Hybridisation
According to the valence bond theory, a covalent bond is formed by the overlapping of half-filled atomic orbitals of the valence shell. This does explain the formation of some molecules, like O2 and N2, but cannot explain the tetravalency of carbon, the bivalency of beryllium, or the trivalency of boron.
- Carbon, with the electronic configuration of \(1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}_x^1, 2 \mathrm{p}_y^1\) has only two unpaired electrons and should have been bivalent. The electronic configuration of boron is \(1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}_x^1\) which means that it has only one half-filled orbital but it forms compounds like BF3. Beryllium, with the electronic configuration of 1s2, 2s2 has no impaired electron. Its covalency should have been zero, but it forms compounds like BeCl2.
- To explain this apparent contradiction, the modem theory of bond formation assumes that these and other atoms acquire excited states before participating in bond formation. In the excited state, one of the electrons in the 2s orbital in the examples we are considering gets promoted to a vacant 2p orbital. Thus, for example, in the excited state, carbon has four half-filled orbitals. The energy used for excitation is compensated for by the energy released during bond formation.
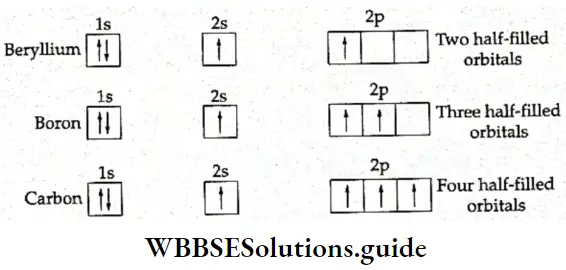
The assumption that atoms acquire an excited state before forming bonds can explain some things like the tetravalency of carbon. But it leaves certain other questions unanswered. Take the formation of methane, for example. The carbon atom has four unpaired electrons in the excited state, one electron in the s orbital, and three in the p orbitals.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
- If these orbitals overlap with the Is orbitals of four hydrogen atoms, three p-s sigma bonds and one s-s sigma bond should be formed. In that case the four bonds should not be equivalent, they should have different strengths, bond lengths, and bond angles. However, it has been seen experimentally that the four bonds are equivalent and that the bond length is 109 pm, while the H—C—H bond angle is 109°28′.
- Pauling and Slater, two American scientists, introduced the concept of hybridisation to explain this apparent discrepancy. According to them, before an atom forms bonds with other atoms, its orbitals get mixed and redistributed to form new, equivalent orbitals.
- These equivalent orbitals are called hybrid orbitals and the phenomenon is called hybridisation. Thus, hybridisation is the intermixing of atomic orbitals of comparable energies to form new orbitals of equivalent energy and identical shape. The energies of the orbitals get redistributed in the process.
Going back to methane, the three 2p orbitals of the carbon atom intermix with the 2s orbital to produce four sp3 hybridised orbitals, which form four equivalent bonds with the Is orbitals of four hydrogen atoms.
Rules of hybridisation: Hybridisation is a theoretical concept that is used to explain some structural properties of molecules. It would be useful to remember the following points about hybridisation.
- The orbitals taking part in hybridisation must have comparable (small difference) energies.
- It is not necessary for an orbital to be half-filled in order to participate in hybridisation. Vacant and completely filled orbitals can also be involved in hybridisation. This means electrons may or may not be promoted from a lower to a higher subshell for the formation of hybrid orbitals.
- The electron density of a hybrid orbital is concentrated on one side of the nucleus, which means one lobe of the orbital is relatively larger than the other. Therefore, it Hybridised orbital can overlap to a large extent than an s orbital or a p orbital.
- Hybridised orbitals have equivalent energies and identical shapes.
- The number of hybrid orbitals formed is always equal to the number of atomic orbitals taking part in hybridisation.
- Hybrid orbitals form stronger bonds than pure atomic orbitals because they can overlap to a greater extent. They always form a bonds because their electron cloud is parallel to the internuclear axis.
- A molecule adopts a particular shape not because of hybridisation but to have the lowest favorable energy.
Types of hybridisation: Hybridisation is a concept which is applicable to all types of atomic orbitals, viz., s, p, d, and f. Let us discuss a few of the simpler types.
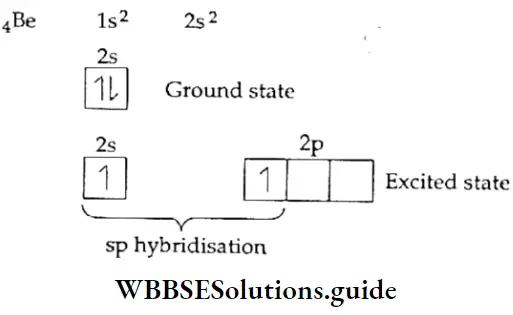
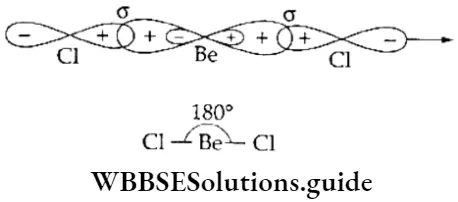
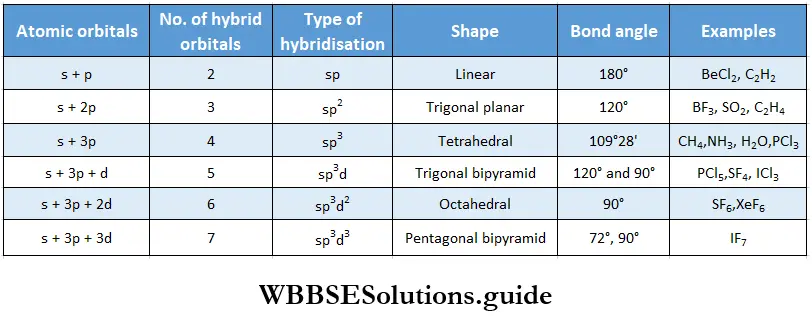
sp hybridisation When one s and one p orbital belonging to the same shell of an atom hybridise to form two new orbitals, the type of hybridisation is called sp hybridisation. The new orbitals formed are called sp hybrid orbitals. The two sp hybrid orbitals orient themselves at an angle of 180° to reduce repulsion to the minimum.
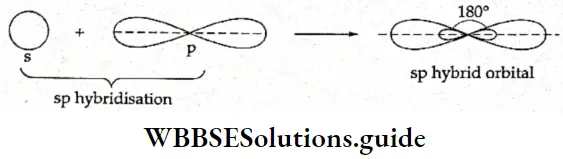
Let us consider beryllium chloride (BeCl2)- The central atom, Be, has two electrons in the 2s subshell. One of these electrons is promoted to the 2p subshell. Then the 2s and 2p orbitals undergo hybridisation to form two sp-hybridised orbitals, which are linear (180° apart) and contain one electron each. The two sp-hybridised orbitals overlap axially with the p orbitals of two chlorine atoms to form two σ bonds.
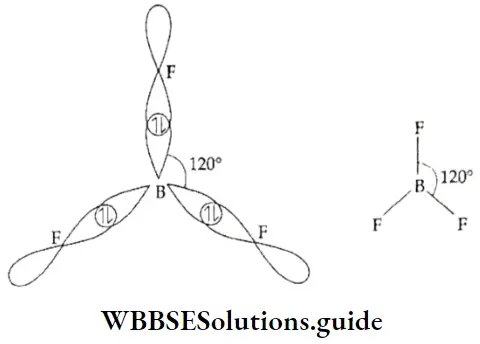
sp2 hybridisation When one s and two p orbitals of the same shell of an atom hybridise to form three hybrid orbitals of equivalent energy and identical shape, the hybrid orbitals are called sp2-hybridised orbitals.
In order to reduce repulsion, the three sp2 hybrid orbitals orient themselves in such a way as to form angles of 120° with each other and lie in the same plane. In other words, the orbitals are directed towards the vertices of an equilateral triangle. If the sp2-hybridised central atom of a molecule is linked directly to three other atoms, the molecule is trigonal planar in shape.
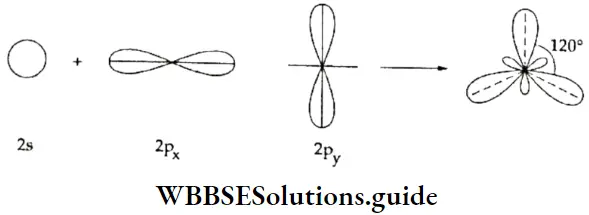
Take the case of BF3, in which the central atom, B, has three electrons in the valence shell (1s2, 2s2, 2p1). One electron is unpaired in the ground state. In the excited state there are three unpaired electrons, one in the s and two in the p orbital. Then one s and two p orbitals undergo sp2 hybridisation, to form three sp- hybridised orbitals, which form σ bonds with the 2p orbitals of three fluorine atoms. The shape of the BF3 molecule is trigonal planar.
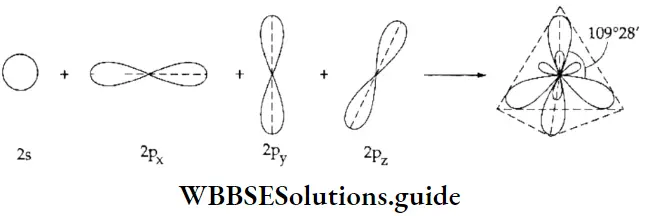
sp3 hybridisation When one s and three p orbitals belonging to the same shell of an atom hybridise to form four hybrid orbitals of equivalent energy and identical shape, the new orbitals are called sp3-hybrid orbitals.
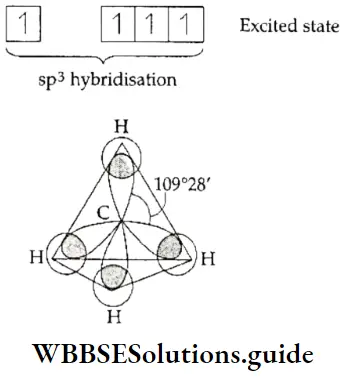
This type of hybridisation is called sp3 hybridisation. Molecules in which the sp3– hybridised orbitals of the central atom form bonds with four other atoms are tetrahedral in shape. This is because the sp3 -hybridised orbitals are directed towards the vertices of a tetrahedron. The angle between sp3 – hybridised orbitals, and consequently the bond angle of tetrahedral molecules, is 109°28′.
In methane, the carbon atom is sp3 – hybridised. It has four sp3 -hybridised orbitals containing one electron each. The carbon atom shares these electrons with four hydrogen atoms. In other words, all the hybridised orbitals of the central atom form bonds with four atoms. This is why the methane molecule has a regular tetrahedral shape and the bond angle is 109°28′.
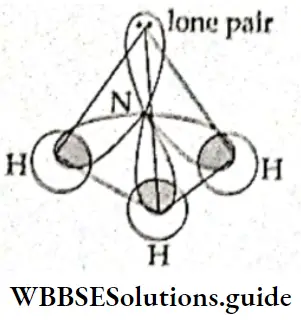
However, all molecules in which the central atom is sp3 hybridised do not have a regular tetrahedral shape. In NH3, for example, the central atom, nitrogen, has three unpaired electrons in its 2p subshell. To make three covalent bonds with three hydrogen atoms, nitrogen needs only three unpaired electrons.
- Therefore, in this case, the question of the promotion of an electron from a lower subshell does not arise. Nitrogen undergoes hybridisation in the ground state. One s and three p orbitals hybridise to form sp3-hybridised orbitals. However, one orbital contains a pair of electrons which the atom does not share. The other three orbitals form sigma bonds with the Is orbitals of three hydrogen Atoms.
- The shape of the molecule Is, thus, not regular tetrahedral because the orbital (of nitrogen) with the lone pair of electrons does not participate in bond formation. On account of unequal repulsions, the bond angle changes from the regular angle of 109°28′ to 107°, and the shape of the molecule is pyramidal.
In water, the central oxygen atom sp3 hybridised, The configuration of the oxygen atom is 1s2, 2s2, 2p4. f wo p orbitals contain unpaired electrons which is all the oxygen atom needs to form bonds with two hydrogen atoms. The oxygen atom undergoes sp3 hybridisation in the ground state.
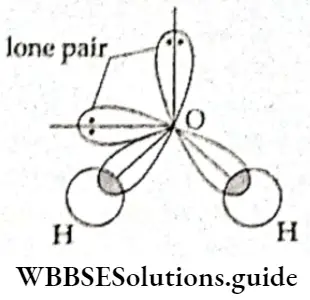
An s orbital containing two electrons, a p orbital containing two electrons, and two p orbitals containing an electron each undergoes hybridisation. Two of the hybrid orbitals contain a pair of electrons each and do not participate in bond formation. The other two (containing one electron each) form σ bonds with the 1s orbitals of two hydrogen atoms. The unequal repulsion on account of the two lone pairs of electrons reduces the bond angle to 105°.
sp3d hybridisation This type of hybridization entails the intermixing of one s, one d, and three p orbitals. Five equivalent sp3d hybrid orbitals are formed. The central atoms of PCl5, SF4, and ICl3 are sp3d hybridised.
In PCI5, the central atom has only three unpaired electrons in its 3p subshell in the ground state.
⇒ \(\left({ }_{19} \mathrm{P}-1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}^6, 3 \mathrm{~s}^2, 2 \mathrm{p}_x^1, 3 \mathrm{p}_y^1, 3 \mathrm{p}_z^1\right) \text {. }\).
To make five covalent bonds with five chlorine atoms, phosphorus needs five unpaired electrons which are present in the excited state. Then one 3s, three 3p and one 3d orbital undergo sp3d hybridisation. The five hybrid orbitals form sigma bonds with the orbitals of five chlorine atoms. The shape of the molecule is regular trigonal bipyramidal and bond angles arc 120° and 90°.
In SF4, the central atom, sulphur, has only two unpaired electrons \(\left(1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}^6, 3 \mathrm{~s}^2, 3 \mathrm{p}_x^2, 3 \mathrm{p}_y^1, 3 \mathrm{p}_z^1\right)\).
To make four covalent bonds the sulphur atom needs four unpaired electrons. Therefore, one electron from the 3px orbital is promoted to the 3d orbital. Then one s, three p, and one d orbitals undergo sp3d hybridisation. Four of the hybrid orbitals form sigma bonds with four fluorine atoms. The fifth hybrid orbital contain a lone pair of electrons.
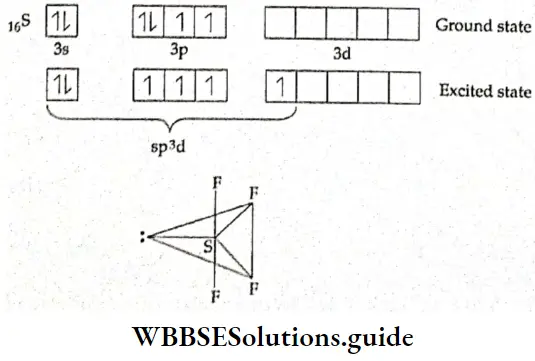
The central atom is, thus, surrounded by a lone pair and four bonded pairs of electrons. In order to reduce repulsion to the minimum, the lone pair occupies an equatorial position. ‘Hie unequal repulsion on account of the presence of a lone pair gives rise to a distorted trigonal bipyramidal geometry.
sp3d2 hybridisation This involves the intermixing of one s, three p, and two d orbitals. The sp3d2 hybridised f central atom of a molecule is linked directly to six other atoms, the shape of the molecule is regular octahedral.
⇒ \({ }_{16} s-1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}^6, 3 \mathrm{~s}^2, 3 \mathrm{p}_x^2, 3 \mathrm{p}_y^1, 3 \mathrm{p}_z^1\)
- Let us consider the formation of sulfur hexafluoride (SF6). The central atom in the ground state has two unpaired electrons in the 3p subshell. In the excited slate, the electrons from the 3s and the 3p subshell are promoted to the 3d subshell.
- Thus one s, three p, and two d orbitals intermix to form six sp3d2– hybridised orbitals. All the hybrid orbitals participate in bond formation with six fluorine atoms. Hence the shape of the SF6 molecule is regular octahedral.
sp3d3 hybridisation This involves the hybridisation of one s, three p, and three d orbitals to form seven hybrid orbitals. When sp3d3 -hybridised central atom of a molecule is bounded with seven other atoms, the geometry of the molecule is pentagonal bipyramidal.
- For example, in the IF7 the central atom has seven valence electrons but only one unpaired electron. To make seven covalent bonds, iodine needs seven unpaired electrons. Therefore, three electrons are promoted to d orbitals. Then one s, three p, and three d orbitals hybridise to form seven hybridised orbitals. The IF7 molecule has a pentagonal bipyramidal shape and the bond angles are 90° and 72°.
- We have already mentioned earlier in the chapter that there are many exceptions to the octet rule. While going through the discussion on hybridization, you would have observed that generally, the molecules in which bonds are formed using s-p-d hybrid orbitals are exceptions to the octet rule. This implies that the octet rule is broken when atoms have an extra energy level, which is close in energy to the p level. This means the octet rule is more likely to be violated in elements having vacant d orbitals.
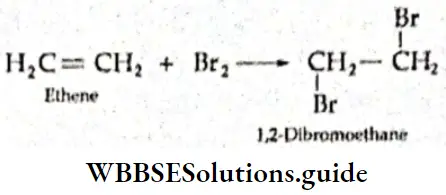
Molecules containing π bond: Pi bonds are formed by the sideways overlapping of atomic orbitals which have electron clouds perpendicular to the internuclear axis. Obviously, hybrid orbitals cannot form π bonds since their electron densities are parallel to the internuclear axis. Only pure p and d orbitals with electron densities perpendicular to the internuclear axis can overlap laterally to form π bonds.
The π bonds are not responsible for the shape of a molecule. That it is determined by the σ bonds. Pi bonds just shorten the bond lengths in a molecule. Let us consider a few examples of the formation of π bonds.
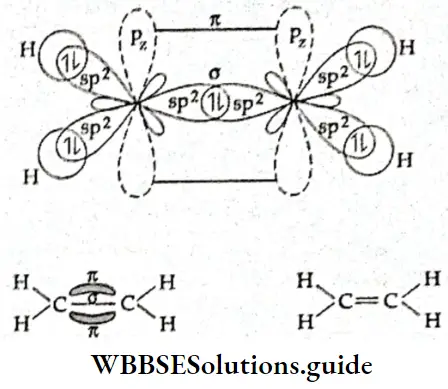
Structure of ethene In the ethene (C2H4) molecule, each carbon atom is sp2 hybridised. One of the 2s electrons gets promoted to the 2p subshell. Then one 2s and two 2p orbitals of the excited carbon atom undergo hybridisation to form three sp2 hybridised orbitals. The remaining p orbital does not participate in hybridisation.
⇒ \({ }_6 \mathrm{C}-1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}_x^1, 2 \mathrm{p}_y^1\)
The three sp2– hybridised orbitals lie In the some pi one and are at an angle of 120° with each other, One sp2 -hybridized orbital of one carbon atom overlaps axially with one sp2– hybridised orbital of the other carbon atom to form a σ bond. The remaining two sp2– hybrld orbitals of each carbon atom overlap with the half-filled Is orbital of H atoms along their respective Internuclear axes to form four σ bonds.
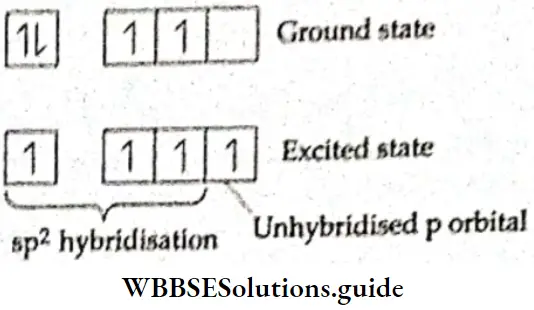
The unhybridised 2p orbital of each carbon atom Is perpendicular to the plane of the hybridised orbitals, or the internuclear axis. These two pure p orbitals overlap sideways to form a rc bond between the two carbon atoms. The C—C bond length in the ethanol molecule is 134 pm as compared to 154 pm in the ethane (C2H2) molecule. In the ethane molecule, like methane, the two carbon atoms are sp3 hybridised. All the bonds in the ethane molecule—one C—C and six C—H— are σ bonds.
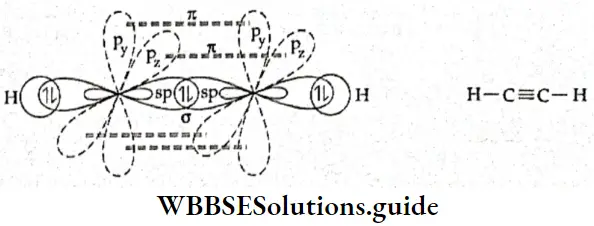
Structure of acetylene (ethyne) In acetylene (C2H2), each carbon atom undergoes sp hybridisation. Each carbon atom has two sp- hybridised orbitals oriented at an angle of 180° to each other and two pure p orbitals which arc perpendicular to each other and to the plane of the sp-hybrid orbitals.
- One sp-hybrid orbital of one carbon atom overlaps axially with one sp-hybrid orbital of the other carbon atom to form a C—C σ bond. The second sp-hybrid orbital of each carbon atom overlaps axially with the half-filled Is orbital of a hydrogen atom to form two C—H σ bonds.
- The unhybridised 2py orbital of the first carbon atom overlaps sideways with the 2py orbital of the second carbon atom, forming a π bond between the two carbon atoms. Similarly, the unhybridised 2pz orbital of one carbon atom overlaps sideways with the 2pz orbital of the other carbon atom to form another n bond between the two carbon atoms.
- All the carbon and hydrogen atoms lie linearly in the same plane. The electron clouds of one n bond lie above and below the internuclear axis, while the electron clouds of the other π bond lie in front and at the back of the axis. The C—C bond length in the ethyne molecule is 120 pm. This shows that pi bonds shorten the bond length in a molecule.