NEET Physics Dual Nature of Radiation and Matter Notes
Dual Nature of Radiation and Matter
The energy gained by an electron when it is accelerated between a potential difference of 1 volt is
called 1eV.
The minimum energy required by an electron to escape from the metal surface is called work
function \(\left(\phi_0\right)\)
Caesium (Cs) has the least work function (2.14 eV)
Read And Learn More: NEET Physics Notes
Platinum (Pt) has the highest work function (5.65 eV)
The phenomenon of emission of free electrons from a metal surface on illumination of electromagnetic radiation of suitable frequency is called Photoelectric Effect.
Maximum kinetic energy of emitted photoelectrons is given by,
\(K_{\max }=e V_0\)where, V0 is stopping potential.
Experimental Observations of Photoelectric Effect
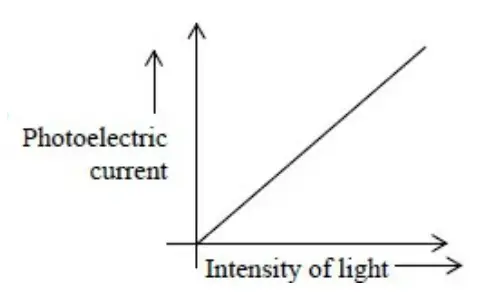
1. Above threshold frequency, the photocurrent is directly proportional to intensity of incident light.
2. Saturation current is proportional to intensity of incident radiation but stopping potential is independent of intensity.
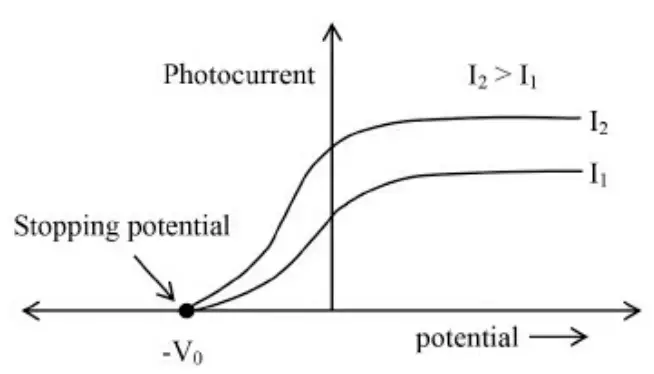
3. There exists a certain minimum frequency below which photoemission is absent is called threshold frequency.
4. Photoelectric effect is an instantaneous process.
Note: Wave theory of light failed to explain experimental observations of photoelectric effect.
Einstein’s Explanation for Photoelectric Effect
According to Einstein when a photon of energy falls on a metal surface, the maximum kinetic energy of emitted photoelectrons is given by,
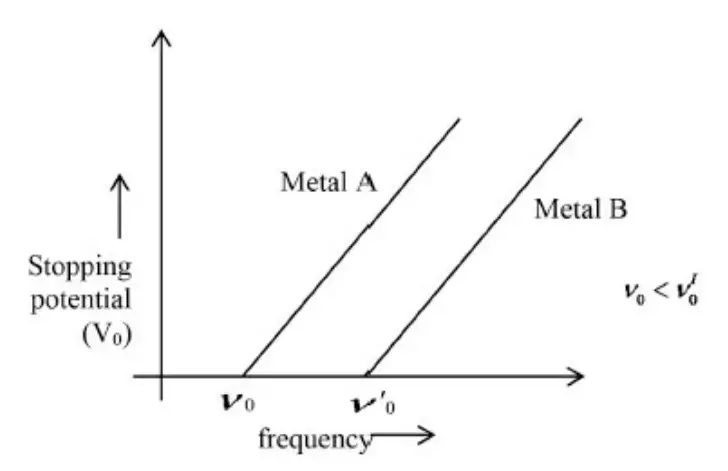
\(K_{\max }=h v-\phi_0 \rightarrow(1)\)Where, is the work function of the metal.
Equation (1) is in the form of y = mx + c
i.e., the graph of \(K_{\max }\)versus will be a straight line with slope h.
\(\begin{aligned}(1) \Rightarrow e V_0 & =h v-\phi_0 \quad\left(∵ K_{\max }=e V_0\right) \\
V_0 & =\left(\frac{h}{e}\right) v-\frac{\phi_0}{e} \rightarrow(2)
\end{aligned}\)
Equation (2) is also in the form of y = mx + c
i.e., the graph V0 of versus is a straight line with slope \(\frac{h}{e}\)
Important properties of photon
- Momentum of the photon is \(\frac{E}{c}=\frac{h v}{c}\)
- The rest mass of a photon is zero.
- Photons are electrically neutral; they are not deflected by electric or magnetic fields.
Matter waves
Waves associated with moving matter are called matter waves.
According to Louis de Broglie wavelength of matter waves is given by,
\(\lambda=\frac{h}{P}=\frac{h}{m v}\)Consider a particle of mass m and charge q accelerated from rest through a potential V. Then
The kinetic energy of the charged particle is given by,
K = qV
\(\text { w.k.t., } \lambda=\frac{h}{P}=\frac{h}{\sqrt{2 m K}}\) \(\Rightarrow \lambda=\frac{h}{\sqrt{2 m q V}}\)For an electron,
\(\lambda=\frac{h}{\sqrt{2 m e V}}=\frac{1.227}{\sqrt{V}} \mathrm{~nm}\)Note: Davisson and Germer’s experiment proved the wave nature of electrons.
According to Heisenberg’s uncertainty principle, it is impossible to measure two canonically conjugate physical quantities like position and momentum of a microscopic entity simultaneously and accurately.
Or in simple form,
It is impossible to measure position and momentum of a microscopic particle accurately and simultaneously.

