Principles And Processes Of Isolation Of Elements
That matter is made up of different elements is something you have known for a long time. Clements are present in the earth’s crust and also in water bodies (seas and oceans). Elements naturally occur in the form of compounds. However, some of the less reactive elements like gold, platinum, carbon, sulphur and noble gases are found in the free state.
The naturally occurring compounds of elements present in the earth’s crust are called minerals. Minerals may be obtained by mining. A metal may be present in many minerals, but it may be profitably extracted from only a few of them. The mineral from which a metal can be extracted on a commercial basis Is called an ore. Ores are generally associated with rocks and sand.
The process of obtaining a pure metal from an ore is referred to as metallurgy.
Occurrence and Abundance of Metals
As already stated, metals are generally present in the earth’s crust in the combined state. Being exposed to air and moisture, many metals occur as oxides. The abundance of oxygen and its high electronegativity also contribute to the formation of natural oxides.
Many metals occur as sulphides and carbonates too. However, halides, sulphates, phosphates and silicates are also not so uncommon. Different metals occur in different chemical combinations because of the differences in their chemical properties. For example, as you can see in Table 6.1, calcium occurs as a carbonate whereas beryllium occurs as a silicate. Despite their chemical similarity (both Be and Ca belong to Group 2), the metals occur in different chemical combinations in nature. The carbonate of beryllium is unstable whereas that of calcium is stable. Differences in the ionic sizes and different enthalpies of the formation of compounds also lead to an enormous variety of minerals in nature.
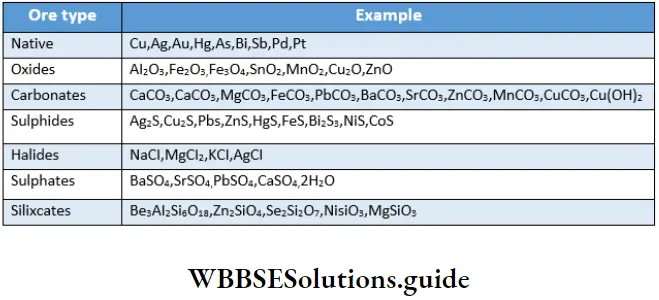
As you can a metal may occur in more than one form but for the purpose of extracting it commercially, only a suitable ore is chosen. Generally, silicate minerals are not chosen because it is difficult to extract metals from them.
The abundance of elements in the earth’s crust or any other region like the oceans and the atmosphere also varies to a great extent. For example, lithium is the thirty-fifth most abundant element in the earth’s crust. The elements belonging to the same group (Group 1)—sodium and potassium—are the seventh- and eighth most abundant elements respectively.
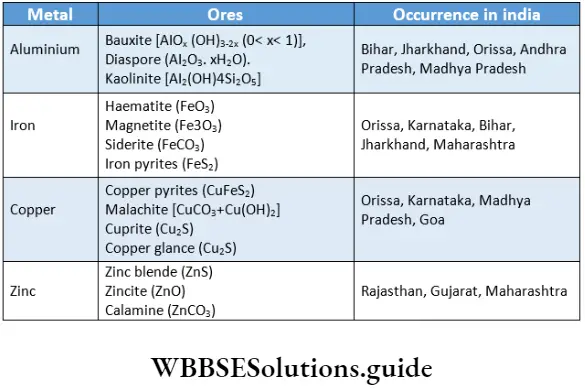
Aluminium is the most abundant metal in the earth’s crust, being present in igneous rocks, clay, and mica and as a constituent of some gemstones. However, it is extracted from a hydrated oxide called bauxite. In other words, bauxite is an ore of aluminium. Likewise, iron is found as oxides, sulphides and carbonate but the most important ores are the oxides. Whenever it is possible to extract metals from them, oxides are preferred to sulphides because the latter have to be roasted and during that process, sulphur dioxide, a polluting gas, is produced.
Extraction of Metals
Metals are generally extracted from their ores by reduction. However, prior to this, the ore is subjected to various operations. Broadly, the three steps involved in metallurgy are as follows.
- Concentration of the ore
- Extraction of the crude metal from the concentrated ore
- Refining of the metal
Different methods and techniques are employed for each stage. The choice of the technique depends on the nature of the ore, the chemical reactivity of the metal to be extracted, the type of impurity present in the ore and the available commercially viable processes.
The big lumps of the ore are crushed into smaller pieces. The crushed ore is then ground into fine powder and this process is called pulverisation. The pulverised ore is then concentrated.
Concentration Of Ores Notes
The ores obtained from the earth’s crust contain impurities (gangue). The removal of these impurities from the ore is called concentration, ore dressing or beneficiation.
Some of the important methods of concentration of ores are discussed.
Hydraulic washing or gravity separation
Hydraulic Separation Method
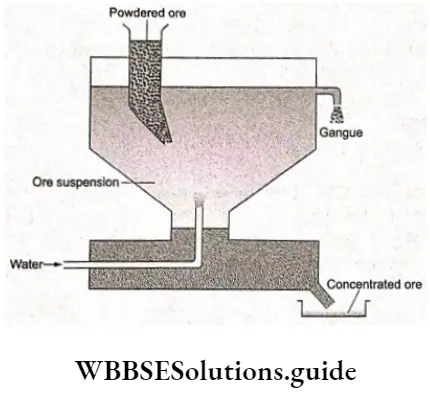
The method is based on the difference between the specific gravities of the ore and the gangue particles. The powdered ore is washed with an upward stream of running water. The lighter gangue particles are washed away, leaving the heavier ore particles behind. The oxide ores of iron and tin and native ores of gold and silver are concentrated by this method.
Concentration Of Ores In Chemistry
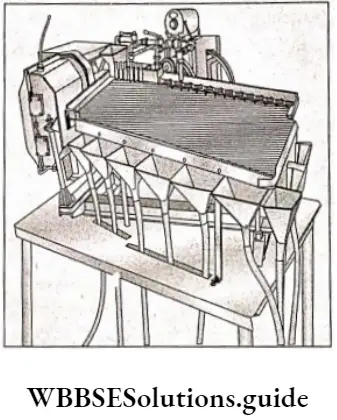
Hydraulic washing of the ore is also done using a Wilfley table. The table is rectangular, sloping at 25°, with ribs on its top. The powdered ore is introduced on the table surface by vibrating hoppers. The water is made to flow over the table surface for washing the ore; the lighter gangue particles are flushed off the table soon whereas the heavier ore particles are retained by the grooves in the table.
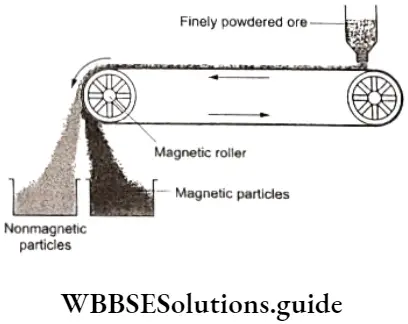
Magnetic separation
This method has limited applicability and is employed when either the ore or the gangue is magnetic in nature. The powdered ore is placed on a conveyor belt which passes over two rollers, one of which has an electromagnet in it. As the ore passes over the belt, the magnetic particles get attracted by the magnetic roller and fall below the roller, while the nonmagnetic particles fall separately, away from the roller.
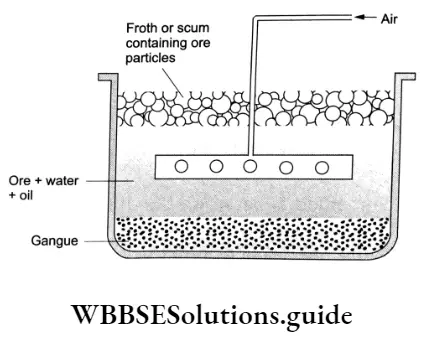
Froth flotation
This process is widely used for the concentration of sulphide ores, for example, those of zinc, copper and lead. The principle behind this technique is that the ore particles are preferentially wetted by oil and that of the gangue by water. The finely powdered ore is suspended in water and certain chemicals called collectors (for example pine oil, xanthates, fatty acids) and froth stabilisers (for example cresols, and aniline) are added. The collectors prevent wetting of the mineral particles by water and froth stabilisers stabilise the froth.
A vigorous stream of air Is blown through the ore-wafer suspension. The ore particles are rendered hydrophobic by collectors so that the air bubbles adhere only to particles of tire ore and carry them to the surface while gangue particles are left behind. This results In the formation of a froth on the surface carrying ore particles. The froth is skimmed off the surface. It is then dried to recover the ore particles.
Sometimes the ore may contain sulphides of two metals. These may be separated from each other by adjusting the relative proportions of oil and water. In some cases, depressants are used, which selectively prevent one type of sulphide from coming with the froth.
A mixture of ZnS and PbS may be separated by using NaCN as the depressant, NaCN forms a computer. zinc and prevents ZnS from being carried with the froth.
⇒ \(4 \mathrm{NaCN}+\mathrm{ZnS} \rightarrow \mathrm{Na}_2\left[\mathrm{Zn}(\mathrm{CN})_4\right]+\mathrm{Na}_2 \mathrm{~S}\)
Leaching (Hydrometallurgy)
This method is employed when the ore is soluble In a suitable solvent One of the most importantappSesfier of leachings is in Bayer’s process, where pure aluminium oxide (Al203) Is obtained from bauxite—the principal ore of aluminium. The main impurities present in the ore are silica and oxides of iron and titanium. The ore is first treated with concentrated sodium hydroxide at 473-523 K at high pressure. Al203 and St02 dissolve as sodmrn. aluminate and sodium silicate respectively, leaving the impurities behind.
⇒ \(\mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})+2 \mathrm{NaOH}(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{Na}\left[\mathrm{Al}(\mathrm{OH})_4\right](a q)\)
The solution is then filtered and cooled and its pH is lowered by passing carbon dioxide through it, and hydrated aluminium oxide gets precipitated. This process is hastened by seeding the reaction mixture with fresh precipitated hydrated aluminium oxide.
⇒ \(2 \mathrm{Na}\left[\mathrm{Al}(\mathrm{OH})_4\right (\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g}) \rightarrow \mathrm{Al}_2 \mathrm{O}_3 \cdot x \mathrm{H}_2 \mathrm{O}(\mathrm{s})+2 \mathrm{NaHCO}_3(\mathrm{aq})\)
Sodium silicate remains in solution. The hydrated oxide is filtered, dried and then heated to give pure alumina.
⇒ \(\mathrm{Al}_2 \mathrm{O}_3 \cdot x \mathrm{H}_2 \mathrm{O} \stackrel{1473 \mathrm{~K}}{\longrightarrow} \mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})+x \mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
Another important application of leaching is in the concentration of silver and gold ores by using sodium or potassium cyanide. The finely powdered ore is treated with a dilute solution of NaCN or KCN in the presence of air. The metal forms a soluble complex leaving the impurities behind.
⇒ \(4 \mathrm{M}(\mathrm{s})+8 \mathrm{CN}^{-}(\mathrm{aq})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{aq})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 4\left[\mathrm{M}(\mathrm{CN})_2 \mathrm{~J}^{-}(\mathrm{aq})+4 \mathrm{OH}^{-}\right.\)
The metal may be recovered by treating the solution with powdered zinc, which replaces the metal in the complex.
⇒ \(2\left[\mathrm{M}(\mathrm{CN})_2\right]^{-}(\mathrm{aq})+\mathrm{Zn}(\mathrm{s}) \rightarrow\left[\mathrm{Zn}(\mathrm{CN})_4\right]^{2-}(\mathrm{aq})+2 \mathrm{M}(\mathrm{s})\)
Extraction Of The Crude Metal From The Concentrated Ore
If the metal in the concentrated ore is present in the oxidised state (i.e., in the form of a compound), the die oxide has to be reduced in order to obtain the metal in the free state. As you know, not all compounds can be reduced with equal ease. Generally, oxides can be most readily reduced. So the extraction of metals is usually done from their oxides. Metals found in water-free and oxygen-poor conditions occur as their sulphides and not oxides. In the extraction of such metals, the process of reduction is preceded by conversion of the ore into respective oxide.
Conversion of ore Into metal oxide
Melnls present in ores as hydrated oxides, carbonates and sulphides are converted into their oxides by either calcination or roasting. The choice of the process employed depends upon the chemical composition of the ore.
Calcination This process is used when the ore is in the form of a carbonate or a hydrated oxide. The concentrated ore is heated in a reverberatory furnace below its melting point, either in the absence of air or in a limited supply of air. During the process, the following changes occur.
- Moisture and volatile impurities present in the ore are removed.
- Impurities like sulphur, phosphorus and arsenic are removed as their volatile oxides.
- Hydrated oxides and hydroxides undergo dehydration to give the oxides.
\(\mathrm{Fe}_2 \mathrm{O}_3 \cdot x \mathrm{H}_2 \mathrm{O} \stackrel{\Delta}{\longrightarrow} \mathrm{Fe}_2 \mathrm{O}_3+x \mathrm{H}_2 \mathrm{O}\)
Limonite - Carbonate ores are converted to oxides by the loss of carbon dioxide.
\(\mathrm{CaCO}_3 \cdot \mathrm{MgCO}_3 \stackrel{\Delta}{\longrightarrow} \mathrm{CaO}+\mathrm{MgO}+\mathrm{CO}_2\)
Dolomite
\(\mathrm{CaCO}_3 \stackrel{\Delta}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2\)
Limestone
\(\mathrm{ZnCO}_3 \stackrel{\Delta}{\longrightarrow} \mathrm{ZnO}+\mathrm{CO}_2\)
Calamine
\(\mathrm{CuCO}_3 \cdot \mathrm{Cu}(\mathrm{OH})_2 \stackrel{\Delta}{\longrightarrow} 2 \mathrm{CuO}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
Malachite - The ore becomes porous.
Roasting This method is employed mostly for sulphide ores. The ore is heated strongly, in an excess of air, at a temperature insufficient to melt it. This is usually done in a reverberatory furnace. In the process of roasting the sulphide ores are converted to oxides. Some examples are as follows.
\(2 \mathrm{PbS}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{PbO}+2 \mathrm{SO}_2\)
Galena
\(2 \mathrm{ZnS}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{ZnO}+2 \mathrm{SO}_2\)
Zinc blende
\(2 \mathrm{Cu}_2 \mathrm{~S}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{Cu}_2 \mathrm{O}+2 \mathrm{SO}_2\)
Copper glance
The sulphur dioxide liberated in the processes is used to manufacture sulphuric added by the contact process.
Reduction of the oxide to the metal
The roasted or the calcined ore contains the metal in the form of its oxide, i.e., the metal is present in the positive oxidation state. To obtain the metal in its free state, the metal oxide has to be reduced. Generally, carbon is used as the reducing agent due to its availability and low cost. However, the process of reduction by carbon requires high temperatures, which is expensive. Another disadvantage is the formation of carbides with metals.
Carbon combines with the oxygen of the metal oxide to give the free metal and carbon monoxide. For example,
⇒ \(\mathrm{ZnO}+\mathrm{C} \stackrel{\Delta}{\longrightarrow} \mathrm{Zn}+\mathrm{CO}\)
The carbon monoxide produced may also bring about the reduction of any unreacted oxide.
⇒ \(\mathrm{ZnO}+\mathrm{CO} \stackrel{\Delta}{\longrightarrow} \mathrm{Zn}+\mathrm{CO}_2\)
In all cases, heating is required. Sometimes, the temperature needed for reduction by carbon is too high to make the process economically viable. Then the reduction is done by another highly electropositive metal. Aluminium i one such metal. It releases a large amount of energy on oxidation to A1203. In the process, the metal to be extracted gets reduced to its free state. The release of a large amount of energy when aluminium reacts with iron(III) oxide is the basis of the thermit process, which you have studied in your earlier class. The temperature attained due to this highly exothermic reaction is enough to melt iron, and it is therefore used in localised welding.
⇒ \(2 \mathrm{Al}+\mathrm{Fe}_2 \mathrm{O}_3 \rightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Fe}\)
TW thermal tivaHwnl of mineral* and ores to bring about physical and chemical transformations enabling extraction of the metal is studied in pyrometallurgy—a branch of metallurgy.
In Older to make the choice of reducing agent to reduce a metal oxide and to know the appropriate temperature m\k\l for reduction, it is helpful to use some basic concepts of thermodynamics, particularly the variation in Gibbs fits’ energy of the reactions.
Thermodynamics in Metallurgy
In the process of extracting a metal from its ore, the main difficulty is generally faced in the reduction of the metal oxide. The Gibbs free energy changes occurring during these processes are of importance in metallurgy. You have already studied that the change in AG (Gibbs free energy) for any reaction at a specified temperature (in Kelvin) is given by the equation:
⇒ \(\Delta G^{\Theta}=\Delta H^{\Theta}-T \Delta S^{\Theta},\)
where AH and AS refer to the enthalpy and entropy changes respectively for that reaction and T is the specified temperature. You already know that the change in Gibbs free energy for a reaction can also be expressed as
⇒ \(\Delta G^\theta=-R T \ln K\)
where K is the equilibrium constant for that reaction. For a reaction to be spontaneous, i.e., for it to proceed in the desired direction, AGe should be negative. A negative value of AGÿ is possible only if AHe is negative and ASÿ is positive. As the temperature increases, the value of TAS° also increases, so that TASe > A and therefore AGe becomes more negative.
Consider a reaction involving the formation of an oxide from a metal and oxygen.
⇒ \(2 x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{M}_x \mathrm{O}(\mathrm{s} \text { or } \mathrm{l})\)
[Both (s) and (1) are used in the reaction as both the metal and the oxide may melt at a very high temperature.]
Oxygen is used up in the reaction in its gaseous form. You know that gases have greater randomness than solids and liquids, and therefore a high entropy. So in this reaction, the entropy decreases (as the products are not gases) and therefore ASe is negative. As the temperature increases TAS’0’ becomes more negative and since TASe is subtracted from AH0, AGe becomes less negative.
Thus the magnitude of AGe decreases with an increase in temperature.
Ellingham diagram
Ellingham diagrams show the values of standard Gibbs free energy changes for chemical reactions in graphical form with AGe as the ordinate and temperature as the abscissa. The most common diagrams are for the metal oxide systems, i.e., showing the reaction of a metal with oxygen. However, diagrams are also known for metal sulphides and halides. These diagrams help us to predict the conditions under which a metal ore will be reduced to the metal.
The free energy changes that occur when 1 g mol of a common reactant (oxygen) is used may be plotted as a function of temperature (with AGe decreasing upwards) for a number of reactions of metals to their oxides as shown in. The graph shown is called an Ellingham diagram for oxides. (Similar Ellingham diagrams can be drawn for sulphides and halides as well.) The Ellingham diagram for oxides has the following important features.
- The graphs for metal-to-metal oxide formation slope upwards as AG° becomes less negative, i.e., free energy change decreases with an increase in temperature.
- Each plot is a straight line and at the kinks in the lines, the slope changes, indicating a change in the phase of the metal, i.e., it either melts or vaporises, and the entropy of the reaction changes accordingly.
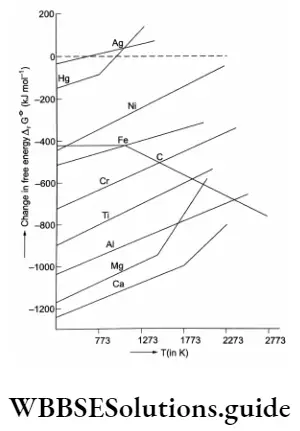
The following are the chemical reactions involved in the formation of the respective oxides
- 2Ag+O2→Ag2O
- 2Hg+O2→2HgO
- 2Ni+O2→2NiO
- 2Fe+O2→2FeO
- C+O2→CO2
- 2C+ O2 -> 2CO
- \(\frac{4}{3} \mathrm{Cr}+\mathrm{O}_2 \rightarrow \frac{2}{3} \mathrm{Cr}_2 \mathrm{O}_3\)
- Ti+O2→TiO2
- \(\frac{4}{3} \mathrm{Al}+\mathrm{O}_2 \rightarrow \frac{2}{3} \mathrm{Al}_2 \mathrm{O}_3\)
- 2Mg+O2→2MgO
- 2Ca+O2→2CaO
Entropy Increases as solid changes to liquid and liquid changes to gas, As you can see in the Slope Of the I lg-1 JgO line changes at the boiling point of mercury (l,e„ 630 K) and that of the Mg-MgO line changes at 1303 K.
3. As the temperature Is raised, a stage will come when the graph for a metal oxide crosses the \(\Delta G^\theta\) = 0 line. If it’s temperature, the free energy of formation of the oxide is negative, i.e., the oxide is stable. Above this temperature, the free energy of formation of the oxide is positive, i,e., the oxide is unstable and it decomposes, Theoretically, all graphs will cross the ΔG = 0 line at some temperature but generally these temperatures are too high and thus thermal decomposition of oxides is not an economically and technologically feasible process for obtaining the metals, In practice, AgO and HgO decompose at attainable temperatures,
4. In a number of extraction processes, one meal Is used to reduce the oxide of another metal, which is to be extracted, If the Oils process is feasible the metal which is used as a reducing agent should form more stable oxide than Ine oxide which Is being reduced, A vertical line drawn on the Ellingham diagram of metal oxides, al any temperature, gives the order of the stability of the metal oxides, which decreases on moving upwards, Any meal can reduce the oxides of those metals which lie above it in the Ellingham diagram of metal oxides, This Is because \(\Delta G^\theta\) becomes more negative by an amount equal to the difference between the two graphs al a certain temperature.
Thus, Al can reduce oxides of Fe, Cr and Ni but It cannot reduce MgO below 1773 K (point of Intersection of 2Mg+O2 →2MgO and \( \frac{4}{3} \mathrm{Al}+\mathrm{O}_2 \rightarrow \frac{2}{3} \mathrm{Al}_2 \mathrm{O}_3\) lines), At tills temperature, AG(t becomes zero and equilibrium results, and an increase In temperature will make the reaction proceed provided no kinetic barriers exist
Carbon As A Reducing Agent
Carbon is an Inexpensive and easily available reducing agent if combined with oxygen to give two oxides, CO (on partial oxidation) and C02 (on complete oxidation).
⇒ \(\mathrm{C}(\mathrm{g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g})\)
⇒ \(\mathrm{C}+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\)
In the case of partial oxidation of carbon, 2 volumes of CO are produced for every volume of oxygen consumed; thus ASe for the reaction is positive. Hence, \(\Delta G^\theta\) becomes increasingly negative as temperature increases. We know that \(\Delta G^{\Theta}=\Delta H^{\Theta}-T \Delta S^{\Theta}\), which is the basis for the appearance of the Ellinghum diagram.
We also know that the enthalpy and entropy of a reaction are, to a reasonable approximation, independent of temperature; therefore the slope of a line in the Ellingham diagram should be equal to the ASe of that reaction.
If a graph is plotted between \(\Delta G^\theta\) and T for the formation of carbon monoxide, we will get a line sloping downwards.
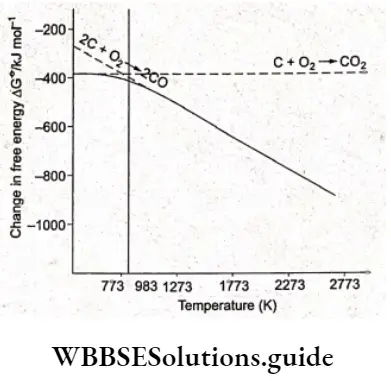
In the case of complete oxidation of carbon, the volumes of oxygen consumed and COZ produced are almost the same, so there is hardly any change in \(\Delta G^\theta\). Therefore, the graph between AGe and T0 is almost horizontal. As we can see the two lines (one for the formation of CO2 and the other for the formation of CO) intersect at 983 K.
Below this temperature, the formation of CO2 is favoured while above this temperature, the formation of CO is favoured. (The more negative the value of \(\Delta G^\\) theta, the more is the reaction favoured.) When the Ellingham diagram for the formation of oxides of carbon is superimposed on the Ellingham diagram for the formation of metal oxides, the line for the formation of carbon monoxide intersects all the metal-metal oxide lines due to its slope in the opposite direction. Hence, carbon can reduce any metal oxide provided the temperature is sufficiently high.
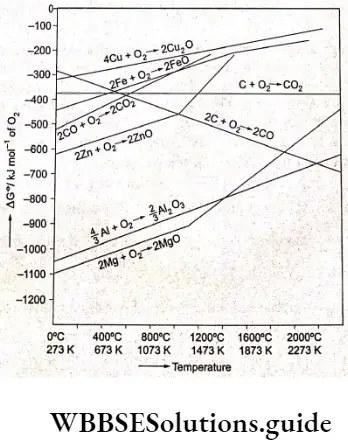
The use of carbon for reduction purposes is not feasible for metal oxides seen towards the bottom of the Ellingham diagram. The reduction of these oxides requires very high temperatures, making the reduction process uneconomical and technologically difficult. One more limitation encountered in carrying out such reduction processes is that the metals may form carbides.
We know that a reaction between two or more elements can be expressed in the form of a chemical equation. The reduction of a metal oxide by carbon or carbon monoxide can be expressed as a combination of various reactions whose AyG values are available in the literature. These reactions can be coupled to determine ArGe. If ArGe for the reaction is negative then the reaction is feasible.
During reduction, the metal oxide decomposes as
⇒ \(\mathrm{M}_x \mathrm{O}(\mathrm{s}) \rightarrow x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g})\)
The reducing agent combines with the oxygen liberated in (a).
⇒ \(\mathrm{C}(\mathrm{s})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g})\)
⇒ \(\mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\)
The values of CO and C02 are available in the literature. The decomposition of MxO is the reverse of its formation.
⇒ \(x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{M}_x \mathrm{O}(\mathrm{s})\)
The AyGe of (d) is known. Thus, ArGe of (a) is the reverse of AyGe of MxO. If Equation (a) is added to Equations (b) and (c) separately then the composite equations respectively are as follows.
⇒ \(\mathrm{M}_x \mathrm{O}(\mathrm{s})+\mathrm{C}(\mathrm{s}) \rightarrow x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\mathrm{CO}(\mathrm{g})\)
⇒ \(\mathrm{M}_x \mathrm{O}(\mathrm{s})+\frac{1}{2} \mathrm{C}(\mathrm{s}) \rightarrow x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\frac{1}{2} \mathrm{CO}_2(\mathrm{~g})\)
These chemical equations represent the chemical reactions occurring between MxO and carbon and ArG” be determined.
While using carbon as a reducing agent, the CO produced during reduction may get oxidised to C02 according to the following equation.
⇒ \(\mathrm{CO}(\mathrm{g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})\)
Thus, CO(g) may also act as a reducing agent as here.
⇒ \(\mathrm{M}_x \mathrm{O}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \rightarrow x \mathrm{M}(\mathrm{s} \text { or } \mathrm{l})+\mathrm{CO}_2(\mathrm{~g})\)
It has been shown that below 983 K, CO is more readily oxidised to COz than carbon and is a better-reducing agent. However, above 983 K, carbon is a better-reducing agent than CO as the latter becomes more stable and less susceptible to oxidation to C02.
Example The values ofAfG* ofMgO and CO respectively at 127 K and 2273 K are as follows.
⇒ \(\frac{1}{2} \mathrm{Mg}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{MgO}(\mathrm{s}) ; \quad \Delta_f G^{\ominus}=-941 \mathrm{~kJ} \mathrm{~mol}^{-1}(1273 \mathrm{~K})=-314 \mathrm{~kJ} \mathrm{~mol}^{-1}(2273 \mathrm{~K})\)
⇒ \(\mathrm{C}(\mathrm{s})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g}) ; \quad \Delta_f G^{\ominus}=-439 \mathrm{~kJ} \mathrm{~mol}^{-1}(1273 \mathrm{~K})=-628 \mathrm{~kJ} \mathrm{~mol}^{-1}(2273 \mathrm{~K})\)
Indicate whether carbon can be used as a reducing agent at 1273 K and 2273 K.
Solution
The overall reaction for the reduction of magnesium oxide is
⇒ \(\mathrm{MgO}(\mathrm{s})+\mathrm{C}(\mathrm{s}) \rightarrow \mathrm{Mg}(\mathrm{s})+\mathrm{CO}(\mathrm{g}) \quad \Delta_r G^\theta=\Delta_f G_{(\mathrm{C} \rightarrow \mathrm{Co})}^{\ominus}-\Delta_f G_{\left(\mathrm{M}_{\mathrm{g}} \rightarrow \mathrm{Mg}_g \mathrm{O}\right)}^{\ominus}\)
⇒ \(\text { At } 1273 \mathrm{~K}, \Delta_r G^{\ominus}=-439-(-941)=+502 \mathrm{~kJ} \mathrm{~mol}^{-1} \text {. }\)
Since \(\Delta_r G^{\Theta}\) is positive, the reaction is not possible,
⇒ \(\text { At } 2273 \mathrm{~K}, \Delta, G^{\Theta}=-628-(-314)=-314 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Since \(\Delta_r G^{\Theta}\) is negative, the reaction can proceed.
Example The reaction is endothermic at low temperatures but becomes exothermic above the melting point of magnesium. Example giving a reason.
⇒ \(\mathrm{MgO}(\mathrm{s})+\mathrm{C}(\mathrm{s}) \rightarrow \mathrm{Mg}(\mathrm{s})+\mathrm{CO}(\mathrm{g})\)
Solution
The entropy of the liquid state is higher than the entropy of the Solid State. Thus above the melting point of magnesium, the entropy of the products is high, i.e., AS is more positive and thus the reaction becomes exothermic, i.e., ΔG becomes negative.
Under what conditions can magnesium be used for the reduction of chromium oxide?
Solution
The equations involved in the process are
⇒ \(\frac{4}{3} \mathrm{Cr}+\mathrm{O}_2 \rightarrow \frac{2}{3} \mathrm{Cr}_2 \mathrm{O}_3\)
⇒ \(2 \mathrm{Mg}+\mathrm{O}_2 \rightarrow 2 \mathrm{MgO}\)
The overall reaction is
⇒ \(\frac{2}{3} \mathrm{Cr}_2 \mathrm{O}_3+2 \mathrm{Mg} \rightarrow 2 \mathrm{MgO}+\frac{4}{3} \mathrm{Cr}\)
According to the Ellingham diagram (Figure 6.5), the reaction will be possible at any temperature above the point of intersection of the Cr203 and MgO curves.
Example The thermite reaction is exothermic, but does not proceed at room temperature and needs initiation by heating. However, once the reaction has started, further heating is not needed. What do you think might be the reason for this?
⇒ \(2 \mathrm{Al}+\mathrm{Cr}_2 \mathrm{O}_3 \rightarrow 2 \mathrm{Cr}+\mathrm{Al}_2 \mathrm{O}_3\)
Solution
The reaction has a very high activation energy as both reactants are in the solid state. Thus initiation is needed. As the reaction is exothermic, the reaction proceeds on its own, once the activation energy is attained.
Reduction Of Sulphides
Although carbon reduces oxides quite effectively, it is not so effective when used directly on sulphides of metals. The reason why carbon reduces oxides at high temperatures is that the AG vs. T plot for CO has a negative slope due to the positive AS value, i.e., the formation of CO becomes more feasible at high temperatures. In the case of sulphides, the reaction should be
⇒ \(\mathrm{MS}(\mathrm{s})+\frac{1}{2} \mathrm{C}(\mathrm{s}) \rightarrow \mathrm{M}(\mathrm{s})+\frac{1}{2} \mathrm{CS}_2(\mathrm{~g})\)
There is no compound formed by the reaction between carbon and sulphur which is analogous to CO (i.e., CS) with a negative slope. The Age vs. T plot for CS2 is almost horizontal.
⇒ \(\frac{1}{2} \mathrm{C}(\mathrm{s})+\mathrm{S}(\mathrm{g}) \rightarrow \frac{1}{2} \mathrm{CS}_2(\mathrm{~g})\)
That is, the plot cannot intersect the plots of metal sulphides. In other words, carbon does not have much affinity for sulphur and thus sulphides are first roasted to oxides and then reduced.
Limitations of the Ellingham diagram The study of the \(\Delta G^{\Theta}\) vs. T plots merely suggests whether or not a reaction is possible. It can only indicate whether or not thermodynamically a reducing agent can be used. It totally ignores the kinetic aspect of a reaction, i.e., the rate of a reaction.
A reaction is thermodynamically feasible if ArG° I; is negative; however, sometimes the rate of the reaction may be so low that despite the reaction being thermodynamically possible, it does not occur. The study of Fllingtwm diagrams does not include the pÿlMtity of any other competing reaction. For example, carbon cannot be used as a reducing agent for some metals which form carbides.
It is also implicitly assumed that the reactants and products are In equilibrium, which Is not necessarily true. Sometimes, if the reactants and products are all solids, the reaction is stow and becomes possible if there is a change in phase, i.e., a compound melts.
This is because the randomness increases in tire liquid state resulting in an increase in ASe. For instance, the reduction of ZnO and MgO by carbon becomes more spontaneous after the melting point of Zn or Mg as this results in greater A$i%.
Some of the common extraction processes are discussed as follows.
Extraction Of Iron From Its Oxides
Iron is extracted from its oxides in a blast furnace. The furnace is a tall refractory-lined cylindrical structure that is charged at the top. The refractory material generally used is MgO. (Refractory materials are those that can withstand high temperatures without melting or softening).
The calcined ore mixed with coke (a reducing agent) and limestone (slag forming) is referred to as charge, which is fed from the top of the blast furnace and a blast of hot air is introduced from the bottom. Coke bums to produce heat and CO.
⇒ \(2 \mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}(\mathrm{g})\)
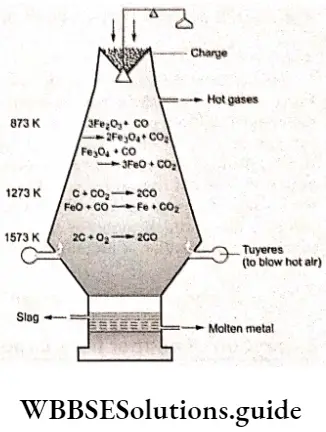
Since the reaction is exothermic, the temperature at the lower part of the furnace is very high. As the gases move up, they meet the descending charge and the temperature drops gradually, on moving towards the top of the furnace, resulting in a temperature gradient in the furnace. The iron oxide is reduced to iron mainly by CO and some reduction takes place by coke.
Knowing the thermodynamics of the process helps us to understand how the reduction occurs and why the blast furnace is chosen for extracting iron from its oxide.
The following is the main reduction step in the process.
⇒ \(\mathrm{FeO}(\mathrm{s})+\mathrm{C}(\mathrm{s}) \rightarrow \mathrm{Fe}(\mathrm{s} / \mathrm{l})+\mathrm{CO}(\mathrm{g})\)
This is the overall reaction obtained by coupling two reactions. One is the reduction of metal oxide to metal and the other is the oxidation of carbon to CO.
⇒ \(\mathrm{FeO}(\mathrm{s}) \rightarrow \mathrm{Fe}(\mathrm{s})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) ; \Delta G_1\)
⇒ \(\mathrm{C}(\mathrm{s})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g}) ; \quad \Delta \mathrm{G}_2\)
Δ, G for the overall reaction is
⇒ \(\Delta_r G=\Delta G_2+\Delta G_1\)
The reaction will proceed when ArG is negative. If we consider the Ellingham diagram showing the change in AG for the formation of oxides then we find that the AG plot for Fe ->• FeO is sloping upwards while that for Q QQ is sloping downwards. The two lines intersect at about 1073 K. Above this temperature the C -> CO line falls below the Fe -» FeO line and a reduction of FeO occurs.
As discussed earlier in this section, there is a temperature gradient in the furnace. We have also studied earlier that below 983 K, carbon monoxide is a better-reducing agent than carbon. The other oxides of iron (Fe203 and Fe30< ) get reduced at relatively lower temperatures by CO, Thus the reactions occurring in the upper parts of the blast furnace/ which are at lower temperatures than that at the bottom, are as follows.
3Fe2O3+CO→2Fe3O4+CO2
Fe3O4+CO→3FeO+CO2
Fe2O3+CO→2FeO+CO2
At a higher temperature in the furnace, coke is the reducing agent
CO2+C →2CO
FeO+CO→Fe+CO2
Adding these reactions, we get
Limestone is added to the charge to form slag; thus, it acts as a flux It decomposes to form CaO. This combines with infusible impurities like silica present in the concentrated ore to form an easily fusible material called slag.
⇒ \(\mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3\)
Flux Cangue Slag
Slag melts at the temperature that prevails in the furnace. It is insoluble in the molten metal and being lighter floats on the surface of the metal therefore it can be skimmed off, and molten iron gets collected at the bottom of the furnace. Slag protects the molten iron from being oxidised.
The iron thus obtained is called pig iron. It contains impurities like carbon (4%) and smaller amounts of sulphur, phosphorus, silicon and manganese. Another form of iron is called cast iron. It has a lower carbon content and is very hard and brittle. The purest form of iron is wrought iron.
It is malleable in nature and is prepared by heating cast iron in the presence of air in a reverberatory furnace lined with haematite. Limestone is added as flux. In the presence of air, impurities like sulphur, silicon and phosphorus are oxidised to the respective oxides. These combine with CaO (obtained by the thermal decomposition of limestone) to form slag. Haematite oxidises carbon to carbon monoxide.
Fe2O3+3C→2Fe+3CO
Thus all impurities from cast iron are removed
Extraction Of Copper From Copper(I) Oxide
Copper(I) oxide is not very stable and can be readily reduced by carbon. (In the Ellingham diagram the Cu→ Cu2 Oline lies above the C→C02and C -> CO lines.)
Generally copper is present as the sulphide along with iron sulphide in the ore. The sulphide ore is first roasted to give the oxide.
2Cu2S+3O2→2Cu2O+2SO2
Iron, if present as a sulphide, is also converted to its oxide.
2FeS+3O2→2FeO+2SO2
The roasted ore is then heated in a reverberatory furnace, using silica as flux. FeO combines with silica to form iron silicate slag, which floats on the surface and therefore can be easily skimmed off leaving behind copper matte, which consists of Cu2O and unreacted Cu2Sand FeS.
⇒ \(\mathrm{FeO}+\mathrm{SiO}_2 \rightarrow \underset{\text { Slag }}{\mathrm{FeSiO}_3}\)
The molten matte is fed into a silica-lined converter through which hot compressed air is blown, which causes partial oxidation. The remaining FeS is converted to FeO, which forms a slag with silica.
2FeS+3O2→2FeO+2SO2
⇒ \(\mathrm{FeO}+\mathrm{SiO}_2 \rightarrow \underset{\text { Slag }}{\mathrm{FeSiO}_3}\)
The supply of hot compressed air is turned off after some time and self-reduction of the oxide and sulphide occurs.
2Cu2O+Cu2S→6Cu+SO2
The molten copper obtained is 99% pure and is called blister copper. The term blister comes from the (fact that as the melt solidifies, the dissolved sulphur dioxide, oxygen and nitrogen escape giving rise to blisters on the surface of the metal.
Extraction Of Zinc From Zinc Oxide
The main ore of zinc is zinc blende (ZnS), which is first concentrated and then roasted to form the oxide. The zinc oxide is made into briquettes with coke and clay and heated In vertical retorts at 1673 K. (The temperature range for the reduction of ZnO is higher than that in the two cases discussed above.) The coke reduces the oxide to zinc.
ZnO+C→Zn+CO
The zinc obtained distils off because its boiling point is lower than the temperature of the retort. The gaseous zinc is prone to reoxidation; therefore it is collected by sudden cooling.
Not all elements can be obtained from their ores by the reduction of their oxides by carbon. Some of them which are active metals are reduced by electrolysis whereas those which are not so active can be reduced by adding some reducing element.
Electrolytic Reduction
Active metals like alkali metals, alkaline earth and aluminium cannot be obtained by the reduction of their oxides by carbon. This is because their oxides are very stable and can be only reduced at extremely high temperatures. However, at such high temperatures, the metals may form carbides.
Example Of Magnetic Separation
Such active metals are obtained by the electrolysis of their fused salts. The relation between Gibbs energy and electrode potential is as follows.
⇒ \(\Delta G^{\Theta}=-n F E^{\Theta}\)
where n is the number of electrons involved in the redox process, Ee is the electrode potential of the redox couple and F is the Faraday constant. Highly reactive metals have large negative Ee values and it is difficult to carry out their reduction chemically.
In electrolysis, the fused salt dissociates to form M”+ ions, which migrate to the cathode and get deposited and discharged (lose their charge by gaining electrons) there. Only such materials are used as electrodes which do not react with the metal.
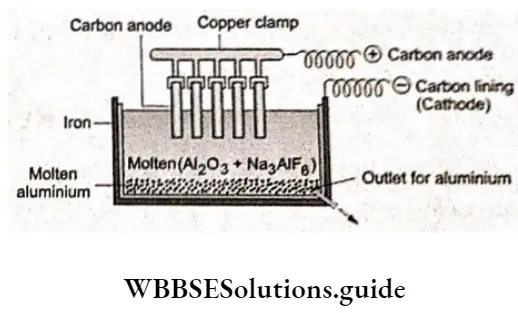
An important example is the electrolysis of A1203 to give aluminium. Purified A1203 is mixed with cryolite (Na3AlF6) or calcium fluoride, which lowers the melting point of the mixture and makes it more conducting. The fused mixture is electrolysed in a cell containing a graphite-lined steel cathode and graphite anode. Aluminium is liberated at the cathode and oxygen at the anode.
At cathode AI2O3→2AI3++3O2-
AI3++3e–→AI (I)
The oxygen combines with the carbon anodes to give CO2 and CO.
At anode C(s)+O2-→CO2(g)+2e–
C(s)+2O2-→CO2(g)+4e–
Thus, the carbon anodes are progressively burnt away.
Metal Displacement
You have studied in your previous class that when a more reactive metal like iron is brought in contact with the solution of a less reactive metal like copper the former goes into the solution and the latter precipitates out.
Cu2+(aq)+Fe(s) →Fe2+(aq)+Cu(s)
This principle may be applied in metallurgy. Low-grade copper ores may be leached using or subjected to the action of certain bacteria. To this solution, scrap iron is added, which displaces copper. (Leaching with bacteria is known as bioleaching.)
Extraction Based on Oxidation
So far you have studied extraction based on reduction. Nonmetals are generally extracted by oxidation. A common example is the extraction of chlorine from seawater. The following reactions show the oxidation of chlorine.
2CI–(aq)+2H2O(I) →2OH–(aq)+H2(g)+CI2(g)
The \(\Delta G^{\ominus}\) for this reaction is +422\(E^\theta=-2.2 \mathrm{~V}\) and £e‘= -2.2 V, You know that for a reaction to become spontaneous£e (the electrode potential) should be positive. To make the net potential positive (in case of negative potential values) an external emf greater than the standard potential value of the die reaction has to be applied. Thus, in tins case the minimum potential needed is +2.2 V. If molten NaCl is electrolysed then sodium metal is produced at the cathode.
The extraction of gold and silver by leaching the native ores with sodium cyanide also involves oxidation in the first step.
4Au(s)+8CN–(aq)+2H2O(aq)+O2(g)→4[Au(CN)2]–(aq)+4OH–
This is because the oxidation state of gold in the elemental state is zero whereas in the cyanide complex is +1 (i.e., the electron is lost). In the next step, gold is recovered from the cyanide complex by displacement by zinc.
2[Au(CN)2]–(aq)+Zn(s)→2Au(s)+[Zn(CN)4]2-(aq)
In this step, zinc acts as a reducing agent as it reduces gold from Au (I) to Au.
Refining Techniques
A metal obtained by extraction is not pure. It is contaminated with various impurities, a few examples of which are as follows.
- Other metals also present in the ore are obtained by simultaneous reduction of their oxides.
- Metalloids like silicon and nonmetals like phosphorus are obtained by the reduction of their oxides.
- Unreacted oxides and sulphides of the metals.
- Substances used in the furnace like flux and slag.
To obtain the pure metal, it is important to subject the crude metal to an appropriate refining technique so that the impurities are removed. The choice of the refining technique depends on the nature of the metal, the nature of impurities present and the purpose for which the pure metal is needed.
Some common methods of refining are as follows.
Distillation
This method is used when the metal being purified has a low boiling point, like mercury, cadmium and zinc. The impure metal is heated in an iron retort and the vapours are chilled to obtain the pure metal. The less volatile impurities are left behind.
Liquation
This technique is used when the melting point of the metal is lower than that of the impurities. Theerode metal is heated on a sloping surface. It melts and flows down the Scope, from where it is collected. Tin and lead are purified by this method.
Electrolytic Refining
This method is used in the refining of metals like copper,anc. aluminium, gold. silver and nickel. A block of the pure metal is made of the anode, while a thin strip of fee pure metal is made of fee cathode. The electrodes are suspended in an electrolyte.
An electrolyte is a Squid that conducts electodtj’ due to the presence of ions. Electrolytes are molten ionic compounds or solutions of ionic salts or compounds that ionise in solution. In electrolytic refining, a fee solution of an ionic salt of fee metal to be extracted is used as a fee electrolyte.
On passing current, fee electrolytes dissociate?, fee metal Ions deposit on the cathode as fee pure metaL while an equivalent amount of fee metal dissolves from fee anode and passes into solution as fee metal ion. The reactions involved in the fee process are as follows.
Anode: M→Mn++ne–
Cathode: Mn++ne–→M
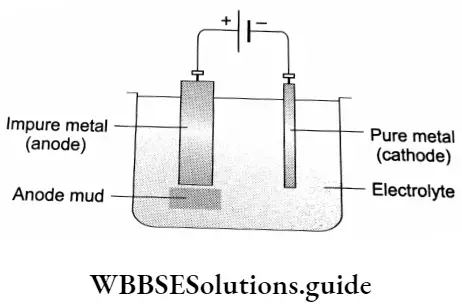
The impurities present may either be more electropositive than the metal being refined, or less electropositive. An optimum voltage is applied such feat fee more electropositive metal impurities remain in solution, while fee less electropositive ores remain un-ionised and deposit under fee anode as anode mud.
In the refining of copper, the electrolyte is copper sulphate solution, the fee anode is a block of blister copper, while the fee cathode is a thin strip of pure copper. Impurities like selenium, tellurium, silver, gold, platinum and antimony form fee anode mud.
Zone Refining
This technique is employed to get metals of very high purity. Germanium, silicon, gallium, indium and boron, which are used in semiconductors, are purified by this method. This method is based on the fact that the solubility impurities are different in the solid and liquid states of a material.
Generally, the solubility is more in the liquid state than in the solid state. Thus, when molten metal is allowed to cool, the impurities remain in the melt, while the pure metal crystallises out.
The impure metal is taken in the form of a rod and a mobile circular heater is fitted at one end of the rod. The portion of the metal rod in contact with the heater melts. The heater is slowly moved forward and the molten zone moves along with the heater.
As the heater moves forward, pure metal crystallises out of the melt and impurities pass on to the adjacent molten zone. The process is repeated several times; the heater is moved in fee same direction. The impurities get concentrated on one end of the rod and this end is cut off to obtain fee pure metal.
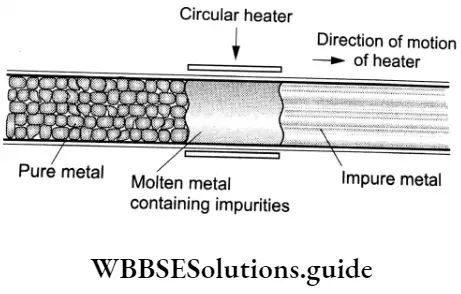
Vapour Phase Refining
This method is used to purify certain metals. The basic requirement of the process is that the metal to be purified should form a volatile compound on being heated with a reagent; furthermore, the volatile compound should undergo thermal decomposition at a high temperature to give the pure metal. There are two important processes based on this technique —the Mond process and the Van Arkel method.
Mond process
This method is used to obtain highly pure nickel. Nickel oxide is heated with water gas to 323 K. The hydrogen reduces nickel oxide, leaving behind impure nickel.
NiO(s)+H2(g) →Ni(s)+H2O(g)
The impure nickel is heated in a current of carbon monoxide at 330-350 K and forms a volatile carbonyl complex, tetracarbonyl nickel. This complex undergoes thermal decomposition at 450-470 K to give pure nickel.
⇒ \(\underset{\text { Impure }}{\mathrm{Ni}}+4 \mathrm{CO} \rightarrow \mathrm{Ni}(\mathrm{CO})_4\)
Van Arkel method
This method is used to prepare highly pure samples of titanium and zirconium. These metals are difficult to extract because they react readily with air, oxygen, nitrogen and hydrogen at elevated temperatures. The respective oxides cannot be reduced by C or CO as the oxides form carbides. The crude metal is heated in an evacuated vessel in the presence of iodine to form the volatile tetraiodide—Til4 or Zrl4.
The iodide is then brought in contact with an electrically heated tungsten filament when it decomposes to give the pure metal which gets deposited on the filament. The iodine liberated in the process is reused.
⇒ \(\underset{\text { Impure }}{\mathrm{Ti}(\mathrm{s})}+2 \mathrm{I}_2(\mathrm{~g}) \stackrel{523 \mathrm{~K}}{\longrightarrow} \mathrm{TiI}_4(\mathrm{~g}) \stackrel{1800 \mathrm{~K}}{\longrightarrow} \underset{\text { Pure }}{\mathrm{Ti}(\mathrm{s})}+2 \mathrm{I}_2(\mathrm{~g})\)
⇒ \(\mathrm{Zr}(\mathrm{s})+2 \mathrm{I}_2(\mathrm{~g}) \stackrel{870 \mathrm{~K}}{\longrightarrow} \mathrm{ZrI}_4(\mathrm{~g}) \stackrel{2073 \mathrm{~K}}{\longrightarrow} \mathrm{Zr}(\mathrm{s})+2 \mathrm{I}_2(\mathrm{~g})\)
An essential requirement of the process is that the metal should not be volatile at the temperature at which the decomposition of the iodide takes place.
Chromatographic Methods
As you know from your previous class, chromatography is a useful process to separate the constituents of a mixture. It is based on the principle that when a mixture is moved through an adsorbent, different components of the mixture are adsorbed to different extents.
Chromatography involves two phases—a stationary phase and a mobile phase. Many different chromatographic processes are available depending on the choice of the two phases. A few examples are paper chromatography, column chromatography and gas chromatography.
In column chromatography, an adsorbent like silica or A1203 is packed in a glass column similar to a burette. This is the stationary phase. A mixture of compounds (in soluble form) is introduced from the top of the column. As the mixture trickles down the column, the different components are adsorbed in different areas of the column and are seen as coloured bands.
The mixture to be purified is dissolved in a suitable solvent and poured through the top of the column. As the liquid percolates through the adsorbent, the different components are adsorbed at different regions of the column.
The strongly adsorbed component is adsorbed readily and is present towards the top of the column and the weakly adsorbed component towards the bottom. The adsorbed components are then removed by using a suitable reagent.
This process is called elution and the reagent used is called the eluate. The weakly adsorbed component is eluted first followed by the strongly absorbed ones. This method is suitable for elements which are available in very small amounts and whose impurities are chemically similar to the element being purified.
Zirconium and hafnium can be separated from each other by passing a solution of their chlorides (NrCI4 and HfCI4) in anhydrous methanol through a silica column followed by elution with 1.9 M HCI whereby NrCI4 is eluted. HfCI4 is later eluted by using 3.5 M H2SO4.
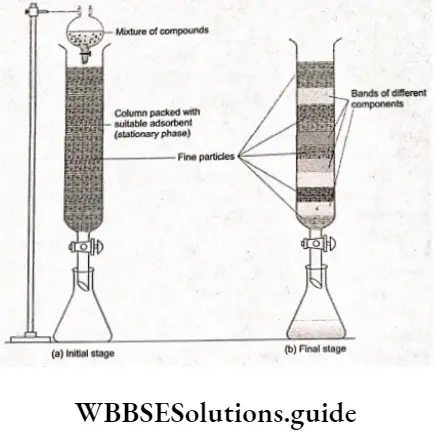
Uses of Aluminium, Copper, Iron and Zinc
Aluminium is a very useful metal due to its properties like high electrical conductivity, lightness, resistance to corrosion, and the fact that it can be recycled. Aluminium wires are used as electrical conductors. Aluminium powder is used for making paints and lacquer.
Aluminium foil is used for wrapping photographic films, medicines, and food items like sweets and chocolates. Aluminium metal is used to extract manganese and chromium from its oxides.
Being corrosion-resistant, the metal is used to make utensils. Alloys of aluminium are light and strong and find a variety of uses. The greatest use of aluminium alloys is in construction.
Copper is a very good conductor of heat and electricity and is highly malleable. It is used to make electrical wires and cables. Alloys of copper like brass (with zinc) and bronze (with tin) are very useful.
Copper compounds like basic copper hydroxide (also known as Bordeaux mixture) are used to prevent potato blight (a fungal disease).
Iron is a widely used metal and many industries are based on iron. The uses of iron are manifold. Cast iron is an important engineering material with a wide range of applications. For example, it is used in making pipes, tanks, railway coaches, machines and car parts.
It is also used to manufacture wrought iron and steel. Examples of items produced from wrought iron include nuts, bolts, rivets, horseshoes, boiler tubes, agricultural implements and furniture. There are various types of steel like nickel steel, chrome steel and stainless steel.
Steel is used in making a variety of things, from the thinnest surgical needle to immense ships. Modern structures like skyscrapers and bridges are based on a steel framework. Nickel steel is used in ship and aircraft building, for automobiles and as support in reinforced concrete and railroad tracks.
Chrome steel is used in making cutting tools while stainless steel is used in making utensils, surgical equipment, and cycle and automobile parts.
Zinc is an important constituent of alloys like brass, solder, bronze and German silver. A large proportion of zinc is used to galvanize metals like iron to prevent corrosion. Zinc metal is used in dry batteries. Zinc oxide is used as a paint pigment and in pharmaceutical products. Zinc sulphide is used for making luminous dials.
Principles And Processes Of Isolation Of Elements Multiple-Choice Questions
Question 1. The impurities present in an ore are called
- Slag
- Flux
- Mineral
- Gangue
Answer: 1. Gangue
Question 2. Which of these metals does not occur in the native state?
- Gold
- Platinum
- Lead
- Silver
Answer: 2. Platinum
Question 3. Which of these is an ore of copper?
- Malachite
- Calamine
- Haematite
- Bauxite
Answer: 1. Malachite
Question 4. Froth floatation is used to concentrate
- Native Ores
- Oxide Ores
- Sulphide Ores
- None Of These
Answer: 3. Sulphide Ores
Question 5. Which of these is a depressant?
- Xanthate
- Pine Oil
- Aniline
- Sodium Cyanide
Answer: 4. Sodium Cyanide
Question 6. Which of these is used as a collector in froth flotation?
- Aniline
- Pine Oil
- Cresol
- None Of These
Answer: 2. Pine Oil
Question 7. Ti02 is separated from bauxite by leaching with
- Acid
- Alkali
- Sodium Cyanide
- Water
Answer: 2. Alkali
Question 8. Which of these ores is roasted?
- ZnCO3
- CaCO3. MgCO3
- ZnS
- AI2O3.xH2O
Answer: 2. CaCO3. MgCO3
Question 9. Which of these is used as a flux in the extraction of iron from its oxide?
- C
- CO
- Fe2O3
- CaO3
Answer: 4. CaO3
Question 10. The purest form of iron is
- Wrought Iron
- Cast Iron
- Pig Iron
- Steel
Answer: 1. Wrought Iron
Question 11. Which metal can be purified by distillation?
- Iron
- Copper
- Lead
- Zinc
Answer: 4. Zinc
Question 12. In the extraction of aluminium, the anode is made of
- Aluminium
- Cryolite
- Carbon
- Iron
Answer: 3. Carbon
Question 13. Copper is refined by
- Distillation
- Liquation
- Zone Refining
- Electrolysis
Answer: 4. Electrolysis
Question 14. Which of these is purified by zone refining?
- Ge
- Pb
- A1
- Fe
Answer: 1. Ge

