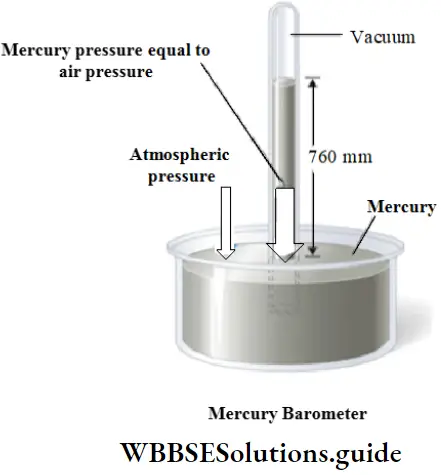
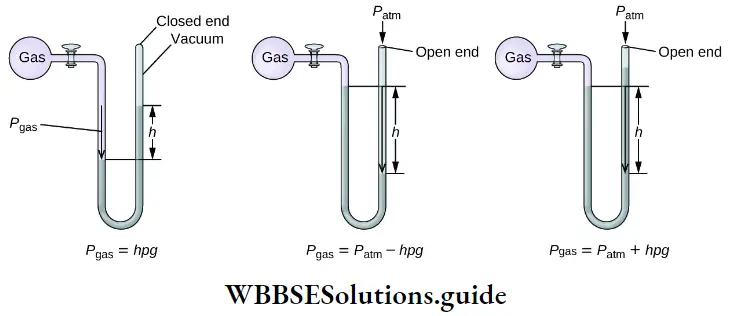
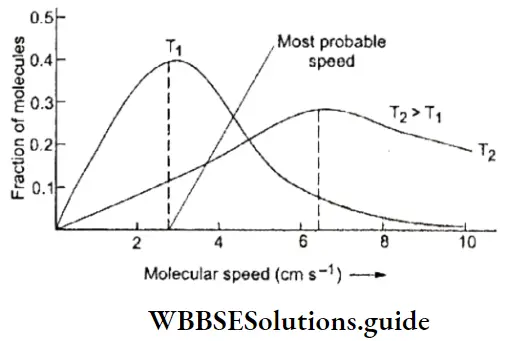
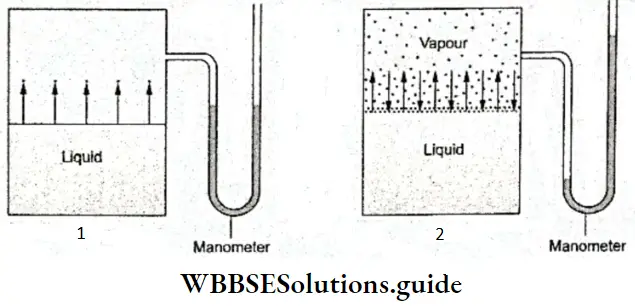
Water
An essential constituent of all animal and vegetable matter, water is the most abundant substance on earth.
It occurs as a colourless liquid at room temperature and is present as water vapour in the air which condenses at low temperatures to form mist, rain, hail or snow, depending on the atmospheric conditions.
Water on the earth’s surface in the form of oceans, rivers, etc., covers about three-quarters of the earth’s crust.
Of the total amount of water on the earth, oceans and other saline water bodies constitute 97.3%.
Most of the freshwater, amounting to about 2.7%, is not readily accessible since it occurs in the form of frozen lakes, glaciers, etc. Water available for human use is far less than 1% of the total water on the earth.
Water – Physical Properties
Water is a colourless, odourless and tasteless liquid. Pure water is neutral to litmus and is a bad conductor of electricity.
“WBCHSE Class 11 Chemistry, properties of water, physical and chemical”
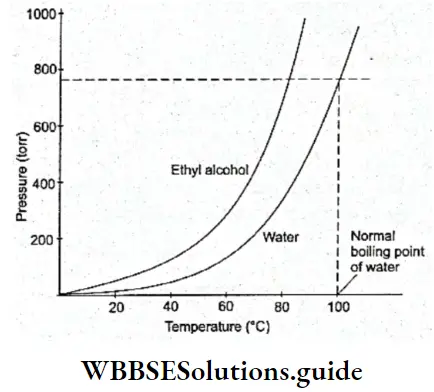
Its b.p. is 373 K at ATM pressure. It has a maximum density (1 g cm-3) of 277 K. Due to the presence of hydrogen bonding, water has a high boiling point, enthalpy of fusion (6.01 kJ Mol-1) and enthalpy of vaporisation (40.66 kJ Mol-1).
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
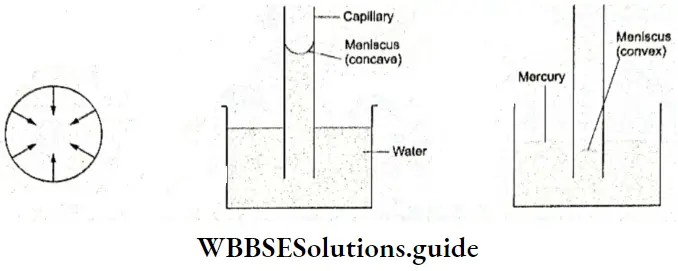
Water has a high specific heat, thermal conductivity and surface tension (compared to most of the other liquids). These properties are responsible for its vital role in the biosphere.
The moderation of the climate and body temperature regulation is due to the high enthalpy of vaporisation and high heat capacity of water. It is polar in nature and an excellent solvent for many other polar and ionic substances.
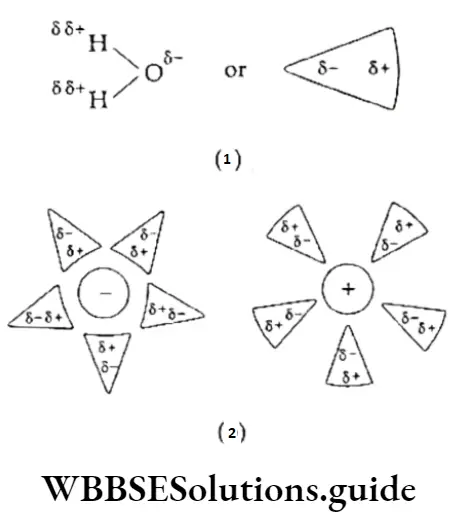
Why is the water molecule polar? The difference in the electronegativity of oxygen and hydrogen in the water molecule makes the covalent bond polar. Thus, the molecule behaves as a tiny dipole.
The slight charges on the water molecules are attracted to the charges on ions.
When an ionic solid is placed in water, the ions from its surface are pulled into the solution.
Since water has the capacity to dissolve most of the inorganic substances, it is regarded as a universal solvent.
A few organic substances such as urea, alcohol, sugar, etc., also dissolve in water. This is due to the presence of intermolecular hydrogen bonding in the molecules of the solute and water.

Water Chemical Properties
1. Dissociation of water Being stable, the water molecule does not dissociate into its constituent elements even at high temperatures. However, pure water has a small electrical conductivity. It dissociates as
Given
Dissociation of water Being stable, the water molecule does not dissociate into its constituent elements even at high temperatures. However, pure water has a small electrical conductivity.
⇒ \(\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq}) hydronium ion\)
From this reaction, it is clear that water acts as an acid as well as a base. In other words, it is amphoteric. The following reactions illustrate the nature of water.
“Properties of water, WBCHSE Class 11, chemistry notes, and key characteristics”
⇒ \(\begin{aligned}
&\mathrm{H}_2 \mathrm{O}+\mathrm{HCl} \longrightarrow \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{Cl}^{-}\\
&\underset{\text { did }}{\mathrm{H}_2 \mathrm{O}}+\underset{\text { base }}{\mathrm{NH}_3} \longrightarrow \underset{\text { acid }}{\mathrm{NH}_4^{+}}+\underset{\text { base }}{\mathrm{OH}^{-}}
\end{aligned}\)
The self-ionisation of water has significance in acid-base chemistry.
2. Reactions with metals Alkali metals like Na and K react with cold water.
⇒ \(2 \mathrm{Na}+2 \mathrm{H}_2 \mathrm{O}(\text { cold }) \longrightarrow 2 \mathrm{NaOH}+\mathrm{H}_2\)
However, alkaline earth metals, e.g., Mg, react with hot water.
⇒ \(\mathrm{Mg}+\mathrm{H}_2 \mathrm{O} \text { (warm) } \longrightarrow \mathrm{MgO}+\mathrm{H}_2\)
Thus, water is easily reduced by highly electropositive metals.
Reactions with nonmetals Fluorine reacts with cold water to form hydrogen fluoride and liberates oxygen. Thus, water is oxidised and fluorine is reduced
⇒ \(2 \mathrm{~F}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{H}_2 \mathrm{~F}_2+\mathrm{O}_2\)
Chlorine forms hydrochloric acid and hypochloric acid upon reacting with water. Bromine is less reactive with water and iodine does not react at all.
⇒ \(\mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{HCl}+\mathrm{HOCl}\)
“WBCHSE Class 11, chemistry notes, on physical and chemical properties of water”
⇒ \(\mathrm{Br}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{HBr}+\mathrm{HOBr}\)
However, in the presence of sunlight, water reacts only with chlorine (among the halogens) forming hydrochloric acid and liberating oxygen.
⇒ \(2 \mathrm{Cl}_2+2 \mathrm{H}_2 \mathrm{O} \stackrel{\text { sunlight }}{\longrightarrow} 4 \mathrm{HCl}+\mathrm{O}_2\)
Carbon decomposes steam, liberating hydrogen. Thus, water is reduced to hydrogen and carbon is oxidised to carbon monoxide.
⇒ \(\mathrm{C}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{CO}+\mathrm{H}_2\)
Reactions with oxides Metallic oxides react with water to form leases. For example,
⇒ \(\mathrm{Na}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{NaOH}\)
Reactions with oxides Metallic oxides react with water to form leases. For example
⇒ \(\mathrm{Na}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{NaOH}\)
However, nonmetallic oxides react with water to form acids
⇒ \(\begin{aligned}
& \mathrm{SO}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}_2 \mathrm{SO}_3 \\
& \mathrm{SO}_3+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}_2 \mathrm{SO}_4
\end{aligned}\)
Hydrolysis of compounds Water hydrolyses many compounds like hydrides, nitrides and carbides, liberating hydrogen, ammonia and acetylene respectively.
⇒ \(\mathrm{CaH}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_2+\underset{\text { ditydrogen }}{2 \mathrm{H}_2}\)
⇒ \(\mathrm{AlN}+3 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Al}(\mathrm{OH})_3+\mathrm{NH}_3\)
⇒ \(\mathrm{Mg}_3 \mathrm{~N}_2+6 \mathrm{H}_2 \mathrm{O} \longrightarrow 3 \mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{NH}_3\)
⇒ \(\mathrm{CaC}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_2+\underset{\text { acetylene }}{\mathrm{C}_2 \mathrm{H}_2}\)
Water also hydrolyses compounds, forming acids.
⇒ \(\mathrm{PBr}_3+3 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{H}_3 \mathrm{PO}_3+3 \mathrm{HBr}\)
Water as a catalyst In many reactions, water acts as an effective catalyst. Perfectly dry gases fail to react and a trace of moisture promotes the reaction.
For example, hydrogen and chlorine do not react in the dry state but do so in the presence of moisture.
Hydrates Water forms compounds with some metal salts called hydrates, of which there are three types.
Complex compounds are formed when water molecules combine with metal ions through coordinate bonds, e.g.,
⇒ \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Li}\left(\mathrm{H}_2 \mathrm{O}\right)_5\right]^{+} \text {and }\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
Compounds in which water molecules are hydrogen bonded to certain oxygen-containing anions, e.g., CuSO45H2O with four water molecules coordinated to the central Cu2+ ion through the lone pairs on the oxygen atoms while the fifth water molecule is hydrogen bonded to a sulphate group Compounds in which water molecules may occupy interstitial sites in crystal lattices, e.g., BaCl2 2H2O
Structure Of The Water Molecule And Its Aggregates
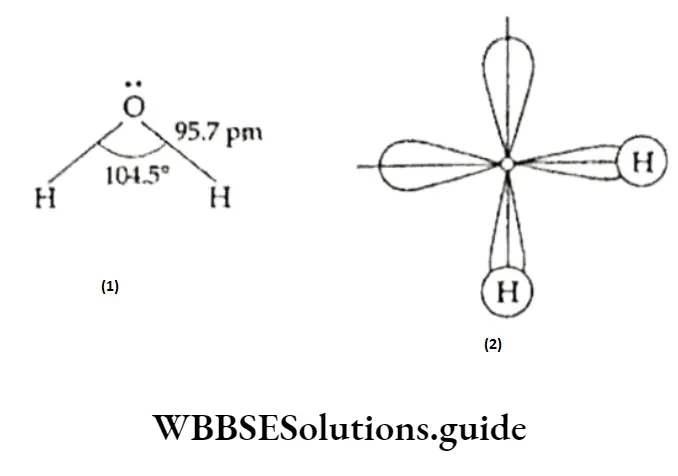
In a tire water molecule, oxygen is the central atom and has six valence electrons. Two of the valence electrons of oxygen form covalent bonds with two hydrogen atoms, thus completing the octet.
The other two pairs of valence electrons of oxygen are nonbonding. Thus, in the water molecule, there are two bond pairs and two lone pairs, and their interactions with each other reduce the bond angle.
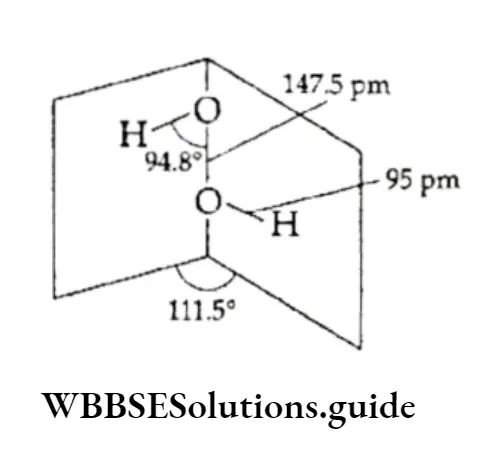
Thus, in the gaseous form, the water molecule has an O —H bond length of 95.7 pm and an HOH bond angle of 104.5°.
As already stated, due to the high electronegativity of oxygen, the water molecule is highly polar.
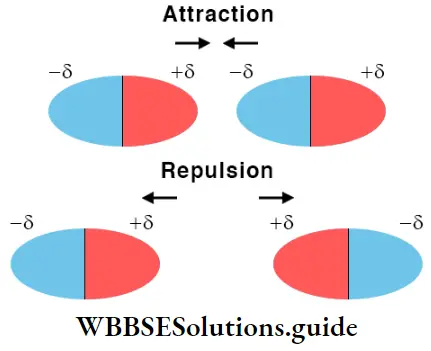
The partial negative charge on the oxygen atom in a water molecule attracts a hydrogen atom (which has a partial positive charge) of another water molecule.
Also, the partial positive charge on a hydrogen atom attracts the partial negative charge on the oxygen atom of still another molecule of water.
“Physical and chemical properties of water, structure, and bonding, WBCHSE syllabus”
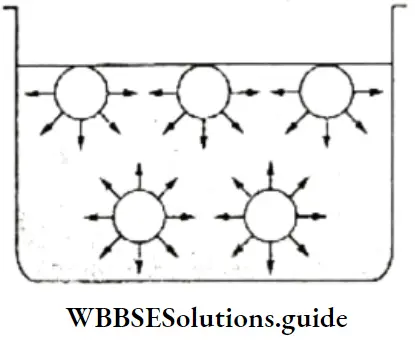
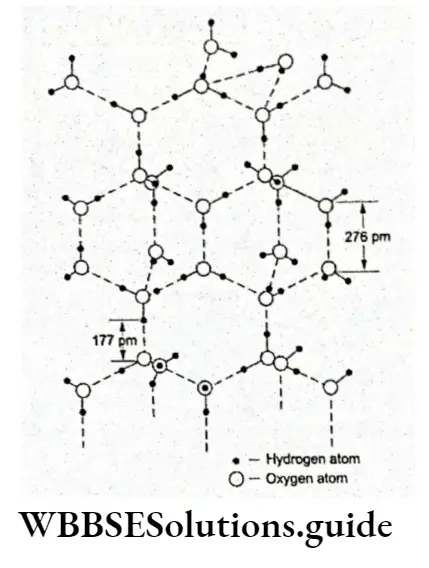
Thus, in the liquid state, the water molecules are held together by hydrogen bonds resulting from a weak electrostatic attraction. The hydrogen bonding in water can be shown as follows.
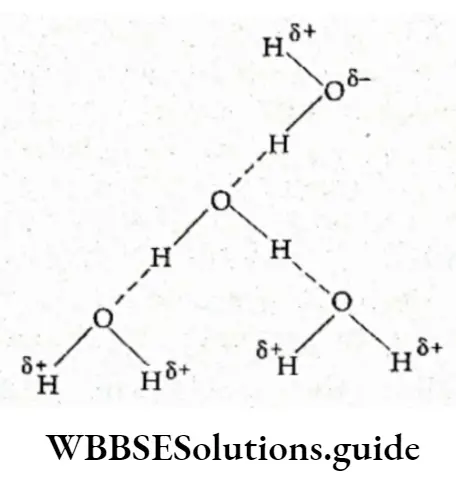
Water is a liquid at room temperature due to hydrogen bonding between the molecules. One after the other the molecules are held together by the hydrogen bonds, to form an aggregate of molecules.
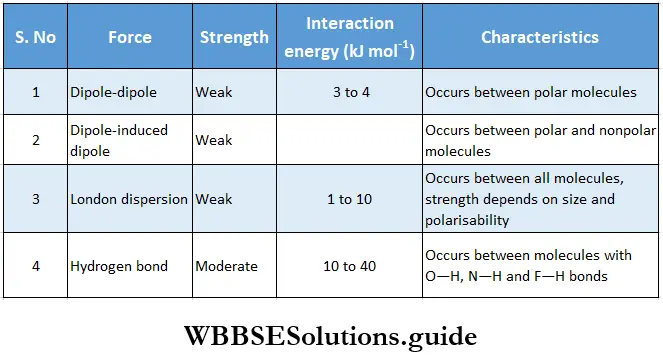
There may be several aggregates of varying numbers of water molecules in liquid water. Since hydrogen bonds are very weak (bond enthalpy is about 10 kJ Mol-1), the water molecules break away from one aggregate to join another in liquid water and maintain a dynamic equilibrium.
Like water, many other polar molecules, e.g., HF and NH3, form intermolecular hydrogen bonds. The water molecules are joined together in a three-dimensional network in which each oxygen atom is bonded to three hydrogen atoms by one hydrogen bond and by two normal covalent bonds in a tetrahedral configuration. This is, however, not the case in molecules like HF and NH3.
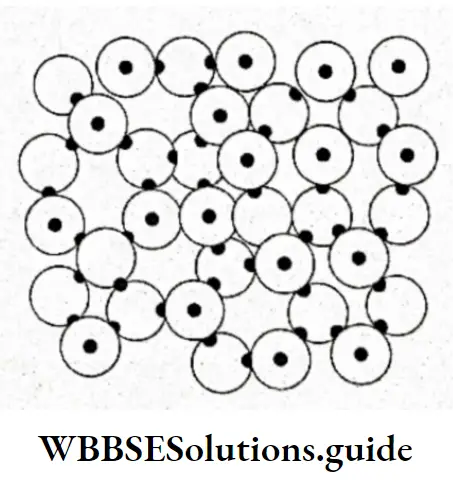
The Structure Of Ice
Ice can exist in nine different crystalline forms. These structures depend on the conditions of temperature and pressure in which water is frozen to make the ice.
In normal hexagonal ice, each oxygen is tetrahedrally surrounded by four other oxygen atoms, there being a hydrogen atom between each pair of oxygen atoms.
Each 2hydrogen atom is covalently bonded to one oxygen atom and linked to another oxygen atom by a hydrogen bond.
Such an arrangement leads to packing with comparatively large open spaces.
Therefore, the density of ice is less than that of liquid water and so ice floats on water.
However, when ice melts, some of the hydrogen bonds are broken and the water molecules become more closely packed.
The result is an increase in density above the melting point (273 K) of ice. Floating blocks of ice prevent or delay the freezing of underlying water, enabling aquatic life to survive in the colder regions of the earth.
Heavy Water
Normal water has one part of heavy water in 6,000 parts of it. molecule of heavy water contains two atoms of heavy hydrogen (deuterium) combined with one oxygen atom. Represented by the formula D20, it is also called deuterium oxide.
Heavy water Preparation
Heavy water is prepared by the exhaustive electrolysis of natural water containing an alkali. Electrolysis is continued in stages till the electrolyte is reduced to one-sixth of the original volume.
The alkali present is neutralised partially by passing carbon dioxide gas through the electrolyte. The solution is then distilled, and the distillate contains about 0.5% DzO.
Distillation is repeated and 99% D2O is obtained after the seventh stage. This is possible because natural water dissociates about three to four times as much as heavy water does.
The equilibrium constant for the dissociation of H2O is10 x10’14 while that for D2O is 3.0 x10-15.
“WBCHSE Class 11 Chemistry, water properties, hydrogen bonding, and solubility”
Protonium bonds are broken more readily than deuterium bonds. Thus, when water is electrolysed, H2 is liberated much faster than D2 and the remaining water gets enriched in D2O.
Water and DzO can also be separated to an extent by fractional distillation, using a fractionating column about 13 metres long. The lighter fraction (H2O) distils first leaving behind a residue richer in D2O
Heavy water Properties
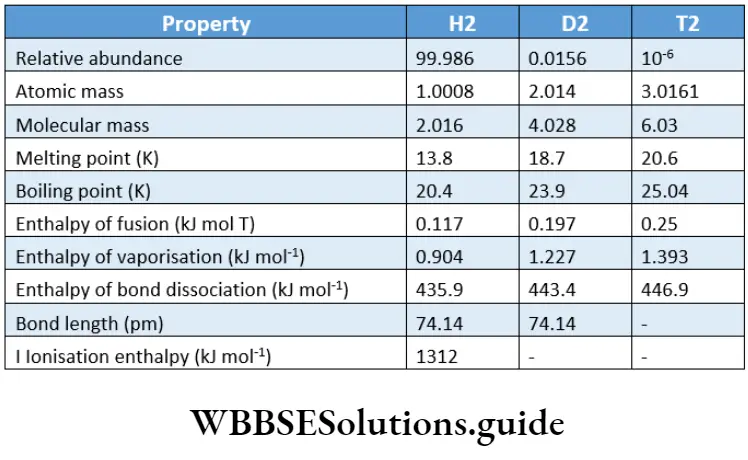
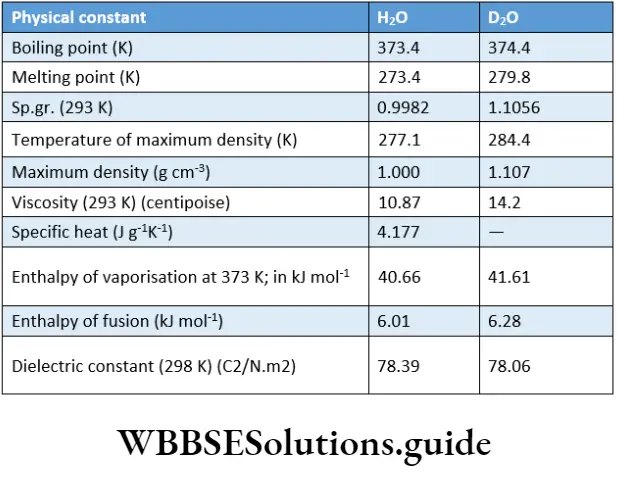
Like normal water, heavy water is a colourless, odourless and tasteless liquid. Almost all the physical constants for heavy water are higher than those for normal water.
This is because of the higher molecular mass of heavy water, and also owing to the strong van der Waals forces between its molecules. A few physical constants of normal water and heavy water are compared.
However, the surface tension of D2O (67.8) is lower than that of ordinary water. Also, the refractive index of heavy water (1.3284) is lower than that of normal water (1.333) at 293 K.
The solubility of salts like barium chloride and sodium chloride is less in normal water than in heavy water. Heavy water is harmful to living organisms. For instance, it hinders plant growth.
D2O and H2O differ very little in chemical behaviour, a rare example being that the reaction rate of D2O reactions is slightly less than that of H2O reactions. One significant property of D2O is that it participates in ‘exchange’ reactions.
For example, D2 reacts with H2 at high temperatures to form FID. Exchange reactions also occur with NH3 and CH4, the products being NH2D, NHD2, ND3, and CH3D and CD4. D2O can also be used directly to prepare deuterium compounds through such reactions. For example,
⇒ \(\begin{aligned}
& \mathrm{NaOH}+\mathrm{D}_2 \mathrm{O} \longrightarrow \mathrm{NaOD}+\mathrm{HDO} \\
& \mathrm{NH}_4 \mathrm{Cl}+\mathrm{D}_2 \mathrm{O} \longrightarrow \mathrm{NH}_3 \mathrm{DCl}+\mathrm{HDO}
\end{aligned}\)
In such reactions, deuterium is exchanged for hydrogen in the formation of compounds. Some of these, e.g., NaOD and D2SO4, are useful in the study of reaction mechanisms, e.g., those of esterification or hydrolysis.
Heavy water Uses
D20 is used to detect the presence of acidic hydrogen. For example, upon reacting with D20, a carboxylic acid (R—COOH) gives the exchanged product (R—COOD) and a phenolic compound (R—OH) gives R—OD.
The presence of D in the exchanged product can be ascertained by the use of spectroscopic techniques (particularly nuclear magnetic resonance).
However, a discussion of these techniques is beyond the scope of this book. Heavy water is used in nuclear reactors as a moderator.
In other words, it slows down the fast-moving neutrons in nuclear reactors so that nuclear fission may take place in a controlled way. When heavy water is made to react with sodium, deuterium is formed. This reaction is used in the production of deuterium.
Hard and Soft Water
Water containing soluble salts of calcium and magnesium (viz., chlorides, bicarbonates and sulphates) is called hard water. Such water does not readily form a lather with soap.
In contrast, soft water, which does not contain calcium or magnesium ions, lathers readily with soap. Soap is the sodium salt of a higher fatty acid, e.g., sodium stearate.
The sodium salt is soluble in water but the corresponding calcium and magnesium salts are not.
When added to hard water (which contains calcium and magnesium ions), soap is precipitated as insoluble salts of calcium and magnesium (scum). Therefore, no lather is formed till all these ions are removed
⇒ \(2 \mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COONa}+\mathrm{CaCl}_2 \longrightarrow 2 \mathrm{NaCl}+\left(\mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COO}\right)_2 \mathrm{Ca}\)
Sodium stearate (In hard water) Calcium Stearate (white ppt)
(soap)
If a sample of hard water can be rendered soft by boiling, it is said to have temporary hardness.
“Properties of water, boiling point, density, and chemical reactions, WBCHSE notes”
Temporary hardness is due to the presence of bicarbonates of calcium and magnesium. If a sample of hard water cannot be made soft by boiling, it is said to have permanent hardness.
Permanent hardness is due to the presence of chlorides and sulphates of calcium and magnesium.
Water Softening
Removal of hardness is called softening. Depending on the type of hardness, water can be softened by suitable processes. Temporary hardness can be removed by the following methods.
1. By boiling As already stated, temporary hardness is due to the presence of bicarbonates of calcium and magnesium.
When hard water is boiled, the bicarbonates decompose to form insoluble carbonates, which can be removed by filtration.
⇒ \(\begin{aligned}
& \mathrm{Ca}\left(\mathrm{HCO}_3\right)_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaCO}_3(\downarrow)+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2(\uparrow) \\
& \mathrm{Mg}\left(\mathrm{HCO}_3\right)_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{MgCO}_3(\downarrow)+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2(\uparrow)
\end{aligned}\)
By the Clark process By adding a calculated quantity of slaked lime to a sample of hard water, the soluble bicarbonates are converted into insoluble carbonates, which are removed by filtration.
⇒ \(\mathrm{Ca}\left(\mathrm{HCO}_3\right)_2+\mathrm{Ca}(\mathrm{OH})_2 \longrightarrow 2 \mathrm{CaCO}_3(\downarrow)+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{Mg}\left(\mathrm{HCO}_3\right)_2+\mathrm{Ca}(\mathrm{OH})_2 \longrightarrow \mathrm{MgCO}_3(\downarrow)+\mathrm{CaCO}_3(\downarrow)+2 \mathrm{H}_2 \mathrm{O}\)
In the above process, an excess of calcium hydroxide (slaked lime) must not be used, since it causes permanent hardness.
Permanent hardness may be removed by the following methods.
1. By adding washing soda The addition of washing soda (sodium carbonate) removes temporary as well as permanent hardness from samples of hard water by the precipitation of carbonates of calcium and magnesium from their chlorides and sulphates.
⇒ \(\mathrm{CaCl}_2+\mathrm{Na}_2 \mathrm{CO}_3 \longrightarrow \mathrm{CaCO}_3 \downarrow+2 \mathrm{NaCl}\)
⇒ \(\mathrm{MgCl}_2+\mathrm{Na}_2 \mathrm{CO}_3 \longrightarrow \mathrm{MgCO}_3 \downarrow+2 \mathrm{NaCl}\)
⇒ \(\mathrm{MgSO}_4+\mathrm{Na}_2 \mathrm{CO}_3 \longrightarrow \mathrm{MgCO}_3 \downarrow+\mathrm{Na}_2 \mathrm{SO}_4\)
By the pennutit process In this process, the ions responsible for the hardness of water (e.g., Ca++, Mg++) are 4 exchanged for sodium ions by using certain complex salts called ion-exchange resins.
These complex salts are also known as zeolites. They can be either naturally occurring or artificially synthesised. The name permit is used for * these zeolites.
A typical zeolite, e.g., sodium aluminium orthosilicate (Na2Al2Si208) is obtained by fusing together sodium carbonate (Na2CO3), alumina (A12O3) and silica (SiO3)
When hard water is passed through a zeolite, the calcium and magnesium ions are absorbed (exchanged) by the zeolite, and an equivalent amount of sodium ions passes into the water.
⇒ \(\mathrm{Na}_2 \mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8+\mathrm{Ca}^{2+} \longrightarrow \mathrm{Ca}\left(\mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8\right)+2 \mathrm{Na}^{+}\)
⇒ \(\mathrm{Na}_2 \mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8+\mathrm{Mg}^{2+} \longrightarrow \mathrm{Mg}\left(\mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8\right)+2 \mathrm{Na}^{+}\)
⇒ \(\mathrm{Na}_2 \mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8+\mathrm{Mg}^{2+} \longrightarrow \mathrm{Mg}\left(\mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8\right)+2 \mathrm{Na}^{+}\)
When all the sodium ions are exchanged for the calcium and magnesium ions, no further removal of hardness is possible.
Therefore, the used zeolite is treated with a sodium chloride solution (10%) so that all the calcium and 4 magnesium ions in the used zeolite are replaced by sodium ions. The zeolite is thus regenerated.
⇒ \(\mathrm{Ca}\left(\mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8\right)+2 \mathrm{NaCl} \longrightarrow \mathrm{Na}_2\left(\mathrm{Al}_2 \mathrm{Si}_2 \mathrm{O}_8\right)+\mathrm{CaCl}_2\)
The regenerated peanut is reused to soften water.
“WBCHSE Class 11, chemistry notes, on water properties, polarity, and pH level”
3. By the Calgon process In this process, calcium and magnesium ions, which are responsible for the hardness of water, are made ineffective by treatment with sodium hexametaphosphate [Na2Na4(P03)6 or Na6P6Ol8], which is known by the trade name Calgon.
The calcium and magnesium ions present in hard water combine with Calgon to form a soluble complex of calcium and magnesium salts. The following reactions take place in this process.
⇒ \(2 \mathrm{CaCl}_2+\mathrm{Na}_2 \mathrm{Na}_4\left(\mathrm{PO}_3\right)_6 \longrightarrow \mathrm{Na}_2 \mathrm{Ca}_2\left(\mathrm{PO}_3\right)_6+4 \mathrm{NaCl}\)
⇒ \(2 \mathrm{MgSO}_4+\mathrm{Na}_2 \mathrm{Na}_4\left(\mathrm{PO}_3\right)_6 \longrightarrow \mathrm{Na}_2 \mathrm{Mg}_2\left(\mathrm{PO}_3\right)_6+2 \mathrm{Na}_2 \mathrm{SO}_4\)
Once the calcium and magnesium ions go into the solution, the water readily forms a lather with soap.
The water softened by this procedure can be used only for washing purposes, and for raising steam in boilers. However, it is not fit for drinking.
4. By synthetic resins Water used in chemical laboratories and industries should be devoid of minerals (cation impurities and anion impurities).
Such water is called deionised or demineralised water and is as good as distilled water. Certain synthetic organic exchangers, also called ion-exchange resins, remove all types of cations and anions from water.
Such resins are preferable to zeolites since the latter remove only calcium and magnesium ions. There are two types of ion-exchange resins.
Cation-exchange resins: These resins have acidic groups like —COOH (carboxylic) or —SOaOH (sulphonic) attached to a large hydrocarbon molecule.
Thus, the molecules are represented as R—COOH or R—SO3OH. Such resins can exchange ions with all cations present in water and are, therefore, called cation-exchange resins.
Anion-exchange resins: These have basic groups like —OH, usually in the form of substituted ammonium hydroxides and are represented as R—NH3OH, R being a large hydrocarbon molecule.
These resins can exchange OH- ions with all anions present in water and are, therefore, called anion-exchange resins.
To obtain deionised water, hard water is passed through a tank packed with a cation-exchange resin supported over gravel.
The water thus obtained is passed through a second tank, which is packed with an anion-exchange resin supported on gravel.
By this treatment, all cations (Ca2+, Mg2+, etc.) are exchanged with H+ in the first tank and all anions (like C1-, SO2-) are exchanged with OH- in the second tank.
The water now obtained is devoid of any cations or anions which were originally present. The reactions involved are as follows.
⇒ \(\mathrm{R}-\mathrm{COOH}+\mathrm{CaCl}_2 \longrightarrow(\mathrm{RCOO})_2 \mathrm{Ca}+2 \mathrm{H}^{+}+2 \mathrm{Cl}^{-}\)
cation-exchange resin orMgClj from water or(RCOO)2Mg exhausted resin
⇒ \(\mathrm{R}-\mathrm{NH}_3 \mathrm{OH}+\mathrm{Cl}^{-} \longrightarrow \mathrm{R}-\mathrm{NH}_3 \mathrm{Cl}+\mathrm{OH}^{-}\)
Anion exchange Resin Or SO2-4 or R—NH3SO4 exhausted resin
The exhausted resins can be regenerated from the first tank by treatment with moderately concentrated HC1 or H2SO4, and those from the second tank by treatment with an NaOH solution.
⇒ \((\mathrm{RCOO})_2 \mathrm{Ca}+2 \mathrm{HCl} \longrightarrow \mathrm{RCOOH}+\mathrm{Ca}^{2+}+2 \mathrm{Cl}^{-}\)
⇒ \(\mathrm{RNH}_3 \mathrm{Cl}+\mathrm{NaOH} \longrightarrow \mathrm{RNH}_3 \mathrm{OH}+\mathrm{Na}^{+}+\mathrm{Cl}^{-}\)
Thus, the cation exchange and the anion exchange resins can be used over and over again.
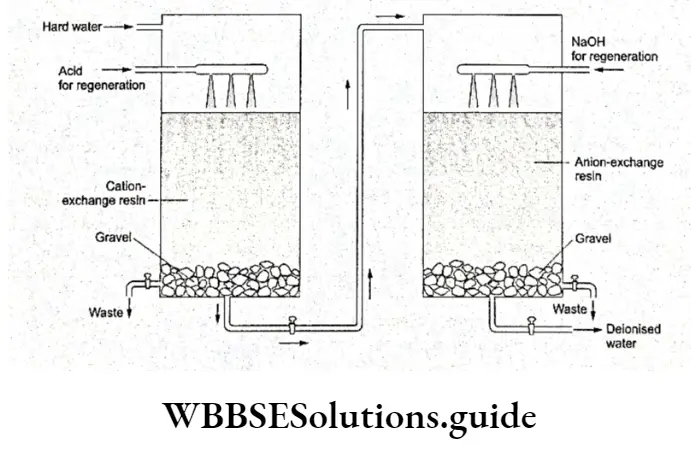
Alternatively, a ‘mixed bed’, i.e., a single column made of two different ion exchange resins, may be used.
Purification of Water for Drinking
Pure water is required for drinking purposes. The suspended impurities have to be removed, and the bacteria present destroyed before water can be used for drinking.
The water to be purified is taken in a large tank and treated with the requisite quantity of aluminium sulphate.
“Physical properties of water, cohesion, adhesion, and heat capacity, WBCHSE Chemistry
This treatment coagulates the impurities, which settle down along with bacteria and other suspended particles.
The water is then filtered through sand filters. The sand filters consist of a top layer of fine sand (1-1.25 m thick), a middle layer of coarse sand (0.3-0.5 m thick) and a bottom layer of graded sand (0.5-1 m thick).
Through this process, all the coagulated and suspended impurities along with most of the bacteria are removed.
The filtered water thus obtained is treated with liquid chlorine in big tanks. This kills the bacteria in the water.
Chlorinated Water is Fit for Drinking.
Some households use water filters. An ordinary water filter is fitted with porous tubes made of unglazed porcelain.
Such a filter, though, has to be cleaned frequently. Some water purifiers are also fitted with mercury vapour lamps. The ultraviolet light from the lamps destroys the bacteria in impure water.
River water is purified by the natural process of aeration. The oxygen present in the air is absorbed in water and oxidises the organic matter.
“WBCHSE Class 11 Chemistry, chemical properties of water, dissociation, and amphoteric nature”
The shortage of drinking water has become an acute problem. Therefore, attempts are being made to convert seawater into drinking water on a large scale.
Some methods which can be used for this purpose are as follows.
Distillation Sea water is distilled by using solar heaters. However, only limited quantities of seawater can be purified using this method.
Reverse osmosis In this process, seawater is passed through a semipermeable membrane under an applied pressure that is greater than normal osmotic pressure. The semipermeable membrane only permits the solvent to pass through leaving the solute behind.
Filtration Special membranes are used to filter water. This allows water to pass through, leaving behind dissolved salts.