NEET Physics Nuclei Notes
Nuclei
Nucleons. Protons and neutrons which are present in the nuclei of the atoms are collectively known as nucleons.
Atomic number. The number of protons present in the nucleus is called the atomic number.
It is denoted by Z.
Mass number. The total number of protons and neutrons present in the nucleus is called the mass number of the element. It is denoted by A.
Read And Learn More: NEET Physics Notes
Number of protons in an atom = Z, Number of electrons in an atom = Z
Number of nucleons in an atom = A, Number of neutrons in an atom = N = A – Z.
Nuclear mass. The total mass of the protons and neutrons present in the nucleus is called the nuclear mass.
Isotopes. Nuclei having the same atomic number but different mass numbers are called isotopes.
Example: Hydrogen has three isotopes
\({ }_1^1 H,{ }_1^2 H \text { and }{ }_1^3 H \text {. }\)Isobars. Nuclei having different atomic numbers but the same mass number are called isobars.
They contain different numbers of protons hence they are atoms of different elements.
\(\text { Example: (1) }{ }_1^3 \mathrm{H} \text { and }{ }_2^3 \mathrm{He}(2){ }_{18}^{40} \mathrm{Ar} \text { and }{ }_{20}^{40} \mathrm{Ca}\)Isotones. Nuclei having an equal number of neutrons but different atomic numbers are called isotones. \({ }_1^3 \mathrm{H} \text { and }{ }_2^4 \mathrm{He}\) are isotones since they contain 2 neutrons.
Isomers. The nuclei having the same atomic number and same mass number but differ from one another in their internal structure and their nuclear energy states are called isomers. For example, the stable nucleus of \({ }_{38}^{87} \mathrm{Sr}\) has an isomer which emits gamma rays with a half life of 2.8 hour.
Mirror nuclei. Nuclei having the same mass number but the proton number and neutron number interchanged are called mirror nuclei. For example, \({ }_1^3 \mathrm{H} \text { and }{ }_2^3 \mathrm{He}\) are mirror nuclei.
Atomic mass unit. Atomic mass and nuclear mass are measured in terms of atomic mass unit (u). One atomic mass unit (1u) is defined as \(\frac{1}{12}^{t h}\) the mass of an atom of carbon- 12. Thus,
\(\begin{aligned}& 1 \mathrm{u}=\frac{\text { mass of one atom of }{ }^{12} \mathrm{C}}{12} \\
& ∴ 1 \mathrm{u}=1.66 \times 10^{-27} \mathrm{~kg} \\
& ∴ \text { Mass of carbon-12 is, } 12 \mathrm{u} .
\end{aligned}\)
\(Mass of proton is, m_p=1.00727 u=1.00727 \times 1.66 \times 10^{-27}\) \(=1.67262 \times 10^{-17} \mathrm{~kg}\) \(Mass of neutron is, \mathrm{m}_n=1.00866 \mathrm{u}=1.6749 \times 10^{-27} \mathrm{~kg}\) \(Mass of electron is, \mathrm{m}_{\boldsymbol{c}}=0.00055 \mathrm{u}=9.13 \times 10^{-31} \mathrm{~kg}\)
Size of the nucleus. From experimental observations, it has been found that the volume of the nucleus is proportional to the number of nucleons present in it (or mass number A). Generally, nuclei are found to have a spherical shape.
\(R=R_0 A^{1 / 3}\)Where Ro is a constant of proportionality. R = Ro when A = 1. Thus Ro represents the radius of the nucleus of a hydrogen atom which is nothing but a proton. From experiments, it has been found that Ro \(1.3 \times 10^{-15} \mathrm{~m}=1.3 \text { fermi }\)
Nuclear charge. If Z is the number of protons present in the nucleus then a charge of the nucleus is given by,
\(\mathrm{q}=+Z e, \mathrm{e}=1.602 \times 10^{-19} \mathrm{C}\)Nuclear density. \(\text { Nuclear density }=\frac{\text { mass }}{\text { volume }}\)
Consider a nucleus of mass number A and let the mass of each nucleon is \(\mathrm{m}_{\mathrm{N}}=1.67 \times 10^{-27} \mathrm{~kg}\)
\(\text { Nuclear density }=\frac{A m_N}{\frac{4}{3} \partial R^3}=\frac{A m_N}{\frac{4}{3} \partial\left(R_0 A^{1 / 3}\right)^3}=\frac{A m_N}{\frac{4}{3} \partial R_0^3 A}\) \(=\frac{3 m_N}{4 \delta R_0^3}=\frac{3 \times 1.67 \times 10^{27}}{4 \delta \times\left(1.3 \times 10^{-15}\right)^3}=1.815 \times 10^{17} \mathrm{kgm}^{-3}\)The nuclear density does not depend on mass number A or the number of nucleons in the nucleus. Hence nuclei of all elements have nearly the same density.
Mass energy relation. Before the special theory of relativity, it was presumed that mass and energy were conserved separately in a reaction. However, Einstein showed that mass is another form of energy and one can convert mass energy into other forms of energy.
Einstein’s mass-energy equivalence relation is given by, E=mc²
Here the energy equivalent of mass m is related by the above equation and c is the velocity of light in vacuum and is approximately equal to \(3 \times 10^8 \mathrm{~m} / \mathrm{s}\)
Energy Equivalent of One Atomic Mass Unit
\(∴\mathrm{lu}=931 \mathrm{MeV}\)Mass defect. The difference between the sum of the masses of the constituent nucleons and the actual mass of the nucleus is called mass defect.
Let M be the mass of the nucleus having mass number A and atomic number Z. If mp is the mass of the proton and mn is the mass of the neutron, the mass defect of the nucleus is,
\(\Delta \mathrm{M}=\left[\mathrm{Zm}_{\mathrm{p}}+(\mathrm{A}-\mathrm{Z}) \mathrm{m}_{\mathrm{n}}\right]-\mathrm{M}\)Binding energy. The binding energy of a nucleus can be defined as the minimum energy required to split the nucleus into its constituent nucleons.
Note. Mass defect is equivalent to binding energy.
\(\mathrm{E}_{\mathrm{b}}=\left[\mathrm{Z} \mathrm{m}_{\mathrm{p}}+(\mathrm{A}-\mathrm{Z}) \mathrm{m}_{\mathrm{n}}-\mathrm{M}\right] \mathrm{c}^2\)The ratio of the binding energy of a nucleus to the number of nucleons in that nucleus is called binding energy per nucleon or specific binding energy.
\(\text { i.e., } E_{b a}=\frac{E_b}{A}\)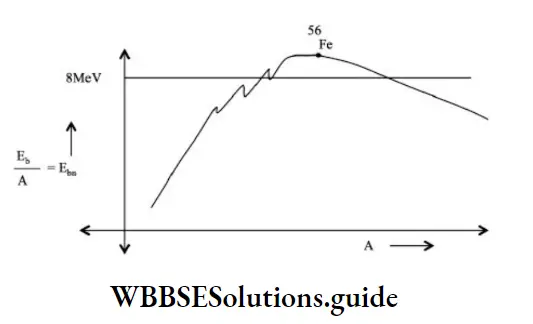
The binding energy per nucleon is close to the maximum value for nuclei in the medium mass number range of 30 to 170. Hence they have great stability. Binding energy per nucleon is maximum for \({ }_{26}^{56} \mathrm{Fe}\)
For higher mass numbers, the specific binding energy is lower and hence they are less stable.
For example, the last few naturally available elements exhibit radioactivity because of lesser stability.
The nature of the binding energy curve gives a clue to the release of energy in a nuclear process.
For example, lighter nuclei having lesser specific binding energy undergo fusion to form a nucleus of higher specific binding energy, resulting in the release of energy. Very heavy nuclei undergo fission to form medium-sized nuclei having greater specific binding energy, resulting in the release of energy.
Packing fraction: The mass defect per nucleon is called the packing fraction.
\(f=\frac{\Delta m}{A}=\frac{M-A}{A}\)M = mass of the nucleus
A = mass number
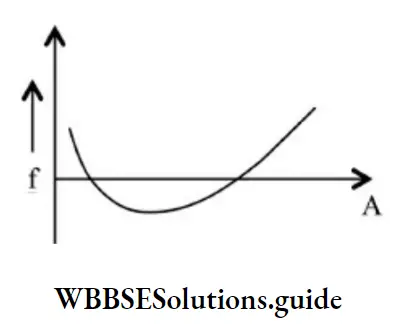
The packing fraction measures the stability of nucleons. Smaller the value of packing fraction, larger is the stability of the nucleons.
Nuclear force. We know that for average mass nuclei the binding energy per nucleon is approximately 8MeV, which is much larger than the binding energy in atoms. Therefore to bind a nucleus together there must be a strong attractive force of a totally different kind. It must be strong enough to overcome the repulsion between the protons to bind both protons and neutrons into a tiny nuclear volume.
Characteristics of Nuclear Force
- Nuclear forces are the strongest known forces in nature.
- Nuclear forces are short-range forces.
- Nuclear forces are charge-independent.
- Nuclear forces have the property of saturation.
- Nuclear forces are spin-dependent.
- Nuclear forces are exchange forces.
- Nuclear forces have a repulsive core.
- Nuclear forces are non-central.
Radioactivity. The phenomenon of spontaneous disintegration of the nuclei of heavy elements with the emission of certain radiation is called radioactivity.
Law of radioactive decay: The rate of disintegration of a radioactive substance at any instant of time is directly proportional to the number of atoms of that substance present at that instant of time.
\(N=N_0 e^{-\lambda t}
when \mathrm{t}=\frac{1}{\lambda}, \quad \mathrm{N}=\mathrm{N}_0 e^{-1} \Rightarrow \mathrm{N}=\frac{1}{e} \mathrm{~N}_0=0.3679 N_0\)
Therefore decay constant of a radioactive substance is defined as the reciprocal of the time during which the number of atoms of the substance decreases times the number of atoms originally present.
Half-life. Half life of a radioactive substance is defined as the time during which half of the original atoms disintegrate.
∴\(\mathrm{T}_{12}=\frac{0.693}{\lambda}\)
Mean life. The mean life or average life of a radioactive substance is the ratio of the sum of lives of all the individual atoms to the total number of atoms present in the sample \((\tau)\).
\(\tau=\frac{1}{\lambda}\)Activity. The activity of a radioactive sample is a measure of the number of disintegrations per second or the rate of disintegrations in it.
Since the magnitude of the rate of disintegration is, \(\left|\frac{d N}{d t}\right|=\lambda N\) , i.e., we have activity, A= \(\mathrm{A}=\lambda N\)
The S.I. unit of activity is becquerel (Bq).
Activity is one becquerel if there is one disintegration per second in the substance.
The commonly used unit is curie (Ci).
One curie is the activity of a radioactive sample in which atoms disintegrate per second. It is also the activity of one gram of radium.
∴\(1 \mathrm{Ci}=3.7 \times 10^{10} \text { disintegrations } / \text { second }=3.7 \times 10^{10} \mathrm{~Bq} \text {. }\)
Note:
1. Radioactivity is a nuclear phenomenon. It is not affected by external factors such as temperature, pressure, electric and magnetic fields, chemical reactions, etc. The activity depends only on the radioactive substance and the number of atoms taken.
2. If A0 is the initial activity and A is the activity of a substance at an instant of time t, then
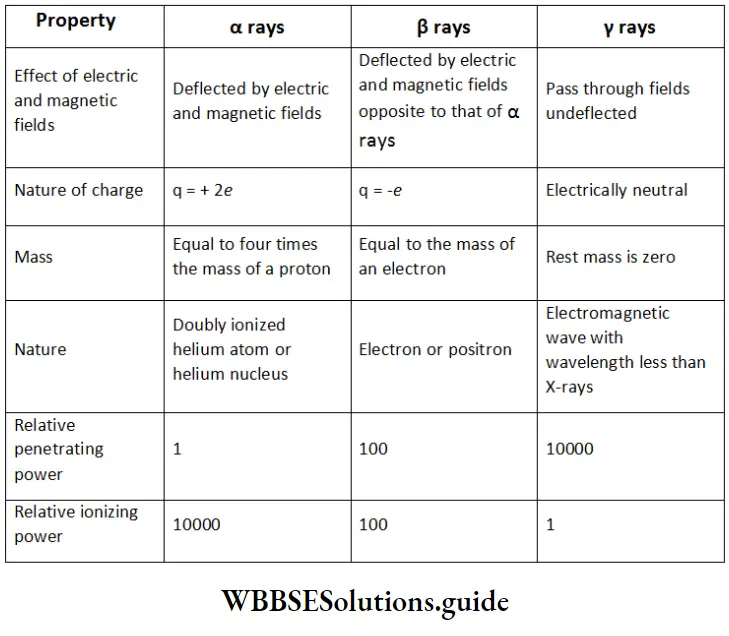
\(\mathrm{A}=\mathrm{A}_0 \mathrm{e}^{\mathrm{it}} \text { and } \mathrm{t}=2.303 \log _{10}\left(\frac{\mathrm{A}_0}{\mathrm{~A}}\right)\)Alpha decay. Alpha decay is a process in which a nucleus decays spontaneously emitting an alpha particle. These α-particles have discrete values of energy. When a nucleus decays with the emission of α-particle (helium nucleus), the product nucleus (daughter nucleus) has atomic number two less and mass number four less than that of the decaying nucleus (parent nucleus).
In general,
\(\begin{aligned}& { }_z^A \mathrm{X} \rightarrow{ }_{\mathrm{z}-2}^{\mathrm{A}} \mathrm{Y}+{ }_2^4 \mathrm{He} \\
& \mathrm{Eg}:{ }_{92}^{238} \mathrm{U} \rightarrow{ }_{90}^{234} \mathrm{Th}+{ }_2^4 \mathrm{He}
\end{aligned}\)
This spontaneous decay is possible only when the total mass of decay products is less than the mass of initial nucleus. The decrease in mass during the decay appears as K.E. of the products.
The disintegration energy or Q-value of the reaction is the difference between the initial mass energy and the total mass-energy of the decay products.
\(\text { i.e., } Q=\left(M_X-M_Y-m_\alpha\right) c^2\)Where MX, MY & mα are the masses of the initial nucleus, product nucleus, and alpha particle. Clearly Q>0 for exothermic processes such as α-decay.
Β Decay
β –decay is a process in which a nucleus decays spontaneously emitting an electron or positron. During the β –decay process of a nucleus, the mass number of the product nucleus remains the same but its atomic number changes by one.
A common example of β decay is
\({ }_{15}^{32} P \rightarrow{ }_{16}^{32} S+e^{-}+\bar{v}
and that of \beta^* decay is
{ }_{11}^{22} \mathrm{Na} \rightarrow{ }_{10}^{22} \mathrm{Ne}+e^{+}+v
\)
Nuclear fission. Nuclear fission is the process in which the nucleus of an atom of a heavy element breaks up into two nuclei of comparable masses with the release of a large amount of energy.
The most important neutron-induced nuclear reaction is fission. An example of fission is when a uranium isotope \({ }_{92}^{235} \mathrm{U}\) bombarded with a neutron breaks into two intermediate-mass nuclear fragments
\({ }_0^1 n+{ }_{92}^{235} U \rightarrow{ }_{92}^{236} U \rightarrow{ }_{56}^{144} \mathrm{Ba}+{ }_{36}^{89} \mathrm{Kr}+3{ }_0^1 n\)The same reaction can produce other pairs of intermediate mass fragments
\(\begin{gathered}{ }_0^1 n+{ }_{92}^{235} \mathrm{U} \rightarrow{ }_{92}^{236} \mathrm{U} \rightarrow{ }_{51}^{133} \mathrm{Sb}+{ }_{41}^{99} \mathrm{Nb}+4{ }_0^1 n \text { or, } \\
{ }_0^1 n+{ }_{92}^{235} \mathrm{U} \rightarrow{ }_{92}^{236} \mathrm{U} \rightarrow{ }_{54}^{140} \mathrm{Xe}+{ }_{38}^{94} \mathrm{Sr}+2{ }_0^1 n
\end{gathered}\)
1. Controlled chain reaction. In this type, the chain reaction is first accelerated so that the neutron population is built up to a certain level and thereafter the number of fission-producing neutrons is kept constant. As a result, the energy is released in a controlled manner at a constant rate. This forms the principle of a nuclear reactor.
2. Uncontrolled chain reaction. In this type, the number of neutrons is allowed to multiply indefinitely by increasing the frequency of fissions so that the entire energy is released in a very short interval of time. The energy released will be uncontrolled and it results in a violent explosion. This forms the principle of an atom bomb.
An average of 2.5 neutrons are released in a single fission of . In order to achieve a self-propagating nuclear reaction, at least one of the neutrons in the fission must be captured by another \(\) nucleus and cause further fission. This possibility is determined by a quantity called reproduction constant or multiplication factor denoted by K. It is defined as the ratio of the
secondary neutrons produced to the original neutrons.
Nuclear reactor. A nuclear reactor is a device in which a controlled nuclear chain reaction can be initiated and sustained to harness nuclear energy for constructive purposes.
The essential components of a nuclear reactor are:
Nuclear fuel. The core of the reactor is the site of nuclear fission. It contains the fuel elements in suitably fabricated form. The fuel may be \({ }_{94}^{239} \mathrm{Pu}\) or enriched uranium (i.e., one that has a greater abundance of \({ }_{92}^{235} U\) than naturally occurring uranium).
1. Moderator. The average energy of a neutron produced in fission of \({ }_{92}^{235} U\) is 2MeV. These neutrons unless slowed down will escape from the reactor without interacting with the uranium nuclei, unless a very large amount of fissionable material is used for sustaining the chain reaction. What one needs to do is to slow down the fast neutrons by elastic scattering with light nuclei. Therefore, in reactors, light nuclei called moderators are provided along with the fissionable nuclei for slowing down fast neutrons. The moderators commonly used are water, heavy water (D2O) and graphite.
2. Control rods. The reaction can be shut down by means of control rods (made of, for example, cadmium) that have high absorption of neutrons. The K value can be varied in a reactor by the proper use of these rods, hence are called control rods.
3. Cooling system. The energy (heat) released in fission is continuously removed by a suitable coolant. The coolant transfers heat to a working fluid which in turn may produce steam. The steam drives turbines and generates electricity. The coolants used are carbon dioxide gas or ordinary water when graphite is the moderator. Heavy water is used also as coolant when it is used as moderator.
4. Reflector. The core is surrounded by a reflector to reduce leakage. This helps in the reduction of critical size. The commonly used reflector is graphite.
5. Safety system & protective shield. The problems of reactor safety are very vast and complex. All reactors are provided with back up cooling system as a safety measure. This system takes over if the regular cooling system fails.
A reactor is provided with adequate shielding to minimize the biological effects of harmful radiation ( -rays and neutrons ). The shield is generally a 2m thick concrete wall surrounding the reactor.
Nuclear fusion. Nuclear fusion is the opposite of nuclear fission. It is the process in which two lighter nuclei fuse together to form a heavier nucleus with the liberation of energy
\({ }_1^1 \mathrm{H}+{ }_1^1 \mathrm{H} \rightarrow{ }_1^2 \mathrm{H}+\mathrm{e}^{+}+\mathrm{v}+0.42 \mathrm{MeV}\)Controlled thermonuclear fusion. In controlled fusion reactors, the aim is to generate steady power by heating the nuclear fuel to a temperature in the range of 108 K. At these temperatures, the fuel is a mixture of positive ions and electrons (plasma). The challenge is to combine this plasma, since no container can stand such a high temperature. This can be done by using a magnetic bottle.