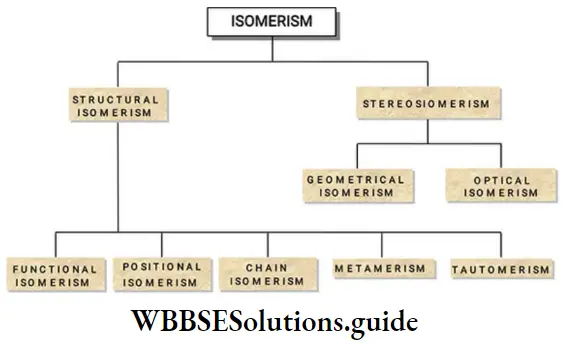
Isomerism
The existence of two or more compounds with the same molecular formula but different physical and chemical properties is known as isomerism and the molecules themselves are called as isomers.
Read And Learn More: NEET General Organic Chemistry Notes, Question And Answers
The term was given by Berzelius. The difference in properties of the two isomers is due to differences in the arrangement of atoms within their molecules.
Isomerism is mainly classified into structural isomerism and stereoisomerism.
Structural Isomerism
Structural isomerism is due to the differences in structures of the isomers. Structural isomerism is further classified into 5 types.
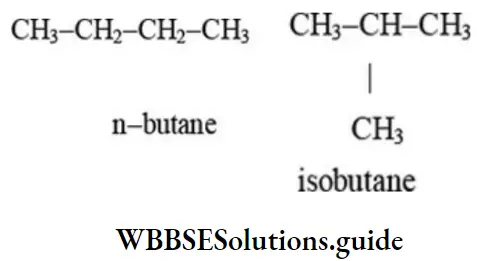
1. Chain isomerism (nuclear isomerism): Compounds with same molecular formula but differ in the arrangement and number of carbon atoms within the molecule are called chain isomers and the phenomenon as chain isomerism.
2. Butane (C4H10) has two isomers – normal butane and isobutene. One isomer has a straight chain and the other has a branched chain.
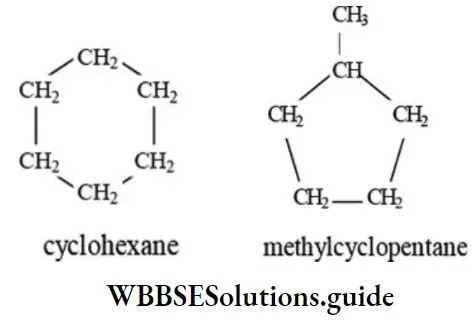
3. Cyclohexane and methyl cyclopentane are nuclear isomers.
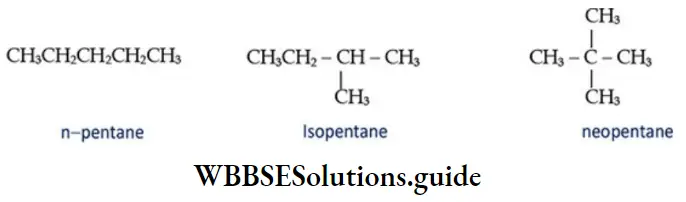
4. C5H12 has three chain isomers.
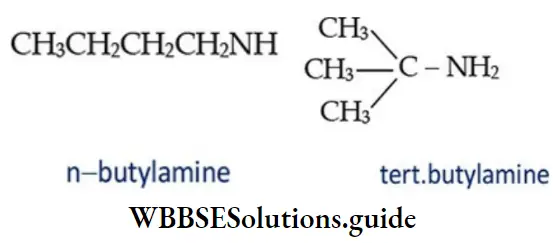
5. C4H9NH2 also shows two chain isomers.
Solve 1: How many chain isomers does butane have?
Solution:
Solve 2: How many chain isomers does propylbenzene have?
Solution: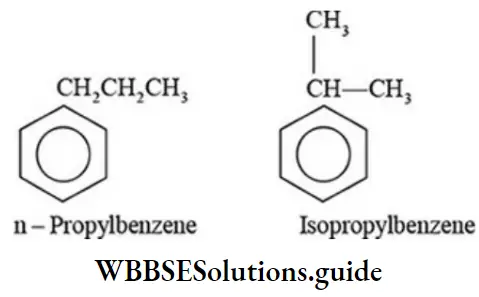
Functional Isomerism
Compounds with same molecular formula but differ in functional groups are called functional isomers and the phenomenon is known as functional isomerism. For example
Diethyl ether butyl alcohol both have the molecular formation C4H6O but contains different functional groups. Thus, functional group in diethyl ether is (-O-), while is butyl alcohol it is (-OH).
C2H5-O-C2H5 (diethyl ether); C4H9-OH (butyl alochol)
Acetone and propionaldehyde both with the molecular formula are functional isomers. In acetone the functional group is (-CO-) while in acetaldehyde it is (-CHO)
CH3-CO-CH3 (acetone); CH3-CH2-CHO (acetaldehyde)
Cyanides are isomeric with isocyanides
RCN (Alkyl cyanide); RNC (Alkyl isocyanide)
Carboxylic acids are isomeric with esters.
CH3CH2COOH (Propanoic acid); CH3COOCH3 (Methyl ethanoate)
Nitroalkanes are isomeric with alkyl nitrites:
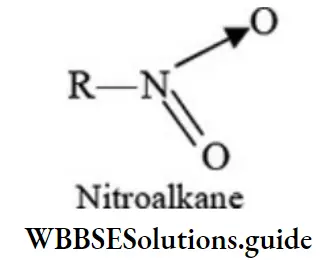
R-O-N = O (Alkyl nitrite)
Sometimes a double bond-containing compound may be isomeric with a triple bond-containing compound. This also is called as functional isomerism. Thus, butyne is isomeric with butadiene (molecular formula C6H6).
CH3-CH6C ≡ CH (1-Butyne);
CH6 = CH-CH = CH2 (1, 3-Butadiene)
Unsaturated alcohols are isomeric with aldehydes. Thus,
CH2=CH-OH (Vinyl alochol); CH3CHO (Acetaldehyde)
Unsaturated alcohols containing three or more carbon atoms are isomeric to aldehydes as well as ketones:
CH2 = CH-CH2OH (Alkul alochol);
CH3CH2CHO (Propionaldehyde);
CH3COCH3 (Acetone)
Aromatic alcohols may be isomeric with phenols
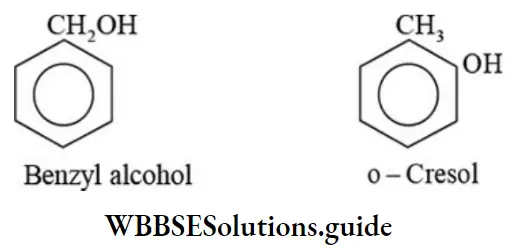
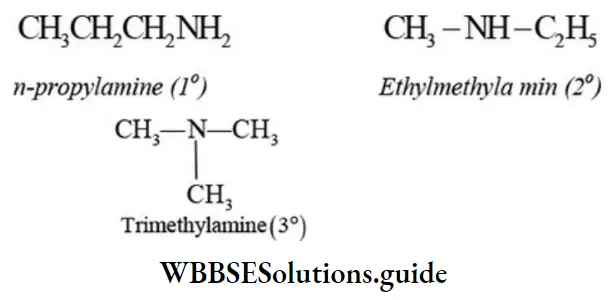
Primary, secondary, and tertiary amines of same molecular formula are also functional isomers.
Alkenes are isomeric with cycloalkanes:
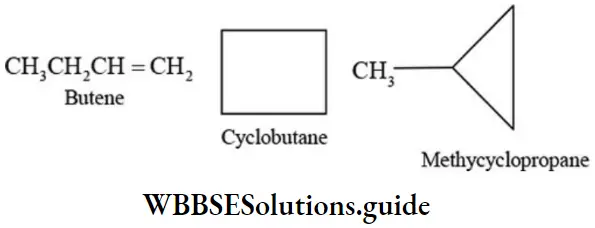
Such isomers in which one is cyclic and the other is open chain are called ring-chain isomers. Alkynes and alkadienes are isomeric with cycloalkanes.
CH3CH2C ≡ CH (1-Butyne);
CH2 =CH-CH = CH2 (1,3 -Butadinene)
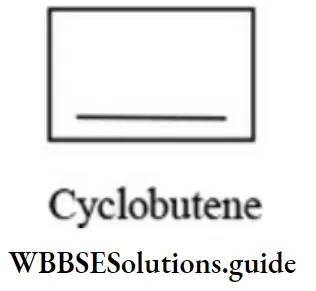
Position isomerism
Compounds which have the same structure (arrangement) of carbon chain (carbon skeleton) but differ in the position of the multiple bond or the functional group are called position isomers and the phenomenon is known as position isomerism.
Thus the following compounds can exhibit position isomerism:
- Alkenes
- Alkynes
- Arenes
- Alkyl halides
- Aryl halides
- Alcohols
- Amines and
- Nitro compounds
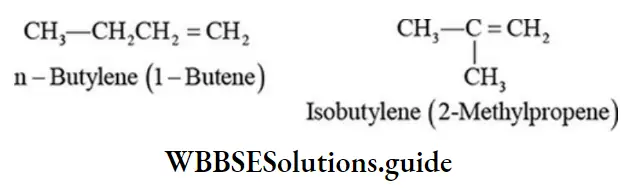
Alkenes containing four or more carbon atoms can exhibit position isomerism due to the difference in the position of the double bond on the same carbon skeleton, For example:
- CH3CH2CH = CH2 (but-1-ene) ; CH3CH =CHCH3 (But-2-ene)
- CH2CH2CH2CH = CH2 (Pent-l-ene); CH3CH2CH = CHCH3 (Pent-2-ene)
- CH3CH2CH2CH = CH2 (Hex-l-ene); CH3CH2CH2CH = CHCH3 (Hex-2-ene); CH3CH2CH = CHCH2CH3(Hex-3-ene)
Alkynes containing four or more carbon atoms can exhibit position isomerism due to the difference in the position of triple bond on the same carbon skeleton. For example:
- CH3CH2C = CH2 (But-l-yne); CH3C = CCH3 (But-2-yne)
- CH3CH2CH2C (Pent-l-ene) = CH; CH3CH2C = CCH3
Arenes containing eight or more carbon atoms exhibit position isomerism due to the difference in the position of alkyl groups on the benzene ring. For example:
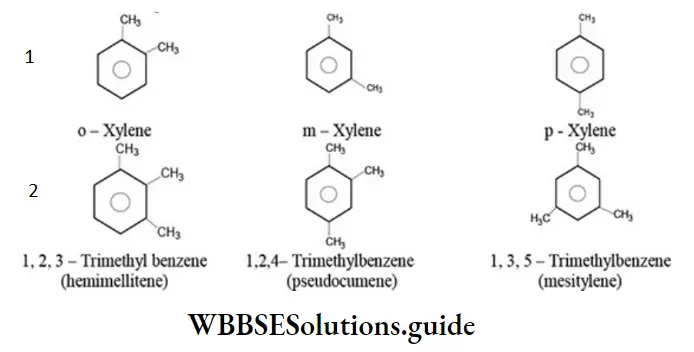
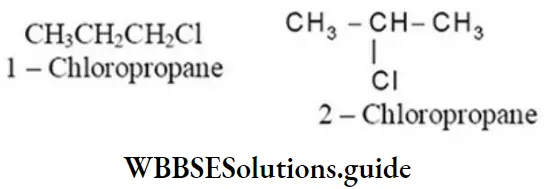
Alkyl halides containing three or more carbon atoms can exhibit position isomerism due to the difference in the position of halogen atom on the same carbon skeleton. For example
1. C3H7Cl has two position isomers:
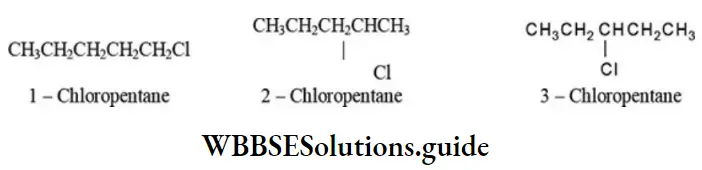
2. n – Pentane on monochlorination gives three isomeric chloromethanes:
Polyhalogen derivatives containing two or more carbon atoms can also exhibit position isomerism. For example:
CH3CHCl2 (1, 1- Dichloroethane)
ClCH2CH2Cl (1, 2-Dichloroethane)
Aryl halides containing two or more benzene rings can exhibit position isomerism due to the difference in the position of halogen atom. For example:
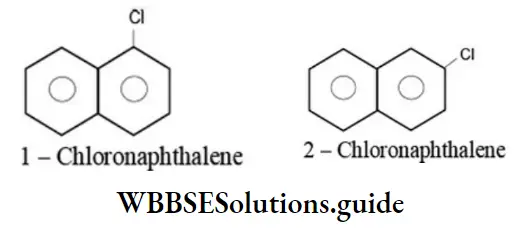
Polyhalogen compounds containing just one benzene ring can also exhibit position isomerism. For example:
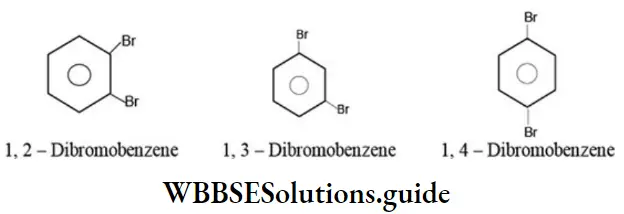
Alcohols containing three or more carbon atoms exhibit position isomerism due to the difference in the position of functional group (-OH). For example:
1. The molecular formula C3H8O represents two isomeric alcohols:
CH3CH2CH2OH (Propan 1-ol)
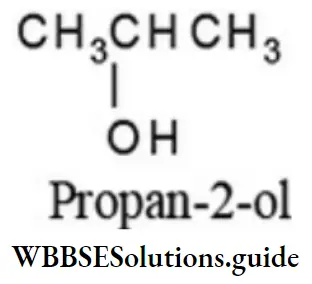
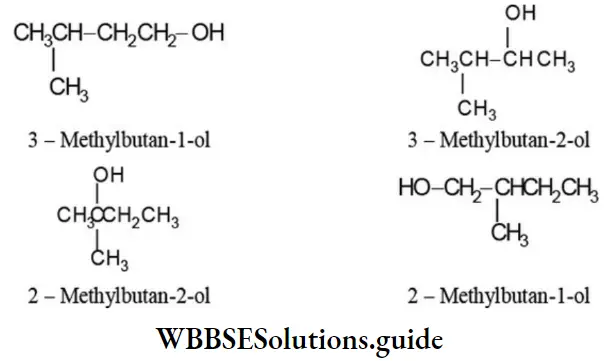
2. Four monohydric alcohols differing in the position of -OH group can be derived from isopentane:
Primary amines (RNH2) containing three or more carbon atoms can exhibit position isomerism due to the difference in the position of amino group on the same carbon skeleton. For example:
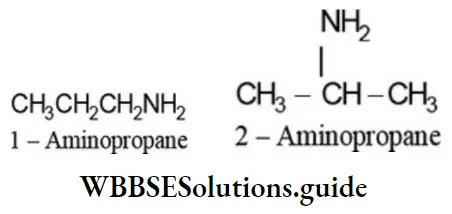
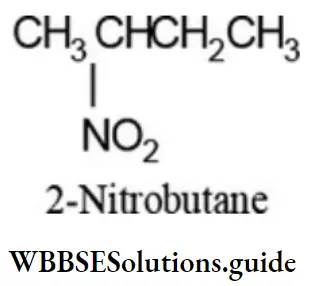
Nitro compounds (RNO2) containing three or more carbon atoms can exhibit position isomerism due to the difference in the position of nitro group on the same carbon skeleton. For example:
CH3CH2CH2CH2NO2 (1-Nitobutane)
Metamerism
Here two or more different compounds having the same molecular formula but different number and arrangements of carbon atoms on either side of the functional group is called metamerism. Such compounds are known as metamers.
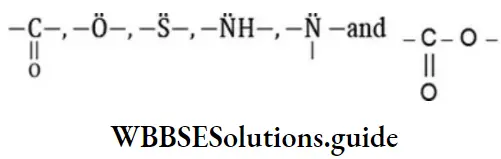
Metamerism is never possible in compounds possessing the univalent functional group. Metamerism is due to the difference in the nature of alkyl groups attached to the same polyvalent is functional group such is Metamerism is exhibited by compounds of the same homologous series.
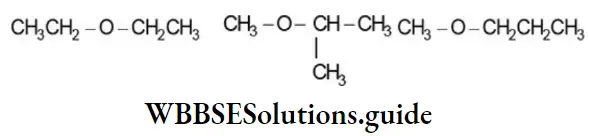
Ethers, R-Q-R, exhibit metamerism due to differences in the nature of the alkyl groups attached to the oxygen atom. Thus, the molecular formula, C4H10O, represents the following metamers:
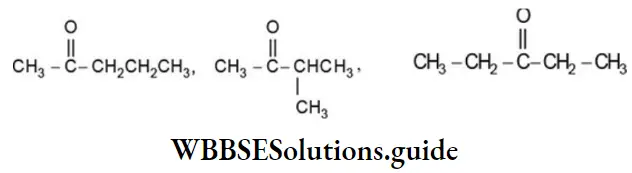
Ketones ![]() , exhibit metamerism due to the difference in the nature of the alkyl groups attached to the carbonyl group. Thus, the molecular formula, C5H10O, represents the following metamers:
, exhibit metamerism due to the difference in the nature of the alkyl groups attached to the carbonyl group. Thus, the molecular formula, C5H10O, represents the following metamers:
Thioethers, R-S-R’, exhibit metamerism due to the difference in the nature of the alkyl groups attached to the sulfur atom. Thus, the molecular formula, C4H10S, represents the following metamers:
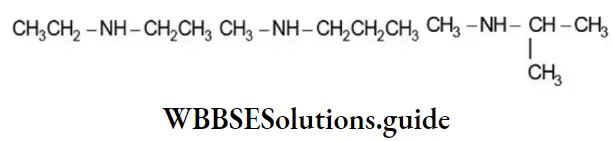
Secondary and tertiary amines exhibit metamerism due to the difference in the nature of the alkyl groups attached to the – NH – group and the ![]() atom respectively. Thus the molecular formula, C4H11N, represents the following metamers:
atom respectively. Thus the molecular formula, C4H11N, represents the following metamers:
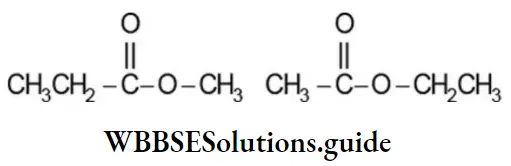
Esters, ![]() exhibit metamerism due to the difference in the nature of the alkyl groups attached to the -C – 0 – group. Thus, the following esters are metamers:
exhibit metamerism due to the difference in the nature of the alkyl groups attached to the -C – 0 – group. Thus, the following esters are metamers:
Note – If same polyvalent functional group is present in two or more organic compounds, then instead of chain or position isomerism, treat the phenomenon as metamerism.
- Pentan – 2- one and pentan – 3- one are metamers and not position isomers. They can be included in position isomerism, if metamerism is not mentioned.
- Similarly, pentan – 2- one and 3 – methylbutan-2-one are metamers and not chain isomers.
Metamers may be considered as position isomers. For instance, pentan – 2- one and penta-3- one may be regarded as position isomers as well as metamers.
Tautomerism
Here a single compound exists in two readily interconvertible structures that differ in position of the hydrogen atom. Tautomer exhibits dynamic equilibrium with each other. A very common form of tautomerism is that between a carbonyl compound containing an a – hydrogen and its enol form. This type of isomerism is also known as keto-enol isomerism.
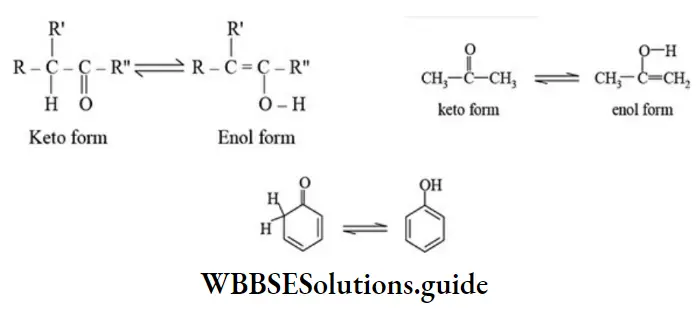
The percentage of enol form increases in the order simple aldehydes and ketones <β-keto esters <β-diketones <β-diketones having phenyl group < phenols. This increase in the enol content is due to the fact that the enol form of the above type of compounds is increasingly stabilized by resonance and hydrogen bonding than the corresponding keto form.
Ring Chain Isomerism
The phenomenon of existence of two or more compounds having the same molecular formula but possessing open chain and closed chain (cyclic structure) is called ring-chain isomerism. This type of isomerism arises due to different modes of linking of carbon atoms. Thus ring-chain isomers possess open chain or closed-chain structures as illustrated by the following examples:
Two ring-chain isomers are possible corresponding to the molecular formula C3H6:
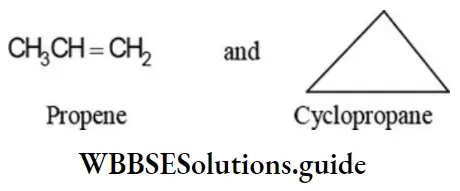
Six pairs of ring-chain isomers are possible for the molecular formula C4H8:
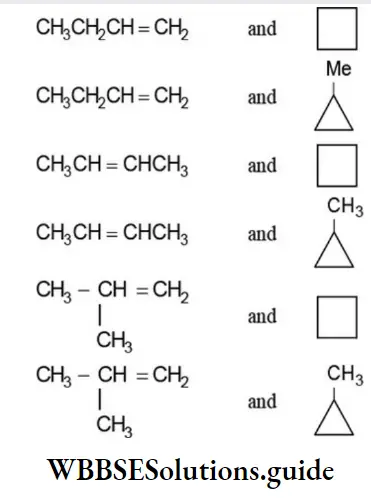
The molecular formula C3H4 represents the two ring-chain isomers:

Ring chain isomerism can be included in functional isomerism, if not considered separately.

