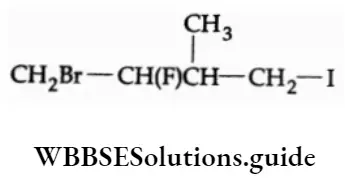
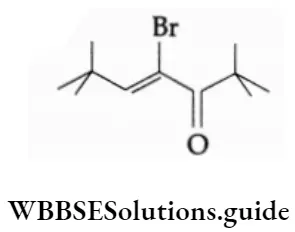
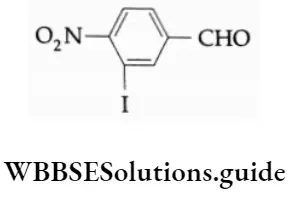
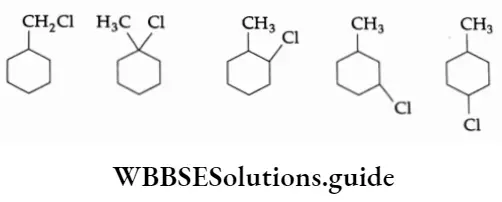
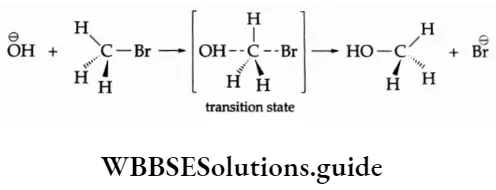
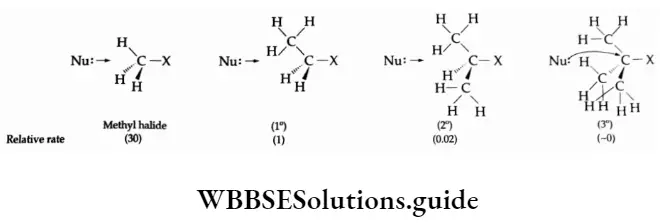
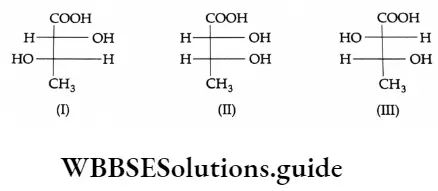
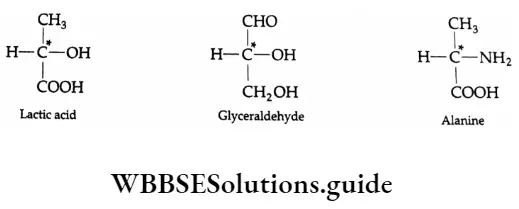
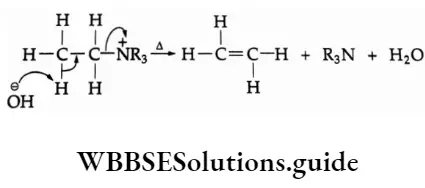
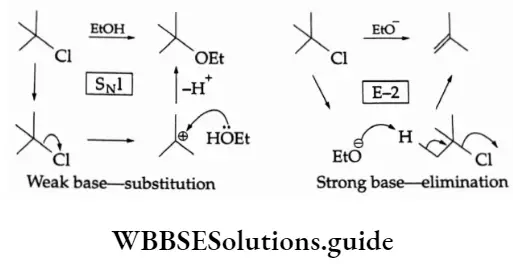
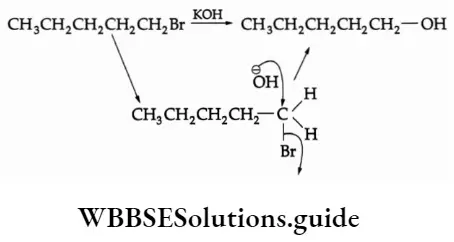
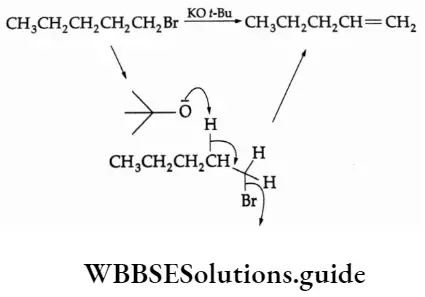
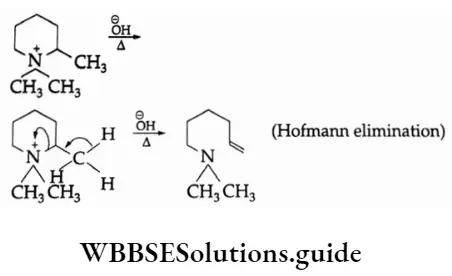
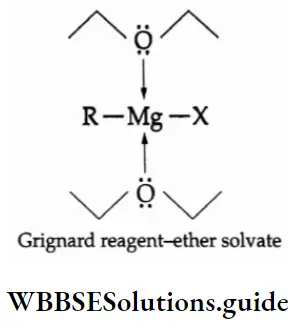
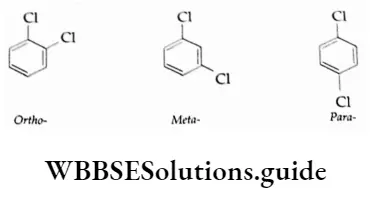
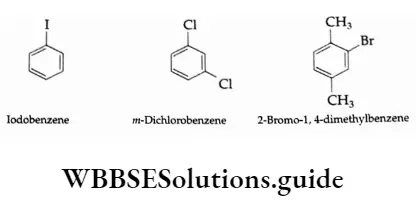
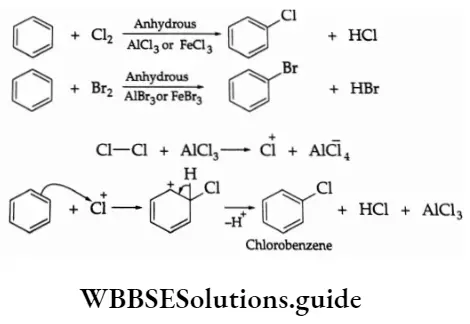
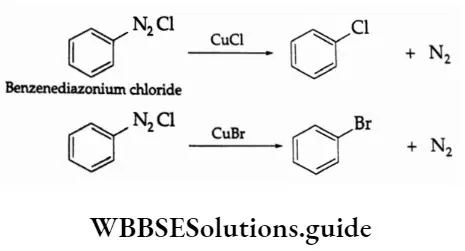
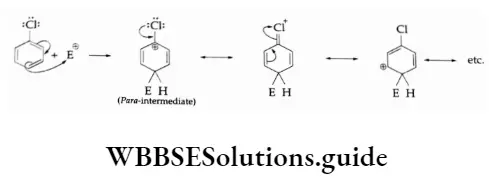
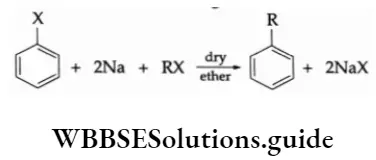
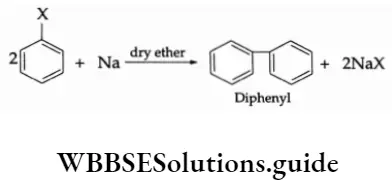
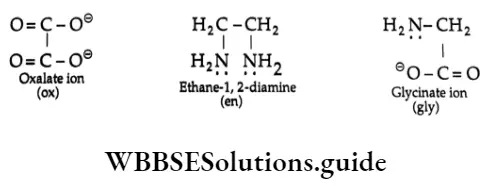
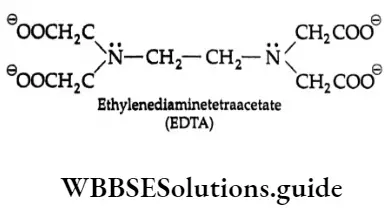
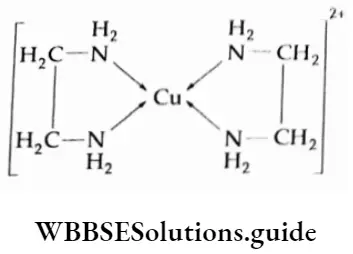
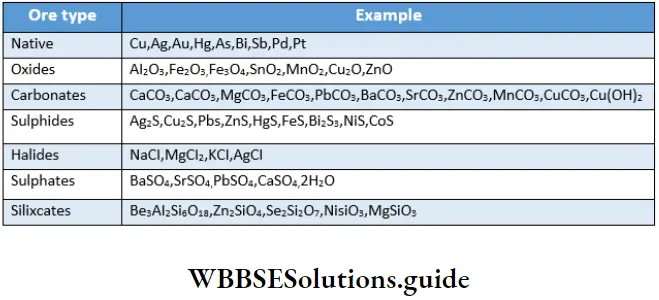
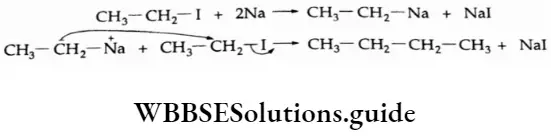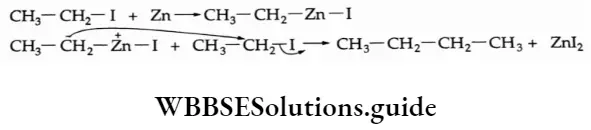The P Block Elements
The long form of the periodic table is divided into s, p, d and f blocks. Elements are grouped in four blocks depending upon the subshell in which the valence electron of the atom of an element enters, the p block on the periodic table constitutes Groups 13 to 18.
In class XI you have already studied the chemistry of the elements of Groups 13 and 14 along with those of Groups 1 and 2. The p-block elements show a much greater variation In properties than that shown by s- and d-block elements.
The variation in properties of p-block elements is tine in varying atomic sizes, ionisation enthalpies, electronegativities, and electron gain enthalpies. You know that the valence shell electronic configuration of the p-block elements is wsJMp’ h.
The first member of the group differs from the subsequent members due to its small size and nonavailability of d orbitals in its atoms. The p-block elements comprise metals, nonmetals and metalloids, thereby showing diversity In their properties.
P-block elements class 12 chemistry notes
Group 15 Elements
This group comprises the elements nitrogen, phosphorus, arsenic, antimony and bismuth. Nonmetnllic character changes to metallic character gradually, on descending the group. Nitrogen and phosphorus are nonmetals, arsenic and antimony are metalloids, while bismuth is a metal. The elements of Group 15 are less metallic than the corresponding elements of Group 14.
Occurrence And Uses
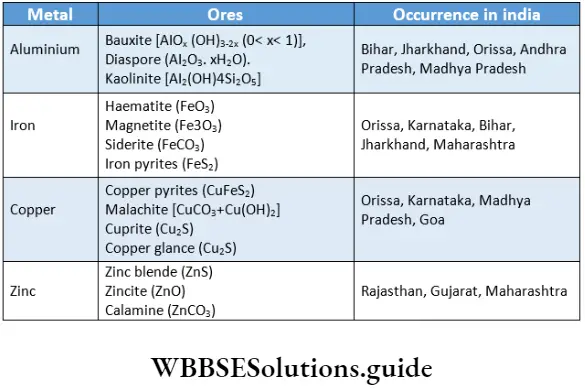
Nitrogen is present to the extent of 78% in the atmosphere. However, it is not very abundant in the earth’s crust as all nitrites and nitrates are water soluble. The major minerals are Indian saltpetre (KNO3) and Chile saltpetre (NaNO3). Nitrogen is an essential constituent of proteins and amino acids. Phosphorus is the eleventh element in order of abundance in the earth’s crust.
The common minerals of phosphorus arc fluorapatite [Ca9(PO4)6 , CaF2] and hydroxyapatite [Ca9(PO4)6Ca(OH)2], which are present in phosphate rocks. Phosphorus is an essential constituent of plants and animals.
It is present in bones, teeth and other hard tissues of the animal body. Arsenic, antimony and bismuth are not abundant and occur in trace amounts as sulphides along with other minerals.
Nitrogen is used in iron and steel industries and oil refineries to maintain an inert atmosphere. Liquid nitrogen is used as a refrigerant. Large amounts of nitrogen are used in the manufacture of ammonia and calcium cyanamide.
The major use of phosphorus is in making phosphatic fertilisers. Phosphates are also used in the food industry, in detergents and as pharmaceuticals. Phosphorus is used in safety matches and to make phosphor-bronze, an alloy.
It is also used to make pesticides and organophosphorus compounds. Arsenic is used to dope semiconductors and to alloy with lead to make it harder. Compounds of arsenic are used as pesticides and weedicides.
Antimony is used in alloys with tin and lead. It is used to electroplate steel to prevent rusting. Bismuth is used to make low-melting alloys which are used in fuses and fire alarms.
Atomic And Physical Properties
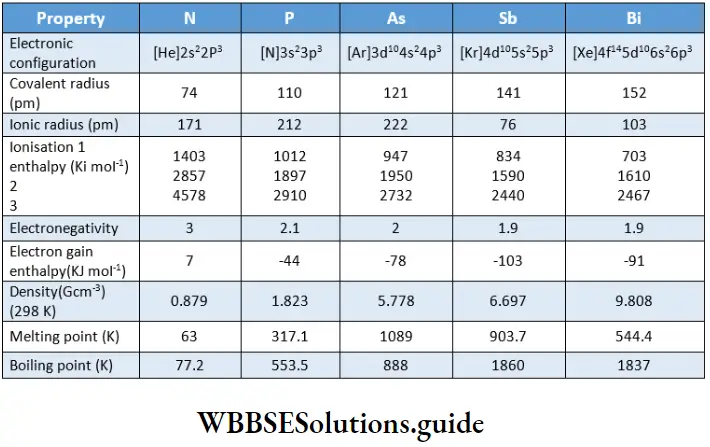
Electronic configuration
The valence shell electronic configuration of these elements is ns2np3. The presence of completely filled s orbitals and half-filled p orbitals confers extra stability to the electronic configuration.
Size
Like in other groups, the atomic and ionic radii of elements increase on moving down the group. There is a substantia] increase in size from nitrogen to phosphorus. However, the increase in size from phosphorus to arsenic and then from antimony to bismuth is less due to the presence of filled d or f orbitals.
Ionisation enthalpy
It decreases down the group due to an increase in atomic size. The first ionisation enthalpy is quite high because of the stable ns2 np3 configuration.
Electron gain enthalpy
In Group 15, the electron gain enthalpy of nitrogen is positive whereas that of other elements is negative. The positive electron gain enthalpy of nitrogen is due to its compact atomic size. In the case of other elements, though the electron gain enthalpy is negative, it is not very high as the elements of the group have stable, half-filled p orbitals.
Properties and characteristics of p-block elements
Electronegativity
Electronegativity decreases down the group with an increase in atomic size and metallic behaviour of the elements. However, the difference in electronegativities is less pronounced with increasing atomic weight.

Boiling and melting points
All elements in this group are polyatomic and display allotropy, except nitrogen. There is an increase in boiling point down the group; however, the variation in melting point is not regular.
Chemical Properties
Oxidation states and trends in chemical reactivity
The elements of this group have five electrons in their outermost shell. The elements exhibit a maximum oxidation state of +5. The stability of the +5 oxidation state decreases down the group due to the increasing inert pair effect. The only well-characterised binary compound of bismuth in the +5 oxidation state is BiF5.
(As you know from your previous class, the inert pair effect is the tendency of s electrons to remain inert, i.e., not to take part in bond formation.) Nitrogen and phosphorus may also form N-3 and P-3 species with electropositive metals, by gaining three electrons. For example, magnesium and calcium nitrides (Mg3N2 and Ca3N2), which constitute N3- ions.
Tire stability of the -3 oxidation state decreases down the group as the electropositive character of the element increases. Nitrogen displays a wide range of oxidation states, ranging from -3 to +5. The common oxidation states of phosphorus and arsenic are -3, +3 and +5.
In the case of nitrogen, disproportionation reactions are common as exemplified by the case of nitrous acid
3HNO2 →HNO3+H2O+2NO
In the case of phosphorus, such reactions are common
4H3PO3→3H3PO4+PH3
You have already studied in your previous class that in a disproportionation reaction, a substance is both oxidised and reduced. In the examples discussed above the reactant contains nitrogen and phosphorous in the +3 oxidation state.
This oxidation state is not very stable and a higher stable oxidation state, i.e., +5 state is known for the elements. For the heavier members of the group, the stability of the +5 oxidation state decreases and that of the +3 state increases (inert-pair effect). Hence the tendency for disproportionation decreases.
Bond type
Group 15 elements generally form covalent compounds. However, there is a decrease in the covalent character in the order P > As > Sb > Bi. Antimony and bismuth form tripositive cations due to an increase in metallic character, on moving down the group.
Formation of penta- and hexa-coordinated derivatives
Due to the nonavailability of d orbitals, nitrogen cannot expand its octet. The heavier members of the group use d orbitals to form species like PCI5, PF6–, and AsF5.
Tendency to form multiple bonds and catenation
The tendency to form multiple bonds relative to single bonds decreases on descending the group. Nitrogen is diatomic two atoms are bound by a triple bond in a molecule. Nitrogen also forms pπ-pπ bonds with other elements having comparable size and electronegativity (like C and O).
The other members of the group do not form multiple bonds as the orbitals are larger and diffuse and therefore do not allow effective overlap. Phosphorus, arsenic and antimony are tetraatomic, the atoms being linked by single bonds.
Recently compounds like R3P= O and R3P= CH2 (R = alkyl group) have been isolated involving dir-pn bonds, i.e., overlap of d orbitals of phosphorus with p orbitals of carbon and oxygen.
P-block elements group-wise classification and examples
The trialkyl and triaryl derivatives of phosphorus and arsenic act as electron-pair donors towards transition metals, where the unshared electron pair on phosphorus and arsenic is donated to the vacant d orbital of the metal.
The bond thus formed is strengthened by an overlap of filled d orbitals of the metal with vacant d orbitals of P or As. This is referred to as a dπ-dπ bond.
The single N-N bond is weaker than the single P-Pbond. This is due to the small size of nitrogen and small N-N bond length, which lends to high Intcreledrunic repulsion between the nonbonding electrons.
Thus the catenation power of phosphorus Is greater than that of nitrogen. This Is manifested in a large number of allotropes of phosphorus.
Anomalous behaviour of nitrogen Nitrogen, the first member of group 15, differs considerably from the rest of the members. These differences arise due to the small size of nitrogen, ils high electronegativity, its tendency to form stable pn pa bonds and the nonavailability of d orbitals in its valence shell.
Some of the anomalous properties of nitrogen are listed as follows.
- Nitrogen exists as a diatomic gaseous molecule, while the lower members of the group exist as polyatomic solids.
- Nitrogen is inert due to the high strength of the NnN bond. The rest of the members of the group are more reactive.
- Nitrogen forms pπ-pπ bonds with itself and with elements like carbon and oxygen. Consequently, it forms a large number of oxides which are monomeric, unlike those of phosphorus which arc dimeric. Also, it forms species N–3 and CN–.
- The maximum covalency of nitrogen is four. The other elements can expand their octet and form species with coordination numbers five and six.
Reactivity towards hydrogen
The elements of Group 15 form trihydric of the general formula EH3. Their ease of formation and stability decrease down the group. This can be explained in terms of E—H bond enthalpy. On descending the group, the size of E increases so that the orbitals become larger and more diffuse.
Consequently, these orbitals do not undergo effective overlap with the small 1 s orbital of hydrogen which leads to a decrease in bond enthalpy on descending the group. Thus, the reducing power of the hydrides increases down the group.
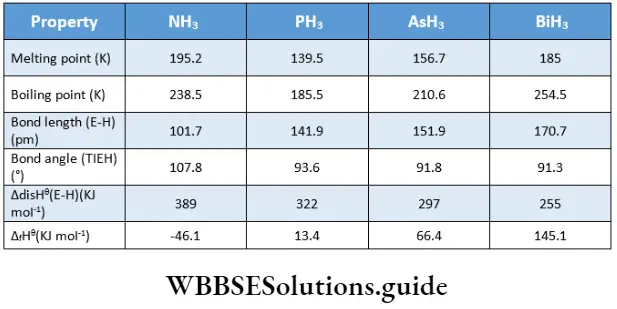
Apart from ammonia, the other hydrides are toxic gases. The low volatility of ammonia is dm* to intermolecular hydrogen bonding.
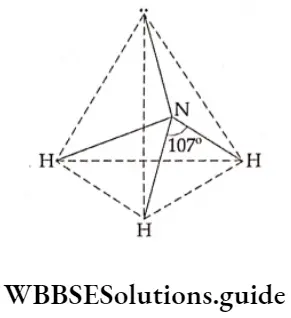
The hydrides of Group 15 elements have a pyramidal structure. The central atom is sp3 hybridised having a lone pair of electrons. Due to the repulsion between the lone pair and a bond pair of electrons, the bond angle reduces from 109°27′ (that of a regular tetrahedron) to 107°48’.
Thus the tetrahedral shape is slightly distorted. (The shape of the ammonia molecule is described as pyramidal since one of the tetrahedral positions is occupied by a lone pair.) Distortion due to lone pairs is even more in PH3, AsH3 and SbH3, causing the bond angle to reduce further up to 91.3°.
Group 13 elements – boron family properties and examples
Hydrides of Group 15 elements donate lone pairs of electrons and thus behave as Lewis bases. The basicity decreases down the group.
Reactivity towards oxygen
Since the Group 15 elements exhibit predominantly two oxidation states +3 and +5, two types of oxides are known— and E205. The +5 oxidation state in bismuth is not stable. Therefore it’s the main oxide! is Bi2Ov Oxides of nitrogen and phosphorus are acidic, those of arsenic and antimony are amphoteric, while that of bismuth is basic.
Thus the basic character of the oxides increases down the group as the metallic character increases. When an element forms two oxides E2O3 and E2O5, the oxide in the higher oxidation state is more acidic.
Reactivity towards halogens
Trihalides, EX3, of all elements are known. Of all nitrogen halides, only NF5 is stable due to the strong N-F bond. The trihalides of other elements are stable and are predominantly covalent, except BiF3. The pentahalides are fewer in number than trihalides.
Nitrogen does not form pentahalides due to the non-availability of d orbitals in its valence shell. The well-characterised pentahalides are PX5 (X = F, Cl, Br), AsF5, SbF5, SbCl5 and BiF5. Pentahalides are more covalent than trihalides.
This is because the central atom in the +5 oxidation state has greater polarising power and can polarise the anion considerably. You have studied in class XI that when the degree of polarization is large, the concentration of electrons increases between the two bonded atoms and the covalent character increases.
Reactivity towards metals
Group 15 elements react with metals to form binary compounds in which they exhibit a -3 oxidation state. Nitrogen forms nitrides with lithium (Li3N), alkaline earth metals (Mg3N2, Ca3N2, etc.) and aluminium (AIN). There are also examples of phosphides (Ca3P2), arsenides (Na3As, Mg3As2), antimonides (Zn3Sb2) and bismuthides (Mg3Bi2).
Nitrogen (N2)
Nitrogen is the most abundant gas in the atmosphere. It comprises about 78.1% of the atmosphere by volume. It is less abundant in the earth’s crust because of the high solubility of nitrates.
Preparation
Nitrogen is obtained commercially by the fractional distillation of liquefied air. Air is first cooled to remove water vapour and carbon dioxide and then liquefied. On fractional distillation, nitrogen distils out first, leaving behind oxygen.
In the laboratory, nitrogen is obtained by warming an aqueous solution of ammonium chloride and sodium nitrite. This produces the thermally unstable ammonium nitrite, which decomposes to give nitrogen.
⇒ \(\mathrm{NH}_4 \mathrm{Cl}(\mathrm{aq})+\mathrm{NaNO}_2(\mathrm{aq}) \stackrel{\Delta}{\longrightarrow} \mathrm{NaCl}(\mathrm{aq})+\mathrm{NH}_4 \mathrm{NO}_2(\mathrm{aq}) \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}\)
Small amounts of nitric acid and nitric oxide are formed as by-products in this reaction and may be removed by passing the gaseous product through a mixture of aqueous sulphuric acid and potassium dichromate.
Other methods of preparation of nitrogen include the oxidation of ammonia by bromine and the thermal decomposition of ammonium dichromate.
⇒ \(8 \mathrm{NH}_3+3 \mathrm{Br}_2 \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2+6 \mathrm{NH}_4 \mathrm{Br}\)
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{Cr}_2 \mathrm{O}_7 \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2+\mathrm{Cr}_2 \mathrm{O}_3+4 \mathrm{H}_2 \mathrm{O}\)
Small quantities of very pure nitrogen can be obtained by the thermal decomposition of sodium azide or barium azide.
⇒ \(2 \mathrm{NaN}_3 \stackrel{\Delta}{\longrightarrow} 3 \mathrm{~N}_2+2 \mathrm{Na}\)
Properties
Nitrogen is a colourless, odourless and nontoxic gas. The gas has low freezing and boiling points and low solubility in water. The two stable isotopes of nitrogen are 14N and 15N.
Nitrogen exists as a diatomic molecule, N =N. It is rather inert at room temperature due to the high bond enthalpy of the N =N bond. However, at elevated temperatures, it becomes increasingly reactive and combines directly with hydrogen oxygen, and some electropositive metals.
⇒ \(\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \underset{\text { catalyst }}{\stackrel{773 \mathrm{~K}}{\rightleftharpoons}} 2 \mathrm{NH}_3(\mathrm{~g})\)
⇒ \(\mathrm{N}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \stackrel{2000 \mathrm{~K}}{\rightleftharpoons} 2 \mathrm{NO}(\mathrm{g})\)
⇒ \(6 \mathrm{Li}(\mathrm{s})+\mathrm{N}_2(\mathrm{~g}) \stackrel{\Delta}{\longrightarrow} 2 \mathrm{Li}_3 \mathrm{~N}(\mathrm{~S})\)
Ammonia
Small amounts of ammonia are present in nature as it is released from the decay of nitrogenous organic matter like urea.
⇒ \(\mathrm{NH}_2 \mathrm{CONH}_2+\mathrm{H}_2 \mathrm{O} \rightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{CO}_3\)
Preparation
Ammonia is obtained by heating ammonium salts with an alkali in the laboratory.
⇒ \(2 \mathrm{NH}_4 \mathrm{Cl}+\mathrm{Ca}(\mathrm{OH})_2 \stackrel{\Delta}{\longrightarrow} \mathrm{CaCl}_2+2 \mathrm{NH}_3+2 \mathrm{H}_2 \mathrm{O}\)
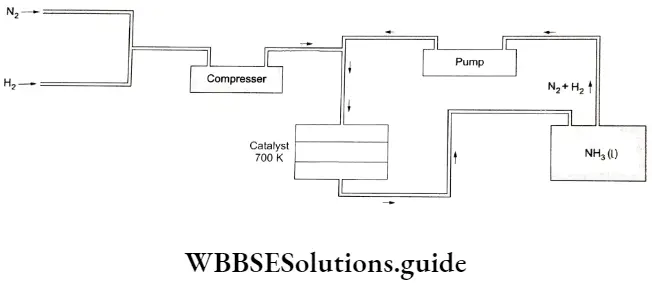
On a large scale, ammonia is prepared by the Haber process.
⇒ \(\underbrace{\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g})}_{4 \text { volumes }} \rightleftharpoons \underbrace{2 \mathrm{NH}_3(\mathrm{~g})}_{2 \text { volumes }} \quad \Delta_{\mathrm{f}} \mathrm{H}^{\ominus}=-46.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
One volume of nitrogen combines with three volumes of hydrogen to give two volumes of ammonia. The reaction is reversible, accompanied by a reduction in volume and is exothermic. According to Le Chatelier’s principle, the forward reactions are favoured by high pressure and low temperature.
This reaction is carried out at a pressure of 150-250 Pa and a temperature of 600-700 Kin the presence of a finely divided iron catalyst with small amounts of oxides of potassium, aluminium and molybdenum.
The gases are passed over four beds of catalyst, with cooling between each pass. On each pass about 15% conversion occurs and the unreacted gases are recycled so that eventually an overall conversion of 98% can be achieved.
Properties
Ammonia is a colourless gas at room temperature (freezing point 198.4 K boiling point 239.7 K) and has a strong, characteristic pungent smell. It can be liquefied easily. The molecules of ammonia are extensively associated with hydrogen bonding both in the liquid and solid states.
Thus, it is less volatile than the other Group 15 hydrides. It is highly soluble in water and its aqueous solution is weakly basic owing to the presence of OH“ ions as shown by the following reaction.
⇒ \(\mathrm{NH}_3(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{NH}_4^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})\)
NH3 and NH4OH both react with acids forming ammonium salts. These salts are thermally unstable and decompose on heating. If the anion is not oxidising, ammonia is evolved.
⇒ \(\mathrm{NH}_4 \mathrm{Cl} \stackrel{\Delta}{\longrightarrow} \mathrm{NH}_3+\mathrm{HCl}\)
If the anion is oxidising then NH+4 is oxidised to N2 or N2O.
⇒ \(\mathrm{NH}_4 \mathrm{NO}_2 \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{NH}_4 \mathrm{NO}_3 \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2 \mathrm{O}+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\left(\mathrm{NH}_4\right)_2 \mathrm{Cr}_2 \mathrm{O}_7 \stackrel{\Delta}{\longrightarrow} \mathrm{N}_2+4 \mathrm{H}_2 \mathrm{O}+\mathrm{Cr}_2 \mathrm{O}_3\)
When aqueous ammonia is added to a metal salt solution, in many cases the metal hydroxide or hydrated oxide is precipitated.
CaCI2(aq)+2NH4OH(aq) →Ca(OH)2(s)+2NH4CI(aq)
Zn(NO3)(aq)+2NH4OH(aq)→Zn(OH)2(s)+2NH4NO3(aq)
2FeCI3(aq)+3NH4OH(aq) →FeO3.xH2O(s)+3NH4CI(aq)
In the ammonia molecule, the nitrogen atom is sp3 hybridised. There are three bond pairs and one lone pair. The resultant structure is pyramidal with an unshared electron pair on nitrogen. The bond angle (107.8°) is less than the bond angle associated with sp3 hybridisation (109.5°) due to distortion caused by the lone pair.
The ammonia molecule donates this lone pair to electron-pair acceptors and thus behaves as a Lewis base. It donates these electrons to many transition metal ions, forming complex compounds. When ammonia solution is added to copper sulphate solution, a deep blue colouration is obtained owing to the formation of [Cu(NH3)4]2+ ions
⇒ \(\mathrm{Cu}^{2+}(\mathrm{aq})+4 \mathrm{NH}_3(\mathrm{aq}) \rightleftharpoons\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}(\mathrm{aq})\)
AgCl(s) dissolves inNH3(aq) to form a complex, [Ag(NH3)2 ]C1. You are familiar with this reaction as it forms the basis of confirming chloride ions in salt analysis.
CI–(aq)+AgNO3(aq)→AgCI(s)+NO–3(aq)
AgCI(s)+2NH3(aq)→[Ag(NH3)2]+(aq)
Uses
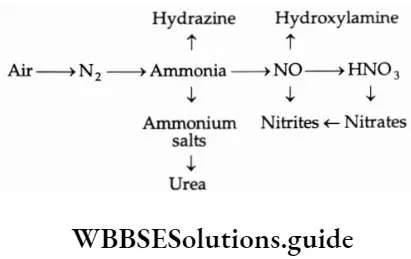
Ammonia is widely used in the manufacture of a large number of nitrogenous fertilisers like urea, ammonium nitrate, ammonium sulphate and ammonium phosphate. It is also used to prepare many inorganic nitrogen-containing compounds, the most important being nitric acid. Ammonia and ammonium compounds are used in the preparation of explosives, fibres and plastics.
Ammonia is also used as a refrigerant and in the manufacture of detergents and numerous inorganic and organic chemicals. Synthetic ammonia is the key to the industrial production of most inorganic nitrogen compounds as shown in the given scheme.
Oxides Of Nitrogen
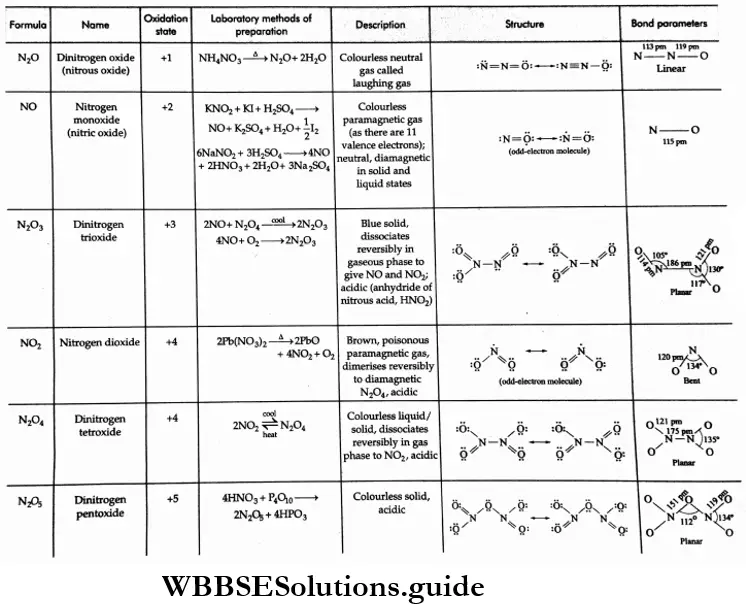
Nitrogen forms a large number of oxides in the oxidation states +1 to +5. The oxides in the lower oxidation states are neutral while those in the higher oxidation states are acidic.
They exhibit pπ- pπ bonding between nitrogen and oxygen and exist as resonance hybrids of different canonical forms. Their formulae, names, methods of preparation and structures are summarized in.
Oxoacids Of Nitrogen
Nitrogen forms several oxoacids, many of which are unstable in the free state and are known only in aqueous solution or as their salts. The principal species are hyponitrous acid (H2N2O2, a weak acid, whose salts are known), hydronitrous acid (H4N2O4, whose sodium salt is known), nitrous acid (HNO2) unstable and weak though salts are known and nitric acid (HNO3) which is the most stable and the important one.
Nitric acid, HNO3
Nitric acid is one of the three most important acids in the modern chemical industry (the others are sulphuric acid and hydrochloric acid).
Preparation In the laboratory nitric add is prepared by heating an alkali metal nitrate with concentrated sulphuric acid.
⇒ \(\mathrm{KNO}_3+\mathrm{H}_2 \mathrm{SO}_4 \stackrel{\Delta}{\longrightarrow} \mathrm{KHSO}_4+\mathrm{HNO}_3\)
It is industrially prepared by the Ostwald process, which involves the catalytic oxidation of ammonia to NO.
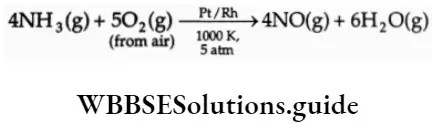
The nitric and air cooled and the mixture of gases is absorbed in a counter-current of water. During this process, nitric oxidised to nitrogen dioxide, which dissolved in water to give nitric acid.
⇒ \(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NO}_2(\mathrm{~g})\)
⇒ \(3 \mathrm{NO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons 2 \mathrm{HNO}_3(\mathrm{aq})+\mathrm{NO}(\mathrm{g})\)
The nitric oxide is recycled and the aqueous nitric acid can be concentrated by distillation to the extent of up to 68% by mass since at this composition a constant-boiling mixture (azeotrope) is formed. Further concentration to 98% may be achieved by dehydrating with concentrated sulphuric acid or phosphorous pentoxide.
Properties Pure nitric acid is a colourless liquid (freezing point 231.4 K, boiling point 355.6 K). It has a specific gravity of 1.504. On exposure to light, it undergoes slight decomposition to N02 and Oz and thus acquires a yellowish-brown colour.
4HNO3→4NO2+O2+2H2O
It is a strong add and is completely dissociated in aqueous solutions
⇒ \(\mathrm{HNO}_3(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{NO}_3^{-}(\mathrm{aq})\)
Nitric add forms a large number of nitrates, which are highly soluble in water. Dilute nitric acid (< 2M) behaves as a typical strong acid. More concentrated aqueous solutions are strongly oxidising and attack most metals except noble metals like gold and platinum.
When nitric acid acts as an oxidising agent, it is reduced and the products of reduction depend upon the concentration of the acid, temperature and the other reactant.
Zinc reacts with dilute nitric acid to give nitrous oxide and with the concentrated acid, it forms nitrogen dioxide. With copper, dilute nitric acid gives nitric oxide and concentrated acid gives nitrogen dioxide.
4Zn+10HNO3(dil.)→4Zn(NO3)2+N2O+5H2O
Zn+4HNO3(conc.)→Zn(NO3)2+2NO2+2H2O
3Cu+8HNO3(dil.)→3Cu(NO3)2+2NO+4H2O
Cu+4HNO3(conc.)→Cu(NO3)+2NO2+2H2O
Metals like aluminium and chromium are rendered passive by concentrated nitric acid due to the formation of a superficial oxide film on the surface of the metal.
Concentrated nitric acid oxidises nonmetals to their corresponding oxides or oxoacids.
C+4HNO3(conc.)→Cu(NO3)2+2H2O
⇒ \(\mathrm{P}_4+20 \mathrm{HNO}_3 \longrightarrow \underset{\text { Phosphoric acid }}{4 \mathrm{H}_3 \mathrm{PO}_4}+20 \mathrm{NO}_2+4 \mathrm{H}_2 \mathrm{O}\)
S8+48HNO3→8H2SO4+48NO2+16H2O
⇒ \(\mathrm{I}_2+10 \mathrm{HNO}_3 \longrightarrow \underset{\text { logic acid }}{2 \mathrm{HIO}_3}+10 \mathrm{NO}_2+4 \mathrm{H}_2 \mathrm{O}\)
A mixture of concentrated nitric and hydrochloric acids (1 : 3 by volume respectively) is referred to as aqua regia; it is a very powerful oxidising mixture and dissolves metals like gold and platinum. However, it does not dissolve silver; instead, it forms an insoluble chloride with it.
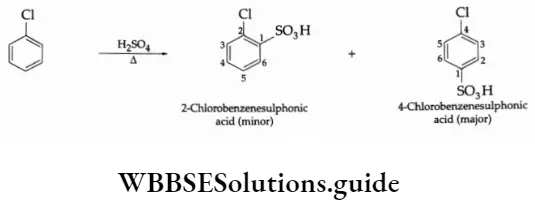
When nitric acid is mixed with concentrated sulphuric acid, the nitronium ion(NO2) is obtained, which is the active species used in the nitration of organic compounds.
Test for nitrates Nitrates are detected by the brown ring test. A freshly prepared ferrous sulphate solution is added to an aqueous solution of the nitrate followed by the slow addition of concentrated sulphuric acid along the side of the test tube so that it forms the layer at the bottom.
A brown ring appears at the interface of the two liquids, which confirms the presence of nitrate ions in the solution. The ferrous ions reduce the nitrate ions to nitrogen monoxide. This reacts with hydrated ferrous ions to form a brown complex.
NO–+3Fe2++4H+→NO+3Fe3++2H2O
⇒ \(\underset{\text { Hydrated ferrous ion }}{\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}}+\mathrm{NO} \longrightarrow \underset{\text { Brown colour }}{\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_5 \mathrm{NO}^{2+}\right.}+\mathrm{H}_2 \mathrm{O}\)
Structure Nitric acid has a planar structure. The bond parameters of a molecule of nitric acid are as follows.
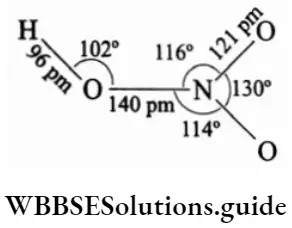
The nitrate ion has a planar structure with equal bond lengths.
Nitric acid is largely used in the production of ammonium nitrate, which is employed for the production of fertilisers and other nitrates, which are used in the manufacture of explosives. It is used to make cyclohexanone
Which is one of the starting materials to prepare the monomers for the polymers nylon 6, 6 and nylon 6. Another major use is in the preparation of organic nitro compounds like nitroglycerine, nitrocellulose and trinitrotoluene. Minor uses include the pickling of stainless steel and the etching of metals. It is also used as an oxidiser in rocket fuel.
Phosphorus—Allotropic Modification
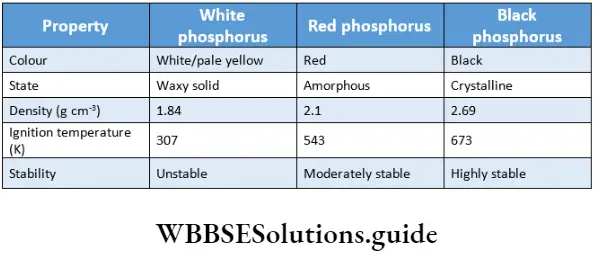
The main allotropes of phosphorus are white phosphorus, red phosphorus and black phosphorus.
White phosphorus is the most common allotrope obtained by the condensation of gaseous or liquid states. It is a waxy, translucent solid, pale yellow in colour and soluble in carbon disulphide and benzene. It is toxic, spontaneously catches fire and is, therefore, stored underwater. It reacts with moist air and gives out a characteristic faint glow and this is referred to as chemiluminescence.
It consists of discrete tetrahedral P4 units. The P-P bond angle is 60° and there is a considerable angular strain in the molecule. This accounts for the low stability and high reactivity of this allotrope. It readily catches fire in the air, forming the pentoxide.
P4+5O2→P4O10
Red phosphorus is obtained by heating white phosphorus at 573 K in the absence of air for several days. It is less reactive than white phosphorous and does not display chemiluminescence. It has a polymeric structure consisting of tetrahedral P4 units joined together.
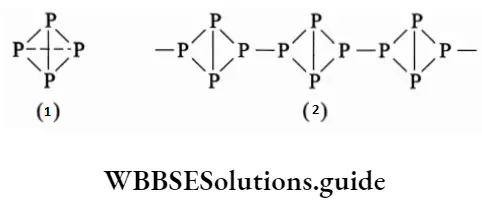
Thermodynamically the most stable form is black phosphorus. It exists in two forms—a-black phosphorus and (3-black phosphorus. The a-form is obtained by heating red phosphorus in a sealed tube at 803 K.
It sublimes in the air and consists of opaque, monoclinic crystals. The |3-form is obtained by heating white phosphorus at 473 K under high pressure. It is inert and has a layered structure. Important physical properties of these allotropes are summarized.
Phosphine
Preparation
Phosphine, PH3, is the hydride of phosphorus. It is obtained by the alkaline hydrolysis of yellow phosphorus or by the reaction of calcium phosphide with water or dilute acid.
⇒ \(\mathrm{P}_4+3 \mathrm{NaOH}+3 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{PH}_3+3 \mathrm{NaH}_2 \mathrm{PO}_2\)
Sodium hypophosphite
Ca3P2+6H2O→2PH3+3Ca(OH)2
Ca3P2+6HCI→2PH3+3CaCI2
The phosphine obtained is purified by passing it through hydrogen iodide, whereby phosphonium iodide is formed. This treatment with alkali gives pure phosphine.
PH3+HI→PH4I
PH4I+NaOH→PH3+NaI+H2O
Phosphine catches fire spontaneously because it contains traces of diphosphine (P2H6), which is inflammable.
Properties
Phosphine is a colourless, toxic gas having an unpleasant odour similar to that of rotten fish. It is sparingly soluble in water and the resultant solution undergoes photodecomposition to give red phosphorus and hydrogen.
Pure phosphine is stable in the air but catches fire above 423 K.
⇒ \(\mathrm{PH}_3+2 \mathrm{O}_2 \stackrel{423 \mathrm{~K}}{\longrightarrow} \mathrm{H}_3 \mathrm{PO}_4\)
It is feebly basic and forms phosphonium salts with acids.
PH3+HX→PH4X
Itbums in chlorine to give phosphorus trichloride and phosphorus pentachloride
PH3+3CI2→PCI3+3HCI
PH3+4CI2→PCI5+3HCI
When phosphine is bubbled through aqueous solutions of copper and mercury(2) salts, the corresponding phosphides are precipitated.
3CuSO4+2PH3(g)→Cu3P2(s)+3H2SO4(aq)
3HgCI2(aq)+2PH3(g)p2(s)+6HCI(aq)
Uses
The spontaneous combustion of phosphine is used in Holme’s signals in deep seas and oceans for signalling danger to ships. Containers containing calcium phosphide and calcium carbide are pierced and thrown into the sea.
Group 14 elements – carbon family in p-block elements
In the presence of water, calcium phosphide hydrolyses to give phosphine which contains traces of inflammable P2H4. As stated earlier, diphosphine catches fire spontaneously. This ignites the ethyne produced by the hydrolysis of calcium carbide, and a luminous flame is obtained. Phosphine is also used in smoke screens.
Phosphorus Halides
All the possible phosphorus trihalides, PX4 (X=F, Cl, Br, I) and phosphorus pentahalides, PX5(X=F, Cl, Br) are known.
Phosphorus trichloride
This is commercially the most important trihalide of phosphorus.
It may be prepared by passing dry chlorine over gently heated white phosphorus.
P4+6CI2→4PCI3
It may also be obtained by treating white phosphorus with thionyl chloride.
P4+8SOCI2→4PCI3+4SO2+2SO2CI2
Phosphorus trichloride is a colourless, pungent-smelling liquid with a boiling point of 349 K. In moist air, i1 undergoes hydrolysis to give fumes of hydrochloric acid.
PCI3+3H2O→H3PO3+3HCI
Phosphorus trichloride reacts with oxygen to form phosphorus oxy-chloride and with chlorine, it forms phosphorus pentachloride
2PCI3+O2→2POCI3
PCI3+CI2→PCI5
PCI3 reacts with organic compounds containing —OH group (carboxylic acids, alcohols) as follows.
3RCOOH+PCI3→3RCOCI+H3PO3
3ROH+PCI3→3RCI+H3PO3
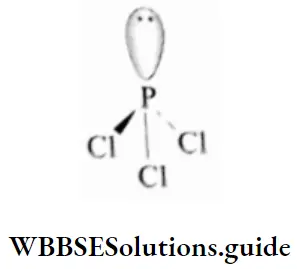
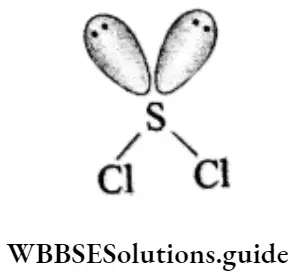
The shape of the PCI3 molecule is pyramidal and the phosphorus atom in the molecule is a sp3 hybridised
Phosphorus pentachloride
This is the most well-characterised pentahalide of phosphorus.
It is prepared by the reaction of chlorine with phosphorus trichloride.
\(\mathrm{PCl}_3+\mathrm{Cl}_2 \stackrel{\mathrm{CCl}_4}{\longrightarrow} \mathrm{PCl}_5\)It can also be obtained by treating white phosphorus with an excess of dry chlorine or thionyl chloride.
P4+10CI2→4PCI5
P4+10SO2CI2→4PCI5+10SO2
PCI5 is a yellowish-white powder.
It sublimes on heating and decomposes at a higher temperature to give the trichloride and chlorine.
\(\mathrm{PCl}_5 \stackrel{\Delta}{\longrightarrow} \mathrm{PCl}_3+\mathrm{Cl}_2\)PCI5 is susceptible to hydrolysis, which is a two-step reaction.
⇒ \(\mathrm{PCl}_5+\mathrm{H}_2 \mathrm{O} \longrightarrow\underset{\begin{array}{c}\text { Phosphorus } \\\text {oxychloride }\end{array}}{\mathrm{POCl}_3}+2 \mathrm{HCl}\)
PCI5 is an excellent chlorinating agent and converts alcohols and carboxylic acids to their respective chloi derivatives.
R.OH+PCI5→RCI+POCI3+HCI
R.COOH+PCI5→RCOCI+POCI3HCI
It converts many metals to the corresponding chlorides.
⇒ \(\mathrm{Sn}+2 \mathrm{PCl}_5 \stackrel{\Delta}{\longrightarrow} \mathrm{SnCl}_4+2 \mathrm{PCl}_3\)
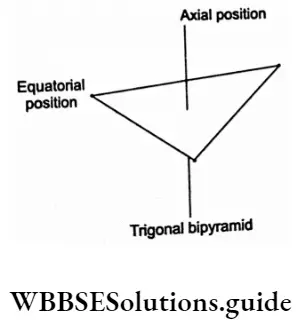
In the gaseous and liquid states, PCI5 has a trigonal bipyramidal structure. The two axial P-Cl bonds are longer (240 pm) than the three equatorial bonds (202 pm). This is because bond-pair-bond-pair repulsions are stronger in the axial atoms than in the equatorial atom.
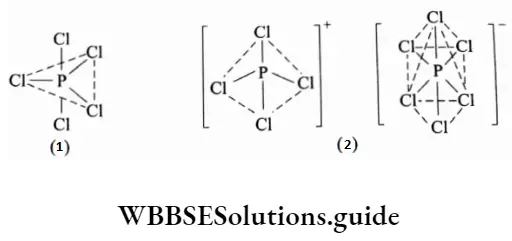
PCI5 dimerises in the solid state and exists as an ionic solid, containing the tetrahedral [PCI4]+ cation and the octahedral [PCI6] anion.
Oxides Of Phosphorus
Phosphorus forms two oxides—the trioxide (P4O6) and the pentoxide (P4O10). The former is prepared by burning white phosphorus in a limited supply of air, while for the other an excess of air is needed.
\(\mathrm{P}_4+3 \mathrm{O}_2 \stackrel{\Delta}{\longrightarrow} \mathrm{P}_4 \mathrm{O}_6\)Both the oxides are soluble in water, forming acidic solutions. This is expected as they are oxides o a nonmetal.
P4O6+6H2O→4H3PO3
P4O10+6H2O→4H3PO4
Due to the inability of phosphorus to form pπ-pπ double bonds with oxygen, its oxides are dimeric (P4O6 and P4O10). This is in sharp contrast to the oxides of nitrogen which are monomeric (N2O3 and N2O5)
Oxoacids Of Phosphorus
Phosphorus forms a large number of oxoacids. Before we discuss the properties and structure of each oxoaci note the following points in this context.
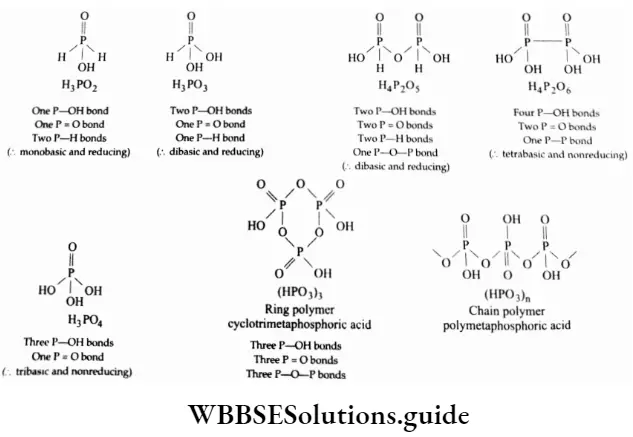
- In a molecule of phosphorus acid, the phosphorus atom is sp3 hybridised and tetrahedrally surrounded by other atoms.
- The ionisable acidic hydrogens in the molecules are attached to oxygen atoms, i.e., every acid contains at least one P-OH bond. These oxygens, which are attached to hydrogen, are called hydroxylic oxygens. All oxoacids also contain nonhydroxylic oxygens where the oxygen atom is linked only to phosphor forming the P = O bond.
- In addition to P-OH and P = O bonds, the acids may contain either P-H or P-P bonds.
- The presence of P-H bonds in the oxoacids confers reducing and not acidic properties.
The names, formulae, methods of preparation and some salient features of the oxoacids of phosphorus are shown.
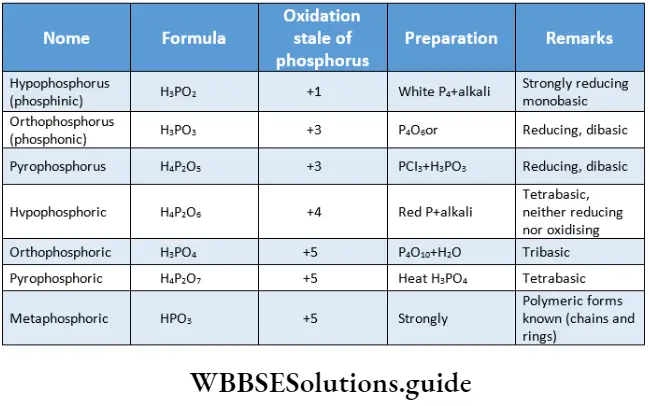
It is seen that oxoacids containing phosphorus in their lower oxidation state are reducing. For example, hypophosphorus acid (H3P02) reduces silver salts to the metal.
⇒ \(4 \mathrm{AgNO}_3+2 \mathrm{H}_2 \mathrm{O}+\mathrm{H}_3 \mathrm{PO}_2 \longrightarrow 4 \mathrm{Ag}+4 \mathrm{HNO}_3+\mathrm{H}_3 \mathrm{PO}_4\)
The oxoacids in the +5 oxidation state do not have reducing or oxidising properties. Disproportionation is common for oxoacids containing phosphorus in the +3 oxidation state.
⇒ \(4 \mathrm{H}_3 \stackrel{+3}{\mathrm{PO}_3} \longrightarrow 3 \mathrm{H}_3 \stackrel{+5}{\mathrm{PO}_4}+\stackrel{-3}{\mathrm{P}} \mathrm{H}_3\)
The molecular structures of the oxoacids of phosphorus along with the types of bonds present in a molecule of the various oxoacids are shown in.
Group 16 Elements
Group 16 of the periodic table comprises oxygen, sulphur, selenium, tellurium and polonium. The elements of the group are called chalcogens or ore-forming elements as most metals occur as their oxides or sulphides. Nonmetallic character is maximum in oxygen and sulphur, weaker in selenium and tellurium whereas the short-lived and radioactive polonium is predominantly metallic.
Occurrence And Uses
The amount of oxygen present in dry air is about 20.946% by volume. Oxygen is the most abundant element in the earth’s crust. Most of the combined oxygen is in the form of silicates, oxides and water.
In contrast, the abundance of sulphur in the earth’s crust is only 0.03-0.1%. It is the 16th most abundant element and occurs mostly in the form of sulphide (zinc blende—ZnS, galena—PbS, cinnabar—HgS, and copper pyrites—CuFeS2) and sulphate (gypsum—CaSO4 2H2O, Epsom salt—MgSO4 -7H20, baryte—BaSO4) ores. Elemental sulphur is also present as hydrogen sulphide in natural gas, crude oil and volcanic ash.
Sulphur is a constituent of proteins and enzymes and some amino acids, for example, cysteine. The other elements of Group 16 show comparatively low abundance. Selenium and tellurium occur among sulphide ores.
The main source of selenium and tellurium is the anode mud obtained during the electrolytic refining of copper. Thorium and uranium minerals contain polonium as a natural decay product.
Oxygen is essential for life—most life processes are based on oxidative metabolism. It is used in various energy generation processes through the combustion of wood and fossil fuels. Rocket fuels have liquid oxygen as the oxidant.
Oxyacetylene flames have very high temperatures and are used in welding. Many chemical industries use oxygen as an oxidant. Mountaineers use oxygen cylinders at high altitudes. Ozone, an allotrope of oxygen, is used as a disinfectant and for water sterilisation. It is also a bleaching agent.
Sulphur is predominantly used for the manufacture of sulphuric acid, which in turn is used in making fertilisers and other chemicals. Elemental sulphur is used in the vulcanisation of rubber and as a disinfectant.
Selenium is used to decolourise glass and as a photoconductor in photocopying machines. Tellurium is used as an additive to steel to increase its ductility. Tellurium and polonium are toxic.
Atomic And Physical Properties
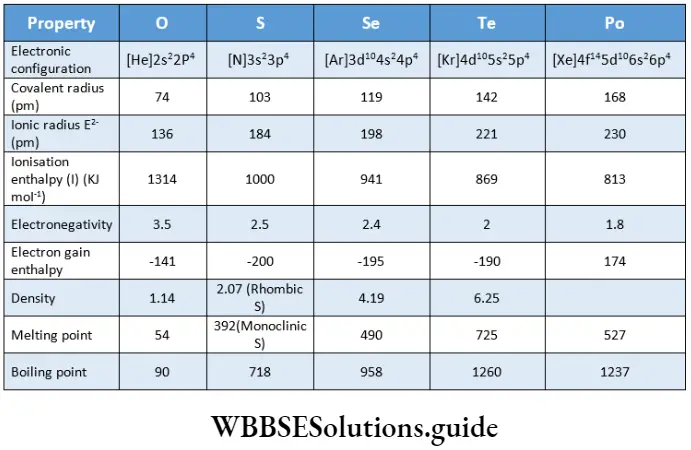
Electronic configuration
The valence-shell electronic configuration of these elements isns2np4. They attain a noble-gas configuration by gaining two electrons, thus forming an E2” anion (E = O, S, Se, Te) or by sharing two electrons and thus forming two covalent bonds.
Size
The atomic size of Group 16 elements increases down the group as extra shells of electrons are added. The small atomic radius of oxygen and the absence of d orbitals in its atom are responsible for the distinctive chemical properties and also the high electronegativity of the element.
Ionisation enthalpy
The ionisation enthalpy of the elements decreases down the group as the size of the atom increases. The ionisation enthalpies of the Group 16 elements are strikingly less than those of the corresponding Group 15 elements. The unexpectedly high first ionisation enthalpies of Group 15 elements is due to the extra stability associated with half-filled p orbitals, which is not there in Group 16 elements.
Electronegativity
Oxygen is the second most electronegative element, fluorine being the most. Within the group, the electronegativity decreases on moving down. This indicates that the metallic character increases down the group.
Electron gain enthalpy
The electron gain enthalpy of oxygen is less negative than that of sulphur due to the small atomic size of oxygen. The electron gain enthalpy becomes less negative from sulphur to polonium.
Boiling and melting points
Due to small size, high electronegativity and absence of d orbitals, in an oxygen molecule, pπ-pπ bonds are formed between two oxygen atoms (0=0). Thus oxygen is stable and exists as a diatomic molecule in the gaseous state.
Oxygen too has a triatomic allotrope—ozone. The other elements of the group do not form multiple bonds and exist as polyatomic solids. In fact, sulphur is octa-atomic (S8). The large difference between the boiling points of oxygen and sulphur and that between their melting points can be explained on the basis of their atomicity (oxygen exists as O2 and sulphur as S8). All elements of the group exhibit allotropy.
Chemical Properties
Oxidation states and trends in chemical reactivity
As you already know, the outermost electronic configuration of the elements of Group 16 is ns2np4 short of the nearest noble-gas configuration by two electrons. The elements can achieve the nearest noble-gas configuration by gaining or sharing two electrons.
Thus, the E2- ion of Group 16 elements exists with highly electropositive elements. Group 16 elements exhibit variable oxidation states due to the presence of empty d orbitals. Oxygen (which has no d orbitals) generally exhibits a-2 oxidation state.
In peroxides, however, it exhibits a -1 oxidation state (O-22)• The stability of the -2 oxidation state decreases down the group. Oxygen usually displays a negative oxidation state, except in some binary compounds with fluorine like OF2 and O2F2 (Fluorine is more electronegative than oxygen.)
Sulphur, selenium and tellurium show a tendency for covalency with formal oxidation states of +2, +4 and +6 in compounds where they are combined with more electronegative elements like oxygen or halogens.
The reactivity of elements generally decreases down the group. Sulphur combines with all elements except noble gases, nitrogen, tellurium, iodine, platinum and gold.
Bond type
Oxygen readily forms the divalent anion, O2-. The tendency for the formation of the divalent anion decreases from sulphur onwards due to an increase in the size of the atom and a decrease in electronegativity. Compounds in a positive oxidation state are generally covalent; the covalent character decreases down the group.
Tendency to form multiple bonds and catenation
The tendency to form multiple bonds decreases down the group. Thus oxygen exists as Oz held by 0=0, while the other elements are polyatomic. The bond energy of the oxygen-oxygen double bond, 0=0, is 498 kJ mol-1.
This makes the 0=0 bond more than three times as strong as the 0-0 bond (bond energy for 0-0 is 142 kJ mol-1). In comparison, the S = S bond is less than twice as strong as the S-S single bond (bond energy for S = S is 434 kJ mol -1, and that for S-S is 264 kJ mol-1 ). The tendency of catenation in sulphur is much higher; this is evident from the large number of allotropes of sulphur.
Anomalous behaviour of oxygen Oxygen differs considerably from the rest of the members of the group. The factors responsible for this anomalous behaviour of oxygen are small size, high electronegativity, nonavailability of d orbitals and the tendency to form pn-multiple bonds.
Some specific differences between the properties of oxygen and the other members of the group are as follows.
- Oxygen is a gas while the other members are solids.
- Oxygen is diatomic while the other members are polyatomic.
- Being highly electronegative, oxygen shows only negative oxidation states of -2 and -1 (except with fluorine) while other elements of the group show positive oxidation states.
- Oxygen tends to form hydrogen bonds.
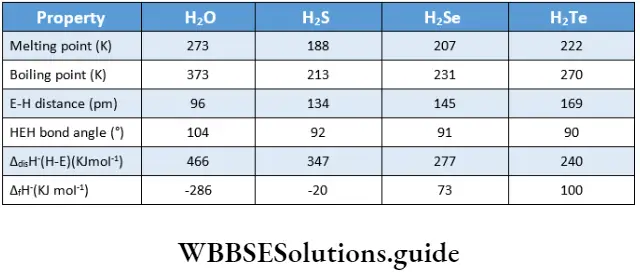
Reactivity towards hydrogen
Binary hydrides of the general formula H2E are known as Group 16 elements. Some of the physical properties of their hydrides are summarised. The thermal stability of the hydrides decreases down the group. As the size of the central atom increases, the strength of the covalent H-E bond decreases. Thus both bond enthalpy and thermal stability decrease.
A consequence of decreasing bond enthalpy is the increase in acidic character. In other words, bond cleavage becomes easier and dissociation of H2E to H+and HE becomes easier. The stability of hydrides decreases on descending the group and H2Te in fact is thermodynamically unstable.
Apart from water, the hydrides are foul-smelling, toxic gases. The high boiling point of water is due to the presence of intermolecular hydrogen bonding. Hydrogen sulphide and the lower hydrides are reducing agents and the reducing power increases down the group. The hydrides are with two lone pairs. angular in shape. The central atom is sp3 hybridised
Reactivity towards oxygen
Group 16 elements mainly form dioxides (EO2) and trioxides (EO3). The dioxides are known for all elements, whereas the trioxides are known for sulphur, selenium and tellurium. The oxides are acidic in nature. The reducing power of the dioxides decreases down the group.
Reactivity towards halogens
The elements of Group 16 form a large number of binary compounds with halogens. The three main types of halides are EX2, EX4 and EX6, but other halides are also known. The halogen compounds of sulphur, selenium, tellurium and polonium are called halides.
Oxygen too combines with halogens but its compound with fluorine only is said to be a halide. The compounds of oxygen with other halogens are oxides and not halides. This is so because of the high electronegativity of oxygen, which is exceeded only by fluorine and not chlorine, bromine and iodine.
The highest oxidation states of the Group 16 elements are realised only in combination with the most electronegative fluorine. Also, for a given oxidation state of Group 16 elements, fluoride is the most stable. Among the hexafluorides, SF6 is extremely stable. Its inertness is due to the sterically hindered sulphur in an octahedral structure.
SF6 is unaffected by water as the protected sulphur atom does not allow hydrolysis, which is a thermodynamically favoured reaction. On the other hand, SeF6 and TeF6 are slightly more reactive and TeF6 is hydrolysed, probably due to the large size of the central atom.
The tetrafluorides of sulphur, selenium and tellurium are stable and exist as a gas (SF4), liquid (SeF4) and solid (TeF4) respectively. The central atom in these compounds has sp3 hybridization and thus the molecular structure is trigonal bipyramidal with one lone pair in the equatorial position. This kind of geometry is called see-saw geometry.


Dimeric monohalides of the type S2X2 (X = F, Cl, Br) and Se2X2(X = Cl, Br) are known. However, they are unstable and tend to be disproportionate as follows.
2SeCI2→SeCI4+3Se
The hydrolysis is also accompanied by disproportionation.
2Se2CI2+2H2O→H2Se+3Se+4HCI
Oxygen
Oxygen occurs as dioxygen (O2) and ozone (O3). Dioxygen (O2) exists as a gas and makes up 21 per cent of air in the atmosphere. It makes up 89 per cent by weight of the water in the oceans. Oxygen also occurs as sulphates, carbonates, nitrates, borates, etc. The three stable isotopes of oxygen are 16O,17O and 18O. We will now discuss the preparation and properties of dioxygen.
Preparation
1. The most convenient method of preparation of oxygen in the laboratory is by the thermal decomposition of potassium chlorate in the presence of manganese dioxide as a catalyst.
⇒ \(2 \mathrm{KClO}_3 \underset{420 \mathrm{~K}}{\stackrel{\mathrm{MnO}_2}{\longrightarrow}} 2 \mathrm{KCl}+3 \mathrm{O}_2\)
In the absence of manganese dioxide, the reaction occurs slowly at 670-720 K. Apart from potassium chlorate, potassium nitrate, potassium permanganate and barium peroxide also give dioxygen on heating.
⇒ \(2 \mathrm{KNO}_3 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{KNO}_2+\mathrm{O}_2\)
⇒ \(2 \mathrm{KMnO}_4 \stackrel{\text { heat }}{\longrightarrow} \mathrm{K}_2 \mathrm{MnO}_4+\mathrm{MnO}_2+\mathrm{O}_2\)
⇒ \(2 \mathrm{BaO}_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{BaO}+\mathrm{O}_2\)
2. Oxygen can also be prepared by the thermal decomposition of oxides of less reactive metals (metals placed low in the electrochemical series) like mercury and silver and also from oxides of some metals in their higher oxidation states.
⇒ \(2 \mathrm{HgO} \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{Hg}+\mathrm{O}_2\)
⇒ \(2 \mathrm{Ag}_2 \mathrm{O} \stackrel{\text { heat }}{\longrightarrow} 4 \mathrm{Ag}+\mathrm{O}_2\)
⇒ \(2 \mathrm{PbO}_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{PbO}+\mathrm{O}_2\)
⇒ \(2 \mathrm{~Pb}_3 \mathrm{O}_4 \stackrel{\text { heat }}{\longrightarrow} 6 \mathrm{PbO}+\mathrm{O}_2\)
⇒ \(3 \mathrm{MnO}_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{Mn}_3 \mathrm{O}_4+\mathrm{O}_2\)
3. Another convenient method of preparation of oxygen is by the action of water on sodium peroxide or by the decomposition of hydrogen peroxide in the presence of manganese dioxide.
2Na2O2+2H2O→4NaOH+O2
2H2O2→2H2O+O2
4. Commercially, oxygen is obtained by the liquefaction of air. Initially, carbon dioxide and water vapour are removed from the air, and then the air is liquefied and subjected to fractional distillation. Nitrogen with a lower boiling point (77 K) distils out first leaving oxygen behind.
5. Large amounts of oxygen are obtained by the electrolysis of water.
⇒ \(2 \mathrm{H}_2 \mathrm{O} \stackrel{\text { electrolysis }}{\longrightarrow} 2 \mathrm{H}_2+\mathrm{O}_2\)
Hydrogen is liberated at the cathode and oxygen at the anode.
Properties
Dioxygen is a colourless, odourless and tasteless gas. It has a freezing point of 65 K and a boiling point of 90 K. It is slightly soluble in water (30.8 g per dm3 of water at 298 K and atmospheric pressure). This small amount of dissolved oxygen in water sustains aquatic life.
Oxygen has an even number of electrons yet it is paramagnetic. You have learnt about this in your previous class under molecular orbital theory.
Oxygen is a supporter of combustion. It reacts with most metals (except some less reactive metals like gold platinum, and noble gases) and nonmetals to form the respective oxides.
2Ca+O2→2CaO
2Mg+O2→2MgO
4AI+3O2→2AI2O3
4Fe+3O2→2Fe2O3
2H2+O2→2H2O
S+O2→SO2
C+O2→CO2
P4+5O2→P4O10
The bond dissociation enthalpy of the O = O bond in an oxygen molecule is high and to initiate the reactions, initial heating is needed. However, these reactions are exothermic in nature and the heat liberated can sustain the reactions.
Oxygen reacts with a variety of compounds as shown.
⇒ \(2 \mathrm{SO}_2+\mathrm{O}_2 \underset{723 \mathrm{~K}, 2 \mathrm{~atm}}{\stackrel{\mathrm{V}_2 \mathrm{O}_5}{\longrightarrow}} 2 \mathrm{SO}_3\)
This reaction is the basis of the contact process for the manufacture of sulphuric acid.
⇒ \(4 \mathrm{NH}_3+5 \mathrm{O}_2 \underset{500 \mathrm{~K}}{\stackrel{\mathrm{Pt}}{\longrightarrow}} 4 \mathrm{NO}+6 \mathrm{H}_2 \mathrm{O}\)
This reaction is involved in the Ostwald process for the manufacture of nitric acid.
Metal sulphides react with oxygen to form oxides. These oxides may then be reduced to give the metal.
⇒ \(2 \mathrm{ZnS}+3 \mathrm{O}_2 \longrightarrow 2 \mathrm{ZnO}+2 \mathrm{SO}_2\)
Both saturated and unsaturated hydrocarbons cause an excess of oxygen to give carbon dioxide and water.
CH4 + 2O2→CO2 + 2H2O
H2C= CH2 + 3O2→2CO2 + 2H2O
2HC = CH+ 5O2→4CO2 + 2H2O
As these reactions are highly exothermic, hydrocarbons are used as fuels.
Oxides
Oxygen reacts with almost all the elements to form binary compounds called oxides. An element may form more than one oxide. For example, nitrogen forms six oxides.
Metal oxides can be simple (for example CaO, ZnO, Fe2O3) where the metal displays one oxidation state or they can be mixed (for example FeO4, Pb3O4). A mixed oxide is made up of two oxides, in which the metal shows two oxidation states.
Thus Pb3O4 may be considered to be a mixture of PbO2 and PbO and may also be formulated as PbO2 2PbO. Depending on how an oxide behaves chemically, the oxides can be classified as
- Acidic,
- Basic,
- Amphoteric And
- Neutral.
The oxide that combines with water to give an acidic solution is referred to as acidic. Generally, oxides of nonmetals are acidic. For example,
CO2+H2O→H2CO3
P4O10+6H2O→4H3PO4
SO2+H2O→H2SO3
CI2O7+H2O→2HCIO4
Acidic oxides react with bases to form salt and water oxides of metals generally basic as they dissolve in water to give a basic solution. for example,
MgO+2H2O→Mg(OH)2
CaO+2H2O→Ca(OH)2
Na2O+H2O→2NaOH
Basic oxides react with acids to form salt and water. Amphoteric oxides are some metallic oxides which show both acidic and basic properties. They react with acids as well as bases to form salts. For example,
ZnO+2HCI→ZnCI2+H2O
ZnO+2NaOH+ H2O→Na[Zn(OH)4]
When a metal forms oxides exhibiting different oxidation states, then the oxides which have metals in their higher oxidation states display acidic properties; for example, V2O5 and Mn2O7 are acidic in nature.
Some oxides like CO, N2O and NO display neither acidic nor basic properties and are called neutral oxides.
Group 15 elements – nitrogen family and their chemical behavior
Ozone
As already stated elemental oxygen exists in two allotropic modifications, dioxygen (O2) and trioxygen or ozone (O3).
Ozone is present in the upper atmosphere at a height of about 20 km from the earth’s surface. It is formed by the action of ultraviolet radiation on oxygen.
⇒ \(3 \mathrm{O}_2 \stackrel{\text { UV light }}{\longrightarrow} 2 \mathrm{O}_3\)
The ozone layer in the atmosphere protects us from the harmful effects of ultraviolet rays.
It has been shown that oxides of nitrogen, particularly nitric oxide, combine rapidly with ozone.
NO+O3→NO2+O2
Nitric oxide emitted by supersonic aircraft is responsible for the slow depletion of the ozone layer. Another threat to the ozone layer is chlorofluorocarbons (CFCs) or freons which are used as refrigerants and as aerosol propellants.
These molecules diffuse into the stratosphere and undergo slow photochemical degradation to produce atomic chlorine which reacts with ozone. Ozone being thermodynamically unstable liberates nascent oxygen, which combines with the CIO* free radical.
⇒ \(\mathrm{CFCl}_3 \stackrel{\text { UV rays }}{\longrightarrow} \dot{\mathrm{C}} \mathrm{FCl}_2+\mathrm{Cl}^{\circ}\)
⇒ \(\mathrm{Cl}^*+\mathrm{O}_3 \longrightarrow \mathrm{ClO}^*+\mathrm{O}_2\)
\(\mathrm{ClO}^*+\mathrm{O} \longrightarrow \mathrm{Cl}^*+\mathrm{O}_2\)Preparation
Ozone can be obtained by the action of a silent electric discharge through pure and dry oxygen.
⇒ \(3 \mathrm{O}_2 \longrightarrow 2 \mathrm{O}_3 ; \Delta H^{\ominus}(298 \mathrm{~K})=+142.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
This is an endothermic reaction. It is essential to use a silent electric discharge as it generates less heat; if the temperature of the reaction is allowed to rise continuously then the ozone formed may decompose back to oxygen. This reaction produces only about 10% ozone. The product is actually? a mixture of dioxygen and is called ozonised oxygen.
Properties
Ozone is a pale blue gas which condenses to give a deep blue liquid and a violet-black solid. It has a strong characteristic smell and is heavier than air. Ozone is slightly soluble in water but readily soluble in organic solvents.
Ozone in low concentrations is not so toxic, but it becomes harmful in concentrations above 100 ppm. Then it may cause respiratory problems, headaches and nausea. Ozone is diamagnetic. It is not very stable and decomposes slowly to give dioxygen.
2O3→3O2
This reaction is exothermic and therefore the enthalpy of the reaction is negative. The reaction is also associated with an increase in entropy (positive ΔS). Thus the reaction is thermodynamically favoured and the Gibbs energy change (ΔG) has a large negative value. Thus high concentration of ozone can lead to an explosion.
Ozone is a very powerful oxidising agent next only to fluorine in oxidising power. It releases atomic oxygen in the reaction which brings about oxidation.
O3→O2+O
2NO2+O3→N2O5+O2
S+HO2+O3→ H2SO4
Ozone oxidises metal sulphides to their respective sulphates.
⇒ \(\mathrm{PbS}+4 \mathrm{O}_3 \longrightarrow \mathrm{PbSO}_4+4 \mathrm{O}_2\)
Thus when ozone is passed through a suspension of lead sulphide (black), the colour changes from black to white, owing to the formation of the white lead sulphate.
Ozone oxidises halogen acids to halogens, potassium iodide to iodine and acidified ferrous salts to ferric salts.
⇒ \(2 \mathrm{HCl}+\mathrm{O}_3 \longrightarrow \mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{O}_2\)
⇒ \(2 \mathrm{KI}+\mathrm{H}_2 \mathrm{O}+\mathrm{O}_3 \longrightarrow \mathrm{I}_2+2 \mathrm{KOH}+\mathrm{O}_2\)
⇒ \(2 \mathrm{Fe}^{2+}+2 \mathrm{H}^{+}+\mathrm{O}_3 \longrightarrow 2 \mathrm{Fe}^{3+}+\mathrm{H}_2 \mathrm{O}+\mathrm{O}_2\)
The reaction of ozone with potassium iodide is used for quantitative estimation of ozone as the iodine liberated can be estimated by titrating with sodium thiosulphate, using starch as the indicator.
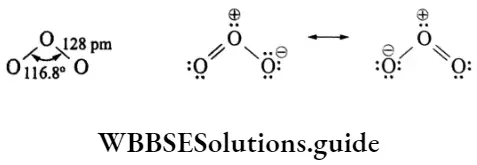
Ozone has an angular structure. The bond length in ozone is 128 pm which is intermediate between the 0-0 single bond length of 148 pm and the 0 = 0 double bond length of 110 pm. The structure is a resonance hybrid of the two resonating forms, as shown in.
Uses
Ozone is used as a disinfectant and for sterilising water. It is used for bleaching delicate fabrics, oils, ivory, starch, etc. It is used in the industry for the manufacture of potassium permanganate, artificial silk, etc. It finds use in organic chemistry, as an oxidising agent and for carrying out ozonolysis.
Allotropic Modifications Of Sulphur
Sulphur has a strong tendency towards catenation and this is manifested in a large number of allotropes of sulphur. The main allotropes of sulphur are rhombic (α-sulphur) and monoclinic ((β-sulphur).
The rhombic form is stable at room temperature whereas monoclinic sulphur is stable over 369 K. Rhombic sulphur is a bright yellow solid, readily soluble in carbon disulphide. It is soluble in ether, alcohol and benzene too but to a lesser extent.
It gets converted to the monoclinic form on slow heating above 369 K. It has a melting point of 385.8 K and a specific gravity of 2.06. It is obtained when a solution of sulphur in carbon disulphide is crystallised.
Monoclinic sulphur is prepared by melting rhombic sulphur in a dish and letting it cool till a superficial crust is formed. Two holes are pierced into the crust and the liquid sulphur lying below the crust (which has not yet solidified) is poured out through one of the holes.
Small needle-like crystals of monoclinic sulphur become visible. Monoclinic sulphur melts at 393 K, has a specific gravity of 1.98 and is soluble in carbon disulphide. Bel this temperature rhombic sulphur is stable and at this temperature, both forms are in equilibrium. This temperature is called the transition temperature.
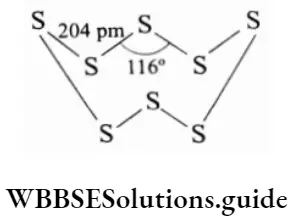
Both rhombic and monoclinic sulphur consist of S8 rings, the sulphur atoms joined together in the shape of a crown. The packing pattern varies leading to different symmetry in crystals. Several other ring sizes from S6 to S20 have been synthesised in the recent past. A form is also known where the S6 rings are arranged in the chair conformation.
Plastic sulphur is an amorphous form of sulphur obtained when molten sulphur is poured into cold water. In addition to this several chain polymers also exist in the liquid and vapour states. Above 1000 K the main species is S2, which like O2 is paramagnetic.
Sulphur Dioxide
When sulphur is burnt in the air, sulphur dioxide is the main product obtained (along with 6-8% sulphur trioxide)
S+O2→SO2
In the laboratory sulphur dioxide is prepared by heating copper turnings with concentrated sulphuric acid.
⇒ \(\mathrm{Cu}+2 \mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{CuSO}_4+\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O}\)
It is also obtained when a sulphite is treated with dilute sulphuric acid.
⇒ \(\mathrm{Na}_2 \mathrm{SO}_3+2 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2\)
Commercially, sulphur dioxide is obtained as a by-product in the roasting of sulphide ores.
⇒ \(2 \mathrm{PbS}+3 \mathrm{O}_2 \longrightarrow 2 \mathrm{PbO}+2 \mathrm{SO}_2\)
⇒ \(4 \mathrm{FeS}_2+11 \mathrm{O}_2 \longrightarrow 2 \mathrm{Fe}_2 \mathrm{O}_3+8 \mathrm{SO}_2\)
Properties
Sulphur dioxide gas has a sharp, choking odour. It can be readily liquefied at room temperature at a pressure of two atmospheres. It is an acidic oxide and dissolves in water to give sulphurous acid.
⇒ \(\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_2 \mathrm{SO}_3\)
When sulphur dioxide reacts with sodium hydroxide, it forms two salts—sodium hydrogen sulphite (i\ahlb(J3) and sodium sulphite (Na2S03). With an excess of sodium hydroxide, sodium sulphite is formed; this reacts with more sulphur dioxide to form sodium hydrogen sulphite.
⇒ \(2 \mathrm{NaOH}+\mathrm{SO}_2 \longrightarrow \mathrm{Na}_2 \mathrm{SO}_3+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{Na}_2 \mathrm{SO}_3+\mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{NaHSO}_3\)
Sulphur dioxide acts as an oxidising agent as well as a reducing agent. However, its role as a reducing agent is more pronounced. It reduces Cr2O2-7 to Cr3+
⇒ \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+2 \mathrm{H}^{+}+3 \mathrm{SO}_2 \longrightarrow 2 \mathrm{Cr}^{3+}+3 \mathrm{SO}_4^{2-}+\mathrm{H}_2 \mathrm{O}\)
It decolorises potassium permanganate solution and reduces ferric salts to ferrous salts.
⇒ \(2 \mathrm{MnO}_4^{-}+5 \mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{Mn}^{2+}+5 \mathrm{SO}_4^{2-}+4 \mathrm{H}^{+}\)
⇒ \(2 \mathrm{Fe}^{3+}+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{Fe}^{2+}+\mathrm{SO}_4^{2-}+4 \mathrm{H}^{+}\)
Sulphur dioxide oxidises hydrogen sulphide to sulphur.
⇒ \(2 \mathrm{H}_2 \mathrm{~S}+2 \mathrm{SO}_2 \longrightarrow 2 \mathrm{H}_2 \mathrm{O}+3 \mathrm{~S}\)
It combines with chlorine in the presence of charcoal as a catalyst to form sulphuryl chloride.
⇒ \(\mathrm{SO}_2+\mathrm{Cl}_2 \stackrel{\mathrm{C}}{\longrightarrow} \mathrm{SO}_2 \mathrm{Cl}_2\)
Sulphur dioxide reacts with oxygen in the presence of vanadium pentoxide as a catalyst to form sulphur trioxide. The reaction, as you know, forms the basis of the contact process for the manufacture of sulphuric acid.
⇒ \(2 \mathrm{SO}_2+\mathrm{O}_2 \stackrel{\mathrm{v}_2 \mathrm{O}_5}{\longrightarrow} 2 \mathrm{SO}_3\)
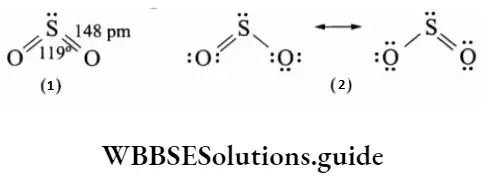
Sulphur dioxide has an angular structure.
Uses
The main use of sulphur dioxide is in the manufacture of sulphuric acid. It is also used as a bleaching agent for wool and silk, as a disinfectant and in petroleum and sugar refining. Liquid sulphur dioxide is used as a nonaqueous solvent.
Oxoacids Of Sulphur
Sulphur forms a significant number of oxoacids. All are not known in the free state but exist in solution and the form of salts. Some of the important oxoacids of sulphur and their structures are listed.
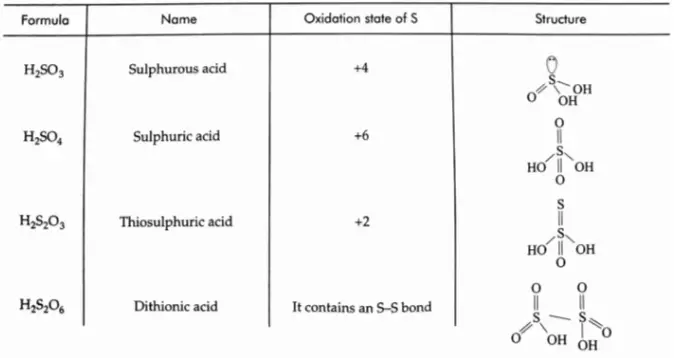
While calculating oxidation states, remember that the oxidation state of oxygen normally is -2; however, it is -1 in peroxides.
Sulphuric Acid
It is the most important industrial chemical produced worldwide. It is manufactured by the contact process. shows a flow diagram for the process.
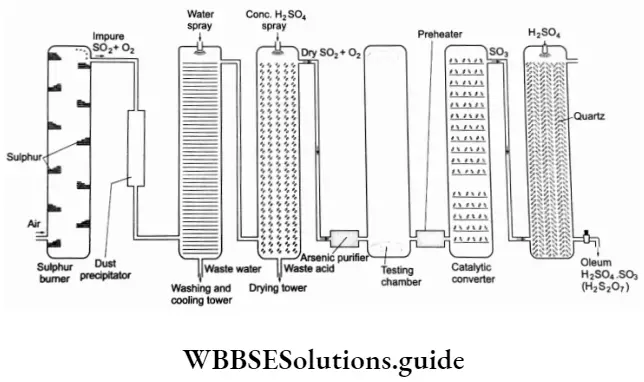
The process involves the following three steps.
Production of SO2 It is obtained by burning sulphur or by roasting iron pyrites.
S+O2→SO2
⇒ \(4 \mathrm{FeS}_2+11 \mathrm{O}_2 \longrightarrow 2 \mathrm{Fe}_2 \mathrm{O}_3+8 \mathrm{SO}_2\)
Oxidation of SO2 to SO3 The SO2 produced contains oxygen with it. The gaseous mixture is purified by passing it first through a dust precipitator and then a washing and cooling chamber. The mixture is then introduced to a drying chamber where concentrated sulphuric acid is sprayed to remove moisture.
Further, the gases are passed through an arsenic purifier containing gelatinous ferric hydroxide, which absorbs arsenic and its compounds present as impurities in the mixture. Now the gases are passed through a testing chamber where they are exposed to a strong beam of light to check whether any impurities are present. Impurities, if present in the mixture, scatter light.
If the gases are found impure, then the initial process is repeated till all the impurities are removed. Now the gaseous mixture is heated to about 720-820 K in a preheater and passed through a catalytic converter where the following reaction occurs.
⇒ \(2 \mathrm{SO}_2+\mathrm{O}_2 \longrightarrow 2 \mathrm{SO}_3 \quad \Delta_r H^{\ominus}=-196.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
The catalyst used is V2O5 at the optimum condition of a temperature of 720 K and pressure of 2 bar. The reaction is exothermic and accompanied by a decrease in volume; thus the forward reaction can proceed if the temperature is low and pressure is high. However, if the temperature is very low, then the reaction becomes too slow.
Absorption of S03 into H2SO4 to give oleum The sulphur trioxide is absorbed in concentrated sulphuric acid to give oleum (H2S2O7).
⇒ \(\mathrm{SO}_3+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7\)
The oleum is diluted with water to give sulphuric acid. The sulphuric acid thus obtained is generally 96-98% pure.
⇒ \(\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{H}_2 \mathrm{SO}_4\)
Sulphur trioxide cannot be directly dissolved in water because the reaction is highly exothermic and the water evaporates from the mixture due to the heat produced.
Properties
Sulphuric acid is a colourless, viscous liquid with a specific gravity of 1.84 at 298 K. It freezes at 283 K and boils at 590 K. It has a strong affinity for water and its dissolution in water is highly exothermic.
Therefore if water is poured into concentrated acid, the heat evolved leads to instant boiling of the water which splashes violently. To prevent this the acid is diluted by slowly adding it to water with constant stirring and not the other way.
Sulphuric acid is a strong dibasic acid. It ionises in two steps:
⇒ \(\mathrm{H}_2 \mathrm{SO}_4+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{HSO}_4^{-} \quad\) K_4>10
⇒ \(\mathrm{HSO}_4^{-}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{SO}_4^{2-} \quad K_{a_2}^{\prime}=1.2 \times 10^{-2}\)
You know that the greater the value of the ionisation constant of an acid, the stronger the acid. The larger value of the acid ionisation constant Ka1(Ka1>10) shows that sulphuric acid is dissociated into H+ and HSO4– to a large extent.
It forms two series of salts—hydrogen sulphates or acid sulphates, for example, NaHS04, and sulphates or normal sulphates, for example, Na2SO4.
Sulphuric acid is a strong dehydrating and oxidising agent. It has a low volatility. Some of its main chemical properties are as follows.
Acidic nature We have already mentioned that sulphuric acid is a strong acid and forms two series of salts. The dilute acid reacts with active metals, liberating hydrogen.
⇒ \(\mathrm{M}+\mathrm{H}_2 \mathrm{SO}_4 \text { (dilute) } \longrightarrow \mathrm{MSO}_4+\mathrm{H}_2\)
M=Active metal, for example, Fe, Zn
Metal oxides and carbonates dissolve in dilute sulphuric acid.
⇒ \(\mathrm{ZnO}+\mathrm{H}_2 \mathrm{SO}_4 \text { (dilute) } \longrightarrow \mathrm{ZnSO}_4+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{SO}_4 \text { (dilute) } \longrightarrow \mathrm{CaSO}_4+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
Low volatility Sulphuric acid is a strong acid with low volatility. The concentrated acid is used to prepare a more volatile acid in reaction with the corresponding salt. For example, when sulphuric acid is treated with sodium chloride, HC1 is evolved.
⇒ \(2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{HCl}\)
Sulphuric acid also liberates hydrogen fluoride from a metal fluoride and nitric acid from a nitrate. Strong dehydrating agent Since concentrated sulphuric acid has a strong affinity for water, it is used to dry gases (provided they do not react with it). It removes water from organic compounds. For example, it chars sugar.
⇒ \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \stackrel{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow} 12 \mathrm{C}+11 \mathrm{H}_2 \mathrm{O}\)
Some other examples of its dehydrating action are shown.
⇒ \(\underset{\text { Formic acid }}{\mathrm{HCOOH}} \stackrel{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow} \mathrm{CO}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\begin{aligned}& \mathrm{COOH} \stackrel{\text { conc. } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow} \mathrm{CO}+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \\& \mathrm{COOH}\end{aligned}\)
Oxalic acid
Oxidising agent Hot concentrated sulphuric acid is a moderately strong oxidising agent. It oxidises metals and nonmetals, itself getting reduced to sulphur dioxide.
⇒ \(\mathrm{Cu}+2 \mathrm{H}_2 \mathrm{SO}_4 \text { (conc.) } \longrightarrow \mathrm{CuSO}_4+\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{C}+2 \mathrm{H}_2 \mathrm{SO}_4 \text { (conc.) } \longrightarrow \mathrm{CO}_2+2 \mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(3 \mathrm{~S}+2 \mathrm{H}_2 \mathrm{SO}_4 \text { (conc.) } \longrightarrow 3 \mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}\)
Uses
Sulphuric Acid Is An Important Industrial Chemical And Is needed in bulk amounts in various processes. It is mainly employed in
- The manufacture of fertilisers, for example., ammonium sulphate and superphosphate,
- The manufacture of dyes, paints, pigments, drugs and detergents,
- Petroleum refining,
- Manufacture of explosives, for example, dynamite and TNT,
- Metallurgical applications (used to clean the surface of metals before electroplating and galvanising), and
- Storage batteries.
Group 17 Elements
This group of the p block comprises fluorine, chlorine, bromine, iodine and astatine, the last member being radioactive. Group 17 elements are called halogens (in Greek, halogen means ‘salt giver’). They are highly reactive nonmetals.
The general characteristics of the elements are similar to those of the elements of Groups 1 and 2 of the periodic table. There is also a regular gradation in physical and chemical properties within the group.
Occurrence And Uses
Due to the high reactivity of the halogens they occur naturally as compounds only. They occur as halides; however, iodine, which is easily oxidised, is also found as iodates. The important ores of fluorine are fluorspar (CaF2), fluorapatite [3Ca3(PO4)2 -CaF2] and cryolite (Na3AlF6).
Chlorine is widely present as sodium chloride (and to a lesser extent as chlorides of potassium, magnesium and calcium) in seawater. The deposits of dried-up seas contain these as well as camalite (KC1- MgCl2 • 6H2O). Bromine and iodine are less abundant.
They occur as bromides and iodides in seawater. Iodine is present in certain forms of marine life like seaweeds. Sodium iodate is present as an impurity in Chile saltpetre (NaNO3) deposits.
Fluorine is used to make UF6 and SF6. The former is used for nuclear power generation and the latter is a dielectric. Important compounds obtained from fluorine are freons and Teflon (polytetrafluoroethylene).
Nonstick cookware is coated with Teflon. Sodium fluoride is used for fluoridation of water. A concentration of 1 ppm of fluoride in drinking water reduces the chances of dental caries. Tin fluoride is used in fluoride toothpastes.
Chlorine is used to purify drinking water and for bleaching textiles, wood pulp and paper. It is also used for the preparation of industrially important organic compounds like chlorinated hydrocarbons (CHCI3, CCI4, feons, DDT) polyvinyl chloride and inorganic compounds like HCI and bleaching powder.
It is also used in the manufacture of dyes and drugs, in the extraction of gold and platinum and in the preparation of some toxic compounds like phosgene (COCl2), tear gas (CCI3NO2) and mustard gas (CICH2CH2SCH2CH2CI).
Bromine is used in the manufacture of silver bromide and potassium bromide, which are respectively used in photography and as a sedative. Iodine is used for the manufacture of potassium iodide and iodoform.
A solution of iodine in alcohol is called a tincture of iodine and is used as an antiseptic. Common salt is iodised by adding sodium or potassium iodide/iodate to it. This is essential as a lack of iodine in the body leads to goitre.
Atomic And Physical Properties
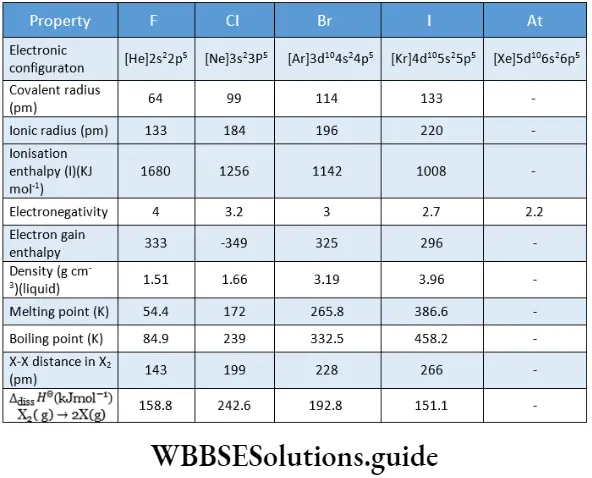
Some important physical constants of the halogens are listed in. The halogens exist as diatomic molecules and a regular gradation in physical properties is observed as we descend the group.
Electronic configuration
The valence-shell electronic configuration of these elements is ns2np5, i.e., only one electron is needed to complete the octet.
Size
The halogens are the smallest atoms in their respective periods in the modern periodic table. This is due to their highly effective nuclear charge. The atomic size increases on moving down the group.
Ionisation enthalpy
Haloeens exhibit high ionisation enthalpies and therefore have little tendency to lose valence-shell electrons and form positive ions. On moving down the group the ionisation enthalpy decreases due to an increase in the atomic size.
Electronegativity
Halogens have very high electronegativity values which decrease on moving down the group, i.e., with an increase in size. Fluorine is the most electronegative element.
Electron gain enthalpy
The halogens have the most negative electron gain enthalpies in their respective periods. This indicates that a lot of energy is evolved when the reaction X -> X” takes place. Halogens need only one electron to acquire a stable noble-gas configuration.
This tendency decreases with an increase in size and the electron gain enthalpy becomes less negative on descending the group. However, the electron gain enthalpy of fluorine is less negative than that of chlorine. This is because the fluorine atom is very small in size and the incoming electron encounters a lot of electron-electron repulsion in a small 2 p subshell.
Physical properties
There is a steady increase in the melting and boiling points of the elements on moving down the group. At room temperature, fluorine and chlorine are gases, bromine is a liquid and iodine is a solid.
Fluorine is light yellow, chlorine is greenish-yellow, and bromine and iodine are reddish-brown and violet respectively. The absorption of visible radiation by atoms of the elements results in the promotion of electrons to higher energy levels.
When these electrons return to the lower energy levels, the energy absorbed is emitted—the frequency of which lies in the visible range. Thus elements exhibit specific colours. All halogens are soluble in water.
However, their reaction to water varies. Fluorine and chlorine both react with water to form the respective halides and oxygen. The reaction is a violent case of fluorine.
Bromine and iodine are sparingly soluble in water and energy has to be supplied to make them oxidise water. Halogens dissolve in organic solvents like carbon disulphide, chloroform and carbon tetrachloride to give coloured solutions.
An anomaly is noted in a variation of bond dissociation enthalpy of the halogen molecules. The X-X bond dissociation enthalpy is expected to decrease down the group with the increase in atomic size resulting in less effective atomic overlap. This trend is well noted from chlorine to iodine in the group.
However, the F-F bond dissociation enthalpy is abnormally low. This is because the fluorine atom is very small. Also, the intemuclear distance (F-F) is small in the F2 molecule. This leads to strong repulsion between the lone pairs of electrons in the small F2 molecule. This factor is responsible for the high reactivity of fluorine.
Chemical Properties
Oxidation states and trends in chemical reactivity
The halogens exhibit a -1 oxidation state in most of their compounds. This is the only oxidation state displayed by fluorine.
The other halogens may display oxidation states of +1, +3, +5 and +7in some of the compounds. The positive oxidation states are displayed in halogen oxides, oxoacids and interhalogens.
Oxidation states of +3, +5 and +7 are realised by the unpairing of the s electron pair and its promotion to the vacant d orbitals. Chlorine and bromine also display oxidation states of +4 and +6 in their oxides.
The halogens are highly reactive and the reactivity decreases down the group.
The high reactivity of halogens is due to the following factors.
- Low enthalpy of dissociation The X-X bond is weak. Therefore, the diatomic molecule dissociates readily.
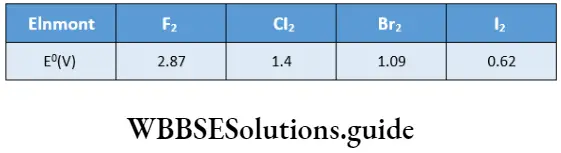
- High oxidising power The halogens have high negative electron gain enthalpies, i.e., they have a strong tendency to acquire electrons and are good oxidising agents. Fluorine is the strongest oxidising agent. Generally speaking, a higher member can displace a lower member of the group from the halide. The following reactions illustrate this.
F2+2X–→2F–+X2
CI2+2X→2CI+X2Br2–+I2
Br2+2I–→2Br–+I2
The oxidising power of halogens doorcases down the group. This is reflected in their decreasing reduction fv4oiUi.il. in descending the group.
As you know, the reduction potential of an element depends on various factors and is shown in the form of a Born-flavour cycle.
⇒ \(\begin{aligned}
\frac{1}{2} \mathrm{X}_2(\mathrm{~s}) \longrightarrow \frac{1}{2} \mathrm{X}_2(\mathrm{~g}) \stackrel{\frac{1}{2} \Lambda_{\mathrm{dit} t} f^{\mathrm{O}}}{\longrightarrow} & \mathrm{X}(\mathrm{g}) \\
\downarrow & \downarrow \Delta_{\mathrm{eg}} H^{\Theta}
\end{aligned}\)
⇒ \(\mathrm{X}^{-} \text {(aq) } \quad \stackrel{\Delta_{\text {hyd }} H^{\Theta}}{\longleftarrow} \quad \mathrm{X}^{-}(\mathrm{g})\)
The reactions of halogens with water indicate their relative oxidising powers. Fluorine oxidises water. Bromine and chlorine dissolve in water, forming hydrohalic and hypohalous acids. The reaction of iodine with water has a positive Gibb’s energy change, i.e., it is nonspontaneous. In fact, an iodide is oxidised to iodine by oxygen. This is the reverse of the reaction observed with fluorine.
⇒ \(2 \mathrm{~F}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 4 \mathrm{H}^{+}+4 \mathrm{~F}^{-}+\mathrm{O}_2\)
⇒ \(\mathrm{X}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{HX}+\mathrm{HOX}\)
⇒ \(4 \mathrm{I}^{-}+4 \mathrm{H}^{+}+\mathrm{O}_2 \longrightarrow 2 \mathrm{I}_2+2 \mathrm{H}_2 \mathrm{O}\)
Anomalous Behaviour Of Fluorine
Fluorine differs from the other halogens because of its exceptionally small size, low F-F bond dissociation enthalpy and the absence of d orbitals in its valence shell.
Some specific examples of its anomalous behaviour are as follows.
- Fluorine is more reactive than other halogens due to the low F-F bond dissociation enthalpy. Also, due to its high electronegativity, it forms strong bonds with other elements.
- Most reactions of fluorine are exothermic due to the formation of strong bonds with other elements.
- Fluorine is the strongest oxidising agent and may oxidise elements to the highest oxidation state. For example, in SF6 and IF7, the oxidation states of sulphur and iodine are +6 and +7 respectively.
- In HF, fluorine forms strong hydrogen bonds. Thus, HF is a liquid while the other hydrogen halides are gases.
- Fluorine forms only one oxoacid while the other halogen forms more oxoacids.
- Due to the high electronegativity of fluorine, fluorides have greater ionic character than other halides.
Reactivity towards hydrogen
All halogens combine with hydrogen to form hydrogen halides, HX. The reaction with fluorine is violent while that with iodine is slow, indicating that the reactivity of the halogens decreases on descending the group. Hydrogen halides are covalent in the gaseous state, but in aqueous solutions, they function as strong acids. Some properties of hydrogen halides are given in.
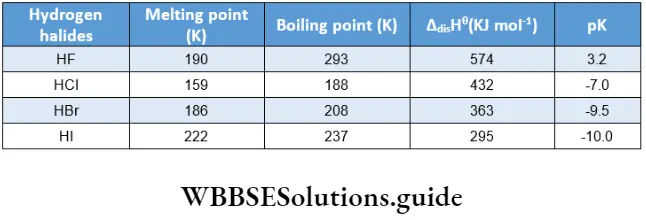
They undergo dissociation in water giving acidic solutions.
⇒ \(\mathrm{HX}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{X}\)
⇒ \(\mathrm{HF}<\mathrm{HCl}<\mathrm{HBr}<\mathrm{HI}\)
Various factors contribute to the acid strength and an important factor is bond enthalpy. The bond enthalpy decreases from HF to HI. Thus HF is the weakest acid, and HI is the strongest acid. Intermolecular hydrogen bonding also contributes to the low acidity of HF.
Reactivity towards oxygen
A large number of binary compounds of halogens and oxygen are known. The important compounds are shown in.
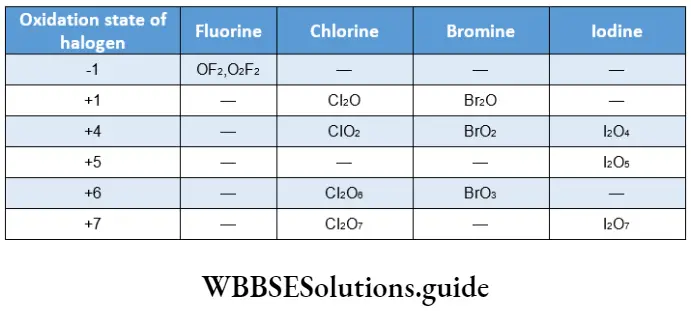
Binary compounds of fluorine with oxygen are called oxygen fluorides due to the higher electronegativity of fluorine, whereas the analogous compounds with other halogens are called halogen oxides. As the electronegativity difference between the halogens and oxygen is small, the bonds are essentially covalent.
Oxygen fluorides are strong fluorinating agents. O2F2 is used to remove plutonium from spent nuclear fuel by converting it to PuF6. Apart from OF2, all oxides have positive Gibbs energy of formation and are unstable with respect to dissociation to the elements.
The oxides of iodine are the most stable followed by the oxides of chlorine. The oxides of bromine are the least stable. This can be explained by taking into consideration both kinetic and thermodynamic factors of the reactions involved. The higher oxides tend to be more stable than the lower ones.
Group 16 elements – oxygen family in p-block elements
Except for that of iodine, all oxides tend to be explosive. Because of its oxidising nature, CIO2 is used as a bleaching agent for paper pulp and textiles and as a germicide in water treatment. I2O5 quantitatively oxidises carbon monoxide to carbon dioxide and is therefore used in the estimation of carbon monoxide.
Reactivity towards metals
The halogens combine with most metals to form metal halides.
⇒ \(2 \mathrm{M}+n \mathrm{X}_2 \longrightarrow 2 \mathrm{MX}_n\)
Fluorine and chlorine react with practically all metals whereas bromine and iodine combine with most metals except the noble metals. The fluorides are all ionic. This is quite obvious as fluorine is the most electronegative element in the periodic table and when it combines with an electropositive metal, owing to the large electronegativity difference an ionic compound is formed. The ionic character of a metal halide follows the order:
fluoride > chloride > bromide > iodide.
Among metal halides in which the constituent elements are the same, the halide in which the halogen is in the higher oxidation state is more covalent than the one in which the halogen is in the lower oxidation state. Thus TICl3, PbCl4, AsCl3, UF4 are more covalent thanTICl, PbCl2, AsCl3 and UF4 respectively.
Reactivity towards other halogens
The halogens combine among themselves to form binary compounds called interhalogen compounds. Interhalogens of the type XX’, XX3, XX’5 and XX’7 are known. (Here X is the higher member and X is the lower member of the group.) We will discuss interhalogens later in this chapter.
Chlorine
Chlorine was first prepared by Scheele (1774) by oxidising HCI with MnO2. Davy (1810) identified it to be an element and coined the name chlorine on account of its colour (chlorosis in Greek means greenish-yellow).
Preparation
Chlorine can be prepared by oxidising HCI with MnO2
⇒ \(\mathrm{MnO}_2+4 \mathrm{HCl} \longrightarrow \mathrm{Cl}_2+\mathrm{MnCl}_2+2 \mathrm{H}_2 \mathrm{O}\)
In practice, a mixture of common salt and concentrated H2SO4 is taken in place of HCI.
⇒ \(\mathrm{MnO}_2+4 \mathrm{NaCl}+4 \mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{MnCl}_2+4 \mathrm{NaHSO}_4+2 \mathrm{H}_2 \mathrm{O}+\mathrm{Cl}_2\)
It is also obtained upon the oxidation of HC1 by potassium permanganate.
⇒ \(2 \mathrm{KMnO}_4+16 \mathrm{HCl} \longrightarrow 2 \mathrm{KCl}+2 \mathrm{MnCl}_2+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{Cl}_2\)
Chlorine is produced commercially by two main processes as follows.
- The electrolysis of brine (concentrated sodium chloride solution) in the manufacture of NaOH
⇒ \(2 \mathrm{NaCl}+2 \mathrm{H}_2 \mathrm{O} \stackrel{\text { electrolysis }}{\longrightarrow} 2 \mathrm{NaOH}+\mathrm{Cl}_2+2 \mathrm{H}_2\) - By the oxidation of hydrogen chloride Gatsby oxygen at 723 Kin the presence of copper chloride as a catalyst
⇒ \(4 \mathrm{HCl}+\mathrm{O}_2 \longrightarrow 2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{Cl}_2\)
Properties
Chlorine is a pungent-smelling, yellowish-green gas which can be liquefied to a greenish-yellow liquid. It reacts with metals and nonmetals to form chlorides.
⇒ \(2 \mathrm{Na}+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{NaCl}\)
⇒ \(\mathrm{Mg}+\mathrm{Cl}_2 \longrightarrow \mathrm{MgCl}_2\)
⇒ \(2 \mathrm{Al}+3 \mathrm{Cl}_2 \longrightarrow 2 \mathrm{AlCl}_3\)
⇒ \(\mathrm{P}_4+6 \mathrm{Cl}_2 \longrightarrow 4 \mathrm{PCl}_3\)
⇒ \(\mathrm{S}_8+4 \mathrm{Cl}_2 \longrightarrow 4 \mathrm{~S}_2 \mathrm{Cl}_2\)
⇒ \(\mathrm{H}_2+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{HCl}\)
It reacts with compounds containing hydrogen (i.e., hydrides, hydrocarbons, etc.) to form hydrogen chloride.
⇒ \(\mathrm{H}_2 \mathrm{~S}+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{HCl}+\mathrm{S}\)
⇒ \(\mathrm{C}_{10} \mathrm{H}_{16}+8 \mathrm{Cl}_2 \longrightarrow 16 \mathrm{HCl}+10 \mathrm{C}\)
The reactions of chlorine with saturated hydrocarbons are substitution reactions and those with unsaturated hydrocarbons are addition reactions.
⇒ \(\mathrm{CH}_4+\mathrm{Cl}_2 \stackrel{\mathrm{uv}}{\longrightarrow} \mathrm{CH}_3 \mathrm{Cl}+\mathrm{HCl}\)
⇒ \(\mathrm{CH}_3 \mathrm{Cl}+\mathrm{Cl}_2 \stackrel{\text { uv }}{\longrightarrow} \mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{HCl}\)
⇒ \(\mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{Cl}_2 \stackrel{\mathrm{uv}}{\longrightarrow} \mathrm{CHCl}_3+\mathrm{HCl}\)
⇒ \(\mathrm{CHCl}_3+\mathrm{Cl}_2 \stackrel{\mathrm{uv}}{\longrightarrow} \mathrm{CCl}_4+\mathrm{HCl}\)
⇒ \(\mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2+\mathrm{Cl}_2 \longrightarrow \underset{\text { 1,2-dichloroethane }}{\mathrm{C}_2 \mathrm{H}_4 \mathrm{Cl}_2}\)
Chlorine reacts with ammonia to give different products depending on the relative proportions of the reactants. With excess ammonia, the products are nitrogen and ammonium chloride, while with excess chlorine, nitrogen trichloride is formed.
⇒ \(8 \mathrm{NH}_3+3 \mathrm{Cl}_2 \longrightarrow 6 \mathrm{NH}_4 \mathrm{Cl}+\mathrm{N}_2\)
⇒ \(\mathrm{NH}_3+3 \mathrm{Cl}_2 \longrightarrow \mathrm{NCl}_3+3 \mathrm{HCl}\)
Chlorine dissolves in water to give a yellow solution. This is called chlorine water. Soon after the solution is prepared it turns colourless due to the following reaction.
⇒ \(\mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{HCl}+\mathrm{HOCl}\)
Hypochlorous acid (HOC1) releases nascent oxygen, which is responsible for the oxidising and bleaching action of aqueous chlorine.
⇒ \(\mathrm{HOCl} \longrightarrow \mathrm{HCl}+\mathrm{O}\)
The bleaching action is permanent. Freshly prepared chlorine water is a strong oxidising agent and oxidises ferrous to ferric.
⇒ \(2 \mathrm{Fe}^{2+}+\mathrm{Cl}_2 \longrightarrow 2 \mathrm{Fe}^{3+}+2 \mathrm{Cl}^{-}\)
It also oxidises sulphur dioxide to sulphuric acid and iodine to iodic acid.
⇒ \(\mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O}+\mathrm{Cl}_2 \longrightarrow \mathrm{H}_2 \mathrm{SO}_4+2 \mathrm{H}^{+}+2 \mathrm{Cl}^{-}\)
⇒ \(\mathrm{I}_2+6 \mathrm{H}_2 \mathrm{O}+5 \mathrm{Cl}_2 \longrightarrow 2 \mathrm{HIO}_3+10 \mathrm{H}^{+}+10 \mathrm{Cl}^{-}\)
Chlorine in reaction with cold and dilute alkalis produces a mixture of chloride and hypochlorite. However, with hot and concentrated alkalis, chlorine forms chloride and chlorate.
⇒ \(\underset{\text { (dilute) }}{2 \mathrm{NaOH}}+\mathrm{Cl}_2 \stackrel{\text { cold }}{\longrightarrow} \underset{\begin{array}{c}
\text { Sodium } \\
\text { hypochlorite }
\end{array}}{\mathrm{NaOCl}}+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\underset{\text { (concentrated) }}{6 \mathrm{NaOH}}+\mathrm{Cl}_2 \stackrel{\text { hot }}{\longrightarrow} \underset{\begin{array}{l}
\text { Sodium } \\
\text { chlorate }
\end{array}}{\mathrm{NaClO}_3}+5 \mathrm{NaCl}+5 \mathrm{H}_2 \mathrm{O}\)
Both these reactions are disproportionation reactions as chlorine (oxidation state zero) undergoes simultaneous oxidation to hypochlorite (oxidation state +1) or chlorate (oxidation state +3) and chloride (oxidation state -1).
The reaction of chlorine with slaked lime yields bleaching powder.
⇒ \(2 \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{Cl}_2 \longrightarrow \mathrm{Ca}(\mathrm{OCl})_2+\mathrm{CaCl}_2+2 \mathrm{H}_2 \mathrm{O}\)
It is a mixture of calcium hypochlorite, calcium chloride and calcium hydroxide and is represented as Ca(OCI)2.CaCI2.Ca(OH)2.2H2O.
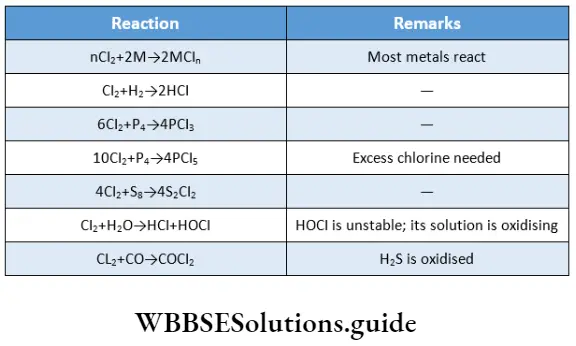
Hydrogen Chloride
It is prepared by heating sodium chloride with concentrated sulphuric acid.
⇒ \(\mathrm{NaCl}+\mathrm{H}_2 \mathrm{SO}_4 \stackrel{\Delta}{\longrightarrow} \mathrm{NaHSO}_4+\mathrm{HCl}\)
⇒ \(\mathrm{NaHSO}_4+\mathrm{NaCl} \stackrel{\Delta}{\longrightarrow} \mathrm{Na}_2 \mathrm{SO}_4+\mathrm{HCl}\)
⇒ \(2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{SO}_4 \stackrel{\Delta}{\longrightarrow} \mathrm{Na}_2 \mathrm{SO}_4+2 \mathrm{HCl}\)
The HCI produced is dried by passing through concentrated sulphuric acid.
The presence of HCI can be detected by holding a glass rod dipped in ammonia at the mouth of the test tube where the reaction has taken place when white fumes of ammonium chloride have evolved.
⇒ \(\mathrm{NH}_3+\mathrm{HCl} \longrightarrow \mathrm{NH}_4 \mathrm{Cl}\)
Properties
Hydrogen chloride is an extremely pungent-smelling, colourless gas which can be liquefied to give a colourless liquid and solidified to give a white solid.
It is highly soluble in water and yields an acidic solution, which is called hydrochloric acid.
⇒ \(\mathrm{HCl}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{Cl}^{-}\)
Hydrochloric acid is a strong acid. It liberates hydrogen from reacting with active metals.
⇒ \(\mathrm{Zn}+2 \mathrm{HCl} \longrightarrow \mathrm{ZnCl}_2+\mathrm{H}_2\)
⇒ \(\mathrm{Fe}+2 \mathrm{HCl} \longrightarrow \mathrm{FeCl}_2+\mathrm{H}_2\)
In reaction with iron, the product formed is ferrous chloride and not ferric chloride as the evolved hydrogen prevents further oxidation of iron. When salts of weak acids (carbonates, sulphites, thiosulphates, sulphides) are treated with dilute hydrochloric acid, the anion is displaced.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
⇒ \(\mathrm{Na}_2 \mathrm{SO}_3+2 \mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2\)
⇒ \(\mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3+2 \mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2+\mathrm{S}\)
⇒ \(\mathrm{Na}_2 \mathrm{~S}+2 \mathrm{HCl} \longrightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{~S}\)
Group 17 elements – halogens and their reactivity
A mixture of three parts of concentrated hydrochloric acid and one part of concentrated nitric acid is called aqua regia Aqua regia combines with noble metals forming soluble chloro complexes.
⇒ \(\mathrm{Au}+4 \mathrm{H}^{+}+4 \mathrm{Cl}^{-}+\mathrm{NO}_3^{-} \longrightarrow\left[\mathrm{AuCl}_4\right]^{-}+\mathrm{NO}+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(3 \mathrm{Pt}+16 \mathrm{H}^{+}+18 \mathrm{Cl}^{-}+4 \mathrm{NO}_3^{-} \longrightarrow 3\left[\mathrm{PtCl}_6\right]^{2-}+4 \mathrm{NO}+8 \mathrm{H}_2 \mathrm{O}\)
Uses
- Hydrochloric acid is used as a laboratory reagent
- It is used in the pharmaceutical industry
- It is used in the manufacture of chlorine and other chemicals.
Oxoacids Of Halogens
Oxoacids of halogens have oxygen attached to the halogen. They have the general formula HOX(O)n where n=0, 1, 2, 3. Due to its small size and high electronegativity, fluorine forms only one oxoacid—the unstable HOF. The other halogens form four acids—hypohalous (HOX), halos (HOXO), halic (HOXO2) and perhalic (HOXO3). Most of the oxoacids are known solutions or salts. The oxoacids of halogens and their structures are shown in.
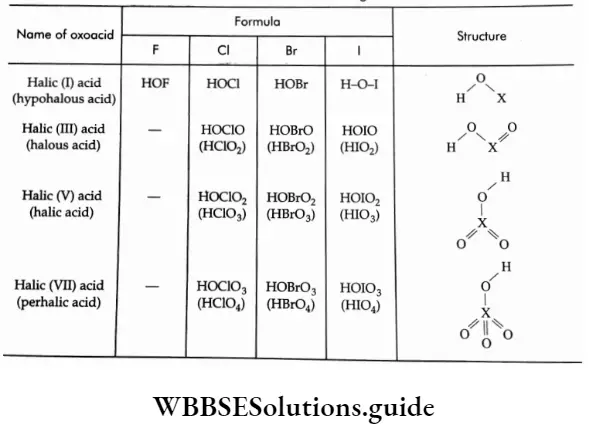
Interhalogen Compounds
These are binary compounds of two different halogen atoms having the general formula AXn where both A and X are halogens and X has a higher electronegativity. The value of n may be 1, 3, 5 or 7. The halogen with the lower e ec onega city is assigned a positive oxidation state.
The total number of halogen atoms is always even as this gives rise to a magnetic species, which is generally more stable. The interhalogens can be prepared by a direct combination of the halogens or by the action of a halogen on an interhalogen compound.
A few examples are as follows.
CI2+F2→2CIF
CI2+3F2→2CIF3
Br2+5F2→2BrF3
CIF3+F2→CIF5
IF5+F2→IF7
Lists some interhalogens of the four types together with their physical state and colour at room temperature. In the interhalogens of the type AX5 and AX7, A is a large halogen (Br or I) and X is fluorine. This type exists as it is easier to pack a large number of small atoms around a large atom.
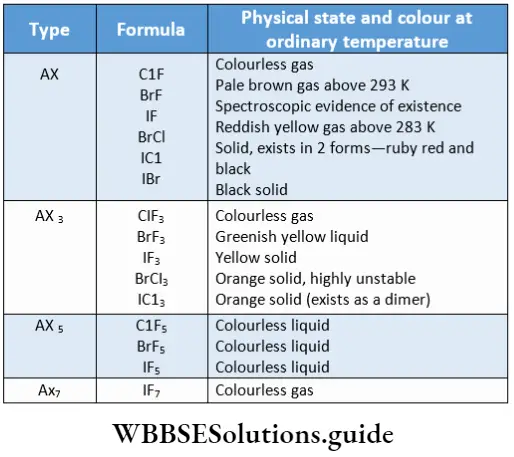
Most of the interhalogens are volatile solids or liquids and their physical properties are intermediate between those of the constituent halogens. The stability of interhalogen compounds increases with an increase in the difference in electronegativity between the two halogen atoms.
Interhalogen compounds are predominantly covalent and are generally more reactive than halogens (except fluorine). This is because the A-X bond in interhalogens is less stable than the X-X bond in halogens, except for the F-F bond.
All interhalogens hydrolyse to give a halide and an oxohalide (hypohalite in case of AX, halitein case of AX3, halate in case of AX5 and perhalate in case of AX7). The smaller halogen is converted to the halide and the larger to the oxohalide.
⇒ \(\mathrm{ClF}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{F}^{-}+\mathrm{OCl}^{-}+2 \mathrm{H}^{+}\)
⇒ \(\mathrm{ICl}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Cl}^{-}+\mathrm{OI}^{-}+2 \mathrm{H}^{+}\)
The VSEPR theory is applied to assign structures to the interhalogen compounds. Let us discuss a few types here.
In CIF3 (AX3 type), the chlorine atom has seven electrons in the outermost shell, out of which three will be used in a bond formation with three fluorine atoms, leaving behind four unused electrons. Thus, there are two lone pairs and three bond pairs, giving rise to sp3d hybridisation, i.e., a trigonal bipyramidal structure.
The two lone pairs will occupy the equatorial positions to minimise repulsions. The molecule is T-shaped with the axial fluorines slightly bent away from the lone pairs and the T is slightly bent.
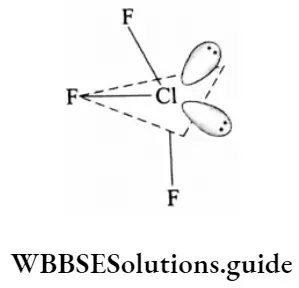
In order to quickly assign the hybridisation of the central atom in the compound to deduce its structure, the following steps will prove to be helpful.
- Count the valence electrons of all the atoms. Add or subtract electrons equal to the charge for anions and cations respectively.
- If the total number of valence electrons in the compound is less than 8, divide by 2. The quotient gives the number of electron pairs. For two electron pairs the hybridisation is sp, for three it is sp2 and for four it is sp3.
- If the total number of valence electrons exceeds 8, divide by 8 to get the quotient Qj and remainder R. If R = 0, then Q: gives a number of electron pairs. If R≠O then further divide R by 2 to get Q2. Add Q1 and Q2 to get the number of electron pairs and assign hybridisation accordingly (Ch = number of bond pairs and Q2 =number of lone pairs).
Let us now deduce the hybridisation of chlorine in CIF3 by following the abovementioned steps in sequence.
- Total number of valence electrons = 7 + 3 x 7 = 28
- ⇒ \(Q_1=\frac{28}{8}=3 ; R_1=4\)
- ⇒ \(Q_2=\frac{R_1}{2}=2 ; \quad ∴ Q_1+Q_2=5\)
There are five electron pairs around the central atom, and the hybridisation is sp3d
In interhalogen compounds of the type AX5, for example, BrF5, the hybridisation is deduced as follows.
- Total number of valence electrons =7+5×7 = 42
- \(Q_1=\frac{42}{8}=5 ; R_1=2\)
- \(Q_2=\frac{R_1}{2}=1 ; \quad ∴ Q_1+Q_2=5+1=6\)
There are six electron pairs around the central atom, giving rise to sp3d2 hybridisation.
There are four bond pairs and one lone pair, giving rise to a square pyramidal structure. Similarly, it can be shown that IF7 has sp3d3 hybridization, giving rise to a pentagonal bipyramidal structure.
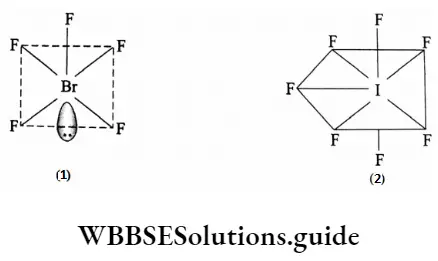
Uses
Interhaloeens are useful as nonaqueous solvents. Many of these undergo self-ionisation, for example.
⇒ \(2 \mathrm{BrF}_3 \longrightarrow \mathrm{BrF}_2^{+}+\mathrm{BrF}_4^{-}\)
C1F3 and BrF3 are good fluorinating agents and are used in the enrichment of converted to hexafluoride.
⇒ \(\mathrm{U}+3 \mathrm{ClF}_3 / 3 \mathrm{BrF}_3 \longrightarrow \mathrm{UF}_6+3 \mathrm{CIF} / 3 \mathrm{BrF}\)
Group 18 Elements
The elements of this group are helium, neon, argon, krypton, xenon and radon. Group 18 elements are called inert gases, rare gases or noble gases. The last member, radon, is radioactive and short-lived. Group 18 elements are chemically inert as their valence-shell orbitals are completely filled. They react with few compounds and are therefore called noble gases.
Occurrence And Uses
Apart from radon, the other noble gases are present in the atmosphere to an extent of about 1% (the major component being argon). Helium is commercially obtained from natural gas, the other sources of helium and neon are radioactive minerals like pitchblende and monazite. Radon is obtained by the radioactive decay of 27h Ra.
⇒ \({ }_{88}^{226} \mathrm{Ra} \longrightarrow{ }_{86}^{222} \mathrm{Rn}+{ }_2^4 \mathrm{He}\)
Helium is noninflammable and light and therefore used to fill balloons employed in meteorological observations. A mixture of helium and oxygen is used by deep-sea divers for respiration and is also used to provide relief to asthmatic patients. Helium has the lowest boiling point among all elements and thus is used to carry out research at very low temperatures. It is nonradioactive has high thermal conductivity, and is therefore used as a heat transfer agent in gas-cooled atomic reactors. Liquid helium is used as a coolant in superconducting coils used to build superconducting magnets which form a part of the NMR spectrometers used in MR! systems for clinical diagnosis.
Neon is used in discharge tubes and fluorescent lamps for advertising purposes. Neon bulbs are used in gardens and greenhouses. Neon can carry high currents at high voltages and is used to protect electrical instruments, e.g., voltmeters and rectifiers.
Argon is used to create an inert atmosphere in metallurgical operations carried out at high temperatures, for example., welding. It is used to fill electric light bulbs. Krypton and xenon are used for filling electric bulbs and phototubes. Radon is used in radioactive research and in the treatment of cancer.
Atomic And Physical Properties
Some physical characteristics of the noble gases are summarised in.
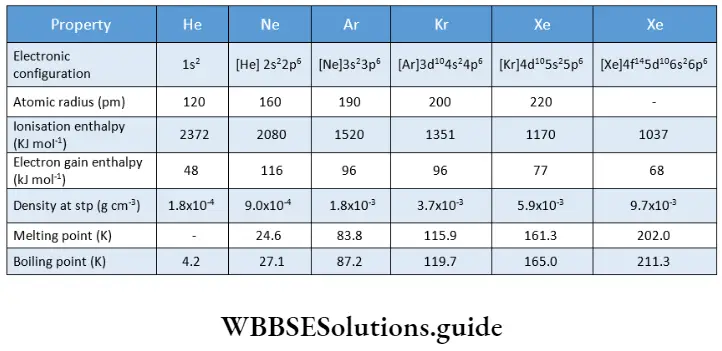
The electronic configuration of helium is Is2 while the other members of the group have a complete octet of electrons in their outermost shell, or the valence-shell configurations in these elements are MS2up6.
All the noble gases are monatomic. They have large atomic radii, which are actually van der Waals radii (van der Waals radii are nonbonded radii, which represent the distance of closest approach) and not ionic or covalent radii.
The atomic radii increase down the group. They have high ionisation enthalpy due to a stable electronic configuration. They have no tendency to accept electrons, as is reflected by large, positive electron gain enthalpy values.
Noble gases are colourless, tasteless odourless and sparingly soluble in water. Noble-gas atoms are held together by weak van der Waals forces, which can be readily overcome and hence they have low melting and boiling points.
As stated earlier, helium has the lowest boiling point among all elements (4.2 K). It has the unique property of diffusing through substances like rubber, glass and plastic.
Chemical Reactivity And Compounds
The stable electronic configuration of the elements leads to the involvement of high energies in the loss or gain of electrons (revealed by high ionisation enthalpy values and positive electron gain enthalpy values). Owing to this the noble gases show very low chemical reactivity.
The reactivity of noble gases was constantly investigated and the first real compound of a noble gas was made in 1962. Bartlett had observed that PtF6 combined with molecular oxygen (Oz) to form 02[PtF6 Since the first ionisation enthalpies of O2 (O2 ->O2+, 1175 kJ mol-1) and Xe (Xe —» Xe+; 1170 kJ mol-1) are comparable, it was predicted that a similar compound could be formed by making Xe react with PtF6. In fact, PtF6 vapours reacted with xenon at room temperature to form a reddish-yellow solid which was incorrectly thought of as xenon hexa fluoroplatinate.
⇒ \(\mathrm{Xe}(\mathrm{g})+\mathrm{PtF}_6(\mathrm{~g}) \longrightarrow \underset{\text { Xenon hexafluoroplatinate }}{\mathrm{Xe}^{+}\left[\mathrm{PtF}_6\right]^{-}(\mathrm{s})}\)
Actually, the product formed was really a more complicated one [XeF]+[Pt2F11]–. After this, it was found that xenon combined with electronegative elements like fluorine and oxygen to form various compounds.
Xenon oxofluorides can be obtained from fluorides. Fluorine and oxygen are strong oxidising agents and highly electronegative and hence can oxidise xenon to display positive oxidation states in compounds.
The ionisation enthalpies of He, Ne and Ar are too high to allow the formation of compounds. The ionisation enthalpy of Kr is lower, and so KrF2 is known, Rn is radioactive and its compounds have not been isolated; the < presence of RnF2 has been confirmed by radiotracer techniques.
Xenon-Fluorine Compounds
Xenon forms three fluorides, XeF2, XeF4 and XeF6 by direct combination with fluorine. The fluoride former depends on the prevailing experimental conditions.
In all the reactions involving the formation of a xenon fluoride, the reactants are taken in a nickel container and subjected to high temperature and pressure. Depending on the proportion of reactants, temperature and pressure that prevails during the reaction, different xenon fluorides are formed, which are shown in the following.
⇒ \(\begin{aligned}
& \mathrm{Xe}(\mathrm{g})+\mathrm{F}_2(\mathrm{~g}) \stackrel{673 \mathrm{~K}, 1 \mathrm{bar}}{\longrightarrow} \mathrm{XeF}_2(\mathrm{~g}) \\
& \quad(2: 1 \text { ratio })
\end{aligned}\)
⇒ \(\begin{aligned}
& \mathrm{Xe}(\mathrm{g})+2 \mathrm{~F}_2(\mathrm{~g}) \stackrel{873 \mathrm{~K}, 6-7 \text { bar }}{\longrightarrow} \mathrm{XeF}_4(\mathrm{~g}) \\
& \quad(1: 5 \text { ratio })
\end{aligned}\)
⇒ \(\begin{aligned}
& \mathrm{Xe}(\mathrm{g})+3 \mathrm{~F}_2(\mathrm{~g}) \stackrel{573 \mathrm{~K}(60-70 \mathrm{bar})}{\longrightarrow} \mathrm{XeF}_6(\mathrm{~g}) \\
& \quad(1: 20 \text { ratio })
\end{aligned}\)
XeF6 can also be made by the fluorination of XeF4 with oxygen fluoride at low temperature
⇒ \(\mathrm{XeF}_4+\mathrm{O}_2 \mathrm{~F}_2 \stackrel{143 \mathrm{~K}}{\longrightarrow} \mathrm{XeF}_6+\mathrm{O}_2\)
The xenon fluorides are colourless, crystalline solids which sublime at 298 K. They are powerful fluorinating agents. They are susceptible to hydrolysis. Hydrogen fluoride is formed during the hydrolysis of all xenon fluorides.
⇒ \(2 \mathrm{XeF}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{Xe}+4 \mathrm{HF}+\mathrm{O}_2\)
⇒ \(6 \mathrm{XeF}_4+12 \mathrm{H}_2 \mathrm{O} \longrightarrow 4 \mathrm{Xe}+2 \mathrm{XeO}_3+24 \mathrm{HF}+3 \mathrm{O}_2\)
XeF6 undergoes partial hydrolysis as well as complete hydrolysis.
⇒ \(\mathrm{XeF}_6+\mathrm{H}_2 \mathrm{O} \longrightarrow \underset{\substack{\text { Xenon } \\ \text { oxofluoride }}}{\mathrm{XeOF}_4}+2 \mathrm{HF}\)
⇒ \(\mathrm{XeF}_6+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{XeO}_2 \mathrm{~F}_2+4 \mathrm{HF}\)
⇒ \(\mathrm{XeF}_6+3 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{XeO}_3+6 \mathrm{HF}\)
The products of partial hydrolysis are xenon oxyfluorides and HF while that of complete hydrolysis is xenon trioxide.
It may be noted here that the oxidation state of xenon in XeF6, XeOF4, XeO2F2 and XeO3 is +6 and thus the hydrolysis of XeF6 whether partial or complete is not a redox reaction, unlike in the other cases, where there is a change in the oxidation state of Xe. [XeF2 gives Xe, the change in oxidation state is from +2 to zero; XeF4 has Xe in the +4 oxidation state, it gives Xe (zero oxidation state).]
Xenon fluoride acts as a fluoride acceptor as well as a fluoride donor. In reactions with fluoride ion acceptors, it forms cationic species whereas in reactions with fluoride ion donors, it forms anionic species.
⇒ \(\mathrm{XeF}_2+\underset{\substack{\text { Fluoride } \\ \text { ion acceptor }}}{\mathrm{PF}_5} \longrightarrow[\mathrm{XeF}]^{+}\left[\mathrm{PF}_6\right]^{-}\)
⇒ \(\mathrm{XeF}_4+\underset{\substack{\text { Fluoride ion } \\ \text { acceptor }}}{\mathrm{SbF}_5} \longrightarrow\left[\mathrm{XeF}_3\right]^{+}\left[\mathrm{SbF}_6\right]^{-}\)
⇒ \(\mathrm{XeF}_6+\underset{\substack{\text { Fluoride } \\ \text { ion donor }}}{\mathrm{MF}} \longrightarrow \mathrm{M}^{+}\left[\mathrm{XeF}_7\right]^{-}\)
Xenon fluorides are strong oxidising and fluorinating agents and combine quantitatively with hydrogen.
⇒ \(\mathrm{XeF}_2+\mathrm{H}_2 \longrightarrow \mathrm{Xe}+2 \mathrm{HF}\)
⇒ \(\mathrm{XeF}_4+2 \mathrm{H}_2 \longrightarrow \mathrm{Xe}+4 \mathrm{HF}\)
⇒ \(\mathrm{XeF}_6+3 \mathrm{H}_2 \longrightarrow \mathrm{Xe}+6 \mathrm{HF}\)
The structures of xenon fluorides can be derived from the VSEPR theory. In XeF2, Xe has eight valence electrons out of which two are involved in bond formation. Thus there are two bond pairs and three lone pairs giving rise to sp3d hybridisation with the lone pairs in equatorial positions.
The resultant structure is linear. On the other hand, in XeF4 the central atom undergoes sp3d2 hybridisation. There are two lone pairs and the resultant structure is square planar. XeF6 has a distorted octahedral structure involving sp3d3 hybridisation. There is one lone pair present at the centre of one of the triangular faces.
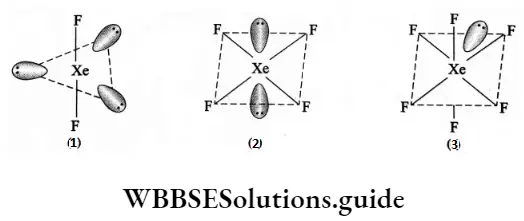
Xenon-Oxygen Compounds
Oxides
Two oxides of xenon—XeO3 and XeO4—are known. XeO3 is more important and is formed during the hydrolysis of XeF4 and XeF6. It is highly explosive and a vigorous oxidising agent. It has a pyramidal structure involving sp3 hybridisation and one lone pair.
Oxyfluorides
Xenon oxyfluoride (XeOF2) is obtained by the partial hydrolysis of XeF4
⇒ \(\mathrm{XeF}_4+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{XeOF}_2+2 \mathrm{HF}\)
Xenon oxytetrafluoride (XeOF4) and xenon dioxydifluoride (XeO2F2) are obtained during the partial hydrolysis of XeF6. The structures of the oxyfluorides are shown in XeOF2 has a T-shaped structure due to sp3d hybridisation and two lone pairs. XeOF4 has a square-pyramid structure because xenon is sp3d2 hybridised with one lone pair. In XeO2F2 xenon is sp3d hybridised with one lone pair, giving rise to a see-saw geometry.
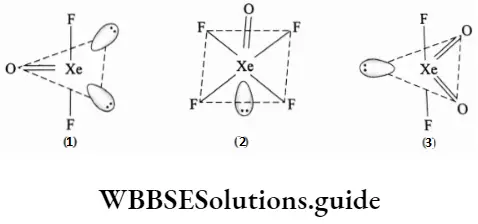
The P Block Elements Multiple-Choice Questions
Question 1. Which of the following is not an acidic oxide?
- N2O
- N2O
- P4O6
- SO2
Answer: 1. N2O
Question 2. In the molecular state, phosphorus exists as
- P
- P2
- P4
- Px
Answer: 3. P4
Question 3. Which of the following is true for PCI5?
- All the P-Cl bonds are equivalent.
- The axial bonds are longer than the equatorial bonds.
- The axial bonds are shorter than the equatorial bonds.
- The five P-Cl bond lengths differ from each other.
Answer: 2. The axial bonds are longer than the equatorial bonds.
Question 4. The strongest base among the following is
- NF3
- NH3
- PF3
- PH3
Answer: 2. NH3
Question 5. The hybridisation of P in PCI3 is
- sp2
- sp2d
- sp3d2
- sp3
Answer: 4. sp3
Question 6. Which of these is paramagnetic?
- N2O
- N2O
- N2O3
- N2O4
Answer: 2. N2O
Question 7. PH3 is evolved when phosphorus is treated with
- H2O
- HCI
- NaOH
- None of these
Answer: 3. NaOH
Question 8. Solid PClg is made up of
- discrete PCI5 units
- [PCI6]+[PCI4]–
- [PCI4]+[PCI6]–
- [P2CI8]2+[P2CI12]2-
Answer: 3. [PCI4]+[PCI6]–
Question 9. Which of these contains an O-O linkage?
- H2SO3
- H2S2O3
- H2SO4
- H2S2O8
Answer: 4. H2S2O8
Question 10. Which of the following is not a diprotic acid?
- H2SO3
- H2SO4
- H3PO3
- H3PO4
Answer: 3. H3PO3
Group 18 elements – noble gases and their properties
Question 11. Which of the following is a reducing agent?
- SO2
- SO3
- NO2
- CO2
Answer: 1. SO2
Question 12. The correct order of stability of Group 15 hydrides is
- H2O>H2S>H2Se>H2Te
- H2Te>H2Se>H2S>H2O
- H2S>H2O>H2Se>H2Te
- H2Te>H2Te>H2Se>H2S
Answer: 1. H2O>H2S>H2Se>H2Te
Question 13. The correct order of bond enthalpy among the following is
- CI2<I2<Br2<F2
- F2<I2<CI2<Br2
- F2<Br2<CI2<I2
- I2<F2<Br2<CI2
Answer: 4. I2<F2<Br2<CI2
Question 14. In the reaction Cl2+ 2X“ —> X2+ 2C1, X is
- Br orI
- I only
- F, Br or I
- F only
Answer: 1. Br orI
Question 15. The reaction 3C10 —> C103 + 2C1 is an example of
- Decomposition
- Disproportionation
- Oxidation
- Reduction
Answer: 2. Disproportionation
Question 16. When concentrated sulphuric acid is added to sodium chloride, the gas evolves is
- SO2
- SO3
- CI2
- HCI
Answer: 4. HCI
Question 17. Hydrogen bonding is not present in
- PH3
- NH3
- H2O
- HF
Answer: 1. PH3
Question 18. Among hydrogen halides, the correct order of acidity is
- HF<HCI<HI<HBr
- HI<HBr<HCI<HF
- HF<HCI<HBr<HI
- HI<HBr<HF<HCI
Answer: 3. HF<HCI<HBr<HI
Question 19. The hybridisation of Cl in CIF3 is
- sp2d
- sp2
- sp3
- sp3d
Answer: 4. sp3d
Question 20. Which of these is paramagnetic?
- CI2
- S2
- N2
- Br2
Answer: 2. S2
Question 21. The hydrolysis product of XeF4 is
- Xe+HF
- XeO3+O2+HF
- Xe+HF+XeO3+O2
- XeOF4
Answer: 3. Xe+HF+XeO3+O2
Question 22. Which of these is a planar molecule?
- XeO3
- XeOF4
- XeO4
- XeF4
Answer: 4. XeF4
Question 23. The hybridisation of Xe in XeF4 is
- sp3d
- sp3d
- sp2
- sp3
Answer: 2. sp3d
Question 24. Which among the following is tetrahedral?
- XeO4
- XeF4
- XeOF4
- XeOF2
Answer: 1. XeO4
Question 25. Which among these does not exist?
- XeF2
- NeF2
- XeO3
- XeF4
Answer: 2. NeF2
Question 26. The N2 molecule is isoelectronic with
- CO–,CN+ and NO+
- CO, CN–and NO+
- CO+,N2O and O2-2
- O2+O2– and CO+
Answer: 2. CO, CN–and NO+
Question 27. Which of these metals is rendered passive on treatment with concentrated HNO3?
- Cr
- Ni
- Cu
- Fe
Answer: 1. Cr
Question 28. Which of these is a dibasic acid and reducing agent?
- H3PO4
- H3PO2
- H3PO3
- HPO3
Answer: 3. H3PO3