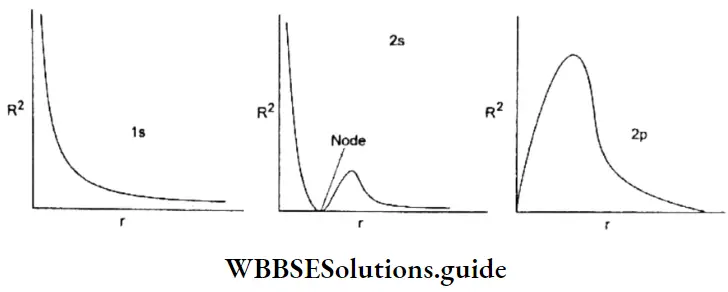
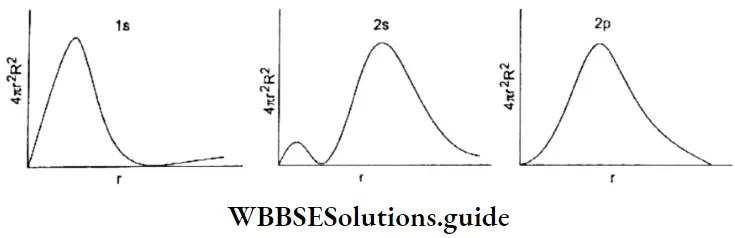
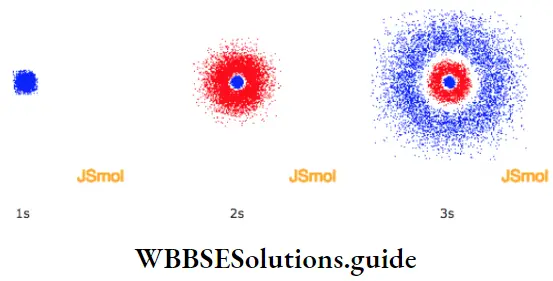
WBCHSE Class 11 Notes On Heisenberg’s Uncertainty Principle
All moving particles follow well-defined paths and the path may be determined if we know the position and velocity of the particle at different instances of time.
In 1927, Heisenberg stated that since matter has a dual nature it is impossible to determine simultaneously both the velocity and the position of a moving object with absolute precision (remember that we are talking about the microscopic world).
‘This, in essence, is Heisenberg’s uncertainty principle. To put it more formally, it is not possible to determine simultaneously the position and momentum of a small moving particle, such as an electron, with absolute accuracy. Mathematically, this may be expressed as follows.
“WBCHSE Class 11 Chemistry, Heisenberg’s Uncertainty Principle, notes, and key concepts”
∴ \(\Delta x \times \Delta p_x \geq \frac{h}{4 \pi} \quad \text { or } \quad \Delta x \times m \Delta v_x \geq \frac{h}{4 \pi}\)
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
where, Δx = uncertainty in determination of position,
Δpx = uncertainty in the determination of momentum,
Δvx = uncertainty in the determination of velocity, and
m = mass of particle.
This mathematical expression means \(\Delta x \propto \frac{1}{\Delta p_x}\), which implies that the greater the precision in the determination of the momentum of a particle, the lesser the precision in defining its position, and vice versa. Heisenberg was awarded the Nobel Prize in Physics in 1932.
It should be made clear that uncertainty is due to the disturbance caused by the microscopic particle or an electron while measuring its dimensions. To observe an electron we need to illuminate it with some electromagnetic radiation, which will make protons strike the electrons and change their velocity.
Example 1. The uncertainty in the momentum of a particle is 6 x 10-2 kg m s-1. Calculate the uncertainty in its position.
Solution:
Given:
The uncertainty in the momentum of a particle is 6 x 10-2 kg m s-1.
According to the uncertainty principle,
⇒ \(\Delta x \times \Delta p_x \geq \frac{h}{4 \pi}\)
or \(\Delta x=\frac{h}{4 \pi \Delta p_x}\).
∴ \(\quad \Delta x =\frac{h}{4 \pi \Delta p_x}=\frac{6.62 \times 10^{-34} \mathrm{~kg} \mathrm{~m}^2 \mathrm{~s}^{-1}}{4 \times 3.14 \times 6 \times 10^{-2} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}}\)
= \(8.76 \times 10^{-34} \mathrm{~m}\).
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
Example 2. An electron has a velocity of 300 m s-1 that is accurate to 0.01%. With what accuracy can one locate the position of this electron (mass of electron = 9.1 x 10-31 kg)?
Solution:
Given:
An electron has a velocity of 300 m s-1 that is accurate to 0.01%. W
Velocity = 300 ms-1.
Uncertainty in velocity = 0.01% of 300
= \(\frac{0.01}{100}\) x 300 = 3 x 10-2 ms-1.
According to the uncertainty principle,
⇒ \(\Delta x m \Delta v_x=\frac{h}{4 \pi}\)
“Heisenberg’s Uncertainty Principle, WBCHSE Class 11, chemistry notes, and explanation”
∴ \(\quad \Delta x=\frac{h}{4 \pi m \Delta v_x}=\frac{6.62 \times 10^{-34} \mathrm{~kg} \mathrm{~m}^2 \mathrm{~s}^{-1}}{4 \times 3.14 \times 9.1 \times 10^{-31} \mathrm{~kg} \times 3 \times 10^{-2} \mathrm{~m} \mathrm{~s}^{-1}}\)
The uncertainty in position and velocity is negligible for macroscopic objects. If it is applied to an object of mass 1 g, i.e., 10-3 kg then
⇒ \(\Delta v_x \Delta x=\frac{h}{4 \pi m}=\frac{6.62 \times 10^{-34} \mathrm{~kg} \mathrm{~m}^2 \mathrm{~s}^{-1}}{4 \times 3.14 \times 10^{-3} \mathrm{~kg}}=5 \times 10^{-32} \mathrm{~m}^2 \mathrm{~s}^{-1}\)
This value is extremely small and therefore insignificant.
Why Bohr’s model failed: If one accepts Heisenberg’s uncertainty principle, one must reject Bohr’s model of the atom. Since it is impossible to determine accurately the position and the momentum of an electron at the same time, it is quite futile to talk about determining its orbit.
- Talking about fixed orbits of electrons, as Bohr did, implies that it is possible to know, with some precision, both the position and the velocity of an electron at the same time. This violates the uncertainty principle, and it should become clear to you why Bohr’s model was not extended further.
- To describe the structure of the atom, what was needed was a concept that took into account the wave-particle duality of matter and at the same time was consistent with the Heisenberg uncertainty principle.
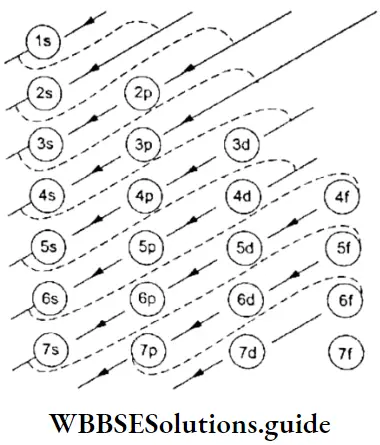
WBCHSE Class 11 Heisenberg’s Uncertainty Principle Notes Energy Sequence Of Orbitals
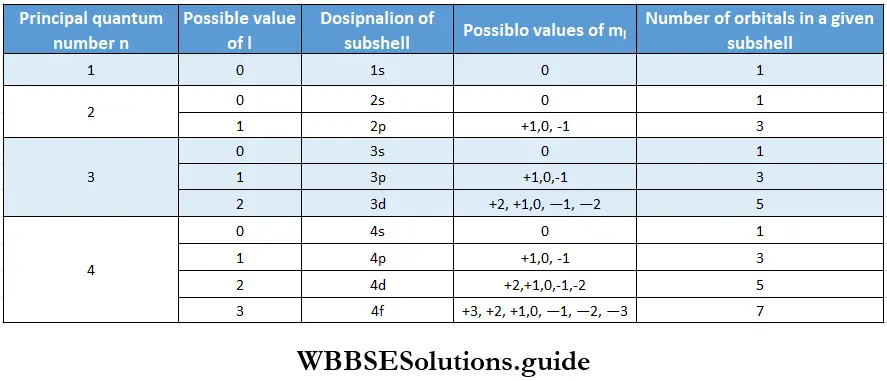
The hydrogen atom has only one electron. In a single-electron system (for example, H, He+, and Li2+), all the orbitals of a given principal shell have the same energy. For instance, the 2s and 2p orbitals have the same energy. The energy of an orbital in such an atom depends only on n and is independent of l.
In multi-electron atoms, the picture is different because of the presence of other electrons in the atom. The energy of an electron dependds not only on its principal quantum number but also on its azimuthal quantum I number.
“WBCHSE Class 11, chemistry notes, on Heisenberg’s Uncertainty Principle, and mathematical expression”
- The stability of an electron in the atom is the net result of attraction between the electron and the nucleus and repulsion between the electron and the other electrons present in the atom. It is not possible to calculate exactly the energies of the orbitals in such an atom, but it is possible to get an idea about these energy levels from spectral data.
- In general, subshells of the same principal shell have different energies and a subshell with a higher value of l has higher energy. For instance, the energy of the 2p shell (1 = 1) is higher than that of the 2s (l = 0) shell.
- Some subshells of a lower energy level (principal shell) may have higher energies than some subshells of a higher energy level (principal shell). The energies of the various subshells are in the following order.
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p …
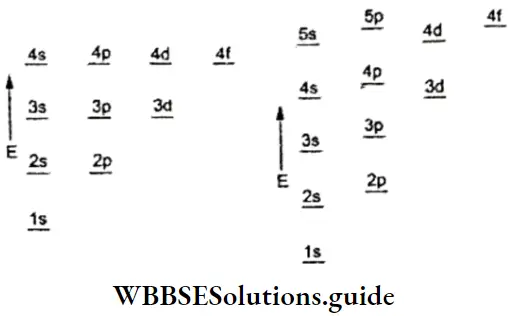
The energy of an orbital depends upon the nuclear charge, the principal quantum number, and the presence of other electrons in the lower energy levels.
- In relative energies of the various orbitals in an atom are called hydrogenlike atoms, there is only one electron and the energy level diagram. attractive force of the nucleus on the single electron is always constant, thus the energy of any orbital occupied by this solitary electron at any instant is given by the principal quantum number.
- In a multi-electron atom, the situation is different because an increase in the number of electrons is also accompanied by an increase in nuclear charge. As the nuclear charge increases, the orbitals are somewhat contracted. The electrons in the lower-lying orbitals tend to reduce the effect of the nuclear charge on the electrons present in the higher-energy orbitals.
- This is referred to as the screening effect or shielding effect. The electrons present in lower-lying orbitals act as a “screen” so that the outer electrons “see” only part of the nuclear charge. Thus the nuclear charge felt by the outer electrons is less than the actual nuclear charge. The reduced charge felt by the outer electrons is called the effective nuclear charge (Zeff).
∴ Zeff =Z-S
where Z = actual nuclear charge and S =shielding constant.
- In neutral atoms, a subshell with a lower value of n + l has lower energy. For example, the 4s(n +1 – 4 + 0 = 4) orbital has lower energy than the3d orbital (n + l =3 + 2 = 5).
- If two subshells have the same value of n + l, the subshell with the lower value of n has lower energy. For instance, 4p(n + l =3 + 2 =5) and 3d(n +Z =3 + 2=5) orbitals have the same value of n + 1, but 3d (5) has lower energy than 4p.
- The energies of the orbitals in the same subshell of two different „ atoms are not the same. They decrease with the increase in atomic number (Zeff). For example, the energy of the 2s orbital of the hydrogen atom is greater than the energy of the 2s orbital of the lithium atom.
Heisenberg’s Uncertainty Principle And Its Application In WBCHSE Chemistry Electronic Configuration
The distribution of electrons in the different orbitals of an atom is known as the electronic configuration of the atom. An atom in its lowest energy state is said to be in the ground state. This is the most stable state of the atom. The orbitals in the ground state are filled according to the following rules.
“Heisenberg’s Uncertainty Principle, definition, equation, and significance, WBCHSE syllabus”
Aufbau principle Aufbau, in German, means building up. This rule of “building up” the atom or filling the orbitals in the ground state says that the orbitals are filled in order of increasing energies, or that electrons occupy the lowest energy orbital available, subject to the Pauli principle.
Pauli exclusion principle You have already read that according to this rule, two electrons of an atom cannot have the same set of (all four) quantum numbers.
Hund rule Also referred to as the Hund rule of maximum multiplicity, this rule deals with the filling of orbitals of the same subshell (degenerate orbitals). According to this rule, the pairing of electrons in the orbitals of a particular subshell (p, d, or f) does not take place until all the orbitals of the subshell are singly occupied.
- Also, the singly occupied orbitals must have electrons with parallel spins. This is because it is energetically preferable to have single electrons in different orbitals than to have a pair of electrons in a particular orbital (two electrons in the same orbital experience more repulsion).
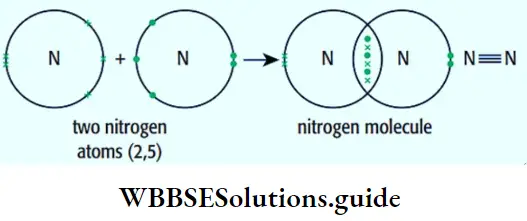
- As for the second part of the rule, when two electrons occupy two different orbitals, energy is lower if their spins are parallel. For example, the electronic configuration of 6C is \({ }_6 \mathrm{C} \text { is } 1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}_x^1 2 \mathrm{p}_y^1\), rather than \(1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}_x^2\). (This is a way of representing the electronic configuration of atoms—you will learn about it in the next section.)
Representation of electronic configuration: There are two ways of representing the electronic configuration of an atom. Of these, the first is the one used most often because the second one is rather cumbersome.
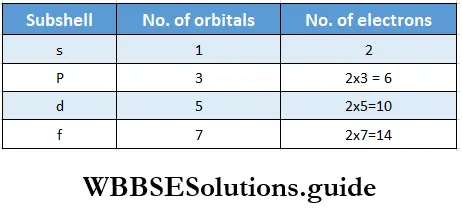
1. The electron population of an orbital is represented symbolically as nlx, where n stands for tire principal quantum number, l for the subshell or orbital, and x for tire number of electrons present in the orbital. For example, if two electrons are present in the s subshell of the first principal shell, it would be represented as 1s2 in this notation.
2. In the second method of representing the electronic configuration of an atom, squares or circles stand for orbitals and electrons are represented by arrows within the squares or circles. For instance, 1s2 would be represented as ↓↑
The electronic configurations of some elements are represented below.
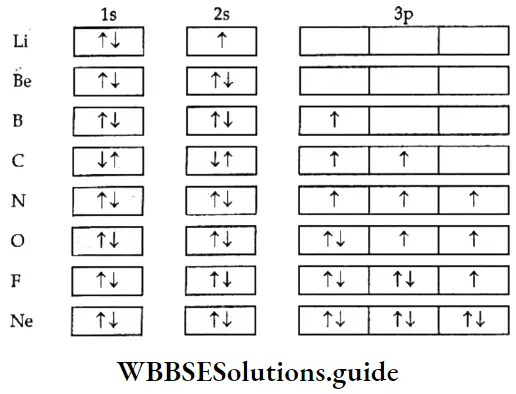
- The electronic configurations of the elements sodium (\(1 s^2 2 s^2 2 p^6 3 s^1 \text { or }[\mathrm{Ne}]\)) to argon (\(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6\) or \(\left.[\mathrm{Ne}] 3 s^2 3 p^6\right)\)) follow the same pattern as the elements horn lithium to neon except that the 3s and 3p orbitals begin to get filled.
- In potassium and calcium, the 4s orbitals, being lower in energy than the 3d orbitals, get occupied. Tire 3d orbitals (being lower in energy than the 4p orbitals) are filled from scandium (atomic number 21) to zinc (30).
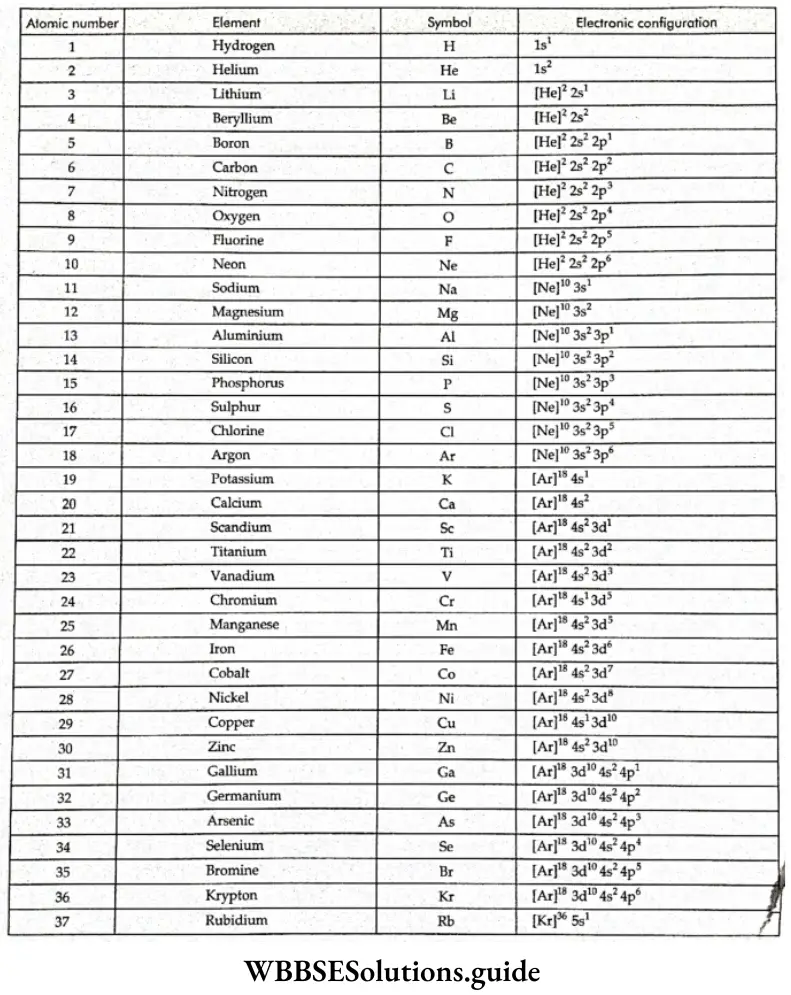
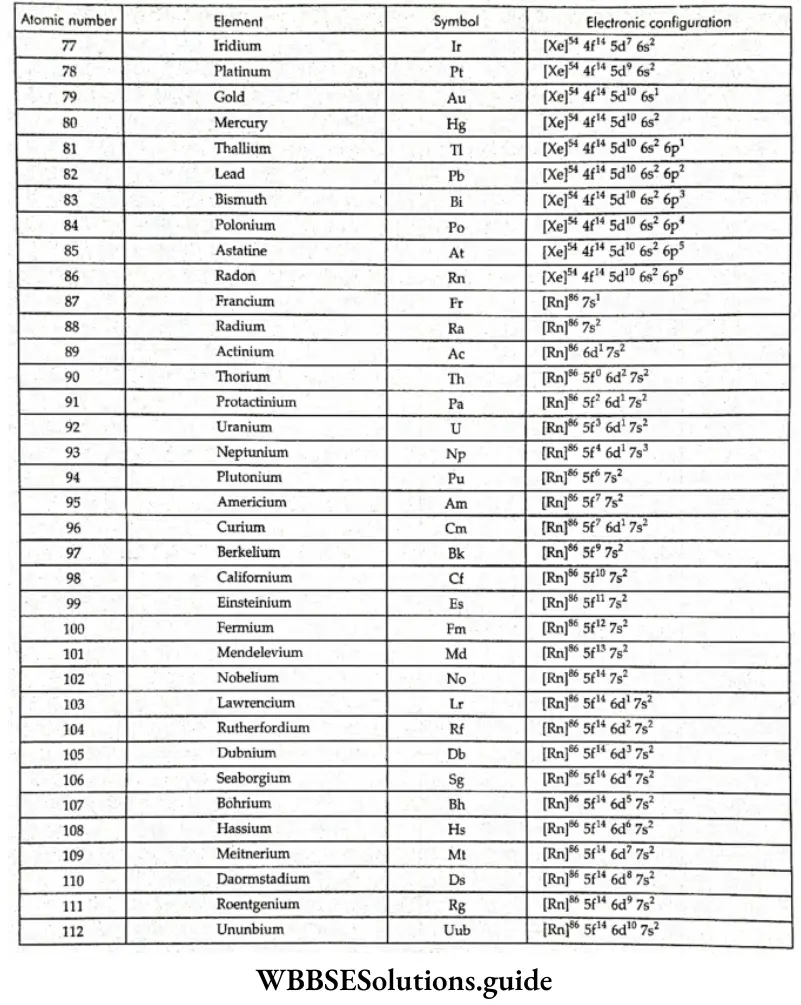
Note: The superscripts on the noble-gas atoms should not be confused with mass numbers. They are the numbers of electrons in the atoms of the noble gases.
- Chromium and copper have five and ten electrons in the 3d orbitals rather than four and nine, as their positions would have indicated. This is because half-filled and filled orbitals are more stable. To acquire this stability, one of the 4s electrons goes into the 3d orbitals.
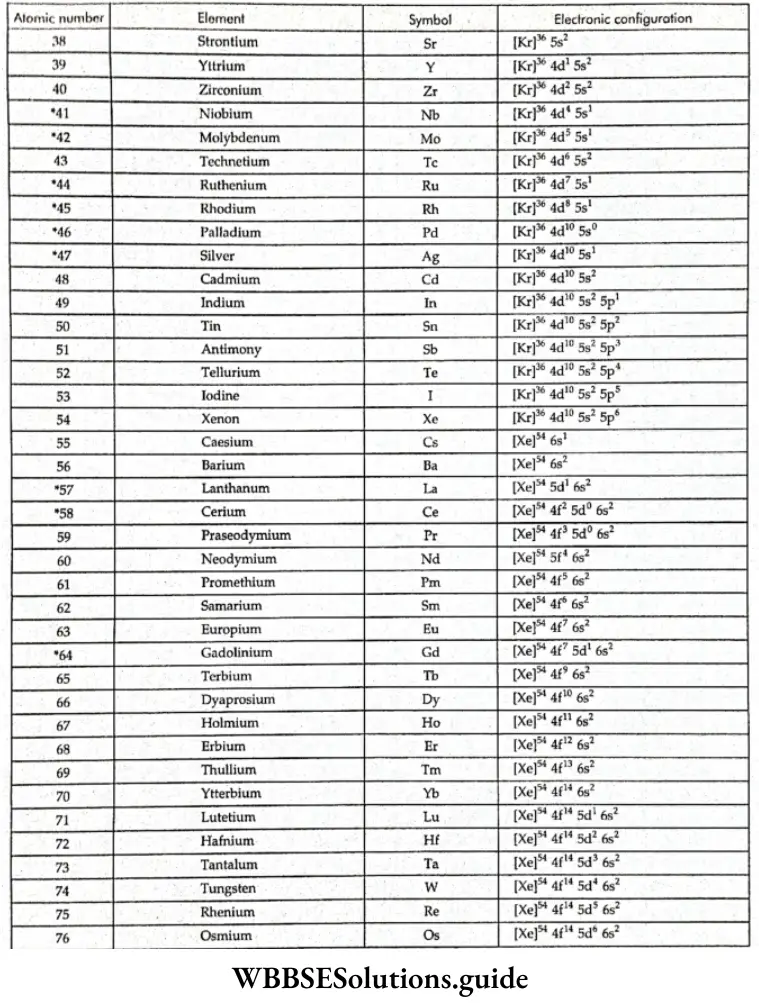
- With the saturation of the 3d orbitals, the 4p orbitals get filled from gallium (Ga) to krypton (Kr). In the next 18 elements, from rubidium (Rb) to xenon (Xe), the pattern of filling of the 5s, 4d, and 5p orbitals is similar to that of the 4s, 3d and 4p orbitals. The 6s orbital gets filled in cesium (Cs) and barium.
- The 44 and 5d orbitals get filled from lanthanum to mercury. The 6p, 7s, 5f, and 6d orbitals get filled (in that order) after this. The elements after uranium are all short-lived and produced artificially.
“WBCHSE Class 11 Chemistry, Heisenberg’s principle, limitations of classical mechanics, and quantum theory”
- It is important to remember that orbitals are not like boxes into which electrons are filled. When we talk about electrons filling orbitals we mean that they have a particular distribution corresponding to a particular level of energy.
- Electrons always try to have the distributions with the lowest energy possible and orbitals merely determine the shape, size, and orientation of the electron distribution.
Stability of half-filled and filled orbitals: While discussing the configurations of chromium and copper it was mentioned that half-filled and filled orbitals are more stable (than those that are almost half-filled or almost full). There are two reasons for this stability.
Class 11 WBCHSE Notes On Quantum Mechanics And Uncertainty Principle
Symmetry Orbitals that are half-filled or filled have a more symmetrical distribution of electrons. Consequently, they have lower energy and greater stability.
Exchange energy Electrons in degenerate orbitals (orbitals of the same energy) can exchange positions with other electrons with the same spin. In the die process, they gain exchange energy. The greater the possibility of exchange, the more stable the configuration. The exchange energy (or stabilization energy) can be calculated from the formula given below.
∴ Exchange energy = \(\frac{n !}{2 \times(n-2) }\)
where n is the number of electrons with parallel spin.
Let us consider the case of chromium.
Expected configuration [Ar] 4s23d4
Actual configuration [Ar]4s1 3d5
Exchange energy for expected configuration =\(\frac{n !}{2 \times(n-2) !}=\frac{4 !}{2 \times 3 !}=\frac{4 \times 3 \times 2 \times 1}{2 \times 3 \times 2 \times 1}\) = 2 units
“Heisenberg’s Uncertainty Principle, derivation, formula, and implications, WBCHSE notes”
Exchange energy for actual configuration = \(\frac{n !}{2 \times(n-2) !}=\frac{6 !}{2 \times 4 !}=\frac{6 \times 5 \times 4 \times 3 \times 2 \times 1}{2 \times 4 \times 3 \times 2 \times 1}\) = 15 units
Thus, the actual configuration has more exchange or stabilization energy and is more stable.
Electronic configuration of ions: Follow the steps given below to obtain the electronic configuration of an ion.
- Write the electronic configuration of the atom in the ground state.
- Rearrange the subshells in increasing order of the values of n and l
- Remove electrons from the outermost subshell.
Example: Write the electronic configurations of Fe2+ and Fe3+.
⇒ \({ }_{26} \mathrm{Fe}=[\mathrm{Ar}] 4 \mathrm{~s}^2 3 \mathrm{~d}^6\) (ground state electronic configuration rearranged according to increasing n and l of Fe)
⇒ \(\mathrm{Fe}^{2+}=[\mathrm{Ar}] 3 \mathrm{~d}^6\) on removing two electrons from valence shell
“WBCHSE Class 11, chemistry notes, on uncertainty principle, electron position, and momentum”
⇒ \(\mathrm{Fe}^{3+}=[\mathrm{Ar}] 3 \mathrm{~d}^5\) on removing two electrons from the 4s subshell and one from the 3d subshell