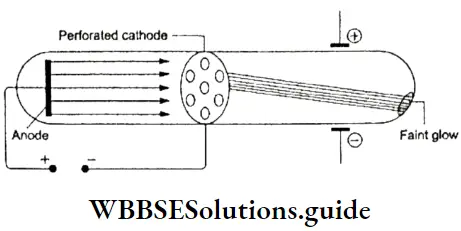
The e/m ratio of the cathode rays is independent of the gas used because these rays comprise electrons. The e/m ratio of the anode rays, on the other hand, depends on the gas used because the mass of the ion depends on the mass of the original atom.
“WBCHSE Class 11 Chemistry, subatomic particles, discovery, and experimental evidence”
Cathode And Anode Rays Discovery Of Proton: Observations showed that the positive particles (ions) produced by hydrogen had the highest e/m ratio. In other words, hydrogen produced the lightest positive particle. This particle was called a proton.
Its charge-to-mass ratio was found to be 9.58 x 104 C g-1. The charge on the proton is equal and opposite to that on the electron, i.e., 16 x 10-19 C and its mass is 167 x 10-24 g. The proton is about 1837 times heavier than the electron and its mass is almost the same as that of a hydrogen atom.
Class 11 WBCHSE Chemistry Atomic Structure And Discovery Of Subatomic Particles PDF
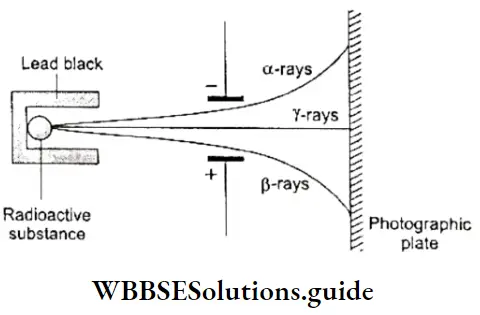
As mentioned earlier, Becquerel’s investigations into the phenomenon of radioactivity further confirmed that the atom is divisible and is made up of charged particles. The spontaneous emission of radiation by some substances like uranium is called radioactivity. Elements that have this property are called radioactive elements.
- When the radiation emitted by a radioactive substance is allowed to pass through a strong electric field or magnetic field, it splits into three parts which are deflected in different directions.
- The rays deflected towards the negative plate are called α (alpha) rays. These consist of positively charged He2+ ions.
- The rays deflected towards the positive plate are called β (beta) rays and consist of negatively charged β particles or electrons.
- The undeflected rays are high-energy electromagnetic radiations called γ (gamma) rays. You will learn more about this phenomenon later.
Thomson’s Model Of The Atom
Once it was discovered that the atom consisted of negatively and positively charged particles (this was before Chadwick discovered the neutron), scientists wished to know how these particles were arranged inside the atom.
“Discovery of subatomic particles, J.J. Thomson, Rutherford, and Chadwick, WBCHSE Chemistry”
In 1898 Thomson proposed a model of the atom in which the positive charge was supposed to be spread over a sphere of radius 10’8 cm. He assumed that electrons were embedded in this sphere, more or less evenly, as plums in a pudding.
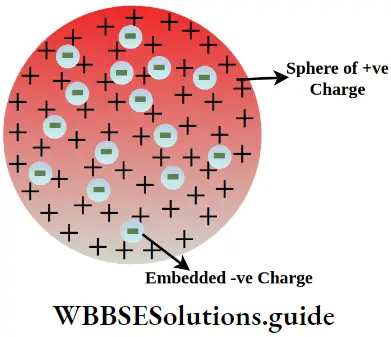
Consequently, Thomson’s model of the atom came to be known as the plum pudding model. Thomson’s model was a pioneering effort to explain the atomic structure and he received the Nobel prize in physics in 1906.