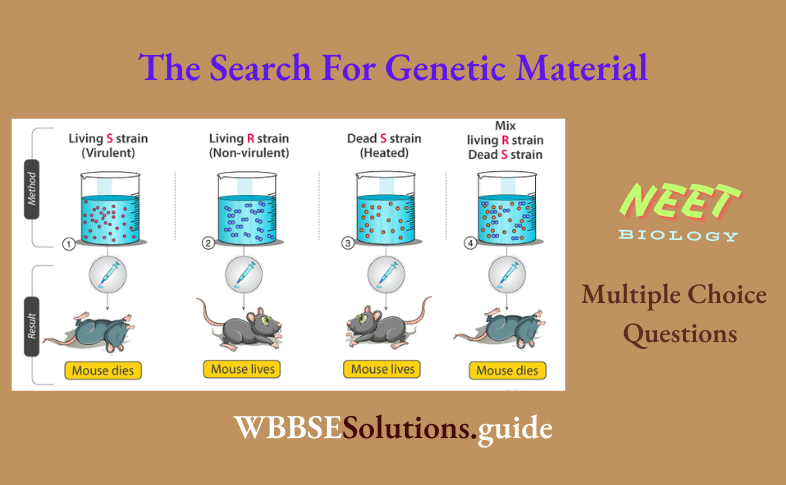
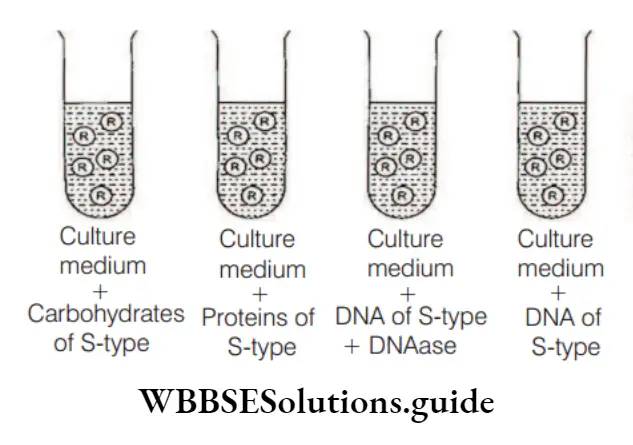
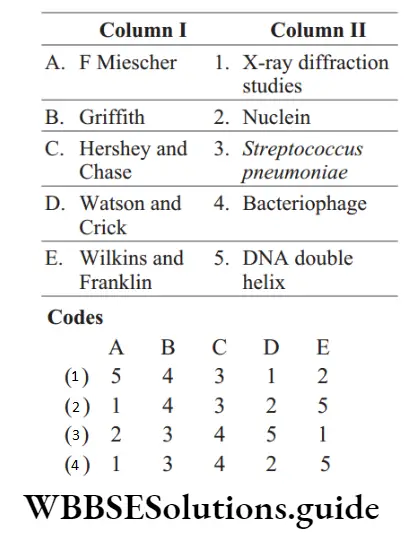
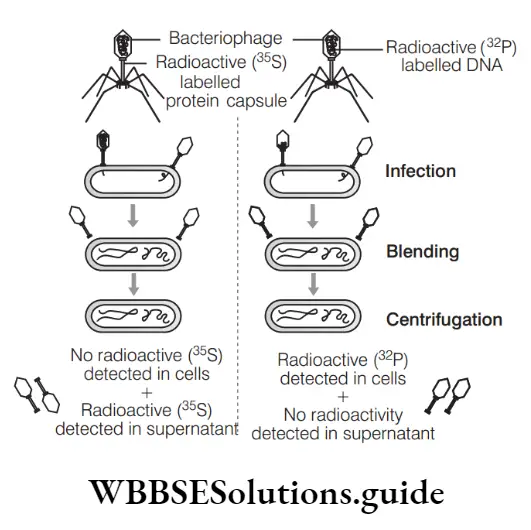
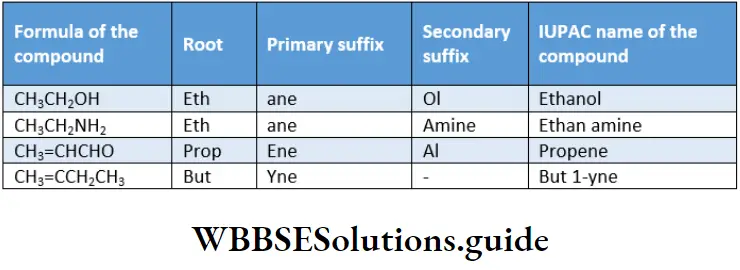
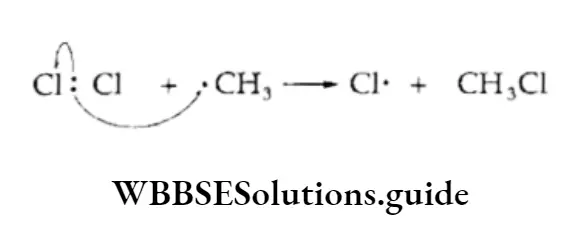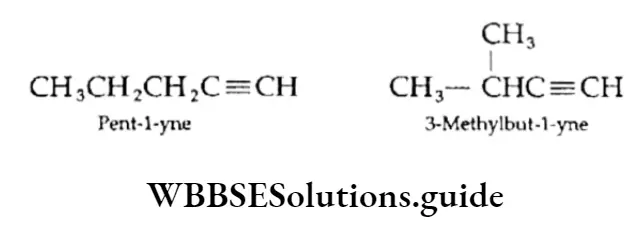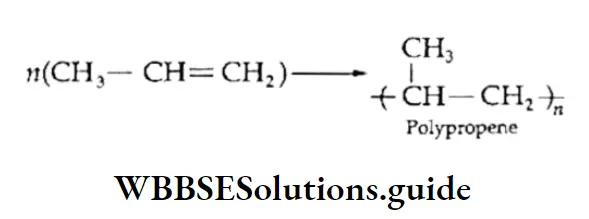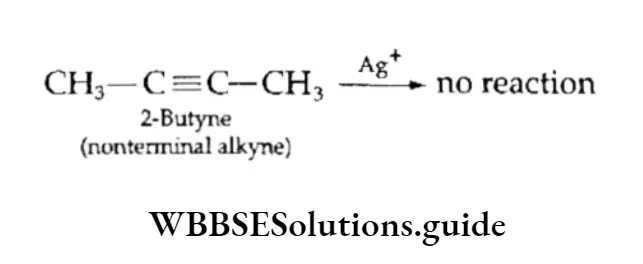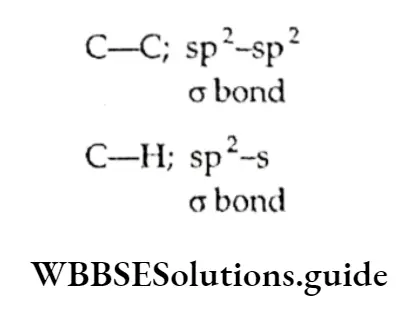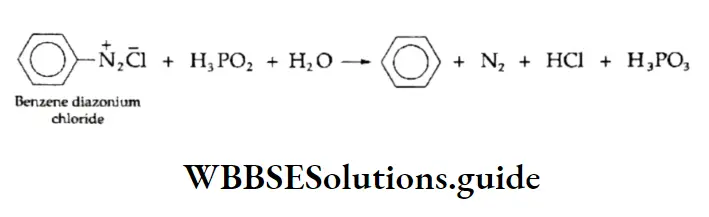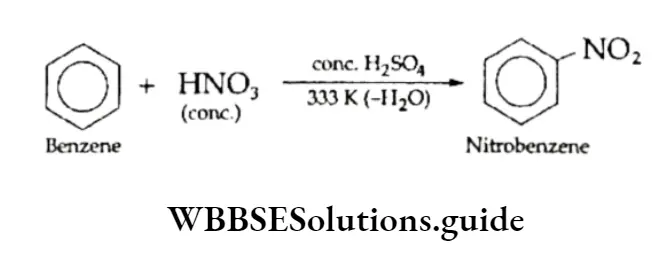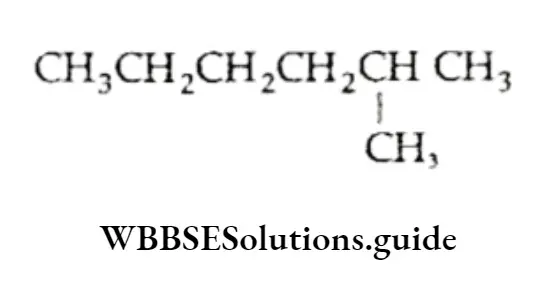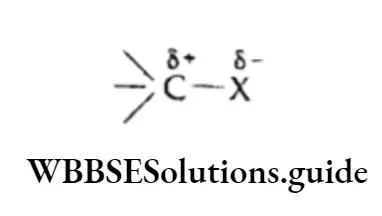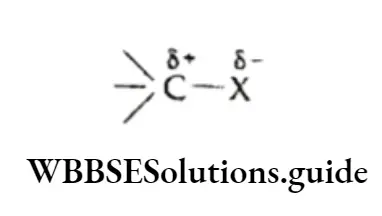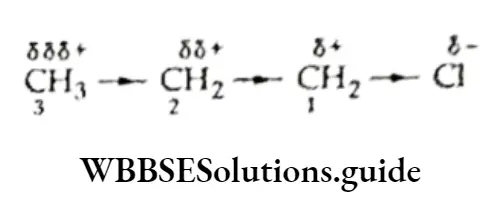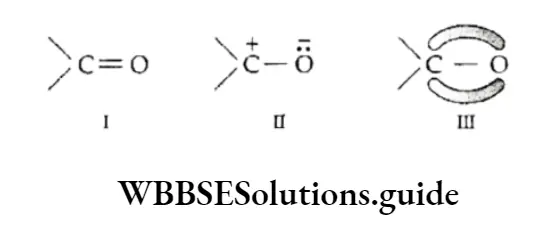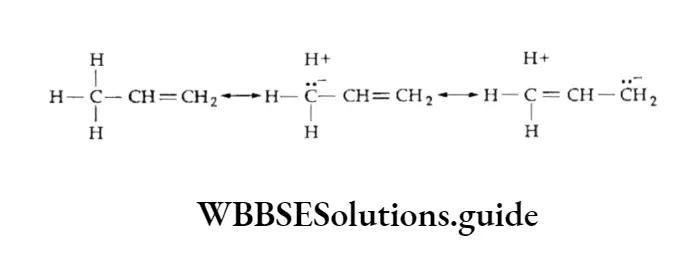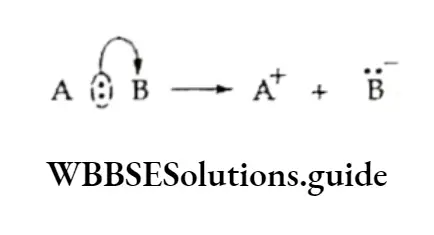Hydrocarbons
Hydrocarbons, or compounds which contain only carbon and hydrogen, are the simplest organic compounds, the main types being alkanes, alkenes, alkynes and arenes.
Hydrocarbons are mainly used as fuels for domestic and industrial purposes. Petroleum, kerosene, CNG, LPG, etc., are all mixtures of hydrocarbons.
All other families of organic compounds can be considered to be derived from hydrocarbons by replacing one or more hydrogen atoms with the appropriate functional group.
Hydrocarbons Classification
Hydrocarbons can be classified either on the basis of their structures or on the basis of whether they are saturated or unsaturated. Structurally, they can be of two types—acyclic and cyclic.
Acyclic Hydrocarbons
Hydrocarbons which have open chains of carbon atoms are called acyclic hydrocarbons.
These are also known as aliphatic hydrocarbons. The name aliphatic comes from the Greek word aliphos, meaning fat. Alkanes, alkenes and alkynes are acyclic hydrocarbons.
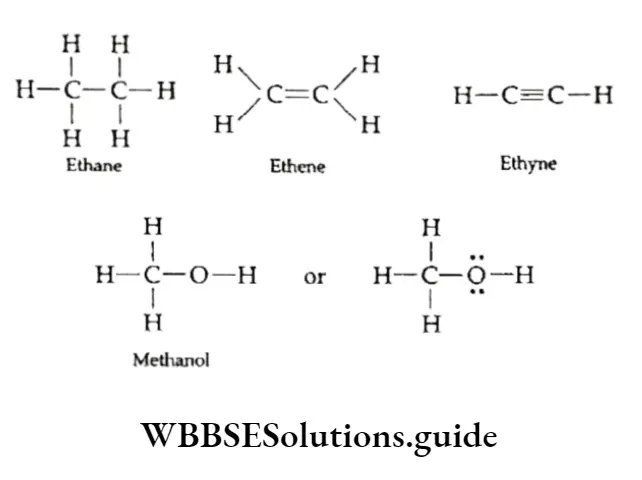
Alkanes are saturated and have only carbon-carbon single bonds. For example, ethane (CH3CH3), propane (CH3CH2CH3) and butane (CH3CH2CH2CH3).
An alkene is an unsaturated hydrocarbon with at least one carbon-carbon double bond.
Some examples are ethene (CH2=CH2), propene (CH3CH=CH2) and but-2-ene (CH3CH=CH—CH3).
Like alkenes, alkynes too, are unsaturated hydrocarbons, but they have at least one carbon-carbon triple bond. Some examples are ethyne (CH=CH), propyne (CH3C≡CH) and but-2-yne (CH3C=CCH3)
Cyclic Hydrocarbons
Hydrocarbons which contain closed chains or rings of carbon atoms are known as cyclic hydrocarbons. These can be of two types—alicyclic and aromatic.
Alicyclic hydrocarbons
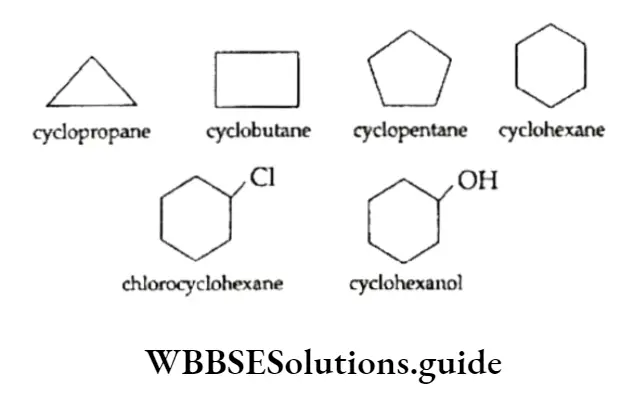
They contain a ring of three or more carbon atoms. They resemble aliphatic hydrocarbons in their properties and are of three types, viz., cycloalkanes, cycloalkenes and cycloalkanes.
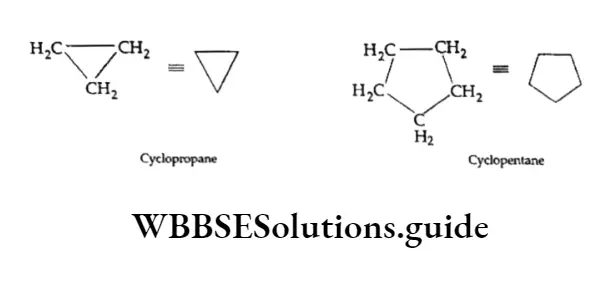
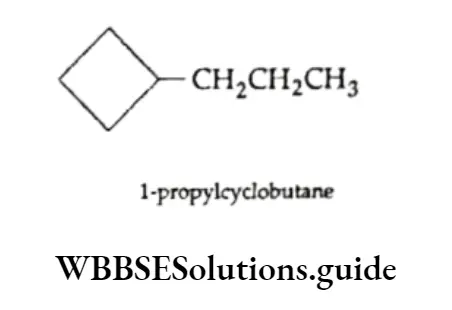
Cycloalkanes are saturated hydrocarbons in which all the carbon atoms are joined by single covalent bonds to form a ring. Cyclopropane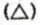 , cyclobutane
, cyclobutane , cyclopentane
, cyclopentane 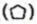 and cyclohexane
and cyclohexane 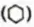 are a few examples.
are a few examples.
Cycloalkenes Unsaturated, alicyclic hydrocarbons containing at least one carbon-carbon double bond are known/ as cycloalkenes.
Some examples of Cyclopropane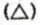 , cyclobutane
, cyclobutane , cyclopentane
, cyclopentane 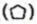 and cyclohexane
and cyclohexane 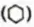
Cycloalkynes Unsaturated alicyclic hydrocarbons containing one carbon-carbon triple bond are called cycloalkynes.
Some examples are Cyclopropane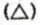 , cyclobutane
, cyclobutane , cyclopentane
, cyclopentane 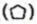 and cyclohexane
and cyclohexane 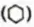 are unstable since they are highly strained.
are unstable since they are highly strained.
Aromatic Hydrocarbons
These are also called arenes. These compounds were obtained from natural resources like balsam and resins. They all had a pleasant odour and so, obtained their name from the Greek word aroma (a pleasing smell).
However, it was later found that not all aromatic compounds had a pleasant smell.
When subjected to various methods of treatment, the aromatic compounds finally produced benzene or its derivatives.
This led to the conclusion that aromatic compounds are hydrocarbons containing one or more benzene rings. These rings may be fused or isolated.
Arenes are also known as benzenoid compounds since their properties are similar to those of benzene. Some examples of aromatic hydrocarbons are as follows.
The classification discussed above may be represented as follows
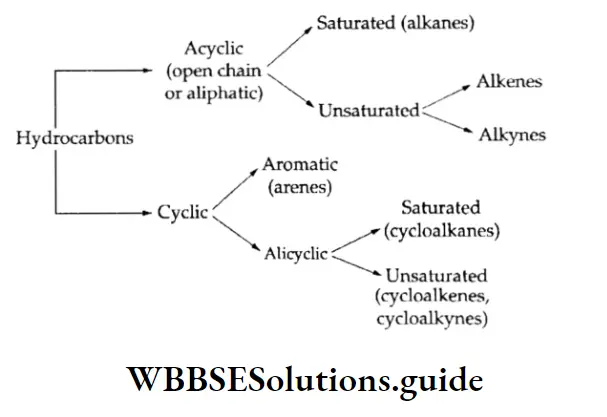
As stated earlier, hydrocarbons can also be classified as
- Saturated And
- Unsaturated.
Saturated hydrocarbons contain only carbon-carbon single bonds and include alkanes and cycloalkanes.
Unsaturated hydrocarbons contain at least one carbon-carbon double or triple bond. Alkenes, alkynes and arenes fall in this class.
Of these, alkenes and alkynes can be either acyclic or cyclic, while arenes are cyclic.
Here it should be made clear that both aromatic and alicyclic compounds are closed-chain structures but their properties are totally different.
This can be attributed to the benzene-based structure of compounds which contains at least six carbon atoms.
Also, the percentage of carbon is higher in aromatic compounds than in the corresponding aliphatic and alicyclic hydrocarbons. For example,
Alkanes
If we examine the molecular formulae of alkanes which, we will see that the successive members of the family differ constantly by —CH2.
In other words, if we replace one H atom in methane (CH4) with CH3, we get C2H6—the next higher member (ethane).
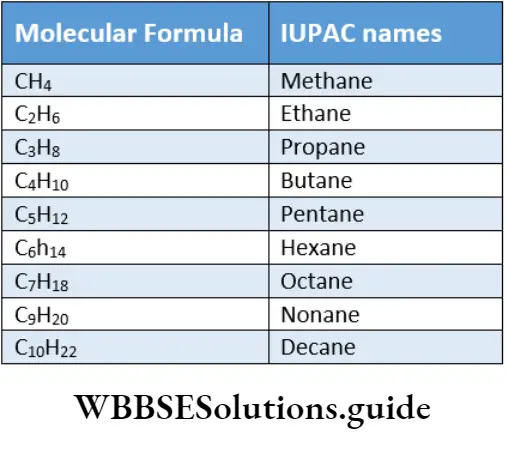
The alkanes, thus, form a homologous series with the general formula CnH2n+2, where n is the number of carbon atoms in the molecule.
A series of compounds in which each member differs from the next member by a constant number of carbon and hydrogen atoms is called a homologous series, and the members of the series are called homologues.
Hydrocarbons Conformations
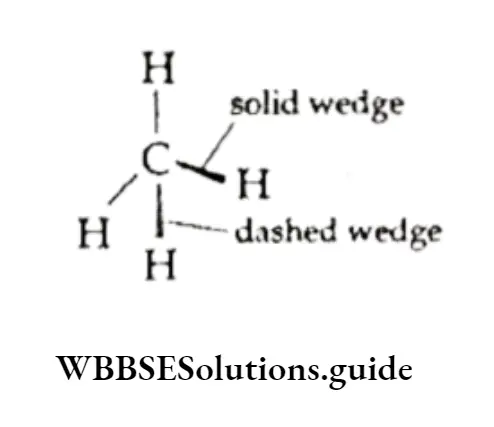
For the sake of convenience, the structures of hydrocarbons are represented in two-dimensional forms or plane-structural formulae as shown below. But you are aware of the fact that most organic molecules have three-dimensional structures.
An important aspect of the three-dimensional shape of molecules is their conformations.
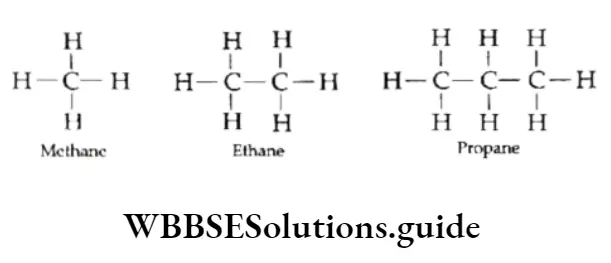
The above structural formulae do not show the spatial arrangement of atoms. This drawback can be overcome by representing the structures in their three-dimensional forms.
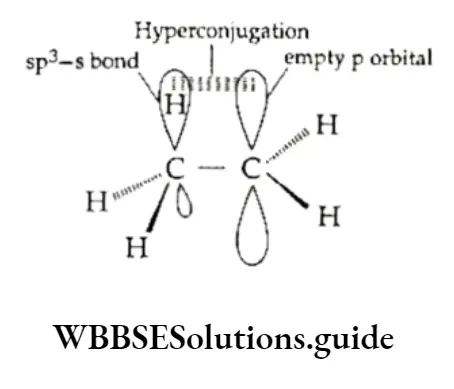
[These are discussed under conformations of alkanes. In hydrocarbons, the carbon-carbon single bond is a bond (formed by the overlapping of sp3 hybrid orbitals of carbon atoms) and has cylindrical symmetry.
This leads to the possibility of free rotation about the carbon-carbon single bond and hence, a large number of different spatial arrangements of atoms or groups of atoms attached to the carbon atoms.
These different spatial arrangements are called conformations. Thus, the large number of different arrangements of atoms in a molecule which result from the rotation of carbon-carbon single bonds are called conformers or rotational isomers.
Certain physical properties show that the rotation of carbon atoms around the C—C bond is not quite free. This is due to the difference in the energy of various arrangements. The rotation is hindered by an energy barrier which may vary from 1 to 20 kJ mol-1.
The difference in energy is caused due to the interaction between electron clouds of the carbon-hydrogen bonds. The repulsive interaction between electron clouds is referred to as torsional strain.
The energy required to rotate the molecule about the C—C bond is torsional energy. Torsional strain and torsional energy arise due to the steric hindrance that occurs whenever bulky portions of a molecule repel other molecules or other parts of the same molecule.
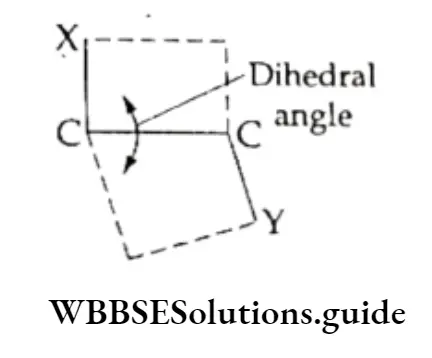
The angle of torsion is a dihedral angle between two planes. Actually, the dihedral angle is the one formed by the intersection of two planes. Conformational isomers or conformers are rapidly interconvertible and nonseparable.
X The energy for this interconversion or rotational motion is acquired from the collisions between the molecules and with the walls of the container.
Remember that no bonds are C broken or made during rotational motion or change of conformation, so there is only one compound. The phenomenon of conformation can be best understood by considering the case of ethane.
In the ethane molecule, the two carbon atoms are joined by a bond. If one of the carbon atoms (methyl group) is fixed and the other is rotated about the C —C bond, a large number of arrangements of the hydrogen atoms attached to one carbon atom with respect to the hydrogen atoms of the second carbon atom are possible.
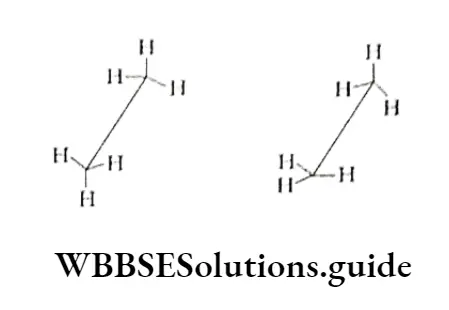
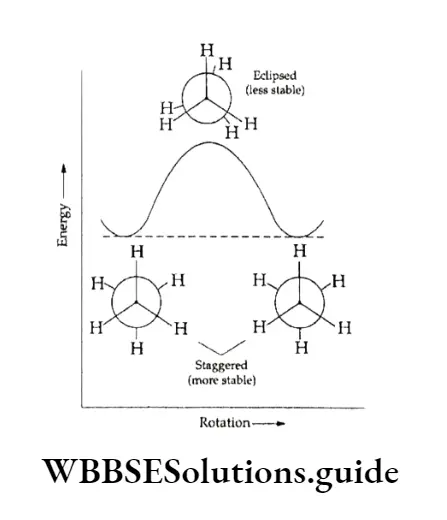
Out of the several arrangements (conformations) possible, only the following two are important. Staggered conformation In this the hydrogen atoms bonded to the two carbon atoms arc staggered with respect to one another.
This implies that the hydrogen atoms of the two methyl groups are a maximum distance apart, making the repulsion between them the minimum possible. Therefore, the conformation is of the lowest potential energy for ethane.
Eclipsed conformation In this the hydrogen atoms bonded to one carbon atom are directly behind those connected to the other, leading to the maximum possible repulsion (maximum energy) between the sets of hydrogen atoms.
Of the two conformations, the staggered conformation is more stable because there is less repulsion between the hydrogen atoms.
All other possibilities between staggered and eclipsed forms are known as skew or gauche forms. Hence the order of stability is staggered > skew > eclipsed.
Conformers are mostly represented in one of two ways. One is called sawhorse projection and the other, is Newman projection.
Sawhorse projection In this method of projecting the three-dimensional structure on paper, the molecule is viewed from above.
The C —C bond is drawn diagonally. The carbon atom shown lower is the one in front and the one drawn above it and to the right represents the carbon atom at the back.
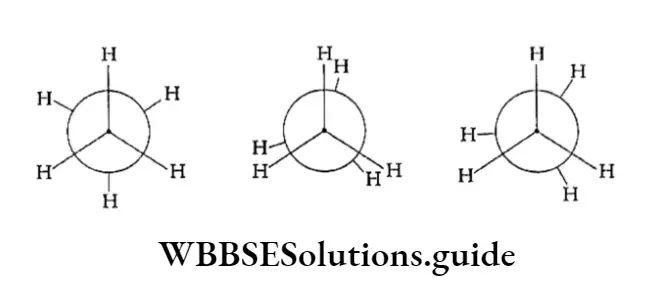
Newman projection Named after M. S. Newman, who first proposed this method of representing the three-dimensional structure on paper, this is easier to visualise than the one described before.
The projection is obtained by viewing the molecule along the C —C bond. The carbon atom closer to the viewer is represented by a circle. The other atom lies behind this and so cannot be seen.
Tire bonds between the first carbon atom and the hydrogen atoms attached to it are shown as equally spaced radial lines from the centre of the circle.
The bonds of the carbon atom which is at the back are shown as lines drawn from the circumference of the circle.
The energy difference between the staggered and eclipsed conformations is about 12.5 kJ mol-1.
This energy difference is not large enough to prevent rotation about the CT bond.
In fact, even at room temperature, the two conformations keep changing from one to the other and it is impossible to isolate either. The variation of energy with rotation about the C —C bond.
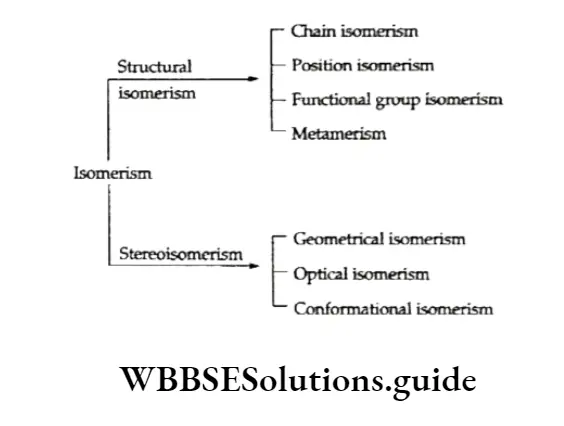
Isomerism
There can be only one way in which the carbon and hydrogen atoms combine in the first three members of the alkane homologous series.
But when the carbons and hydrogens in higher members combine, more than one structure can result.
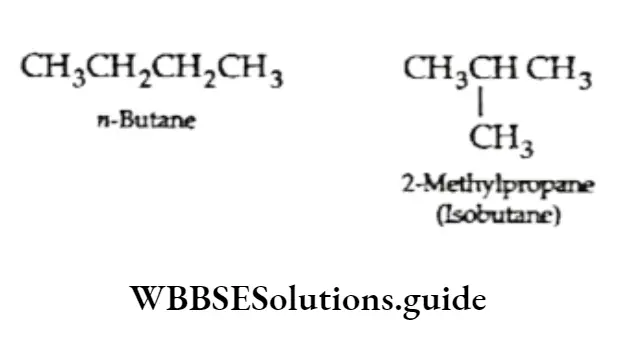
For example, in butane (C4H10) the carbon atoms can be joined either in a continuous chain or in a branched chain.
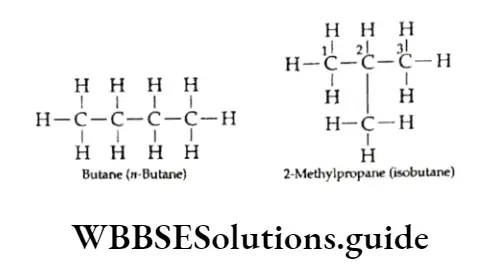
Since the two butanes have the same molecular formula but different structures, they exhibit structural isomerism.
u-Butane has a continuous-chain structure and isobutane, has a branched-chain structure. Isomers which differ in the way their carbon chains are arranged are known as chain isomers.
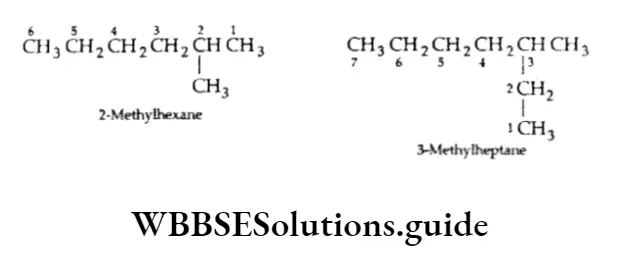
Now consider the next homologue, pentane (C5H12). The carbon chain in this molecule can be arranged in three different ways.
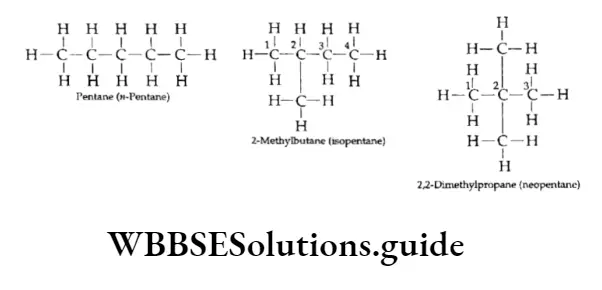
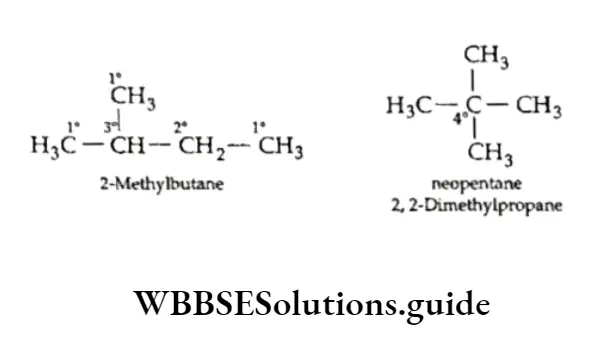
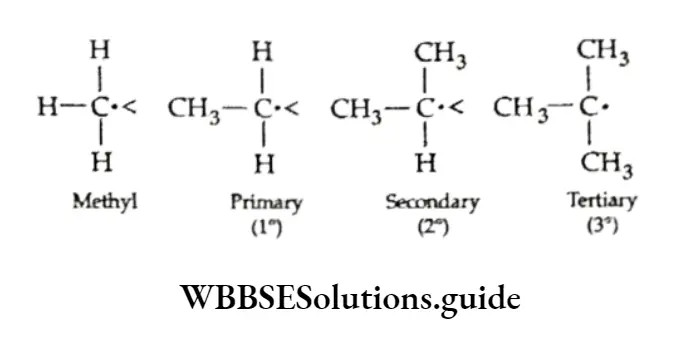
As you can see, all three structures are chain isomers of pentane. We have already discussed the classification of carbon atoms as primary, secondary, tertiary and quaternary in a molecule. Can you identify the 1°, 2°, 3° and 4° carbon atoms in the above molecular structures?
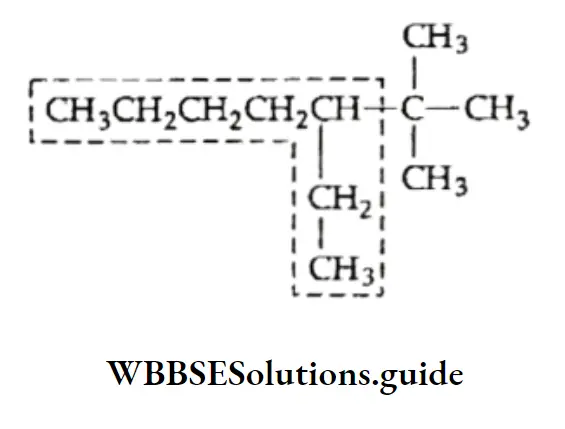
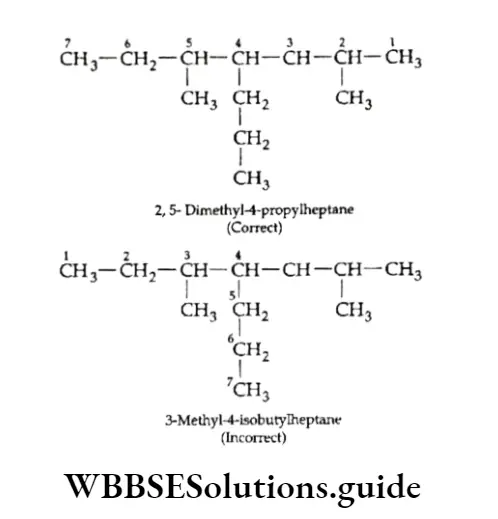
Example An alkane with the molecular formula C8H18 can have the following structures. Identify which of them represents the same molecule and which is the isomer.
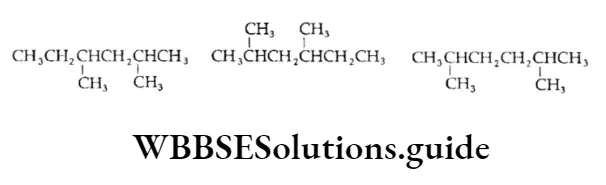
Solution:
Let us first write the IUPAC names for all three structures.
- 2,4-Dimethylhexane
- 2,4-Dimethylhexane
- 2,5-Dimethylhexane
Thus, the IUPAC names suggest that structures represent the same molecule and are the isomers of and.
It is important to note that different isomers are different chemical compounds, and so they have different chemical and physical properties. For example, the b.p. of n-butane is 273 K whereas that of isobutane is 261 K.
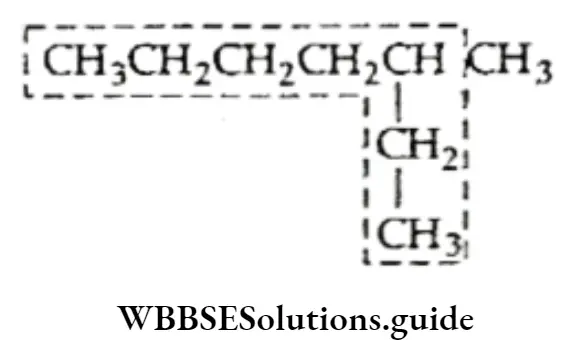
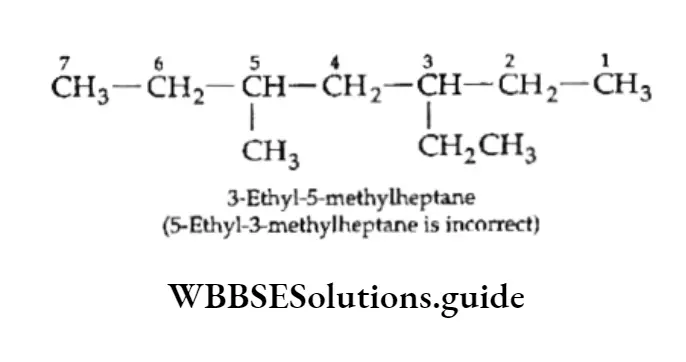
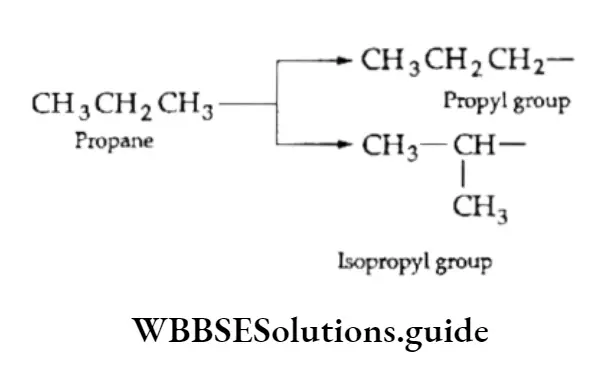
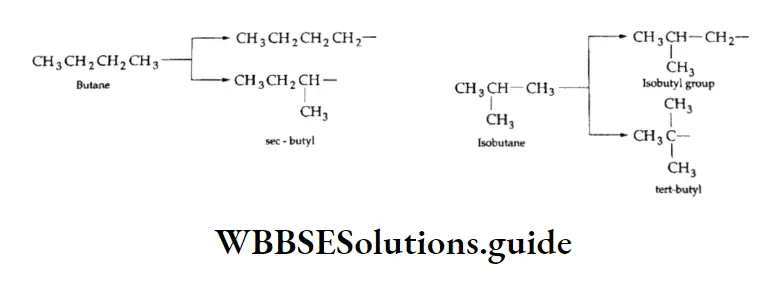
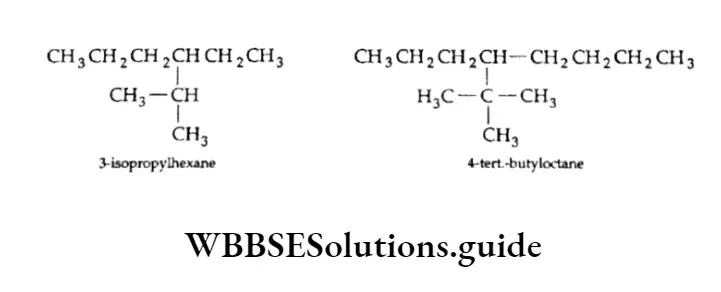
We have already discussed the alkyl groups that are attached to carbon atoms in alkanes or other classes of compounds. These substituent alkyl groups have the general formula CnH2n+1.
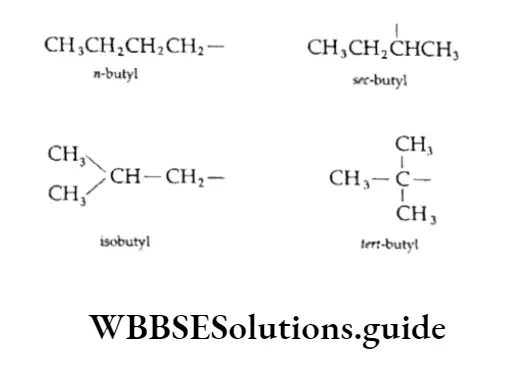
Depending upon which carbon atom (of the alkyl group) is attached to the other group, the alkyl groups can be straight-chain or branched-chain. In other words, different types of alkyl groups result, depending upon which hydrogen is removed from the parent alkane.
For Example, there are four different kinds of butyl groups. Two (n-butyl and sec-butyl) are derived from the l straight-drain butane and two (isobutyl and ferf-butyl) are derived from the branched-chain butane.
The alkyl groups themselves are not stable compounds and are simply parts of molecules that help us name compounds.
The prefixes sec- and tert- refer to the kind of carbon (2° and 3° respectively) of the alkyl group which gets attached to the other group. We have already discussed the nomenclature of alkanes and other hydrocarbons.
The examples given in this chapter in the subsequent sections will help you to further understand the IUPAC 2 nomenclature.
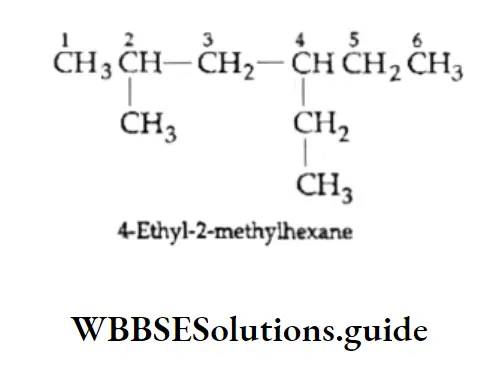
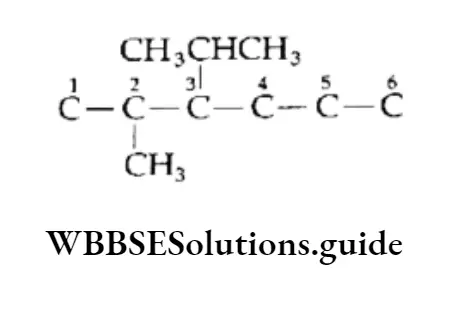
Example Write the structure of 3-lsopropyl-2-methylhexane.
Solution:
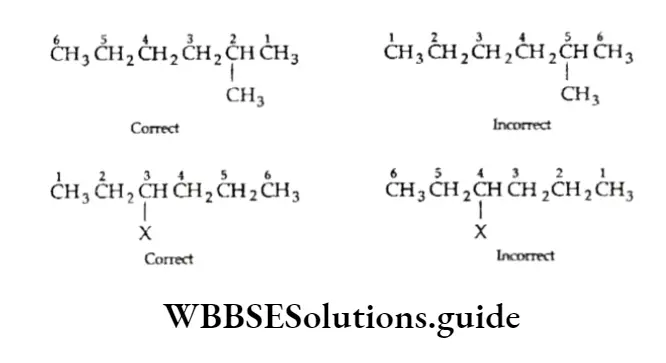
1. Draw the carbon chain of the parent alkane (hexane) and number the carbon atoms.
⇒ \(\stackrel{1}{\mathrm{C}}-\stackrel{2}{\mathrm{C}}-\stackrel{3}{\mathrm{C}}-\stackrel{4}{\mathrm{C}}-\stackrel{5}{\mathrm{C}}-\stackrel{6}{\mathrm{C}}\)
2. Attach isopropyl at C-3 and a methyl group at C-2
3. Add the requisite number of hydrogen atoms to each carbon of the parent chain.
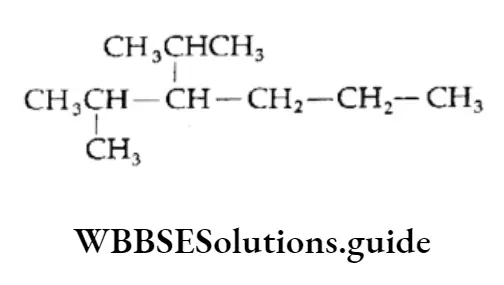
Thus, we get the correct structure of 3-isopropyl-2-methylhexane.
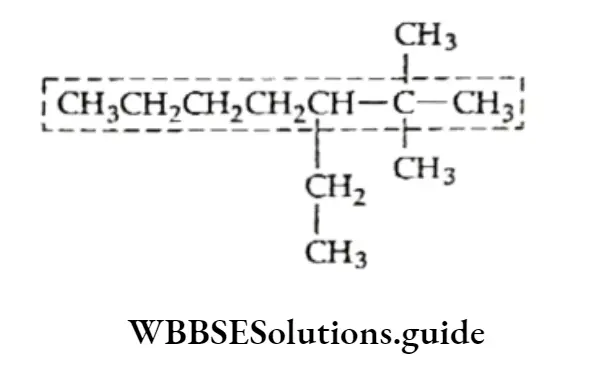
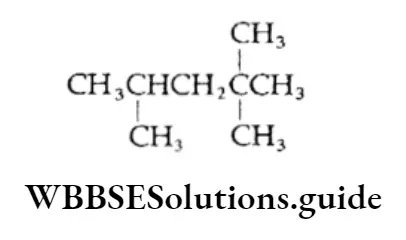
Example Draw the structural formula of the following compounds:
- 3,3-Diethyl-5-isopropyl-4-methyl octane
- 2,2,4-Trimethylpentane
Solution:
1. Draw the carbon chain of the parent alkane (octane) and number the carbon atoms in it
⇒ \(\underset{1}{\mathrm{C}}-\mathrm{C}_2-\mathrm{C}_3-\mathrm{C}_4-\mathrm{C}_5-\underset{6}{\mathrm{C}}-\underset{7}{\mathrm{C}}-{ }_8^{\mathrm{C}}\)
Attach two ethyl groups at C-3, one isopropyl at C-5 and one methyl group at C-4.
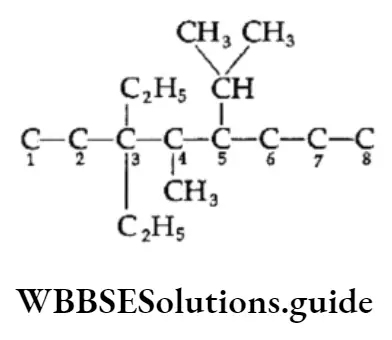
Add the requisite number of hydrogen atoms to each carbon of the parent chain.
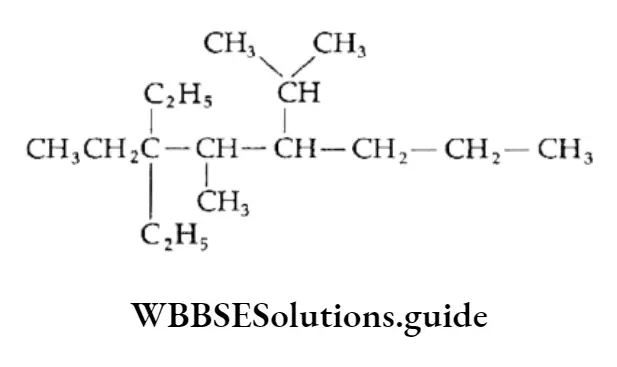
This is the structure of 3,3-Diethyl-5-isopropyl-4-methyloctane.
In this case, the parent alkane is pentane with three substituent methyl groups attached.
Thus, the structure of the compound will be:
Preparation
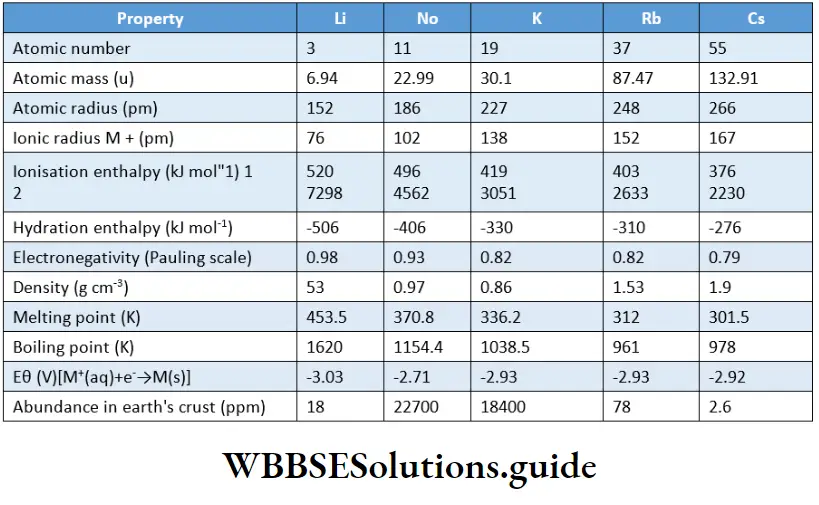
The main source of alkanes is petroleum, together with the accompanying natural gas.
Natural gas, of course, contains low molecular weight (volatile) alkanes, i.e., 80 per cent of methane and 10 per cent of ethane, the remaining 10 per cent being a mixture of next higher homologues.
Crude petroleum, on the other hand, is a mixture of higher alkanes. Alkanes can be prepared by the following methods.
From Unsaturated Hydrocarbons
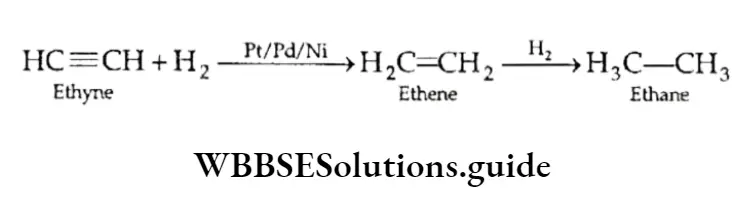
Alkanes are obtained by passing a mixture of unsaturated hydrocarbons (e.g., alkenes and alkynes) and hydrogen over a catalyst.
The catalyst is a finely divided metal, usually platinum, palladium or nickel.
A relatively higher temperature and pressure are required with nickel. The catalyst causes the addition of molecular hydrogen to the unsaturated bond.
⇒ \(\underset{\text { Ethene }}{\mathrm{CH}_2=\mathrm{CH}_2}+\mathrm{H}_2 \stackrel{\mathrm{Pt} / \mathrm{Pd} / \mathrm{Ni}}{\longrightarrow} \underset{\text { Ethane }}{\mathrm{CH}_3-\mathrm{CH}_3}\)
⇒ \(\underset{\text { Propene }}{\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2}+\mathrm{H}_2 \stackrel{\mathrm{Pt} / \mathrm{Pd} / \mathrm{Ni}}{\longrightarrow} \underset{\text { Propane }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3}\)
⇒ \(\underset{\text { Propyne }}{\mathrm{CH}_3 \mathrm{C} \equiv \mathrm{CH}}+2 \mathrm{H}_2 \stackrel{\mathrm{Pt} / \mathrm{Pd} / \mathrm{Ni}}{\longrightarrow} \underset{\text { Propane }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3}\)
From alkyl halides
Reduction of alkyl halides (except fluorides) with zinc and dilute acid (viz., hydrochloric acid) leads to the replacement of a halogen atom by a hydrogen atom.
Alkyl halides, on treatment with sodium metal in dry ether, give higher, symmetrical alkanes
⇒ \(\underset{\text { Bromomethane }}{\mathrm{CH}_3 \mathrm{Br}}+2 \mathrm{Na}+\mathrm{CH}_3 \mathrm{Br} \stackrel{\text { dryether }}{\longrightarrow} \underset{\text { Ethane }}{\mathrm{CH}_3-\mathrm{CH}_3+2 \mathrm{NaBr}}\)
This reaction is called the Wurtz reaction. If two different alkyl halides are used in this reaction (e.g., CH3Br and C2H5Br), a mixture of alkanes (CH3CH3C2H5C2H5, CH3C2H5) is obtained.
Decarboxylation of carboxylic acids
Sodium salts of carboxylic acids, on being heated strongly with soda lime (NaOH + CaO), give alkanes.
Since the carbon dioxide is removed, the corresponding alkane obtained has one carbon atom less than the carboxylate salt.
⇒ \(\underset{\text { Sodium acetate }}{\mathrm{CH}_3 \mathrm{COONa}}+\mathrm{NaOH} \underset{\text { heat }}{\stackrel{\mathrm{CaO}}{\longrightarrow}} \mathrm{CH}_4+\mathrm{Na}_2 \mathrm{CO}_3\)
Such a reaction in which carbon dioxide is eliminated from a carboxylic group is called decarboxylation.
Kolbe’s electrolytic method
This method involves the electrolysis of an aqueous solution of the sodium or potassium salt of a carboxylic acid.
A higher alkane containing an even number of carbon atoms is obtained.
⇒ \(\underset{\text { Sodium acrtate }}{2 \mathrm{CH}_3 \mathrm{COONa}}+2 \mathrm{H}_2 \mathrm{O} \stackrel{\text { dectrolysis }}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_3+2 \mathrm{CO}_2+2 \mathrm{NaOH}+\mathrm{H}_2\)
The reaction proceeds as follows.
⇒ \(2 \mathrm{CH}_3 \mathrm{COO} \mathrm{Na}^{+} \stackrel{\text { electrolysis }}{\rightleftharpoons} 2 \mathrm{CH}_3-\stackrel{\mathrm{O}}{\rightleftharpoons}-\overline{\mathrm{O}}+2 \mathrm{Na}^{+}\)
At anode:
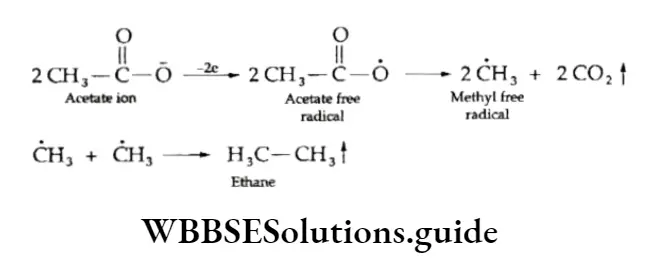
At cathode:
⇒ \(2 \mathrm{Na}^{+} \stackrel{+2 \mathrm{e}}{\longrightarrow} 2 \mathrm{Na} \stackrel{2 \mathrm{H}_2 \mathrm{O}}{\longrightarrow} 2 \mathrm{NaOH}+\mathrm{H}_2 \uparrow\)
Since the two free radicals produced as a result of the decarboxylation of the acetate radical combine to form alkanes, methane, which contains only one carbon atom, cannot be prepared by this method.
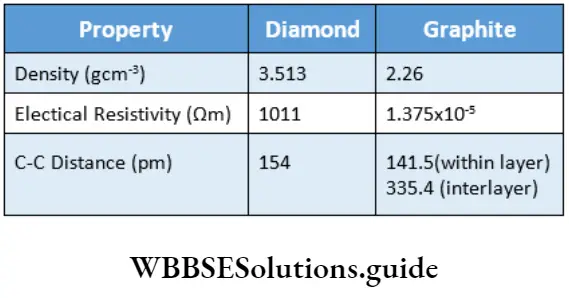
Physical Properties
Alkanes, being covalent in nature, are held together by weak van der Waals forces. The intermolecular forces are strong in the large molecules.
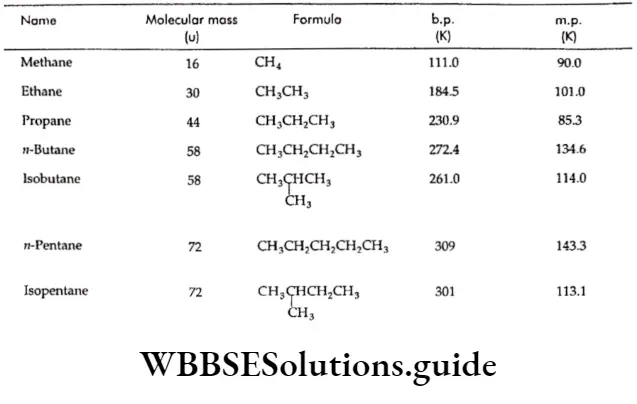
Thus, the first four members of the alkane homologous series are colourless gases, and the next thirteen members from C-5 to C-17 are colourless liquids. The higher members, from C-18 onwards, are waxy solids.
The alkane molecule is nonpolar or very weakly polar due to the very low electronegativity difference between carbon and the hydrogen atom.
Being nonpolar, alkanes are insoluble in water, which is polar. However, they are soluble in nonpolar solvents like carbon tetrachloride and benzene.
The boiling point of n-alkanes increases with an increase in molecular weight.
In the case of alkanes which exist in straight-chain as well as branched-chain forms, the branched-chain isomer has a lower boiling point than the corresponding straight-chain isomer.
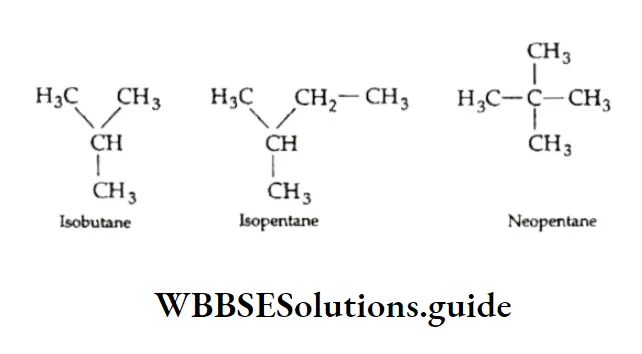
Thus, n-butane has a higher boiling point than that of isobutane. The boiling point is still lower if the branching is more.
Thus, neopentane boils at a temperature lower than the boiling point of isopentane.
Why does the increase in branching of the carbon chain lead to the lowering of the boiling point of the alkane?
Because branching in a carbon chain reduces the surface area of the molecule and therefore brings down intermolecular attractive forces, resulting in a decrease in boiling point.
The melting points of alkanes also increase with the increase in molecular weight but the increase is not so regular.
This is because the intermolecular forces in a crystal depend not only on the size of the molecule but also on how it fits within a crystal lattice.
However, alkanes with an even number of carbon atoms have a higher melting point than those with an odd number of carbon atoms.
This is largely due to the better crystal lattice packing of the alkanes with an even number of carbon atoms.
Chemical Properties
Alkanes are inert in nature and do not undergo any chemical reactions under ordinary conditions with adds, alkalis, oxidising and reducing agents.
However, under certain conditions, alkanes undergo substitution reactions and some others like halogenation, combustion and isomerisation.
The nonreactivity of alkanes with ionic reagents can be attributed to the nonpolar nature of alkanes. We will now discuss some of the reactions of alkanes.
Halogenation
Alkanes react with halogens at 523-673 K or in the presence of sunlight to form halogen-substituted alkanes and hydrogen halide.
Reactions in which the hydrogen atoms of alkanes are substituted by other groups are known as substitution reactions.
The higher alkanes, apart from halogenation, also undergo nitration and sulphonation substitution reactions.
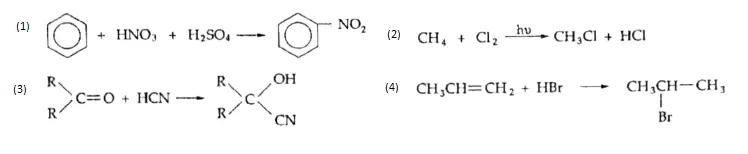
Methane reacts with chlorine to form chloromethane, which, on further reaction with chlorine, forms dichloromethane.
⇒ \(\mathrm{CH}_4+\mathrm{Cl}_2 \longrightarrow \underset{\text { Chloromethane }}{\mathrm{CH}_3 \mathrm{Cl}}+\mathrm{HCl}\)
⇒ \(\mathrm{CH}_3 \mathrm{Cl}+\mathrm{Cl}_2 \longrightarrow \underset{\text { Dichloromethane }}{\mathrm{CH}_2 \mathrm{Cl}_2}+\mathrm{HCl}\)
When the chlorination is continued further, trichloromethane (chloroform) and tetrachloromethane (carbon tetrachloride) are formed.
⇒ \(\mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{Cl}_2 \longrightarrow \underset{{\text { Trichloromethane } \\ \text { (chloroform) }}}{\mathrm{CHCl}_3}+\mathrm{HCl}\)
⇒ \(\mathrm{CHCl}_3+\mathrm{Cl}_2 \longrightarrow \underset{{\text { Tetrachlomomethane } \\ \text { (earbon tetrachloride) }}}{\mathrm{CCl}_4}+\mathrm{HCl}\)
Thus, the chlorination of methane gives a mixture of products. The composition of the product mixture depends on the ratio in which the reactants (methane and chlorine) are used.
If chlorine is used in excess then the percentage of carbon tetrachloride obtained is more. The mixture obtained can be separated into its constituents by fractional distillation.
The chlorination of methane is an example of a chain reaction, a reaction that involves a series of steps. Each step in the chain reaction generates a reactive substance that brings about the next step.
The reactivity of bromine with alkanes is less than that of chlorine. Unlike chlorination and bromination of alkanes, the iodination of alkanes is a reversible process.
However, the reaction can be made to proceed in one direction in the presence of an oxidising agent, which decomposes the hydrogen iodide produced.
⇒ \(\mathrm{CH}_4+\mathrm{I}_2 \rightleftharpoons \mathrm{CH}_3 \mathrm{I}+\mathrm{HI}\)
⇒ \(5 \mathrm{HI}+\underset{\text { lodic acid }}{\mathrm{HIO}_3} \longrightarrow 3 \mathrm{I}_2+3 \mathrm{H}_2 \mathrm{O}\)
The fluorination of alkanes is explosive and even in the dark the reaction needs to be controlled by mixing the reactants in an inert solvent.
The reactivity of alkanes with halogens is of the order: F2 > Cl2 > Br2 > I2. The ease of abstraction of hydrogen atoms follows the sequence 3°> 2°> 1°> CH3 —H.
Mechanism Let us discuss the mechanism of the chlorination of methane as an example.
The halogenation of other alkanes proceeds according to the same mechanism as that of methane.
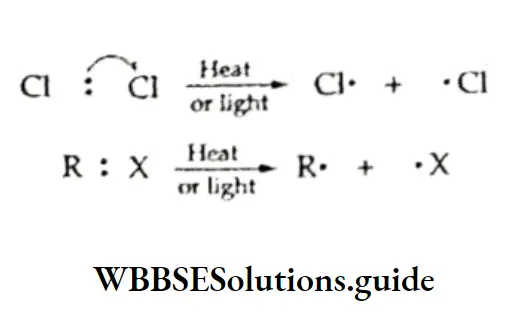
The chlorination of methane is believed to proceed via a free-radical mechanism involving three steps—initiation, propagation and termination.
Initiation The reaction is initiated by the homolysis of the chlorine molecule to form free radicals. The Cl—Cl bond is weaker than the C —C and C—H bonds and breaks up easily in the presence of sunlight or heat.
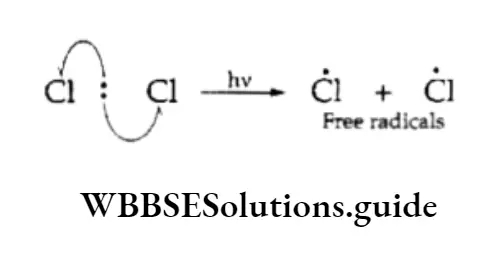
Propagation The chlorine free radical attacks the C—H bond of the methane molecule to generate a methyl radical along with the formation of a molecule of hydrogen chloride.
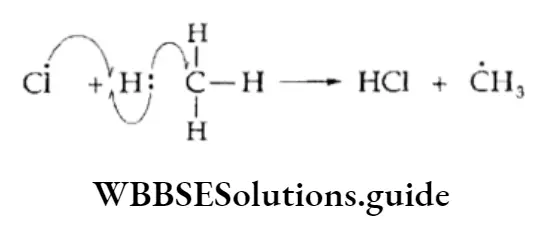
The methyl free radical thus produced attacks the second molecule of chlorine to yield methyl chloride and another chlorine free radical.
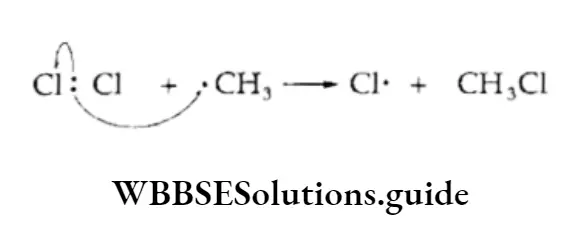
Each of the two chain-propagation steps 1 and 2 consumes a reactive substance and generates another.
Termination Finally, when the chain reaction terminates, the reactive intermediates are not generated but consumed.
The two reactive intermediates like two chlorine radicals or two methyl radicals or one methyl and one chlorine radical combine.
⇒ \(\begin{aligned}
& \mathrm{Cl} \cdot+\mathrm{Cl} \cdot \longrightarrow \mathrm{Cl}_2 \\
& \mathrm{CH}_3+\mathrm{CH}_3+\mathrm{CH}_3 \mathrm{CH}_3 \\
& \mathrm{CH}_3+\mathrm{Cl} \cdot \longrightarrow \mathrm{CH}_3 \mathrm{Cl}
\end{aligned}\)
Oxidation
Combustion On being heated, alkanes completely oxidise in the presence of air or oxygen, producing C02, H2O and a large amount of heat.
⇒ \(\mathrm{CH}_4(\mathrm{~g})+(\text { excess }) 2 \mathrm{O}_2(\mathrm{~g}) \longrightarrow \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) ; \Delta_c \mathrm{H}^{\ominus}=-890 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
The more the molecular weight of the alkane, the more is the heat produced on combustion. Alkanes (methane, ethane, propane, butane, etc.) are used as fuel, e.g., LPG.
In an insufficient amount of air, alkanes undergo incomplete combustion, producing carbon in a very finely divided state —carbon black.
Carbon black is used to make paints and printers’ ink. It is also used in the rubber industry to make tyres.
⇒ \(\mathrm{CH}_4(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \longrightarrow \mathrm{C}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
Controlled oxidation The controlled oxidation, i.e., oxidation in a regulated supply of air at high temperature and pressure, under various conditions, of alkanes leads to different products. Some examples are as follows.
⇒ \(2 \mathrm{CH}_4+\mathrm{O}_2 \underset{\text { heat }}{\stackrel{\mathrm{Cu} .523 \mathrm{~K}, 1000 \mathrm{~atm}}{\longrightarrow}} \underset{\text { Methanol }}{2 \mathrm{CH}_3 \mathrm{OH}}\)
⇒ \(\mathrm{CH}_4+\mathrm{O}_2 \underset{\text { heat }}{\stackrel{\mathrm{Mo}_2 \mathrm{O}_3}{\longrightarrow}} \underset{\text { Methanal }}{\mathrm{HCHO}}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{CH}_3 \mathrm{CH}_3+3 \mathrm{O}_2 \stackrel{\left(\mathrm{CH}_3 \mathrm{COO}\right)_2 \mathrm{Mn}}{\longrightarrow} \underset{\text { heat }}{2} \underset{\text { Ethanoic adid }}{2 \mathrm{CH}_3 \mathrm{COOH}}+2 \mathrm{H}_2 \mathrm{O}\)
Normally, alkanes are resistant to strong oxidising agents but alkanes with a tertiary hydrogen atom can be oxidised to the corresponding alcohol by potassium permanganate.
⇒ \(\underset{\text { 2-Methylpropane }}{\left(\mathrm{CH}_3\right)_3 \mathrm{CH}}+[\mathrm{O}] \stackrel{\mathrm{KMnO}_4}{\longrightarrow} \underset{\text { 2-Methyl-propan-2-ol }}{\left(\mathrm{CH}_3\right)_3 \mathrm{COH}}\)
Isomerisation On heating in the presence of aluminium chloride and hydrogen chloride, normal alkanes get converted to their corresponding branched-chain isomers. For example,
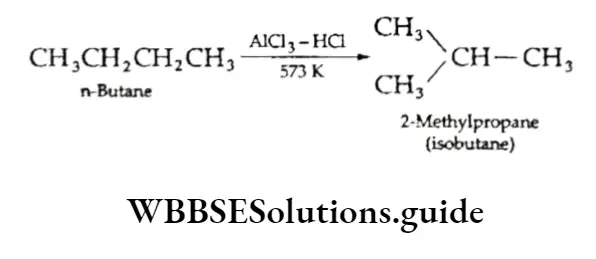
The process is used to increase the branched-chain content of lower alkanes produced by cracking since branched-chain isomers in petrol are more valuable than n-alkanes.
Aromatisation Alkanes containing six to ten carbon atoms can be converted into benzene and its homologues at a high temperature in the presence of a catalyst.
The process is known as aromatisation and takes place through the simultaneous dehydrogenation and cyclisation of the alkane to give an aromatic compound containing a number of carbon atoms.
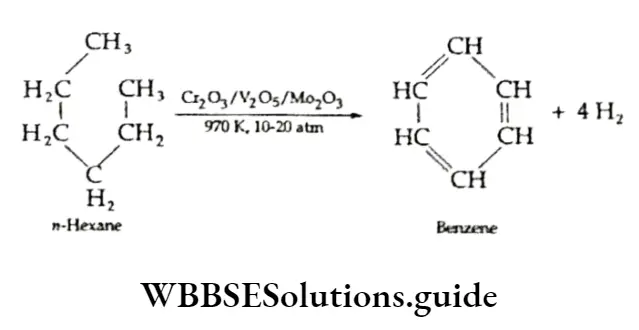
Reaction with steam Methane reacts with steam at 1273 K in the presence of nickel, which acts as a catalyst, to give carbon monoxide and dihydrogen. This mixture of carbon monoxide and hydrogen is known as water gas.
⇒ \(\mathrm{CH}_4+\mathrm{H}_2 \mathrm{O} \underset{\text { heat }}{\stackrel{\mathrm{Ni}}{\longrightarrow}} \mathrm{CO}+3 \mathrm{H}_2\)
This procedure is used for the industrial preparation of dihydrogen
Pyrolysis (Cracking)
When heated to high temperatures (773 K-873 K), alkanes decompose into smaller molecules.
This is known as cracking. The thermal decomposition of organic compounds is known as pyrolysis.
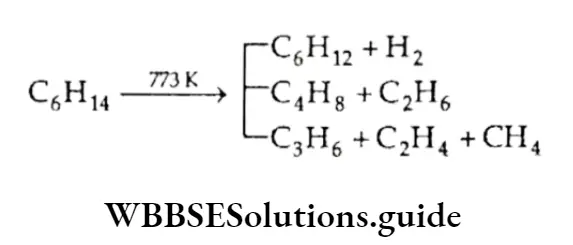
The products obtained from the cracking of alkanes depend on the structure of the alkane, pressure conditions and the catalyst present.
For example, dodecane, a constituent of kerosene oil, when heated to 973 K, in the presence of catalysts platinum palladium or nickel, gives a mixture of products (mainly heptane and pentene)
⇒ \(\underset{\text { Dodecane }}{\mathrm{C}_{12} \mathrm{H}_{26}} \underset{\mathrm{P} / \mathrm{Pd} / \mathrm{Ni}}{\stackrel{973 \mathrm{~K}}{\longrightarrow}} \mathrm{C}_7 \mathrm{H}_{16}+\mathrm{C}_5 \mathrm{H}_{10}+\text { other products }\)
Alkenes
Alkenes are unsaturated hydrocarbons with at least one carbon-carbon double bond. Their general formula is CnH2n. They are also called olefins (Greek: oil forming) since the lower members of this series produce oily products in reaction with halogens.
Structure Of Double Bond
The presence of the double bond in alkenes gives rise to certain special structural features which can be better understood if we consider the structure of the simplest alkene, ethene (ethylene).
Each carbon atom in ethene is sp2 hybridised, which means that each carbon atom has three sp2 orbitals lying in the same plane and inclined to one another at an angle of 120°, and an unhybridised 2p2 orbital which is perpendicular to the plane of the sp2 orbitals.
Two of the three sp2 hybrid orbitals of each carbon atom overlap with the Is orbitals of two hydrogen atoms (forming C—H bonds).
The remaining sp2 orbital of each carbon atom overlaps along the internuclear axis to form a (C —C) bond.
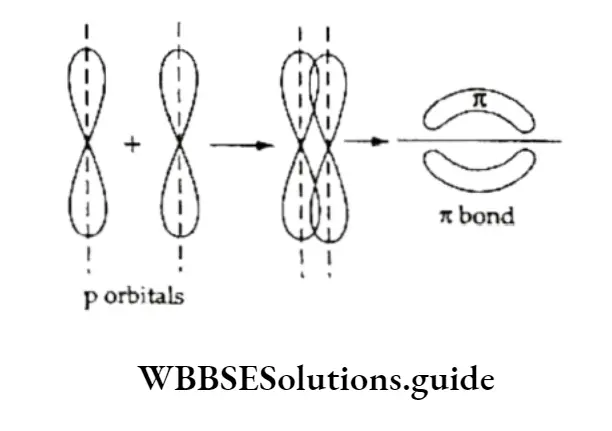
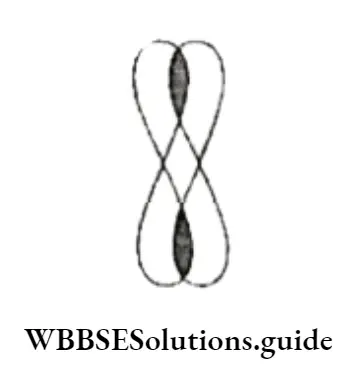
The unhybridised orbitals of the two carbon atoms (dash lines), which are perpendicular to the plane of the sp2 orbitals, overlap sideways to form a π bond.
Thus, the C—C double bond consists of an o bond and an π bond. The n bond is weaker and makes alkenes reactive.
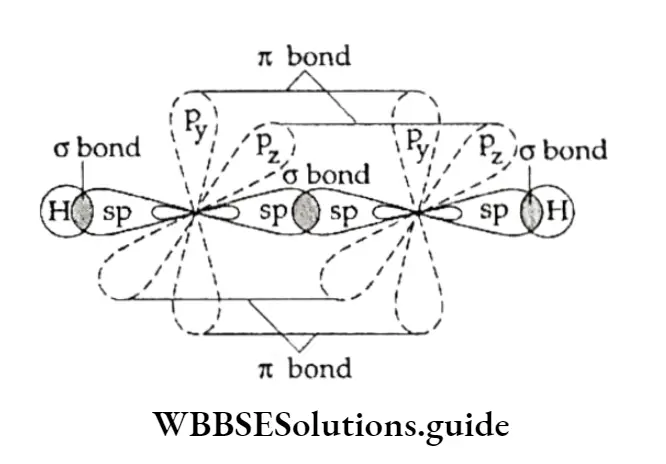
What makes TC a weaker bond? The n electrons are less involved in holding together the two carbon nuclei than the CT electrons are.
They form a cloud of π electrons above and below the plane of the atoms
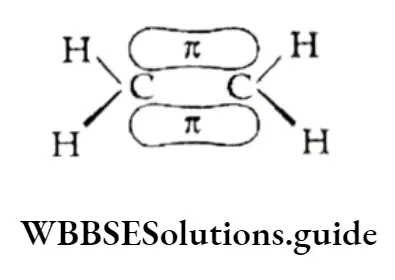
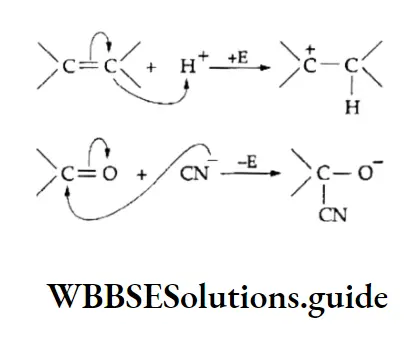
During a reaction, these loosely held n electrons are particularly available to a reagent that is seeking electrons.
The electron-deficient reagents or compounds which are seeking a pair of electrons are called electrophilic reagents.
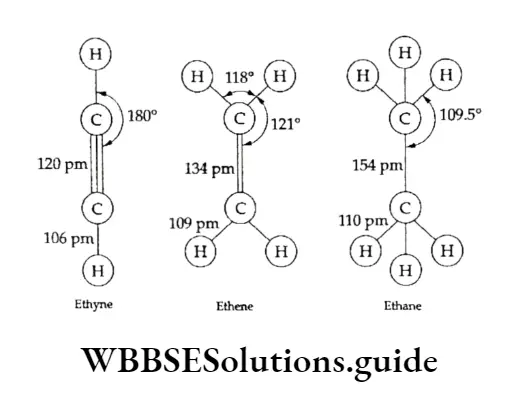
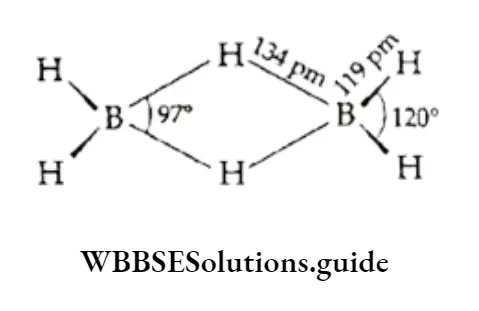
Electrophilic addition is the typical reaction of an alkene. The C —C bond length in ethene is 134 pm, which is less than that in ethane (154 pm). The C—C bond length in ethyne is still shorter (120 pm).
However the bond enthalpy of C=C (ethene) is greater (678 kJ mol-1) than that of C—C (ethane), which is 370 kJ mol-1.
Hence, the alkene molecule is more reactive than its corresponding alkane molecule, due to the presence of the double bond.
Example Calculate the number of it bonds in the following structures and write their IUPAC names
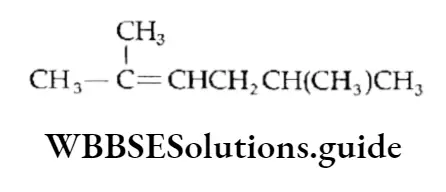
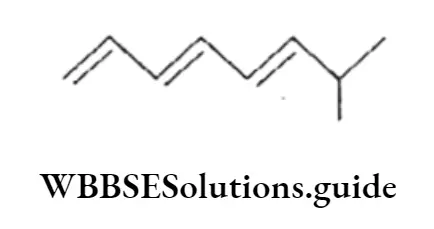
Solution:
1. The structure can be drawn as:
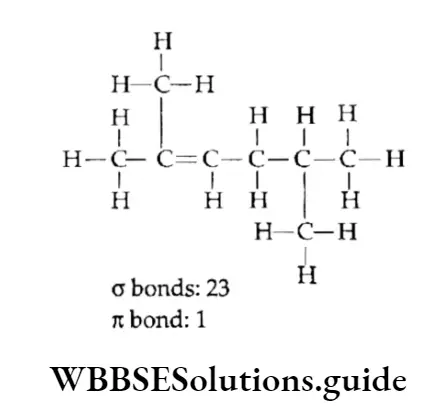
The IUPAC name is 2,5-Dimethylhex-2-ene.
2. The structure can be drawn as:
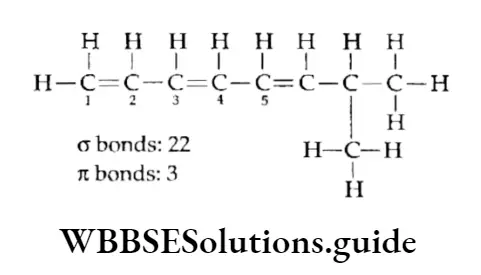
The IUPAC name of the structure is 7-Methyl-l,3,5-octatriene.
Isomerism
Alkenes normally show the following types of isomerism.
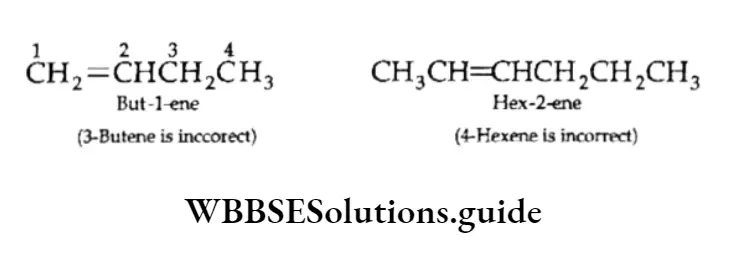
Position isomerism The first two members of the alkene series (viz., ethene, propene) do not show isomerism.
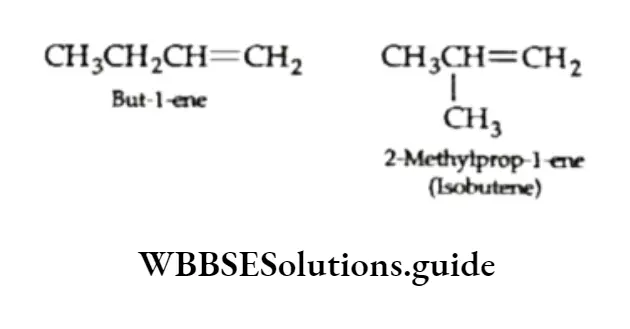
However, butene exhibits position isomerism as but-l-ene and but-2-ene. They differ in the position of the double bond.
⇒ \(\mathrm{H}_3 \mathrm{CCH}_2 \mathrm{CH}=\mathrm{CH}_2
But-1ene\)
⇒ \(\mathrm{H}_3 \mathrm{CCH}=\mathrm{CHCH}_3
But-2-ne\)
Chain isomerism Butene also shows chain isomerism as isobutene has a branched-chain structure and n-butene has a straight-chain structure
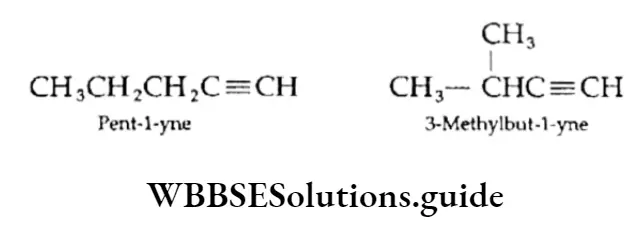
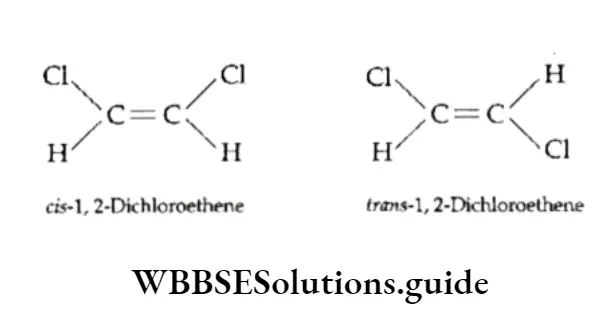
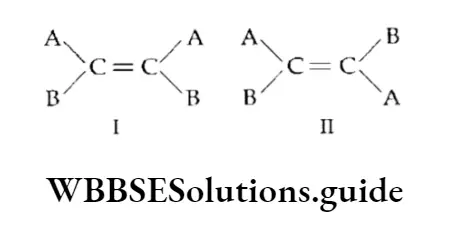
Geometrical isomerism Compounds which have the same structural formula, but in which atoms or groups have a different spatial arrangement about a double bond are called geometrical isomers and the phenomenon is known as geometrical isomerism.
Geometrical isomerism is exhibited by alkenes and their derivatives in which the double-bonded carbon atoms are linked to two different groups. In other words, all compounds containing a carbon-carbon double bond do not exhibit geometrical isomerism.
An alkene molecule exhibits geometrical isomerism only when the atoms or groups attached to each double-bonded carbon atom are different.
That is to say, geometrical isomerism is exhibited by molecules of the kind ABC=CAB or of the kind ABC=CDE but not of the kind AAC=CDE or of the kind ABC=CDD.
You have already studied the infinite number of conformational isomers which arise due to the rotation of carbon atoms connected through bonds in alkanes.
However, this is not the case in alkenes which have a double bond containing a cr bond and π bond. Rotation is possible in the case of a bond but not at all in that of a n bond.
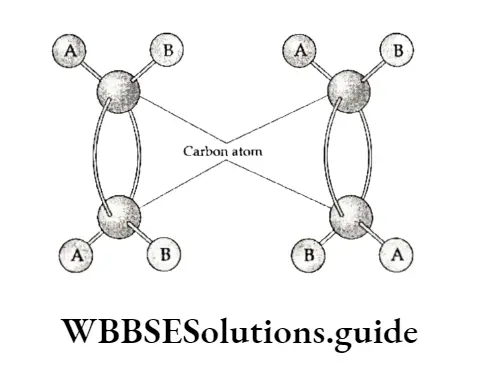
The reason for this simply lies in the formation of π bonds. An π bond is formed by the overlap of two p orbitals.
The p orbitals lie perpendicular (above and below) to the plane of A orbitals(sp2 hybrid orbitals in this case) forming a CT bond.
Conversion of 1 into 2 is possible only when the molecule is twisted, which would definitely break the 71 bond. The n bond is formed by the sideways overlapping of p orbitals, the groups or atoms attached to the sp2 hybridised carbon atom cannot rotate about it.
Rotation is therefore possible by breaking the n bond, which would require energy of the order of 251 kJ mol-1.
This energy is not available at room temperature. Because of the energy barrier, there is hindered rotation about the double bond, which gives rise to geometric isomers.
Using a ball and stick model, geometrical isomers can be represented
Isomers in which similar atoms or groups lie on the same side of the double bond are called cis-isomers and those in which similar atoms or groups lie on opposite sides of the double bond are called trans-isomers.
The trans-isomers are usually more stable than the corresponding cis isomers. This will become clear if we consider cis- and trans-isomers with the formula ABC=CAB, in which A is the bulkier of the two groups A
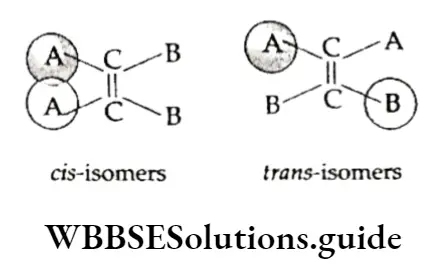
And B (e.g., CH3 —CH=CH—CH3). In the cis-isomer, the two bulky A groups are very close to each other. The repulsion between the overlapping electron clouds of the A groups makes this isomer less stable than the trans isomer, in which the A groups are far apart.
There is no absolute method for determining whether a particular isomer is a cis- or trans-isomer.
However, the following characteristics are useful.
1. The cis-isomer is more polar than the trans-isomer. In other words, the cis-isomer has a greater dipole moment than the trans-isomer.
This will become clear if we consider the cis- and trans-isomers of 1,2-dichloroethene.
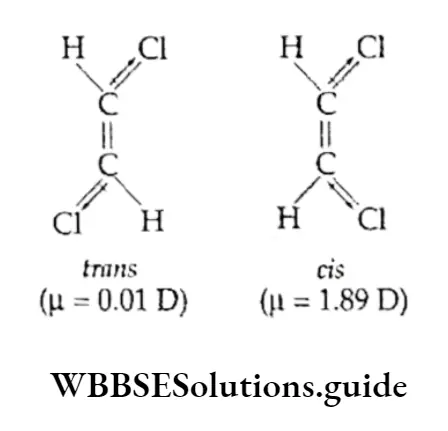
The trans-isomer has a small dipole moment. This is because the dipole moments of the two C—Cl bonds are opposed due to the symmetry of the molecule. The cis-isomer, being nonsymmetrical, has a larger dipole moment.
2. The cis-isomer has a lower melting point than the corresponding trans-isomer. For example, the m.p. of maleic acid, cis-HOOCCH=CHCOOH, is 130°C, while that of fumaric add trans-HOOCCH=CHCOOH, is 286º0.
3. The cis-isomer has a higher boiling point than the corresponding trans-isomer. For example, the b.p. of cis-2-butene is 4°C and that of trans-2-butene is 1ºC.
4. If the cis- and trans-isomers of an alkene have a COOH group then the cis compound forms an anhydride on heating while the trans compound does not. To give an example, this is how we distinguish between maleic acid and fumaric acid.
Example Draw cis and trans isomers of those which are capable of exhibiting cis-trans isomerism, of the following compounds.
- Hex-l-ene
- Hex-2-ene
- Hex-3-ene
Solution: Hex-2-ene and Hex-3-ene will exhibit cis-trans isomerism.

Preparation
Alkenes are generally obtained from saturated compounds by the elimination of atoms or groups from the two adjacent carbon atoms.
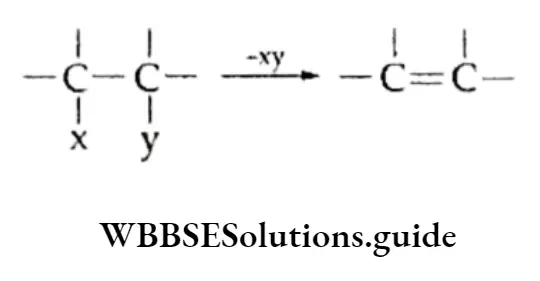
Some common methods of preparation are discussed in the following.
Dehydration Of Alcohols
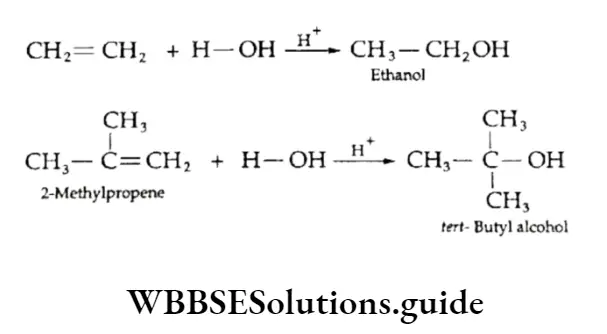
The elimination of a water molecule from a compound is called dehydration. On being heated with concentrated sulphuric acid or phosphoric acid, alcohols form the corresponding alkenes by the elimination of a water molecule.
This reaction is since the hydrogen attached to the p-carbon atom is removed. The carbon atom attached to the functional group is and the one next to it is p.
⇒ \(\underset{\text { Ethanol }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}} \underset{-43-453 \mathrm{~K}}{\stackrel{\operatorname{conc} \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \underset{\text { Ethere }}{\mathrm{CH}_2}=\mathrm{CH}_2+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\underset{\text { Propanol }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}} \underset{43-453 \mathrm{~K}}{\stackrel{\text { Ponc } \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow}} \underset{\text { Prop-1 -ene }}{\mathrm{CH}_3 \mathrm{CH}}=\mathrm{CH}_2+\mathrm{H}_2 \mathrm{O}\)
The mechanism of the dehydration of alcohols by sulphuric arid is as follows.
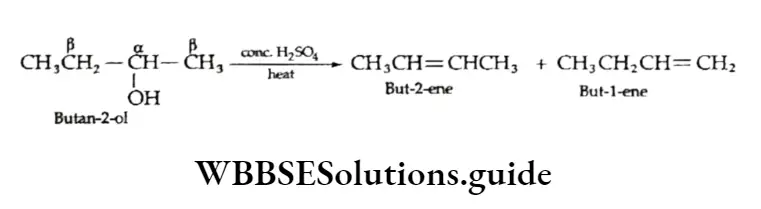
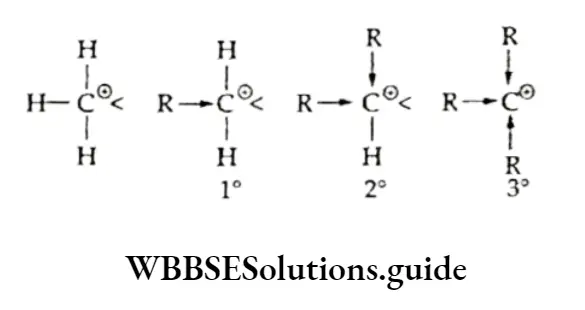
The order of reactivity with respect to dehydration is 3° > 2° > 1°. Tertiary alcohols undergo rapid dehydration as they form the most stable carbocations (3°), which in turn form the most stable alkenes.
In the case of secondary or tertiary alcohols, the elimination of the water molecule gives rise to more than one alkene.
For example, but-2-ol on dehydration gives but-2-ene as the major product and but-l-ene as the minor product. As you can see, there are two p carbons in the structure of butane-2-ol.
The course of the reaction in such cases is determined by the Sayiezeff rule, according to which hydrogen is abstracted preferentially from the carbon atom attached to fewer hydrogen atoms.
Dehydrohalogenation of alkyl halides
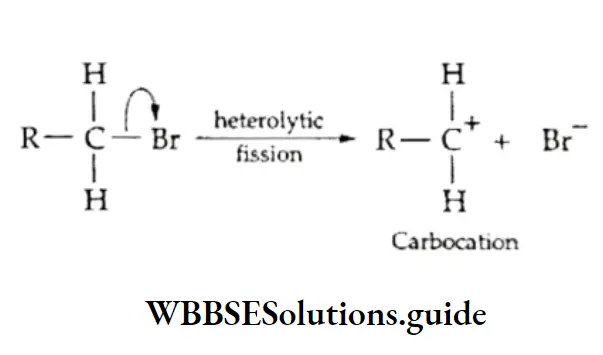
The elimination of a molecule of hydrogen halide (H—X) from an alkyl halide in the presence of alcoholic potash forms an alkene.
This is dehydrohalogenation; it involves the elimination of one halogen atom and one hydrogen atom from the adjacent carbon atoms of a molecule of an alkyl halide.
This reaction is called p elimination. Ethyl bromide, on being heated with alcoholic potassium hydroxide, undergoes dehydrohalogenation.
⇒ \(\underset{\text { Ethyl bromide }}{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Br}} \stackrel{\text { KOH, } \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}{\longrightarrow} \underset{\text { Ethene }}{\mathrm{CH}_2}=\mathrm{CH}_2+\mathrm{KBr}+\mathrm{H}_2 \mathrm{O}\)
The mechanism of dehydrohalogenation of alkyl halides is shown below:
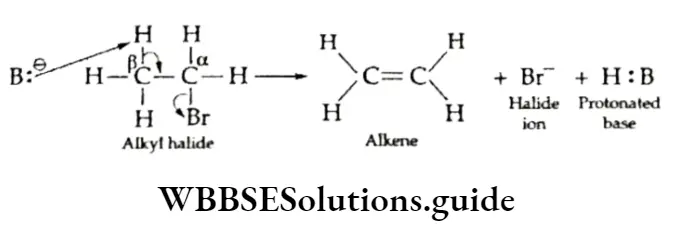
B’ is a base like C2H2O–.
Depending on the structure of the starting alkyl halide, more than one product can be obtained in this case.
For example, 1-chlorobutane yields one product whereas 2-chlorobutane or sec-butyl chloride yields two products.
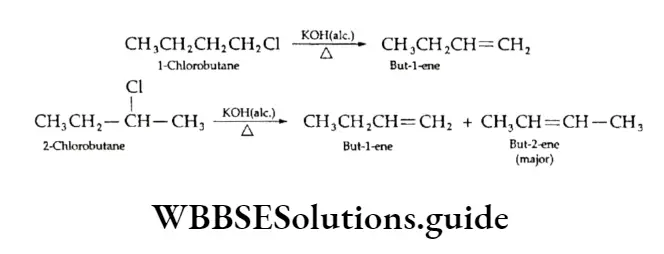
The ease of elimination of halogen atoms is of the order RF < RC1 < RBr < RI.
Dehalogenation of vicinal dihalides
Vicinal dihalides are those alkyl halides in which halogen atoms are present on adjacent carbon atoms. When treated with zinc, a vicinal dihalide gives an alkene.
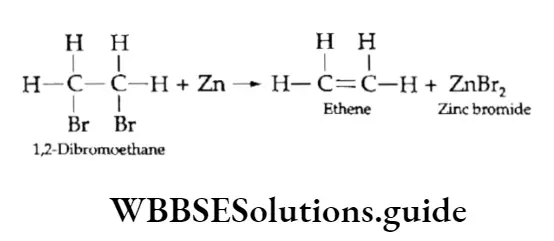
Partial reduction of alkynes
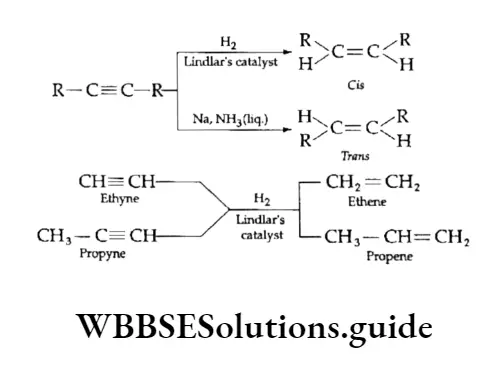
On partial reduction with hydrogen in the presence of a catalyst (Lindlar’s catalyst), alkynes give alkenes.
A specially prepared palladium (palladised charcoal partially deactivated with poisons like quinoline) is Lindlar’s catalyst.
Unless the triple bond in the alkyne undergoing reduction is at the end of a chain, the probability of obtaining either cis- or a trans-alkene cannot be ruled out.
The choice of reducing agent decides the isomer which predominates. With Lindlar’s catalyst cis-alkenes are obtained. However, on reduction with sodium in liquid ammonia, alkynes form trans-alkenes.
Electrolysis of salts of carboxylic acids
Alkenes are obtained by the electrolysis of an aqueous solution of potassium salts of dicarboxylic acid.
Thus potassium succinate on electrolysis gives a carboxylate anion and a potassium cation. Carbon dioxide is released at the anode and hydrogen at the cathode.
At Anode:
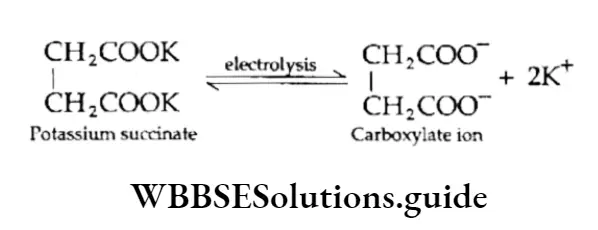
At Cathode:
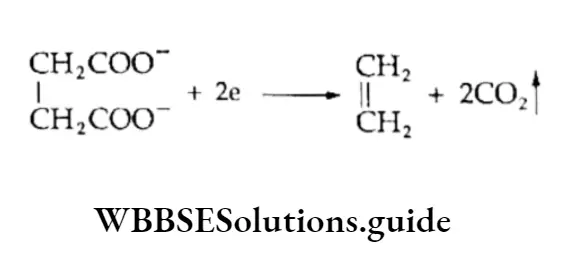
⇒ \(\begin{aligned}
& 2 \mathrm{~K}^{+}+2 \mathrm{e} \longrightarrow 2 \mathrm{~K} \\
& 2 \mathrm{~K}+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{KOH}+\mathrm{H}_2 \uparrow
\end{aligned}\)
Cracking or pyrolysis of alkanes.
On being heated to 773-873 K or in the presence of a catalyst to 498 K, higher alkanes yield alkenes.
Physical Properties
The physical properties of alkenes are similar to those of alkanes. The lower members of this class (up to C4) are colourless gases, the higher members (C6-C18) are liquids and the rest are solids at room temperature.
They are less dense than water and insoluble in it but soluble in common organic solvents like benzene and chloroform.
The boiling points of alkenes, as is the case with alkanes, rise with the increase in the number of carbon atoms.
The boiling point increases by 20°C to 30°C for each carbon atom added to the molecule. Also, branching in an alkene lowers the boiling point.
Chemical properties
We know that the C=C double bond in alkenes consists of a bond and a n bond. Alkenes owe their reactivity to the weaker T2 bonds. They usually undergo addition reactions, the most important being electrophilic and free radical additions.
As discussed earlier in the chapter, the addition of hydrogen to alkenes, in the presence of a catalyst, yields the corresponding alkanes.
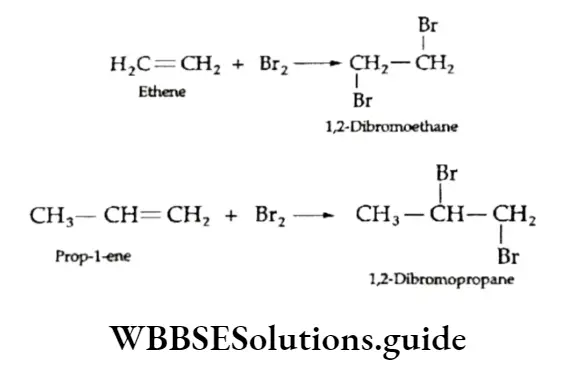
Addition of halogens
Alkenes on treatment with halogens yield 1,2-halogenated alkanes (adduct). Alkenes react readily with chlorine and bromine at room temperature without any exposure to ultraviolet light.
The reaction is carried out in an inert solvent like carbon tetrachloride. The reaction of an alkene with bromine is used as a test for unsaturation.
A solution of bromine in carbon tetrachloride is red, which slowly decolourises as a colourless vicinal dihalide is formed.
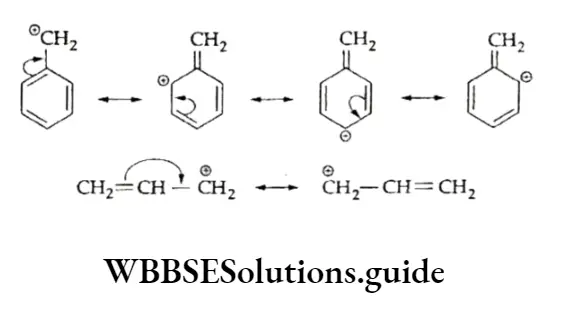
The reaction is an example of electrophilic addition and involves two steps. In the first step, the electrophile (a positively charged species) adds to the carbon-carbon double bond, resulting in the formation of a carbocation intermediate.
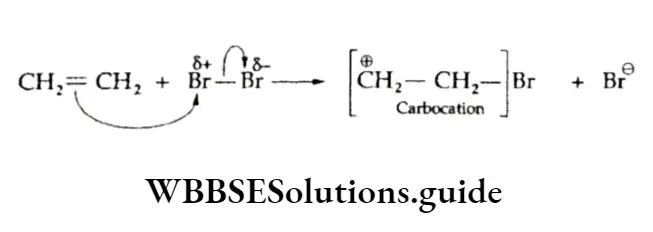
The second step involves the addition of the nucleophile (a negatively charged species) to the positively charged carbon forming the adduct.
⇒ \(\stackrel{\oplus}{\mathrm{C}} \mathrm{H}_2-\mathrm{CH}_2-\mathrm{Br}+\mathrm{Br}^{\ominus} \longrightarrow \underset{\text { 1,2-Dibromoethane }}{\mathrm{CH}}-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br}\)
Addition of hydrogen halides
On reaction with hydrogen halides, alkenes give the corresponding alkyl halides.
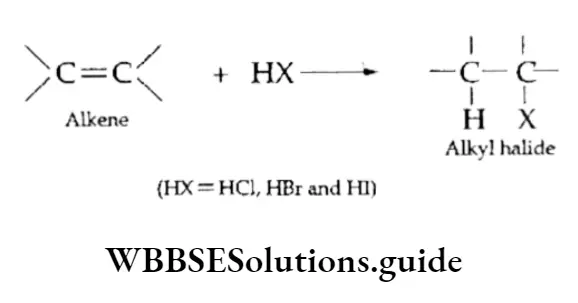
In the case of symmetrical alkenes (like ethene), only one product is obtained
⇒ \(\mathrm{CH}_2=\mathrm{CH}_2+\mathrm{HBr} \longrightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Br}
Ethene Ethyl bromide\)
However, in the case of unsymmetrical alkenes like propene, two products may be formed.
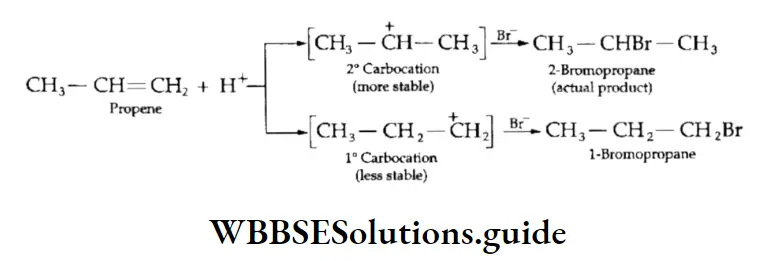
The above reaction shows that out of two possible products, only one product is formed.
Such reactions are called regiospecific reactions. The exclusive formation of one product can be explained by the Markovtiikov rule.
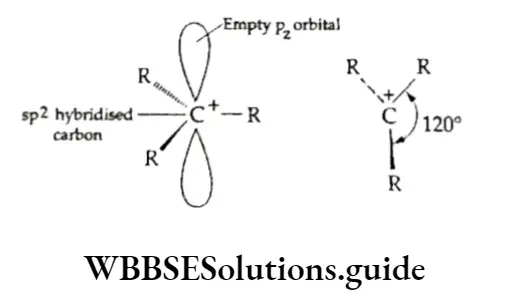
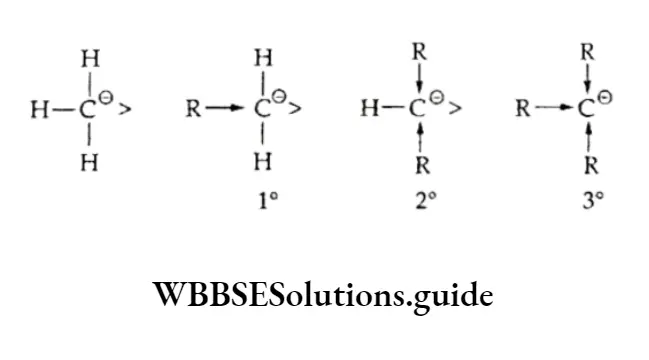
According to this rule (formulated by the Russian chemist Vladimir Markovnikov in 1870), a molecule of a hydrogen halide adds to an unsymmetrical alkene in such a way that the negative part of the reagent (X6-) goes to that carbon atom of the alkene which is bonded to fewer hydrogen atoms. The Markovnikov rule was explained on the basis of the relative stability of carbocations.
Thus, in the above example, when hydrogen bromide is added to propene there is a possibility of formation of two carbocations — primary and secondary.
However, the secondary carbocation is more stable than the primary one. Therefore, the actual product formed is 2-bromopropane.
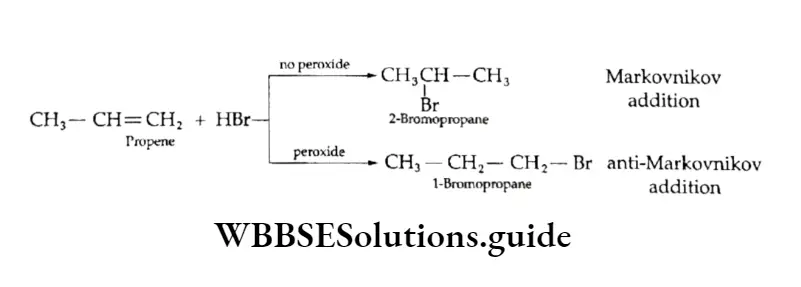
Sometimes, it has been seen that the addition of hydrogen bromide to unsymmetrical alkenes is in contradiction to the Markovnikov rule.
Kharasch and Mayo (1933) first observed and found the cause for such addition.
They observed the anti-Markovnikov addition of hydrogen bromide to propene in the presence of benzyl peroxide. This is also known as the Kharasch effect or peroxide effect.
You have already studied that peroxides are compounds with —O—O— linkage. Certain organic peroxides like benzoyl peroxide (C6H5COO)2 are synthesised and used as reagents.
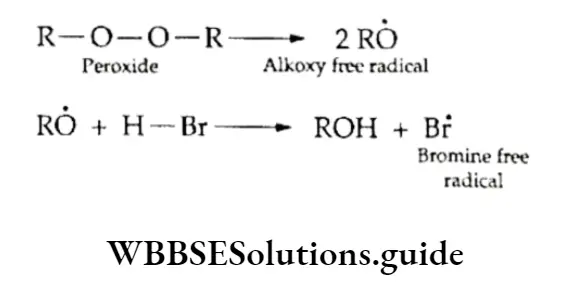
It is believed that the peroxide dissociates to give two alkoxy free radicals, which attack hydrogen bromide to form a bromine free radical.
The bromine free radical generated can add to any of the two carbon atoms of the double bond in an unsymmetrical alkene giving rise to either a primary or a secondary free radical.
The order of stability of free radicals is tertiary > secondary > primary. Therefore, in the presence of a peroxide the secondary free radical is predominantly formed and reacts with HBr to form an anti-Markovnikov product.
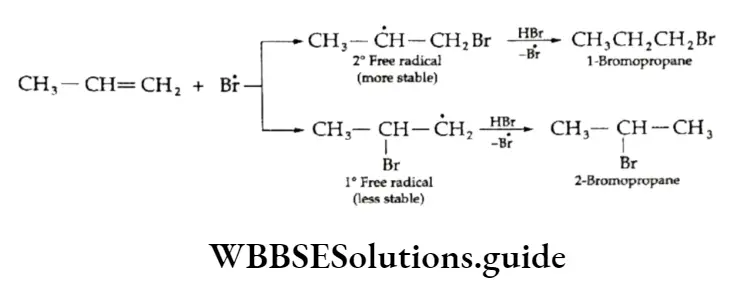
HC1 and HI give the normal Markovnikov addition products even in the presence of peroxides.
The chances of the homolytic cleavage of the H—Cl bond are lower than that of the H—Br bond because it is stronger than the H —Br bond.
The iodine free radical is easily formed since the H—I bond is weak (297 kJ mol-1) and combines with another iodine free radical to form an iodine molecule instead of the relatively unstable carbon-iodine bond.
Addition of sulphuric acid
An alkene reacts with cold concentrated sulphuric acid to form alkyl hydrogen sulphate, which on heating with water gives the corresponding alcohol. The method is used to manufacture ethyl alcohol.
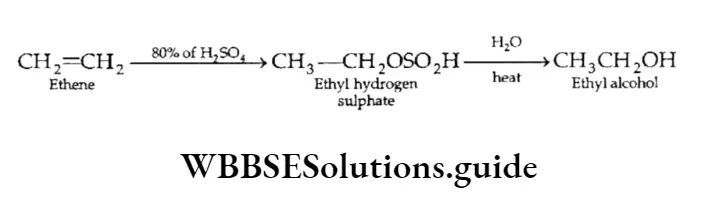
In the case of unsymmetrical alkenes, the addition of sulphuric acid is consistent with the Markovnikov rule. Like the addition of alkyl halides, this reaction is also an example of electrophilic addition.
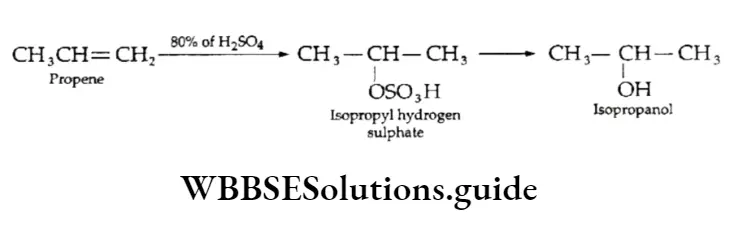
Addition of water
Water adds to the more reactive alkene only in the presence of an acid catalyst (H3P04) to form alcohols. The addition follows the Markovnikov rule.
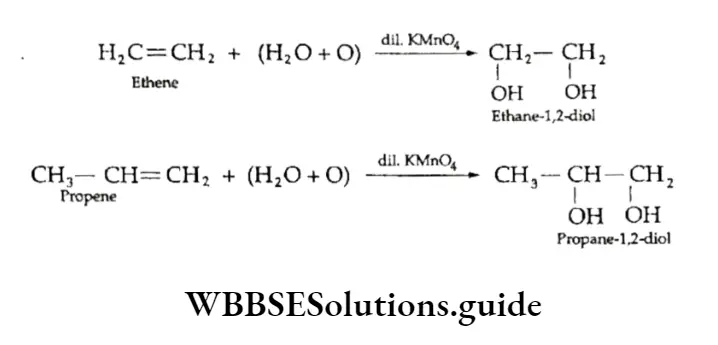
Oxidation
Alkenes are more readily oxidised than alkanes are. This is due to the presence of a double bond, which is the site of attack. The nature of the oxidation products depends on the oxidising agent employed.
1. Hydroxylation On treatment with cold aqueous dilute potassium permanganate solution, a 1,2-dihydroxy compound (diol) is obtained. The reaction is known as hydroxylation of alkenes.
2. Oxidative cleavage of alkenes Oxidation by sodium periodate (NalO4) in the presence of a permanganate solution yields a carboxylic add or a ketone. In the reactions that follow the double bond undergoes oxidative damage.
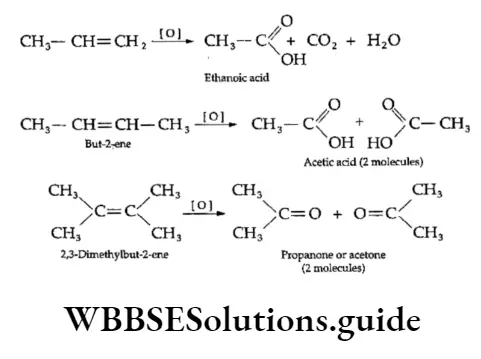
3. Ozonolysis When ozone gas is passed into a solution of an alkene in an inert solvent like carbon tetrachloride or chloroform at low temperatures, a cyclic unstable compound called ozonide is formed.
Ozonide is then treated with water or hydrogen in the presence of a reducing agent like zinc to give compounds containing the >C=0 group (carbonyl compounds), i.e., aldehydes and ketones.
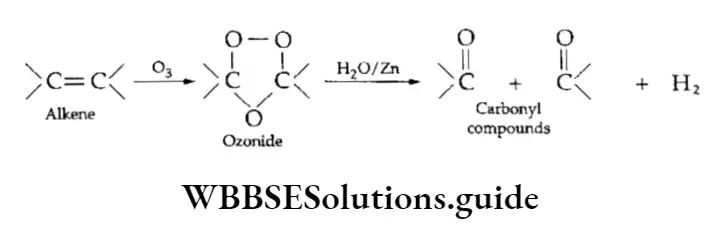
The process of addition of ozone to an alkene-forming ozonide and then hydrolysis of the ozonide to form carbonyl compounds is called ozonolysis.
In this addition reaction, first, an unstable molozonide is formed, which rearranges spontaneously to form the ozonide. Some other examples of ozonolysis of alkenes are given below.
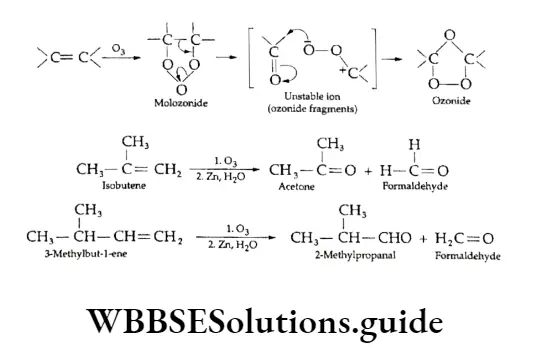
Ozonolysift is used for locating the position of the double bond in an alkene.
The carbon atoms in the carbonyl group of the two carbonyl compounds obtained after hydrolysis of ozonide are the ones bonded to each other by a double bond in the original alkene.
Polymerisation
A polymer is a substance with large molecules consisting of repeating units called monomers.
Polymers are formed by polymerisation, which is a chemical reaction in which the molecules join each other by addition or condensation (elimination of a small molecule, each time a molecule joins the other).
Some simple alkenes can also be converted into polymers. For example, ethylene (a monomer) polymerises on heating under pressure in the absence of air and the presence of a catalyst to give polyethene, a highly useful industrial polymer.
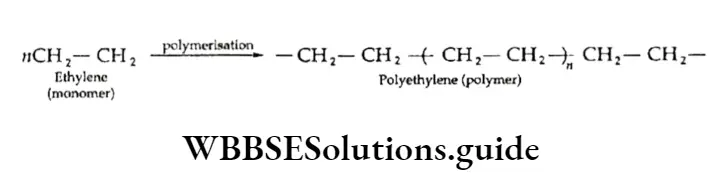
This polymer is formed by an addition reaction and is, therefore, called an addition polymer or chain-growth polymer. In the same way, propene polymerises into polypropene.
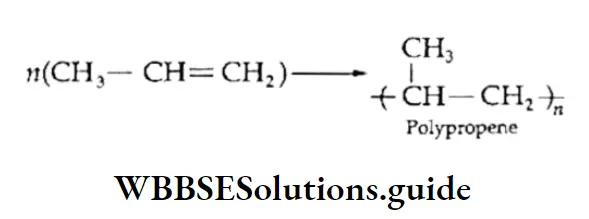
Nowadays, the excessive use of polythene and polypropylene has created environmental hazards, which is a matter of great concern.
Alkynes
Like alkenes, alkynes are also unsaturated hydrocarbons, but they contain a carbon-carbon triple bond (C=C).
The general formula of alkynes is CnH2n-2, which shows that they contain two hydrogen atoms less than the corresponding alkenes and four hydrogen atoms less than the corresponding alkanes.
Isomerism
Unlike alkenes, alkynes do not exhibit cis-trans isomerism since the molecules are linear. We normally encounter three types of isomerism in alkynes.
1. Chain isomerism: Alkynes exhibit chain isomerism. For example, Pent-l-yne is a straight-chain and 3-Methylbut-l-yne is a branched-chain isomer.
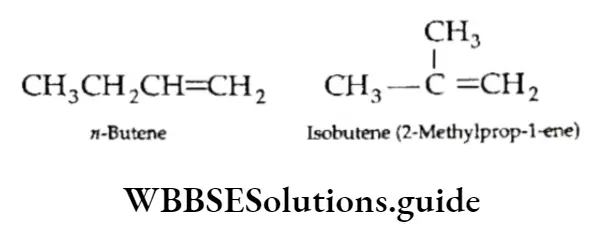
2. Position isomerism: This arises due to different positions of the triple bond.
⇒ \(\begin{gathered}
\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{C} \equiv \mathrm{CH} \\
\text { But-1-yne } \\
\text { (terminal) }
\end{gathered}\)
⇒ \(\mathrm{CH}_3 \mathrm{C} \equiv \mathrm{CCH}_3
But-2-yne (nonterminal)\)
3. Functional isomerism Alkynes exhibit functional isomerism with alkadienes. The tire functional group in But- 2-one is a triple bond whereas, in Buta-1,3-diene, it is a double bond.
⇒ \(\underset{\text { But-2-yne }}{\mathrm{CH}_3 \mathrm{C} \text { ant }} \mathrm{CCH}_3\)
⇒ \(\mathrm{CH}_2=\underset{\text { Buta-1,2-diene }}{\mathrm{CH}}-\mathrm{CH}=\mathrm{CH}_2\)
Structure Of Triple Bond
To understand the structure of a triple bond, let us consider acetylene, the first member with the simplest structure in the alkyne homologous series. Tire two carbon atoms in ethyne are sp hybridised.
That is to say, each carbon atom has two sp hybrid orbitals, which are collinear and two unhybridised orbitals (2pyand 2pz), which are perpendicular to each other and to the sp orbitals.
One sp hybrid orbital of one carbon atom overlaps with one sp orbital of the second carbon atom, forming a C—C CT bond. The other sp hybrid orbital of each carbon atom forms a bond with a hydrogen atom.
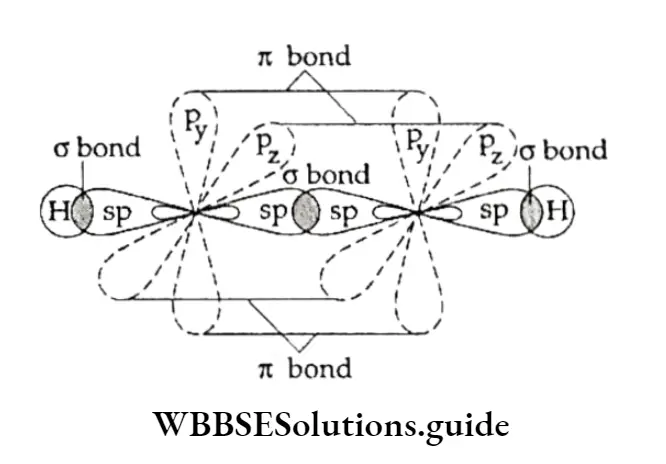
Since the two sp hybrid orbitals of the carbon atoms are collinear and overlapping of the orbitals takes place along the internuclear axis, the two carbon and the two hydrogen atoms lie along a straight line. In other words, the acetylene molecule is linear.
The two unhybridised orbitals of one carbon atom form two n bonds with the two unhybridised orbitals of the other carbon atom.
The orbital structure of acetylene is Thus, the carbon-carbon triple bond in acetylene consists of one strong bond and two weak n bonds.
The H —C—H bond angle is 180°, since die Hacetylene molecule is linear. Two n bonds formed together make a single cylindrical sheath about the line joining the two nuclei.
Preparation
From calcium carbide
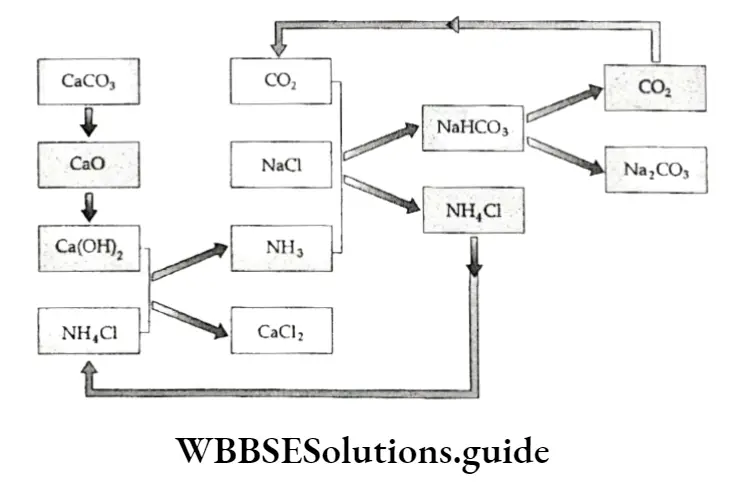
Acetylene is manufactured by treating calcium carbide (CaC2) with water.
⇒ \(\mathrm{CaC}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}+\mathrm{Ca}(\mathrm{OH})_2\)
For this purpose, calcium carbide is obtained by heating a mixture of coke and limestone in an electric furnace.
⇒ \(\begin{gathered}
\mathrm{CaCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2 \\
\mathrm{CaO}+3 \mathrm{C} \stackrel{2273-3273 \mathrm{~K}}{\longrightarrow} \mathrm{CaC}_2+\mathrm{CO}
\end{gathered}\)
Generally, there can be two processes for the synthesis of alkynes. One of them involves the formation of a triple bond by elimination reactions and the other involves the addition of alkyl groups to molecules containing a triple bond.
Dehydrohalogenation Of Dihalides
Alkynes can be prepared by the elimination of two molecules of hydrogen halides from a dihalide.
The dihalide may be geminal (in which both the halogen atoms are attached to the same carbon atom) or vicinal (in which the two halogen atoms are attached to adjacent carbon atoms).
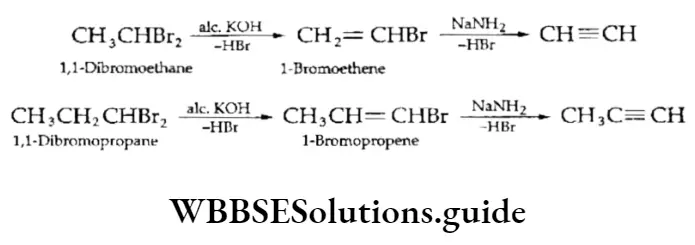
1. Vicinal dihalides On treatment with suitable bases, vicinal dihalides undergo an elimination reaction whereby two molecules of hydrogen halide are eliminated from the adjacent carbon atoms to give an alkyne. A stronger base like sodamide is used for unreactive vinylic halides.
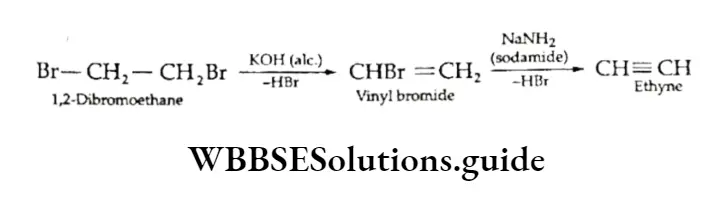
The dehydrohalogenation of dihalides is a useful method to obtain alkynes as dihalides can be readily obtained by the addition of halogens to the corresponding alkenes.
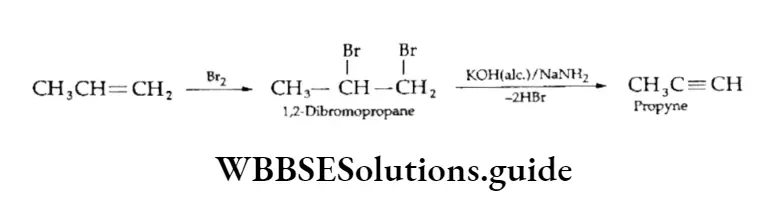
2. Geminal dihalides (1,1-dihalides) On treatment with a base, geminal dihalides also undergo dehydrohalogenation to give alkynes.
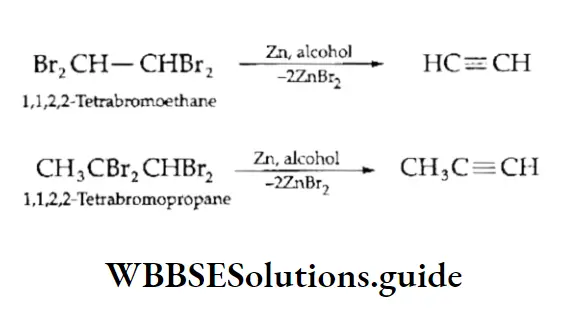
Dehalogenation of tetrahalides
Alkynes may also be obtained by the dehalogenation of tetrahalides.
Physical Properties
Alkynes resemble alkenes and alkanes in their physical properties. They are all colourless and odourless, with the exception of ethyne, which has a characteristic odour.
The first three members are gases, the next eight members are liquids and the higher members are solids at room temperature.
The melting points, boiling points and densities increase gradually with an increase in molecular weight and show the usual effects of chain branching.
Alkynes have slightly higher boiling points than the corresponding alkenes and alkanes.
They are weakly polar and dissolve readily in low-polarity solvents like ether, benzene and carbon tetrachloride. Alkynes are insoluble in water.
Chemical properties
The reactivity of alkynes is mainly due to the presence of two 7t bonds and their acidic nature.
The acidic nature of alkynes
Alkynes are acidic organic compounds but, unlike other acidic inorganic compounds, they do not turn blue litmus red or neutralise aqueous bases.
Yet, they are acidic because they have the tendency to lose hydrogen ions. A hydrogen atom can be released as a positive hydrogen ion by the breaking of a bond if it is bonded with an electronegative atom which can accommodate the bonded electron pair left behind.
If we consider the second period of the p block of the periodic table, the electronegativity of elements decreases as F > O > N > C.
From this order of electronegativity, we may conclude that HF is a strong acid, H2O is a weak acid, NH3 is still weaker and CH4 is not considered an acid at all.
But in the case of a compound containing a triply bonded carbon atom, like acetylene, the relative acidity is of the order H2O > HC=CH > NH3.
It seems that the carbon atom in acetylene is a different element than the one in methane, CH4 (single bond) and ethene (double bond).
Also, the alkynes, which contain hydrogen attached to triply bonded carbon atoms (known as terminal alkynes) show comparable acidity.
Why is the triple bond responsible for the acidity of alkynes? This can be explained on the basis of the hybridisation state of the carbon atom in the compound.
Electrons in the s orbital have lower energy than those in the p orbital. Since the s orbital is spherical the electrons in it are much closer to the nucleus than the electrons in the p orbital, which is lobed on either side of the nucleus.
Therefore, in the case of hybrid orbitals, more s character means more electronegativity and a more stable anion.
The sp hybrid orbital of the triply bonded carbon atom in ethyne has 50 per cent s character whereas it is 33.3 per cent in the sp2 hybrid orbital of ethene and 25 per cent in the sp3 hybrid orbital of ethane.
Therefore, the electrons forming the C —H bond in ethyne are closer to carbon, making the carbon electronegative.
So, hydrogen has the tendency to leave as a positive ion, thereby showing acidity.
Acetylene and other terminal alkynes containing acidic hydrogen atoms can be converted into metal acetylides and metal alkynes on treatment with sodium and sodamide respectively in liquid ammonia.
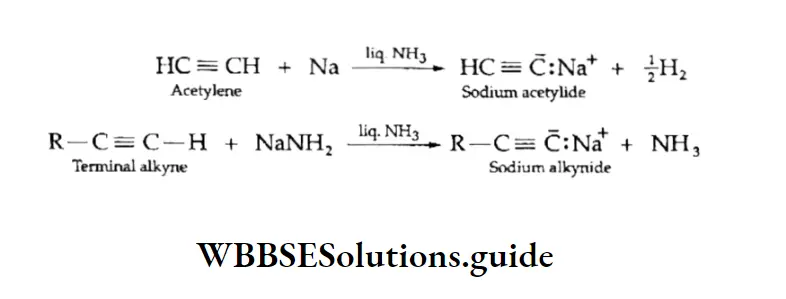
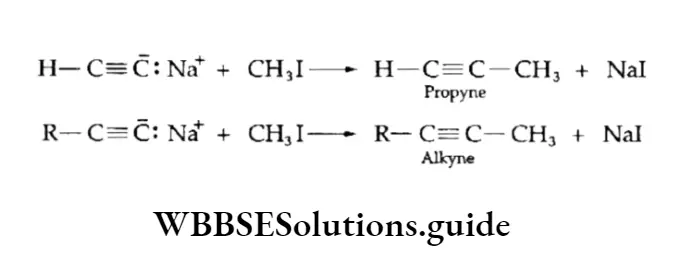
The sodium derivatives obtained above on treatment with alkyl halides give alkynes. By this method, the lower alkynes can be converted to higher alkynes. Alkanes and alkenes do not undergo these reactions.
Acidic alkynes react with an ammoniacal solution of copper sulphate and an ammoniacal solution of silver nitrate (Tollen’s reagent) to give the corresponding copper and silver alkylides, which are red and white precipitates respectively.
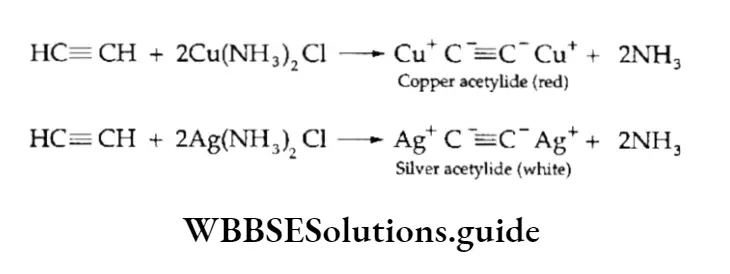
The reaction can be used to distinguish between terminal and nonterminal alkynes and is used as a diagnostic test for the —C=CH group.
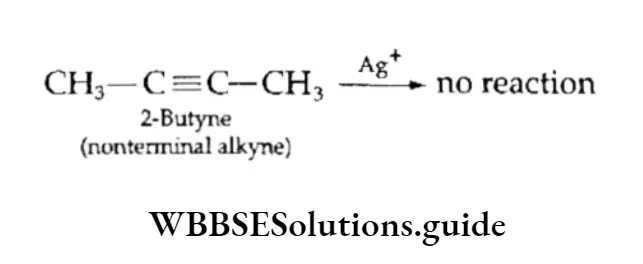
Addition Reactions
Like alkenes, alkynes undergo electrophilic addition reactions. However, with alkynes, the addition may occur once or twice depending on the number of molar equivalents of the reagents employed.
1. Addition of hydrogen (reduction) Alkynes can be reduced, first to alkenes and then to the corresponding alkanes in the presence of catalysts—finely divided platinum or palladium or nickel.
The above reaction can be stopped at the alkene stage by using Lindlar’s catalyst (Pd-BaSO4 poisoned with quinoline)
\(\mathrm{HC} \equiv \mathrm{CH} \underset{\mathrm{Pd}-\mathrm{BuSO}_4}{\stackrel{\mathrm{H}_2}{\longrightarrow}} \mathrm{H}_2 \mathrm{C}=\mathrm{CH}_2\)
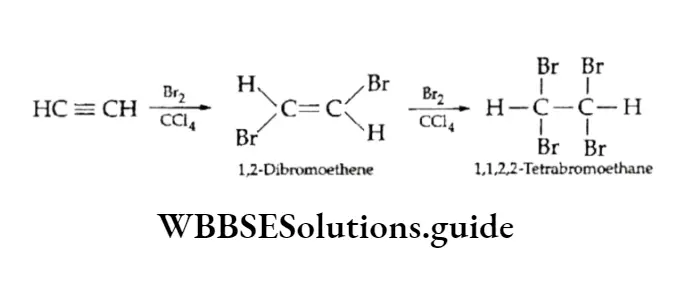
2. Addition of halogens Alkynes add one mole of bromine to give a dibromo and two moles of bromine to give a tetrabromo product.
A solution of bromine in carbon tetrachloride, which is red, gets decolourised in addition to an alkyne. This is used as a test to detect unsaturation.
Chlorine reacts in a similar fashion.
3. Addition of hydrogen halides Alkynes react with two moles of hydrogen bromide or hydrogen chloride to first form the corresponding haloalkene and finally geminal dihalide. Both the electrophilic addition reactions follow the Markovnikov rule.
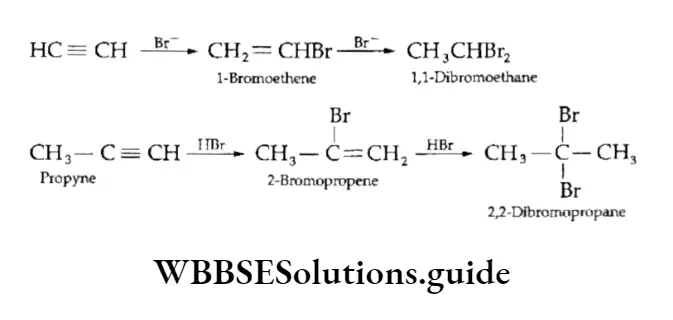
The anti-Markovnikov addition of hydrogen bromide to alkynes takes place in the presence of peroxides. The reaction occurs through a free-radical mechanism.
\(\underset{\text { Propyne }}{\mathrm{CH}_3 \mathrm{C} \equiv \mathrm{CH}} \frac{\mathrm{HBr}}{\text { peroxide }}-\underset{\text { 1-Bromopropene }}{\mathrm{CH}_3 \mathrm{CH}=\mathrm{CHBr}}\)
4. Addition of water Alkynes, although immiscible with water, can be hydrated in the presence of mercuric sulphate dissolved in dilute sulphuric acid. The triple bond adds a molecule of water to form vinyl alcohol, an unstable product, which isomerises to an aldehyde or a ketone.
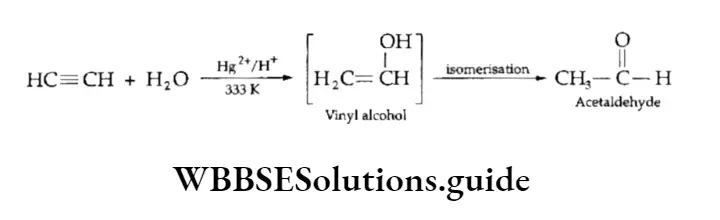
In the case of the substituted alkynes, the addition follows the Markovnikov rule and ketones are formed.
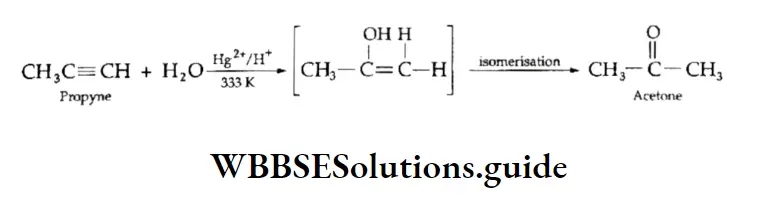
Oxidation Of Alkynes
When oxidised with an alkaline potassium permanganate solution, the acetylene or terminal alkyne molecule undergoes cleavage at the site of the triple bond to give carboxylic acids and carbon dioxide. But nonterminal alkynes give only carboxylic acids on oxidation.
⇒ \(\mathrm{R}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}+4[\mathrm{O}] \stackrel{\mathrm{KMnO}_4}{\longrightarrow} \mathrm{R}-\mathrm{COOH}+\mathrm{CO}_2\)
⇒ \(\mathrm{R}-\mathrm{C} \equiv \mathrm{C}-\mathrm{R}+4[\mathrm{O}] \stackrel{\mathrm{KMnO}_4}{\longrightarrow} \mathrm{R}-\mathrm{COOH}+\mathrm{RCOOH}\)
Thus, by identifying the products formed during oxidation by a potassium permanganate solution, it is possible to understand the structure of alkynes.
However, on treatment with ozone, alkynes form ozonides (their structure is different from the ozonides obtained in the case of alkenes), which on treatment with water yield carboxylic acids.
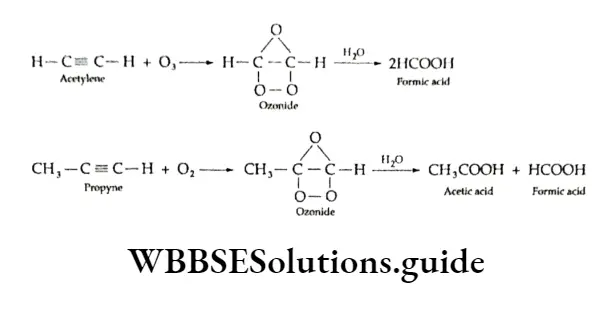
Polymerisation
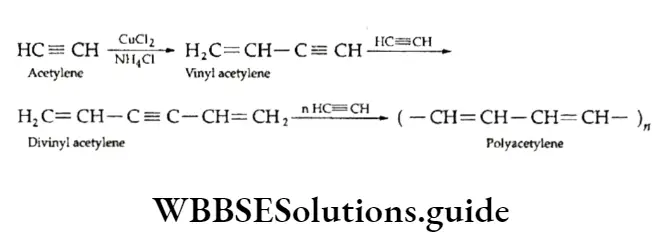
Alkynes undergo polymerisation to form linear or cyclic polymers. Linear polymers are obtained in the presence of cuprous chloride and ammonium chloride, which, together, catalyse the reaction.
The linear polymer polyacetylene is a conductor of electricity and is used as an electrode in batteries.
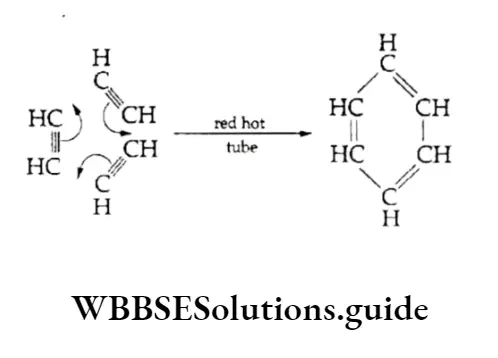
When passed through a red hot tube, acetylene polymerises to form a cyclic product, benzene.
Aromatic Hydrocarbons
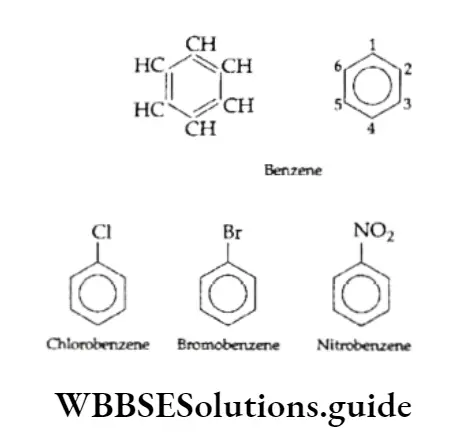
We already know that aromatic hydrocarbons, also known as arenes or benzenoid compounds, contain one or more benzene rings.
In fact, the scope of the term ‘aromatic’ is now not limited to benzenoid compounds, but also includes non-benzenoid compounds which do not have a carbon sextet and yet possess aromatic character. We will discuss aromaticity.
Isomerism
Position isomerism
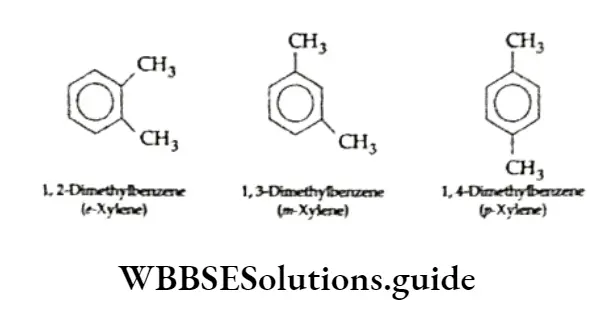
Isomerism is not observed in the case of monosubstituted benzene compounds since all the six hydrogen atoms which may be substituted are equivalent.
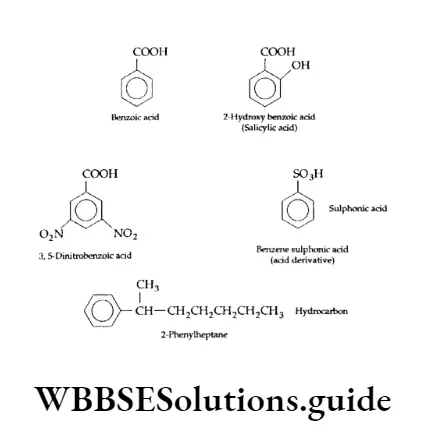
Disubstituted and trisubstituted benzene compounds exhibit isomerism. Because substituents attached to the benzene ring have different relative positions. For example, dichlorobenzene can have the following three possible isomers.

Similarly, a trisubstituted benzene exhibits three isomers having different relative positions of the substituents.
Ring-chain isomerism
If two compounds have the same molecular formula but one has an open chain and the other a cyclic structure, they are called ring-chain isomers.
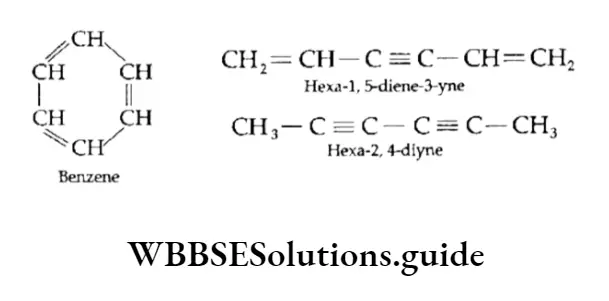
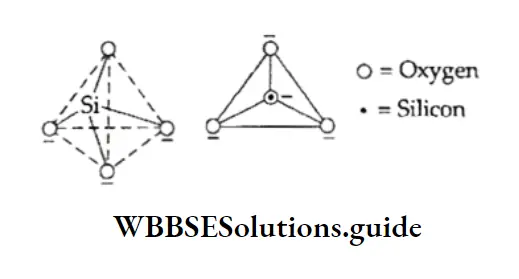
Structure Of Benzene
The number of hydrogen atoms in benzene (C6H6) is eight less than that in the corresponding saturated alkane, hexane (C6H14).
Benzene, therefore, should be expected to be an unsaturated compound. This expectation is supported by the fact that benzene adds three molecules of hydrogen in the presence of Raney nickel or platinum at 473-523 K when cyclohexane, C6H12, is formed.
⇒ \(\underset{\text { Bentene }}{\mathrm{C}_6 \mathrm{H}_6}+3 \mathrm{H}_2 \underset{473-523 \mathrm{~K}}{\stackrel{\text { RaneyNi }}{\longrightarrow}} \underset{\text { Cyclohecane }}{\mathrm{C}_6 \mathrm{H}_{12}}\)
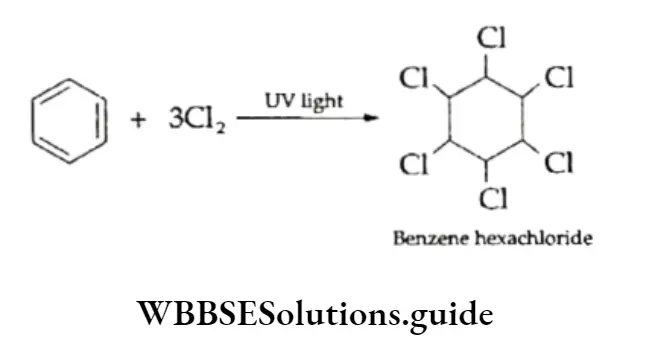
The formation of benzene hexachloride (C6H6C16) from benzene in the presence of sunlight is another reaction which proves the unsaturation of benzene.
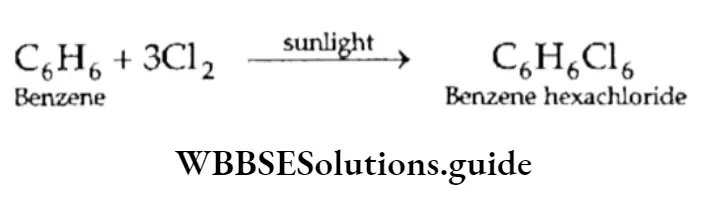
On the other hand, certain reactions show that benzene unexpectedly behaves like a saturated compound. It undergoes substitution reactions and unlike alkenes and alkynes, it does not decolourise bromine in a solution of carbon tetrachloride. Instead, when treated with Br2 in the presence of FeBr3, it forms monobromobenzene, which is a substitution product.
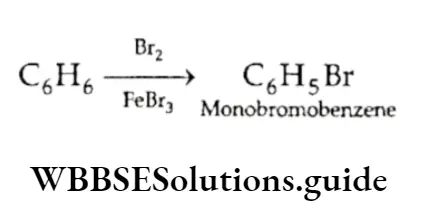
Also, like saturated compounds, benzene is resistant to oxidation. Unlike alkenes and alkynes, benzene does not undergo oxidation readily. Even with strong oxidising agents like chromic acid and potassium permanganate, benzene oxidises only very slowly to CO2 and H2O.
Kekule Structure
Until 1865 no scientist could come up with a satisfactory structure for benzene, though they knew its molecular formula.
Then Kekule, a German chemist, proposed that the six carbon atoms of benzene are joined to each other by alternate single and double bonds to form a six-membered ring and that each carbon atom is also bonded to a hydrogen atom.
Strangely enough, Kekuld got this insight into the structure of benzene in a dream. The structure he proposed was a major breakthrough in science, but it could not explain a few characteristics of benzene.
- It could not explain, for example, why despite containing three double bonds, benzene is not easily oxidised by oxidising agents like KMnO2 and why it undergoes substitution reactions.
- If the Kekultf structure is valid, there should be two o-disubstituted products.
- One with a single bond between the two chlorine atoms and the other with a double bond between the two chlorine atoms. However, in reality, there is only one o-dichlorobenzene.
- Kekutd tried to account for this by proposing a dynamic equilibrium between the two structures. That is to say, the positions of the single and double bonds are not fixed but oscillate back and forth between the alternate positions.
- According to Kekule, there should be two kinds of bonds in benzene and the C—C bond length should be greater than the C=C bond length.
- However, X-ray diffraction studies show that benzene is a regular hexagon with an angle of 120° (all the bonds are alike) and that all the C —C bond lengths are equal (139 pm).
- The C—C bond length in benzene lies between the single-bond length (154 pm) and the double-bond length (134 pm).
- Thus, the Kekul6 structure could not explain the fact that the carbon-carbon bond lengths in benzene are equal, or account for the unusual stability of benzene. These were later explained on the basis of molecular orbitals and resonance.
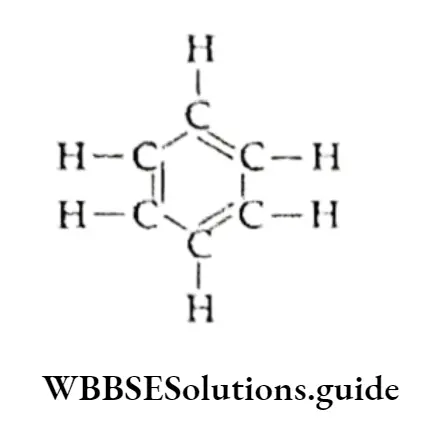
Molecular Orbital Structure
According to the molecular orbital concept, each carbon atom in benzene is sp2 hybridised, which means that each carbon atom has three sp2 orbitals.
Of these orbitals, two ores are involved in forming a bond with two adjacent carbon atoms and the remaining orbital forms a CT bond with a hydrogen atom, meaning that there are six C—C o bonds and six C—H bonds which lie in one plane. This explains why the angle between any two adjacent a bond (C-C—C) is 120°
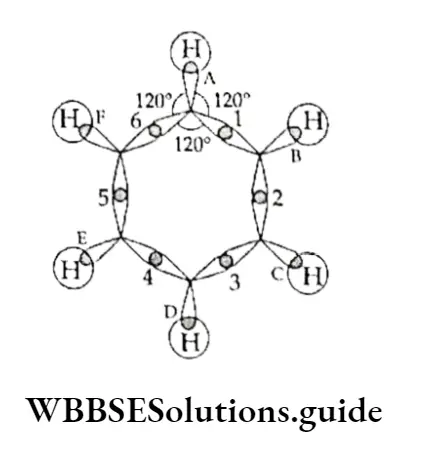
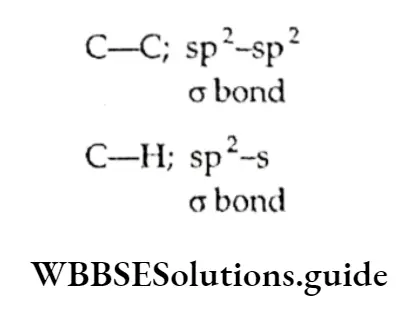
Each carbon atom in the molecule, being sp2 hybridised, is left with an unhybridised p2 orbital.
The six p. orbitals (with one electron each) are parallel to one another and perpendicular to the plane of the a-bonded ring of carbon atoms.
Each of these p orbitals overlaps with the p orbital of the adjacent carbon atom in equally good ways, forming three n bonds in all.
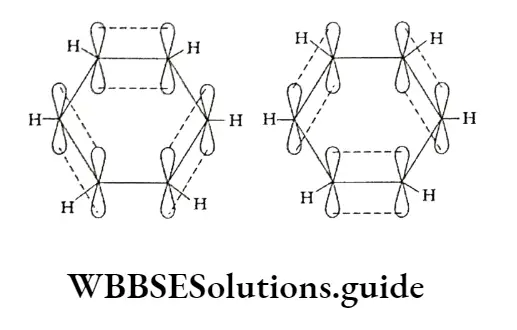
The electron cloud of one p2 orbital spreads to the neighbouring p2 orbitals equally well.
This type of participation of n electrons in more than one bond is called delocalisation of electrons.
All the six carbon atoms in benzene attract these delocalised electrons equally, thus leading to the formation of two n electron clouds—one above and the other below the plane of the carbon and hydrogen atom.
Due to this all the C—C bond lengths are equal, all the C—H bonds are equivalent and the dipole moment of the molecule is zero. All these factors contribute to the stability of benzene.
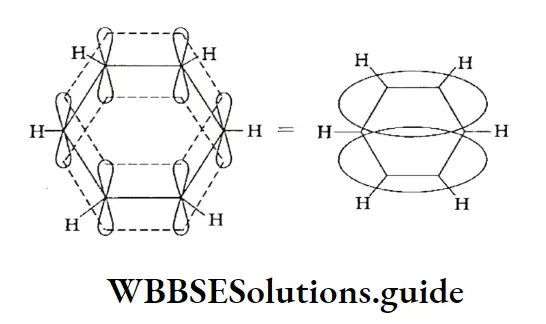
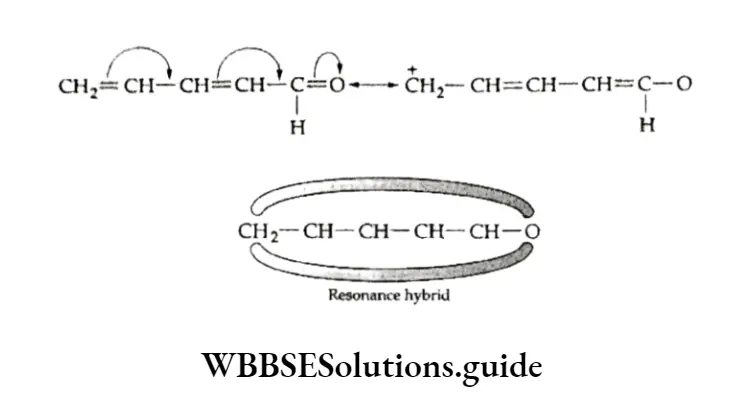
Resonance
The concept of resonance can also be used to explain the stability of benzene.

The real structure of benzene can be represented as a resonance hybrid of the two Kekule structures. Structures 1 and 2 are known as resonating or canonical structures.
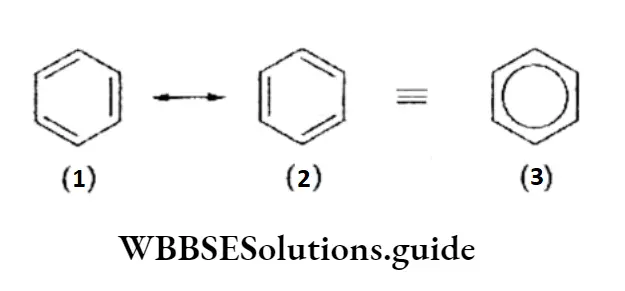
The resonance structure of benzene explains the following observations.
- The C—C bond length in benzene is 139 pm which lies between the C—C bond length (154 pm) and the C=C bond length (134 pm).
- The existence of only one o-dichlorobenzene.
- The stability of benzene or the fact that it behaves like a saturated hydrocarbon. Because of resonance, the n-electron charge in benzene gets distributed over a large area; in other words, it gets delocalised. This leads to a decrease in the energy of the resonance hybrid by about 50 kJ mol”1 as compared to that of the contributing structures. The difference in energy is called resonance energy.
Aromaticity
Despite being unsaturated, benzene resists addition and oxidation reactions. Thus, benzene shows characteristic stability. Earlier the term ‘aromatic character’ or ‘aromaticity’ was used to signify the characteristic chemical behaviour of benzene and its related compounds.
Robinson was the first to point out that the presence of alternate double and single bonds conferred aromaticity to the benzene ring. This was attributed to the delocalisation of the six n electrons over the planar carbon hexagon.
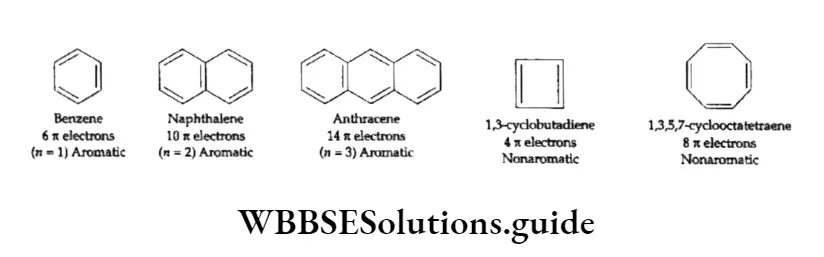
The modem theory of aromaticity was advanced by Eric Hiickel (1931). The basic concepts of this theory are as follows.
- The complete delocalisation of the n electrons in the ring system is necessary to make the system aromatic I in character.
- Ample or complete delocalisation of the n electrons is possible only if the ring is flat or coplanar so as to allow cyclic overlap of TC electrons. For example, benzene, which has a coplanar ring, is aromatic whereas 1,3,5,7-cyclooctatetraene, being nonplanar, lacks aromaticity.
- Hiickel’s rule or the (4n + 2) rule states that a planar, cyclic system should have n electrons to exhibit aromatic character. Here n is a natural number. Thus, in other words, to be aromatic, a molecule should have 6 7t(n =1), 10 n(n = 2) electrons and so on. Some examples of both aromatic and nonaromatic cyclic hydrocarbons are given below.
Preparation Of Benzene
Enormous quantities of aromatic compounds are obtained from coal and petroleum, petroleum being the chief source of benzene and toluene.
Still, larger quantities of aromatic hydrocarbons are synthesised from alkanes through the process of catalytic reforming. The process involves dehydrogenation, cyclisation and isomerisation.
As stated earlier in the chapter, acetylene (or ethyne) when passed through a heated tube polymerises to a small extent to benzene.
Recently, large-scale production of benzene from acetylene (from petroleum sources) has been achieved by using cobalt carbonyls and other metal complexes.
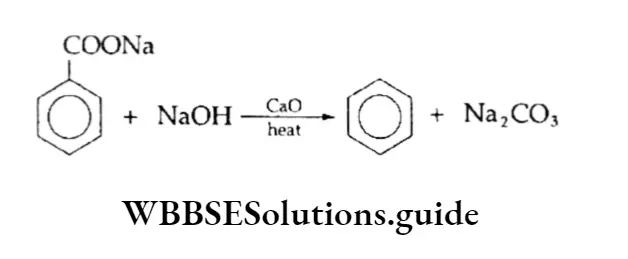
Decarboxylation of aromatic acids
On being heated with soda lime, sodium salts of benzoic acids give benzene.
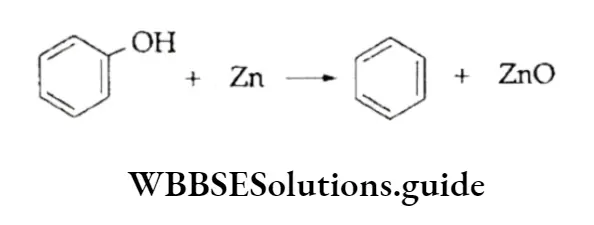
By Reduction Of Phenol
When passed over heated zinc dust, phenol in vapour form is reduced to benzene.
Reduction of diazonium salts
Aryldiazonium salts, on reduction with hypophosphorous acid, yield the corresponding arene. The diazo group is replaced by H.
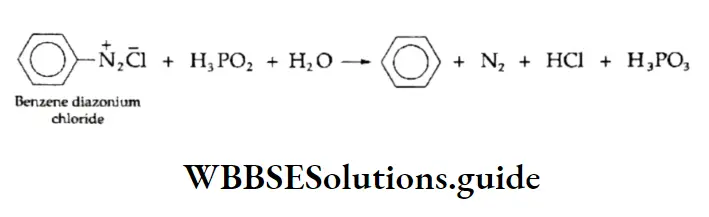
Physical Properties
Being nonpolar, aromatic hydrocarbons are insoluble in water but soluble in organic solvents. Benzene is a colourless liquid, b.p. 353 K. It freezes to a solid at 278.5 K.
It has a characteristic odour. It is highly inflammable and bums with a sooty flame. It is slightly soluble in water. Being toxic and carcinogenic in nature, its use as a solvent has been now prohibited in most advanced countries.
Chemical Properties
Arenes, the derivatives of benzene, do not undergo addition and oxidation reactions under normal conditions, though the benzene ring contains three double bonds. Benzene and its derivatives are characterised by electrophilic substitution reactions.
The stability of benzene has been attributed to resonance. It can also be explained by considering its enthalpies of hydrogenation and combustion, which are lower than expected.
The enthalpy of hydrogenation is the amount of energy that evolves when one mole of an unsaturated compound is hydrogenated. In most alkenes, this value is about 120 kJ mol _1 for each double bond.
On the basis of this value, one expects the enthalpy of hydrogenation for cyclohexatriene or benzene to be 120 x 3 = 360 kJ mol-1 whereas the actual value for benzene is only 208 kJ mol-1, which is 152 kJ mol-1 less than the expected value. This means that benzene is more stable than expected.
Similarly, the enthalpy of combustion of benzene is also lower than expected.
Electrophilic Substitution Reactions
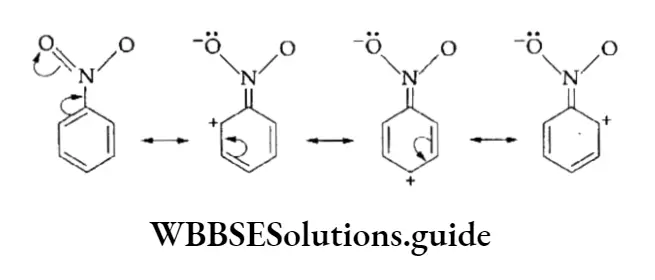
The attacking reagent in the reaction is electron deficient, as the name electrophilic suggests, whereas the benzene ring serves as a source of electrons.
In the reaction, a hydrogen atom of the benzene ring is replaced by an active species, the electrophile (E+).
Depending upon the type of electrophile, electrophilic substitution reactions include nitration, halogenation, sulphonation, Friedel-Craft alkylation and acylation reactions.
1. Nitration of Benzene and its homologues, on reaction with a mixture of concentrated nitric acid and sulphuric acid, form nitrobenzene. In the mixture of the two acids, sulphuric acid serves as an acid and nitric acid as a base.
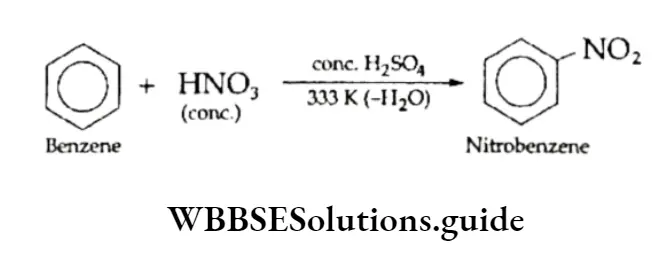
The mechanism of the reaction involves the generation of the electrophilic nitronium ion by protonation and its reaction with benzene to form a resonance-stabilised carbocation. The carbocation rapidly loses a proton to give nitrobenzene. The following steps exhibit the sequence of reactions.
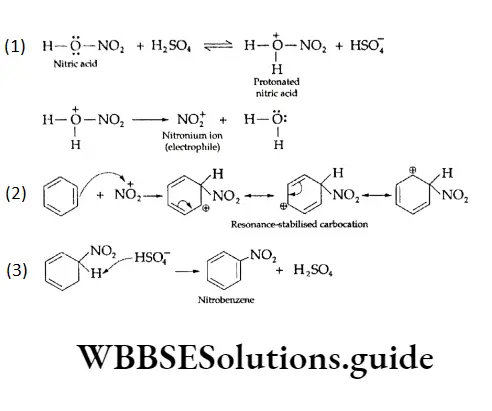
2. Halogenation Arenes react with halogens (bromine or chlorine) in the presence of a Lewis add catalyst—ferric bromide, ferric chloride or aluminium chloride—to give aryl halides.
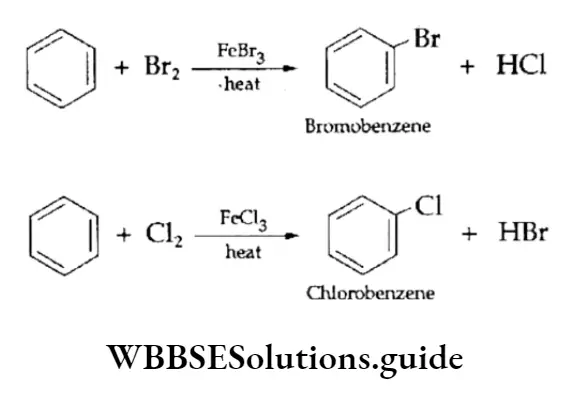
The mechanism of halogenation follows the same steps as that of nitration. The electrophile X+ is generated with the help of the Lewis acid.
⇒ \(\mathrm{Br}-\mathrm{Br}+\mathrm{FeBr}_3 \longrightarrow \underset{\text { Bromonium ion }}{\mathrm{Br}^{+}}+[\mathrm{FeBr}]_4^{-}\)
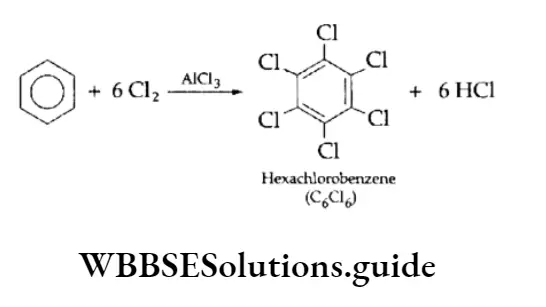
If an excess of the electrophilic reagent is used, all the hydrogen atoms can be replaced with the electrophile. For example, on treatment with an excess of anhydrous aluminium chloride in darkness, benzene gives hexachlorobenzene.
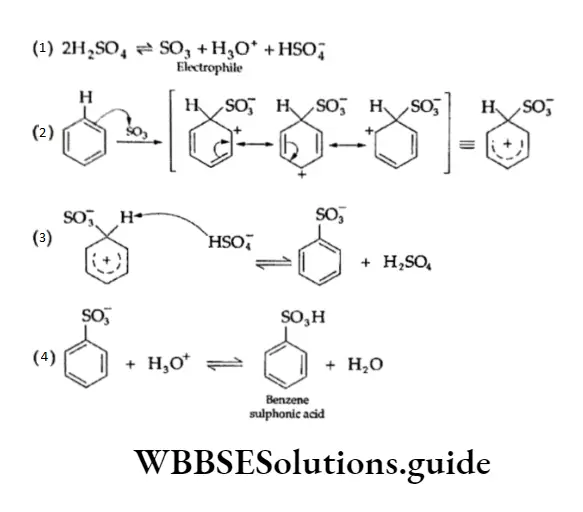
3. Sulphonation Benzene reacts with fuming sulphuric acid (H2S2O7) to give benzene sulphonic acid. (Fuming sulphuric add is obtained by passing S03 through 98% H2SO4.) The electrophilic reagent in this reaction is the electron-deficient sulphur trioxide.
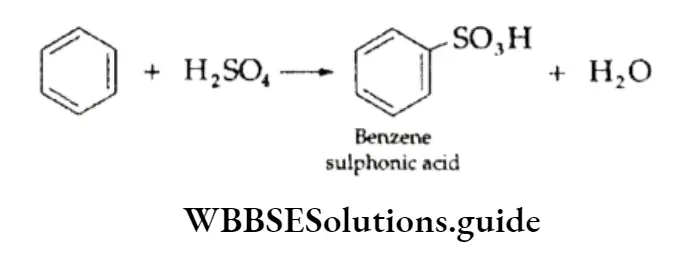
The mechanism of the reaction involves the generation of the electrophilic reagent SO3, which attaches itself to the benzene ring to form the carbocation.
The intermediate carbocation formed rapidly loses a proton to give the anion of benzene sulphonic acid. The acid is strong and remains highly dissociated.
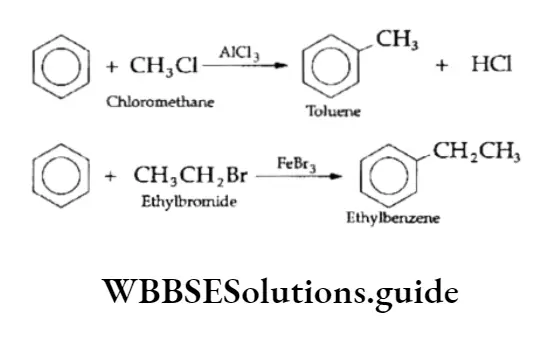
4. Friedel-Crafts reaction This electrophilic substitution reaction involves the introduction of an alkyl group in the benzene ring.
In the presence of a Lewis acid, which acts as a catalyst, an alkyl halide reacts with benzene. The products obtained are alkyl benzenes, which are not otherwise easily synthesised. The reaction is known as Friedel-Crafts alkylation.
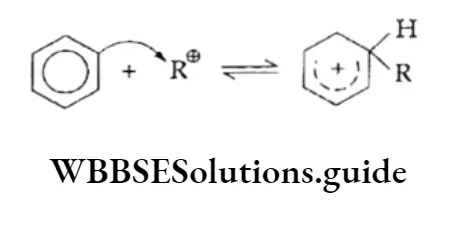
The mechanism of the Friedel-Crafts reaction involves the following steps:
1. Generation of the electrophile (a carbocation in this case)
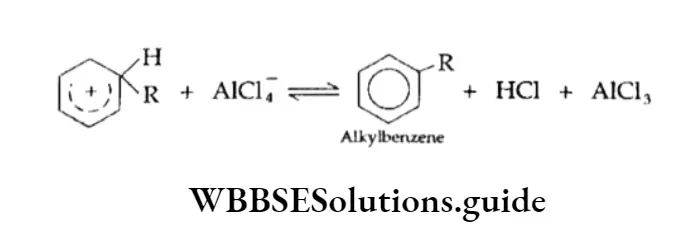
⇒ \(\mathrm{RCl}+\mathrm{AlCl}_3 \rightleftharpoons \mathrm{AlCl}_4^{-}+\mathrm{R}^{\oplus}\)
Elimination of a proton (H+)
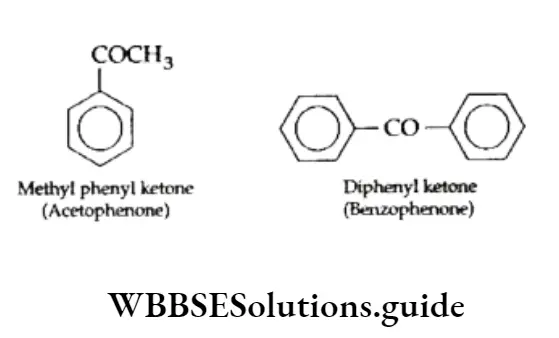
In Friedel-Crafts alkylation, if in place of alkyl halides (RX), an acyl halide (e.g., acetyl chloride or benzoyl chloride) is used, the product obtained is the corresponding ketone. This reaction is known as Friedel-Crafts acylation.
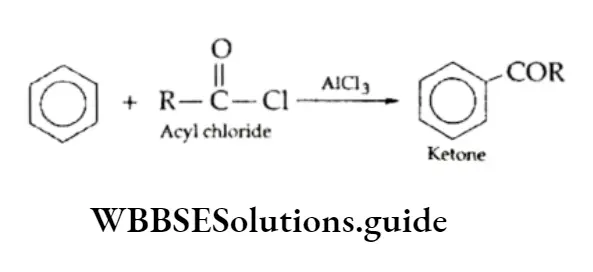
Acetophenone (C6H5COCH3) and benzophenone (C6H5COC6H5) can be prepared from acetyl chloride and benzoyl chloride respectively.
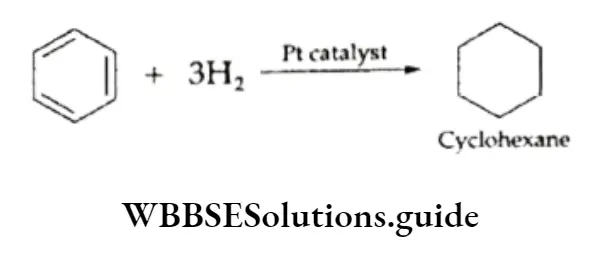
Addition Reactions
- Addition reactions of hydrogen In the presence of finely divided platinum metal,
benzene adds hydrogen to give cyclohexane.
- Addition of halogens Benzene adds three molecules of chlorine or bromine under the influence of UV light to form the corresponding hexahalide. The chlorination of benzene gives benzene hexachloride, also called lindane, which has insecticidal properties.
Oxidation
Benzene bums with a sooty flame in oxygen, giving CO2 and H2O. A large amount of heat is liberated.
⇒ \(2 \mathrm{C}_6 \mathrm{H}_6+15 \mathrm{O}_2 \longrightarrow 12 \mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O} ; \quad \Delta H^{\Theta}=-6530 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
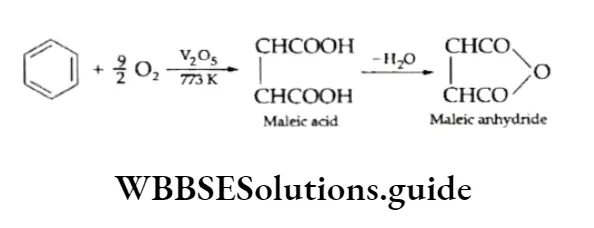
Benzene can also be oxidised in presence of vanadium pentoxide catalyst at 773 K to give maleic acid and maleic anhydride.
Directive Influence Of Substituents In Monosubstituted Benzene
We have seen that benzene forms only one monosubstituted product. However, when the substituted benzene undergoes electrophilic attack, the group already present on the ring influences the rate of the reaction as well as the site of attack.
We, therefore, say that the substituent group affects the reactivity as well as orientation in electrophilic aromatic substitution reactions.
Depending on the reactivity or how readily the reaction occurs, the substituent groups can be divided into the following two classes.
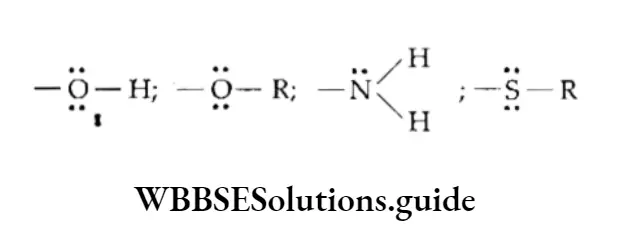
- Activating groups These groups cause the ring to be more reactive than benzene. For example, —CH3 and —NH2.
- Deactivating groups These groups cause the ring to be less reactive than benzene. For example, —COOH and —NO2.
According to orientation, the substituent groups can be divided into the following two classes.
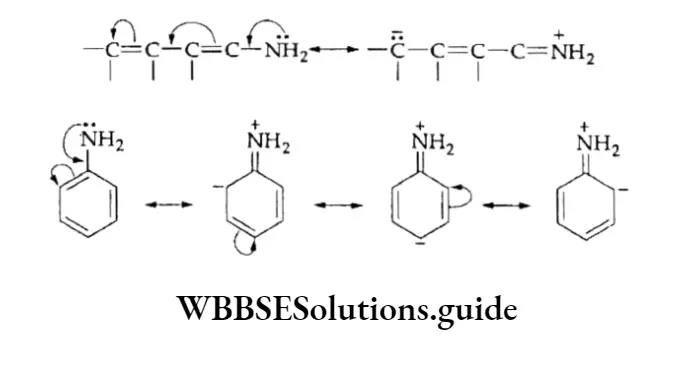
1. Ortho-para directing The substituent groups that release electrons activate the benzene ring and direct the incoming groups to ortho and para positions. For example, —CH3, —NH2 and —OH. In general, the activating groups are a-, and p-directing.
2. Meta directing The substituent groups that withdraw electrons deactivate the ring and direct the incoming groups to the meta position. For example, —NO2, —CN and —COOH. In general, the deactivating groups are directed.
Halogens are exceptions—they are deactivating and still o-, p-directing.
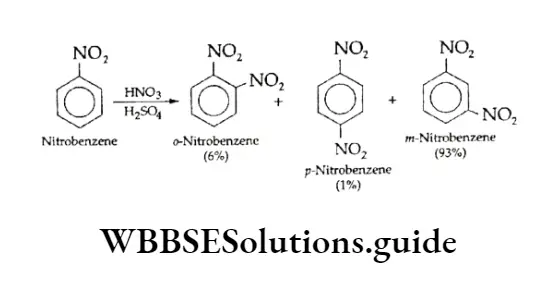
Nitration Of Toluene
Toluene on nitration gives a mixture of all three isomers, o,p and m. The yield of o,p-nitrotoluenes is almost 96%, and a very small amount of the m-isomer (4%) is formed.
The example illustrates that the CH3 group is o-, p-directing. As we have just said, the yield of o-, and p-isomers is much more than that of the m-isomer.
On the other hand, when benzene undergoes nitration, the yield is only 45- 50% which means it is less reactive than toluene.
The greater reactivity of toluene is also indicated by the fact that toluene can be nitrated under milder conditions i.e., lower temperature and lower concentration of HN03.
The alkyl group releases electrons into the ring, thus increasing the nucleophilicity of the ring and stabilising the intermediate carbocation.
To understand the reason behind the o-, p-directing nature of the alkyl group, let us observe the relative stabilities of the different carbocations formed by the electrophile attack at the o- p- and m-positions of toluene.
When the attack occurs at the ortho position, out of three canonical structures, the structure (1) is relatively stable because the positive charge is placed on the carbon atom immediately next to the alkyl group.
The canonical form (2) in case of the para attack, has a positive charge on the carbon atom adjacent to the alkyl group. There is no such equivalent structure formed by the attack at the meta position.
So the carbocations resulting from the attack at the ortho and para positions are more stable than the carbocations formed from the attack at the meta position. Thus, the alkyl groups are ortho- and para-directing. In the structures shown below, E denotes an electrophilic group.
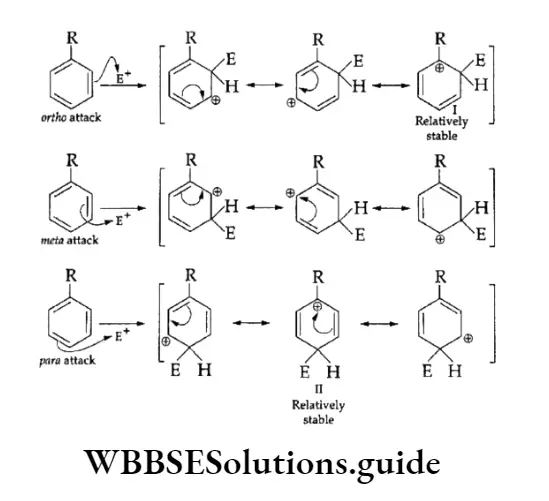
The structures shown below depict the o- and p- p-directing nature of the —OH group in the case of a phenol. The stability of structures is due to the complete octet of electrons in every atom.
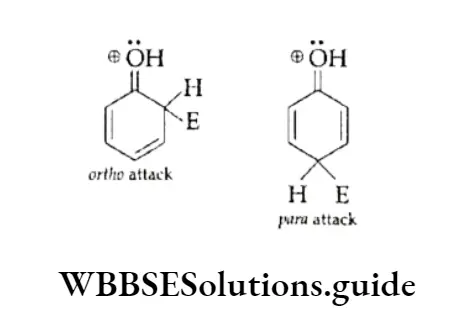
Nitration Of Nitrobenzene
Nitration of nitrobenzene gives the m-isomer in a large amount (93%).
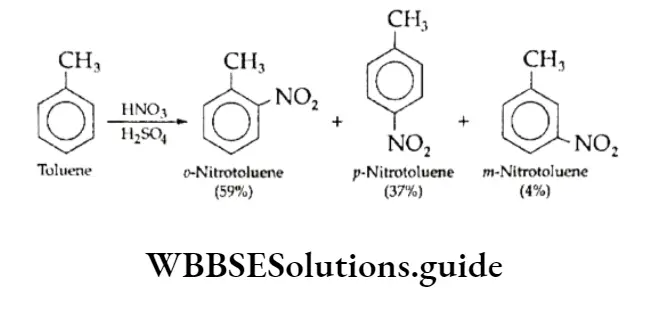
Nitrobenzene undergoes nitration at the rate of only 10″4 times that of benzene. This shows that the NO2 group is a strong deactivating group.
The nitro group is electron-withdrawing, and when present on an aromatic ring, tends to intensify the positive charge and destabilise the intermediate carbocation.
The destabilisation is more prominent in the intermediates arising from ortho and para attacks.
Thus, the meta attack is favoured. If we compare the resonance structures of carbocations formed by attacks at the ortho and para positions of nitrobenzene, the structures (1) and (2) are destabilised, because the positive charge is placed on the carbon atom next to the electron-withdrawing nitro group.
There is no such structure arising from the meta attack and so the meta attack is favoured.
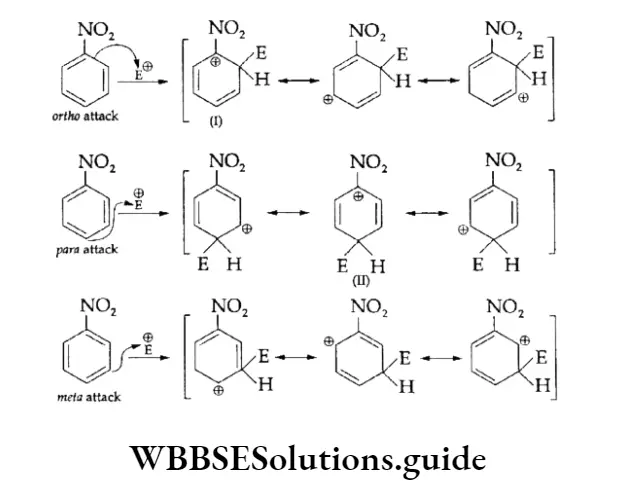
Haloarenes
Halogens deactivate the ring through the inductive effect by direct substitution to the ortho and para positions.
Halogens, being more electronegative than carbon, withdraw (-1 effect) the electrons from the aromatic ring, leaving it less nucleophilic.
However, halogens have unshared electron pairs which can delocalise the electrons in the ring by extended n bonding. This is possible only when the attack occurs at the ortho and para positions.
The resonance structures (1) and (2) formed from ortho and para attacks have the halogens bonded directly to the carbon on which the positive charge is placed.
The halogen atom is able to effect a further delocalisation of positive charge by sharing one of its nonbonding electron pairs to give two resonance structures, (3) for ortho attack and (4) for para attack.
Halogens cannot exert their stabilising influence on the intermediate when the meta position is attacked. The result is that they deactivate the ring by one effect and direct substitution to ortho and para positions by another.
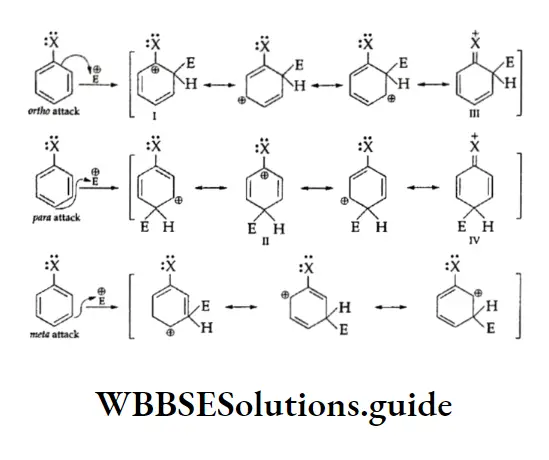
In general, except for the alkyl and phenyl substituents, all of the ortho-para directing groups have at least one pair of nonbonding electrons on the atom adjacent to the benzene ring.
On the basis of studies carried out with a large number of substituted benzene derivatives, the effect of substituents on electrophilic aromatic substitution.
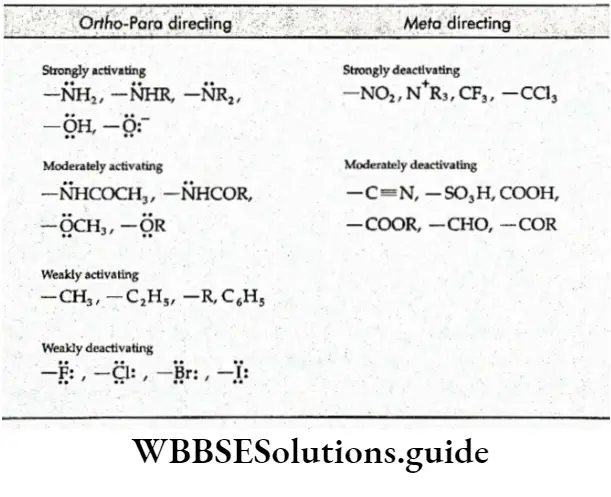
Polynuclear Hydrocarbons
Polynuclear hydrocarbons are divided into two groups—one containing isolated rings and the other containing fused rings.
Biphenyl and diphenylmethane are examples of isolated rings. When two or more rings are fused together they are known as condensed polynuclear hydrocarbons, for example, naphthalene, anthracene, phenanthrene and pyrene.
Toxicity Of Polynuclear Hydrocarbons
Although many aromatic compounds are essential for life (e.g., vitamins, hormones, steroids, purines, pyrimidines), many others are quite toxic, and several benzenoid compounds, including benzene itself, are carcinogenic. Two other examples are benzo [a] pyrene and 7-methyl [a] anthracene.
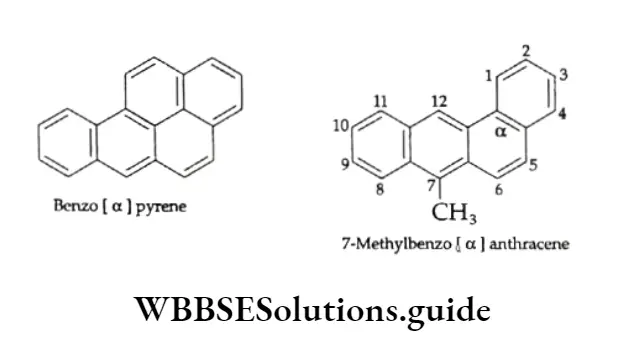
Certain molecules from the environment become carcinogenic by activation through metabolic processes.
Two such examples are dibenzo (a, 1) pyrene, a polycyclic aromatic hydrocarbon, and aflatoxin BJ, a compound formed during the metabolism of certain fungi.
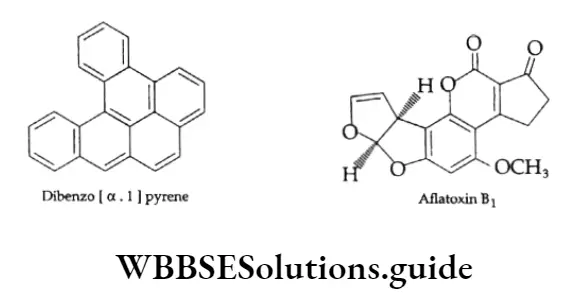
Some alkyl or alkyl derivatives of 3,4-benzphenanthrene (also called benzoic phenanthrene) have also been found to be carcinogenic. For instance, 2-methyl- 3,4-benzophenanthrene is mildly carcinogenic. People who work in industries dealing with such polynuclear aromatics are at a high risk of cancer. Also, prolonged exposure to coal tar leads to cancer. Though some hydrocarbons are toxic, many of them are useful too.
As already stated, hydrocarbons are used as fuels. In fact, as of now, they are one of the earth’s most important energy resources. For example, petroleum, LPG and CNG. Petroleum The word petroleum is derived from the Greek word ‘petra’ meaning ‘rock’ and ‘oleum’ meaning ‘oil’.
Petroleum is oily, viscous and usually dark. It is found deep below the earth’s crust, entrapped within rocks and below the surface of the oceans.
It is believed to have been formed from the dead remains of animals and trees which got buried deep inside the earth millions of years ago.
The absence of air, presence of moisture, high pressure and high temperature under the earth’s crust facilitates the conversion of dead remains to petroleum.
Petroleum is also formed from the decomposition of marine animals at the bottom of the sea.
In India, petroleum is obtained from the sea bed at Bombay High. It is found trapped in sedimentary rocks, in the oil centres situated at Assam and Gujarat. The crude oil is pumped out from the wells and is called black gold.
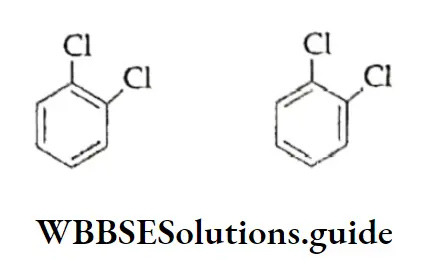
The composition of crude petroleum varies with its occurrence. However, they all contain alkanes (straight and branched-chain hydrocarbons from C1 to C40), cycloalkanes, naphthalenes and aromatic hydrocarbons. The low-boiling fractions of petroleum are composed of alkanes.
It is the composition of higher-boiling fractions which differs according to the source of petroleum Besides hydrocarbons, petroleum also contains compounds containing oxygen, nitrogen and sulphur.
LPG Liquefied petroleum gas (LPG) contains hydrocarbons like propane and butane and is obtained as a product from the refining of petroleum. The gas mixture is liquefied by compression and is used mostly as household cooking fuel. LPG is odourless, and small amounts of foul-smelling substances are added to detect any leakage.
It is also used in organic synthesis, especially of synthetic rubber. CNG (Compressed natural gas) Natural gas is a mixture of gases occurring in pockets below the earth’s surface along with petroleum. This mixture usually contains methane and ethane along with small amounts of higher hydrocarbon gases like propane and butane.
Like petroleum, it is believed to have been formed from the decomposition of organic matter trapped in layers of sedimentary rocks. CNG is widely used as a fuel. In India, it occurs in large amounts in Bombay High, the Gulf of Cambay and Ankleshwar in Gujarat.
Earlier, natural gas was considered a hazard in the petroleum industry, as it forms an explosive mixture with air. So it was ‘flared’, or burnt away. Natural gas is now recovered from the source through pipelines.
It is compressed and filled in cylinders for use. In India, CNG is used as a fuel for motor vehicles, buses, etc. The use of CNG, particularly in Delhi, has considerably reduced air pollution. Natural gas also serves as a starting material for making useful petrochemicals.
Hydrocarbons Multiple Choice Questions
Question 1. Which of the following statements is/are correct about methane?
- The carbon atom is sp3 hybridised.
- The H-C—H bond angle is109° 28′.
- It has four bonds.
- The carbon atom is sp2 hybridised.
Answer: 1. The carbon atom is sp3 hybridised.
Question 2. How many 1°, 2°, 36 and 4° carbon atoms are present in 2,2,4-trimethylpentane?
- Five 1°, one 2°, one 3°, one 4°
- Four 1°, two 2°, two 3°, zero 4°
- Four 1°, two 2°, one 3°, one 4°
- Three 1°, two 2°, two 3°, one 4°
Answer: 1. Five 1°, one 2°, one 3°, one 4°
Question 3. How many a and it bonds are present in ethene?
- 5ct, 2n
- 5a, lit
- 4a, 3it
- 6a, zero it
Answer: 2. 5a, lit
Question 4. Which of the following statements is/are correct about acetylene?
- It contains two and three bonds.
- The H—C—C bond angle is 180°.
- It contains two it and two bonds.
- The molecule is nonlinear.
Answer: 1. It contains two and three o bonds.
Question 5. Which of the following statements is/are correct about benzene?
- Each C atom in benzene is sp2 hybridised.
- All the C—C bond lengths are equal.
- All the C—Co bonds and C—Ha bonds lie in the same plane.
- Each C atom in benzene is sp3 hybridised.
Answer: 1. Each C atom in benzene is sp2 hybridised.
Question 6. Which of the following is/are correct?
- The eclipsed conformation of ethane is less stable than the staggered conformation.
- The eclipsed conformation is more stable than the staggered conformation.
- Both are equally stable.
- Both the conformations can be separated.
Answer: 1. The eclipsed conformation of ethane is less stable than the staggered conformation.
Question 7. A compound on ozonolysis gives propanal and benzaldehyde. The compound can be
- 1-Phenylbut-l-en
- Pent-2-en
- 2-Ethylbut-ene
- None of the above
Answer: 1. 1-Phenylbut-l-en
Question 8. Propanal and Pentan-3-one are the products obtained by the ozonolysis of which of the following alkenes?
- 3,4-Dimethylhept-3-ene
- 1-Phenylbut-l-ene
- 4-Ethylhex-3-ene
- None Of The Above
Answer: 3. 4-Ethylhex-3-ene
Question 9. But-l-ene and but-2-ene are
- Geometrical isomer
- Chain isomers
- Position isomers
- Optical isomers
Answer: 2. Chain isomers
Question 10. How many structures can a compound with the molecular formula C4H8 have?
- 1
- 2
- 3
- 4
Answer: 3. 3
Question 11. Which of the following statements is/are true of geometrical isomers?
- The frans-isomers of alkenes are more stable than the cis-isomers.
- The cis-isomers of alkenes are more stable than the frans-isomers.
- The c/s-isomer is more polar than the trans-isomer.
- The frans-isomer is more polar than the cis-isomer.
Answer: 1. The frans-isomers of alkenes are more stable than the cis-isomers.
Question 12. Which of the following compounds will exhibit geometrical isomerism?
- Hex-3-ene
- 3-Ethylpent-2-ene
- But-2-ene
- 1,1,2-Tribromoethene
Answer: 1. Hex-3-ene
Question 13. An aqueous solution of a compound gives ethane on electrolysis. The compound can be
- Sodium acetate
- Sodium ethoxide
- Sodium propanoate
- None of these
Answer: 1. Sodium acetate
Question 14. Among the following, the most reactive compound in the context of electrophilic nitration is
- Benzene
- Toluene
- Benzoic Add
- Nitrobenzene
Answer: 2. Toluene
Question 15. In a reaction of QH5A, the major product is an m-isomer. So A is
- -Cl
- —COOH
- —OH
- All
Answer: 4. All
Question 16. Which of the following is aromatic in nature?
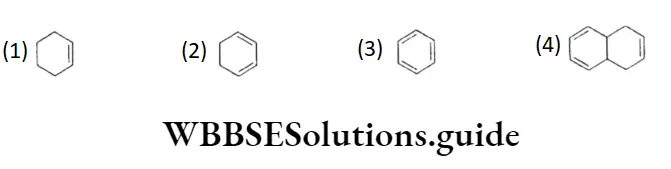
Answer: 3. 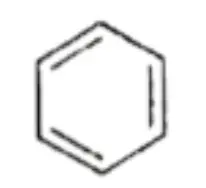
Question 17. The C—C bond distance is maximum in
- Ethane
- Ethyne
- Benzene
- All Are Equal
Answer: 1. Ethane
Question 18. The number of n electrons in naphthalene is
- 3
- 4
- 5
- 10
Answer: 4. 10