Alcohols Phenols And Ethers
Alcohols:
Alcohols can be regarded as derivatives of hydrocarbons, saturated or unsaturated, where one or more of the hydrogens have been replaced by hydroxyl group(s).
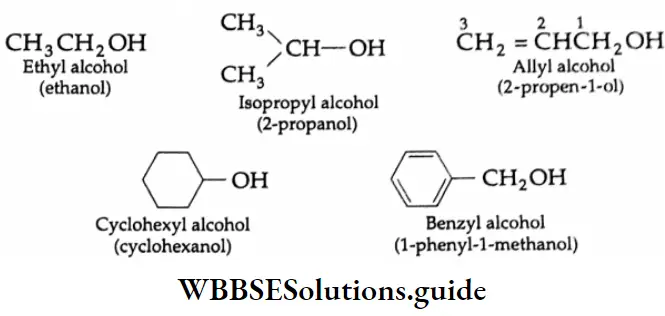
Ethyl alcohol (C2H5OH) has been known since ancient times. It is an important constituent of alcoholic beverages, like beer, wine and brandy. It is also used in making tincture iodine (I2+ C2H5OH), cough syrups and tonics.
Isopropyl alcohol [(CH3)2 CHOH] is the common ‘rubbing alcohol’ which is used as a 70% solution in water for its antibacterial properties. Methyl alcohol is widely used as an industrial solvent. Glycol is widely used as a solvent and antifreeze for fuels. Glycerol is used in medicines and cosmetics.
When the hydroxyl group is connected to a carbon atom of a benzene ring, the compound is known as a phenol. Phenols possess the general formula Ar-OH where Ar is a phenyl or substituted phenyl group. Phenol containing a small amount of water is known as carbolic acid, which is used as a disinfectant. Phenol was the firs compound to be used as an antiseptic (1867).
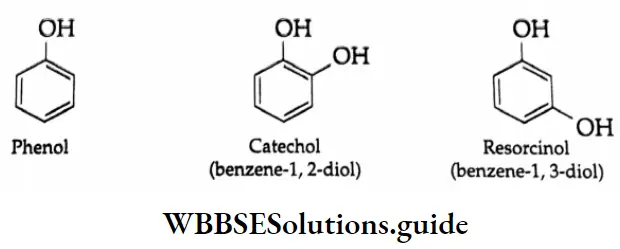
Phenols are considered to be different from alcohols because their chemical properties are rather different.
Ethers: Ethers are compounds in which two carbon atoms are connected to a single oxygen (C-O-C). Diethyl eth has since long been used as a general anaesthetic. It is also used as a solvent.
⇒ CH3– O- CH3
(Dimethyl ether)
⇒ CH3-CH2OCH3
(Ethyl Methyl Ether)
If the C-O-C unit is part of a ring, the molecule is known as a cyclic ether, examples being (tetrahydrofuran) and dioxane. If the two ether-linkage carbons are directly bonded to each other to form a t-membered ring, the molecule is known as an oxirane (or epoxide).

Example 1: Can you what petroleum ether is
Solution: Petroleum ether is not an ether, but a mixture of alkanes.

Classification Alcohols Phenols And Ethers
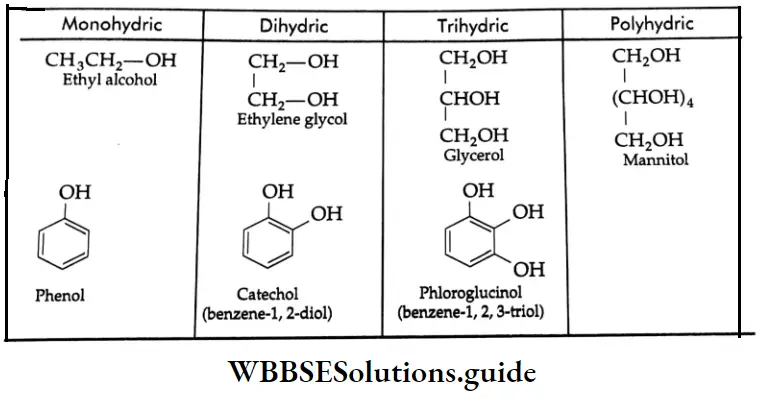
Depending upon the number of hydroxyl groups, alcohols and phenols are classified into monohydric (one-OH group) dihydric (two-OH groups) and trihydric (three-OH groups) and polyhydric (more than three -OH groups) compounds.
Structures of some monohydric, dihydric, trihydric and polyhydric compounds:
Monohydric alcohols may be classified based on the hybridisation of the carbon atom to which the hydroxyl group is attached.
Compounds Containing An Sp3 C-OH Bond
Primary, secondary and tertiary alcohols:
Monohydric alcohols are classified into primary, secondary and tertiary alcohols, depending upon the number of alkyl groups attached to the carbon atom carrying the hydroxyl group.
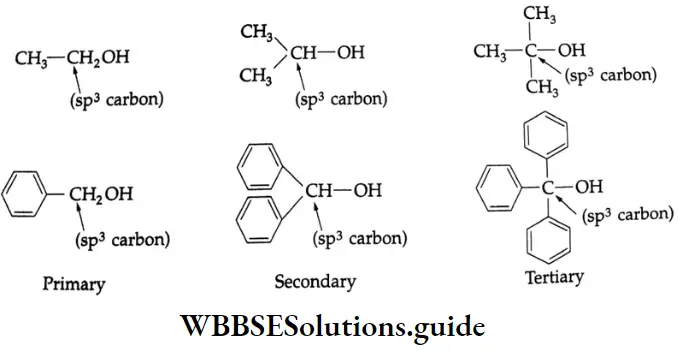
Thus, a primary alcohol contains a monovalent —CH2 OH group, a secondary alcohol contains a bivalent ->CHOH group and a tertiary alcohol has a trivalent ->C-OH group.
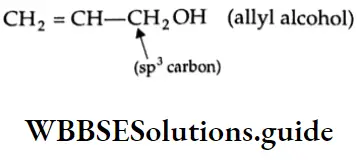
Allyl alcohol:
In an allyl alcohol, the hydroxyl group is attached to that sp3-hybridised carbon which is bonded to the carbon-carbon double bond.
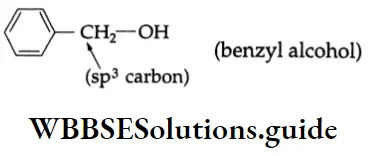
Benzyl alcohol:
In benzyl alcohol, the hydroxyl group is attached to that sp3-hybridised carbon which is bonded to the benzene ring.
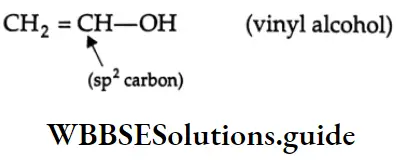
Alcohols Containing An Sp2 C-OH Bond
Vinyl alcohol:
In vinyl alcohol, the hydroxyl group is bonded to the sp2 -hybridised carbon atom of a carbon-carbon double bond.
Phenol:
In phenol, the hydroxyl group is attached to the sp2-hybridised carbon atom of a benzene ring.

Example 2: Classify the following as primary (1°), secondary (2°) and tertiary (3°) alcohols.
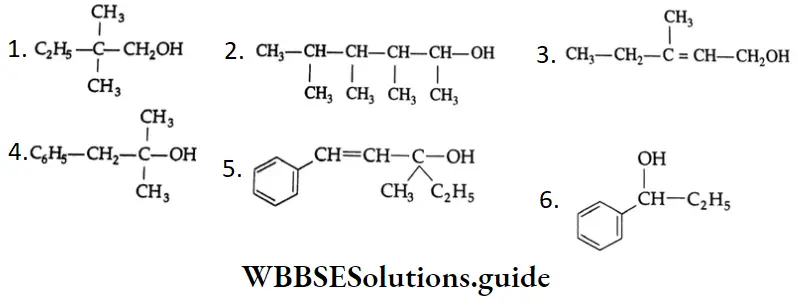
Solution:
- 1 and 3 are primary alcohols.
- 2 and 6 are secondary alcohols.
- 4 and 5 are tertiary alcohols.
Ethers:
Ethers are said to be symmetrical or simple when the two alkyl groups are the same and unsymmetrical or mixed when they are different.
Symmetrical Ethers:
⇒ CH3 – O- CH3
(Dimethyl ether)
⇒ C2H5 – O- C2H5
(Diethyl ether)
Unsymmetrical Ethers:
⇒ CH3 – O- C2H5
(Ethyl methyl ether)
⇒ CH3 – O- CH2– CH2 -CH3
(Methyl n – propyl ether)
Nomenclature
There are three different systems of naming alcohols, and two systems of naming phenols and ethers.
Trivial name
Simple alcohols are commonly known by their trivial names or common names. According to this system, the name of an alcohol is derived by writing the name of the alkyl group and then the word alcohol. The name is always written as two separate words.
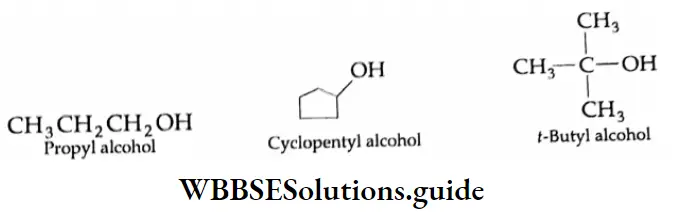
In the common system of nomenclature, the position of an additional substituent is indicated by letters of the Greek alphabet rather than by numbering, the carbon attached to the OH group being labelled α.
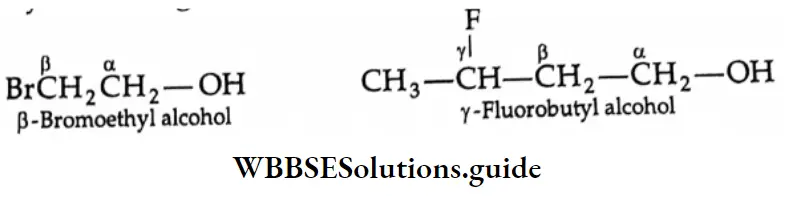
Any simple group that has a common name may be used in the alkyl alcohol system, with one exception. The group C6H5-is phenyl, but the compound C6H5-OH is phenol and not phenyl alcohol.

Substituted phenols are considered to be derivatives of the parent compound phenol.
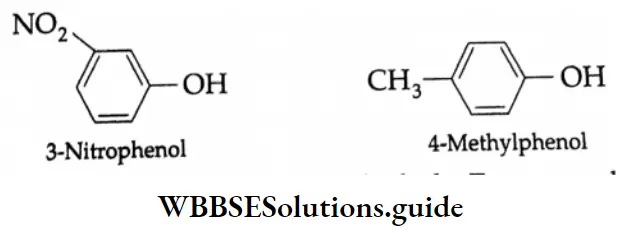
However, phenyl-substituted alkyl alcohols are normal alcohols. For example,
CH2OH
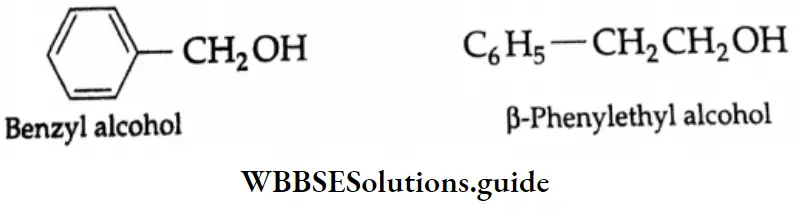
The Carbinol System
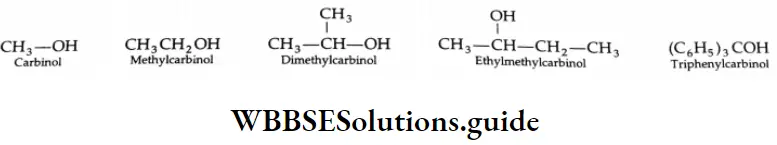
In the carbinol system, the simplest alcohol, CH3OH, is called carbinol. More complex alcohols are considered to be alkyl-substituted carbinols. The whole name is written as one word.
The IUPAC System
Alcohols:
In the IUPAC system, alcohols are named by replacing the ‘e’ of the corresponding alkane by the suffix ‘ol’.
CH3OH – Methane-e + ol = Methanol
CH3CH2OH – Ethane-e+ol Ethanol
The positions of the hydroxyl group and other substituents are indicated by numbers, the lowest possible number being given to the carbon atom attached to the hydroxyl group.
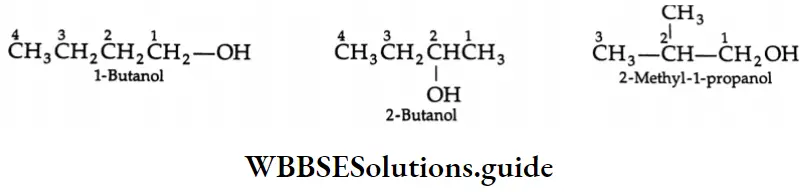
In the IUPAC system, if two-OH groups are present in a compound (as in CH2OH CH2OH), the suffix becomes ‘diol’ instead of ‘ol’. If three – OH groups are present (as in CH2OH – CHOH- CH2OH), the suffix becomes’triol’ instead of ‘ool’. Thus:

(Note that terminal ‘e’ of the parent alkane has not been removed because the suffix ‘diol’ or triol begins with a consonant.)
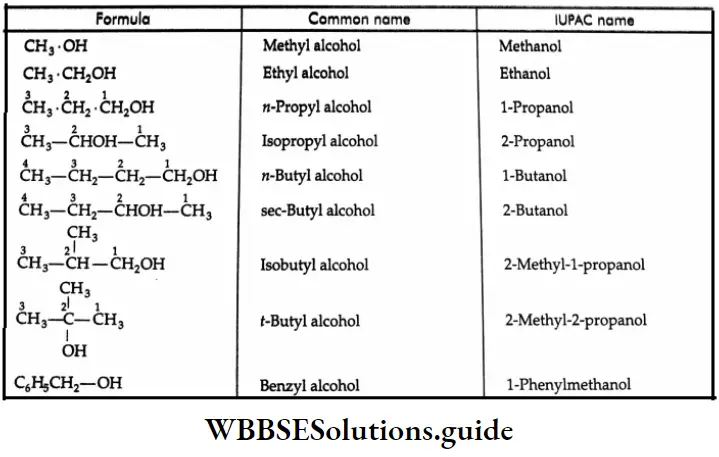
Gives the common and IUPAC names of certain typical alcohols:
Monocyclic alcohols are named using the prefix cyclo and considering the -O-H group to be attached to C-1.
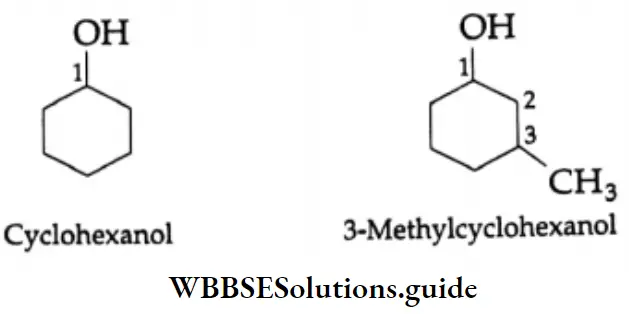
Phenols:
In the IUPAC system, a compound with the hydroxyl group directly attached to a benzene ring is called phenol.
Substituted phenols are named as derivatives of the parent compound phenol as illustrated by the following IUPAC names.
The common and IUPAC names of some phenols:
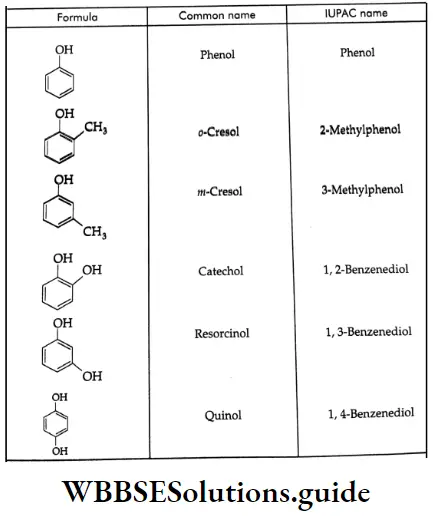
Ethers:
In the common system, ethers are named by first writing the names of the two alkyl groups in alphabetical order and adding the word ether.
⇒ CH3OCH2CH
( Ethyl Methyl Ether)
⇒ CH3OC(CH3)
(t-Butyl methyl ether)
In symmetrical ethers, the prefix ‘di’ is used.
![]()
Diethyl Ether
⇒ (CH3)2 CHOCH(CH3)2
(Disopropyl ether)
In the IUPAC system ethers are considered to be alkoxyalkanes. For complex molecules, the simplest organic group along with the oxygen atom is named as an alkoxy group and used as a substituent on a more complex chain.
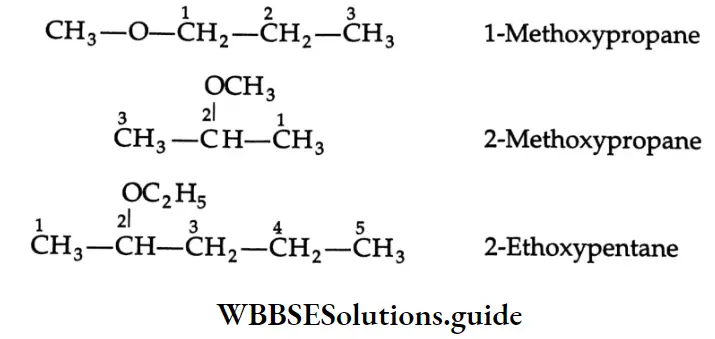
The Common And IUPAC names of some ethers:
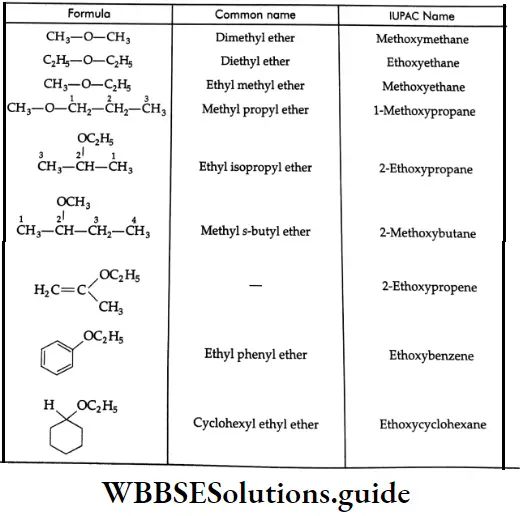
A few examples of IUPAC nomenclature:
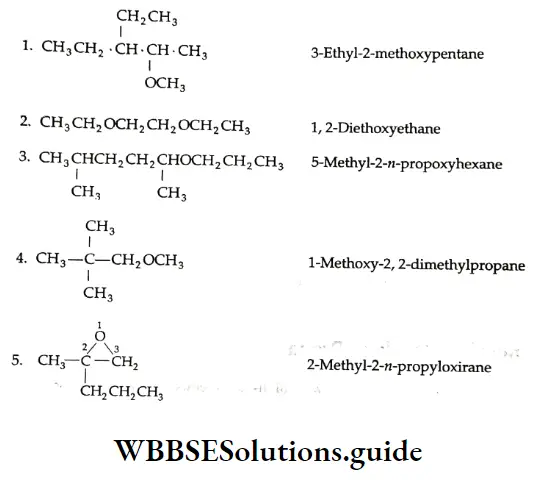
Cyclic ethers are known as epoxides The term ‘epoxy’ is derived from epoxide
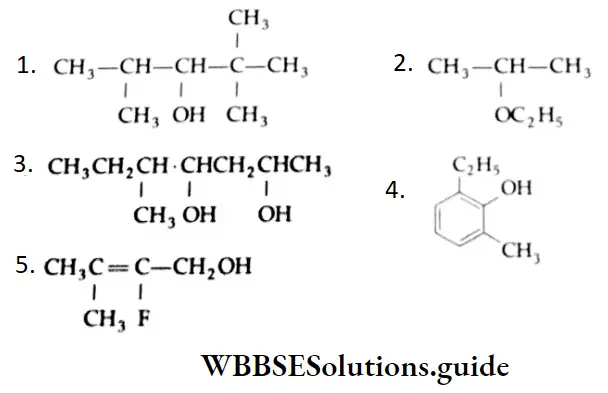
Example 3: Draw the structures of the folloeing compounds.
- 2,2,4 – Trimethyl – 3 pentanol
- 2- Ethoxypropane
- 5 – Methyl – 2, 4 – Hepatanediol
- 2 – Ethyl – 6- Methylphenol
- 2- Fluoro – 3- Methyl – 2 buten-1 – ol
Solution:
Structures of Alcohols, Ethers And Phenols
In alcohol, the hybridisation of carbon is approximately sp3. So is the hybridisation of oxygen. Oxygen share one bond with carbon and one with hydrogen. The two lone pairs of electrons on oxygen occupy orbitals that ar each approximately sp2-hybridised.
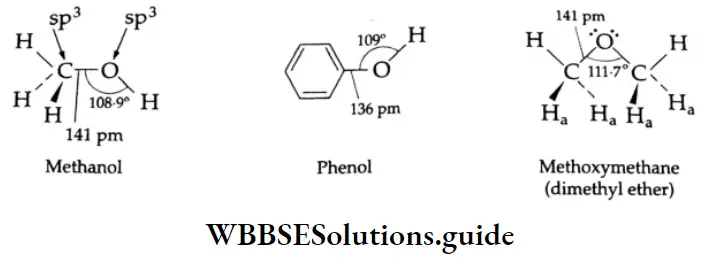
The ![]() bond angle in an alcohol is 108.9° (a little less than the tetrahedral angle 109° 28′). This is due to the repulsion between the two lone pairs of electrons on oxygen.
bond angle in an alcohol is 108.9° (a little less than the tetrahedral angle 109° 28′). This is due to the repulsion between the two lone pairs of electrons on oxygen.
In dimethyl ether the ![]() bond angle is 111.7° (a little more than the value of the tetrahedral angle). This is due to repulsion between the hydrogens marked H, in the above structural formula.
bond angle is 111.7° (a little more than the value of the tetrahedral angle). This is due to repulsion between the hydrogens marked H, in the above structural formula.
The C-O bond length of (136 pm) in phenol is slightly less than the C-O bond length (141 pm) in methanol. This is because the hydroxyl group in phenol is directly attached to the sp2-hybridised carbon of the benzene ring, which acts as an electron-withdrawing group.
General Methods Of Preparing Alcohols
Alcohols can be prepared by several methods. In this chapter, we will discuss methods which are generally used to prepare alcohols in the laboratory.
From Alkenes
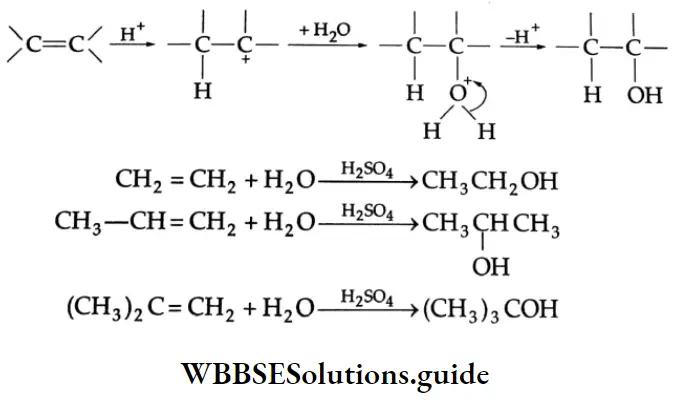
By hydration:
Alcohols are prepared commercially by the hydration of alkenes (obtained cheaply from the cracking of crude oil) in the presence of an acid catalyst. The addition of a molecule of water to an alkene takes place according to the Markovnikov rule. Except for the hydration of ethylene, the reaction produces secondary and tertiary alcohols.
By hydroboration oxidation:
Alkenes react with diborane to give trialkyl boron compounds, which yield alcohols on oxidation with alkaline hydrogen peroxide.
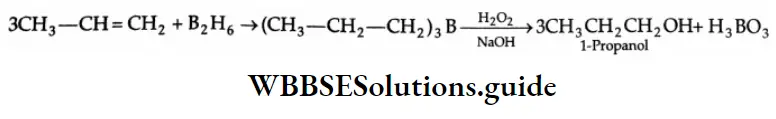
Hydroboration Mechanism:
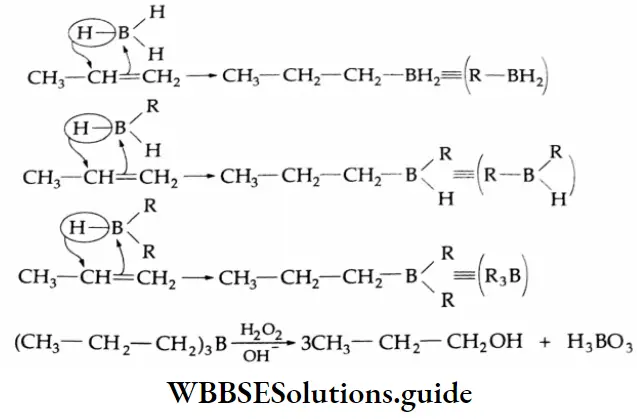
Because of the vacant orbital on boron, borane may be regarded as an electrophile and attacks the electrons of an alkene in accordance with the Markovnikov rule. But the chief product of the overall reaction, n-propyl alcohol, arises from the anti-Markovnikov addition of a molecule of water at the site of the double bond.
By The Hydrolysis Of Alkyl Halides
Alkyl halides can easily be hydrolysed to the corresponding alcohols by boiling with an aqueous alkali or moist silver oxide (AgOH).
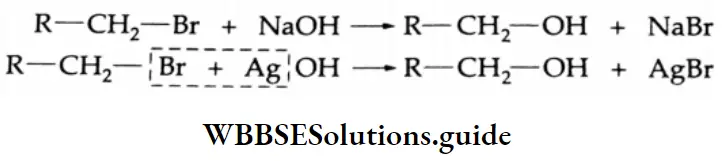
The hydrolysis of alkyl halides in an aqueous alkali may occur by either the SN1 or the SN2 mechanism. The hydrolysis of most primary halides occurs by the SN2 mechanism and yields alcohols in appreciable quantities.
Tertiary halides also undergo hydrolysis, but these reactions occur by the SN1 rather than the SN2 mechanism.
By The Reduction Of Aldehydes And Ketones
Carbonyl compounds can be reduced to the corresponding alcohols by a number of methods, either catalytically or by the use of chemical reagents.
Catalytic reduction:
Aldehydes and ketones are reduced by hydrogen in the presence of Ni, Pt or Pd catalyst at room temperature and moderate pressure to the corresponding alcohols.
⇒ \(\mathrm{RCHO} \stackrel{\mathrm{H}_2 / \mathrm{Ni}}{\longrightarrow} \mathrm{RCH}_2 \mathrm{OH}\)
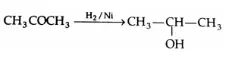
During the process, the double bond is converted into a single bond.
⇒ \(\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}-\mathrm{CHO} \stackrel{\mathrm{H}_2 / \mathrm{Ni}}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\)
Reduction by metal hydrides:
Aldehydes and ketones are easily converted to alcohols in dry ether by LIAIH4. LIAIH4 is a specific reagent an does not ordinarily reduce the ethylenic double bond in the molecule to a single bond.
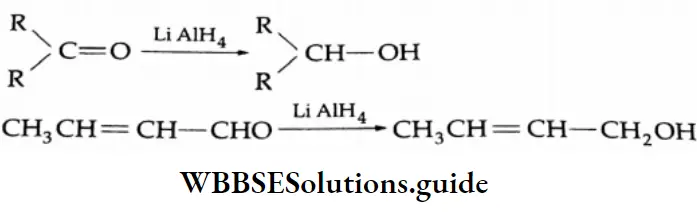
Sodium borohydride is a milder reducing agent and reduces aldehydes and ketones only.
⇒ \(\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}-\mathrm{CHO} \stackrel{\mathrm{Li} \mathrm{AlH}_4}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}-\mathrm{CH}_2 \mathrm{OH}\)
Aldehydes are reduced to primary alcohols. Ketones are reduced to secondary alcohols.
By The Reduction Of Carboxylic Acids And Esters
Carboxylic acids and esters are usually reduced by LiAlH4 in dry ether.
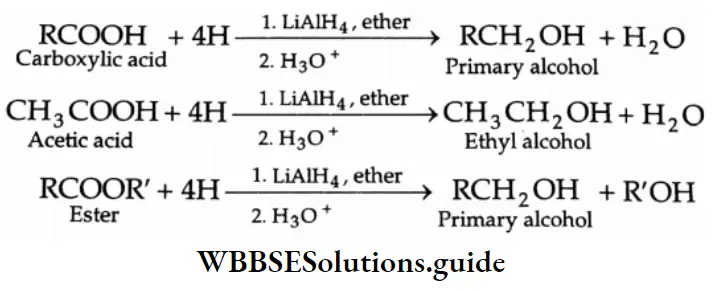
In order to reduce a carboxylic acid to an alcohol, the carboxylic acid is first converted to an ester and then reduced catalytically by hydrogen to yield an alcohol.
⇒ \(\mathrm{RCOOH}+\mathrm{R}^{\prime} \mathrm{OH} \stackrel{\mathrm{H}^{+}}{\longrightarrow} \mathrm{RCOOR} \stackrel{\mathrm{H}_2}{\longrightarrow} \mathrm{RCH}_2 \mathrm{OH}+\mathrm{R}^{\prime} \mathrm{OH}\)
Grignard Reagents
Primary, secondary and tertiary alcohols can be prepared by the use of Grignard reagents.
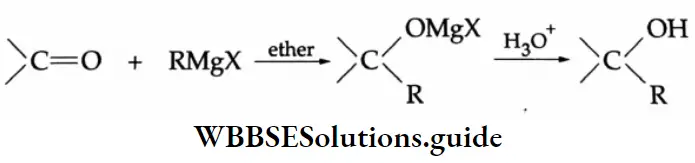
The addition of a Grignard reagent to formaldehyde yields a primary alcohol
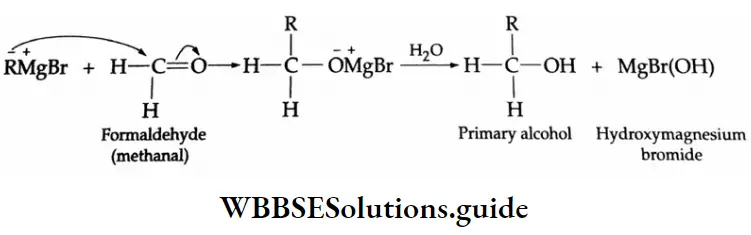
The addition of a Grignard reagent to formaldehyde yields a Secondary alcohol
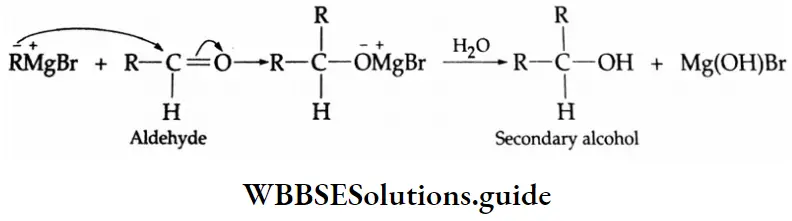
The addition of a Grignard reagent to formaldehyde yields a Tertiary alcohol
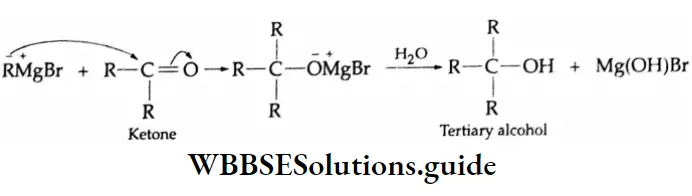
Physical Properties Of Alcohols
The introduction of a hydroxyl group in a hydrocarbon brings about a marked change in its physical properties like melting point, boiling point and solubility in water.
Boiling point:
The boiling and melting points of alcohols show a regular increase with the increase in the number of carbon atoms. The boiling point rises by about 20 K for each additional carbon atom.
This is due to the increase in van der Waals attraction. In the case of isomeric alcohols, the boiling points show a regular decrease with an increase in branching as there is a decrease in van der Waals attraction due to branching (less surface area).
The boiling points of some alcohols:
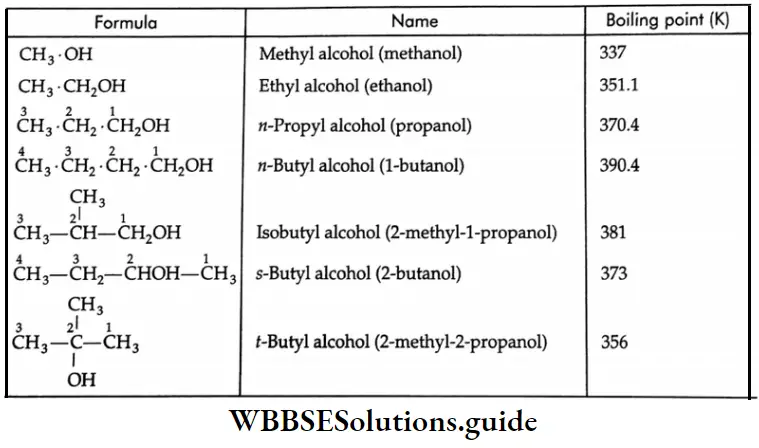
When compared to hydrocarbons, alkyl halides and ethers of comparable molecular weights the boiling points of alcohols are unusually high due to the association of their molecules through intermolecular hydrogen bonding. The high boiling point of alcohols may be attributed to the high energy required to break the hydrogen bond.
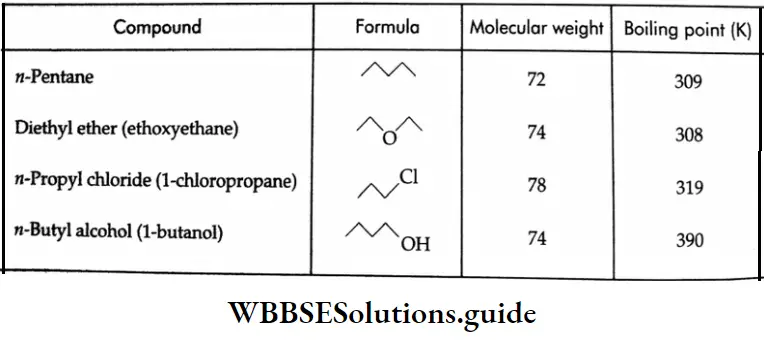
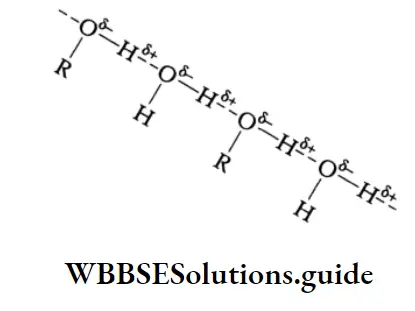
Solubility:
The lower members of the class of alcohols like methyl, ethyl, n-propyl, t-butyl and many polyhydric alcohols are completely soluble in water. This may be attributed to the intermolecular hydrogen bonding between the molecules of alcohol and water.
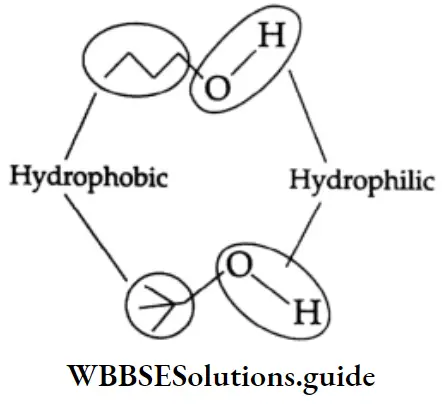
The solubility of alcohols decreases in water as the alkyl chain increases in length. Alcohols have both hydrophilic (waterlike, due to the OH group) and hydrophobic (alkane like, due to the carbon chain) moieties. With the increase in molecular weight, the hydrophobic character of alcohols increases. This reduces their solubility in water.
Among isomeric alcohols, tertiary alcohols are more soluble than primary and secondary alcohols. This can be attributed to a decrease in the relative volume of the hydrophobic portion.
Chemical Properties Of Alcohols
Alcohols react both as nucleophiles and electrophiles.
The O-H bond is broken when alcohols react as nucleophiles.
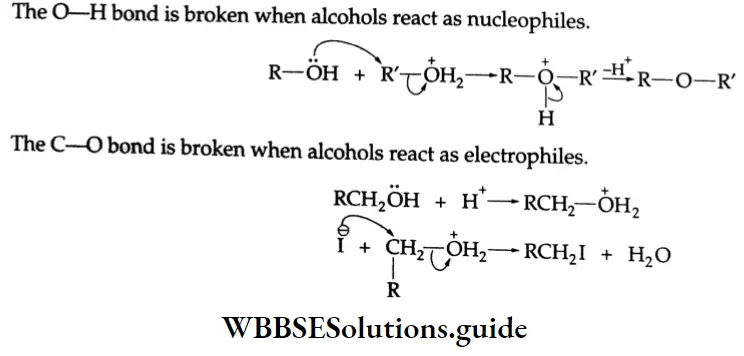
The reactions of alcohol can be divided into the following four types.
- Those in which the O-H bond is cleaved,
- Those in which the C-O bond is cleaved,
- Those in which the oxygen acts as a base, and
- Oxidation.
Reactions Due To Fission Of O-H Bond
Reactions with metals-Acidic nature:
Alcohols behave as weak acids. This characteristic can be explained on the basis of the fact that the hydrogen atom is attached to the electronegative oxygen atom, which attracts the pair of electrons of the O-H bond.
Due to this attraction, there is a tendency for the loss of hydrogen as a proton. Thus, alcohols react with strong electropositive metals, evolving hydrogen
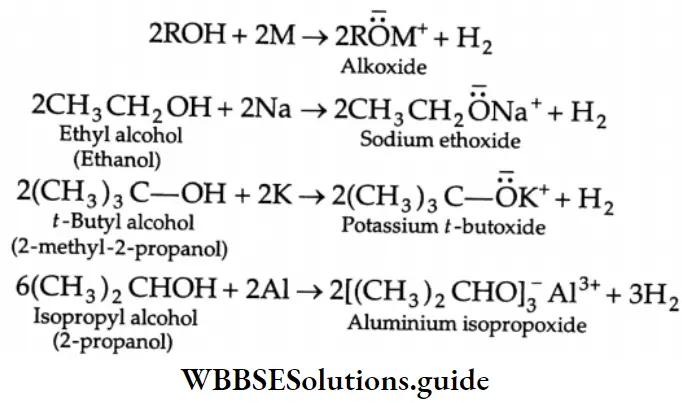
The order of acid strength of various types of alcohols is primary > secondary > tertiary.
This is because oxygen is considerably more electronegative than either carbon or hydrogen. Therefore the C-O and O-H bonds are polarised towards the oxygen atom.
The tertiary alcohol is the weakest because of the presence of three electron-releasing alkyl groups on the carbon atom attached to oxygen. This increases the electron density on oxygen tending to decrease the polarity of the O-H bond. This causes a decrease in acid strength.
Reaction with Grignard reagent
Alcohols react with Grignard reagent to form alkanes.
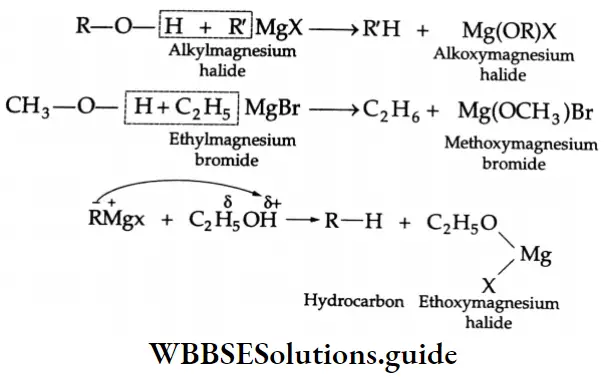
In these reactions, the order of reactivity of alcohols is primary>Secondary> Tertiary
Esterification
Alcohols react with
- Carboxylic acids
- Acid halides and
- Acid anhydrides to form esters
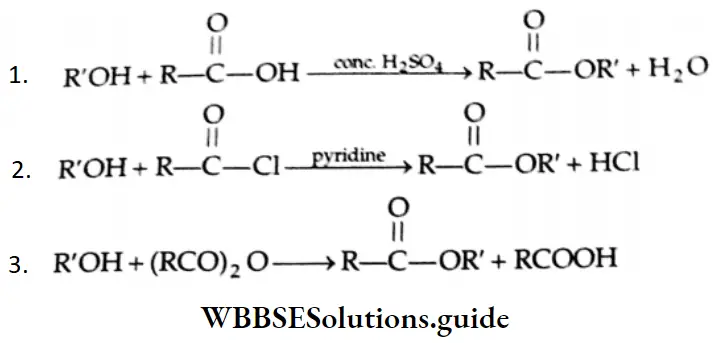
The reaction of an alcohol with carboxylic acid is reversible. It is catalysed by concentrated H2SO4. Concentrated H2SO4 removes water as soon as it is formed so that the forward reaction is favoured.
Esterification Mechanism:
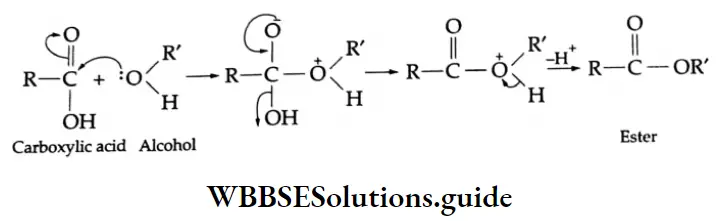
The reaction of an alcohol with an acid chloride occurs rapidly and does not require an acid catalyst. Pyridine (a base) is usually added to the reaction mixture to neutralise HCl as soon as it is formed during the reaction. Alcohols react with acid anhydrides to form esters in the absence of a catalyst.
Reactions Of Alcohols Involving C-O Bond Cleavage
Formation of an alkyl halide
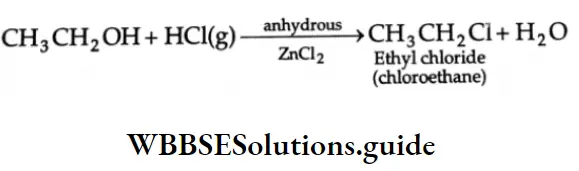
Alcohols react with a variety of reagents to yield alkyl halides. The most commonly used reagents are hydrogen halides (HCl, HBr or HI), phosphorus tribromide (PBr3) and thionyl chloride (SOCI2).
Reactions with hydrogen halides
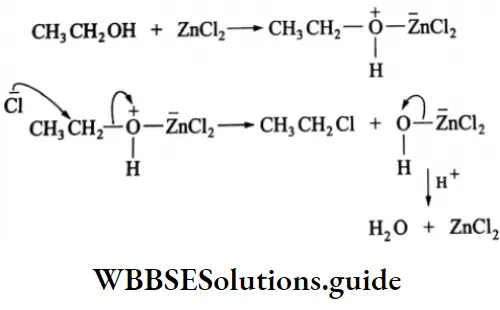
Alcohols react with dry HCl(g) in the presence of anhydrous ZnCl2 as a catalyst. CH3CH2OH + HCl(g)- anhydrous →CH3CH2Cl+ H2O ZnCl2 Ethyl chloride (chloroethane)
Hydrogen halides Mechanism
Lucas reagent
Low-molecular-weight primary, secondary and tertiary alcohols react at different rates with a solution of anhydrous zinc chloride in concentrated hydrochloric acid (Lucas reagent) (alcohols are soluble in the Lucas reagent) to give alkyl halides which are not soluble in the reaction mixture and can be seen as a separate oily layer (and on shaking give a cloudy appearance).
The ease with which the alkyl halide forms depends upon the ease with which the alcohol is converted into a carbocation. Tertiary alcohols react instantly, secondary alcohol within two or three minutes, and primary alcohols do not produce turbidity at room temperature.
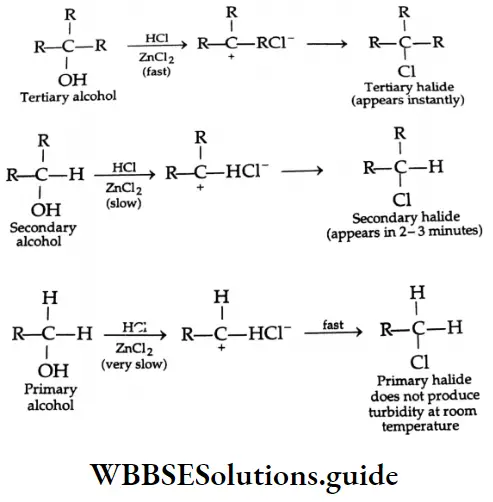
The difference in reaction rates described above is the basis of the Lucas test by means of which primary, secondary and tertiary alcohols are distinguished.
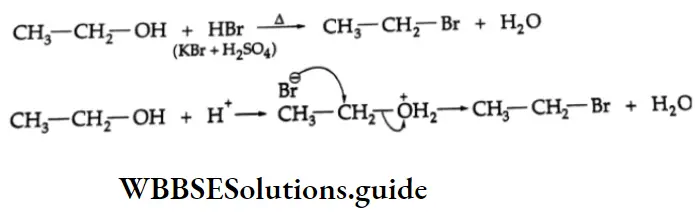
Alcohols react with HBr (formed by the reaction between KBr and concentrated H2SO4) to yield alkyl bromides.
Alcohols give alkyl iodides on reaction with hydrogen iodide.
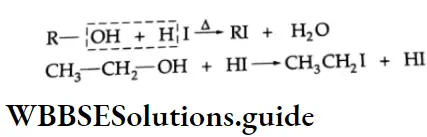
Reaction with phosphorus halides
Alcohols react with phosphorus halides to form alkyl halides.
⇒ \(\mathrm{R}-\mathrm{OH}+\mathrm{PX}_5 \rightarrow \mathrm{RX}+\mathrm{POX}_3+\mathrm{HX}\)
⇒ \(3 \mathrm{R}-\mathrm{OH}+\mathrm{PX}_3 \rightarrow 3 \mathrm{RX}+\mathrm{H}_3 \mathrm{PO}_3\)
For example:
⇒ \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{PCl}_5 \rightarrow \underset{\text { Ethyl chloride }}{\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}}+\mathrm{POCl}_3+\mathrm{HCl}\)
⇒ \(3 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\underset{\left(2 \mathrm{P}+3 \mathrm{Br}_2\right)}{\mathrm{PBr}_3} \rightarrow \underset{\text { Ethyl bromide }}{3 \mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}}+\mathrm{H}_3 \mathrm{PO}_3\)
⇒ \(3 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{PI}_3 \rightarrow \underset{\text { Ethyl iodide }}{3 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{I}}+\mathrm{H}_3 \mathrm{PO}_3\)
Victor Meyer Test (Distinction between primary, secondary and tertiary alcohols):
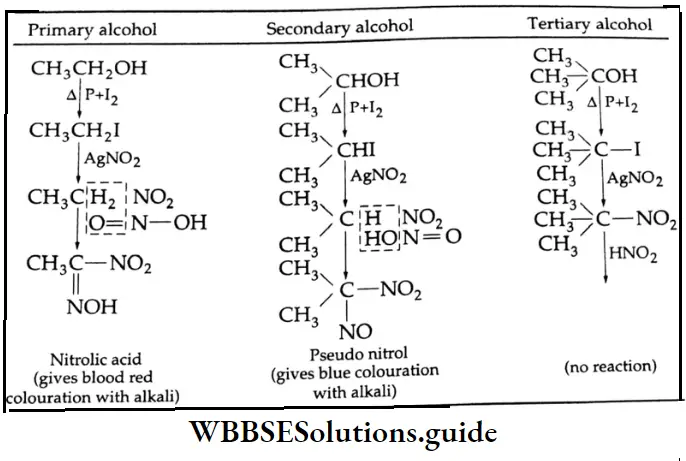
Reaction with thionyl chloride
Alcohols react with thionyl chloride in the presence of pyridine to form alkyl chlorides.
⇒ \(\mathrm{ROH}+\underset{\begin{array}{c}
\text { Thionyl } \\
\text { chloride }
\end{array}}{\mathrm{SOCl}_2} \stackrel{\text { Pyridine }}{\longrightarrow} \mathrm{RCl}+\underbrace{\mathrm{SO}_2+\mathrm{HCl}}_{\text {gas }}\)
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}+\mathrm{SOCl}_2 \stackrel{\text { Pyridine }}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Cl}+3 \mathrm{SO}_2 \uparrow+\mathrm{HCl} \uparrow\)
Reaction with concentrated H2SO4
Like other acids, concentrated H2SO4 also reacts with an alcohol to form an ester (alkyl hydrogen sulphate).
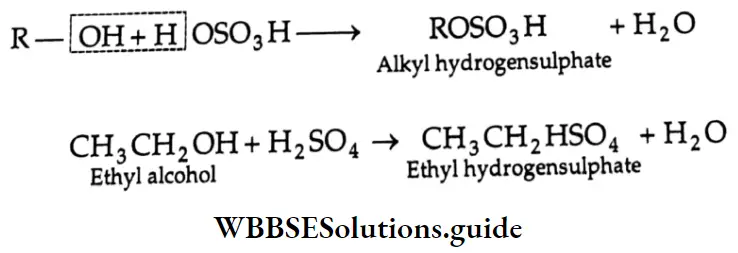
Reaction with Ammonia:
A mixture of primary, secondary and tertiary amines is formed when vapours of alcohol and ammonia are passed through heated alumina at 633 K.
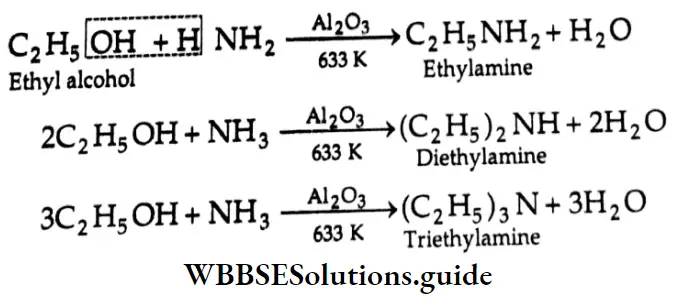
Dehydration
Alcohols can easily be dehydrated to olefins at high temperatures in the presence of an acid
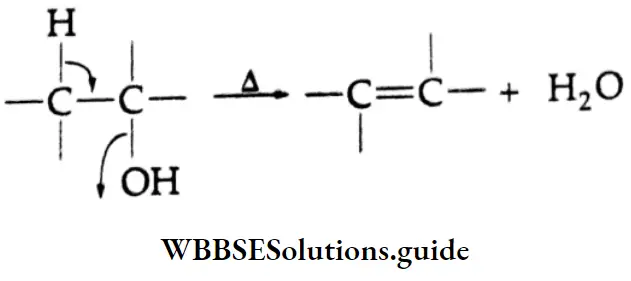
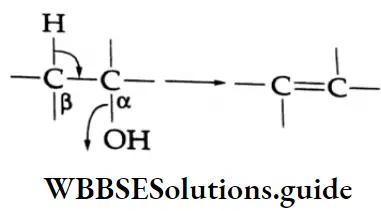
This is a ẞ-elimination reaction and is favoured by high temperature.
An elimination reaction in which a proton is lost from one carbon (B-carbon) and a nucleophile is lost from the adjacent carbon (a-carbon) is called a -elimination reaction or 1, 2-elimination reaction. The most common examples of B-elimination reactions include the dehydrohalogenation of alkyl halides and the dehydration of alcohols
In order of decreasing acidity, the acids commonly used in the reaction are H2SO4>H3PO4 >(COOH)2 >HCOOH> KHSO4 > Al2O3
The dehydration of primary alcohols requires a higher temperature than is needed for the dehydration of secondary or tertiary alcohols.
Secondary alcohols undergo dehydration in milder conditions.
Tertiary alcohols undergo dehydration at a much lower temperature.
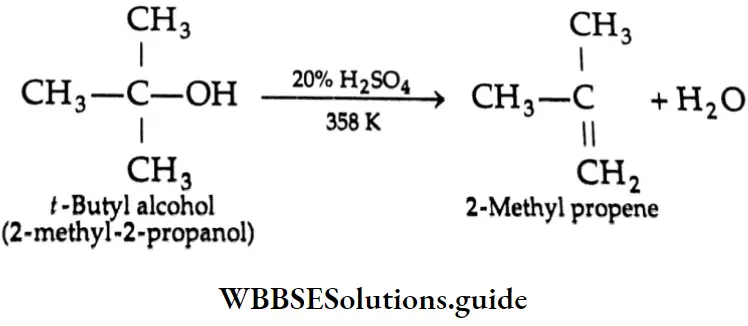
Thus, the relative ease with which alcohols undergo dehydration is in the following order.
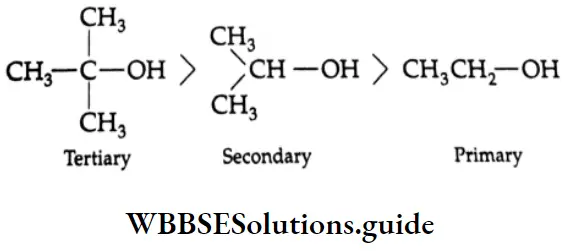
Dehydration Mechanism:
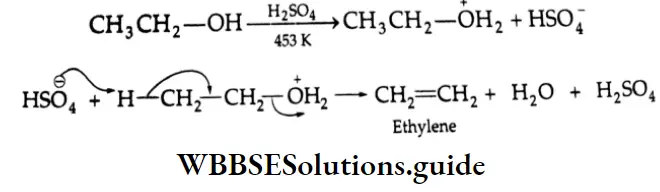
In case the olefin concerned has an isomer, the more stable isomer is formed (Saytzev rule).
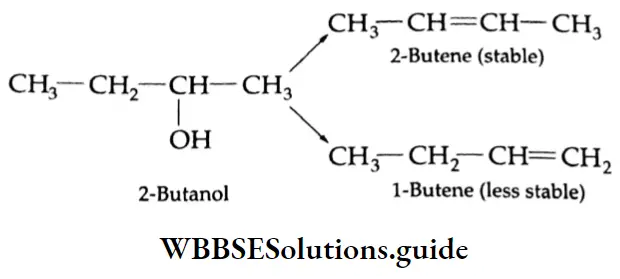
Some Primary and secondary alcohols undergo rearrangement during dehydration. For example,
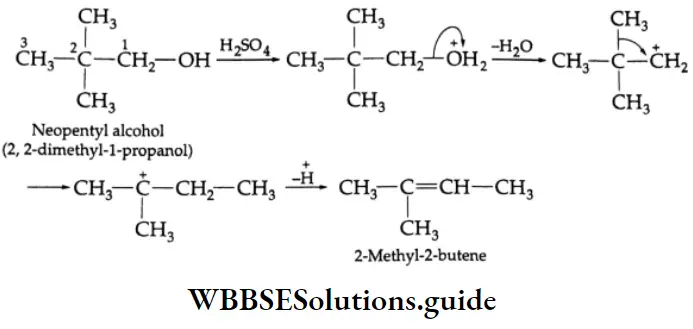
Oxidation And Dehydrogenation Of Alcohols
The oxidation of alcohol takes place with the cleavage of O-H and C-H bonds to form the C = O bond.
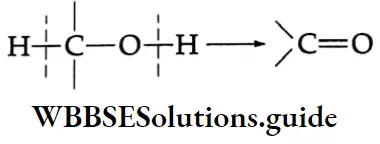
This type of cleavage and formation of bonds occurs in oxidation and dehydrogenation reactions.
Primary, secondary and tertiary alcohols differ in their behaviour towards oxidising agents. In general, primary alcohols yield aldehydes, which are readily oxidised further to carboxylic acids. Secondary alcohols give ketones, which are relatively resistant to further oxidation.
Tertiary alcohols do not have a hydrogen atom on the carbon attached to the hydroxyl group and are resistant to oxidation.
The orange-red colour of a solution of chromic anhydride (CrO3 in aqueous sulphuric acid) is immediately discharged when this solution is added dropwise to a solution of a primary or secondary alcohol in acetone. Tertiary alcohols fail to react immediately.
Reactions of primary, secondary and tertiary alcohols with CrO3 in aqueous H2SO4:
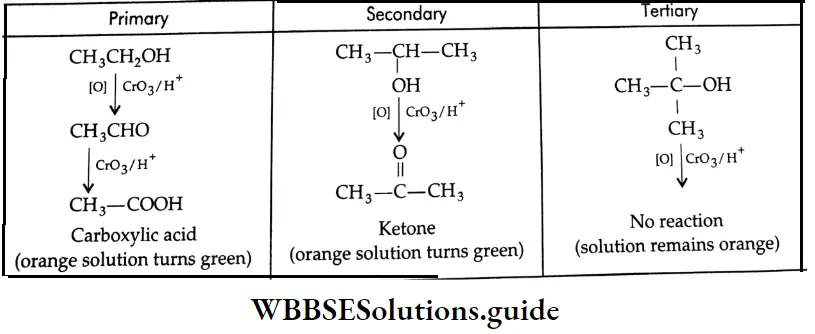
Acidic or basic aqueous potassium permanganate is often used to oxidise primary alcohols to carboxylic acids directly.
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH} \frac{\mathrm{KMnO}_4 / \mathrm{H}_2 \mathrm{SO}_4}{[\mathrm{O}]} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{COOH}\)
Tertiary alcohols are not easily oxidised by acidified KMnO4. However, at elevated temperatures, the use of strong oxidising agents (acidified KMnO4 ) leads to the breaking of C-C bonds and the formation of a carboxylic acid containing a fewer number of carbons.
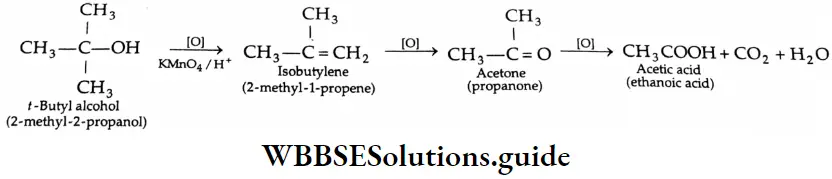
Primary alcohols are mainly oxidised to aldehydes by a weak oxidising agent, pyridinium chloromate (PCC).
⇒ \(\mathrm{R}-\mathrm{CH}_2-\mathrm{OH} \underset{[\mathrm{O}]}{\stackrel{\mathrm{PCC}}{\longrightarrow}} \mathrm{R}-\mathrm{CHO}\)
Pyridinium chlorochromate is a complex compound of chromium trioxide, pyridine and concentrated HCl.
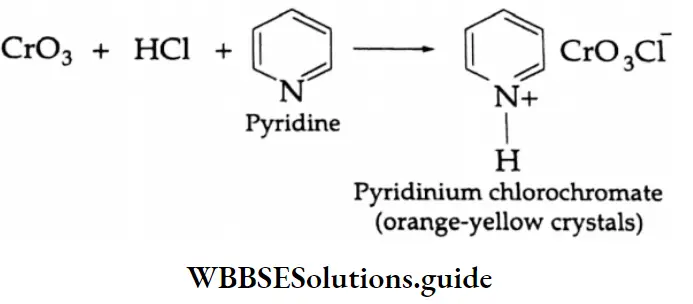
This compound oxidises a primary alcohol to an aldehyde and the reaction stops at this stage. Pyridinium chlorochromate does not attack double bonds.
When alcohol vapour is passed over copper at 573 K, dehydrogenation occurs; a primary alcohol yields an aldehyde and a secondary alcohol yields a ketone. A tertiary alcohol undergoes dehydration to yield an alkene.
⇒ \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH} \underset{573 \mathrm{~K}}{\stackrel{\mathrm{Cu}}{\longrightarrow}} \underset{\text { Acetaldehyde }}{\mathrm{CH}_3-\mathrm{CHO}}+\mathrm{H}_2\)
⇒ \(\underset{\text { Isopropyl alcohol }}{\mathrm{CH}_3 \mathrm{CHOHCH}_3} \underset{573 \mathrm{~K}}{\stackrel{\mathrm{Cu}}{\longrightarrow}} \underset{\text { Acetone }}{\mathrm{CH}_3 \mathrm{COCH}_3}+\mathrm{H}_2\)
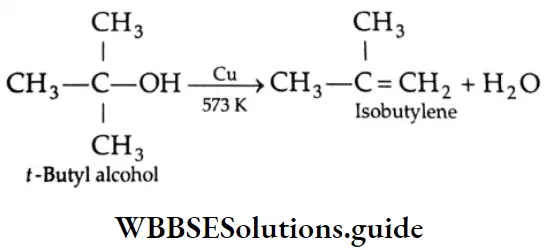
This reaction may be used for differentiating primary, secondary and tertiary alcohols.
Example 4: Give the structures of the products of the following reactions. Give reasons for your answer.
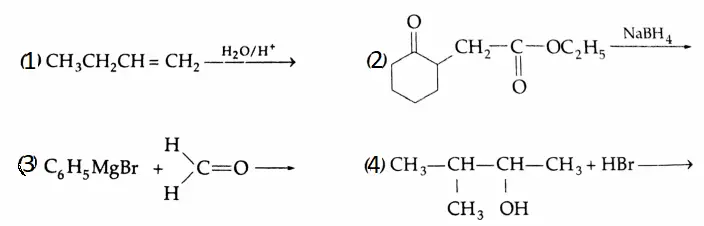
Solution:
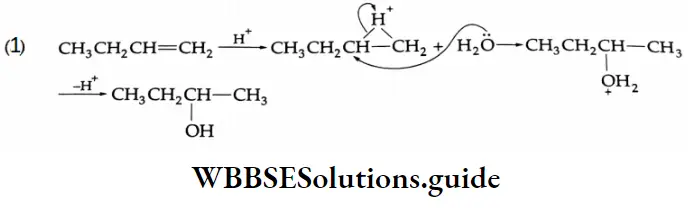
This is an electrophilic addition reaction. In an acid medium water adds according to the Markovnikov rule.
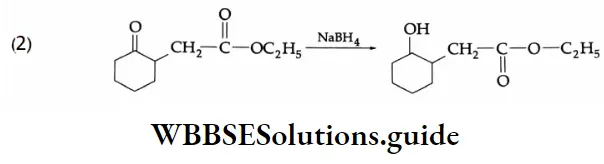
NaBH4 is a weak reducing agent>It reduces the ketonic group selectively.
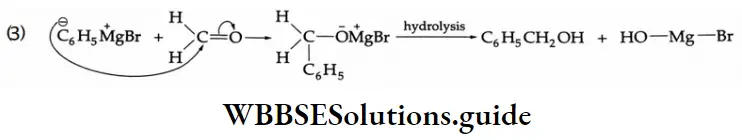
This s the nucleophilic addition reaction of a Grignard regent with a carbonyl compound
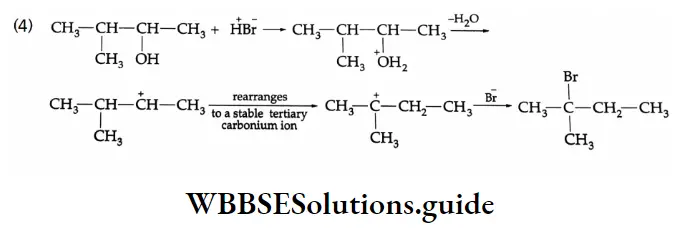
Some Commercially Important Alcohols
Methanol
Methanol is known as wood alcohol or wood spirit because it was first obtained by the destructive distillation of wood. Today methanol is produced commercially by passing a mixture of carbon monoxide and hydrogen over a catalyst containing oxides of chromium, copper and zinc at a temperature of 623-723 K and a pressure of 200 atm.

Methanol is highly toxic. Ingestion of even small quantities of methanol can cause blindness; large quantities cause death. It is used as a laboratory reagent and as a solvent for paints and varnishes. It is also used in the preparation of formaldehyde.
Ethanol
Ethanol is manufactured on a large scale from molasses, a brown syrup prepared from raw sugar during the sugar-manufacturing process. Molasses is diluted with water whereby some of the cane sugar dissolves. The diluted solution is fermented with yeast.
The enzymes invertase and zymase are present in yeast. Invertase converts sugar into a mixture of the isomers glucose and fructose. Zymase converts this mixture into ethanol and carbon dioxide. After fermentation is over the alcohol is distilled. The alcohol so obtained is 95% pure, the rest being water. This is referred to as rectified spirit.
⇒ \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}+\mathrm{H}_2 \mathrm{O} \stackrel{\text { invertase }}{\longrightarrow} \underset{\text { Glucose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}+\underset{\text { Fructose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}\)
⇒ \(\underset{\text { Glucose and fructose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6} \stackrel{\text { zymase }}{\longrightarrow} \underset{\text { Ethyl alcohol (ethanol) }}{2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}+2 \mathrm{CO}_2\)
Pure ethanol (absolute alcohol) cannot be obtained by fractionation. It is difficult and expensive to remove the last traces of water. Water can be removed by distilling the rectified spirit with anhydrous benzene. A ternary azeotropic mixture of 7.5% water, 18.5% alcohol and 74% benzene is formed, which distils out first. The residual liquid in the distilling flask is absolute alcohol (99.9%).
The last traces of water can be removed by distilling it over metallic magnesium. Absolute alcohol is hygroscopic and should be carefully preserved away from moisture. Rectified spirit and absolute alcohol, like alcoholic drinks, are taxed at a very high rate. For many industrial purposes pure alcohol is not needed and it is made undrinkable by adding various other chemicals.
This industrial methylated spirit or denatured alcohol is a mixture of 95% rectified spirit and 5% methanol, which is extremely poisonous. Sometimes CuSO4 is added to give a blue colour to methylated spirit, so that people do not drink it by mistake. Alcohol is mainly used as a solvent for paints, varnishes, perfurmes, and so on. It may be a useful fuel some day, since it burns to form CO2 and water, producing a considerable amount of heat.
Wine production
- Wine is made from grapes, which contain sugar. The grapes are crushed to squeeze out the juice. A fungus (yeast) grows naturally on the skins of the grapes. Its enzymes start the fermentation of the sugar in the juice as soon as the grapes have been crushed.
- Fermentation is carried out in the absence of air (anaerobic condition). This is because the oxygen of air oxidises ethanol to acetic acid, which destroys the taste of alcoholic drinks.
- Fermentation is complete when all the sugar has been converted into alcohol after several days. The wine is stored for several months, or even years, to mature before being bottled for sale.
- Red wine is produced from black grapes by mixing some of the skins of black grapes into the juice during fermentation so that the colouring matter from the skins passes into the wine. Sparkling wines, like champagne, are made by bottling the wine before fermentation is complete. Fermentation continues inside the bottle and the carbon dioxide produced dissolves in the wine.
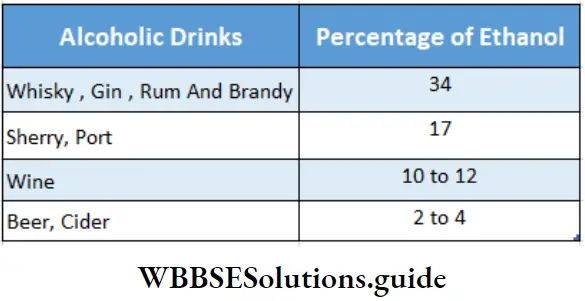
- When the bottle is opened, the pressure is released and the carbon dioxide bubbles out slowly. Wine contains between 10% and 12% of alcohol by weight. To make stronger wines like sherry and port, brandy (34% of alcohol by weight) is added to increase the ethanol content to about 17% by weight. The average strengths of some alcoholic drinks are given in the following table.
The alcohol content of some common alcoholic drinks:
The effects of alcohol on the body
In small quantities, ethyl alcohol (generally called alcohol) is a stimulant and can be beneficial. In small quantities, alcohol increases the flow of the digestive juices and stimulates the appetite. In larger quantities, alcohol makes the whole nervous system less sensitive. An excessive intake of alcohol over a long period can cause addiction (alcoholism), affect the liver and kidneys, and eventually cause damage to the brain.
Phenols
Phenol was discovered in the middle oil fraction during the distillation of coal tar sometime in the nineteenth century and was named carbolic acid.
Phenol Methods of Preparation
Phenol is prepared by the following methods.
1. Laboratory methods:
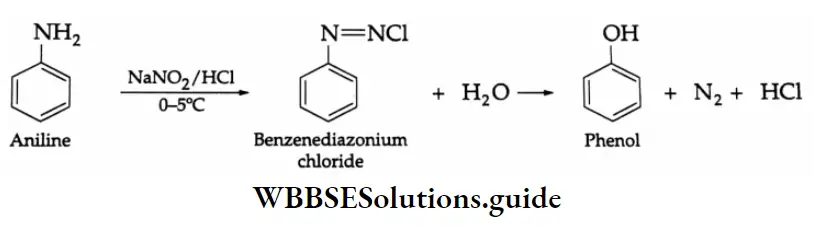
From benzene diazonium salts: Aniline is diazotised with NaNO2 and HCl at 0-5°C (278 K) and the resulting benzene diazonium salt forms phenol with water.
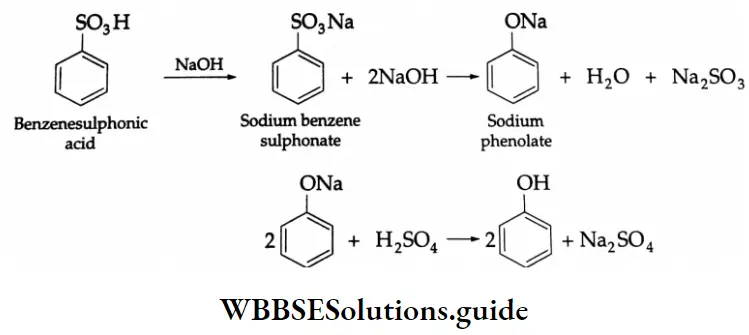
From benzene sulphonic acid: The fusion of the sodium salt of benzene sulphonic acid with solid NaOH at 623 K followed by acidification yields phenol.
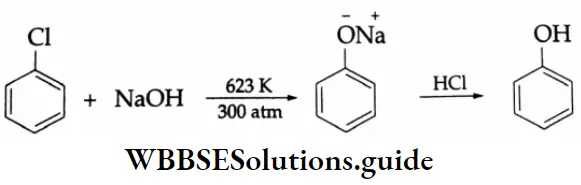
From chlorobenzene: Chlorobenzene reacts with aqueous sodium hydroxide solution at 623 K and 300 atm to produce sodium phenoxide, which on acidification yields phenol.
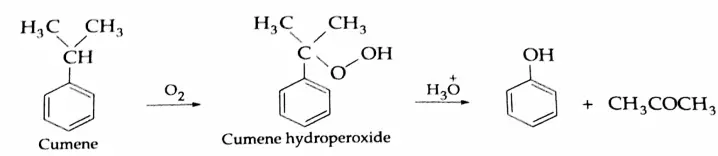
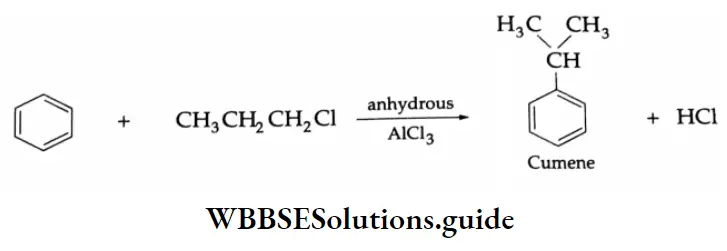
2. Commercial method (from cumene):
The oxidation of isopropylbenzene (cumene) with air in the presence of an acid catalyst (H2SO4) gives cumene hydroperoxide which on hydrolysis with an acid yields phenol and acetone.
Cumene itself is prepared by the Friedel-Crafts alkylation of benzene with propyl chloride
Physical Properties of Phenols
Phenol is a colourless, crystalline, low-melting (315 K) solid. The O-H bond of phenol is polar and therefore involved in intermolecular hydrogen bonding as shown below.
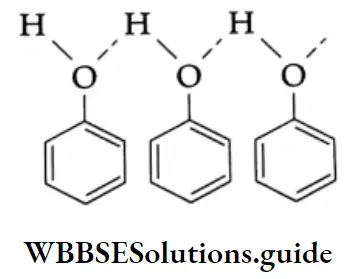
Due to intermolecular hydrogen bonding, the boiling point of phenol (455 K) is higher than that of toluer (383 K), which has a comparable molecular weight.
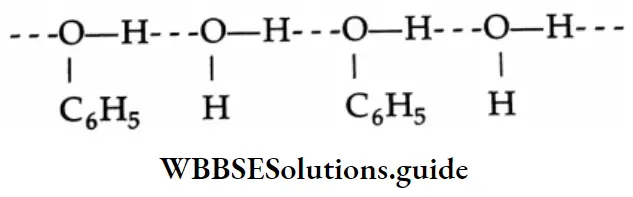
Phenol is moderately soluble in water because it forms hydrogen bonds with water.
Chemical Properties of Phenols
Reactions of the phenolic (-OH) group
Acidic nature: The acidic nature of phenol is indicated by the following reactions.
1. Phenol turns blue litmus red.
2. Phenol reacts with sodium metal to evolve hydrogen gas.
⇒ 2C6H5OH + 2Na →2C6H5ONa+ H2
3. Phenol reacts with sodium hydroxide to give sodium phenoxide.
⇒ C6H5 OH + NaOH→ C6H5ONa+ H2O
4. Phenols fail to react with Na2CO3 or NaHCO3. In fact, phenols are precipitated from an aq solution of sodium phenoxide by bubbling CO2 gas.
⇒ C6H5ONa + CO2+ H2O→ C6H5OH+NaHCO3
The hydroxyl group of phenol is directly attached to the sp2-hybridised carbon of the benzene ring. Due to the higher electronegativity of the sp2-hybridised carbon of phenol, the electron density decreases on the oxygen atom. This increases the polarisation of the O-H bond and results in an increase in the ionisation of phenol.
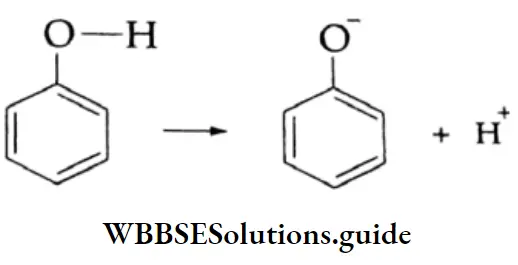
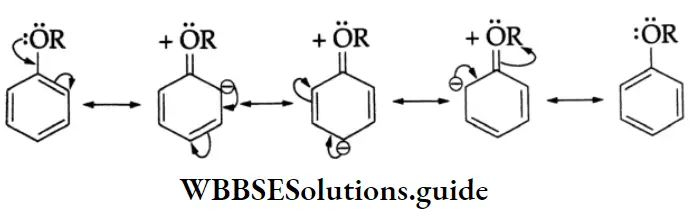
The negative charge on the oxygen atom of the phenoxide anion gets delocalised in the benzene ring. This delocalisation makes the phenoxide anion more stable and favours the ionisation of phenol.
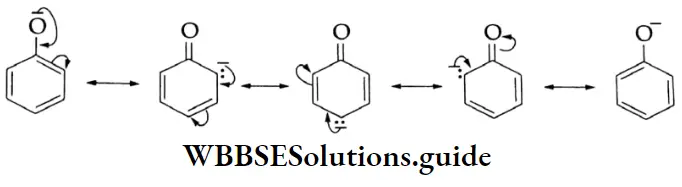
The delocalisation, no doubt, is also possible in phenol as shown below.
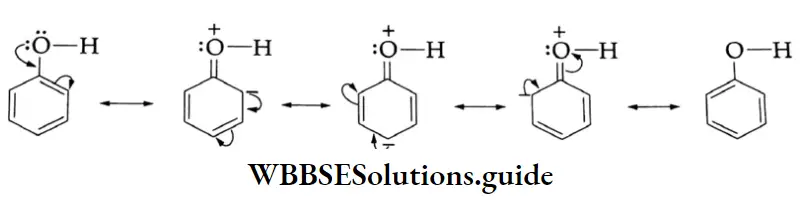
As these resonating structures involve charge separation, phenol is much less stable than the phenoxide anion. The presence of an electron-withdrawing group (NO2) at the o and p positions enhances the acidic strength of phenol.
Thus, p-nitrophenol is more acidic than phenol. On the other hand, an electron-releasing group attached to the benzene ring decreases the acid strength. Cresols, for example, are less acidic than phenol.
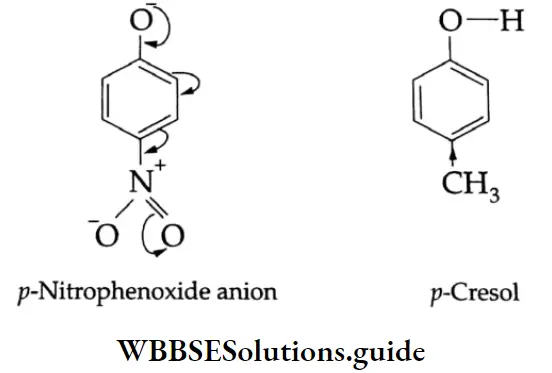
Phenol is more acidic than ethyl alcohol. Let us see how.
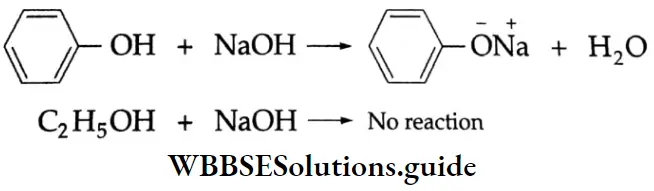
Phenol is soluble in aqueous sodium hydroxide solution and forms sodium phenoxide. Ethyl alcohol, on the other hand, does not react at all with sodium hydroxide.
In phenol, the hydroxyl group is bonded directly to an sp2-hybridised carbon atom of the aromatic ring. Being more electronegative than the sp3-hybridised carbon, the sp2-hybridised carbon polarises the O-H bond. This means that the ionisation of phenol is greater than that of ethyl alcohol (in which the OH group is attached to a -hybridised carbon).
The greater acidity of phenol as compared to ethyl alcohol is attributed to the greater stability of the phenoxide anion than that of the ethoxide anion. In the ethoxide (C2H5, O–) anion, the full negative charge is localised on the oxygen atom and therefore the anion is not stable.
As a consequence, this anion is strongly basic and the corresponding acid (C2 H5 OH) is very weak. On the other hand, in the phenoxide anion, the negative charge is delocalised over the entire molecule as shown below.
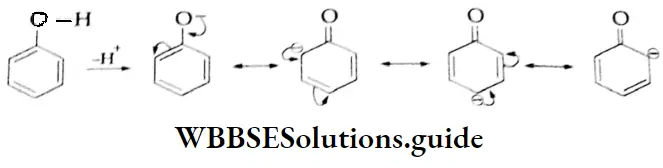
The delocalisation of charge stabilises the phenoxide anion and favours ionisation of the phenol to the phenoxide anion and H.
pk, values of some phenols and ethanol:
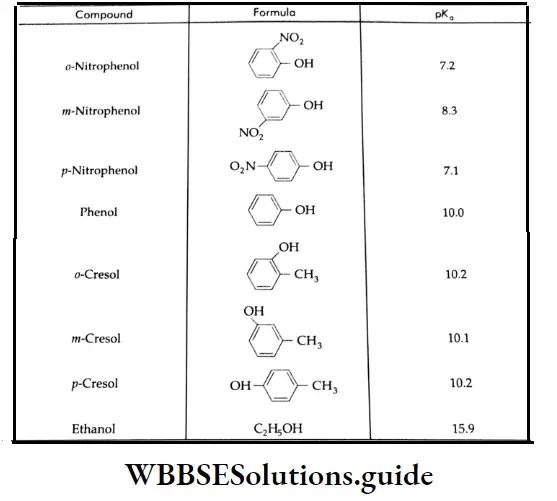
The greater the pK, value, the weaker the acid.
Reaction with Zn dust:
On distillation with Zn dust, phenol is converted to benzene.
⇒ C6H5OH + Zn→ C6H6 + ZnO
Alkylation The sodium salt of phenol (sodium phenoxide) reacts with an alkyl halide to form the corresponding ether.
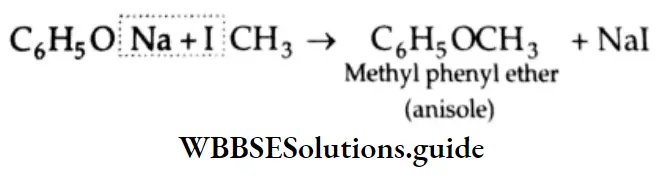
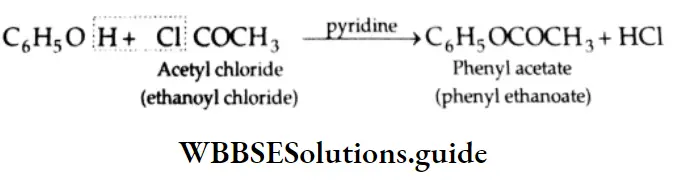
Acylation Phenol reacts with acetyl chloride in the presence of pyridine (a base) to yield phenyl acetate
Reaction with Ammonia
Phenol reacts with ammonia at 573 K in the presence of anhydrous ZnCI2 to yield aniline.
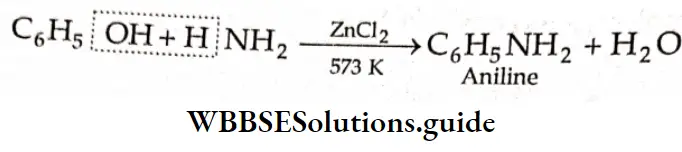
Reaction with phosphorus pentachloride:
On treatment with phosphorus pentachloride, phenol gives chlorobenzene (poor yield)
⇒ C6H5OH+ PCI5→ C6H5CI+ HCI (Chlorobenzene)
Mainly, triphenyl phosphate is formed in the side reaction.
⇒ 3C6H6OH + POCI6 → (C6H5)3 PO4 +3HCI Triphenyl phosphate
Substitution reactions in the benzene nucleus
The presence of the OH group in phenols activates the benzene ring and electrophilic substitution becomes possible. Phenols undergo electrophilic substitution reactions more readily than benzene. The hydroxyl group directs the incoming group to ortho- and para-positions as these positions become electron-rich.
Phenol undergoes nitration, halogenation, sulphonation and Friedel-Crafts reaction readily.
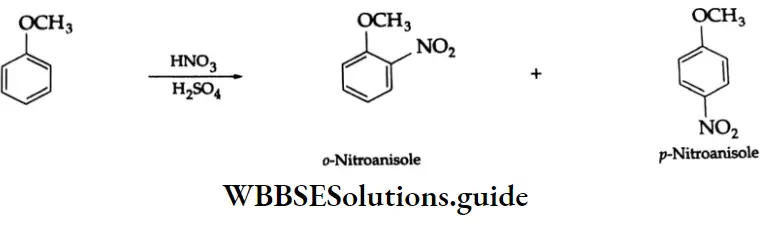
Nitration Phenol can be nitrated in dilute aqueous nitric acid even at room temperature. Ortho- as well as para- para-nitrophenols are formed.
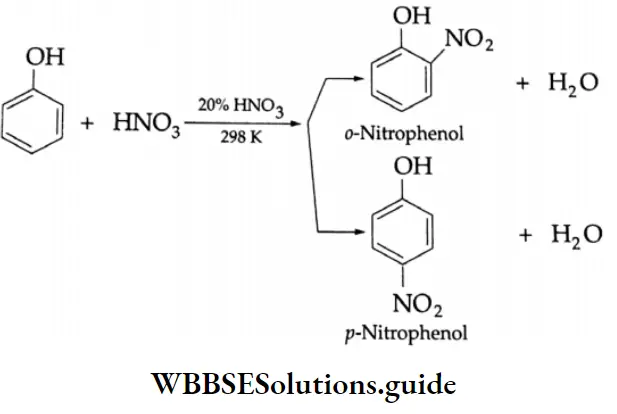
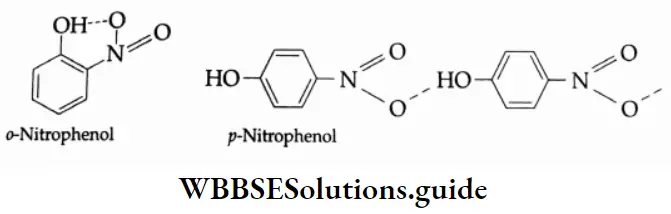
The ortho- and para-isomers can be separated by steam distillation. o-nitrophenol is steam volatile. It has higher volatility because of the intramolecular hydrogen bonding between the hydroxyl group and the nitro group.
p-nitrophenol is less volatile because intermolecular hydrogen bonding causes association between its molecules. Thus, o-nitrophenol passes over with the steam, and p-nitrophenol remains in the distillation flask.
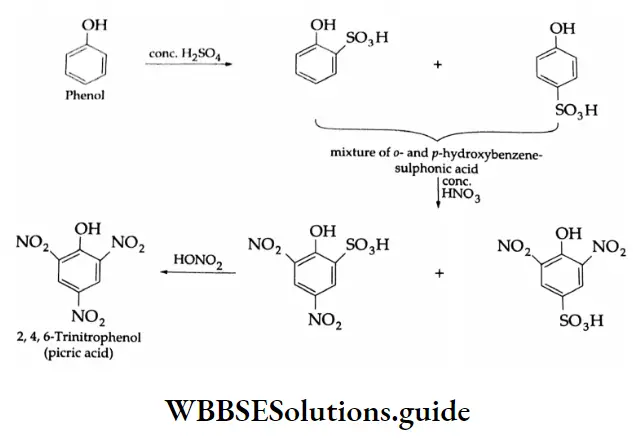
Phenol is first sulfonated and then nitrated to form picric acid. It is not treated with nitric acid first because phenol is easily oxidised by nitric acid.
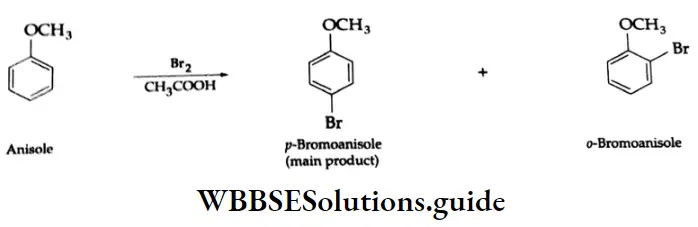
Halogenation: When bromine water is added to phenol, a white precipitate of 2, 4, 6-tribromophenol is formed
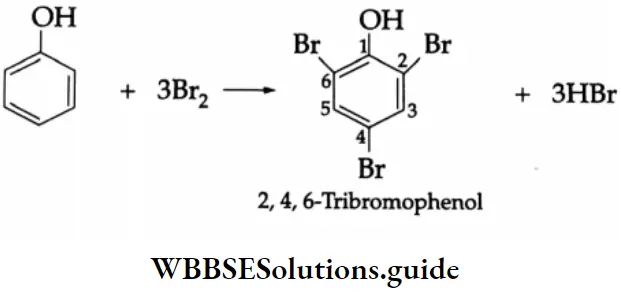
Bromination in a nonpolar solvent (carbon disulphide) affords p-bromophenol as the main product.
Halogenation takes place even in the absence of a Lewis acid because the hydroxyl group in phenol activates the benzene ring towards electrophilic substitution.
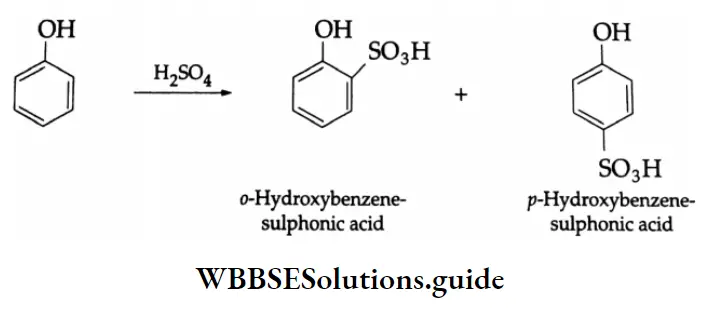
Sulphonation:
Phenol reacts with concentrated H2SO4, to form a mixture of ortho- and para-hydroxybenzene sulphonic acid. At higher temperatures, predominantly the p-isomer is formed because it is less reactive than the ortho-isomer due to less steric interference of the bulky sulphonic acid group.
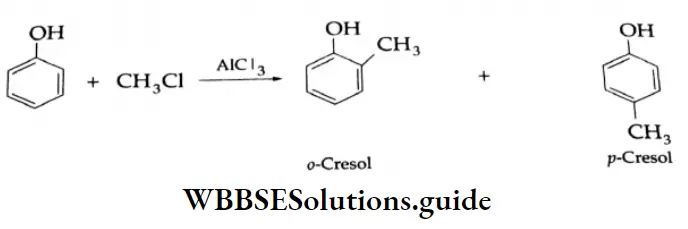
Friedel-Crafts reaction:
Phenol reacts with methyl chloride in the presence of anhydrous AICI3 to give o-cresol and p-cresol.
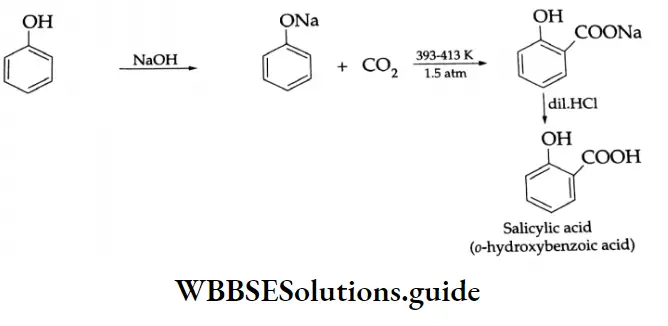
Kolbe reaction:
On being heated with CO2 at 393-413 K and 1.5 atm the sodium salt of phenol yields the sodium salt of salicylic acid. On acidification, the latter salt gives salicylic acid. This entire sequence of reactions comprises what is known as the Kolbe reaction.
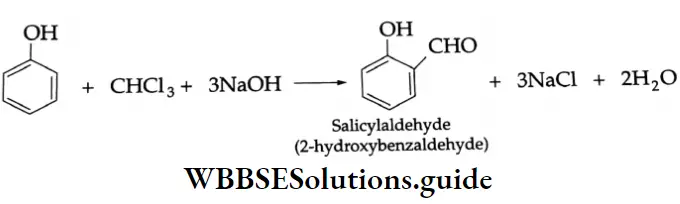
Reimer-Tiemann reaction:
On being heated with chloroform and caustic alkali, phenol gives salicylaldehyde.
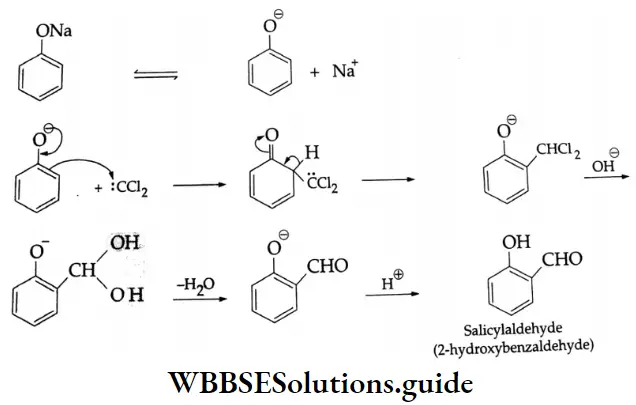
In this reaction, dichlorocarbene (: CCl2) is a reactive intermediate, which is formed by the alkaline hydrolysis of chloroform.
⇒ \(\stackrel{\ominus}{\mathrm{O}}+\mathrm{H}-\mathrm{CCl}_3 \rightleftharpoons \mathrm{H}_2 \mathrm{O}+: \stackrel{\ominus}{\mathrm{C}} \mathrm{Cl}_3 \rightarrow \stackrel{\ominus}{\mathrm{C}}+: \mathrm{CCl}_2\)
: CCl2 is an electrophilic reagent. It reacts with the phenoxide anion to form salicylaldehyde
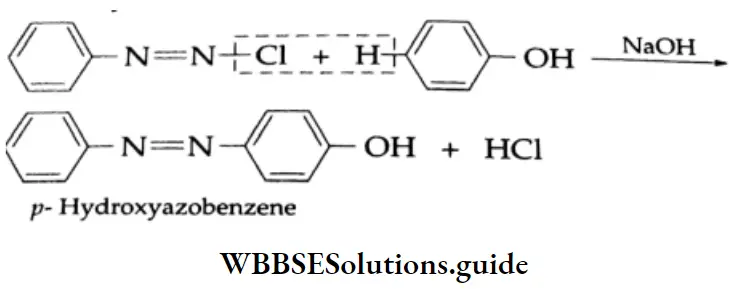
Coupling reaction: In the presence of an alkali, phenol couples with benzene diazonium chloride to form p-hydroxy azobenzene, which is a red dye.
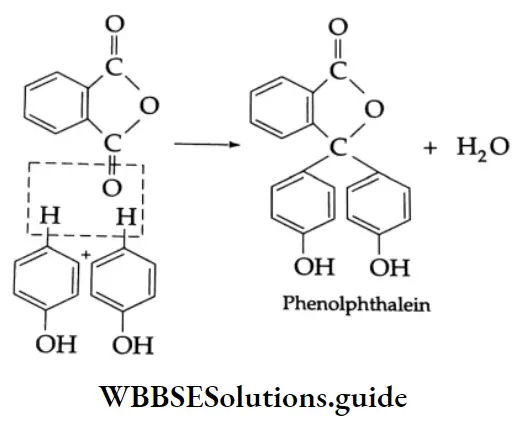
Reaction with phthalic anhydride:
On being heated with phthalic anhydride in the presence of concentrated H2SO4, phenol gives phenolphthalein. Phenolphthalein gives a red colour with an alkali. It is used as an indicator in acid-base titration.
Reaction with FeCl3: On treatment with a neutral FeCl3 solution, phenol gives a violet colouration. The violet colour is due to the formation of a water-soluble iron complex.
6C6H5OH + FeCl3 →3H+ [Fe(OC6H5)6]-3+3HCl [complex ion (violet)]
This reaction is used as a test for the presence of phenol.
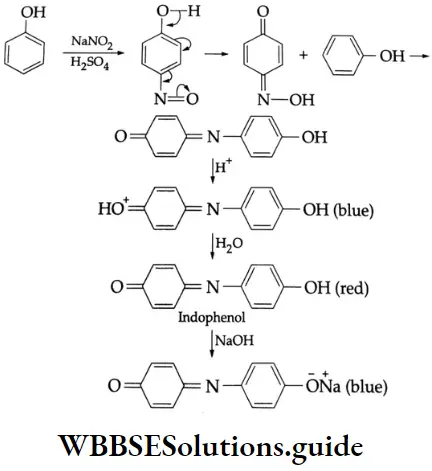
Liebermann’s nitroso reaction:
Phenol forms a deep blue solution on treatment with sodium nitrite and concentrated H2SO4 in cold conditions.
The colour turns red when the solution is diluted with water. The red solution becomes blue when it is made alkaline. This reaction is known as Liebermann’s nitroso reaction and is used as a test for the presence of phenol.
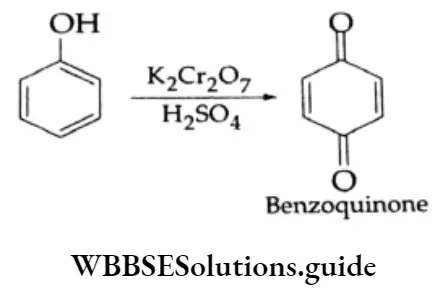
Oxidation: On oxidation with chromic acid, phenol gives benzoquinone.
Phenol Uses
Phenol is used in the preparation of
- Bakelite (synthetic resin),
- Picric acid (as an explosive),
- Phenolphthalein (as an indicator), and
- Salol, aspirin, salicylic acid (as drugs).
Ethers
Ethers Methods of preparation
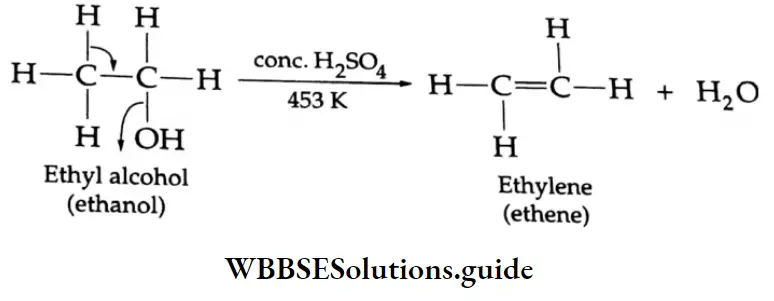
By the dehydration of alcohol:
Ethers are usually prepared by heating alcohols with concentrated H2SO4.
⇒ \(2 \mathrm{ROH} \stackrel{\Delta, \mathrm{H}_2 \mathrm{SO}_4}{\longrightarrow} \mathrm{R}-\mathrm{O}-\mathrm{R}+\mathrm{H}_2 \mathrm{O}\)
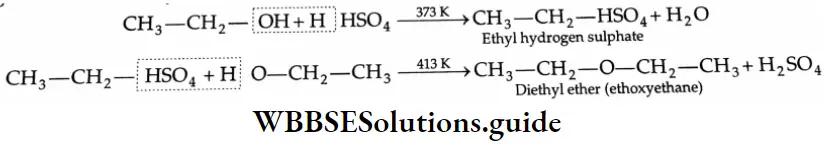
Diethyl ether is prepared by heating ethyl alcohol with concentrated H2SO4 at 413 K. The reaction occurs in two steps. At a low temperature, ethyl alcohol reacts with concentrated H2SO4 to form ethyl hydrogen sulphate which again reacts with ethyl alcohol at 413 K to yield diethyl ether
Ethers Mechanism
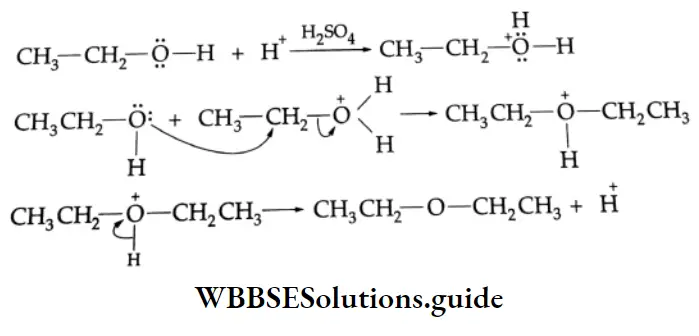
This is a nucleophilic bimolecular substitution (SN2) reaction.
This method is only used in the preparation of ethers having unhindered primary alkyl group dehydration secondary or tertiary alcohols, yield alkenes as elimination competes with substitution.
From alcohol vapours and heated alumina:
Diethyl ether is formed when vapours of alcohol are passed through heated alumina at 513-533 K. anhydrous
⇒ \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH}+\mathrm{H} \mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3 \frac{\text { anhydrous } \mathrm{Al}_2 \mathrm{O}_3}{513-533 \mathrm{~K}} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3+\mathrm{H}_2 \mathrm{O}\)
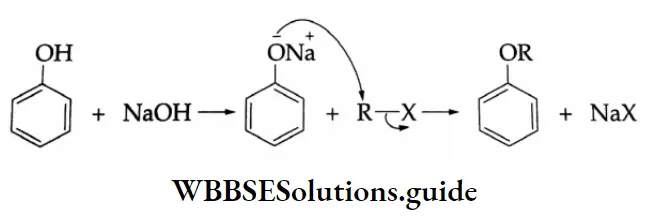
By Williamson Synthesis
Diethyl ether is formed when ethyl iodide is heated with an alcoholic solution of sodium ethoxide. (produced by the action of sodium on ethyl alcohol).
⇒ \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{I}+\mathrm{Na}-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3 \rightarrow \mathrm{CH}_3-\underset{\text { Diedium ethoxide }}{\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3+\mathrm{NaI}}\)
This is an example of Williamson’s synthesis.
Williamson’s synthesis Mechanism
Williamson synthesis involves the displacement of a halide ion from an alkyl halide by an alkoxide. It follows the SN2 mechanism.
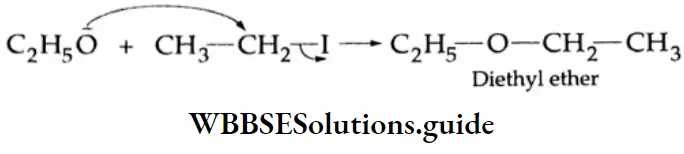
Symmetrical as well as unsymmetrical ethers can be synthesised. However, the alkyl halide must be a primary alkyl halide. For example, sodium f-butoxide reacts with methyl bromide to yield t-butyl methyl ether.
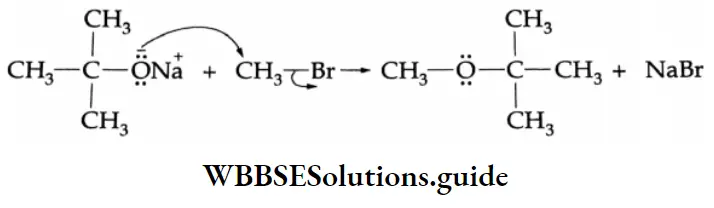
If a tertiary alkyl halide is treated with sodium ethoxide, then an alkene is formed due to the dehydrohalogenation of the alkyl halide. For example, the reaction of CH3CH2ONa with (CH3)3 C-Br gives 2-methylpropene exclusively.
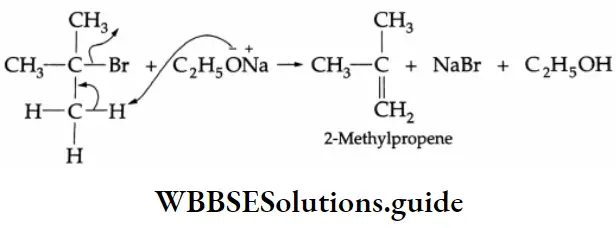
The ethoxide anion (a strong base) reacts with t-butyl bromide, leading to an elimination reaction. Phenol can also be converted into an ether by Williamson synthesis.
From an alkyl halide and dry silver oxide:
Diethyl ether is formed on heating ethyl iodide with dry silver oxide.
⇒ \(2 \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{I}+\mathrm{Ag}_2 \mathrm{O} \rightarrow \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_3+2 \mathrm{AgI}\)
Physical Properties of Ethers
Diethyl ether is a colourless, volatile liquid. The C–O bonds in ethers are polar and ethers do have a dipole noment (for diethyl ether, HD = 1.18), revealing the angular nature of the molecule. The bond angle in diethyl ther is around 110°.
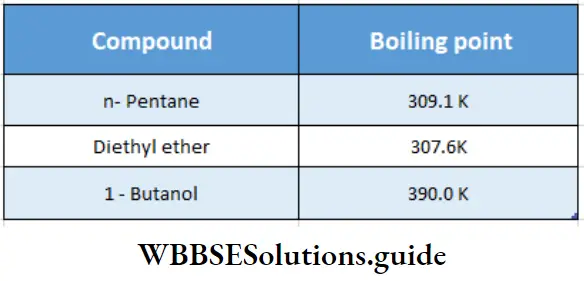
Boiling point:
Ethers have lower boiling points than the corresponding isomeric alcohols since there is no association between the molecules due to hydrogen bonding. The boiling points of ethers are close to those of alkanes of comparable molecular weight.
Solubility:
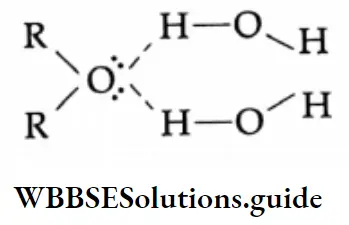
The rule of thumb which states that compounds having no more than four carbons per oxygen are water-soluble holds for ethers as well as for alcohols. The solubility of ethers in water is comparable to that of alcohols of nearly the same molecular weights.
The solubility of diethyl ether and 1-butanol is about 10 g per 100 g of H2O at 298 K. This is because of their capability to form hydrogen bonds with water.
Chemical Properties of Ethers
Ethers are relatively inert to most reagents. They are stable to bases, to catalytic hydrogenation, and to most other reducing agents.
Reactions with acids
Ethers are stable to dilute acids but do react with hot concentrated acids. Strong HBr or HI causes cleavage of the C-O bond in ethers.
Reaction with HI:
Two molecules of an alkyl iodide are formed when an ether is boiled with HI. Initially a molecule of an alcohol is also formed, which reacts further to form a second molecule of an alkyl iodide.
⇒ \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{O}-\mathrm{C}_2 \mathrm{H}_5+2 \mathrm{HI} \stackrel{\Delta}{\longrightarrow} 2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{I}+\mathrm{H}_2 \mathrm{O}\)
Ether Mechanism:
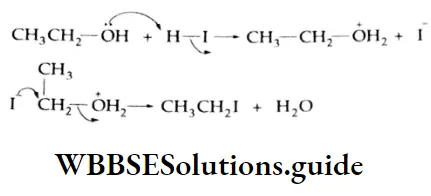
The ether first dissolves in the acid due to its basic nature with the formation of an oxonium ion. This is followed by an SN2 reaction with the iodide ion.
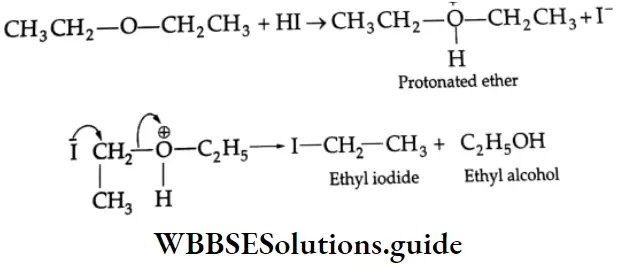
In the next step, the ethyl alcohol formed reacts with HI to produce a second mole of ethyl iodide.
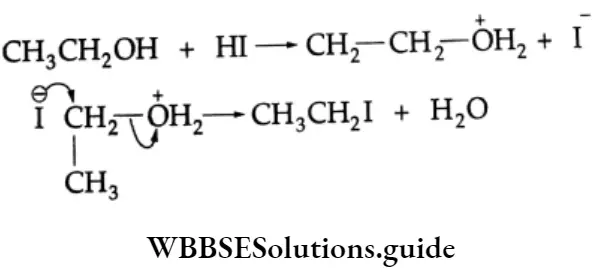
In the case of an unsymmetrical ether, the iodide ion (a nucleophile) attacks the least substituted carbon (carbon the smaller alkyl group) of the oxonium ion and displaces an alcohol molecule by the SN2 mechanism.
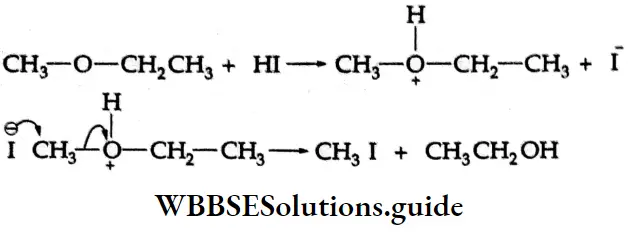
At High Temperature, the excess HI reacts with Ethyl Alcohol to yield ethyl iodide
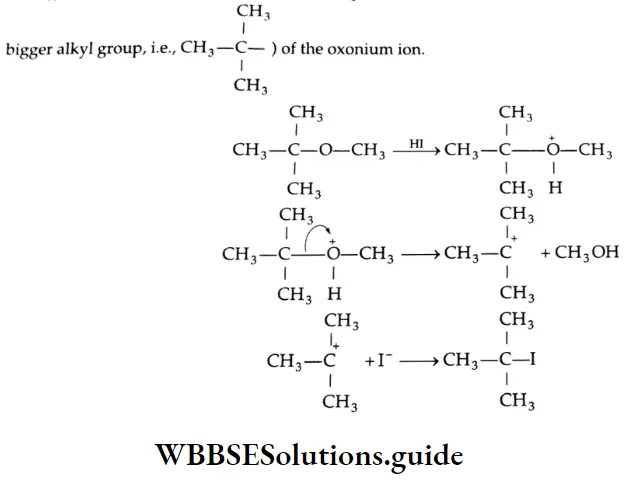
However, when one of the alkyl groups is the tertiary butyl group, the iodide formed is t-butyl iodide through the S 1 mechanism. In this reaction, the nucleophilic iodide ion attacks the more substituted carbon (carbon of the bigger alkyl group i.e.,
Anisole (an aromatic ether) reacts with HI to form methyl iodide and phenol. Alkyl aryl ethers are cleaved at the alkyl-oxygen bond because neither SN1 nor SN2 processes can normally occur at the aromatic carbon. The phenyl group is electron-rich and tends to repel any nucleophile.
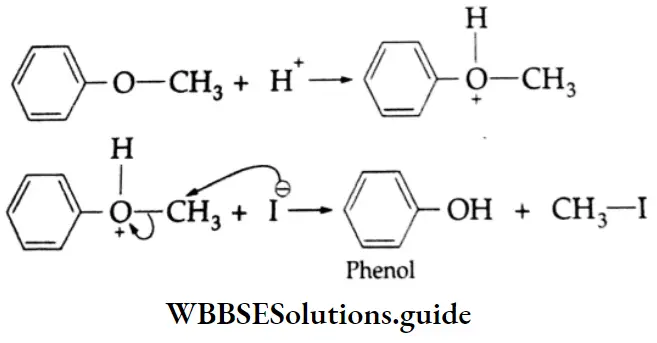
Phenol does not react further to give a halide as the nucleophilic substitution reaction of phenol is rather difficult.
The C-O bond in ethers can also be cleaved upon their reactions with HBr and with HCl. Since these acids are less reactive than HI (because CI and Br are poorer nucleophiles than I), higher concentrations and temperatures are required for them to be effective.
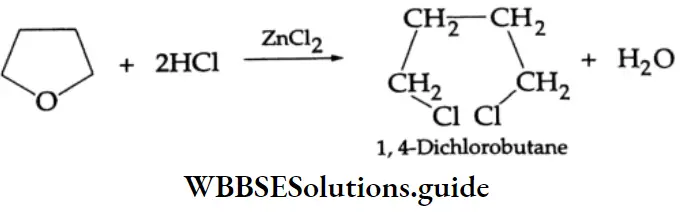
The cyclic ether tetrahydrofuran is cleaved by HCI in the presence of ZnCl2 to yield 1, 4 dichlorobutane, a valuable intermediate in the manufacture of nylon.
Reaction due to ethereal oxygen:
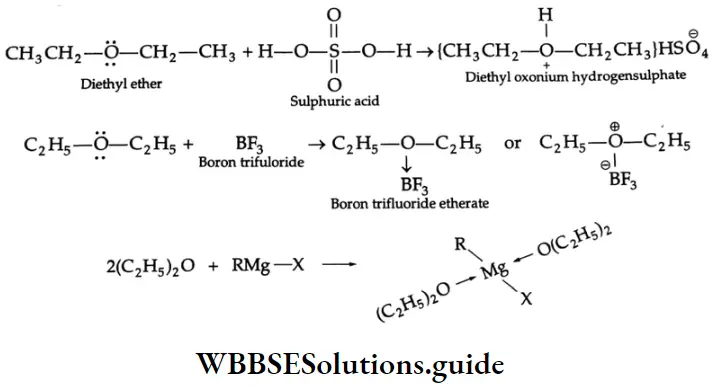
Ethers are bases and can react with acids such as sulphuric acid, boron trifluoride, and Grignard reagents.
Auto – Oxidation:
Ethers react with atmospheric oxygen to form peroxides
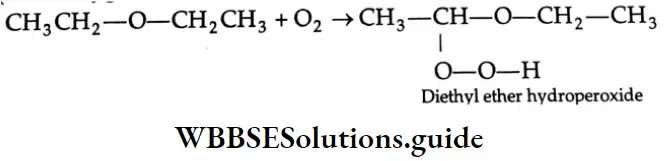
Peroxides explode violently on heating. During the distillation of an ether, the residue in the distilling flask becomes rich in peroxides. Therefore, ethers should not be distilled to dryness.
The presence of peroxide in an ether can be detected by shaking a small volume of an ether mixed with an aqueous KI solution. A purple colour confirms the presence of peroxide.
Electrophilic substitution reactions:
The alkoxy group (OR) activates the benzene ring towards electrophilic substitution. It directs the incoming group to ortho- and para-positions as these positions become electron-rich as shown below.
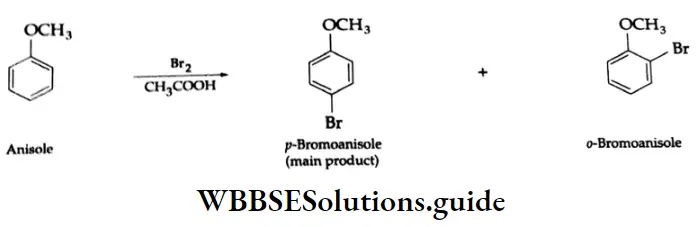
Halogenation: Bromination of anisole (methyl phenyl ether) in an acetic acid medium mainly gives p-bromoanisole
Nitration: In the presence of concentrated H2SO4, anisole reacts with concentrated HNO3 at 323-333 K to give ortho- and para- para-nitro anisole.
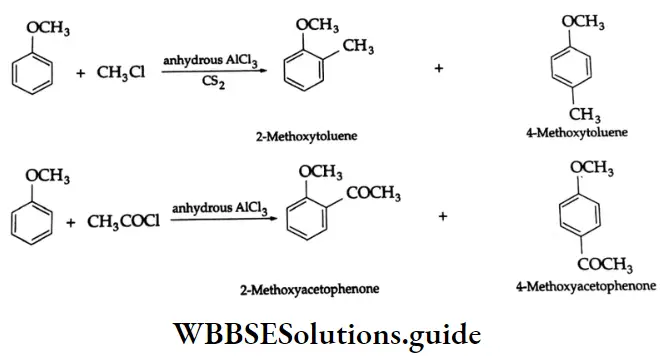
Friedel-Crafts reaction: Anisole reacts with an alkyl halide or acyl halide in the presence of anhydrous AICI3 as a catalyst. The alkyl or acyl groups are introduced at the ortho- and para-positions
Example 5: Give the structures of the products of the following reactions. Explain your answers.
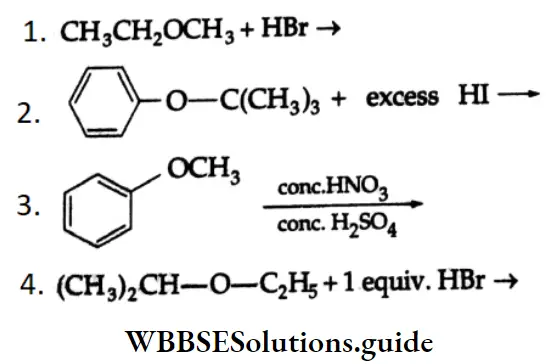
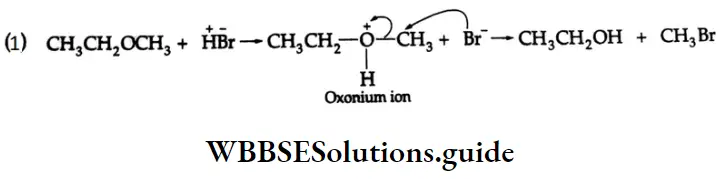
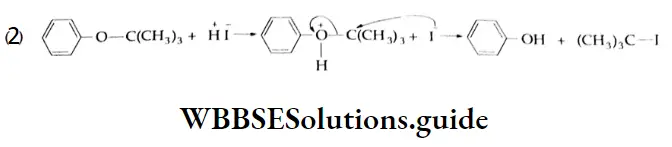
Solution:
This is an SN2 reaction. The nucleophile Br attacks the less substituted alkyl group.
In this case, the nucleophile (I) cannot attack the electron-rich carbon of benzene. It preferentially attacks the more stable tertiary carbonium ion.
This is an electrophilic aromatic substitution reaction. Since the OCH, group is o- and p- directing, NO2 (HNO3 + 2H2SO4→2HSO4 + NO2 + H2O) is substituted at the o- and p-positions.
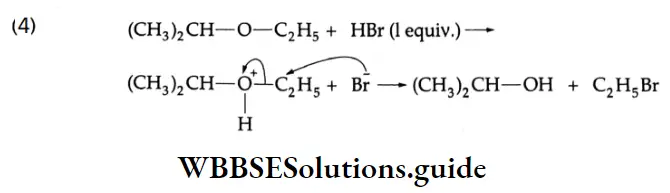
This is an SN2 reaction. The nucleophile Br attacks the less-substituted alkyl group.
Alcohols Phenols And Ethers Multiple-Choice Questions
Question 1. Glycerol is a
- Monohydric alcohol
- Trihydric alcohol
- Dihydric alcohol
- None of these
Answer: 3. Dihydric alcohol
Question 2. Methyl alcohol and ethyl alcohol are distinguished by the reaction with
- I2/NaOH
- Na
- CH3COOH
- None of these
Answer: 1. I2/NaOH
Question 3. C2H2OH and CH3-O-CH, are
- Position isomers
- Chain isomers
- Functional isomers
- Metamers
Answer: 2. Functional isomers
Question 4. Which of the following compounds will give an ester with an acid?
- Paraffin
- Alcohol
- Alkene
- Alkyl halide
Answer: 2. Alcohol
Question 5. The first component formed on the oxidation of a primary alcohol is a/an
- Ketone
- Ester
- Carboxylic acid
- Aldehyde
Answer: 4. Aldehyde
Question 6. Alcohols contains the functional group
- -OH
- -CHO
- C=O
- -NH2
Answer: 1. -OH
Question 7. Lucas reagent is
- Concentrated HCl + anhydrous ZnCl2
- Dilute HCl + Hydrated ZnCl2
- Concentrated HNO3+ anhydrous ZnCl2
- Concentrated HNO3 + anhydrous MgCl2
Answer: 1. Concentrated HCl + anhydrous ZnCl2
Question 8. Ethanol and methanol are distinguished by
- The chloroform test
- The Victor Meyer test
- Their rates of esterification
- The iodoform test
Answer: 4. The iodoform test
Question 9. A primary alcohol contains a
- CHOH group
- -CH2OH
- ->C-OH group
- None of these
Answer: 2. -CH2OH
Question 10. On distillation with zinc, phenol gives
- Nitrobenzene
- Aniline
- Benzene
- Zinc Phenoxide
Answer: 3. Benzene
Question 11. On reaction with bromine-water, phenol gives
- Bromobenzene
- Dibromorphenol
- 2,4,6 – tribromophenol
- Picric acid
Answer: 3. 2,4,6 – tribromophenol
Question 12. Methyl propyl ether is
- Asymmetrical ether
- An unsymmetrical ether
- A simple ether
- None of these
Answer: 2. An unsymmetrical ether
Question 13. In reaction with a mineral acid, an ether gives an
- Oxonium salt
- Ether peroxide
- Alkane
- Aldehyde
Answer: 1. Oxonium salt
Question 14. Diethyl ether and methyl propyl ether are
- Functional isomers
- Metamers
- Position isomers
- Chain isomers
Answer: 2. Metamers
Question 15. The IUPAC name of CH3OC2H5 is
- Methoxy ethane
- Propoxymetane
- Ethyl methyl ether
- Diethyl ether
Answer: 1. Methoxy ethane
Question 16. To prepare diethyl ether, bromoethane is heated with which of the following compounds?
- Ethanol
- Sodium ethoxide in dry ether
- C2H2OH/Na
- HI
Answer: 2. Sodium ethoxide in dry ether
Question 17. Diethyl ether and 1-butanol are
- Position isomers
- Functional Isomers
- Optical isomers
- Metamers
Answer: 2. Functional Isomers
Question 18. The correct order of the boiling points of primary (1), secondary (2°) and tertiary (3°) alcohols is
- 1°> 2° >3°
- 3° 2° 1°
- 2>1° >30
- 2° >30 > 1°
Answer: 1. 1°> 2° >3°
Question 19. Among the following, which is the most acidic?
- Phenol
- Benzyl alcohol
- m-Chlorophenol
- Cyclohexanol
Answer: 1. Phenol
Question 20. Which of the following forms hydrogen bonds to a greater extent?
- Ethanol
- Diethyl ether
- Triethyl amine
- Ethyl chloride
Answer: 1. Ethanol
Question 21. Which of the following will respond positively to the iodoform test?
- CH2OH
- (CH3)2CHOH
- (CH3), COH
- CH3 OH
Answer: 2. (CH3)2CHOH
Question 22. Formaldehyde reacts with CH3MgBr to give
- C2H2OH
- CH3COOH
- CH3CHO
- HCHO
Answer: 1. C2H2OH
Question 23. On treatment with B2H6/H2O2, R-CH=CH2 gives
- RCOCH3
- RCHOH CH2OH
- RCH2 CHO
- RCH2CH2OH
Answer: 4. RCH2CH2OH
Question 24. Which of the following will give a yellow precipitate with I2/NaOH?
- CH3COCH2CH3
- CH3COOCOCH3
- CH3CONH2
- CH3CH(OH)CH2CH3
Answer: 1 and 4 – CH3COCH2CH3 and CH3CH(OH)CH2CH3
Question 25. Phenol reacts with CHCl3/aqueous NaOH. The electrophilic reagent which attacks the benzene nucleus is
- CHCl3
- CHC12
- COCI2
- CCl2
Answer: 4. CCl2
Question 26. Upon reacting with neutral FeCl3, phenol gives a
- Green colour
- Violet colour
- Red colour
- Blue colour
Answer: 2. Violet colour
Question 27. The reaction of

Answer: 1 and 4
Question 28. The central oxygen atom of ether is
- Sp-hybridised
- Sp2-hybridised
- Sp3d2-hybridised
- sp-hybridised
Answer: 3. Sp3d2-hybridised
Question 29. What is the order of dehydration of the following?
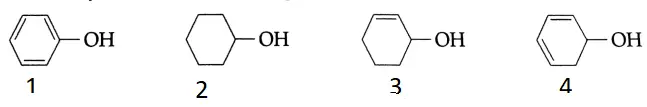
- 1 < 2 < 3 <4
- 2<3<4<1
- 1< 3< 2<4
- 1 < 4 < 2 = 3
Answer: 1. 1 < 2 < 3 <4
Question 30. How many isomeric acyclic alcohols and ethers are possible for C4H8O?
- 7
- 9
- 5
- 8
Answer: 4. 8
Question 31. Among the following reagents, phenol can be distinguished from ethyl alcohol by all except
- NaOH
- FeCl3
- Br2/H2O
- Na
Answer: 4. Na
Question 32. The order of esterification of alcohol is
- 3° > 2° > 1°
- 1° > 2° >3°
- 2° >3° 1°
- None of these
Answer: 3. 2° >3° 1°
