Solutions
Solutions Definition:
A solution is a homogeneous mixture of two or more substances. Homogeneity implies uniformity of appearance throughout the mixture. Even though the substances (also called components) forming the solution can be in any state (solid, liquid or gas), the solutions, i.e., two-component solutions.
However, a solution may contain more than two components. Three-component solutions are known as tertiary solutions, four-component solutions as quaternary solutions, and so on.
In a solution, the component which is present in smaller quantities is called the solute and the one present in larger amounts is called the solvent. The state of the solvent determines the physical state in which the solution exists. Different types of binary solutions and their examples are given in the table.
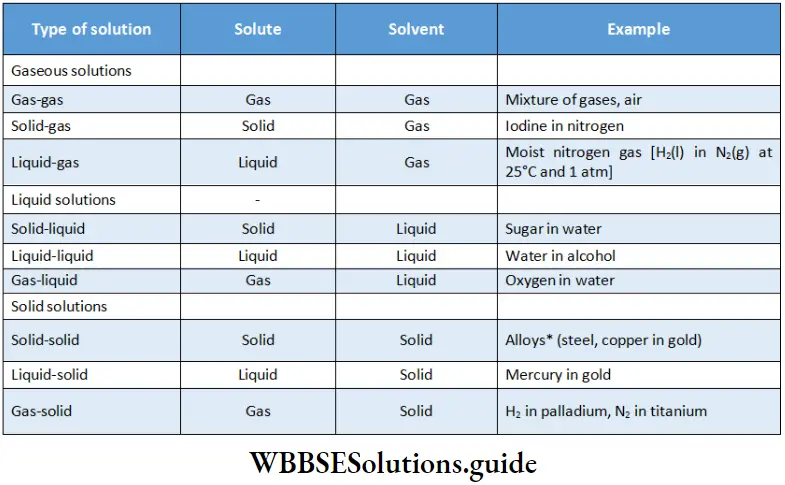
Solid solutions of two more metals are called alloys.
Out of all the categories listed in the table, the most common binary solutions are solid-liquid and liquid-liquid solutions. A solid-liquid solution in which the solvent is water is called an aqueous solution. There is usually some interaction between the solvent and the solute molecules.
Two liquids that can mix at the molecular level are said to be miscible, e.g., water and alcohol. In this case, the molecules interact with each other strongly. In contrast, water and oil molecules are very weak and are, therefore, immiscible.
There is also a third category which is that partially describes liquid; in such cases, the two liquids are miscible at certain ratios and at others, they are immiscible, e.g., a phenol-water system.
Composition Of A Solutions
When we talk about the composition of a solution, we refer to the respective amounts of solute and solvent mixed together to form a certain amount of the solution. The concentration of a solution is the ratio of the solute to either the volume or the mass of the solution or the solvent in which the solute is dissolved.
A homogeneous solution has the same concentration throughout. Concentration can be expressed in several ways in terms of mass percentage, volume percentage, parts per million, and morality. The composition of a solution may also be expressed in mole fractions.
Mass percentage:
The mass percentage of a component of a solution is the mass of that component in grams per 100 grams of the solution. For example, a 10 per cent solution of sodium chloride in water by mass is indicated as 10% (w/w) and implies that 10 g of sodium chloride is dissolved in 90 g of water to make 100 g of solution. Commercially available concentrated aqueous reagents like acids and bases are usually labelled in terms of mass percentage.
Factors affecting solubility of solids in liquids class 12 chemistry
Volume Percentage:
In the case of liquid-liquid solutions, concentration is generally expressed as volume percentage. The volume percentage of a component of a solution is the volume of that component in mL per 100 mL of the solution.
For example, a 10% solution of alcohol in water by volume implies that 10 mL of alcohol has been added to the requisite amount of water so as to obtain 100 mL of the solution. A 35% (v/v) solution of ethylene glycol is marked as permanent antifreeze. It lowers the freezing point of water to a great extent.
Mass by volume percentage:
We may also express the concentration of a solution in mass by volume percentage, i.e., the mass of the solute dissolved in 100 mL of the solution. For example, a 5% (w/v) solution of sodium chloride in water means 5g of solute (NaCl) has been dissolved in the requisite quantity of water to make 100 mL of the solution. Concentrations are often expressed this way in medicine and pharmacy.
Parts per million:
When a solute is present in trace quantities, the concentration is expressed in parts per million, abbreviated as ppm. It is the number of parts of a solute per million parts of the solution. The term parts could mean either mass or volume. concentration in ppm can thus be expressed in mass, volume to volume to volume or mass to volume grams of the solute in 10 6 mL of the solution or micrograms per mL of the solution (m g.mL-1 ).
You know that 10% NaCl solution means 10 g of NaCl in 100 g of the solution. Therefore, in 106 g of the solution, 10 x 10 g of NaCl will be present. Hence the concentration of the solution will be 105 ppm.
Example 1. 5 g of NH4Cl is dissolved in water to obtain 500 mL of a solution. Express the concentration in ppm.
Solution:
Given
5 g of NH4Cl is dissolved in water to obtain 500 mL of a solution.
Since ppm denotes one unit of a substance for 999,999 units of the other substance we must find out the number of grams of NH4Cl dissolved in 10 6 mL of the solution. The concentration is derived in terms of mass by volume.
500 mL of solution contains 5 g of solute.
Therefore, 106 mL of solution will contain \(\frac{5}{500} \times 10^6=10^4\) of solute.
Hence, the concentration of ammonium chloride in the solution is 104 ppm
Example 2. Express the concentration in ppm of a 10% solution of ethanol in water by volume.
Solution:
Ten per cent by volume means 10 mL of ethanol has been mixed with enough water to obtain 100 mL of the solution.
100 mL of solution contains 10 mL ethanol.
∴ 106 mL of solution would contain \(\frac{10}{100} \times 10^6=10^5\) of ethanol.
Hence, the concentration is 105 ppm.
Mole fraction (γ):
The mole fraction of a particular component is the number of moles of that component divided by the total number of moles of all the components present in a solution
⇒ \(x_i=\frac{n_i}{\sum_i n_i}\)
where n, is the number of moles of the ith component and Σn, indicates the summation of the number of moles of all the components. For a binary solution made up of components A and B,
v \(\chi_{\mathrm{A}}=\frac{n_{\mathrm{A}}}{n_{\mathrm{A}}+n_{\mathrm{B}}} \text { and } \chi_{\mathrm{B}}=\frac{n_{\mathrm{B}}}{n_{\mathrm{A}}+n_{\mathrm{B}}}\)
Solubility of a solid in a liquid – definition and explanation
where χ and % are the mole fractions of components A and B respectively, and n and ng are the numbers of moles of the two components.
It may also be noted that in a given solution, the sum of the mole fractions of all the components is equal to one, i.e.,

Example 3. Calculate the mole fractions of the two components in a 10% by-mass solution of common salt in water.
Solution:
A 10% solution of common salt will contain 10 g of NaCl and 90 g of water.
The molar mass of NaCl = 23+35=58 g.
∴ number of moles of NaCl = \(\frac{10}{58}=0.1724\)
Molar mass of H2O=2×1+16 = 18g.
∴ number of moles of H2O = \(\frac{90}{18}=5\)
Total number of moles of NaCl + H2O=5+0.1724-5.1724.
⇒ \(\chi_{\mathrm{NaCC}}=\frac{0.1724}{5.1724}=0.0333\)
⇒ \(\chi_{\mathrm{H}, \mathrm{O}}=1-\chi_{\mathrm{NaCl}}=1-0.0333\)
= 0.9667.
Molarity The molarity (M) of a solution is defined as the number of moles of the solute per litre of the solution.
If n is the number of moles of solute in V litres of solution, then M = n/V. The unit for molarity is mol L-1 or M.
Example 4. The molar concentration of glacial acetic acid is 17 M. Find the number of grams of acetic acid in half a litre of this solution.
Solution:
Given
The molar concentration of glacial acetic acid is 17 M.
17 M of a solution implies that 17 mol of the solute (glacial acetic acid) is present in 1 litre of the solution.
1 litre of the solution contains 17 mol of acetic acid.
∴ \(\frac{1}{2}\) litre of solution will contain \(\frac{17}{2}\) mol of acetic acid.
The molar mass of acetic acid (C2H4O2) = 60 g.
∴ number of grams of acetic acid in half a litre of 17 M glacial acetic acid = \(\frac{17}{2} \times 60\)
= 510.
Molality (m):
The molality of a solution is defined as the number of moles of the solute dissolved in 1 kg of the solvent. To calculate the molality of a solution the individual weights of the solution and the solvent should be known. If ng moles of solute are dissolved in WA kilograms of solvent then mB = ng/WA. The unit of molality is mol kg -1.
Example 5. If 27 g of ammonium chloride is dissolved in 500 g of water, calculate the molality of the solution. One mole of NH4Cl weighs 54 g.
Solution:
Given
If 27 g of ammonium chloride is dissolved in 500 g of water
Number of moles of \(\mathrm{NH}_4 \mathrm{Cl}, n_{\mathrm{NH}_4 \mathrm{Cl}}=\frac{27}{54}=0.5\)
Amount of water, \(w_{\mathrm{H}_2 \mathrm{O}}=500 \mathrm{~g}\)
∴ \(m_{\mathrm{NH}_4 \mathrm{Cl}}=\frac{0.5}{500} \times 1000=1\)
The concentration of the given ammonium chloride solution is 1 m (read as a molal solution).
Nature of Solutions
When sugar is added to water in a beaker, it dissolves to give a solution. If we keep adding sugar to water, a stage will be reached when the sugar will stop dissolving. Such a solution in which no more of the solute can be dissolved is called a saturated solution.
If crystals of sugar are added to a saturated sugar solution, they settle at the bottom of the beaker, showing that dissolution has stopped. Actually what happens is that molecules of sugar from the crystals continue to leave the surface of the crystal and dissolve in water.
At the same time, molecules of sugar from the solution collide with the crystals and occupy positions in the crystal (crystallisation). In a given period of time, the number of molecules that return to the crystals is roughly equal to the number of molecules that dissolve in water.
Important factors influencing solubility in chemistry
Thus, a state of dynamic equilibrium exists between the solid sugar and the solution. The concentration of the solute in the solution remains constant at a particular temperature and pressure and is called its solubility.
Thus the solubility of a solute is the quantity that will dissolve in a given amount of solvent to produce a saturated solution. An unsaturated solution is one in which more of the solute will dissolve.
Factors Affecting The Formation Of A Solution
Generally, the solubility of a solute in a solvent depends on the nature of the solute and the solvent (like dissolves like), particle size of the solute (the smaller the size, the faster is the dissolution), temperature and pressure.
Solubility of a solid in a liquid:
The solubility of solutes with a positive enthalpy of solution (Asol H>0, endothermic process) increases as the temperature is increases. If we prepare a saturated solution and then cool it, some solute usually crystallises out so that the solution remains saturated as the temperature decreases.
However, if we prepare a saturated solution at a higher temperature and remove all the undissolved solutes, we can sometimes cool the solution without crystallisation of the solute. When the cooled solution contains more solute than it would have contained if the dissolved solute were in equilibrium with the undissolved solute, it is said to be supersaturated.
In the case of solutes with a negative enthalpy of solution (AH<0, exothermic process), an increase in temperature reduces solubility. Polar substances dissolve in polar solvents whereas nonpolar substances dissolve in nonpolar solvents. Some solvents in increasing order of their polarity are CCI4, <CHCI3, <C2H5OH < H2O.
Inorganic salts such as NaCl are polar. They are therefore soluble in water, which is also polar. The solubility of an ionic solid in water depends on its lattice energy and ion-dipole interactions. The ion from the solute interacts with the dipole from the water molecule.
When an ionic solid dissolves, the ion-dipole interactions pull out the ions from the solid into the solution. The ion thus separated from its neighbours is surrounded by water molecules, forming a sphere of hydration.
These ions are said to be hydrated (if the solvent is water) or in general, solvated. The ionic solid dissolves when the ion-dipole interactions are stronger than ion-ion interactions in the solid.
The large covalent molecules may also be soluble in water provided they contain sufficiently strong polar bonds. Sucrose (sugar) contains eight polar \(8-\mathrm{O}-\mathrm{H}^{8+}\) groups per molecule and can therefore easily form hydrogen bonds with water. It is thus soluble in water.
However, within a series of organic compounds, solubility in water decreases as the number of carbon atoms in the chain increases. For example, while ethanoic acid is completely miscible with water, in all proportions, hexanoic acid is nearly insoluble in water.
In spite of possessing the highly polar >CO and O-H groups, the latter is insoluble because of large-scale London dispersion forces acting between the hydrocarbon chains of the solute molecules.
As you have already studied in class XI, London forces or dispersion forces are the intermolecular forces caused by temporary dipoles induced by neighbouring atoms or molecules in nonpolar substances. These forces are weaker than ion-ion and ion-dipole forces.
Solubility of a gas in a liquid:
You know that gases dissolve in liquids; for instance, oxygen dissolves in water. Another common example is soda water, which contains carbon dioxide dissolved in water under high pressure. The dissolved gas can be removed from the solution by heating the solution or releasing the pressure.
In such cases (H2, O2, N2 and CH,) the equilibrium can be represented as
⇒ \(\text { gas molecules } \stackrel{\text { water }}{\rightleftharpoons} \text { dissolved molecules }\)
Gases such as NH3, HCl and NO2 chemically react with water. As a result, their solubility in water is more than that of gases which do not react with water. Here the equilibrium at dissolution may be expressed as
Solubility concept with examples and applications
⇒ \(\text { gas molecules } \stackrel{\text { Water }}{\rightleftharpoons} \text { dissolved molecules } \stackrel{\text { water }}{\rightleftharpoons} \text { product }\)
⇒ \(\mathrm{NH}_3(\mathrm{~g}) \stackrel{\text { water }}{\rightleftharpoons} \mathrm{NH}_3(\mathrm{aq})\)
⇒ \(\mathrm{NH}_3(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{NH}_4^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})\)
A gas can dissolve in a solid too, e.g., hydrogen dissolves in palladium.
Variation of solubility of gases with partial pressure At high altitudes, atmospheric pressure and temperature is low. A person climbing a mountain feels weak when he reaches the top because of the low concentration of oxygen in his blood at higher altitudes.
This is because of the lower partial pressure of oxygen at such heights. Thus, the solubility of a gas in a liquid depends not only on its nature but also on the temperature and pressure of the system.
A quantitative relation between the solubility of a gas in a liquid and its partial pressure was established by Henry and is known as Henry’s law. It states that the solubility of a gas in a liquid is proportional to the partial pressure of the gas.
Dalton, a contemporary of Henry, also independently reached the same conclusion. If the mole fraction of the gas is taken as a measure of its solubility then
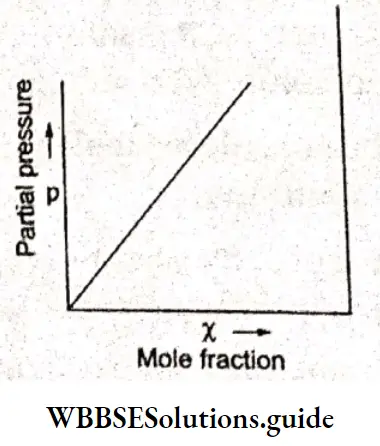
⇒ \(p=K_{\mathrm{H}} \chi\),
where p is the partial pressure of the gas, K is Henry’s law constant for the gas in a particular solvent and X is the mole fraction of the gas. According to this equation, the partial pressure of a gas in the vapour phase is directly proportional to the mole fraction of the gas.
A plot of partial pressure of the gas versus mole fraction is a straight line. The slope of the line is Henry’s law constant K. Different gases have different K values at the same temperature.
It is interesting to note that in order to compensate for the decreased solubility of oxygen for people living at high altitudes, their body adapts to the prevailing environmental conditions by producing more haemoglobin, the iron-containing protein which binds oxygen.
Deep underwater, the solubility of oxygen is very high. Scuba divers experience a change in pressure of about 1 atm for every 33-foot increase in depth; this results in increased pressure in the lungs as compared to that at the surface and the divers should not come to the surface quickly after being underwater for 20 minutes.
Doing so may cause the formation of bubbles of nitrogen in the blood as the decrease in pressure will cause gases dissolved in the blood to rapidly revert to the gaseous state. Nitrogen gas in the blood blocks capillaries and creates a medical condition called “bends”, which is life-threatening.
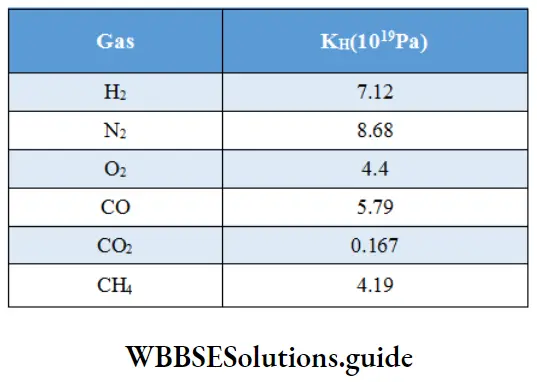
Henry’s law constants for the solubility of gases in water at 298 K:
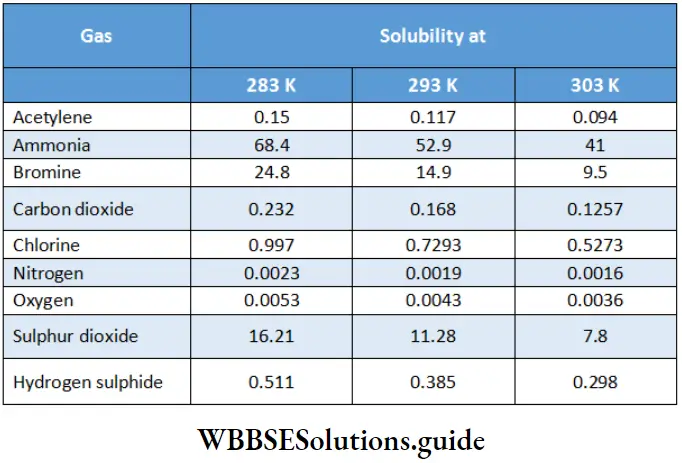
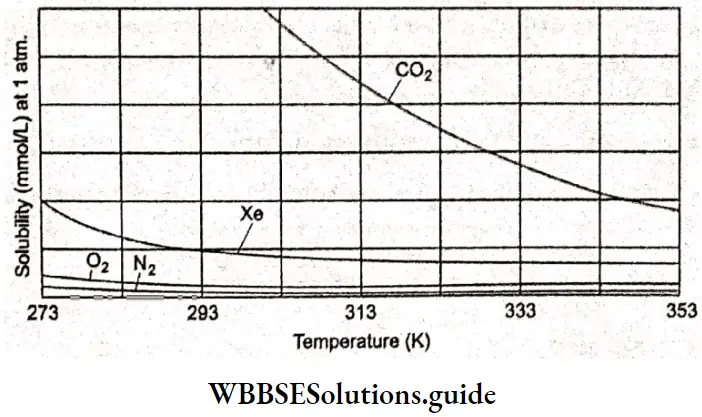
Variation of solubility of gases with temperature The values given in Table show that solubility decreases with an increase in temperature though the extent varies from one gas to another. The solubility decreases since heat is generally evolved during dissolution. There are exceptions, however, especially with solvents like liquid ammonia and many organic liquids. A plot of the solubility of some gases as a function of temperature.
One application of this decreased solubility at higher temperatures is in the case of carbonated drinks; these bubble continuously as they warm up to room temperature after being refrigerated for some time and consequently become “flat”. The heating of lakes and rivers can harm aquatic animals due to the decrease in dissolved oxygen.
Solubilities of some gases at different temperatures (solubilities are expressed as weights of gases in grams dissolved in 100 g of water when the pressure of the gas plus that of water vapour is 1 atm):
Example 1. The volume of blood in a normal human being is 5 L. Henry’s law constant for the solubility of N2 in water is 9.04×10 bar at 298 K. Assume Henry’s law constant for Nf in blood to be the same as that in water. Also assume the density of blood to be the same as that of water, i.e., 1 kg L-1. Calculate the number of moles of nitrogen absorbed in this amount of blood in air containing 80% N2 at sea level, where the pressure is 1 bar, and also at a pressure of 50 bar deep under the sea.
Solution:
Given
The volume of blood in a normal human being is 5 L. Henry’s law constant for the solubility of N2 in water is 9.04×10 bar at 298 K. Assume Henry’s law constant for Nf in blood to be the same as that in water. Also assume the density of blood to be the same as that of water, i.e., 1 kg L-1.
⇒ \(p_{\mathrm{N}_2}=\chi_{\mathrm{N}_2} \cdot K_{\mathrm{H}}\)
⇒ \(\frac{n_{\mathrm{N}_2}}{n_{\mathrm{N}_2}+n_{\mathrm{H}_2 \mathrm{O}}} \cdot K_{\mathrm{H}}\)
The denominator may be approximated as nH2o since N2 is sparingly soluble in water.
Therefore, \(p_{\mathrm{N}_2}=\frac{n_{\mathrm{N}_2}}{n_{\mathrm{H}_2 \mathrm{O}}} \cdot K_{\mathrm{H}}\)
Or \(n_{\mathrm{N}_2}=n_{\mathrm{H}_2 \mathrm{O}} \cdot \frac{p_{\mathrm{N}_2}}{K_{\mathrm{H}}}=\frac{5 \times 10^3 \mathrm{~g} \times 0.8 \mathrm{bar}}{18 \mathrm{~g} \mathrm{~mol}^{-1} \times 9.04 \times 10^4 \mathrm{bar}}\)
Or \(n_{\mathrm{N}_2}=2.5 \times 10^{-3} \mathrm{~mol}\)
At 50 bar, PN2 = 0.8 x 50 = 40 bar.
But \(p_{\mathrm{N}_2}=\frac{n_{\mathrm{N}_2}}{n_{\mathrm{H}_2 \mathrm{O}}} \times K_{\mathrm{H}}=\frac{n_{\mathrm{N}_2}}{5000 / 18} \times 9.04 \times 10^4\)
⇒ \(p_{\mathrm{N}_2}=\frac{n_{\mathrm{N}_2}}{n_{\mathrm{H}_2 \mathrm{O}}} \times K_{\mathrm{H}}=\frac{n_{\mathrm{N}_2}}{5000 / 18} \times 9.04 \times 10^4\)
⇒ \(40=\frac{n_{\mathrm{N}_2}}{500} \times 18 \times 9.04 \times 10^4\)
or, \(n_{\mathrm{N}_2}=\frac{40 \times 5000}{18 \times 9.04 \times 10^4}=0.12 \mathrm{~mol}\)
The solubility at 50 bar is 48 times more than that at 1 bar.
Example 2. Calculate the amount of oxygen dissolved in 5 L of the blood of a mountaineer at a pressure of 0.5 bar at 18000 feet. Assume that K for oxygen in the blood is 4.95 x 10 bar and that the density of blood is 1000 kg m3. Compare the value obtained with the amount of O2 dissolved in blood at 1 bar at sea level. The mole fraction of O2 in air is 0.21.
Solution:
⇒ \(p_{\mathrm{O}_2}=\chi_{\mathrm{O}_2} \cdot K_{\mathrm{H}}\)
Given \(\chi_{\mathrm{O}_2}^{\prime}=0.21 \text { (in air), } K_{\mathrm{H}}=4.95 \times 10^4 \text { bar, } \rho_{\text {blood }}=1000 \mathrm{~kg} \mathrm{~m}^{-3} \text {. }\)
⇒ \(p_{\mathrm{O}_2}=\chi_{\mathrm{O}_2}^{\prime} \cdot p_{\text {total }}=0.21 \times 0.5\)
⇒ \(p_{\mathrm{O}_2}=0.105 \text { bar. }\)
∴ \(p_{\mathrm{O}_2}=\chi_{\mathrm{O}_2} \cdot K_{\mathrm{H}}\)
Or \(0.105=\frac{n_{\mathrm{O}_2}}{n_{\mathrm{O}_2}+n_{\mathrm{H}_2 \mathrm{O}}} \times 4.95 \times 10^4\)
Neglecting no, in comparison to nн2o, we have
⇒ \(0.105=\frac{n_{\mathrm{O}_2}}{n_{\mathrm{H}_2 \mathrm{O}}} \times 4.95 \times 10^4\)
⇒ \(\frac{n_{\mathrm{O}_2}}{5000 / 18} \times 4.95 \times 10^4\) [… 5L = 5kg and p = 1000 kg dm-3=1 kg L-1]
Or \(n_{\mathrm{O}_2}=\frac{0.105 \times 5000}{18 \times 4.95 \times 10^4}=5 \times 10^{-4} \mathrm{~mol}\)
At 0.5 bar, 5 x 10 -4 mol of O2 is dissolved in 5 L of blood.
At sea level,
⇒ \(p_{\mathrm{O}_2}=\chi_{\mathrm{O}_2}^{\prime} \cdot p_{\text {total }}\)
= 0.21 x 1
= 0.21har.
But \(p_{\mathrm{O}_2}=\chi_{\mathrm{O}_2} \cdot K_{\mathrm{H}}=\frac{n_{\mathrm{O}_2}}{n_{\mathrm{H}_2 \mathrm{O}}} \cdot K_{\mathrm{H}}\)
Or \(n_{\mathrm{O}_2}=\frac{p_{\mathrm{O}_2} \cdot n_{\mathrm{H}_2 \mathrm{O}}}{K_{\mathrm{H}}}\)
⇒ \(\frac{0.21 \times 5000}{4.95 \times 10^4 \times 18}\)
Оr \(n_{\mathrm{O}_2}=0.0012=1.2 \times 10^{-3} \mathrm{~mol}\)
At sea level 1.2 × 10-3 mol of oxygen is dissolved in 5 L of blood, which is 2.4 times (1.2x 10-3/5×104) that at 0.5 bar.
Example 3. Henry’s law constant for the solubility of methane (CH) in water is 4.19 x 10° Pa at 25°C. Estimate its molar solubility at 25°C and a partial pressure of 3.4 kPa.
Solution:
Given
Henry’s law constant for the solubility of methane (CH) in water is 4.19 x 10° Pa at 25°C.
According to Henry’s law,
⇒ \(p=K_{\mathrm{H}} \cdot \chi\)
Or \(\chi=\frac{p}{K_{\mathrm{H}}}\)
Given that p = 3.4 kPa = 3.4 x 10³ Pa
and KH = 4.19× 109 Pa.
⇒ \(\chi_{\mathrm{CH}_4}=\frac{3.4 \times 10^3}{4.19 \times 10^9}=8.114 \times 10^{-7}\)
⇒ \(\chi_{\mathrm{CH}_4}\) may be approximated as follows,
⇒ \(\chi_{\mathrm{CH}_4}=\frac{n_{\mathrm{CH}_4}}{n_{\mathrm{CH}_4}+n_{\mathrm{H}_2 \mathrm{O}}} \approx \frac{n_{\mathrm{CH}_4}}{n_{\mathrm{H}_2 \mathrm{O}}}\)
Or \(n_{\mathrm{CH}_4}=\chi_{\mathrm{CH}_4} \cdot n_{\mathrm{H}_2 \mathrm{O}}\)
In 1 L of solution, the volume of water = 1 L.
If the density of water is assumed to be 1 kg/L, then the mass of 1 L of water = 1 kg.
Therefore, \(n_{\mathrm{H}_2 \mathrm{O}}=\frac{1000 \mathrm{~g}}{18 \mathrm{~g} / \mathrm{mol}}=55.6 \mathrm{~mol}\)
⇒ \(n_{\mathrm{CH}_4}=\gamma_{\mathrm{CH}_4} \cdot n_{\mathrm{H}_2 \mathrm{O}}\)
= 8.114 x 10-7 x 55.6
= 4.511 x 10-5 mol.
The concentration of methane in water is 4.51 x 105 mol L-1.
Example 4. Find the concentration of CO2 in a 1-litre can of soda under 2.5 bar of vapour pressure of CO2 at 25°C. Henry’s law constant for the dissolution of CO2 in water is 1.65 x 10³ bar. Assume the density of the solution to be 10³ kg/m³. What will be the concentration when the can is opened and exposed to the atmosphere at 25°C, the partial pressure of CO2 in air being 4×10 atm?
Solution:
According to Henry’s law,
⇒ \(p=K_{\mathrm{H}} \chi\)
Given \(p=2.5 \text { bar }, K_H=1.65 \times 10^3 \text { bar. }\)
Therefore, \(2.5=1.63 \times 10^3 \cdot x_{\mathrm{CO}_2}\)
Or \(x_{\mathrm{CO}_2}=\frac{2.5}{1.63 \times 10^3}=1.515 \times 10^{-3}\)
⇒ \(\χ \mathrm{CO}_2\) may be approximated as follows.
⇒ \(x_{\mathrm{CO}_2}=\frac{n_{\mathrm{CO}_2}}{n_{\mathrm{CO}_2}+n_{\mathrm{H}_2 \mathrm{O}}} \approx \frac{n_{\mathrm{CO}_2}}{n_{\mathrm{H}_2 \mathrm{O}}}\)
Or \(n_{\mathrm{CO}_2}=\chi_{\mathrm{CO}_2} \cdot n_{\mathrm{H}_2 \mathrm{O}}\)
The density of the solution = \(=\frac{10^3 \mathrm{~kg}}{\mathrm{~m}^3}=\frac{10^3 \mathrm{~kg}}{(10)^3 \mathrm{dm}^3}\) (∵ 10 dm = 1m)
⇒ \(1 \mathrm{~kg} / \mathrm{dm}^3 \text { or } 1 \mathrm{~kg} / \mathrm{L}\)
Mass of 1 litre of solution = 1 kg.
Since water is the solvent used, the mass of water in 1 L of solution = 1 kg.
Number of moles of water = \(\frac{1 \mathrm{~kg}}{18 \mathrm{~g} \mathrm{~mol}^{-1}}\)
⇒ \(\frac{1000}{18} \mathrm{~mol}\)
= 55.6 mol.
Substituting from Equations (1) and (3) in Equation (2),
⇒ \(n_{\mathrm{CO}_2}=1.515 \times 10^{-3} \times 55.6\)
= 0.084 mol.
Therefore, the solution contains 0.084 mol of CO2 in 1 L of water, or the concentration is 0.084 mol L-1. When the can is exposed to the atmosphere, the partial pressure of CO2 in the air
= 4 x 10-4 atm (given)
Or \(p_{\mathrm{CO}_2}=4 \times 10^{-4} \times 1.03125 \mathrm{bar}\)
⇒ \(4.05 \times 10^{-4} \text { bar }\)
Using Henry’s law and proceeding as before, we get
⇒ \(n_{\mathrm{CO}_2}=\frac{p_{\mathrm{CO}_2}}{K_{\mathrm{H}}} \times 55.6=\frac{4.05 \times 10^{-3} \times 55.6}{1.65 \times 10^3}\)
⇒ \(1.36 \times 10^{-5} \mathrm{~mol}\)
or concentration = 1.36 x 10-5 mol L-1, which is much less than that when the bottle was closed.
Solid solutions are formed by mixing up solid substances. The solution so formed is also a solid. A mixture of lithium chloride and sodium chloride when melted and cooled forms a solid solution.
It contains an array of chloride ions with a random distribution of lithium and sodium ions. This solution is homogeneous and neither LiCl nor NaCl separates out on standing.
Some ionic substances appear to be nonstoichiometric; their chemical formulae deviate from the ideal ratios. In other respects, they resemble pure compounds. They are in fact solid solutions.
There are two types of solid solutions-substitutional and interstitial. In a substitutional solid solution, atoms, molecules or ions of one substance substitute the species in the lattice of the other substance.
Steel, brass and bronze are examples of substitutional solid solutions. When Al2O3 and Cr2O, are mixed together at high temperatures they form a solid solution. The ions that replace the original ions must have the same charge and must be fairly similar in size.
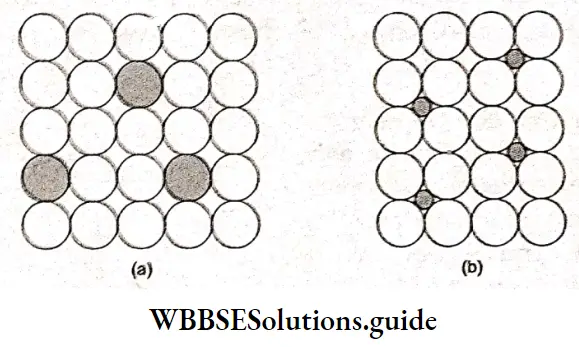
Interstitial solid solutions, as the name suggests, involve the placing of the atom of one substance in the interstices or holes in the crystal of the other substance. Tungsten carbide is an example of such a solid solution. It is a hard substance and has many industrial uses, e.g., it is used to make grinding and cutting tools.
Liquid Solutions
Solutions of a solid, liquid or gas in a liquid are called liquid solutions as the solvent is a liquid. We will consider the case of a liquid in a liquid and a solid in a liquid in the following sections.
Solution of a liquid in a liquid:
If a liquid is placed in a closed container, after some time a dynamic equilibrium between the liquid and its vapour will be established. The pressure exerted by the vapour also reaches a constant value, which, as you already know, is called the vapour pressure of the liquid.
Now let us see what happens in a binary solution (a solution containing two components) made up of two volatile liquids. As both the liquids are volatile, both of them contribute to the vapour pressure. The pressure exerted by the vapour of each component is called the partial vapour pressure of that component.
If the two volatile components are denoted by A and B, the mole fractions and the partial pressures of the two components will be % and %, and PA and PB respectively. The French chemist Francois Raoult performed a series of experiments on structurally related liquids and from the results obtained concluded that the ratio of the partial vapour pressure of each component to its vapour pressure as a pure liquid is approximately equal to the mole fraction of that component in the liquid.
Thus, \(\frac{p_{\mathrm{A}}}{p_{\mathrm{A}}^{\circ}}=\chi_{\mathrm{A}} \text { and } \frac{p_{\mathrm{B}}}{p_{\mathrm{B}}^{\circ}}=\chi_{\mathrm{B}}\)
where p is the partial vapour pressure of a component in the solution and p° is its vapour pressure as a pure liquid. X is the mole fraction of a component.
This relationship can also be expressed as
⇒ \(p_{\mathrm{A}}=p_{\mathrm{A}}^{\circ} \chi_{\mathrm{A}}\).
This is called Raoult’s law.
Thus Raoult’s law states that, in a solution of volatile components, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction (applicable only when a homogeneous solution is obtained).
When pA or pB is plotted against the mole fraction of any one component A or B of a solution, then a straight line is obtained that meets the y-axis at pf and p°.
A line passes through the point pf, which corresponds to the partial vapour pressure of pure component A, and the other passes through y B, the partial vapour pressure of pure component B. The total vapour pressure varies between p ° and p it is given by the line joining p ° and p °. Thus, the total vapour pressure of the solution is given as
⇒ \(p_{\text {total }}=p_{\mathrm{A}}+p_{\mathrm{B}}\)
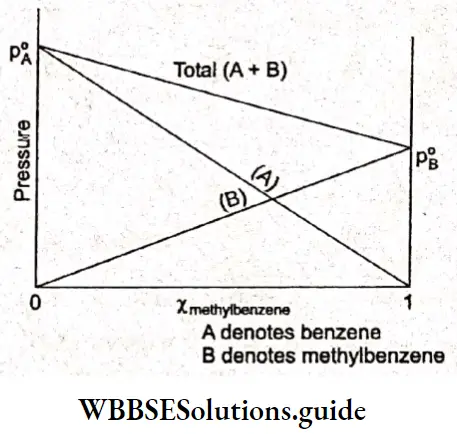
Note that the y-axis is drawn on both sides in the figure. This is to show that the value of the variable in the x-axis cannot exceed the value corresponding to the point where the right-hand side y-axis meets the x-axis.
The mole fraction is the x-variable and it can have a maximum value of 1. In a two-component system of A and B x B = zero refers to the component A being pure; the LHS y-axis corresponds to pure A. When xB is 1, it means only B is present and the RHS y-axis corresponds to pure B. In between these two extremes, both A and B are present.
The partial vapour pressures of the two components benzene and methylbenzene are proportional to their respective mole fractions, the total vapour pressure of the solution is the sum of the two partial vapour pressures.
Benzene and methylbenzene were found to obey Raoult’s law under all conditions of temperature and concentration. Such solutions are called ideal solutions and their vapour pressures are intermediate between the partial vapour pressures of the two components. We can define an ideal solution as one in which each component obeys Raoult’s law over a large range of concentrations.
Role of pressure in solubility of solids in liquids
This behaviour is observed when the components are structurally similar. In a solution formed by say A and B, if the nature of the intermolecular forces and their extent between A and B molecules (A-B interaction) is the same as that between A-A or B-B molecules, then the solution behaves ideally. Strictly speaking, no solution is ideal.
However, there are several nearly ideal solutions; for example, benzene and methylbenzene, chlorobenzene and bromobenzene, hexane and heptane and ethylene bromide and propylene bromide. Generally, all solutions show nearly ideal behaviour when progressively diluted. For an ideal solution, there is no change in enthalpy and volume on mixing the two components.
For an ideal solution \(\Delta_{\text {mix }} H=0\)
and \(\Delta_{\text {mix }} V=0\)
It is also possible to calculate the composition of the vapour phase. If yA and yB are the mole fractions of A and B in the vapour phase then according to Dalton’s law of partial pressures,
⇒ \(p_{\mathrm{A}}=y_{\mathrm{A}} p_{\text {total }}\)
and \(p_{\mathrm{B}}=y_{\mathrm{B}} p_{\text {total }}\)
Example 1. When equal masses of two liquid components A and B are mixed, their partial pressures are equal. If the total vapour pressure of the solution is 0.32 bar, the vapour pressures of pure A and pure B are 0.24 bar and 0.48 bar respectively, and the molar mass of A is 100, what is the molar mass of B?
Solution:
Given,
When equal masses of two liquid components A and B are mixed, their partial pressures are equal. If the total vapour pressure of the solution is 0.32 bar, the vapour pressures of pure A and pure B are 0.24 bar and 0.48 bar respectively, and the molar mass of A is 100,
PA = PB and PA + PB = 0.32
Or, \(2 p_A=0.32\)
⇒ \(p_A=\frac{0.32}{2}=0.16 \mathrm{bar}\)
We know that P = \(p_A^{\circ} x_A\)
On substituting for PA and \(p_A^0\), we get
⇒ \(0.16=0.24 \times \chi_{\mathrm{A}}\)
Or, \(\chi_A=\frac{0.16}{0.24}=0.67\)
Since
⇒ \(χ_A+χ_B=1\)
⇒ \(\chi_B=1-\chi_A=1-0.67=0.33\)
⇒ \(\chi_A=\frac{\frac{w_A}{M_A}}{\frac{w_A}{M_A}+\frac{w_B}{M_B}}\)
and \(\chi_{\mathrm{B}}=\frac{\frac{w_{\mathrm{B}}}{M_{\mathrm{B}}}}{\frac{w_{\mathrm{A}}}{M_{\mathrm{A}}}+\frac{w_{\mathrm{B}}}{M_{\mathrm{B}}}}\)
Or, \(\frac{x_A}{\psi_B}=\frac{w_A}{M_A} \cdot \frac{M_B}{w_B}\)
Given that wA=w, and MA =100.
∴ \(\frac{\chi_{\mathrm{A}}}{\chi_{\mathrm{B}}}=\frac{M_{\mathrm{B}}}{M_{\mathrm{A}}}\)
⇒ \(\frac{0.67}{0.33}=\frac{M_{\mathrm{B}}}{100}\)
⇒ \(M_B=\frac{100 \times 0.67}{0.33}=203\)
Thus, the molar mass of B is 203 g mol-1.
Example 2. What will be the vapour pressure of a solution of 5 mol of sucrose in 1 kg of water if the vapour pressure of pure water is 4.57 mmHg?
Solution:
⇒ \(\frac{p_{\text {water }}^\sigma-p_{\text {solution }}}{p_{\text {water }}^\sigma}=\chi_{\text {sucrose }}\)
Given, n=5 moles, W = 1000 g and \(p_{\text {water }}^{\circ}=4.57 \mathrm{mmHg}\)
⇒ \(\chi_{\text {sucrose }}=\frac{n}{n+N}=\frac{n}{n+\frac{W}{M_{\text {water }}}}=\frac{5}{5+\frac{1000}{18}}=\frac{5}{60.5}=0.083\)
Now \(\frac{p^0-p}{p^0}=0.083\)
Or, \(p^{\circ}-p=0.083 \times p^{\circ}\)
= 0.083 x 4.57
= 0.38
∴ \(p=p^0-0.38\)
= 4.57 -0.38
= 4.19.
The vapour pressure of the solution is 4.19 mmHg.
Vapour pressure of a solution of a solid in a liquid
You have already seen that a pure solvent has a characteristic vapour pressure at any given temperature and if a volatile liquid is added to it, the partial vapour pressure is proportional to the mole fraction. The vapour pressure of many solid solutes is negligible at temperatures at which they are present in solutions. Such solids are called nonvolatile solutes.
If a nonvolatile solute is added to a solvent to make a solution, the vapour pressure of the solution is only due to the solvent and is also less than that of the pure solvent.
This is because, in such a solution, the surface is covered with molecules of the solute as well as the solvent as compared to the pure solvent where only solvent molecules are present on the surface. As a result, the number of solvent molecules escaping into the vapour phase is lower in the solution than in the pure solvent.
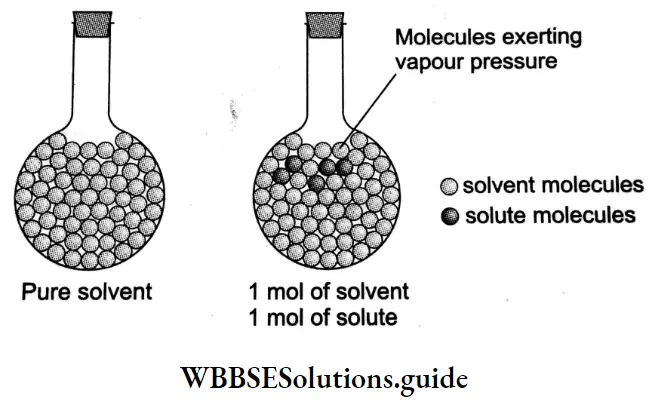
According to Raoult’s law, if p, denotes the vapour pressure of the solvent in solution (which is also simply the vapour pressure of the solution, as the solute is nonvolatile), then
⇒ \(p_{\text {solution }}=p_{\mathrm{A}}=p_{\mathrm{A}}^0 \chi_{\mathrm{A}}\)
A plot of PA versus χA will be a straight line with slope po A.
Nonideal solutions
Solutions that obey Raoult’s law at all concentrations are called ideal solutions while those that do not obey Raoult’s law are called nonideal solutions. Both enthalpy and volume change on mixing the components of a nonideal solution. Some solutions deviate significantly from Raoult’s law.
The deviation from Raoult’s law can be of two types-positive and negative. When the vapour pressure curves of the two components as well as that of the solution lie above the ones predicted by Raoult’s law, the system is said to exhibit positive deviation from Raoult’s law (e.g., acetone and carbon disulphide).
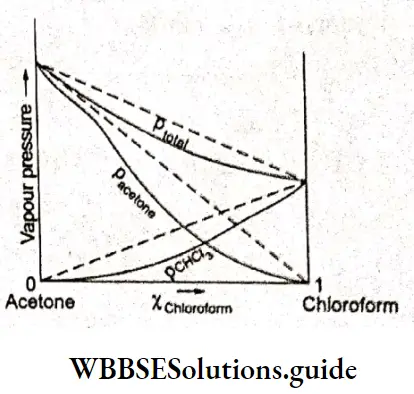
Other examples are ethanol-acetone and carbon tetrachloride-methanol. On the other hand, if the vapour pressure curves lie below (i.e., measured vapour pressure is lower) the ones predicted by Raoult’s law, the negative deviation is exhibited; for example, in an acetone-chloroform system.
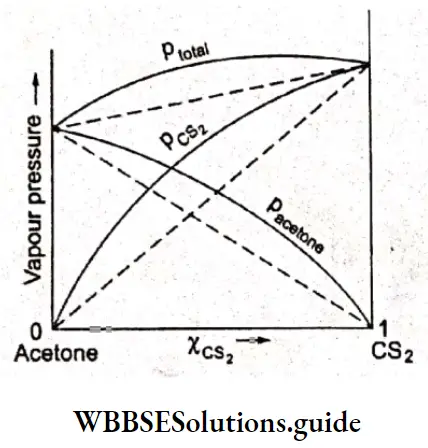
The solutions that exhibit positive deviation from Raoult’s law usually consist of one component whose molecules are associated such as water and alcohols (hydrogen bonding) and another component which is more or less inert, i.e., the structures of the two components are dissimilar.
The mixing of components tends to break some of the association between molecules and hence the enthalpy of mixing (Amix H) is positive. If the system is composed of components A and B, then the intermolecular forces between A and B in the solution are weaker than those between A and A, and B and B, as a consequence of which the molecules of A or B can escape more
easily from the solution than from the respective pure liquids. This causes an increase in the vapour pressure resulting in a positive deviation. The change in volume on mixing (Amix V) is positive. In a mixture of ethanol and acetone, which shows positive deviation, ethanol is hydrogen bonded and some of these bonds are broken on the mixing of ethanol with acetone, thus resulting in nonideal behaviour.
In the case of an acetone-chloroform system, the solution exhibits negative deviation from Raoult’s law, i.e., the vapour pressure curves lie below those suggested by Raoult’s law. Here the A-B interactions (ie., intermolecular interactions between acetone and chloroform) are much stronger than A-A or B-B interactions. Acetone and chloroform are held together by hydrogen bonding.

The result is that the escaping tendency of acetone or chloroform is less than that from the pure liquids and hence the vapour pressures are lower. On mixing the two components, the changes in enthalpy and volume are negative.
Some solutions show positive deviation and some show negative deviation from Raoult’s law:
Ideal Dilute Solutions
Many solutions do not show ideal behaviour. However, it must be noted that even in such cases Raoult’s law is obeyed by the solvent when the solution is dilute. This is because when the solution is dilute, the solvent molecules are far more in number, much like that in the pure solvent, as there are very few solute molecules. For dilute solutions, the solute obeys Henry’s law.
⇒ \(p=\chi K_{\mathrm{H}}\)
Such solutions are therefore called ideal dilute solutions; the solvent obeys Raoult’s law and the solute obeys Henry’s law.
Raoult’s law is a special case of Henry’s law:
According to Raoult’s law, the vapour pressure of a volatile component is proportional to its mole fraction in solution. Similarly, if one of the components is a gas as seen in the case of Henry’s law, the vapour pressure is again proportional to the mole fraction of the gas in the solution.
The only difference in the two cases is the proportionality constant, being på in the former and Henry’s law constant in the latter. Thus Raoult’s law may be considered as a special case of Henry’s law.
Boiling Of Liquid Solutions
A pure liquid boils at a temperature at which its vapour pressure becomes equal to the atmospheric pressure and the composition of the liquid phase and that of the vapour phase are the same. However, the boiling of a liquid solution differs from the boiling of a pure liquid; the composition of the vapour is different from the composition of the boiling solution.
The components of a solution can be separated by fractional distillation. In a simple distillation process, the vapour of the volatile component is collected and condensed, helping to separate it from the nonvolatile component, say a solid from a volatile liquid.
In fractional distillation, the process of boiling and condensation is repeated till the two components are completely separated.
After every step of boiling and condensation, the remaining liquid becomes richer in the less volatile component while the vapour becomes richer in more volatile component. After the distillation is complete, we get the less volatile component as the residue and the more volatile component as the vapour which is then condensed to get the liquid.
Such liquid mixtures which distil with a change in composition are called zeotropic mixtures. We are thus able to separate the two components completely from their solution at all compositions.
However, this is not possible in some nonideal solutions as they have the same composition in the liquid and vapour phases and boil at a constant temperature. In such solutions, the two components cannot be separated completely by fractional distillation. These mixtures are called azeotropic mixtures or simply azeotropes.
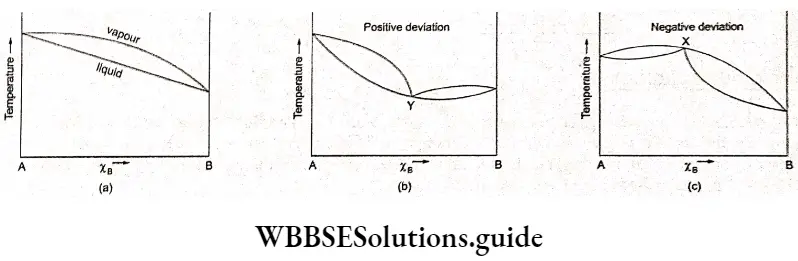
They are of two types-minimum-boiling and maximum-boiling. A solution showing negative deviation from Raoult’s law, on repeated distillation, leaves a residue with the highest boiling point instead of a pure liquid.
This residue has a boiling point higher than that of all other compositions and even the pure liquid. This liquid mixture boils at a constant temperature without further change in composition. Therefore, further distillation is not useful in separating the components. It is called a maximum boiling point azeotrope.
On the fractional distillation of solutions showing positive deviation from Raoult’s law, liquid residues with increasing boiling points are obtained and finally one of the components is obtained in the pure form. Which component is obtained depends upon the composition of the starting liquid solution.
Why pressure has negligible effect on solubility of solids
The vapours collected, on fractionation, give rise to a constant-boiling mixture of composition Y, which is the azeotrope and has the minimum boiling point. As before, further distillation is futile and separation of the azeotrope mixture into its components is impossible.
Thus, a minimum in the vapour-pressure composition curve corresponds to a maximum in the boiling-point composition curve and vice versa.
Colligative Properties
In a dilute solution, there are certain properties which depend only on the number of solute particles present in it and not on their nature or identity.
Such properties are called colligative properties (derived from the Latin word co meaning ‘together’ and ligare meaning ‘to bind’). The relative lowering of the vapour pressure of the solvent, the elevation of the boiling point of the solvent, the depression of the freezing point of the solvent and the osmotic pressure of the solution are examples of colligative properties.
Relative Lowering Of Vapour Pressure
As you have already studied, the vapour pressure of a solvent decreases with the addition of a nonvolatile solute to it. The vapour pressure of the solution is solely due to the solvent and is given by
⇒ \(p_{\text {solution }}=p_{\mathrm{A}}=p_{\mathrm{A}}^{\circ} \psi_{\mathrm{A}}\)
where the subscript A refers to the volatile solvent.
If the nonvolatile solute is denoted by B, then in a binary solution of A and B,
⇒ \(χ_A+χ_B=1\)
Or \(\chi_A=1-\chi_B\)
Substituting Equation 2.2 in Equation 2.1, we have
⇒ \(p_A=p_A^g\left(1-\chi_{1 \beta}\right)\)
Or \(\frac{p_A}{p_A^5}=1-\chi_B \text { or } 1-\frac{p_A}{p_A^0}=χ_B\)
Or \(\frac{p_A^0-p_A}{p_A^0}=\chi_B\)
Here \(p_A^0-p_A\) is the lowering of vapour pressure and \(\left(p_A^0-p_A\right) / p_A^0\) is called the relative lowering of vapour pressure. Thus, we arrive at a relation according to which the relative lowering of the vapour pressure of a solvent in a solution containing a nonvolatile solute is equal to the mole fraction of the solute in the solution. This is the extension of Raoult’s law to solutions containing nonvolatile solutes.
The mole fraction of the solute B can be written as
⇒ \(x_B=\frac{n}{n+N^{\prime}}\)
where n is the number of moles of the solute and N is that of the solvent.
For dilute solutions, n<< N. Therefore, neglecting n in the denominator, we get
⇒ \(\chi_B=\frac{n}{N}=\frac{\frac{w}{M_B}}{\frac{W}{M_A}}\)
On equating Equations (2.3) and (2.4),
⇒ \(\frac{p_{\Lambda}^0-p_{\Lambda}}{p_{\Lambda}^0}=\frac{w}{M_B} \times \frac{M_{\Lambda}}{W}\)
where W and w are the amounts of the solvent and solute respectively in grams in the solution, and MA and M are the molar masses of the solvent and solute respectively. Thus Equation 2.5 can be employed to determine the molar mass of the solute.
Elevation Of Boiling Point
When a liquid is heated, its temperature rises and the vapour. pressure increases gradually. Finally, a temperature is reached when the vapour pressure of the liquid becomes equal to the external pressure above the liquid.
This temperature is called the boiling point of the liquid. As you know, the vapour pressure of a solution is always less than the vapour pressure of a pure solvent. Consequently, the presence of a nonvolatile solute makes the boiling point of a solution higher than that of the pure solvent in which it is prepared.
For example, the boiling point of water is 373 K and the vapour pressure of water at this temperature is 1.013 bar, which is equal to one atmospheric pressure.
But on adding sucrose, the vapour pressure of the water (or sucrose solution) lowers, i.e., it becomes less than 1.013 bar. Now, in order to make the solution boil, it has to be heated to a greater extent so that its vapour pressure equals the atmospheric pressure.
This means that the boiling point of the solution is greater than that of the pure solvent. The greater the concentration of the solute, the higher the boiling point of the solution.
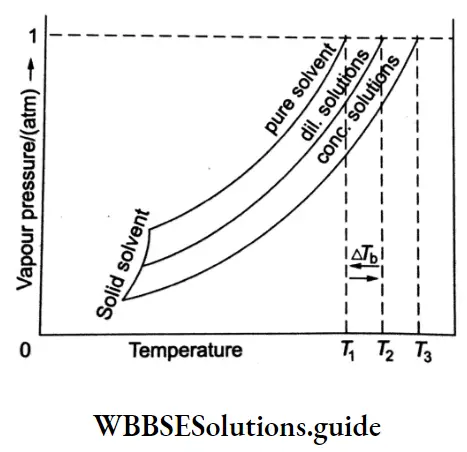
plot of vapour pressure of the pure solvent, the dilute solution and the concentrated solution against temperature. As you can see from the graph T1<T2 <T3 and ΔTb is the elevation in boiling point.
Experimental findings have proved that for a dilute solution the boiling point elevation AT, of a solvent is directly proportional to the number of moles of the nonvolatile, nondissociated solute dissolved in 1000 g of solvent.
⇒ \(\Delta T_b \propto m\)
Or \(\Delta T_{\mathrm{b}}=K_{\mathrm{b}} m\)
where m is the molality of the solution and K, is a constant, characteristic of the solvent, called the molal boiling point elevation constant.
If a known weight of a solute (w) is dissolved in a known quantity of a solvent (W), the elevation in boiling point can be determined and thus molality can be calculated.
Molality can also be written as
⇒ \(m=\frac{\frac{w}{M_{\mathrm{B}}}}{\frac{W}{1000}}=\frac{1000 \times w}{M_{\mathrm{B}} \times W}\)
where w is the weight of the solute in grams dissolved in W grams of solvent and M, is the molar mass of the solute. Thus, the molar mass of a solute can be determined if a known mass of it is taken in a known mass of a solvent and AT, is determined experimentally for a known solvent.
Substituting for the molality of the solution in the equation to determine the elevation of boiling point, we get
⇒ \(\Delta T_{\mathrm{b}}=K_{\mathrm{b}} \cdot\left(\frac{1000 \times w}{M_{\mathrm{B}} \times W}\right)\)
Or \(M_{\mathrm{B}}\left(M_{\text {solute }}\right)=K_{\mathrm{b}} \cdot \frac{1000 \times w}{\Delta T_{\mathrm{b}} \times W}\)
The elevation in boiling point is always positive since the boiling point of a solution is always greater than the boiling point of the pure solvent.
The constant K, which is the characteristic of a particular solvent, can be expressed in terms of its heat of vaporization, \(\Delta_{\text {vap }} H\).
⇒ \(K_{\mathrm{b}}=\frac{R T_0^2 M_{\text {solvent }}}{1000 \Delta_{\text {vap }} H} \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\)
Note that the molar mass of the solvent is given in units of g mol-1; on dividing by 1000, we get it in kg mol-1. Thus, the SI unit of K is K kg mol-1. R is the gas constant (8.314 JK1 mol-1), To is the boiling point of the pure solvent and Msolvent is its molar mass.
Example 1. A solution containing 29 g of a nonvolatile solute in 250 g of water boils at 373.52 K. The boiling point of pure water is 373 K. Find the molar mass of the solute. Given, K1 = 0.52 K kg mol1.
Solution:
Given
A solution containing 29 g of a nonvolatile solute in 250 g of water boils at 373.52 K. The boiling point of pure water is 373 K.
Elevation of boiling point, Δ Tb = 373.52-373= 0.52 K.
⇒ \(\Delta T_b=K_{\mathrm{b}} \cdot m\)
0.52 = 0.52 x m
Or \(m=\frac{0.52}{0.52}=1\)
But, \(m=\frac{w}{M_{\text {solute }}} \times \frac{1000}{W}\)
⇒ \(1=\frac{29}{M_{\text {solute }}} \times \frac{1000}{250}\) [∵ w= 29g, W= 250g]
Or \(M_{\text {solute }}=\frac{29 \times 1000}{250}=116 \mathrm{~g} \mathrm{~mol}^{-1}\)
Example 2. When 54 g of glucose (CH12O6, molar mass = 180) is dissolved in 100 g of water, it results in an elevation of the boiling point of water by 1.56 K. How many grams of another nonelectrolyte of molar mass 90 is required to be added to 100 g of water to elevate the boiling point by 1 K?
Solution:
Given
When 54 g of glucose (CH12O6, molar mass = 180) is dissolved in 100 g of water, it results in an elevation of the boiling point of water by 1.56 K.
The elevation in the boiling point of water on dissolving glucose, Δ Tb = 1.56 K.
Molality of glucose = \(\frac{\frac{54}{180}}{100} \times 1000=3 \mathrm{~m}\)
Since \(\Delta T_{\mathrm{b}}=K_{\mathrm{b}} \cdot m,\)
⇒ \(1.56 \mathrm{~K}=K_{\mathrm{b}} \times 3\)
Or \(K_{\mathrm{b}}=\frac{1.56}{3}=0.52 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\)
Thus, the boiling point elevation constant K of water is 0.52 K kg mol-1. The elevation in boiling point on dissolving another solute is 1.
∴ \(\Delta T_{\mathrm{b}}=1=K_{\mathrm{b}} \cdot m\)
Or 1 = 0.52 x m
⇒ \(m=\frac{1}{0.52}=1.92\)
But molality m = \(\frac{w}{\frac{w}{M_{\text {solute }}}} \times 1000\)
∴ \(m=\frac{w}{90 \times 100} \times 1000=1.92\)
w = 1.92 x 9
= 17.28 g
Therefore, 17.28 g of the other nonelectrolyte has to be added to 100 g of water to elevate its boiling point by 1 K.
Depression Of Freezing Point
While the addition of a nonvolatile solute or a solvent enhances the boiling point of the solvent, its freezing point is reduced. Solutions, in general, freeze at lower temperatures than pure liquids do.
Solutions of antifreeze compounds like ethylene glycol have been used in automobile radiators to avoid freezing. Sea water, due to its salt content, freezes at a lower temperature than does fresh water.
Like the elevation of the boiling point of a solvent, the depression of its freezing point is also a consequence of the lowering of its vapour pressure upon adding a nonvolatile solute. The temperature at which the solid and liquid phases of the solvent are in equilibrium and have the same vapour pressure is called the freezing point of the solvent.
For example, the freezing point of water is 273 K, the temperature at which both water and ice have the same vapour pressure. However, water in a solution has a lower vapour pressure than that of ice (pure solid solvent) at 273 K. Therefore, when ice and an aqueous solution of a compound are kept in contact, ice melts.
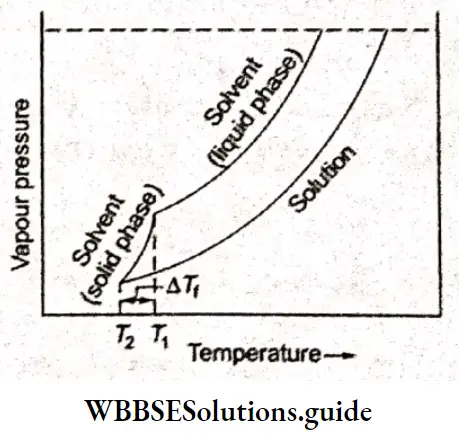
If the temperature is allowed to fall, the vapour pressure of the ice decreases more rapidly than that of the water in the solution and at a specific temperature below 273 K ice and water have the same vapour pressure. This is the point at which ice and solution are in equilibrium or the freezing point of the solution.
In general, 1 mol of a nonelectrolyte such as sucrose, glycerine or alcohol when dissolved in 1 kg of water gives a solution that freezes at -1.86°C. The difference AT, between the freezing point of a pure solvent and the freezing point in the presence of a solute is directly proportional to the molality of the solution, the proportionality constant being K, the molal freezing point depression constant.
Thus, \(\Delta T_f \propto m\)
Or, \(\Delta T_f=K_i m\)
With the help of the following relationship, the molar mass of the solute can be determined, if the quantities \(\Delta T_f, K_f\) are known and w grams of solute is present in W grams of solvent.
⇒ \(M_{\mathrm{B}}=M_{\text {solute }}=K_{\mathrm{f}} \cdot \frac{1000 \cdot w}{\Delta T_{\mathrm{f}} \cdot W} .\)
Like Kb, Kf can also be expressed in terms of thermodynamic quantities.
⇒ \(K_{\mathrm{f}}=\frac{R T_0^2 M_{\text {solvent }}}{1000 \Delta_{\text {fus }} H} \mathrm{Kkg} \mathrm{mol}^{-1}\)
where R is the gas constant and Msolvent is the molar mass of the solvent. A fuH is the change in enthalpy for the fusion of the pure solid solvent and To is its freezing point in the pure state.
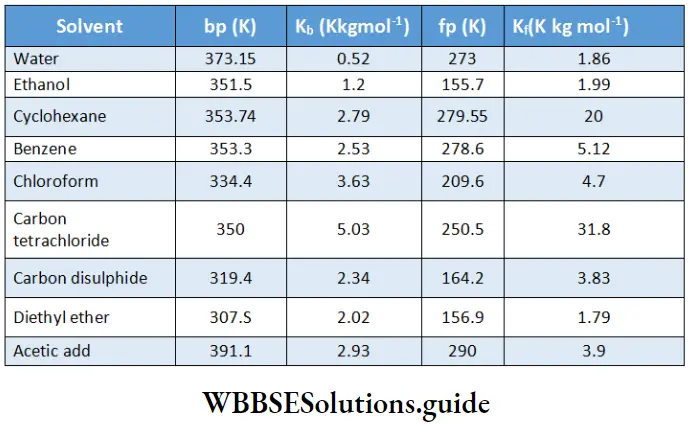
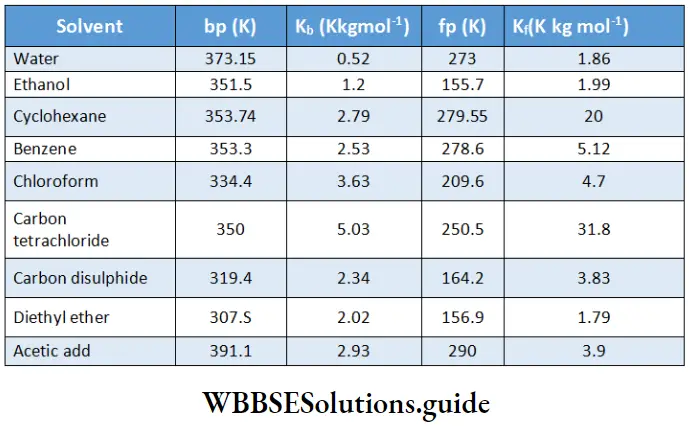
Values of K, and K, for some solvents:
Example 1. 27.5 g of a nonelectrolyte of molar mass 100 g mol is dissolved in 500 g of water. What is the freezing point of this solution if Kf of water is 1.86 K kg mol and the freezing point of water is 0°C?
Solution:
Given
27.5 g of a nonelectrolyte of molar mass 100 g mol is dissolved in 500 g of water.
Molality m of the solution = \(\frac{\frac{27.5}{100}}{500} \times 1000=0.55 \mathrm{~m}\)
Given, Kf of water = 1.86.
⇒ \(\Delta T_{\mathrm{f}}=K_{\mathrm{f}} \cdot m=1.86 \times 0.55\)
= 1.02 k
i.e., Tf-T=1.02 (Tf and T are the freezing points of the solvent and the solution respectively)
273.15-T=1.02
Or T=272.13 K.
Therefore, the freezing point of the solution is 272.13 K.
Osmosis And Osmotic Pressure
When a solution and the solvent are separated by a semipermeable membrane, the spontaneous passage of the solvent through the membrane to the solution side is called osmosis. A semipermeable membrane is one which allows the passage of solvent molecules and prevents the passage of molecules of dissolved substances, i.e., solutes.
Cell membranes in plants and animals, certain parchment papers and some gelatinous inorganic compounds are a few examples of semipermeable membranes. The transportation of water in a tree from the roots to the leaves takes place by the process of osmosis, the cell membranes being the semipermeable membranes.
The swelling of dried vegetables when kept in water and the shrinking of vegetables due to loss of water, when kept in concentrated salt solutions, are some other examples. The functioning of the kidney is also based on this process. This process is also made use of to concentrate fruit juices, and to purify water.
Osmosis occurs when two solutions of different concentrations in the same solvent are separated by a semipermeable membrane. The solvent in this case flows spontaneously from the solution of lower concentration to the solution of higher concentration till the concentrations of both the solutions on either side of the membrane become the same.
Osmotic pressure:
Let us consider the following example to understand what osmotic pressure is. Two long-necked vessels are connected at the bottom through a semipermeable membrane. The two vessels contain an equal quantity of pure water in one and sugar solution in the other so that their levels in the vessels are the same.
Due to the difference in concentration of the solute on the two sides, osmosis takes place and after some time the level of the solution rises and that of the water lowers. The process continues on its own till the concentrations on the two sides become equal.
But if the membrane is strong enough to withstand the pressure and if the tube is long enough, the solution will rise until its hydrostatic pressure (due to the weight of the column of the solution in the tube) is great enough to prevent further osmosis of the pure solvent into the solution.
Pressure and Henry’s Law in gas solubility
The pressure required to stop the passage of solvent molecules into the solution, i.e., to prevent osmosis, is called the osmotic pressure, II, of the solution. Osmotic pressure has the same units as those of pressure. The osmotic pressure of a dilute solution can be calculated from the following expression based on experimental findings.
π=CRT,
where C is the molarity of the solution, R is the gas constant and T is the temperature.
But \(\Pi=C R T\)
where n is the number of moles of the solute and V is the total volume of the solution.
But \(C=\frac{n}{V}\)
Therefore, ΠV = nRT. This equation is called the Van’t Hoff equation and corresponds to the ideal gas equation. If w grams of solute (of molar mass M) is present in the solution then
⇒ \(n=\frac{w}{M_{\text {solute }}}\)
Thus, \(\Pi V=\left(\frac{w}{M_{\text {solute }}}\right) R T\)
Or \(M_{\text {solute }}=\frac{w}{\Pi V} R T\)
Two solutions having the same osmotic pressure at a given temperature are called isotonic solutions. When such solutions are separated by a semipermeable membrane, osmosis does not occur across the membrane.
The fluids present in the human body have a certain osmotic pressure. And therefore the fluids that are injected into the body must be isotonic with the body fluids. A hypotonic solution (less concentrated) results in the swelling of blood cells and causes rupture. A hypertonic solution (of higher concentration) may lead to the death of blood cells due to loss of water.
Example 2. A 5% solution of cane sugar (molar mass 342 g mol-1) is isotonic with a 0.877% solution of a substance X. Find the molar mass of X at 300 K.
Solution:
Given
A 5% solution of cane sugar (molar mass 342 g mol-1) is isotonic with a 0.877% solution of a substance X.
The osmotic pressure of cane sugar solution,
⇒ \(\Pi_1=\frac{n_1 R T}{V_1}=\frac{5 \times 0.082 \times 300}{342 \times 100}\)
The osmotic pressure of a solution containing X,
⇒ \(\Pi_2=\frac{n_2 R T}{V_2}=\frac{\frac{0.877}{M_\chi}}{100} \times 0.082 \times 300\)
But П1 =П2.
Therefore, \(\frac{5}{342 \times 100} \times 0.082 \times 300=\frac{0.877}{M_X \times 100} \times 0.082 \times 300\)
Or \(\frac{5}{342}=\frac{0.877}{M_X}\)
Or \(M_{\mathrm{X}}=\frac{0.877}{5} \times 342\)
Or \(M_X=60\)
The molar mass of X is 60 g.
Reverse osmosis:
If a pressure greater than the osmotic pressure is applied on the solution side, then the process of osmosis gets reversed, i.e., the solvent from the solution passes to the solvent side, thereby increasing its volume. This is called reverse osmosis.
High pressures are required for reverse osmosis to occur. The process has many applications, such as in desalinating sea water. The semipermeable porous membrane used in the desalination of seawater is cellulose acetate, which is permeable to water but not to the ions and other impurities present in seawater.
The drinking-water purifying systems based on reverse osmosis include a thin film composite membrane made of polyamide or cellulose triacetate.
The other uses of reverse osmosis include the purification of rainwater collected from storm drains for use in irrigation, the purification of boiler water in power plants, and in dialysis.
Measurement of osmotic pressure:
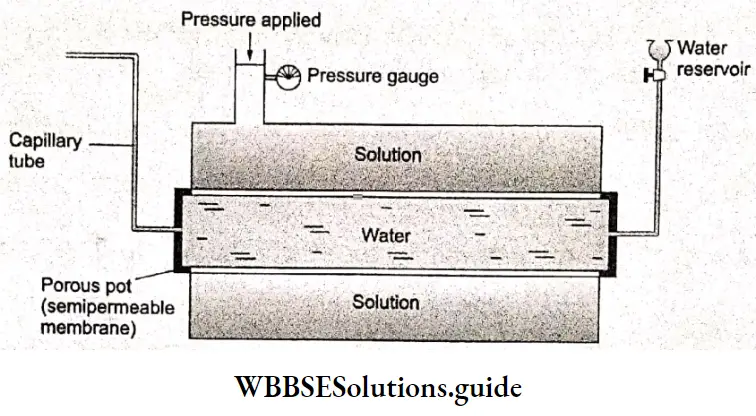
The apparatus used for measuring osmotic pressure is called an osmometer. Shows a schematic diagram of the Berkeley and Hartley osmometer. The central or the inner compartment contains water and the two outer compartments contain solutions.
The compartments are connected to each other on the sides by a semipermeable membrane, which allows the passage of water only. The capillary tube attached to the central compartment indicates any flow of water from the central to the outer compartments.
If the water level in the capillary decreases, it means water has flowed out of the inner compartment to the solution side. On one of the solution sides, there is a pump to exert pressure and a pressure gauge to measure the pressure applied.
To stop osmosis, pressure is applied to the solution to keep the water level constant in the capillary. The applied pressure is read directly from the pressure gauge. This is the osmotic pressure.
Abnormal Molar Mass
As stated earlier, the colligative properties of a solution depend on the number of solute particles present in it. If the solute is a nonelectrolyte, which does not undergo dissociation, the number of particles is the same as the number of molecules.
However, when electrolytes (i.e., almost all acids, bases and salts) are dissolved in water, dissociation takes place and two or more ions are produced.
Each ion then acts as an independent particle and depending on the extent of dissociation, the observed values of colligative properties are higher than the theoretically expected values.
Since the molar mass of the solute is inversely proportional to the colligative property of the solution, the experimentally determined value of the colligative property is far less than the actual value in dissociation cases.
For example, one molal aqueous solution of sodium chloride, an electrolyte, freezes at -3.37°C as compared to one molal aqueous solution of a nonelectrolyte such as sugar, in which case the freezing point is -1.86°C.
The calculation of the molar mass of the electrolyte (here NaCl) gives a value which is nearly twice that of the normal molar mass of 58.5. This is because NaCl ionises to a large extent in water to give Na* and CI ions and the number of particles is two instead of one in one molecular formula of the solute. Hence, the number of particles (and therefore the depression in freezing point) is twice that obtained from a nonelectrolyte.
Factors affecting solubility of solids vs gases in liquids
In 1886, the Dutch scientist Can’t Hoff introduced a factor, known as the Van’t Hoff factor, to account for abnormal molar masses.
⇒ \(\text { van’t Hoff factor, } i=\frac{\text { actual molar mass based on formula of compound }}{\text { observed molar mass }}\)
For such solutions, the respective equations for the colligative properties become
⇒ \(\frac{p_{\text {solvent }}^{\circ}-p_{\text {solution }}^{\circ}}{p_{\text {solvent }}^{\circ}}=i\left(\frac{n}{N+n}\right)\) (relative lowering of vapour pressure)
⇒ \(\Delta T_{\mathrm{b}}=i K_{\mathrm{b}} \cdot m\) (elevation of boiling point)
⇒ \(\Delta T_{\mathrm{f}}=i K_{\mathrm{f}} m\) (depression of freezing point)
⇒ \(\Pi V=i n R T\) (osmotic pressure of solution)
The value of I gives the number of particles obtained from one formula unit of the compound. When dissociation of the solute occurs in solution, i is greater than 1. If a solute is associated with a liquid solvent, then the observed molar mass is more than the theoretical molar mass.
If two molecules associate to form a single particle then the molar mass is nearly double that of the normal molar mass. For example, benzoic acid is found to dimerise in benzene.
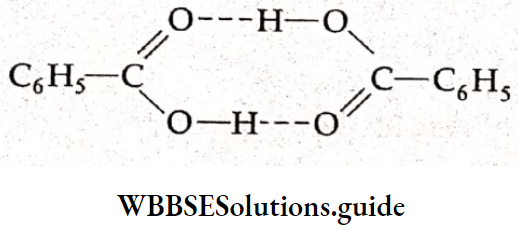
In the case of an association, the can’t Hoff factor is less than unity. The modified equations for the colligative properties are the same as given in the case of the dissociation of solutes.
The can’t Hoff factor may be defined as the ratio of the observed value of a colligative properly to the theoretically calculated value of the property.
Values of can’t Hoff Factor I, at various concentrations, for NoCI, KCI, MgSO, and K2SO4
Example 1. A2m solution of NaCl in water causes an elevation in the boiling point of water by 1.88 K. What is the value of the can’t Hoff factor I? What does it suggest? Normal molar mass of NaCl = 58.5 & K = 0.52 K kg mol -1.
Solution:
Given
A2m solution of NaCl in water causes an elevation in the boiling point of water by 1.88 K.
Expected elevation in boiling point,
⇒ \(\Delta T_{\mathrm{b}}(\text { expected })=0.52 \times 2\)
= 1.04 K.
Observed elevation = 1.88 K.
⇒ \(\text { van’t Hoff factor } i=\frac{\text { observed elevation }}{\text { expected elevation }}\)
⇒ \(\frac{1.88}{1.04} \approx 2\)
A value of 2 for the can’t Hoff factor suggests that 1 molecule of NaCl dissociates into two particles- Na+ and CI–.
Example 2. Calculate the amount of NaCl that must be added to 100 g of water so that the freezing point of water is depressed by 2 K. Given that K, for water, is 1.86 K kg mol-1, can’t Hoff factor, i, is 2 for NaCl and the molar mass of NaCl is 58.5.
Solution:
Since \(\Delta T_f=i K_f m\)
2=2 x 1.86 x m,
⇒ \(m=\frac{2 \times 1.86}{2}=1.86\)
But \(m=\frac{\frac{w_{\mathrm{NaCl}}}{M_{\mathrm{NaCl}}}}{100} \times 1000\)
Where \(w_{\mathrm{NaCl}}\) is the amount of NaCl to be added and \(M_{\mathrm{NaCl}}\) is its molar mass.
Therefore, \(m=\frac{w_{\mathrm{NaCl}}}{58.5 \times 100} \times 1000\)
Ог \(1.86=\frac{w_{\mathrm{NaCl}}}{58.5 \times 100} \times 1000\)
⇒ \(w_{\mathrm{NaCl}}=\frac{1.86 \times 58.5 \times 100}{1000}\)
⇒ \(w_{\mathrm{NaCl}}=10.08\)
Therefore, 10.08 g of NaCl must be added to 100 g of water to cause a depression in the freezing point of 2 K
Solutions Multiple Choice Questions
Question 1. An amalgam of mercury with sodium is an example of a solution whose solvent is a
- Solid
- Liquid
- Gas
- None Of The Above
Answer: 1. Solid
Question 2. One litre of a sample of water contains 0.001 g of sodium ions. Its concentration in ppm is
- 1×10-6
- 0.001
- 1000
- 1
Answer: 4. 1
Question 3. The sum of the mole fractions of all the components
- Less Than One
- Greater Than One
- Equal To One
- Either (1) Or (2)
Answer: 3. Equal To One
Question 4. What is the molality of 4 g of NaOH in 2000 g of water?
- 0.002
- 5×10-5
- 2
- 0.05
Answer: 4. 0.05
Question 5. The dissolution of a particular solid in a liquid is endothermic. Its solubility
- Increases With Temperature
- Decreases With Temperature
- Does Not Depend On The Temperature
- Either (1) Or (2)
Answer: 1. Increases With Temperature
Question 6. What happens to the solubility of gases in liquids with an increase in temperature?
- Increases
- Does not change
- Decreases
- May increase or decrease depending on the gas
Answer: 3. Decreases
How external pressure influences solubility equilibrium
Question 7. Henry’s law gives the relation between
- The Solubility Of A Gas In A Solvent And The Pressure
- The Solubility Of The Gas And Concentration Of The Solution
- The Solubility Of The Gas And The Temperature
- None Of The Above
Answer: 1. The Solubility Of A Gas In A Solvent And The Pressure
Question 8. The vapour pressure of a solvent is…… by the addition of a nonvolatile solute.
- Increased
- Decreased
- Not Affected
- Any Of The Above
Answer: 2. Decreased
Question 9. An ideal solution is one
- Which Obeys Raoult’s Law
- For Which [Latex]\Delta_{\operatorname{mix}} H=0[/Latex]
- For Which [Latex]\Delta_{\operatorname{mix}} V=0[/Latex]
- All Of The Above
Answer: 4. All Of The Above
Question 10. A solution is said to show a positive deviation from Raoult’s law if
- It Shows A Minimum In The Plot Of Vapour Pressure As A Function Of Mole Fraction
- It Shows A Maximum In The Plot Of Vapour Pressure As A Function Of Mole Fraction
- It Shows A Minimum In The Plot Of Temperature As A Function Of Mole Fraction
- It Shows A Maximum In The Plot Of Temperature As A Function Of Mole Fraction
Answer: 2. It Shows A Maximum In The Plot Of Vapour Pressure As A Function Of Mole Fraction
Question 11. A solution that is formed by mixing two liquids has the same composition in the liquid and the vapour phase and boils at a constant temperature is called a/an
- Zeotropic Mixture
- Equilibrium Mixture
- Azeotropic Mixture
- Nonequilibrium Mixture
Answer: 3. Azeotropic Mixture
Question 12. Mixtures with azeotropic composition
- Cannot Be Separated By Fractional Distillation
- Can Be Separated By Fractional Distillation
- Can Be Separated By Simple Distillation
- Either (2) Or (3)
Answer: 1. Cannot Be Separated By Fractional Distillation
Question 13. Solutions which show positive deviation from Raoult’s law form
- Maximum Boiling Azeotropes
- Maximum Boiling Zeotropes
- Minimum Boiling Zeotropes
- Minimum Boiling Azeotropes
Answer: 4. Minimum Boiling Azeotropes
Question 14. Colligative properties are those properties that depend on the
- Nature Of The Solute
- Size Of The Solute Particles
- Nature Of The Solvent
- Number Of Solute Particles
Answer: 4. Number Of Solute Particles
Question 15. A nonvolatile solute is added to a volatile solvent. Its boiling point
- Remains The Same As That Of The Pure Solvent
- Increases
- Decreases
- None Of These Happens.
Answer: 3. Decreases
Question 16. A nonvolatile solute is added to a solvent. Its freezing point
- Remains The Same As That Of The Pure Solvent
- Decreases
- Increases
- None Of These Happens.
Answer: 2. Decreases
Question 17. The freezing point of a substance is defined as the temperature at which the
- Vapour Pressure Of The Substance In The Liquid Phase Is Equal To That In The Solid Phase
- Vapour Pressure Of The Liquid Is Equal To The Atmospheric Pressure
- Vapour Pressure Of The Solid Is Equal To The Atmospheric Pressure
- None Of These
Answer: 1. Vapour Pressure Of The Substance In The Liquid Phase Is Equal To That In The Solid Phase
Question 18. The decrease in the freezing point of a solvent in the presence of a nonvolatile solute is a consequence of
- Zero Vapour Pressure Of The Solute
- The Elevation Of The Vapour Pressure Of The Solvent Because Of The Presence Of The Solute
- The Lowering Of The Vapour Pressure Of The Solvent Because Of The Presence Of The Solute
- None Of These
Answer: 3. The Lowering Of The Vapour Pressure Of The Solvent Because Of The Presence Of The Solute
Question 19. A semipermeable membrane is one which
- Allows The Passage Of Only Solute Molecules
- Does Not Allow The Passage Of Matter Through It
- Allows A Small Amount Of Solute And Solvent Molecules To Move Through It
- Allows the passage of only solvent molecules through it
Answer: 4. Allows the passage of only solvent molecules through it
Question 20. In osmosis, the flow of solvent molecules takes place from
- Concentrated To Dilute Solution
- Dilute To The Concentrated Solution.
- Solvent To The Solution Side
- Either (2) Or (3)
Answer: 2. Dilute To The Concentrated Solution.
Question 21. Osmotic pressure depends on
- The Molarity Of The Solution
- Temperature
- Both (1) And (2)
- The Molar Mass Of The Solvent
Answer: 3. Both (1) And (2)
Question 22. Two solutions that have the same osmotic pressure at
- Hypertonic Solutions
- Isotonic Solutions
- Hypotonic Solutions
- None Of These
Answer: 2. Isotonic Solutions
Question 23. The shrinking of vegetables kept in salt water is due to
- The Depression In The Vapour Pressure Of Water
- The Elevation In The Boiling Point Of Water
- The Depression In The Freezing Point Of Water
- Osmosis
Answer: 4. Osmosis
Question 24. In reverse osmosis,
- A Pressure Larger Than The Osmotic Pressure Is Applied On The Solution Side
- A Pressure Larger Than The Osmotic Pressure Is Applied On The Solvent Side
- A Pressure Smaller Than The Osmotic Pressure Is Applied On The Solution Side
- A Pressure Smaller Than The Osmotic Pressure Is Applied On The Solvent Side
Answer: 1. A Pressure Larger Than The Osmotic Pressure Is Applied On The Solution Side
Question 25. When a solute associates with a solution the value of the can’t Hoff factor is
- Equal To One
- Less Than One
- Greater Than One
- Either (2) Or (3)
Answer: 2. Less Than One
Question 26. When a solute dissociates in solution, the observed molar mass is…… the actual molar mass
- Greater Than
- Less Than
- Equal To
- May Be (1) Or (2)
Answer: 1. Greater Than
