WBCHSE Class 11 Chemistry Notes On Covalent Bonding And Its Applications Covalent Bond
Ionic bonds are formed when one atom loses electrons to achieve a stable configuration and the other atom accepts those electrons to complete the octet in its outermost shell. But what about atoms that have 4 electrons in their valence shell?
- It would be difficult for such an atom to either lose or gain 4 electrons. And what happens when both the atoms are short of electrons? Neither can gain electrons from the other to complete its octet, for example, when two chlorine atoms combine to form a Cl2 molecule.
- Lewis suggested that such atoms can complete their octets by sharing electrons. The bond formed between atoms of the same or different elements due to the mutual sharing of electrons is called a covalent bond.
- In the formation of a covalent bond between two atoms, both atoms contribute an equal number of electrons. When two atoms contribute one electron each to form a bond, this shared pair of electrons is common to both atoms.
- These two electrons which are responsible for the formation of the bond are called the bonded pair or shared pair of electrons. You have already studied the Lewis structures earlier in the chapter. Let us draw Lewis dot structures for a few covalent molecules.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
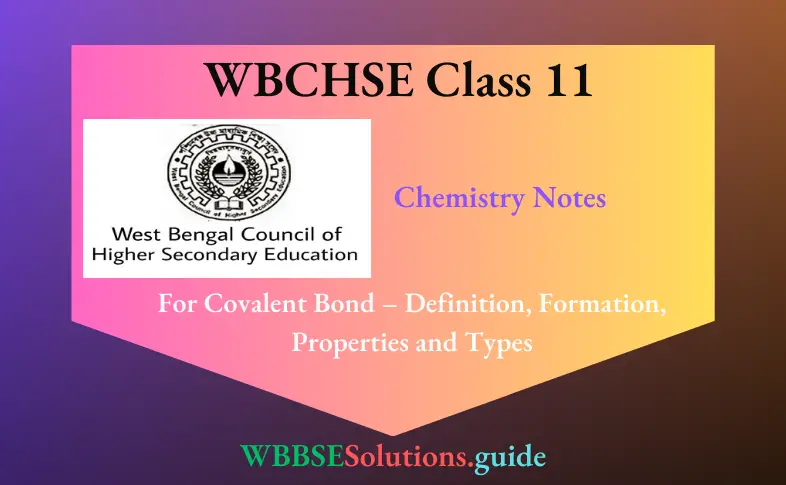
The chlorine molecule Each chlorine atom (Z = 17) has seven electrons in its valence shell (2, 8, 7) and needs one more electron to complete its octet. In the formation of the chlorine molecule, two chlorine atoms contribute one electron each and share two electrons.
“WBCHSE Class 11 Chemistry, covalent bond, definition, formation, properties, and types”
In this way both the chlorine atoms achieve the stable configuration of eight electrons in their outermost shell. The sharing of electrons by two chlorine atoms or the formation of a bond between them is shown in Figure.
- The shared pair of electrons completes the outermost shells of both the bonded atoms and is present in a region between the two bonded atoms. These two electrons are attracted by the nuclei of both atoms. Thus they are held together by a strong attractive force, and form a covalent bond.
- In the formation of a covalent bond, like the one between two chlorine atoms, there is a partial overlapping of atomic orbitals. In other words, a part of the electron cloud of each of the two half-filled atomic orbitals overlaps. Thus, the covalent bond is formed in the direction of overlapping, and unlike an ionic bond, it is directional.
- All the electrons in the valence shell may or may not be involved in bonding. In the formation of the chlorine molecule, for example, there is only one shared pair of electrons. Each chlorine atom is left with three pairs of unshared electrons. Such electron pairs are called nonbonding, unshared, or lone pairs of electrons.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
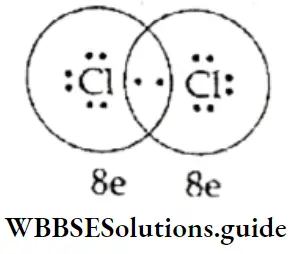
The Ammonia Molecule A nitrogen atom has five electrons in the outermost shell. It shares three of these and attains an octet. Hydrogen has only one electron, it needs one more electron to complete its Is orbital.
The nitrogen atom and each of the hydrogen atoms contribute one electron each to the bonded pair. The electron pair left unshared on the nitrogen atom is the lone pair.
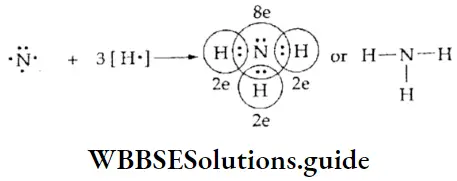
The Carbon Tetrachloride Molecule A carbon atom has four valence electrons. It shares all four electrons with four chlorine atoms to attain an octet in the carbon tetrachloride molecule. Each of the chlorine atoms also attains an octet.
Multiple Covalent Bonds: When two atoms share only one pair of electrons, the bond formed between them is called a single covalent bond. The chlorine, ammonia, and carbon tetrachloride molecules contain single covalent bonds.
There is one single covalent bond in a chlorine molecule. The ammonia and carbon tetrachloride molecules contain three and four single covalent bonds respectively.
“Covalent bond, WBCHSE Class 11, chemistry notes, and key concepts”
Properties Of Covalent Bond In Class 11 Chemistry WBCHSE
Now, two atoms can share more than one pair of electrons. When two atoms share more than one pair of electrons, the bond between the atoms is called a multiple covalent bond.
When two atoms share two pairs of electrons, the bond between the atoms is called a double covalent bond and when three pairs of electrons are shared by two atoms, the bond formed between them is called a triple covalent bond.
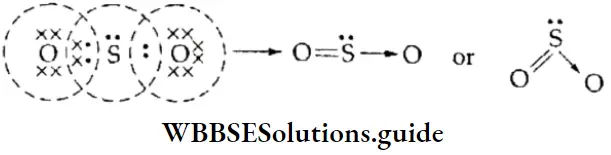
The Oxygen Molecule The oxygen atom (Z = 8) has six electrons in its valence shell (2,6), so it needs two more electrons to complete its outermost cell. In the formation of the 02 molecule, the two oxygen atoms, therefore, contribute two electrons each and share two pairs of electrons. Thus, in the oxygen molecule, the two oxygen atoms are held by a double bond.
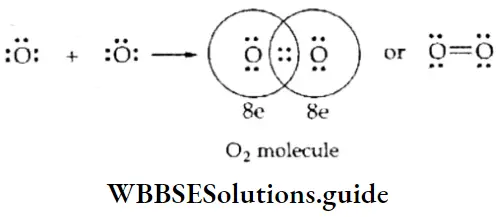
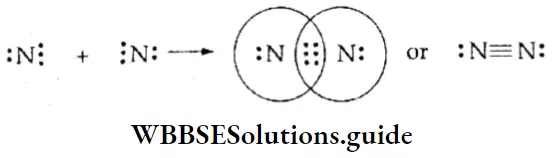
The Nitrogen Molecule The nitrogen atom (Z = 7) has five electrons in its valence shell (2,5) and needs three more electrons to complete its outermost shell. In the nitrogen molecule, therefore, each nitrogen atom contributes three electrons and the two atoms share six electrons, or three electron pairs. Thus, the nitrogen atoms in the N2 molecule are held by a triple covalent bond.
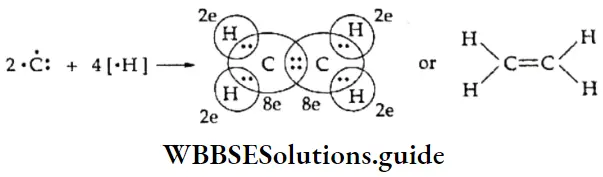
The Ethene Molecule An ethene molecule is formed by bonding between two carbon atoms and four hydrogen atoms. Each of the two carbon atoms combines with two hydrogen atoms forming two single covalent bonds (by sharing two of its electrons). The remaining two electrons of each carbon atom form a double bond between e two carbon atoms.
The valency of an element in a covalent compound is known as covalency or the number of electrons that it contributes to form a covalent bond in that compound. For example, the valency of Cl in Cl2 is 1, of O in O2 is 2. Similarly, the covalency of C in CCl4 is 4 and that of N in N2 is 3.
“WBCHSE Class 11, chemistry notes, on covalent bond, formation, and characteristics”
WBCHSE Class 11 Chemistry Notes On Covalent Bond Definition And Formation
Coordinate Covalent Bond: Certain atoms that have a complete octet and contain a lone pair of electrons can donate this pair to another atom that is short of electrons. When two atoms participate in this kind of sharing of electrons they are said to be bound by a coordinate bond. The coordinate bond can be looked upon as a special type of covalent bond.
- The difference is that in the formation of a coordinate bond, the pair of electrons is contributed by only one of the bonded atoms. The atom that donates an electron pair is called the donor, while the atom that only shares the electron pair is called the acceptor. The bond is represented by an arrow (→) pointing from the donor to the acceptor.
- From the point of view of the orbital theory, a coordinate bond involves the overlapping of an orbital (of an atom) containing a lone pair of electrons with a vacant orbital of another atom.

Formation of O3 A molecule of oxygen contains two oxygen atoms that share two pairs of electrons to complete their octets. In the formation of ozone, one of these oxygen atoms donates a pair of electrons to a third oxygen atom, which contains only six electrons.
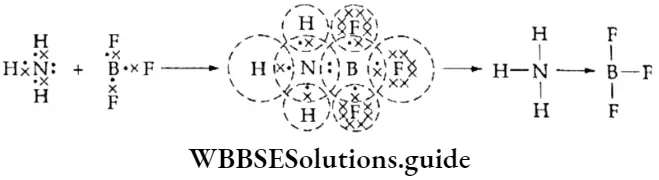
A combination that contains only NH3 and BF3 In ammonia, nitrogen has five valence electrons. Three of these are shared with three hydrogen atoms to form three covalent bonds. Ammonia still has a lone pair of electrons which can be donated to any electron-deficient atom or molecule like BF3.
Formation of SO2 Sulphur and oxygen both have six electrons in their valence shell. Each needs two more electrons to complete its octet, so they share two electrons each, thus forming a double bond with each other.
- Now the sulfur atom still has two unshared pairs of electrons. It donates one of these pairs to another oxygen atom, which is short of two electrons.
- Thus, in the sulfur dioxide molecule, there is a covalent bond between one of the oxygen atoms and the sulfur atom and a coordinate covalent bond between the other oxygen atom and the sulfur atom.
- We have used Lewis dot structures to represent bonding in molecules in this chapter. These structures help to understand bonding in molecules in terms of the number of shared pairs of electrons. Thus, we get an idea about the formation of a molecule, so that a few of its properties can be predicted.
- Remember the following basic steps while writing the Lewis dot structures of a molecule or ionic species for which the molecular formula, along with the charge (in the case of ionic species) has been given.
- Write the valence shell configurations of the combining atoms. Add their valence electrons to obtain the total number of electrons required for representing the structure.
- For cations, subtract one electron for each positive charge from the total number of valence electrons. For anions add one electron for each negative charge to the total number of valence electrons.
- Write the skeletal structure of the molecule. Generally, the least electronegative atom occupies the central position in the molecule. The hydrogen atom usually occupies a terminal position in a molecule.
- Distribute the total number of electrons as shared pairs (for single bonds) between the atoms. The remaining electron pairs are either involved in multiple bonds or constitute lone pairs.
- Ensure that each bonded atom gets an octet of electrons. Now, let us write the Lewis dot structures for a few molecules.
“Covalent bond, definition, how it forms, properties, and examples, WBCHSE Chemistry”
The Nitric Acid Molecule
1. To find the total number of valence electrons available for bonding in a nitric add (HNO3) molecule, write the valence shell configurations of the combining atoms.
⇒ \(\mathrm{H}\left(1 \mathrm{~s}^1\right), \mathrm{N}\left(2 \mathrm{~s}^2 2 \mathrm{p}^3\right), \mathrm{O}\left(2 \mathrm{~s}^2 2 \mathrm{p}^4\right)\)
The total number of valence electrons is [1 + 5 + 3 x (6)] = 24.
2. The skeletal structure of HNO3 is
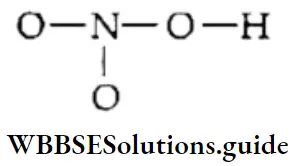
You can write the above skeletal structure by considering the atomic structure and elemental properties of the combining atoms, which we have already studied in previous classes. Although hydrogen is the least electronegative atom, nitrogen is placed in the center of the molecule. The valency of nitrogen is 3, whereas that of hydrogen is 1. For the same reason, N is bonded with three oxygen atoms, and the hydrogen atom is bonded to one of the oxygen atoms.
3. Draw a single bond between each pair of bonding atoms and complete the octets on each oxygen atom.

4. This does not complete the octet on the nitrogen atom. Therefore, we resort to multiple bonding between one of the oxygen atoms and the nitrogen atom so that each bonded atom gets an octet of electrons.

The carbonate ion \(\left(\mathrm{CO}_3^{2-}\right))\)
1. To find the total number of valence electrons available for bonding, write the valence shell configurations of the combining atoms.
∴ \(C\left(2 s^2 2 p^2\right) O\left(2 s^2 2 p^4\right)\)
The total number of valence electrons is [4 + 3 x (6)] = 22. The total number of electrons available is 24 since the carbonate anion carries two negative charges.
“WBCHSE Class 11 Chemistry, covalent bonding, types, and molecular structure”
2. The skeletal structure of carbonate is
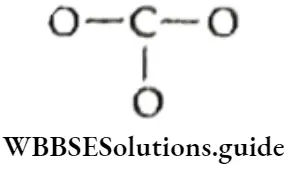
Here carbon is the least electronegative atom and therefore centrally located in the ion.
3. Draw a single bond between each pair of bonding atoms and complete the octet on each of the oxygen atoms.
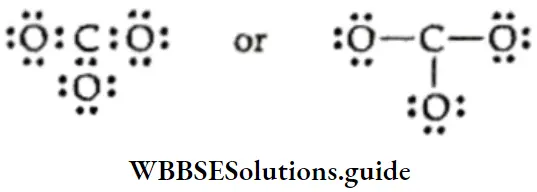
4. In the structure, the octet of carbon is incomplete. Therefore, we resort to multiple bonding between carbon and one of the oxygen atoms.
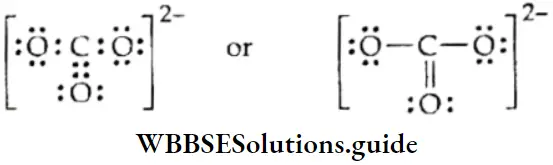
The octet of each bonded atom is now complete in the structure of the carbonate ion.
- In the carbonate anion, the net negative charge is possessed by the ion as a whole and not by any individual atom (here carbon or oxygen). It is however feasible that each atom carries a charge and the charges on each atom of the polyatomic ion sum up to give the net charge.
- Thus each atom constituting a polyatomic ion or a molecule has a formal charge. The total of formal charges in the case of a polyatomic ion should be equal to the net charge on the ion. The total of the formal charges of each atom in a neutral molecule should be equal to zero. The formal charge on an atom is the number of electrons of that atom involved in bonding. While working out the formal charge on an atom,
- Any nonbonding electrons associated with an atom are considered to belong to that atom, and
- The electrons in a bond are assigned half and half to the two atoms in the bond.
- There is a difference between the number of valence electrons of an atom in the isolated state and the number of electrons assigned to that atom when it combines with other atoms in a molecule. This number is counted “by assuming that the atom in a molecule owns one electron of each shared pair and both the electrons of the lone pair.
- This number varies for the same atom in different molecules. Therefore, the formal charge on an atom will not always be the same, though the number of valence electrons in the free atom is always the same. Also, different atoms of the same element in a molecule may have different formal charges.
Types Of Covalent Bonds And Their Properties WBCHSE Class 11
Consider the example of the ozone molecule. Its Lewis dot representation is
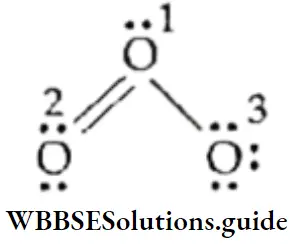
Let us calculate the formal charge on each oxygen atom in the molecule. All the oxygen atoms have six valence electrons. The central oxygen atom marked 1 has one lone pair and three bond pairs.
∴ formal charge on oxygen atom marked 1 = 6 – 2- 1/2 x 6 = +1
The oxygen atom marked 2 has two lone pairs and two bond pairs,
∴ formal charge on oxygen atom marked 2 = 6 – 4- 1/2 x 4 = 0.
The oxygen atom marked 3 has three lone pairs and one bond pair.
∴ formal charge on oxygen atom marked 3 = 6 – 6 -1/2 x 2 = -1
Since O3 is a neutral molecule, the sum of the formal charges is zero.
In general, this simple formula can be used to calculate the formal charge (FC) on an atom in a molecule:
FC = (total number of valence electrons in the free atom) – (total number of electrons in the lone pairs) – 1/2 (total number of shared electrons)
The formal charge is calculated for the species involved in covalent bonding. If there are several possible Lewis structures for a molecule, then the values of the formal charges on its atoms help to determine the lowest energy structure. Generally, the structure with the smallest formal charges on the atoms is the one with the lowest energy.
“Types of covalent bonds, single, double, triple bonds, WBCHSE Class 11 notes”
Properties of covalent compounds, or compounds formed by covalent bonding, have the following common characteristics.
State Covalent compounds, unlike ionic compounds, exist as individual molecules in which the atoms are held together by the sharing of electrons. The intermolecular force of attraction in such compounds is generally weak. So most of these compounds exist in the liquid or gaseous state at room temperature.
Melting and boiling points Covalent compounds generally have low melting and boiling points because the inter-molecular force of attraction in such compounds is weak and not much energy is required to overcome this force.
Conductivity They are generally poor conductors of electricity because they do not contain free electrons or ions to conduct electricity.
“WBCHSE Class 11, chemistry notes, on covalent bond, polarity, and bonding strength”
Solubility They are usually insoluble in water because of the lack of interaction between the polar molecules of water and the nonpolar molecules of such compounds. But they dissolve in nonpolar solvents like benzene.
Molecular reactions Covalent compounds do not produce ions when dissolved. So, when such compounds react with other reagents, the reaction does not involve the combination of ions. It involves the cleavage of the covalent bond in the reacting species and the formation of new covalent bonds in the product molecules. Such reactions are naturally much slower than ionic reactions.
