Concepts In Organic Reaction Mechanism
The organic reactions involve the breaking of covalent bonds in the reacting molecules and formation of new bonds to give product molecules. “A study of the sequential account of each step, describing details of formation energetics during bond cleavage and bond electron movement and the rate of transformation of reactant into products (kinetics) is referred to as reaction mechanism”.

Read And Learn More: NEET General Organic Chemistry Notes, Question And Answers
The organic molecule which reacts with attacking reagent is called substrate. In multistep organic reactions, the substrate react with reagent and leads to the formation of one or more reaction intermediates. The general reaction path involving the formation of one reaction intermediate is depicted as follows.
Fission Of Covalent Bond
The fission of covalent bond can take place in two ways depending on the nature of covalent bond, nature of the attacking reagent, and conditions of the reaction.
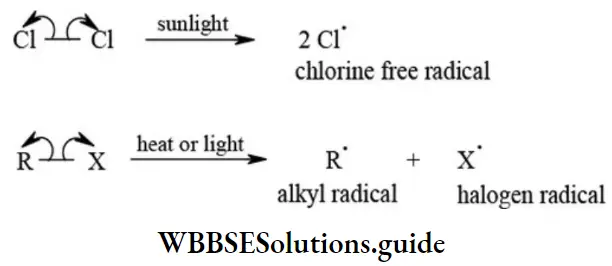
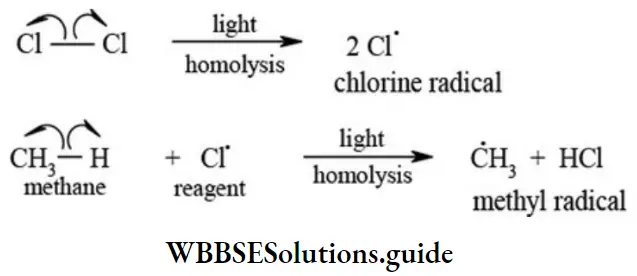
Homolytic Cleavage (Homolytic fission): In this type of fission each fragment formed as a result of cleavage of covalent bond gets on electron from the shared bond pair electrons”. This cleavage results in the formation of specie(s) with unshared electron called free radicals. The homolytic fission of a σ bond is shown as follows.
The free radicals contain unpaired electron (with odd number of electrons), electrically neutral and paramagnetic.
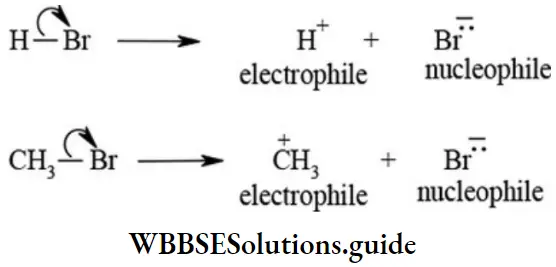
Heterolytic Cleavage (Heterolytic Fission): In this type of cleavage both the bond pair electrons of the covalent bond are taken away by electron-withdrawing fragment which results in the formation of electron-deficient and electron-rich fragments. The electron-deficient fragment is called electrophile while electron rich fragment is known as a nucleophile. The heterolytic fission is shown below
Both electrophiles and nucleophile contain even number of electrons, influenced by strong electrical fields as they possess positive or negative charge and they are diamagnetic.
Substrate + Reagent ⇔ [reaction intermediate] → Product
Reaction Intermediates
A highly reactive, short-lived, and energetic intermediate formed in multistep organic reactions (reactions involving more than one step) by the action of reagent on substrate and readily transformed into the product(s) is called a reaction intermediate.
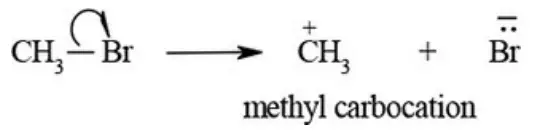
1. Carbocations: A reaction intermediate formed by heterolytic fission of a covalent bond that contains one positively charged carbon with three bond pair electrons (sextet of electrons) is called carbocation. The heterolytic fission of bromomethane yields methyl carbocation and bromide ion as shown below
The carbocations are classified into different groups depending upon the nature of carbon-bearing the positive charge. Alkyl carbocations may be primary (1°), secondary (2°), or tertiary (3°) carbocations.
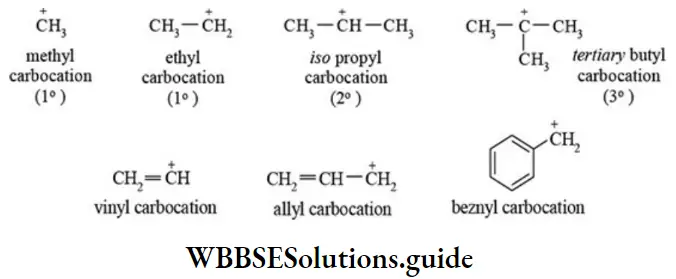
Structure of carbocation: The carbocations are electron deficient and contain six electrons (three bond pair electrons). In strong electrical field, carbocations move toward cathode. The carbocations have a trigonal planar structure and the positively charged carbon in sp2 hybridized.
Stability of carbocations: The carbocations are electron deficient and stabilised by electron releasing group. The participation of empty p-orbital in lateral overlap with completely filled orbital is the major contributing factor for stability to stability in which positive charge is delocalised.
Alkyl group directly bonded to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects. The dispersal of the positive charge due to hyperconjugation stabilise the carbocation. The observed order of carbocation stability is
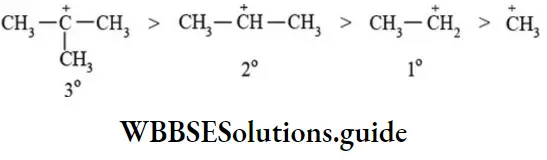
The greater the number of a-hydrogen atoms higher is the stability of carbocations. The reaction intermediates are highly reactive, usually order of reactivity of such intermediates is reverse that of its stability. Therefore order of reactivity of carbocations follows the sequence:
∴ CH3 >1°>20>3°
Hybridised state of carbon: Higher the s-character, lesser the stability. The order of stability is
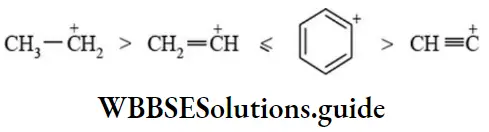
Carbocations Resonance: The greater the delocalization of positive charge of carbocations, the higher is the stability. The stability of benzyl carbocation is comparable with allyl carbocation as resonance energies is nearly the same
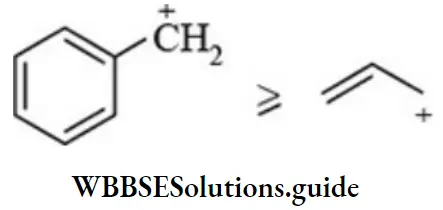
Carbocations undergo rearrangement forming more stable carbocations.
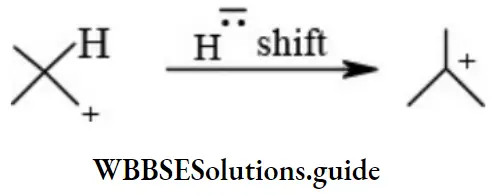
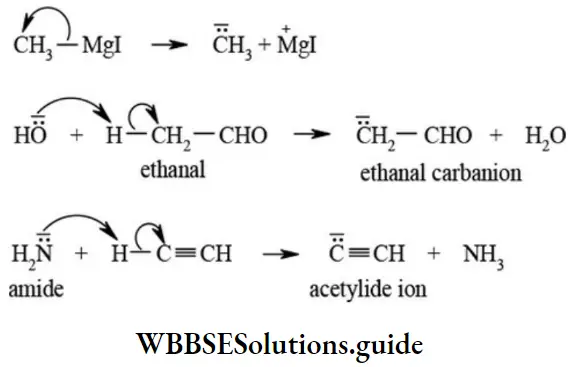
2. Carbanions: A reaction intermediate formed by heterolytic fission of a covalent bond which results in negatively charged carbon with eight electrons in its valence shell is called carbanion.
The heterolytic cleavage of a covalent bond as indicated in the following reactions gives carbanions. The carbanions are classified as primary (1°), secondary (2°), and tertiary (3°) depending upon the nature of carbon bearing negative charge.
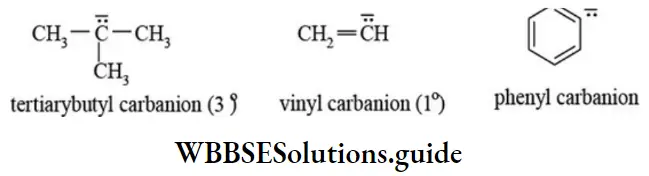
Structure of carbanion: The carbanions are electron-rich with complete octet configuration. In strong electrical field, they move toward anode. The shape of alkyl carbanion is usually pyramidal like ammonia. The carbon atom carrying the negative charge is sp3 hybridized. In contrast, carbanions which are stabilized by resonance are planar and the carbon atom carrying a negative charge is sp2 hybridized. The vinyl carbanion, phenyl carbanion, and cyclopentadiene carbanion are sp2 hybridised as acetylide carbanion is sp hybridised.
Stability of carbanions: Alkyl group bonded to negatively charged carbon increases the intensity of negative charge due to +1 effect and destabilise the carbanion. The order of stability of alkyl carbanions is:
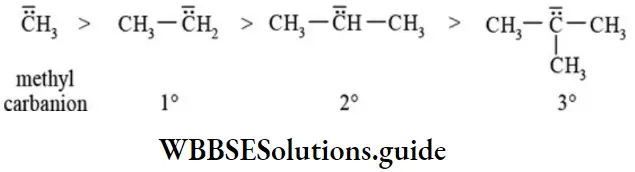
The order of reactivity of carbanions is reversed that of its stability. Therefore order of reactivity of carbanions follows the sequence:
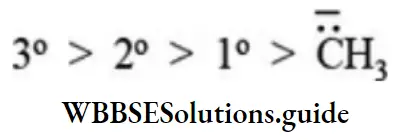
Hybridised state of carbon: Higher the s character greater the stability of carbanion. Order of stability is
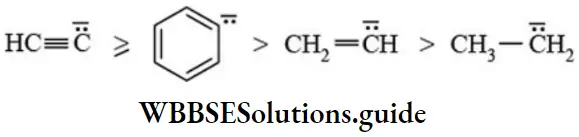
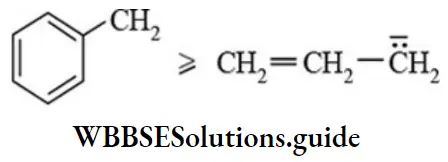
Carbanions Resonance: Greater the delocalisation of negative charge by resonance, higher the stability The stability of benzyl carbanion is comparable with allyl carbanions.
3. Free radicals: A reaction intermediate formed by the homolytic cleavage of a covalent bond that contains an unpaired electron is called free radical. The homolytic cleavage is favoured for nonpolar covalent bonds. Such cleavage for the bond is initiated by the action of heat, light, or a reagent. For example,
Depending upon nature of carbon atom carrying the unpaired electron, free radicals are also classified as primary (1°), secondary (2°), and tertiary (3°) free radicals.
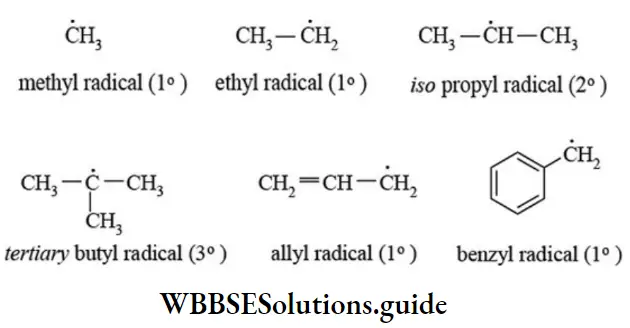
Structure of free radicals: The free radicals are electron deficient since they contain seven electrons on carbon atom. They are electrically neutral and paramagnetic. The structure of alkyl radicals is not known with certainty. For alkyl radicals two possible structures have been proposed. The first is a planar sp2 hybridised radical similar to a carbocation. The second one is a pyramidal sp3 hybridized radical similar to a carbanion.
- Resonance stabilised free radicals such as allyl radicals and benzyl radicals are planar sp2 hybridised.
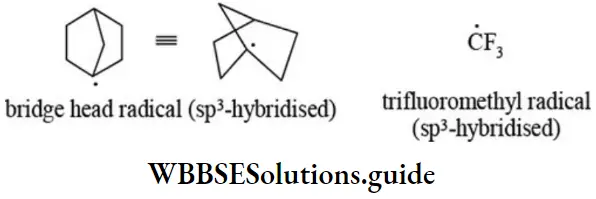
- The bridgehead free radicals are pyramidal (sp3-hybridised) because they cannot assume planar geometry due to angle strain. Further, the free radicals in which carbon is bonded to highly electronegative atoms are pyramidal.
Stability of free radicals: The relative stability of alkyl free radicals is explained on the basis of hyperconjugation and inductive effects. Greater the number of alkyl groups attached to the carbon atom carrying unpaired electron, higher the delocalisation and hence more stable is the alkyl radical.
The order of stability of a few alkyl radicals is given below

Like carbocations and carbanions, free radicals are highly reactive and short-lived intermediates because of the strong tendency of the carbon atom carrying the unpaired electron to acquire one more electron from an atom or a group to complete its octet. The reactivity of alkyl radical is reverse the order of stability CH3> 1 > 2 > 3 resonance. The stability of allyl and benzyl radicals is comparable.
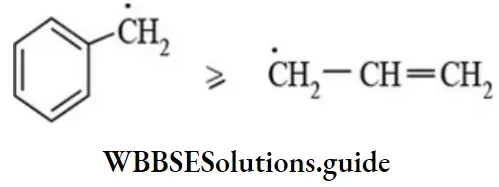
The allyl or benzyl radicals are more stable than alkyl radicals.
Carbenes: The carbenes are neutral and highly reactive species generally obtained by successive elimination of an electrophile and a nucleophile from the same carbon atom (a-elimination). The carbon atom of carbene has six electrons in valence shell, out of which two constitute unshared electrons and two bond pair electrons. So they are divalent carbon species containing two unshared electrons and electrically neutral. Examples of different types of carbenes are given below.
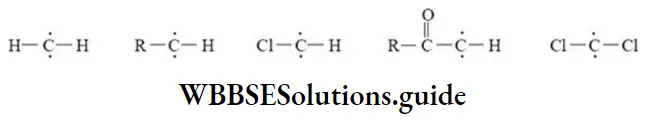
Electrophiles And Nucleophiles
The organic reactions proceed by the attack of highly reactive reagents on the substrate molecule. These reagents are called attacking reagents which may be electron deficient or electron rich. They are classified into two groups.
Electrophiles: The electron-deficient molecules or positively charged ions which are capable of accepting an electron pair from substrate molecule are called electrophiles. These species act as Lewis acids and attack the electron-rich centre of the organic molecules.
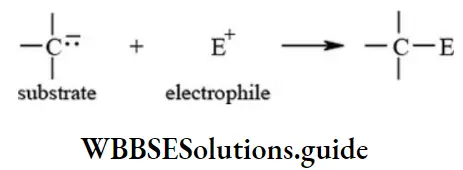
Following are common examples
Positive electrophiles: H+, Cl+, Br+, N+O2, N+ = O, (R+) carbocations, etc.
Neutral electrophiles: SO3, BF3, AlCl3, etc.
It may be noted that all the positively charged species do not act as electrophiles. The positively charged species which can accept an electron pair can act as electrophiles. The positively charged ions such as H3O+, NH+4, Na+, Ca2+ do not act as electrophile as they cannot accept electron pair, since all the ions have an octet configuration.
Nucleophiles: The molecules or negatively charge ions which are capable of donating an electron pair to the electron-deficient centre of the substrate are called nucleophiles. These species act as Lewis bases and attack on the electron-deficient center of organic molecules.

The common examples of nucleophiles are given below:
Negative nucleophiles:
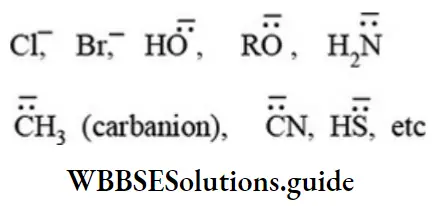
Neutral nucleophiles:

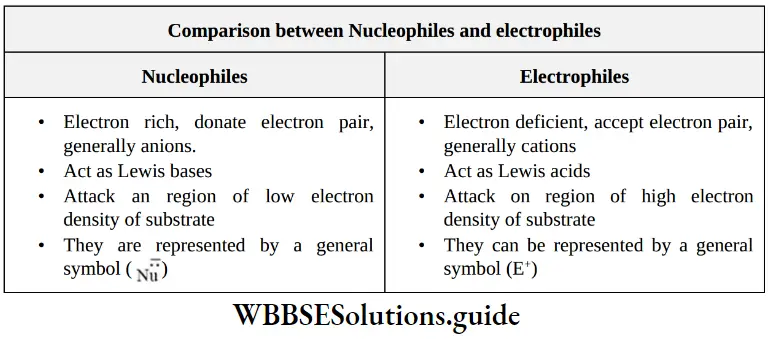
Comparison between Nucleophiles and electrophiles
Electron Displacement Effects in Covalent Bonds: The electron pair displacement in organic molecules takes place under the influence of a hetero atom/group or by the attacking reagent. The displacement or shift of electron pair in the organic molecule under the influence of substituent makes the molecule permanently polar.
1. Inductive effect (I effect): The inductive effect is defined as “the permanent displacement of sigma (σ) bond pair of electrons towards more electronegative atom or group and as a result molecule becomes permanently polar”. Larger the displacement of σ bond pair electrons greater the polarity. Consider the carbon chain in which terminal carbon is bonded to a chlorine atom. Since chlorine is more electronegative bond pair of electrons are displaced towards chlorine.

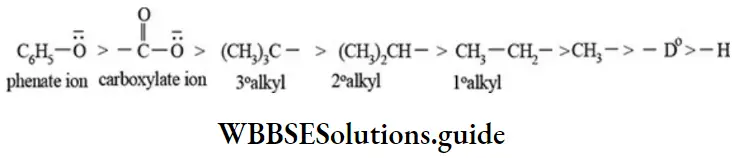
Positive inductive effect (+I effect): In this effect the substituent (Y) releases electron pair away from itself. In other wordso bond pair of electrons are displaced away from the substituent.
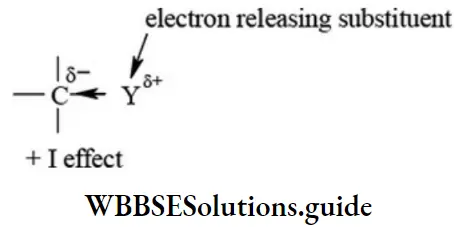
The order of electron releasing or releasing ability of substituent is given below.
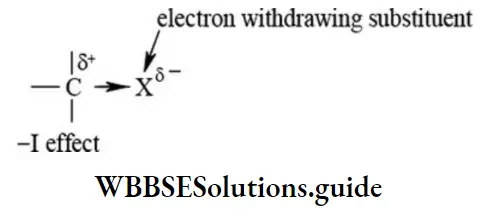
Negative inductive effect (-I effect): In this effect, the sigma bond pair of electrons are displaced towards the electron-withdrawing substituent (X).
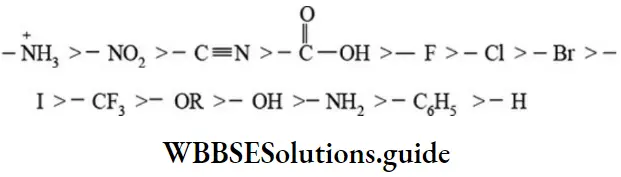
The order of electron-withdrawing ability (intensity of -I effect) of a few substituents is given below.
The inductive effect is a permanent effect operating in the ground state of the organic molecules. It explains facts such as the effect on dipole moment, reactivity of alkyl halides, acidity of carboxylic acids, and basicity of amines and alcohols.
2. Resonance effect (R−effect) or Mesomeric effect: The presence of alternate single and double bond or heteroatom-containing one or more lone pair electrons linked to multiple bonded atom (with single bond in an open chain or cyclic system) is called a conjugate system. Examples are 1,3-butadiene, aniline, phenol, nitrobenzene, etc.
In such systems, π or lone pair electrons are delocalised and the molecule develops polarity. “The permanent polarity produced in the molecule by the shift of pi(π) or lone pair electrons in the conjugate system creating electron deficient and electron-rich centres called resonance effect (R-effect). The effect is transmitted through the entire conjugate system. Depending upon the direction of shift of electron pair in the conjugate system, the R-effect is classified into two types.
Positive resonance effect (+R effect / +M effect): In this effect, of electron pair (π or lone pair) moves away from the substituent attached to conjugate system. The +R effect in aniline as shown below.
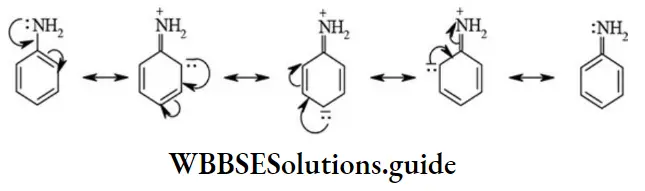
The substituents (atom/group) that exert only +R effect are as follows: -OH, -OR, -OCOR, -NH2, -NHR, -NR2, -NHCOR, -X (halogen ; X = -Cl, Br, I, etc.
The substituent which exerts +R effect are called electron-releasing groups. The resonance effect in which resonance structure violates the octet rule should not be considered. For example, structure (2) cannot be considered as resonance structure since it violates the octet rule because oxygen has 10 electrons in the valence shell.

Negative resonance effect (-R effect / -M effect): In this effect, the shift of π or lone pair electrons is towards the substituent attached to the conjugate system. The electron displacement depicted in nitrobenzene represents -R effect.
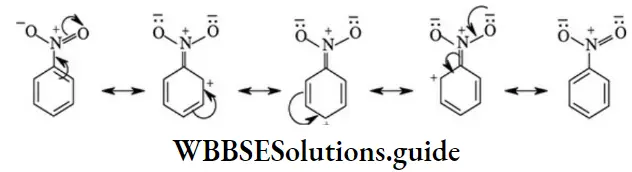
The substituents, which exert only -R effect are given below.
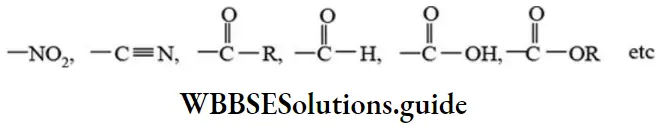
The substituent which exert-R effect is called electron withdrawn group. Resonance effect provides explanation to least reactivity of haloalkenes and aryl halides towards nucleophilic substitution reactions, acidic nature of phenols and carboxylic acids, mechanism of electrophilic substitution reactions of benzene.
Substitutes like 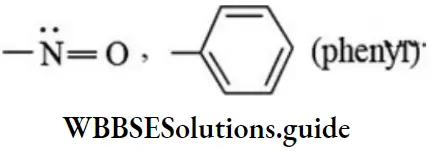 etc. exert both +R and -R effects.
etc. exert both +R and -R effects.
3. Hyperconjugation: The phenomenon of hyperconjugation is also known as the Baker-Nathan effect as it was proposed by Baker and Nathan. This effect is an extension of resonance in which C-H sigma (σ) bond pair electrons are involved in delocalization. The electron release of alkyl group bonded to the unsaturated system in which delocalization of electrons takes place through overlap between C-H sigma (σ) orbital and Pi(π) bond orbital or vacant p-orbital is known as hyperconjugation.
The hyperconjugation is a permanent effect and a stabilizing interaction. The delocalization of electrons by hyperconjugation in propene molecule is depicted as in the figure.
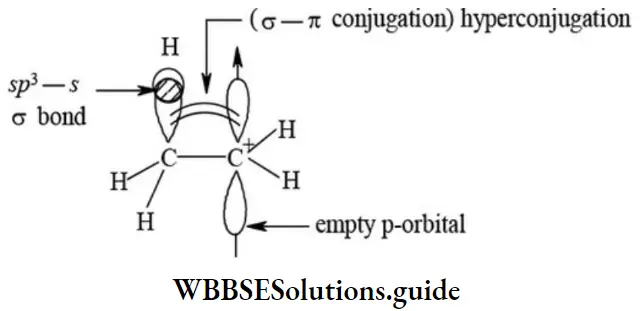
Propene molecule may be regarded as the resonance hybrid of the following hyperconjugative structures.
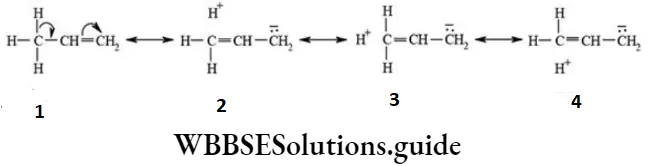
- Since there is no bond between carbon and hydrogen atoms in these structures (2 -4), hyperconjugation is also called no bond resonance. It may be noted that although a free proton (H+) has been shown in the above structures, it is still bound firmly to the π- cloud and hence is not free to move.
- The hyperconjugation effect is much weaker compared to the resonance effect, yet it is quite useful is explaining relative stability, and physical and chemical properties of organic molecules. Larger the number of hyperconjugative structures higher the delocalization of electron pairs and greater the stability of alkene. Number of hyperconjugative structures is equal to number of α-hydrogen atoms plus one. The relative stability of a few alkenes is given.
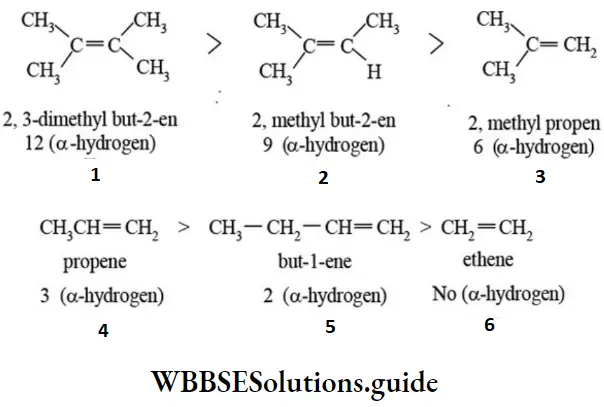
This order of stability is because of greater number of hyperconjugative contributing structures causing larger delocalization of n-electrons solve and hence accounts for higher stability of alkene. To understand the hyperconjugation effect in carbocations, let us take an example of ethyl carbocation (CH2-CH+2), in which the positively charged carbon atom has an empty p orbital. One of the C-Hασ bond orbital of methyl group align in the plane of empty p orbital and this bond pair electrons delocalise into the empty p-orbital as shown in figure.
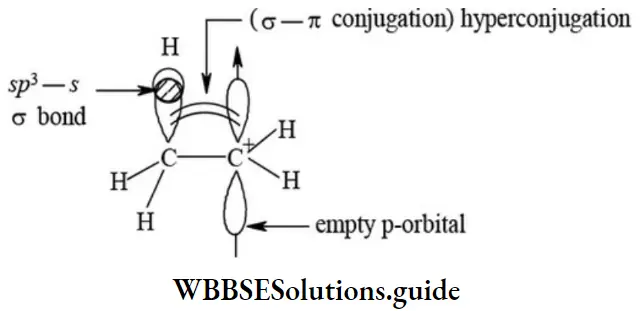
The overlap of completely filled C-H σ bond orbital with empty p-orbital of carbocation causes dispersion of positive charge and stabilize the carbocation. The ethyl carbocation is resonance hybrid of following contributing structures.
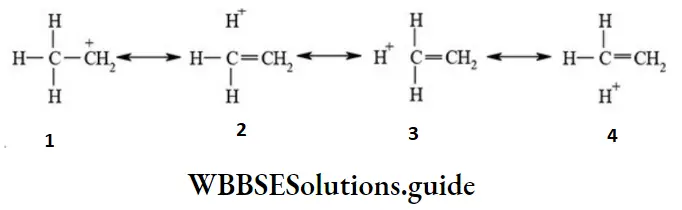
In general, larger the number of a-hydrogen atoms of alkyl groups attached to a positively charged carbon atom greater the stability of carbocations.
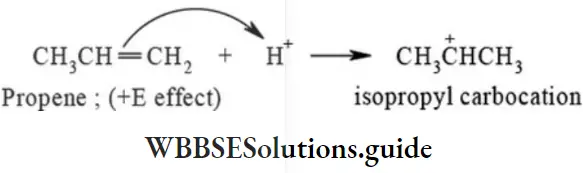
4. Electromeric effect (E effect): The electromeric effect is a temporary effect shown by organic compounds containing multiple bonds. The complete transfer or shift of n electron pair of a multiple bond to one of the bonded atoms during the attack of positively or negatively charged reagent is called electromeric effect or E effect.
This temporary effect takes place only in the presence of an attacking reagent. As soon as the reagent is removed, the molecule reverts back to original position. Depending upon the direction of displacement, the E effect is also of two types.
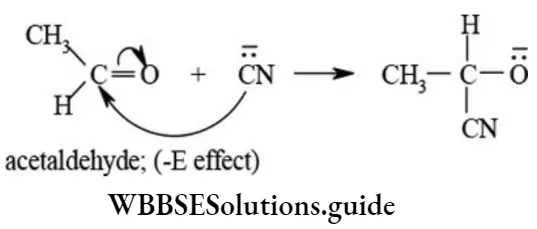
-E effect: An attacking reagent is said to have -E effect when the direction of π electron pair transfer of multiple bond is away from the attacking reagent. The -E effect operates during nucleophilic addition reaction of aldehydes and ketones.
+E effect: An attacking reagent is said to have +E effect when the direction of π electron pair transfer of a multiple bond is towards the attacking reagent. The +E effect is observed during electrophilic addition reaction of alkenes and alkynes