Isotopes
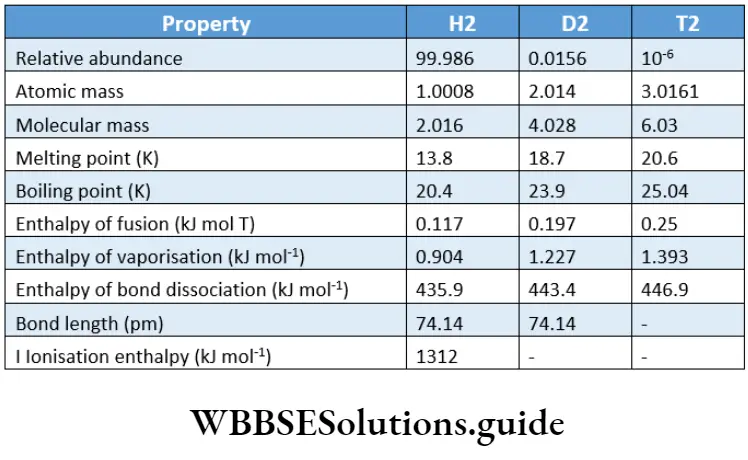
Hydrogen has three isotopes—protium, deuterium and tritium—of mass number 1, 2 and 3 respectively.
The natural abundance of these isotopes is in the ratio 1: 1.56 x 10″2:1 x 10″16. As you will learn later, the properties of the different isotopes of hydrogen are significantly different. Thus, giving them different names is justified.
This is not the case with the isotopes of other elements, e.g., C-12 and C-14, N-14 and N-15.
Proliant (11H) has one proton in the nucleus and one electron in the extranuclear part. Highly stable and nonradioactive, it constitutes approximately 99.986% of hydrogen gas.
Deuterium (21H) has one proton and one neutron in the nucleus, and one electron in the extranuclear part.
Like hydrogen, it is stable and nonradioactive. Represented as D, it is called heavy hydrogen. It is present to the extent of approximately 0.014% of hydrogen gas.
Tritium (31H) has one proton and two neutrons in the nucleus, and one electron in the extranuclear part. In contrast to protium and deuterium, it is unstable and radioactive.
Tritium emits low-energy p-particles (t1/2= 1Z26 years). It occurs in traces in hydrogen gas (approximately one in 1017 parts). Tritium is also represented as.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
The isotopes of an element have the same electronic configuration and so exhibit similar chemical behaviour. However, a chemical reaction may occur at different rates for each isotope.
Due to their different masses, their physical properties are different. Since deuterium is heavier than protium, reactions involving deuterium are slower than those involving protium.
Due to the same reason the boiling point of deuterium is higher than that of protium. The physical and atomic properties of the isotopes of hydrogen.
Dihydrogen Preparation
Dihydrogen (H2), the stable form of elemental hydrogen, is prepared by the following methods.
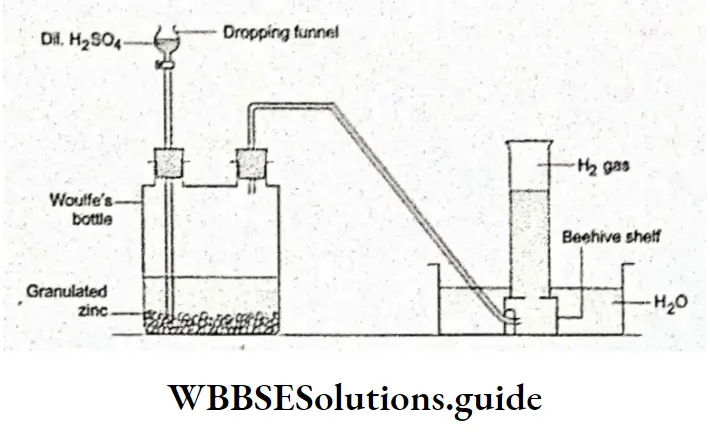
From reactions between metals and acids Metals above hydrogen in the electrochemical series react with dilute acids to produce hydrogen.
Sodium and potassium are much too reactive. However, zinc and magnesium react moderately enough.
⇒ \(\begin{aligned}
& \mathrm{Mg}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{MgSO}_4+\mathrm{H}_2 \\
& \mathrm{Mg}+\underset{\text { d }}{2 \mathrm{HCl}} \longrightarrow \mathrm{MgCl}_2+\mathrm{H}_2 \\
& \mathrm{Zn}+\mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{ZnSO}_4+\mathrm{H}_2 \\
&
\end{aligned}\)
The last of these reactions, i.e., the one involving granulated zinc and dilute H2SO4, is used to produce hydrogen in the laboratory. The use of concentrated H2SO4 in the reaction evolves SO2 gas rather than hydrogen.
⇒ \(\mathrm{Zn}+2 \mathrm{H}_2 \mathrm{SO}_4 \longrightarrow \mathrm{ZnSO}_4+2 \mathrm{H}_2 \mathrm{O}+\mathrm{SO}_2\)
Also, magnesium and manganese react with dilute HN03 to produce hydrogen.
⇒ \(\mathrm{Mg}+2 \mathrm{HNO}_3 \longrightarrow \mathrm{Mg}\left(\mathrm{NO}_3\right)_2+\mathrm{H}_2\)
From reactions between metals and strong alkalis Metals like aluminium, zinc, lead and powdered silicon react with hot solutions of strong alkalis like NaOH and KOH to evolve hydrogen.
⇒ \(\begin{aligned}
2 \mathrm{Al}+2 \mathrm{NaOH}+2 \mathrm{H}_2 \mathrm{O} & \longrightarrow 2 \mathrm{NaAlO}_2+3 \mathrm{H}_2 \\
\mathrm{Zn}+2 \mathrm{NaOH} & \longrightarrow \mathrm{Na}_2 \mathrm{ZnO}_2+\mathrm{H}_2 \\
\mathrm{Si}+2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} & \longrightarrow \mathrm{Na}_2 \mathrm{SiO}_3+2 \mathrm{H}_2
\end{aligned}\)
The reaction of an alkali with aluminium is also used in the laboratory preparation of hydrogen. From reactions between metal hydrides and water Hydrides of metals react with water to produce hydrogen.
⇒ \(\begin{gathered}
\mathrm{CaH}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{H}_2 \\
\mathrm{NaH}+\mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{NaOH}+\mathrm{H}_2
\end{gathered}\)
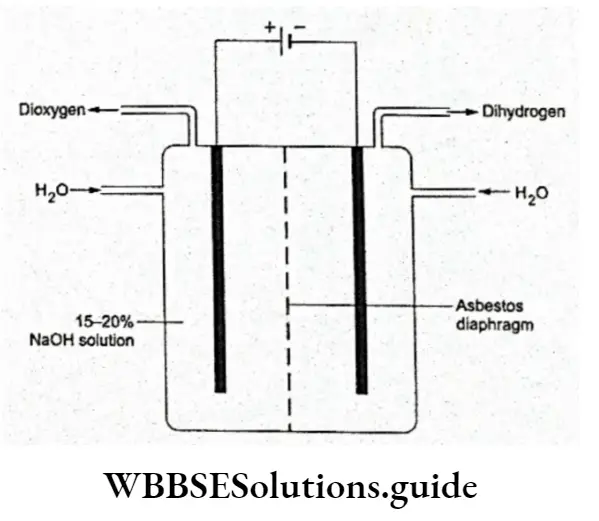
Hydrogen is manufactured on a large scale by the following methods. By the electrolysis of water Pure dihydrogen (99.9% pure) may be prepared by the electrolysis of water in the presence of a small amount of sulphur using an iron cathode and a nickel anode.
⇒ \(2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { electrolysis }}{\longrightarrow} 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})
(acidic or basic)\)
The reaction is represented as
⇒ \(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
⇒ \(\text { At anode } \quad 4 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g})+4 \mathrm{e}^{-}\)
⇒ \(\text { At cathode } \quad 4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_2(\mathrm{~g})\)
The two compartments housing the anode and cathode are separated by an asbestos diaphragm so that the gases evolved in both compartments do not mix. However, this method is generally expensive and is commercially viable only where electricity is cheap.
By passing steam over red-hot coke (Bosch process) When steam is passed over red-hot coke, a mixture of equal volumes of carbon monoxide and dihydrogen is obtained. This mixture is known as water gas.
⇒ \(\mathrm{C}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \longrightarrow \mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g})\)
The carbon monoxide is difficult to remove. Therefore, the gas mixture, mixed with steam, is passed over an F0jO3 catalyst (and a Cr203 promoter) at 673 K, when carbon monoxide is oxidised to carbon dioxide. The carbon dioxide is removed by dissolving the mixture in cold water under pressure.
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) \stackrel{\mathrm{Fe}_2 \mathrm{O}_3 / \mathrm{Cr}_2 \mathrm{O},}{\underset{673 \mathrm{~K}}{\longrightarrow}} \mathrm{CO}_2(\mathrm{~g})+2 \mathrm{H}_2(\mathrm{~g})\)
From natural gas, Natural gas contains hydrogen (20%), methane (70%) and some other hydrocarbons.
When natural gas along with steam is passed over a nickel catalyst at 1270 K, a mixture of carbon monoxide, carbon dioxide and dihydrogen is obtained. The mixture of gases is cooled to 770 K and passed over catalysts (Fe/Cu) to convert carbon monoxide into carbon dioxide.
⇒ \(\begin{gathered}
\mathrm{CH}_4+\mathrm{H}_2 \mathrm{O} \stackrel{\mathrm{Ni}, 1270 \mathrm{~K}}{\longrightarrow} \mathrm{CO}+3 \mathrm{H}_2 \\
\mathrm{CH}_4+2 \mathrm{H}_2 \mathrm{O} \stackrel{\mathrm{Ni}, 1270 \mathrm{~K}}{\longrightarrow} \mathrm{CO}_2+4 \mathrm{H}_2 \\
\mathrm{CO}+\mathrm{H}_2 \mathrm{O} \stackrel{\mathrm{Fe} / \mathrm{Cu}}{\underset{70 \mathrm{~K}}{\longrightarrow}} \mathrm{CO}_2+\mathrm{H}_2
\end{gathered}\)
The carbon dioxide is then removed by passing the mixture of gases through a potassium carbonate solution.
⇒ \(\mathrm{CO}_2(\mathrm{~g})+\mathrm{K}_2 \mathrm{CO}_3(\mathrm{l})+\mathrm{H}_2 \mathrm{O} \longrightarrow 2 \mathrm{KHCO}_3\)
Instead of using natural gas, one can use methane obtained by the cracking of hydrocarbons to produce dihydrogen. The mixture of hydrogen and carbon monoxide obtained by steam reforming of natural gas is called ‘syngas’.
To produce hydrogen, syngas are further processed using a water gas shift reactor to convert carbon monoxide to carbon dioxide and increase the amount of hydrogen, which is eventually separated. Partial oxidation of coal is also one of the clean methods of producing syngas.
This process is known as coal gasification. Rather than burning coal directly, gasification breaks down coal, which is exposed to steam and controlled amounts of oxygen, into a mixture that mainly contains carbon monoxide and hydrogen.
The environmental benefit from the process is that the syngas obtained is virtually free of oxides of nitrogen. Nowadays attempts are being made to build gasifiers which are capable of processing municipal waste to produce syngas.
As a by-product in the manufacture of sodium hydroxide Sodium hydroxide is manufactured by the electrolysis of an aqueous solution of sodium chloride. Hydrogen is obtained as a by-product in this process.
Dihydrogen Physical Properties
Dihydrogen is a colourless, odourless and tasteless gas. It is the lightest gas (density 0.0899 g cm-3) known. Highly inflammable, it should be handled with care.
It is practically insoluble in water and neutral towards litmus. An interesting property of hydrogen is its adsorption or occlusion by certain metals like platinum or palladium. Adsorption is the process of soaking up only on the surface.
(This should not be confused with absorption, in which soaking takes place through the entire mass.)
It is found that one volume of platinum black adsorbs 100 volumes of hydrogen at 925 K, while palladium adsorbs about 500-800 volumes of hydrogen at this temperature.
This property is made use of in the purification of hydrogen since only pure hydrogen is adsorbed by these metals. The adsorbed hydrogen is released when the metals are heated in a vacuum.
Dihydrogen Chemical Properties
In spite of its high bond energy (435.9 kJ mol-1), dihydrogen reacts with almost all elements under appropriate conditions, except noble gases. Some reactions of dihydrogen are given below.
1. Dihydrogen is quite stable and can be broken up into its constituent atoms only when heated to above 2000 K.
⇒ \(\mathrm{H}_2 \stackrel{2000 \mathrm{~K}}{\longrightarrow} 2 \mathrm{H}\)
Due to the high H—H bond enthalpy, dihydrogen is almost inert at room temperature. Therefore, the use of heterogenous catalysis and high temperatures in chemical reactions involving hydrogen can be attributed to the strength of that H—H bond.
2. Dihydrogen is combustible and inflammable. In fact, it bums in the air to form water, and a large amount of heat is liberated.
⇒ \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) ; \quad \Delta H=-485 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
This reaction is utilised in the oxy-hydrogen flame.
3. Reactions with metals Dihydrogen combines with alkali metals (Na, K, Li, etc.) and alkaline earth metals, e.g., Ca, to form hydrides.
⇒ \(2 \mathrm{Na}+\mathrm{H}_2 \stackrel{573 \mathrm{~K}}{\longrightarrow} 2 \mathrm{NaH}\)
⇒ \(\mathrm{Ca}+\mathrm{H}_2 \stackrel{573 \mathrm{~K}}{\longrightarrow} \mathrm{CaH}_2\)
Metal hydrides are extremely reactive and have many applications in organic reactions. Hydrides like CaH2 and LiAlH4 are used for drying solvents, and LiAlH4 is also used to reduce groups like —CHO (aldehydes), C=C, etc. With transition metals like platinum, nickel and cobalt, hydrogen forms what are known as interstitial hydrides.
That is, they occupy the interstitial spaces in the crystal lattices of the metals. The hydrides have been further described later in the chapter.
4. Reactions with nonmetals Under appropriate conditions, dihydrogen reacts with most nonmetals like dioxygen, halogens, dinitrogen, sulphur and carbon. Hydrogen reacts with halogens.
The reaction with fluorine is violent and that with chlorine is explosive in sunlight.
The reaction with bromine occurs at a high temperature. A direct combination of hydrogen with chlorine is used to prepare hydrogen chloride.
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{F}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{HF}(\mathrm{g})\)
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \stackrel{\text { sunlight }}{\longrightarrow} 2 \mathrm{HCl}(\mathrm{g})\)
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Br}_2(\mathrm{~g}) \stackrel{670 \mathrm{~K}}{\longrightarrow} 2 \mathrm{HBr}(\mathrm{g})\)
The reaction of hydrogen with nitrogen produces ammonia. This is used in the manufacture of ammonia by the Haber process.
⇒ \(3 \mathrm{H}_2(\mathrm{~g})+\mathrm{N}_2(\mathrm{~g}) \stackrel{\mathrm{Fe} / \mathrm{Mo}, 200 \mathrm{~atm}}{\underset{673 \mathrm{~K}}{\rightleftharpoons}} 2 \mathrm{NH}_3(\mathrm{~g})\)
Hydrogen reacts with sulphur and carbon at high temperatures to yield hydrogen sulphide, methane and ethyne. The reactions also form the basis of the manufacture of these compounds.
⇒ \(\mathrm{H}_2(\mathrm{~g})+\mathrm{S}(\mathrm{s}) \stackrel{710 \mathrm{~K}}{\longrightarrow} \mathrm{H}_2 \mathrm{~S}(\mathrm{~g})\)
⇒ \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{C}(\mathrm{s}) \stackrel{1275 \mathrm{~K}}{\longrightarrow} \mathrm{CH}_4(\mathrm{~g})\)
⇒ \(\mathrm{H}_2+2 \mathrm{C}(\mathrm{s}) \stackrel{\text { electric art }}{\longrightarrow} \mathrm{C}_2 \mathrm{H}_2(\mathrm{~g})\)
5. Reaction with metallic oxides Dihydrogen reduces the metallic oxides of some less electropositive metals (less active than iron) to the corresponding metals at high temperatures
⇒ \(\mathrm{H}_2+\mathrm{ZnO} \stackrel{\text { heat }}{\longrightarrow} \mathrm{Zn}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{PbO}+\mathrm{H}_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{Pb}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{CuO}+\mathrm{H}_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{Cu}+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{Ag}_2 \mathrm{O}+\mathrm{H}_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{Ag}+\mathrm{H}_2 \mathrm{O}\)
6. Reaction with carbon monoxide Dihydrogen reacts with carbon monoxide in the presence of heated metal oxides at 573 K and 200 atm to form methyl alcohol.
⇒ \(\mathrm{CO}+2 \mathrm{H}_2 \underset{573 \mathrm{~K}, 200 \mathrm{~atm}}{\stackrel{\mathrm{ZnO}+\mathrm{Cr}_2 \mathrm{O}_3}{\longrightarrow}} \mathrm{CH}_3 \mathrm{OH}\)
The above reaction is used in the manufacture of methyl alcohol.
Dihydrogen Uses
Dihydrogen has the following uses.
Being the lightest gas, it is used for filling balloons. However, since it is inflammable, hydrogen is mixed with another light gas—helium—for this purpose.
It helps extract iron, tungsten and molybdenum from their oxide ores.
The catalytic hydrogenation of organic compounds is an industrial process for the manufacture of aldehydes alcohols and other products. For example, the hydroformylation (reaction in the presence of H2 and CO is known as hydroformylation) of olefins gives aldehydes, which can further be reduced to give alcohols.
⇒ \(\underset{\text { olefin }}{\mathrm{RCH}}=\mathrm{CH}_2+\mathrm{H}_2+\mathrm{CO} \stackrel{\text { catalyst }}{\longrightarrow} \underset{\text { RCH }}{\mathrm{RCH}_2 \mathrm{CH}_2 \mathrm{CHO}}\)
⇒ \(\mathrm{RCH}_2 \mathrm{CH}_2 \mathrm{CHO}+\mathrm{H}_2 \stackrel{\text { catalyst }}{\longrightarrow} \mathrm{RCH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\)
Hydrogen is also employed for the hydrogenation of vegetable oils (like cottonseed, coconut and groundnut oilskin the presence of a nickel catalyst to convert them into solid fats like vanaspati, which are used as cooking mediums. They are produced on a large scale in our country by the hydrogenation process.
Vegetable oils ⇒ \(\stackrel{\mathrm{H}_2 / \mathrm{Ni}, 473 \mathrm{~K}}{\longrightarrow}\)solid fats (hydrogenated edible oils)
The vegetable oils are unsaturated (since they contain double bonds in their molecules). When dihydrogen is passed through the oils at 473 K in the presence of a catalyst, solid fats are produced.
As we already know, hydrogen is used in the manufacture of ammonia (Haber’s process), methyl alcohol, hydrogen fluoride, hydrogen chloride, hydrogen bromide, acetylene and metal hydrides.
The oxy-hydrogen torch is used for welding and cutting iron and other metals. The temperature of the flame is approximately 2270-2710 K. The atomic-hydrogen torch gives a temperature of 3770-4270 K and is used for welding.
It is used as a rocket fuel, in combination with oxygen and fluorine.

