WBCHSE Class 11 Chemistry Notes On Chemical Bonding And Molecular Structure
The atom is considered to be the fundamental particle of an element, but apart from a few cases atoms cannot exist in the free state in nature. They combine to form molecules, which are capable of independent existence. This implies that a molecule must be more stable than the individual atoms. The properties of a molecule are different from those of the constituent atoms.
- What holds these atoms together in a molecule? There must be some force that does so. When atoms are held together in a molecule, we say that there is a chemical bond between them.
- Not all atoms are held together by the same type of force of attraction. All atoms do not combine. It seems that only certain combinations are allowed in nature. Only certain atoms combine in certain specific ways to form molecules.
- For example, two oxygen atoms combine to form an oxygen molecule (O2), and two hydrogen atoms combine to form a hydrogen molecule (H2), and though O3 exists, H3 does not. Noble gas atoms generally do not combine.
“Molecular structure, bonding, Class 11 Chemistry, WBCHSE, and key formulas”
Octet Rule
Scientists were curious to know the cause of the combination of atoms and made several attempts to explain how atoms are held together. The first satisfactory explanation was provided based on the configuration of the noble gases. These gases do not combine and are known to be monatomic, i.e., they exist as single atoms. Their atomic numbers and electronic configurations are as follows.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
Helium: 2 – 2
Neon: 10 – 2,8
Argon: 18 – 2,8,8
Krypton: 36 – 2,8,18,8
Xenon: 54 – 2,8,18,18,8
Radon: 86 – 2, 8,18,32,18,8
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
“WBCHSE Class 11 Chemistry, bonding and molecular structure, MCQs and notes”
- As you can see, other than helium, all the noble gases have 8 electrons in their outermost shell. Since all other elements have less than 8 electrons in their outermost shell, scientists concluded that the chemical inertness of the noble gases is related to the presence of 8 electrons in their outermost shell.
- In other words, they concluded that if an element has 8 electrons in its outermost shell, it is chemically unreactive, or its electronic configuration is stable.
- These observations led an American chemist, G N Lewis, to put forward a generalization known as the octet rule.
- According to this rule, atoms of elements combine so as to attain the stable configuration of 8 electrons in their outermost shell. An atom may attain this configuration by gaining, losing, or sharing electrons with other atoms.
Valency Of Nitrogen
Exceptions to the octet rule: The octet rule is a simple and useful generalization that can explain the formation of a large number of compounds. However, it cannot be applied in some cases.
1. Hydrogen has only one electron in its valence or outermost shell, and it needs only one more electron to fill this shell and attain a stable configuration. This is possible because by acquiring one more electron, the hydrogen molecule attains a configuration like that of the noble gas helium. It may be noted that there is no octet here as the first shell can accommodate only two electrons.
2. According to the octet rule, elements of groups 1, 2, and 13 should not form covalent bonds because, having less than 4 electrons in their valence shells, they should not be able to attain stable configurations by sharing electrons. But some elements of these groups do form covalent compounds which are called electron-deficient compounds, for example, BeCl2 (beryllium has only 4 electrons in the outermost shell) and BF3 (boron has only 3 electrons).
“WBCHSE Class 11 Chemistry, bonding and molecular structure, notes and explanations”
3. The octet rule cannot explain the formation of compounds such as PCI5 and SF6 in which the central atom contains more than eight electrons. The central atom is said to have an expanded octet. An expanded octet is mostly found in the elements beyond the third period when d orbitals are available for bonding.
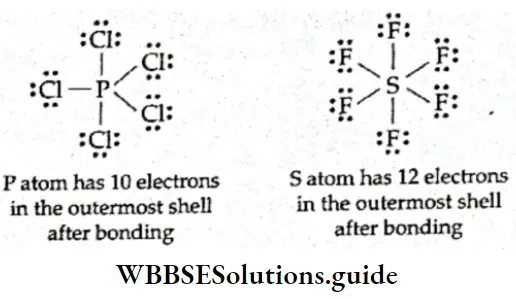
4. The basic assumption on which the octet rule is formulated is that noble gases are inert. However, compounds of xenon like XeF2, XeF4, XeF6 have been prepared.
5. Some molecules like NO and NO2 are called odd-electron molecules as the octet rule does not apply to all the atoms in the molecule.
“WBCHSE Class 11, chemistry notes, on bonding, VSEPR theory, and hybridization”
The Lewis dot representation of these two molecules shows that the octet of the nitrogen atom in both molecules is incomplete (there are 7 electrons).
⇒ \(\ddot{\mathrm{N}}=\ddot{O} \quad \ddot{O}=\dot{\mathrm{N}}^{+}-\ddot{\mathrm{O}}^{-}\)
Lewis Structure The Concept Of Valence Electrons
Another observation that helped scientists understand how atoms combine was that atoms which f have the same number of electrons in their outermost shells exhibit similar chemical properties. This led them to conclude that only the electrons in the outermost shell participate in the process of chemical combination.
- The electrons in the inner shells do not participate in bonding. The outermost shell electrons are called valence electrons. The chemical properties of an element depend on the number of valence electrons.
- Lewis introduced a simple notation to represent the valence electrons in an atom. In this notation, the valence electrons are represented by dots (or crosses) surrounding the chemical symbol of the element.
- These symbols, called Lewis symbols or electron dot symbols, do not show the electrons in the inner shells. The chemical symbol of the element represents the nucleus and the inner electrons. The Lewis symbols for the elements of the second period are given below.
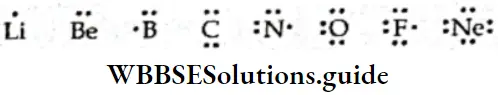
The number of dots in the Lewis symbol of an element indicates the number of electrons in the outermost shell (valence shell) electrons. Consider the symbols for Li, Be, B, and C. The number of dots in these symbols indicates the usual valence of these elements. Thus lithium is monovalent, beryllium is divalent, boron is trivalent and carbon is tetravalent.
- The valencies of nitrogen, oxygen, and neon are 3,2 and 0 respectively. The valence of fluorine is 1. That is to say the valence of these elements can be obtained from the Lewis symbols by subtracting the number of dots from 8.
- Whatever may be the drawbacks of the octet rule, most atoms do tend to combine to attain a more stable configuration of 8 electrons in the valence shell. They do so by the redistribution of their valence electrons.
This can occur in one of two ways:
- By the complete transfer of one or more electrons from one atom to another. The chemical bond thus formed is called an ionic bond.
- By the sharing of electrons between atoms. The bond formed is called a covalent bond.
Electrovalent Or Ionic Bond
Kossel, while studying chemical bonding, observed that the highly electronegative halogens form a negative by gaining an electron whereas the highly electropositive alkali metals form a positive ion by readily losing an electron. The elements of the two groups (halogens and alkali metals) gain or lose electrons respectively and attain a stable outer shell configuration of eight electrons.
The noble gases (Group lb elements) have the stable outer shell configuration of eight electrons except helium, which has just two electrons. Kossel explained that the negative and positive ions (formed by gain or loss of electrons) are held together by electrostatic attraction among them. This electrostatic attraction was later termed the electrovalent bond.
- Kossel’s observations led to the understanding of ionic bonding in ionic compounds. It also provided the basis of the concept of electron transfer in ion formation and thereafter the formation of ionic compounds. Besides providing a concept of ionic bonding, Kossel realized that not all compounds are formed by electron transfer between atoms.
- Atoms that contain 1, 2, or 3 electrons in their valence shell tend to lose electrons to acquire a stable configuration. It is easier for such atoms to lose 1,2 or 3 electrons than to gain 7,6 or 5 electrons respectively. Similarly, atoms that have 5, 6, or 7 valence electrons tend to complete their valence shell by gaining 3,2 or 1 electron respectively.
- Atoms are electrically neutral as they possess an equal number of electrons and protons. However, when an atom loses electrons to attain a stable configuration, it becomes positively charged because it contains a greater number of protons than electrons.

A positively charged atom is called a cation. When, on the other hand, an atom gains electrons to attain a stable configuration, it becomes negatively charged because it has a greater number of electrons than protons. A negatively charged atom is called an anion.
“Bonding and molecular structure, WBCHSE, Class 11 Chemistry, short and detailed notes”
- When one atom acquires the electrons that another atom loses, the oppositely charged ions so formed are drawn together by an electrostatic force of attraction. When two ions are held together in this way, an ionic bond or an electrovalent bond is said to exist between them. In other words, the bond between oppositely charged ions, formed by the tire transfer of electrons from one atom to another, is called an ionic bond.
- Such bonds are generally formed between a metal and a nonmetal (as discussed above, alkali metals and halogens respectively).
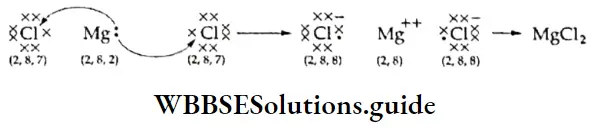
- For example, the sodium atom (atomic number 11) has only one electron in its valence shell. To acquire the nearest noble gas configuration (2, 8) it has to lose one electron.
- The chlorine atom (atomic number 17) has seven electrons in its valence shell and it is easier for it to gain an electron to acquire the nearest noble gas configuration (2, 8, 8). So, the sodium atom transfers its valence electron to the chlorine atom, resulting in tire formation of a sodium ion (Na+) and a chloride ion (Cl–), which are held together by a force of electrostatic attraction.
Class 11 WBCHSE Chemistry Notes On Ionic And Covalent Bonds
The number of electrons that an atom loses or gains while forming an ionic bond is called its electrovalency. Atoms that tend to lose electrons are called electropositive because they form positively charged ions after losing electrons. Atoms that gain electrons are called electronegative because they form negatively charged ions after gaining electrons. One more example of an ionic compound is given here.
Ionic bonds are formed due to electrostatic forces of attraction, which are non-directional. An ion has a uniform electrostatic field of influence around it, i.e., it attracts ions all around it. The attraction between two ions depends on the distance between them, and not on the direction of one from the other.
- Each positively charged ion attracts several negatively charged ions around it and each negative ion attracts several positive ions around it. The number of oppositely charged ions a particular ion will attract depends on its size and charge.
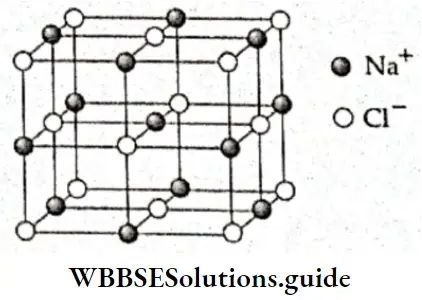
- This aggregation of oppositely charged ions around one ion results in the formation of a three-dimensional aggregate of ions called an ionic crystal. Oppositely charged ions alternate in a regular geometrical pattern in such a crystal. The cations and anions are held together in the crystal by attractive forces.
- An ionic compound consists of an aggregate of ions. These ions held together by an ionic bond cannot be looked upon as a molecule. Thus, an ionic compound cannot be assigned a molecular formula. For example, the formula of sodium chloride can be written as NaCl or Na+Cl–. This is its empirical formula and not its molecular formula.
- The empirical formula of an ionic compound can be derived from the valency of the elements or the ratio of cations to anions. In NaCl, the ratio is 1:1. So, the (empirical) formula of sodium chloride is NaCl.
“Bonding and molecular structure, WBCHSE Class 11, chemistry notes, and key concepts”
Formation of an ionic bond: The formation of an ionic bond broadly involves the formation of the gaseous cation, the gaseous anion, and the packing of ions to form the ionic solid.
Formation of cations and anions You have already studied in Chapter 3 that energy is required to remove an electron from an isolated neutral gaseous atom, and is defined as the ionisation enthalpy of the element. Thus, the formation of a positive ion or removal of electron(s) involves the process of ionisation. Let us consider the formation of NaCl.
⇒ \(\mathrm{Na}(\mathrm{g}) \rightarrow \mathrm{Na}^{+}(\mathrm{g})+\mathrm{e}^{-} ; \Delta H\) (Ionisation enthalpy)
On the other hand, chlorine atom accepts an electron to form a negative ion (Cl–). The enthalpy change in this reaction involving addition of electron(s) is termed as electron gain enthalpy.
⇒ \(\mathrm{Cl}(\mathrm{g})+\mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}(\mathrm{g}) ; \Delta H\)(Electron gain enthalpy)
The ionisation enthalpy of an atom is always positive (energy is always required to remove an electron) but the electron gain enthalpy of an atom may be positive or negative, depending on the nature of the element.
- Considering the enthalpy changes during formation of ions, we may conclude that ionic bonding is most likely to occur between atoms with low ionisation enthalpies and high negative electron gain enthalpies.
- Generally, ionic compounds are formed by cations from metallic elements (metals have low ionisation enthalpies) and anions from nonmetals (nonmetals have high negative electron gain enthalpies).
The conditions required to form an ionic bond are best satisfied in alkali halides. The cation NH+4 (ammonium ion) is an exception, it is made up of two nonmetals.
Packing of oppositely charged ions The last step in the formation of an ionic compound is the packing of negative and positive ions to form a lattice. The energy released in this process is known as lattice energy.
More formally, the energy released in the formation of 1 mole of an ionic solid by the packing of oppositely charged ions is called lattice enthalpy. The lattice enthalpy may also be defined as the energy needed to separate one mole of a solid ionic compound into the constituent gaseous ions. The lattice enthalpy of sodium chloride is 788 kJ mol-1. This means that 788 kJ of energy is needed for the following reaction to occur.
⇒ \(\mathrm{NaCl}(\mathrm{s}) \rightarrow \mathrm{Na}^{+}(\mathrm{g})+\mathrm{Cl}^{-}(\mathrm{g})\)
The higher the value of the lattice enthalpy of an ionic compound, the more stable it is, and the more readily will such a compound be formed. Ionic compounds are held together by electrostatic attraction and the force of attraction depends directly on the magnitude of the charges and inversely on the square of the distance between the charges.
F = \(\frac{q_1 q_1}{d^2}\)
The greater this force, the more the chance of formation of the ionic compound. In other words, a greater magnitude of charge and a smaller ionic size favours the formation of an ionic solid. A smaller ionic size would mean a smaller internuclear distance.
- For stable ionic bonding, the total energy released during the formation of the crystal lattice should be greater than the energy required for the formation of ions.
- In the case which we have just considered, the first ionisation enthalpy for sodium is 495.8 kJ mol-1 and the electron gain enthalpy for chlorine is -348.7 kJ mol-1. Their sum comes to 147.1 kJ mol-1 which is actually the energy required for the formation of ions. This energy is much less than that released during the formation of the crystal lattice, which is equal to 788 kJ mol-1. Thus, a greater lattice enthalpy implies greater stability of the compound.
Properties of ionic compounds: By and large, ionic compounds have certain common characteristics.
“Class 11 Chemistry, WBCHSE, chemical bonding, molecular structure, and revision notes”
Physical state They are usually crystalline solids, in which the ions are arranged in a well-defined geometrical pattern. The force of attraction between the oppositely charged ions is very strong, which is why these compounds exist in the solid state at room temperature.
Melting and boiling points Ionic compounds generally have high melting and boiling points. This is because a large amount of energy is required to overcome the strong electrostatic force of attraction which holds the ions together in such compounds.
Electrical conductivity Ionic compounds are not good conductors of electricity in the solid state because the ions are held in fixed positions in the crystal lattice by electrostatic force of attraction between ions. The ions are, thus, not free to move. However, they become free to move in the molten state and in aqueous solutions, and carry charge. So ionic compounds are good conductors of electricity in the molten or dissolved state.
“WBCHSE Class 11 Chemistry, bonding theories, molecular orbitals, and notes”
Solubility Ionic compounds are soluble in water and other polar solvents. Such solvents can weaken the force of attraction that holds together the ions in an ionic compound. Such compounds are insoluble in nonpolar solvents like benzene and carbon tetrachloride.
Ionic reactions Chemical reactions between ionic compounds are called ionic reactions. Such reactions take place between the ions of the two compounds produced in an aqueous solution. These reactions involve oppositely charged ions which combine readily, so they occur almost instantaneously.
Hydrogen Bond
Hydrogen Bond:
The hydrogen atom has a unique structure. It has only one proton and one electron. When a hydrogen atom forms a covalent bond with a highly electronegative atom, the bonded electron pair shifts towards the latter. Consequently, the nucleus of the hydrogen atom gets exposed and behaves like a proton.
It exerts a strong electrostatic force of attraction on the electronegative atom of an adjacent molecule. This force of attraction between the (covalently bonded) hydrogen atom of one molecule and the electronegative atom of another molecule is called a hydrogen bond. It is represented by a dotted line.
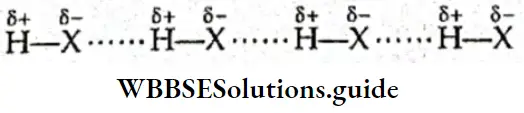
The following conditions are necessary for hydrogen bonding.
1. The molecule must contain a highly electronegative atom linked to the hydrogen atom. The greater the electronegativity of the atom linked to the hydrogen atom, the stronger is the hydrogen bond.
2. The size of the electronegative atom should be small. The smaller the size, the greater is the attraction between the atom and the hydrogen atom and the stronger is the bond.
Only F, O, and N satisfy both these conditions.
Fluorine, having the highest value of electronegativity, forms the strongest hydrogen bond.
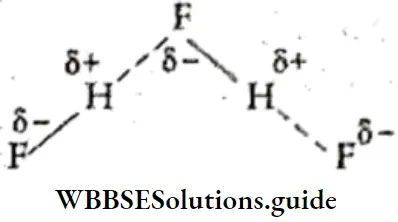
The water molecule contains the highly electronegative oxygen atom linked to the hydrogen atom. The oxygen atom attracts the shared pair of electrons more strongly and this end of the molecule becomes negative, while the hydrogen-end becomes positive.
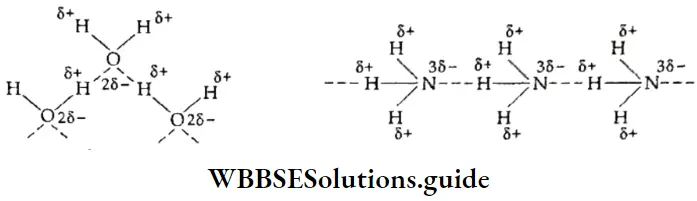
In ammonia, the more electronegative nitrogen atom attracts the shared electrons towards itself, leaving the hydrogen atom positively charged. This hydrogen atom forms a hydrogen bond with the nitrogen atom of another molecule.
“WBCHSE Class 11, bonding and molecular structure, study notes, and solved examples”
Nature of hydrogen bond: The hydrogen bond is a weak bond. Unlike the covalent bond, which involves the sharing of electrons by two atoms of elements of the same or different electronegativities, the hydrogen bond arises due to the interaction between dipoles.
The strength of the hydrogen bond lies between those of the van der Waals force and the covalent bond. The dissociation energy of the hydrogen bond depends upon the electronegativity of the other atom. The bond energy is highest in H—F because fluorine is the most electronegative element.
Impact of hydrogen bonding: Although the hydrogen bond is weak, it influences the physical properties of the substances in which it acts. Association The hydrogen bond links molecules to form aggregates of molecules.
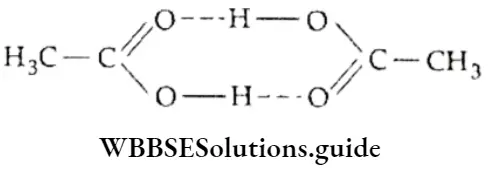
For example, HF molecules become linked by hydrogen bonding and the formula of hydrogen fluoride can be written as (HF)π. This changes the molecular mass of the compound. For instance, carboxylic acids exist as dimers because of hydrogen bonding and their molecular mass is double the molecular mass predicted by their simple formula. The molecular mass of acetic acid is 120 but its mass according to its simple formula should be 60.
Dissociation In an aqueous solution, hydrogen fluoride dissociates and produces the difluoride ion (HF–2) instead of the fluoride ion (F–). This is due to H-bonding in HF. This explains why KHF2 exists, while KHCl2, KHBr2, and KHI2 do not. The other halogens cannot form hydrogen bonds because of low electronegativity and bigger size.
High melting and boiling points Compounds in which the molecules are linked by hydrogen bonds have much higher melting and boiling points than compounds formed by other members of their groups. Tins is because a greater amount of energy is required to break the hydrogen bonds.
- Let us consider the hydrogen halides, for example. Hydrogen fluoride exists as a liquid (high boiling point) at room temperature, while HCl is a gas. The boiling points of the hydrogen halides decrease as molecular mass decreases, but there is a sudden increase in the boiling point of HF because of the presence of the hydrogen bond.
- Similarly, H2O is a liquid, but H2S is a gas at room temperature. In fact water has an abnormally high boiling point as compared to the other hydrides of its group. NH3 has an abnormally high boiling point as compared to the hydrides of the other elements of its group.
- This difference is seen among organic compounds too. For example, ethanol has a higher boiling point than diethyl ether because of the presence of hydrogen bonding in the former. Both compounds have oxygen atoms, but in diethyl ether, the oxygen atom is not bonded to hydrogen.
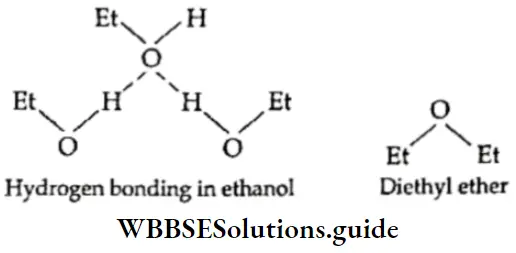
Solubility Covalent compounds are generally insoluble in polar solvents like water. However, compounds which can form hydrogen bonds with water are soluble in water, example, alcohol, acetone, and urea.
Similarly, ammonia (NH3) dissolves in water but PH3 (phosphine) does not. This is because of the ability of the nitrogen atom to form a hydrogen bond with water.
Viscosity Viscosity is the resistance offered to the flow of a liquid. The viscosity of a liquid increases if there is hydrogen bonding between its molecules because this is an additional intermolecular force which aggregates molecules. For example, glycerol is more viscous than ethylene glycol and ethyl alcohol. All alcohols have at least one —OH group which can form a hydrogen bond. Glycerol has three —OH groups, ethylene glycol has two, while ethyl alcohol has only one.
Density of ice Ice has a lower density than water, though hydrogen bonding is present in both water and ice. In ice, each oxygen atom is tetrahedrally bonded with four hydrogen atoms. Two hydrogen atoms are bonded by covalent bonds and another two through hydrogen bonds. This gives rise to an open cage-like structure, which occupies a larger volume than does water. In the liquid, the water molecules are more closely packed. When ice melts, this cage-like structure collapses and the water molecules come closer to each other. Thus, for the same mass of water, the volume decreases and density increases.
Types of hydrogen bonding: There are two types of hydrogen bonding—intermolecular and intramolecular.
Intermolecular When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding. For example, HF, H2O, NH3, and alcohol.
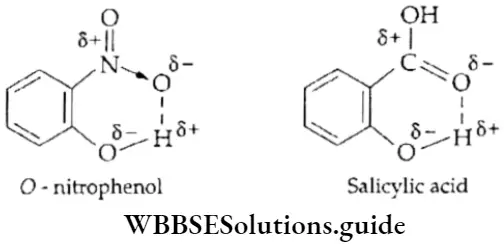
Intramolecular Hydrogen bonds between atoms of the same molecule are called intramolecular hydrogen bonds. Such bonds are formed in many organic compounds, example, o-nitrophenol and o-hydroxy benzoic acid (salicylic acid).
Intramolecular H-bonding prevents the association of molecules, making the molecule contract. Hence, this kind of H-bonding decreases melting and boiling points and solubility. For example, o-nitrophenol has a lower boiling point than p-nitrophenol.
Resonance
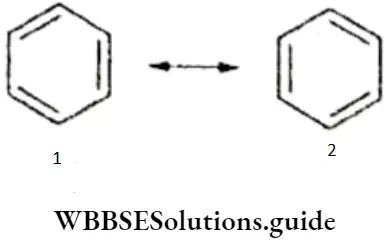
The properties of certain molecules containing a π bond cannot be explained by writing one Lewis structure. For example, the structure of ozone can be written as either (1) or (2) here, but neither can explain its experimentally determined properties.
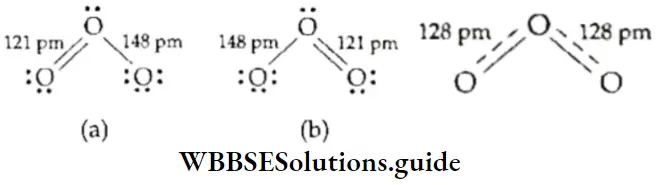
- According to either of these structures, the central oxygen atom is bonded to one oxygen atom by a double bond and the other oxygen atom by a single bond. Since a double bond (121 pm) is shorter than a single bond (148 pm), the two bond lengths in this molecule should be different.
- However experimental evidence shows that the bonds are equal and that the bond length is 128 pm, between the length of a single bond and that of a double bond. Thus, neither structure (1) nor (2) can explain why O3 has two equal bonds.
- It is assumed that tire actual structure is between the two Lewis structures. This phenomenon is called resonance and the two (or more) individual structures are called resonating structures or canonical forms. To define this more clearly, when a molecule can be represented by more than one Lewis structure none of which can individually explain all its observed properties, the actual structure of the molecule is the intermediate of the various Lewis structures and is called the resonance hybrid.
WBCHSE Class 11 Notes On VSEPR Theory And Molecular Geometry
This is a theoretical concept. Tire-resonating structures have no physical reality, that is to say, they do not actually exist. Only the hybrid structure exists. There is no equilibrium between the canonical forms. In theory the various resonating structures have the following characteristics.
- The same arrangement of atoms
- The same of number of paired and unpaired electrons, but different electronic arrangement
- They have nearly the same energy but the energy of the resonance hybrid is less than that of each of the canonical forms. Thus, resonance stabilises the molecule
- The same positions of sigma bonds but different positions of pi bonds
Some examples are given below.
1. Carbon dioxide (CO2) The C—O bond length in the molecule is experimentally determined as 115 pm. The value is between the length of C=O (121 pm) and C≡O (110 pm). Therefore, the structure of CO2 can be described as a resonance hybrid of three canonical forms.
∴ \(: \mathrm{O}=\mathrm{C}=\mathrm{O}: \longrightarrow \stackrel{+}{\mathrm{O}} \equiv \mathrm{C}-\overline{\mathrm{O}}: \longrightarrow: \overline{\mathrm{O}}-\mathrm{C} \equiv \stackrel{+}{\mathrm{O}}:\)
“Chemical bonding, molecular structure, WBCHSE Class 11, and important questions
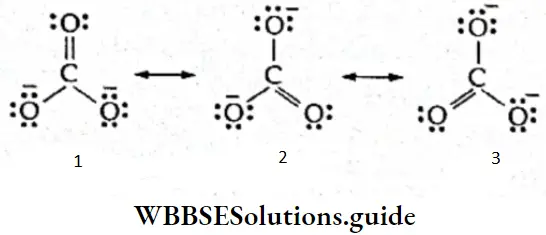
2. Carbonate ion (\(\mathrm{CO}_3^{2-}\)) Since the experimentally determined bond length values show that all the carbon- oxygen bonds are equal in a carbonate ion, its structure can be described as the resonance hybrid of the three canonical forms as follows.
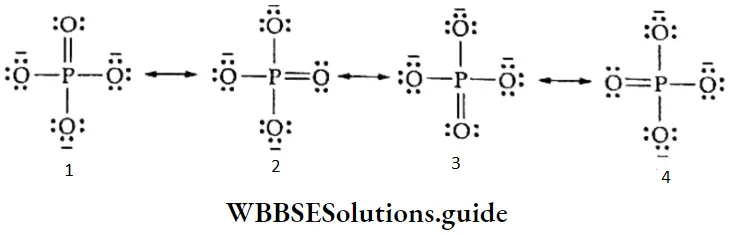
3. Phosphate ion \((\left(\mathrm{PO}_4^{3-}\right))\) Experimental findings show that all the phosphate oxygen bonds are equal in the phosphate ion. Therefore, the structure of the ion is best described as the resonance hybrid of the following four canonical forms.
4. Benzene (C6H6) It has been found that all carbon-carbon bond lengths are identical and the two important canonical forms are