General Organic Chemistry Introduction
Chemistry
‘The branch of chemistry dealing with these compounds, which are widely distributed in nature and play an important role in our daily lives’, is called organic chemistry.
In the earlier period of development of chemistry, compounds were classified into two types:
- Organic compounds derived from ‘living matter’ (plants and animals).
- Inorganic compounds prepared from ‘non-living matter’ (mineral sources).
Read And Learn More: NEET General Organic Chemistry Notes, Question And Answers
Berzelius, a Swedish chemist proposed the mistaken notion that a ‘vital force’ present in living matter was essential for the synthesis of organic compounds. However, the synthesis of urea an organic compound present in urine, from ammonium cyanate, an inorganic compound by Frederich Wohler in 1828 effectively destroyed the myth of organic compounds being associated with a ‘vital force’.
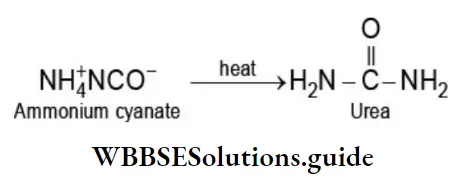
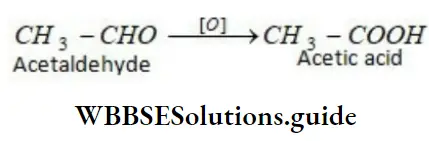
Soon afterwards the pioneering work of Herman Kolbe who synthesized acetic acid and of Berthelot who synthesized methane showed conclusively that organic compounds are essentially the compounds formed by carbon with itself and other elements and that they can be synthesized in a laboratory as easily as inorganic compounds.
NEET organic chemistry introduction notes
The chemistry of hydrocarbons and their derivatives constitutes organic chemistry.
The number of organic compounds available today are more compare to total inorganic compounds of all elements except carbon. This is due to unique catenation property of Carbon.
Catenation: is the property of an element where a large number of its own atoms join together through covalent bonds. Due to which it forms single as well as multiple covalent bonds with other carbon atoms.
It is further supplemented by the fact that it also forms covalent bonds with atoms of other elements like hydrogen, oxygen, nitrogen, Sulphur, phosphorus, and halogens in a variety of ways (i.e., single and multiple bonds). This property gives a scope for the Chemists to synthesize new compounds.

General organic chemistry NEET notes
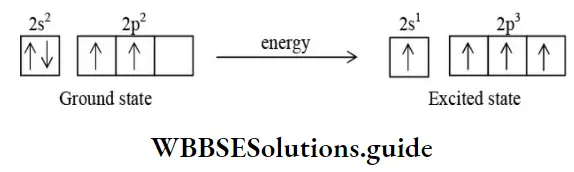
Tetravalency Of Carbon Atom
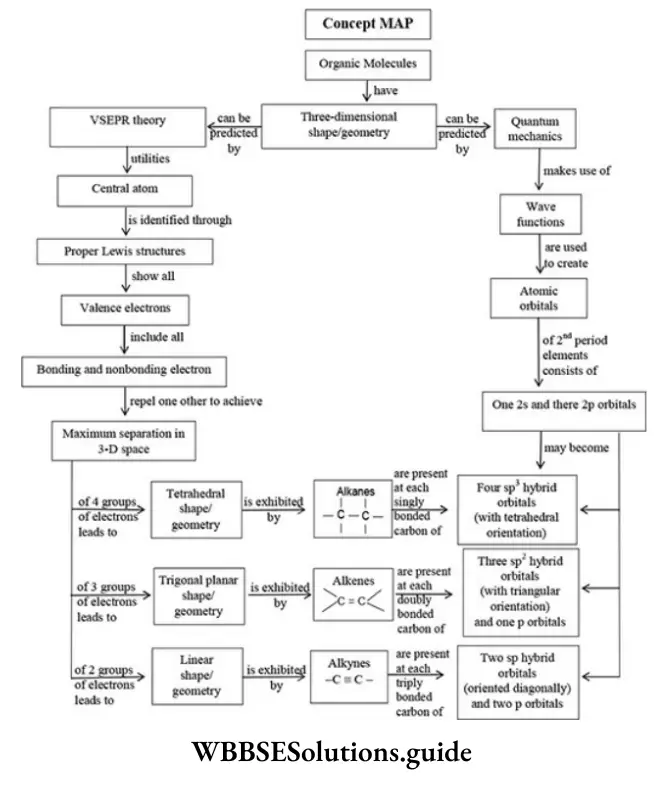
The atomic number of carbon is 6 and it has four electrons in its valence shell. In order to acquire a stable noble gas configuration, it can share its 4 electrons with the electrons of its atom or electrons of other atoms to form four covalent bonds. The bonds can be sigma(σ) or pi(π).
Introduction to general organic chemistry for NEET
The type of bond determines the hybridization of carbon atoms and the geometry of the molecules.
Hybridisation In Carbon Compounds
Hybridization is defined as the intermixing of degenerate orbitals (orbitals at nearly the same energy) to produce an entirely equivalent number of new orbitals of same energy, identical shapes and symmetrically disposed of in planes. The orbitals formed are called hybrid orbitals.
NEET chemistry GOC (General Organic Chemistry) study material
- The orbitals of an isolated atom can undergo hybridization.
- Number of hybrid orbitals generated are equal to number of contributing atomic orbitals.
- The hybrid orbitals orient in space providing definite geometry to molecules or ion.
- Like atomic orbital, a hybrid orbital cannot have more than two electrons of opposite spins.
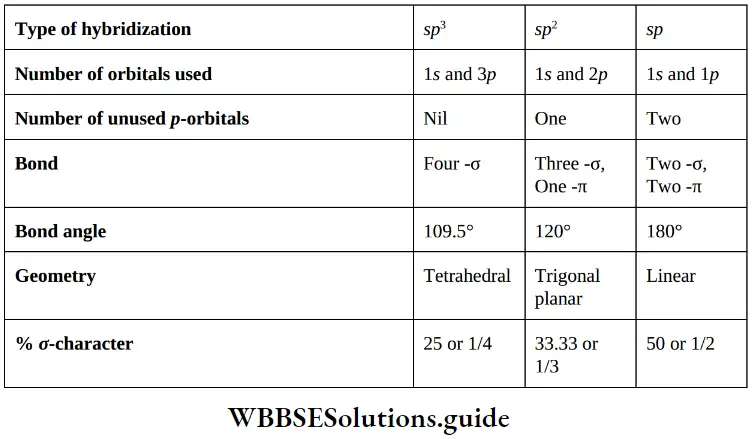
There are three types of hybridization,
- sp3 hybridization (contain saturated organic compounds with only single covalent bonds)
- sp2 hybridization (here organic compounds having carbon atoms linked by double bonds)
- sp hybridization (here organic compounds having carbon atoms linked by a triple bonds).
Basics of organic chemistry for NEET
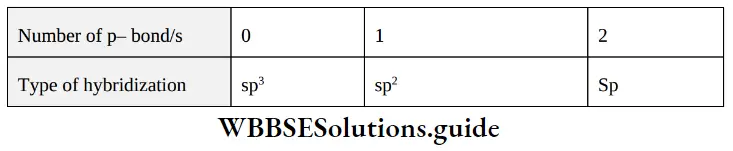
Prediction of hybridization It can be done by two methods,
1. First Method: In this method hybridization can be known by the number of bonds present on that particular atom.
First Method Examples:
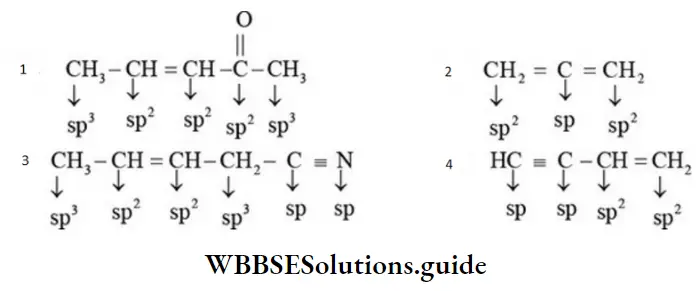
NEET organic chemistry concepts and basics explained
2. Second Method: (Electron pair method)
The hybridized state of an atom of a molecule or an ion or radical can be predicted by calculating number of orbitals or electron pairs involved in hybridization (H) which is evaluated as follows.
H = (number of a bonds formed with adjected atom/s + number of lone pairs of electrons)
∴ ep = bp + lp;
where, ep = electron pair present in hybrid orbitals,
bp = bond pair present in hybrid orbitals
Number of bp = Number of atoms attached to the central atom of the species (do not include π electron pairs).
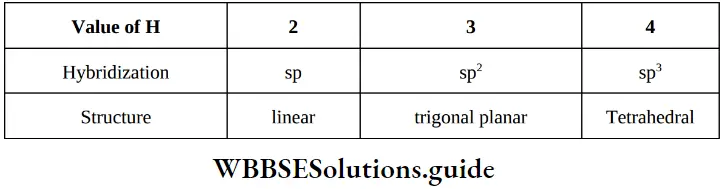
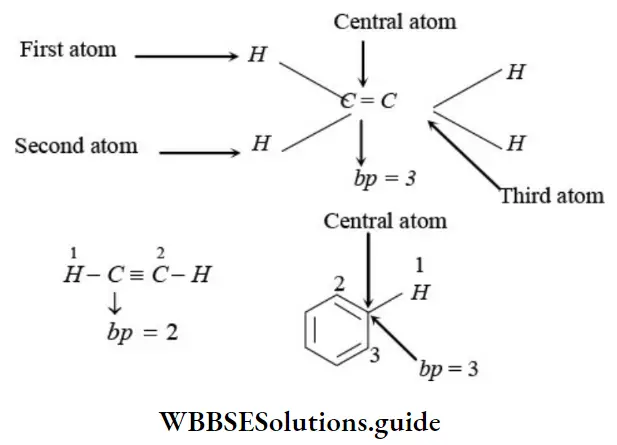
Number of lp’s can be determined as follows,
- If carbon has – bonds or positive charge or odd electron, thanlp on carbon will be zero.
- If carbon has negative charge, then Ip will be equal to one.
Second Method Example:
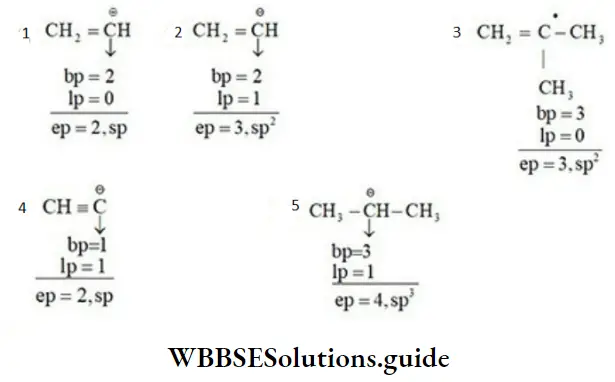
Applications Of Hybridisation
1. Size of the hybrid orbitals: Since – orbitals are closer to the nucleus than – orbitals, it is reasonable to expect that greater the character of an orbital the smaller it is. Thus, the decreasing order of the size of the three hybrid orbitals is opposite to that of the decreasing order of orbital character in the three hybrid orbitals. sp3 > sp2 > sp
2. Electronegativity of different orbitals
- The electronegativity of s-orbital is maximum.
- Electronegativity of hybrid orbital ∝ % s-character in hybrid orbitals
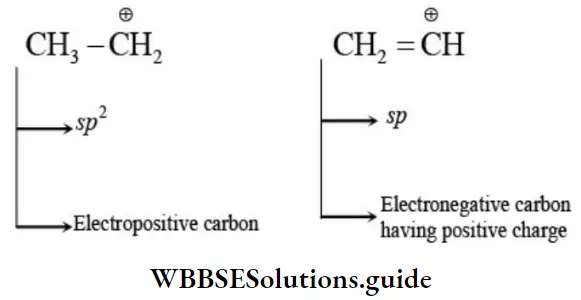
- Thus Sp-hybrid carbon is electronegative and it is electropositive in character and sp3-hybrid carbon can behave as electropositive in character sp2 -hybrid carbon can behave as electropositive (in carbocation) as well as electronegative (in carbanion) in character.
- Electronegativities of different hybrid and nonhybrid orbitals in decreasing order is as follows

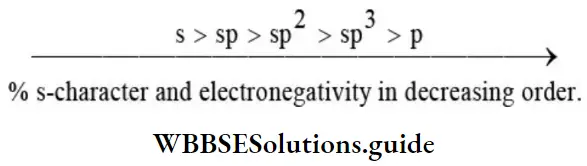
General organic chemistry important topics for NEET
3. ond length variation in hydrocarbons
⇒ \(\% \text { of s orbital character } \propto \frac{1}{\mathrm{C}-\mathrm{C} \text { bond length }} \propto \frac{1}{\mathrm{C}-\mathrm{H} \text { bond length }}\)
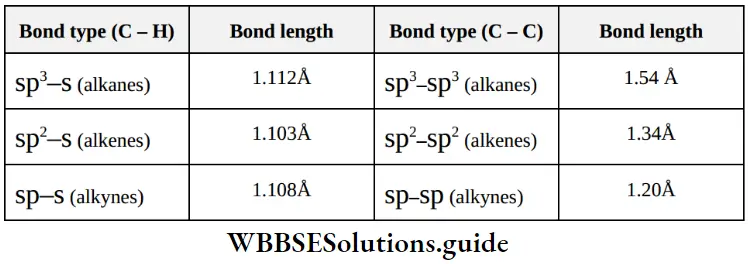
4. Bond strength in hydrocarbons: The shorter is the bond length, the greater is the compression between atomic nuclei, and hence greater is the strength of that bond.
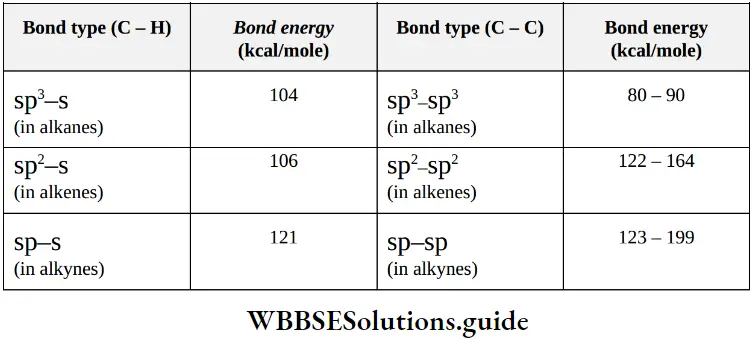
Structural Representation Of Organic Compounds
Structural formula (structure) is the sequence in which different atoms constituting the molecule are bonded to one another. Structures of molecules of organic compounds can be described in various ways.
The most common types of representations are:
1. Lewis structure (or electron dot structure) Here dots are used to represent all of the valence electrons of all the bonded atoms in the molecule:
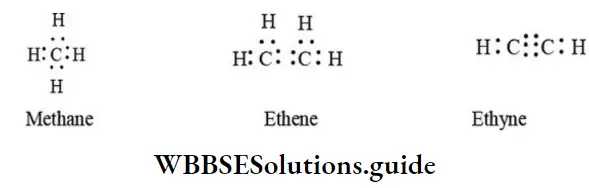
General organic chemistry tricks and shortcuts for NEET
Writing dot structure is tedious and time-consuming. The other representations are more convenient and are, therefore, more often used.
2. Dash structural formula The Lewis structure can be simplified by representing a shared electron pair by a ‘stick’ (dash, -) between the bonded atoms. When there is one dash between two atoms, the atoms are said to be bonded by a single covalent bond.
- A double covalent bond, in which two pairs of electrons are shared, is shown by two dashes between the atoms. A triple bond is represented by three dashes between the atoms. The valence electrons that are not included in covalent bonds are called nonbonding electrons (lone pairs). These are assigned to specific atoms and are represented by dots drawn next to the symbols for these atoms.
- Lone pair of electrons on hetero atoms (for example, oxygen, nitrogen, sulphur, phosphorus, halogens) may or may not be shown. Such structural formulae which focus only on the valence electrons involved in bond formation are called complete structural formulae.
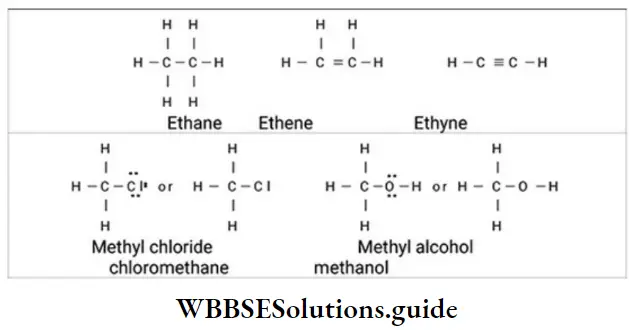
3. Condensed structural formula Complete structural formulae can be shortened by leaving out some or all of the covalent bonds and by denoting the number of identical groups attached to an atom by a subscript. The resulting description of the molecule is called a condensed structural formula. Thus,
CH3CH3 or C2H6 Ethane
H2C = CH2 or C2H4 Ethene
HC ≡ CH or C2H2 Ethyne
CH3CH2Cl or C2H5Cl Ethyl Chloride
CH3CH2CH2OH or CH3(CH2)2OH Methanol
CH3CH2CH2CH2CO2H or CH3(CH2)3CO2H Pentanoic Acid
Condensed structural formulae are easier to write than dash formulae. In condensed formulae, all of the hydrogen atoms that are attached to a particular carbon are usually written immediately after the carbon. In fully condensed formulae, all of the atoms that are attached to the carbon are usually written immediately after that carbon, listing hydrogens first.
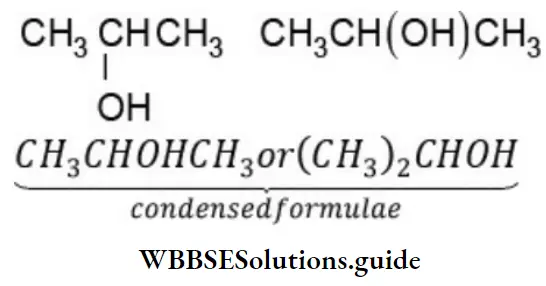
How to study organic chemistry for NEET beginners
For example, the condensed formula for isopropyl alcohol can be written in four different ways:
4. Bond-line structural formula: For further simplification, only lines are used to represent the structures of organic molecules. In this bond-line structural formula of organic molecules, carbon, and hydrogen atoms are not shown. The lines denoting the carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms especially written are the hetero atoms (oxygen, nitrogen etc). They are neither carbon nor hydrogen bonded to carbon.
The termini describe methyl (CH3-) groups (unless denoted otherwise by a functional group). The line junctions indicate carbon atoms bonded to suitable number of hydrogens needed to satisfy the covalency of the carbon atoms: Thus
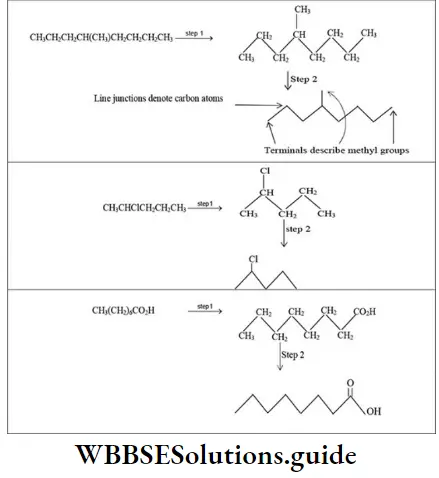
The bond-line representation is the quickest of all to write because it shows only the carbon skeleton.
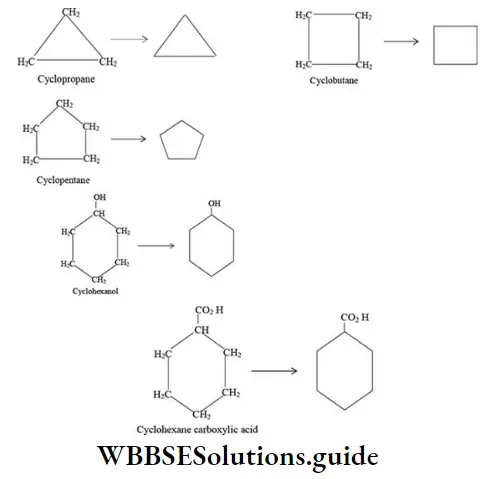
Ring or cyclic compounds: An organic compound in which carbon atoms are not bonded in chain but are bonded in closed structures called rings are known as cyclic compounds. Such a compound containing one or more rings is represented by drawing the suitable ring (polygon) without indicating the carbon and hydrogen atoms. The corner of the polygon denotes a carbon atom and its sides represent a carbon-carbon bond. An atom or a group of atoms (other than hydrogen) bonded to the carbon is however shown in the structure.
Bond-line formulae of some cyclic compounds are:
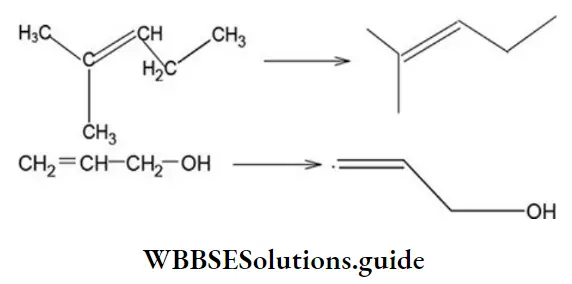
Multiple bonds are also indicated in bond-line formulae.
Multiple bonds example:
Structural Representation Of Organic Compounds Examples
Example 1: Convert each of the following Lewis structures into complete structural formulae:
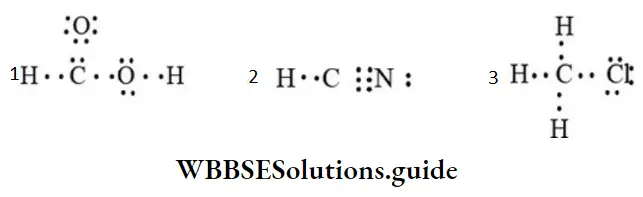
Solution: Use the dash to represent the shared electron pair.
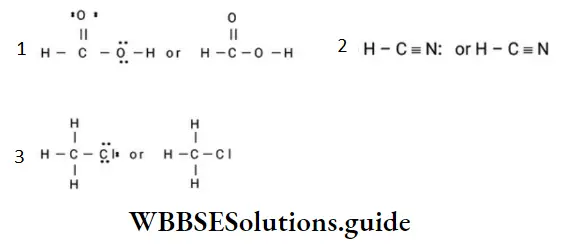
Example 2: Convert each of the following complete structural formulae into condensed formulae.
Solution: Omitting some or all of the dashes and indicating the number of identical groups by a subscript we get
- HO(CH2)2NH2
- CH3C=C(CH2)3CH3
- H3CCOCH3
- CH3(CH2)3NO2
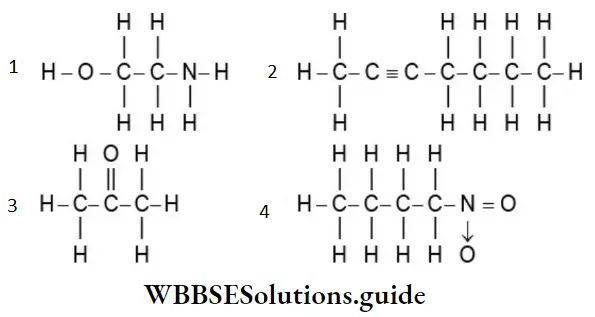
Example 3: Write the condensed structural formulae for the compound that follows in different ways.
Solution:
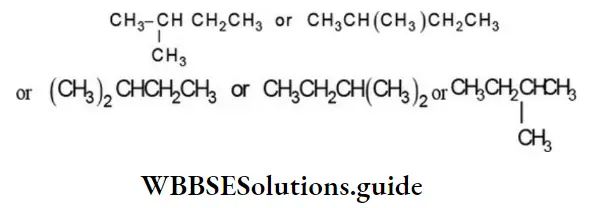
Example 4: Write the bond-line formula for
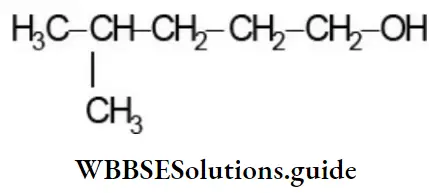
Solution: First, outline the carbon skeleton, including the OH group as follows:
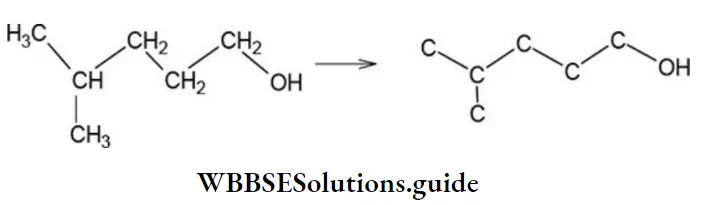
Thus, the bond-line formula is
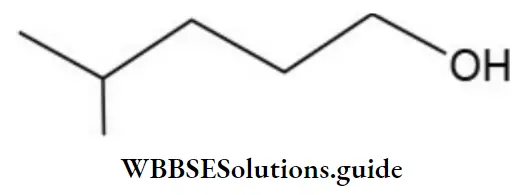
Example 5: For each of the following condensed formulae write the corresponding bond-line formula.
- (CH3)2CH(CH2)2CH2OH
- CH3(CH2)4CHICH2CHO
- (CH3)2CHCH3CH(CH2)3NO2
- (CN)2CHCH2COCl
Solution:
In the bond-line formula carbon and hydrogen atoms except those that are part of the functional groups are not shown. We show only the carbon skeleton. The number of hydrogen atoms necessary to fulfill the carbon atoms’ valences are assumed to be present, but we do not write them in.
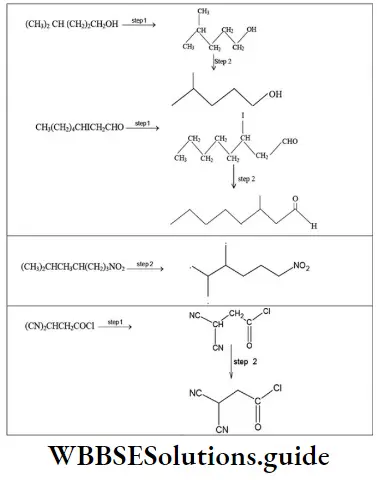
Other atoms (for example, Cl, O, N) are written in. Each intersection of two or more lines and the end of a line represent a carbon atom unless some other atom is written in.
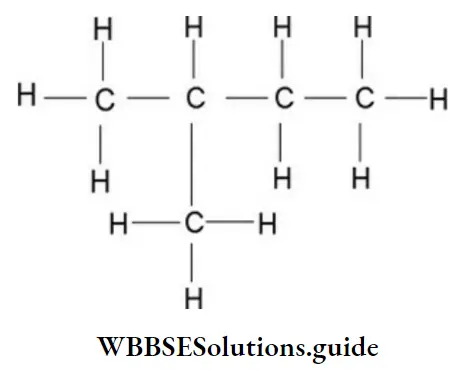
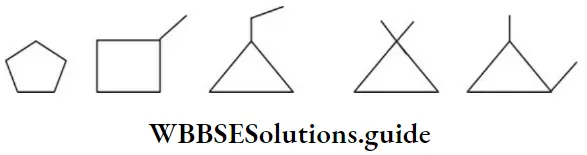
Example 6: Draw all possible bond-line formulae for a cyclic compound, C5H10.
Solution: Start with the maximum number of carbon atoms in the ring and move towards the ring of 3 carbon atoms. Explore all sorts of possibilities on this route:
Three-dimensional representation of organic molecules
None of the formulae that we have described so far convey any information about how the atoms of a molecule are arranged in space. Shape or the three-dimensional (3-D) structure of organic molecules can be described on a paper (two-dimensional) by exploring certain conventions. For instance, by using solid and dashed wedge formula the 3-D image of an organic molecule can be perceived on a two-dimensional paper.
In solid-wedge ![]() and dashed-wedge
and dashed-wedge ![]() description the solid-wedge denotes a bond projecting out of the plane of the paper towards the viewer. The dashed wedge depicts the bond projecting behind the plane of the paper and going away from the viewer. Both the wedges are drawn in such a way that the broad end of the wedge is near the viewer. The other two bonds lying in the plane of the paper are shown by using a normal line (________).
description the solid-wedge denotes a bond projecting out of the plane of the paper towards the viewer. The dashed wedge depicts the bond projecting behind the plane of the paper and going away from the viewer. Both the wedges are drawn in such a way that the broad end of the wedge is near the viewer. The other two bonds lying in the plane of the paper are shown by using a normal line (________).
Let’s consider the wedge- and dashed-wedge representation of methane (CH4) molecule: The two carbon-hydrogen bonds represented by normal lines are in the plane of paper, whereas the carbon-hydrogen bond represented with a solid wedge is aimed to be in front of the plane of paper.
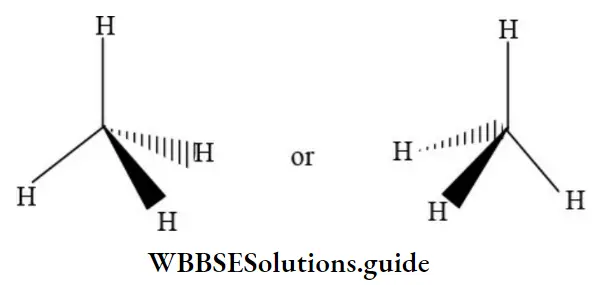
The hydrogen bonded to carbon by dashed edge is intended to be behind the plane of paper. Note that the carbon atom is lying in the plane of paper. Wedge and dashed-wedge formulae are important tool for clearly showing three dimensions.