The S-Block Elements
You have already learnt that elements in the periodic table are arranged in order of increasing atomic number.
The long form of the periodic table is divided into four blocks—s, p, d and f—depending on the type of the orbitals (s, p, d or f) that are being filled with valence electrons.
The elements belonging to the s block can have one or a maximum of two s-electrons in their outermost shell.
The s-block of the periodic table comprises two groups—Group 1 and Group 2. The elements of Group 1 have the general ground state electronic configuration of ns1 and are called alkali metals.
“S-block elements, properties, periodic trends, and electronic configurations”
The elements of Group 2 have the general ground state electronic configuration of ns2 and are called alkaline earth metals.
Elements in which the p orbitals are being filled with valence electrons fall in the p block.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
This block constitutes Groups 13, 14, 15, 16, 17 and 18 with 1, 2, 3, 4, 5 and 6 electrons, respectively, in the p orbitals of the outermost shell, while the s orbitals are already filled.
General Characteristics of s-Block Elements
Some important characteristics of s-block elements are given below. A comparison with the p-block elements is also made, which will be helpful.
- The s-block elements have the general outermost electronic configuration ns1 (for alkali metals) or ns2 (for alkaline earth metals). However, in the case of p-block elements the electronic configuration of the outermost shell is ns2 np1-6.
- The s-block elements are metals and form ionic compounds with nonmetals whereas p-block elements are nonmetals and form predominantly covalent compounds with each other.
- The s-block elements show only one oxidation state, which is equal to their group number.
- Thus, alkali metals (Group 1) and alkaline earth metals (Group 2) show oxidation states of 1 and 2 respectively. On the other hand, most of the p-block elements show more than one positive as well as a negative oxidation state.
- Diagonal relationship The first member of each group of the s-block and those of the other groups differ markedly from the rest of the members. Consider the elements of periods 2 and 3.
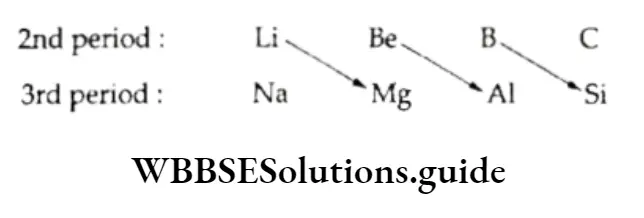
The first element of a group in the second period shows similarities with the second element of the neighbouring group on the right (diagonally opposite element). This is referred to as a diagonal relationship.
Thus, lithium resembles magnesium, beryllium resembles aluminium and boron resembles silicon.
This diagonal relationship is attributed to similarity in the size of the ions (e.g., Li+ =76 pm and Mg2+ =72 pm), and in the electropositive character and polarising power of the elements.
Alkali Metals (Group 1 Elements)
The elements of Group 1 are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr).
All these elements are metallic in nature and are known as alkali metals. The last element of this group, francium, is radioactive and has a very short half-life and so, very little is known about it. All the alkali metals are silvery-white, soft and light.
Occurrence
Alkali metals are very reactive and react with oxygen and water. Therefore, they do not occur in the free state.
However, they occur in the combined state in the form of salts like NaCl and KC1, which occur in large amounts in seawater.
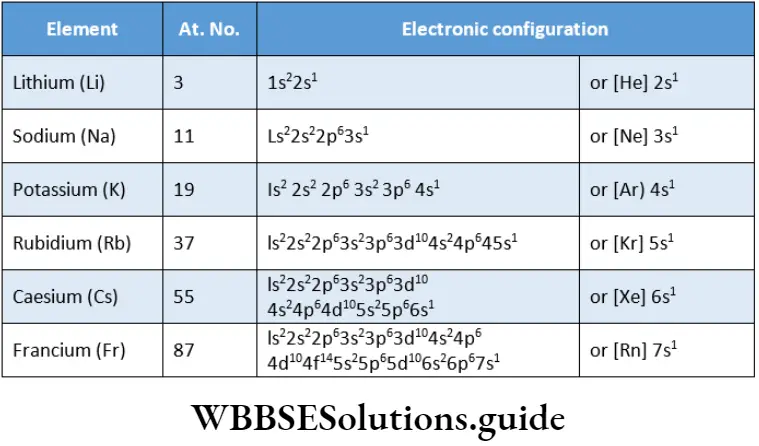
Electronic Configuration
Alkali metals have only one electron (ns1) in the s-orbital of their valence shell. The valence-shell electronic configuration of the alkali metals can be generalised as ns1, where n = 2 to 7.
Atomic And Ionic Radii
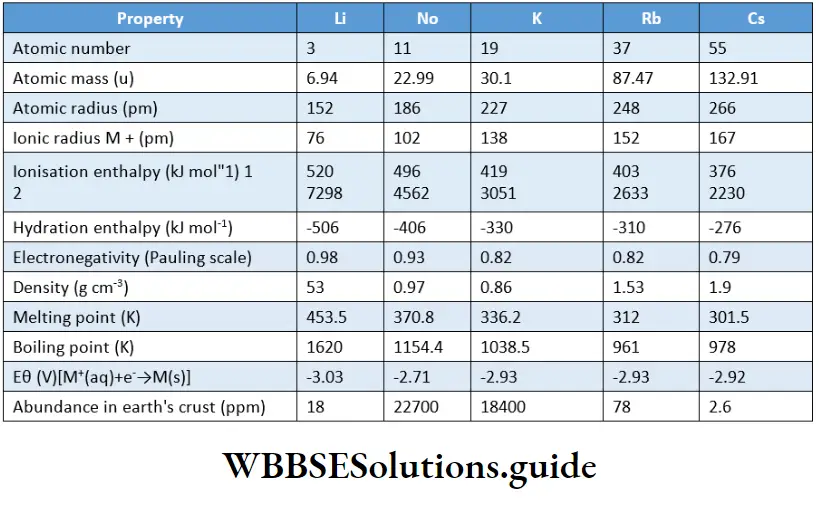
The atoms of alkali metals have the largest size in their respective periods. The atomic radii increase as we go down the group (from Li to Cs) because there is a progressive addition of new energy shells.
This implies that the distance between the nucleus and the last shell increases and the valence electrons are farther from the nucleus. So the atomic radius increases with an increase in atomic number.
“S-block elements, characteristics, periodic table trends, and configurations”
However, with the increase in atomic number the nuclear charge also increases. This tends to decrease the atomic radius by attracting the electron cloud inward with greater force.
However, the effective nuclear charge experienced by valence electrons is less than the actual nuclear charge because of the screening of the outermost electrons by the inner core of electrons.
For example, in the case of the sodium atom, the outermost 3s electron is screened by the inner Is and 2s electrons.
The alkali metals tend to lose their valence electrons and change to monovalent cations in order to achieve the noble-gas configuration.
So the radius of the positive ion is smaller than that of the parent atom. However, within the group, the ionic radius increases with an increase in atomic number.
This is accounted for by the addition of electron shells at every step as we go down the group.
Ionisation enthalpies
The first ionisation enthalpies of the elements of Group 1 are the lowest. This is because the valence electrons are loosely held, and can be easily lost by the atom to achieve the nearest noble-gas configuration.
The second ionisation enthalpies of alkali metals are very high. This is because it is difficult to remove an electron from a monovalent cation (formed by the removal of an electron from an atom of an alkali metal) having a noble-gas configuration.
Hydration Enthalpies
The standard enthalpy of hydration of an element is the energy released when 1 mol of gaseous ions of that element become hydrated (surrounded by water molecules) under standard conditions.
The higher the charge on the ions and the smaller their size, the more negative is the hydration enthalpy.
The hydration enthalpies of metal cations increase on moving down the group. Lithium has the most negative hydration enthalpy. ft Therefore, it mostly forms hydrated salts, e.g., LiCl2H2O.
Electropositive Character And Oxidation State
Elements that are electropositive tend to lose electrons and form positive ions. The alkali metals are typical electropositive elements.
The electropositive character increases from Li to Cs as the tendency to lose an electron increases on moving down the group.
While combining with other elements in a reaction, alkali metals have a strong tendency to lose the single valence electron to form a unipositive ion.
Thus, they show an oxidation state of +1 in their compounds and are strongly electropositive.
⇒ \(\mathrm{M} \rightarrow \mathrm{M}^{+}+\mathrm{e}^{-}\)
Melting And Boiling Points
The alkali metals have low melting and boiling points, which decrease down the group. This indicates weak inter¬ atomic bonding or metallic bonding.
In a metallic bond negatively charged electrons hold the positive ions together. The bonding is weak in alkali metals due to the presence of only a single valence electron for each atom. On moving down the group, atomic size increases, which progressively weakens intermolecular bonds.
Density
The densities of alkali metals are low and increase on moving down the group. However, potassium is an exception and is lighter than sodium.
The low density is attributed to the large atomic size. On moving down the group, atomic size as well as atomic mass increases.
However, the corresponding increase in atomic mass is not compensated by an increase in atomic volume. Thus, the density, which is the ratio of mass to volume, gradually increases.
Flame Colouration
All alkali metals impart a characteristic colour to a Bunsen flame.
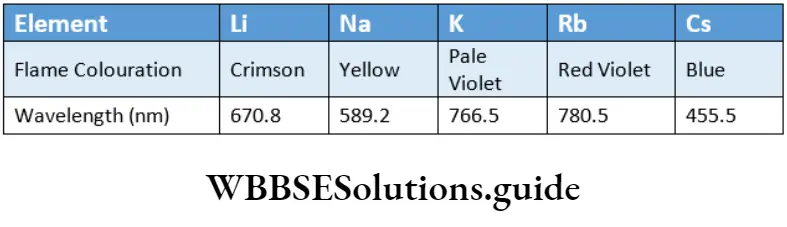
When a sample of any metal salt is heated in a Bunsen burner (a laboratory gas burner) flame it imparts a characteristic colour to the flame.
The orbital electrons of the metal atoms absorb the heat energy supplied and get excited to higher energy states. When the excited electron loses the extra energy, it falls back to its ground state.
The extra energy lost is emitted in the form of radiation, which falls in the visible region, imparting a characteristic colouration to the flame.
Photoelectric effect
All alkali metals except lithium exhibit a photoelectric effect. Due to the low ionisation enthalpies of the metals, the metal surfaces emit electrons when exposed to visible light. Lithium has high ionisation enthalpy values and so does not exhibit a photoelectric effect.
Chemical properties
Alkali metals are very reactive. This is attributed to their low first ionisation enthalpies. We will now discuss some important chemical properties of alkali metals.
Reducing character
Alkali metals are good reducing agents. This is because they have a strong tendency to lose electrons and have large negative values of reduction potential. The reducing character increases from Na to Cs.
However, lithium, in spite of its highest ionisation enthalpy, is the strongest reducing agent among all alkali metals, as indicated by its reduction potential value (3.03 V).
“Properties of s-block elements, periodic variations, and electron configuration”
The unusual behaviour of lithium is due to the exceptionally small size of the lithium atom and the high charge-to-radius ratio of the ion.
Being reducing agents, alkali metals react with compounds containing acidic hydrogen atoms (hydrogen atoms generally attached to oxygen or a triple bond are acidic and they can be replaced by metals), such as water, alcohol and acetylene, liberating hydrogen gas.
The standard electrode potential (Ee’)/ which measures the reducing power and is expressed as M+(aq)/M(s), represents the overall change as follows
⇒ \(\begin{aligned}
& \mathrm{M}(\mathrm{s}) \rightarrow \mathrm{M}(\mathrm{g}) \quad \text { (Sublimation enthalpy) } \\
& \mathrm{M}(\mathrm{g}) \rightarrow \mathrm{M}^{+}(\mathrm{g})+\mathrm{e}^{-} \quad \text { (Ionisation enthalpy) } \\
& \mathrm{M}^{+}(\mathrm{g})+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{M}^{+}(\mathrm{aq}) \quad \text { (Hydration enthalpy) } \\
&
\end{aligned}\)
The reactivity of alkali metals towards compounds containing acidic hydrogen increases from Li to Cs
⇒ \(\begin{aligned}
2 \mathrm{Li}+2 \mathrm{H}_2 \mathrm{O} & \rightarrow 2 \mathrm{LiOH}+\mathrm{H}_2 \\
2 \mathrm{Na}+2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH} & \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{ONa}+\mathrm{H}_2
\end{aligned}\)
⇒ \(2 \mathrm{Na}+\underset{\text { acetylene }}{\mathrm{HC}} \equiv \mathrm{CH} \rightarrow \underset{\text { sodium acetylide }}{\mathrm{HC}} \equiv \mathrm{C}-\mathrm{Na}+\mathrm{H}_2 acetylene sodium acetylide\)
Reaction With Oxygen
Alkali metals tarnish rapidly in dry air. Lithium forms a mixture of its oxide and nitride on the surface.
When burnt in air or oxygen, alkali metals form different types of oxides. For example, on a reaction with a limited quantity of oxygen, these metals form normal oxides (M2O).
⇒ \(\begin{gathered}
2 \mathrm{M}+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{M}_2 \mathrm{O} \\
(\mathrm{M}=\mathrm{L}, \mathrm{Na}, \mathrm{K}, \mathrm{Kb}, \mathrm{Cs})
\end{gathered}\)
However, on being heated with an excess of air or oxygen, lithium forms the normal oxide or monoxide, sodium forms its peroxide (Na202) whereas potassium, rubidium and caesium form superoxides (MO2).
⇒ \(2 \mathrm{Li}+\frac{1}{2} \mathrm{O}_2 \rightarrow \underset{\text { lithium oxide }}{\mathrm{Li}_2 \mathrm{O}}\)
“S-block elements, periodic trends, reactivity, and atomic structure”
⇒ \( 2 \mathrm{Na}+\mathrm{O}_2 \rightarrow \mathrm{Na}_2 \mathrm{O}_2 sodium peroxide\)
⇒ \(\mathrm{K}+\mathrm{O}_2 \rightarrow \quad \mathrm{KO}_2 potassium superoxide\)
Being the smallest in size, Li+ has a small, strong positive field around it. This implies that Li+ can stabilise only a small anion, O2–. However, Na+, being larger in size has a weak positive field around it.
Therefore, it can stabilise a bigger peroxide ion, O22+-, which has a weak negative field around it.
On the other hand, K+, Rb+, and Cs+ being still larger, stabilise the still bigger superoxide ion (O2+) to form superoxides.
The normal oxides and peroxides are colourless solids. However, superoxides are paramagnetic in nature (due to the presence of one impaired electron in the O2–ion) and are yellow solids.
The oxides of alkali metals dissolve readily in water to form hydroxides. The reaction is exothermic.
⇒ \( \mathrm{M}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{MOH}+\text { heat } normal oxide \mathrm{Na}_2 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O}_2+\text { heat } \)
These reactions show that the oxides of alkali metals are basic.
Reaction With Hydrogen
In general, alkali metals react with dihydrogen at about 673 K to give hydrides of the type MH. Lithium, however, reacts at 1073 K.
⇒ \(2 \mathrm{M}+\mathrm{H}_2 \rightarrow \underset{\text { metal hydride }}{2 \mathrm{MH}}\)
The reactivity of alkali metals with hydrogen decreases from Li to Cs. Also, the stability of the hydrides decreases from Li to Cs because, as the size of the alkali metal increases, the M—H bond becomes weak. The hydrides of alkali metals are strong reducing agents and are used in organic synthesis.
Reaction with halogens
Alkali metals react with halogens to form the corresponding halides, M+X
⇒ \(2 \mathrm{M}+\mathrm{X}_2 \rightarrow 2 \mathrm{MX}(\mathrm{X}=\mathrm{F}, \mathrm{Cl}, \mathrm{Br} \text { or } \mathrm{I})\)
The reactivity of alkali metals towards a particular halogen increase from Li to Cs. The reactivity of the halogens towards a particular alkali metal is of the order F2 >C12 > Br2 >I2.
Tire alkali metal halides (MX) are colourless, crystalline solids with high melting points. They can also be prepared conveniently from the corresponding oxide, hydroxide or carbonate by reaction with an aqueous hydrohalic acid (HX)
⇒ \(\begin{gathered}
\mathrm{Li}_2 \mathrm{O}+2 \mathrm{HCl} \rightarrow 2 \mathrm{LiCl}+\mathrm{H}_2 \mathrm{O} \\
\mathrm{Li}_2 \mathrm{CO}_3+2 \mathrm{HCl} \rightarrow 2 \mathrm{LiCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \\
\mathrm{LiOH}+\mathrm{HCl} \rightarrow \mathrm{LiCl}+\mathrm{H}_2 \mathrm{O}
\end{gathered}\)
Alternatively, the nitrates of alkali metals can be decomposed on heating to give the corresponding oxide, which is then converted into a halide.
⇒ \(4 \mathrm{LiNO}_3 \rightarrow 2 \mathrm{Li}_2 \mathrm{O}+4 \mathrm{NO}_2+\mathrm{O}_2\)
Alkali metal halides are ionic compounds. However, Lil is slightly covalent due to polarisation (Li has the maximum polarising power as it is the smallest cation and the iodide ion can be polarised to the maximum extent as it is the largest anion).
“S-block elements, alkali and alkaline earth metals, properties, and trends”
Except for LiF, alkali metal halides are soluble in water. The insolubility of LiF is attributed to its high lattice enthalpy, as a result of the combination of the small anion (F-) and the small cation (Li+).
Melting and boiling points of alkali metal halides All the halides of alkali metals are colourless crystalline solids with high melting points because they contain ionic bonds. The melting and boiling points of these halides follow certain trends.
1. For the same alkali metal, the melting and boiling points decrease in the following order:
fluoride > chloride > bromide > iodide
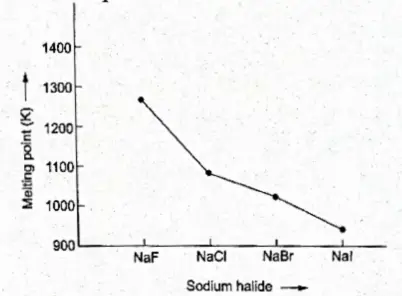
This Can Be Explained On The Basis Of The Lattice Enthalpies Of These Halides. For The Same Alkali Metal Ion, The Lattice Enthalpies Decrease As The Size Of The Halide Ion Increases.
As The Lattice Enthalpies Decrease, The Energy Required To Break The Lattice (Melt The Solid) Decreases. Thus The Melting Points Of Sodium Halides Decrease From Naf To I.
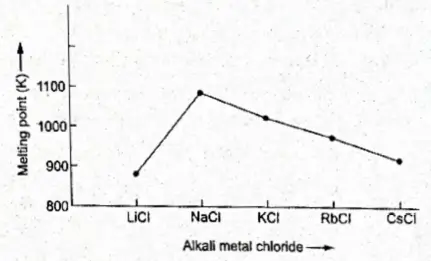
2. For The Same Halide Ion, The Melting Point Of The Lithium Halide Is Lower Than That Of The Sodium Halide. Then There Is A Progressive Decrease In Melting Point From Na To Cs.
The low melting point of LiCl compared to that of NaCl, for example, is due to the fact that LiCl is covalent in nature, while NaCl is held together by ionic bonding.
The lattice enthalpy decreases from NaCl to CsCl as the size of the alkali metal ion increases. So the melting point decreases from NaCl to CsCl.
Reaction With Water
In reaction with water, alkali metals form hydroxides, liberating hydrogen gas.
⇒ \(\begin{aligned}
2 \mathrm{Na} & +2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2 \\
2 \mathrm{~K}+2 \mathrm{H}_2 \mathrm{O} & \rightarrow 2 \mathrm{KOH}+\mathrm{H}_2
\end{aligned}\)
The reaction is so vigorous that the hydrogen evolved catches fire. The reaction becomes more violent on descending down the group. Therefore, alkali metals should be stored in an inert medium, usually kerosene oil.
Lithium is the only alkali metal which reacts gently with water. This is because it has the highest charge-to-radius ratio.
Owing to this it can polarise the water molecule more and with the highest hydration energy form a stronger metal-to-oxygen bond.
But its gentle behaviour towards water lies not in thermodynamics but in the kinetics of the reaction. Potassium, which liberates less energy, has a low melting point.
So it melts by the heat of the reaction, spreads and exposes a larger surface to the water, thus catching fire.
The hydroxides of the alkali metals are strongly basic and the strength of the base increases down the group.
On descending the group, the decrease in ionisation enthalpy results in weaker M —OH bonds from LiOH to C2OH, and this accounts for the increase in the basic strength of the hydroxides. Alkali metal hydroxides in solution ionise easily to form M+ and OH ions due to the weak M—OH bond. This accounts for the strong basic nature of the hydroxides.
Reaction with oxoacids
Being electropositive in nature, alkali metals form oxo salts by reacting with oxoacids. For example, HOC1, H2CO3 and HNO3 react with alkali metals to give salts of oxoacids like MOCI, M2CO3 and MNO3 respectively.
These salts are soluble in water and stable to heat. Li2CO3 is considerably less stable. Being strongly basic, Group 1 metals form solid bicarbonates.
Solutions of metals in liquid ammonia
All Group 1 metals and a few Group 2 metals dissolve in liquid ammonia, forming a deep blue solution. One reason why metals dissolve in liquid ammonia is ionisation.
⇒ \(\mathrm{M} \rightarrow \mathrm{M}^{+}+\mathrm{e}^{-}\)
The cations and electrons combine with ammonia to form ammoniated cations and ammoniated electrons respectively. The cations and electrons actually interact with the molecules of the solvent (ammonia). The overall reaction is
⇒ \(\mathrm{M}+(x+y) \mathrm{NH}_3 \rightarrow\left[\mathrm{M}\left(\mathrm{NH}_3\right)_x\right]^{+}+\left[\mathrm{e}\left(\mathrm{NH}_3\right)_y\right]^{-}
ammoniated cation ammoniated electron\)
The ammoniated electron is responsible for the blue colour of the solution. The solution absorbs energy corresponding to the red region of visible light and the ammoniated electrons get excited to higher energy levels.
When the electrons return to lower energy levels they transmit light which imparts a blue colour to the solution.
The solution is also conducting mainly due to the presence of solvated or ammoniated electrons. The solution fades on standing, slowly liberating hydrogen and forming a metal amide
⇒ \(\mathrm{M}^{+}+\mathrm{e}^{-}+\mathrm{NH}_3(\mathrm{l}) \rightarrow \mathrm{MNH}_2+\frac{1}{2} \mathrm{H}_2(\mathrm{~g})\)
The ammoniated electron is responsible for the blue colour of the solution. The solution absorbs energy corresponding to the red region of visible light and the ammoniated electrons get excited to higher energy levels.
When the electrons return to lower energy levels they transmit light which imparts a blue colour to the solution.
The solution is also conducting mainly due to the presence of solvated or ammoniated electrons. The solution fades on standing, slowly liberating hydrogen and forming a metal amide.
⇒ \(\mathrm{M}^{+}+\mathrm{e}^{-}+\mathrm{NH}_3(\mathrm{l}) \rightarrow \mathrm{MNH}_2+\frac{1}{2} \mathrm{H}_2(\mathrm{~g})\)
If the concentration of the solution is increased, the blue colour changes to copper-bronze with a metallic lustre.
This is because, with increased concentration, metal ion clusters are formed. The solutions of alkali metals in liquid ammonia act as powerful reducing agents and are used in organic reactions.
Solubility In Mercury
The alkali metals dissolve readily in mercury to form amalgams. The process is highly exothermic. The amalgams are also used as reducing agents in organic synthesis.
Anomalous Behaviour Of Lithium
The first element, lithium, of Group 1 differs from the other members of this group in many respects. The main reasons for the anomalous behaviour of lithium are as follows.
- The exceptionally small size of the lithium atom and its ion.
- The higher polarising power (charge to radius ratio) of Li+ is due to its smaller size
and the covalent character of its compounds. - The high ionisation enthalpy and low electropositivity of lithium as compared to other alkali metals.
- absence of vacant d-orbitals in its valence shell.
Some of the important properties in respect of which lithium differs from other members of the group are as follows.
- Lithium is harder than the other alkali metals.
- The melting point and boiling point of lithium are much higher than those of the other alkali metals.
- Lithium is the least reactive as observed by its gentle behaviour towards water. In contrast/ the other alkali metals react violently with water.
- In reaction ‘with oxygen, lithium forms only the monoxide (Li2O) while other alkali metals form the peroxide (as in the case of sodium) or the superoxide (as in the case of potassium).
- The ionic character of lithium salts is less pronounced than that of the salts of other alkali metals. This is because of the high polarising power of Li+. (The partial covalent character of ionic bonds. Due to its small size, Li+ has the maximum tendency to draw electrons towards itself from the negative ion in the salt molecule. This results in the distortion of the electronic cloud of the anion.
- This distortion is known as polarisation. When the degree of polarization is large, the concentration of electrons increases between the two atoms. Therefore, the covalent character of the molecule increases.
- Lithium is the only alkali metal that combines directly with the nitrogen of air to form the corresponding nitride.
- \(6 \mathrm{Li}+\mathrm{N}_2 \rightarrow 2 \mathrm{Li}_3 \mathrm{~N}\)
- The hydroxides, carbonates and nitrates of lithium decompose on heating to form lithium oxide. (Other alkali metal hydroxides and carbonates are stable and the nitrates decompose to give the corresponding nitrite.)
- \(2 \mathrm{LiOH} \stackrel{\text { heat }}{\longrightarrow} \mathrm{Li}_2 \mathrm{O}+\mathrm{H}_2 \mathrm{O}\)
- \(\mathrm{LiCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{Li}_2 \mathrm{O}+\mathrm{CO}_2\)
- \(4 \mathrm{LiNO}_3 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{Li}_2 \mathrm{O}+4 \mathrm{NO}_2+\mathrm{O}_2\)
- \(2 \mathrm{NaNO}_3 \longrightarrow 2 \mathrm{NaNO}_2+\mathrm{O}_2\)
- The hydroxide of lithium (LiOH) is a weak base, whereas the hydroxides of other alkali metals are strong bases.
- Due to their covalent nature, lithium halides (e.g., LiCl) are soluble in organic solvents, unlike the halides of other alkali metals. Also, LiCl is deliquescent and crystallises as LiCl.2H20.
- Due to its smaller size, the lithium-ion (Li+) is more strongly hydrated in an aqueous solution than other alkali metal ions.
Diagonal Relationship Of Lithium With Magnesium
As you know, the first element (Li) of Group 1 shows similarities in properties with the second element (Mg) of Group 2 (the diagonally opposite element).
This is referred to as a diagonal relationship.
Lithium resembles magnesium with respect to the following important properties.
- Both lithium and magnesium are equally hard and light.
- The similarities in lithium and magnesium are mainly due to their similar ionic size (Li+ =76 pm, Mg2+ = 72 pm). Both have nearly equal atomic radii, electronegativities and polarising powers.
- The melting and boiling points of both lithium and magnesium are fairly high.
- On heating in nitrogen both lithium and magnesium form nitrides, which are ionic compounds.
- \(\begin{aligned}
6 \mathrm{Li}+\mathrm{N}_2 & \rightarrow 2 \mathrm{Li}_3 \mathrm{~N} \\
3 \mathrm{Mg}+\mathrm{N}_2 & \rightarrow \mathrm{Mg}_3 \mathrm{~N}_2
\end{aligned}\)
- \(\begin{aligned}
- The hydroxides, carbonates and nitrates of both lithium and magnesium decompose on heating to give the corresponding oxides. The chemical reactions of lithium compounds have been discussed in the previous section.
- \(\mathrm{Mg}(\mathrm{OH})_2 \stackrel{\text { heat }}{\longrightarrow} \mathrm{MgO}+\mathrm{H}_2 \mathrm{O}\)
- \(\mathrm{MgCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{MgO}+\mathrm{CO}_2\)
- \(2 \mathrm{Mg}\left(\mathrm{NO}_3\right)_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{MgO}+4 \mathrm{NO}_2+\mathrm{O}_2\)
- The hydroxides of both lithium and magnesium are weak bases.
- The hydroxides, carbonates and fluorides of both lithium and magnesium are sparingly soluble in water.
- The halides of lithium and magnesium are soluble in organic solvents (they are covalent compounds).
- The chlorides of both lithium and magnesium are deliquescent and separate from aqueous solutions as hydrated salts (LiCl2–2H2O and MgCl2-6H20).
Uses
Some of the important uses of alkali metals are as follows.
- Lithium finds use in making useful alloys like the lithium-lead alloy used for making toughened bearings.
- The lithium-magnesium alloy has high tensile strength and is used to manufacture aircraft components.
- Lithium is also employed in the production of thermonuclear energy required for propelling rockets and guided missiles.
- Sodium metal (molten) or its alloys with potassium are used as a coolant in nuclear reactors. The sodium-lead alloy is used for the preparation of lead tetraethyl [Pb(C2H5)4], which is used antiknock agent in petrol. However, its use has nowadays been discouraged as it causes environmental problems. Potassium chloride is used as a fertiliser and potassium hydroxide is used in the manufacture of soft soap.
- Caesium finds use in the manufacture of photoelectric cells.
Compounds of Sodium
Sodium is the most abundant of the alkali metals. Being extremely reactive, it does not occur in the free state.
Sodium chloride (common salt) is the most common compound of sodium, but many others are also known. Sodium is commercially the most important metal of all alkali metals.
It is used in the manufacture of sodium carbonate, sodium hydroxide, sodium chloride and sodium bicarbonate, which are of great use in the chemical industry.
The process of manufacture, properties and uses of some of these compounds are as follows.
Sodium carbonate
It is also known as washing soda or soda ash. Soda ash is the white powder of sodium carbonate which aggregates on exposure to air due to the formation of monohydrates.
Na2CO3-H20 is a monohydrate and Na2CO310H2O is a decahydrate known as washing soda.
Since pre-historic times, it has been found to occur as natural deposits called trona (Na2CO3-NaHCO3-2H2O) in dried-up lake beds in Egypt. It is also mined in the USA and Kenya. The deposit of trona is converted into sodium carbonate by heating.
⇒ \(2\left(\mathrm{Na}_2 \mathrm{CO}_3 \cdot \mathrm{NaHCO}_3 \cdot 2 \mathrm{H}_2 \mathrm{O}\right) \stackrel{\text { heat }}{\longrightarrow} 3 \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+5 \mathrm{H}_2 \mathrm{O}\)
The Solvay (or ammonia-soda) process
Sodium carbonate is manufactured by the Solvay or the ammonia-soda process. In this process, a purified concentrated solution of sodium chloride (brine) is saturated with ammonia gas.
The ammoniacal brine solution is then carbonated with carbon dioxide, forming sodium bicarbonate, which is insoluble in brine solution due to the common-ion effect and is filtered off.
The sodium bicarbonate on heating decomposes to give anhydrous sodium carbonate. We cannot prepare potassium carbonate by this process as the solubility of KHCO3 is large in brine solution. The reactions taking place during the process are as follows.
⇒ \(\mathrm{NH}_3+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \rightarrow \mathrm{NH}_4 \mathrm{HCO}_3\)
⇒ \(\mathrm{NaCl}+\mathrm{NH}_4 \mathrm{HCO}_3 \rightarrow \mathrm{NaHCO}_3 \downarrow+\mathrm{NH}_4 \mathrm{Cl}\)
⇒ \(2 \mathrm{NaHCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}\)
Initially, the carbon dioxide required in the first step is generated by heating limestone.
⇒ \(\mathrm{CaCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2\)
Subsequently, the CO2 generated by heating sodium bicarbonate is used. The ammonia gas required for the reaction forming ammonium bicarbonate is obtained from CaO2 (quicklime), which is shaken with water and then boiled with the ammonium chloride produced when sodium chloride reacts with ammonium bicarbonate.
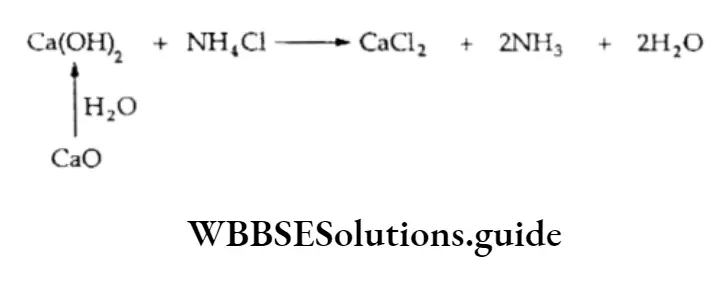
The only raw materials used in the Solvay process are sodium chloride (common salt), limestone (to produce C02 initially) and ammonia. The by-product obtained in this process is calcium chloride, which is not of much use.
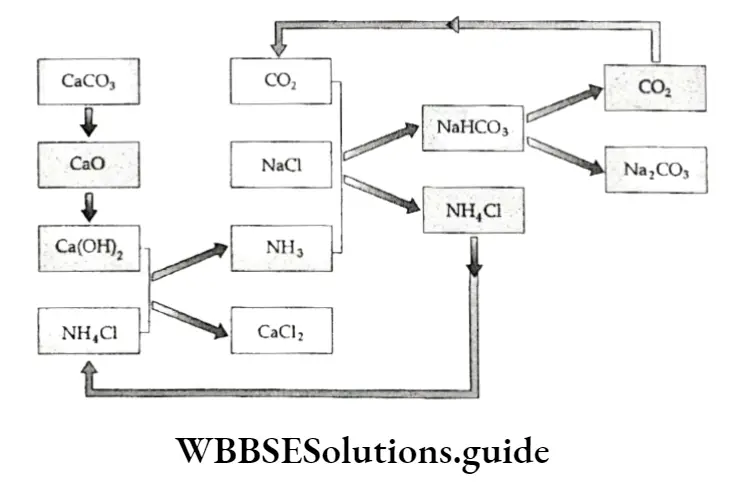
The plant used consists of an ammoniation tower (in which the brine solution is saturated with ammonia and a little carbon dioxide), a filter (in which the ammoniacal brine along with carbon dioxide is filtered), a carbonating tower (in which the ammoniacal liquor is carbonated), a vacuum filter (in which the sodium bicarbonate obtained is filtered) and the ammonia recovery tower (in which calcium hydroxide and ammonium chloride is heated to give ammonia gas).
The diagrammatic representation of the plant used for the Solvay process
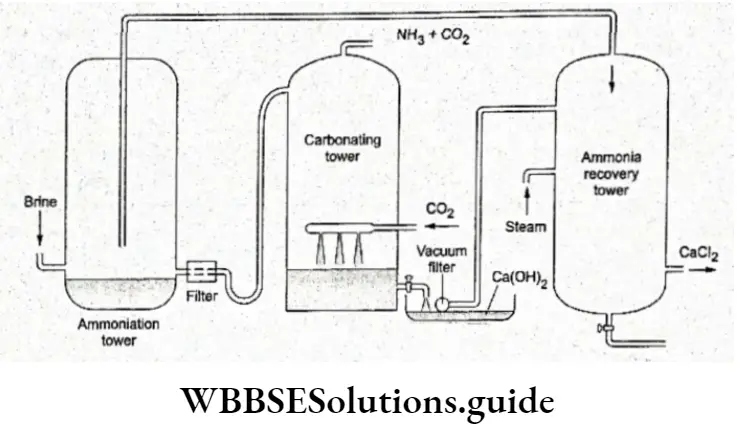
Properties Of Sodium carbonate
Sodium carbonate is a white, crystalline substance and is obtained as a decahydrate (Na2CO3TOH2O). On heating at 373 K, the decahydrate changes to the monohydrate. At a temperature above 373 K, the monohydrate becomes completely anhydrous.
⇒ [Latex]\mathrm{Na}_2 \mathrm{CO}_3 \cdot 10 \mathrm{H}_2 \mathrm{O} \stackrel{373 \mathrm{~K}}{\longrightarrow} \mathrm{Na}_2 \mathrm{CO}_3 \cdot \mathrm{H}_2 \mathrm{O}+9 \mathrm{H}_2 \mathrm{O}[/Latex]
⇒ [Latex]\mathrm{Na}_2 \mathrm{CO}_3 \cdot \mathrm{H}_2 \mathrm{O} \longrightarrow 3 / 3 \mathrm{~K} \longrightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{H}_2 \mathrm{O}[/Latex]
The anhydrous sample absorbs moisture from the air and gives Na2C03-H20. It reacts with ads to give carbon dioxide with effervescence.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{HCl} \rightarrow 2 \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
Sodium carbonate, when hydrolysed with water, forms an alkaline solution.
⇒ \(\mathrm{CO}_3{ }^{2-}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{HCO}_3{ }^{-}+\mathrm{OH}^{-}\)
“S-block elements, valency, ionization energy, and periodic behavior”
Sodium carbonate reacts with hot milk of lime to form sodium hydroxide.
⇒ \(\mathrm{Ca}(\mathrm{OH})_2+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow 2 \mathrm{NaOH}+\mathrm{CaCO}_3\)
Uses Of Sodium carbonate
- Sodium carbonate is used in the manufacture of soap, detergents, paper, glass, phosphates and silicates.
- It is also used in the manufacture of textiles, paints and dyes.
- Sodium carbonate is employed for water softening, laundering and cleaning.
- It is used in the laboratory as a reagent and as a primary standard in acid-base titrations. A mixture of sodium carbonate and potassium carbonate is used as a fusion mixture.
Sodium hydroxide
Sodium hydroxide, also known as caustic soda, is the most important alkali used iri the chemical industry.
It is manufactured by the electrolysis of a saturated solution of sodium chloride. In the past, it was also made by the causticising process, front sodium carbonate. This process is not in much use nowadays as other methods are cheaper.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{Ca}(\mathrm{OH})_2 \rightarrow 2 \mathrm{NaOH}+\mathrm{CaCO}_3\)
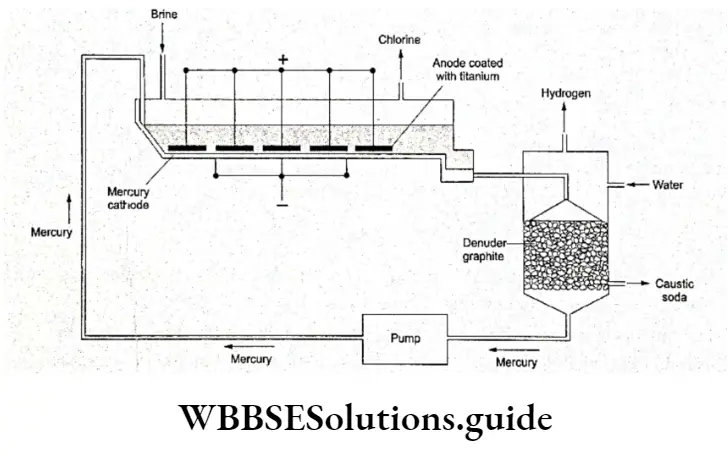
Electrolysis of sodium chloride solution in a Castner-Kellner Cell
In this cell, a solution of sodium chloride (brine) is electrolysed. The mercury cathode is made to flow along the bottom of the cell. The brine also moves along the cathode and the sodium ions are reduced to form sodium metal.
⇒ \(At cathode:
\begin{aligned}
& \mathrm{Na}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Na} \\
& \mathrm{Na}+\mathrm{Hg} \rightarrow \underset{\text { sodium amalgam }}{\mathrm{Na}-\mathrm{Hg}}
\end{aligned} \)
The sodium metal formed dissolves in mercury to form an amalgam (a loose alloy), which is pumped to a different compartment (called the denuder).
In this compartment H20 trickles over lumps of graphite (here acting as an inert solid); this results in the reaction of water with the amalgam, producing an NaOH solution. The strength of the NaOH collected from this compartment is up to 50%.
⇒ \(\mathrm{Na} \text { (amalgam) }+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{NaOH}+\frac{1}{2} \mathrm{H}_2+\mathrm{Hg}\)
The mercury is recycled back to the electrolysis tank. Hydrogen and chlorine are the two by-products formed. Chlorine is liberated at the anode.
⇒At anode:\(\begin{aligned}
\mathrm{Cl}^{-} & \rightarrow \mathrm{Cl}+\mathrm{e}^{-} \\
\mathrm{Cl} & +\mathrm{Cl} \rightarrow \mathrm{Cl}_2
\end{aligned}\)
In the past, the anodes were made of graphite. However, due to traces of dioxygen produced in a side reaction, the graphite anode became pitted (holes were formed) because of the formation of C02.
Nowadays, in the Castner-Kellner cell, the anodes are made of steel coated with titanium. Titanium is very resistant to corrosion, so the problem of pitting is avoided. An additional advantage is that titanium lowers the electrical resistance.
Nowadays, a nafion membrane is also used in the cell to separate the anolyte and the catholyte. The nation is a 4 polymer of certain organic compounds.
Properties Of Sodium hydroxide
Sodium hydroxide is a white, deliquescent solid and absorbs moisture from the air. It can even react with traces of carbon dioxide present in the air to form a solid hydrated carbonate.
⇒ \(2 \mathrm{NaOH}+\mathrm{CO}_2 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3 \cdot \mathrm{H}_2 \mathrm{O}\)
It is soluble in water and forms a caustic alkali, which is one of the strongest bases known in an aqueous solution.
(Potassium hydroxide is another strong base.) Its solution is soapy to the touch and being basic in nature gives a pink colour with phenolphthalein. Some of the other properties of sodium hydroxide are as follows.
It reacts with acids to form salt and water.
⇒ \(\mathrm{NaOH}+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}\)
It precipitates hydroxides of metals from metallic salt solutions.
⇒ \(\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3+6 \mathrm{NaOH} \rightarrow 2 \mathrm{Al}(\mathrm{OH})_3+3 \mathrm{Na}_2 \mathrm{SO}_4\)
⇒ \(\underset{\text { ferric sulphate }}{\mathrm{Fe}_2\left(\mathrm{SO}_4\right)_3}+6 \mathrm{NaOH} \rightarrow \underset{\text { ferric hydroxide(red ppt) }}{2 \mathrm{Fe}(\mathrm{OH})_3}+3 \mathrm{Na}_2 \mathrm{SO}_4\)
⇒ \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3+6 \mathrm{NaOH} \rightarrow 2 \mathrm{Cr}(\mathrm{OH})_3+3 \mathrm{Na}_2 \mathrm{SO}_4\)
On being heated with ammonium salts, sodium hydroxide liberates ammonia.
⇒ \(\mathrm{NH}_4 \mathrm{Cl}+\mathrm{NaOH} \rightarrow \mathrm{NaCl}+\mathrm{NH}_3+\mathrm{H}_2 \mathrm{O}\)
On treatment with caustic soda, certain metals like aluminium, zinc, tin and silicon liberate hydrogen.
⇒ \(\mathrm{Zn}+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{ZnO}_2+\mathrm{H}_2
sodium zincate\)
⇒ \(2 \mathrm{Al}+2 \mathrm{NaOH}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \underset{\text { sodium aluminate }}{2 \mathrm{NaAlO}_2}+3 \mathrm{H}_2\)
⇒ \(\mathrm{Si}+2 \mathrm{NaOH}+\mathrm{H}_2 \mathrm{O} \rightarrow \underset{\text { sodium silicate }}{\mathrm{Na}_2 \mathrm{SiO}_3}+2 \mathrm{H}_2\)
Uses Of Sodium hydroxide
- Sodium hydroxide is used in the manufacture of soaps, paper, viscose rayon (artificial silk) and dyes.
- It is used in the petroleum industry for refining crude oils. It is also used as a reagent in laboratories.
- Sodium hydroxide is also employed in the extraction of metals like aluminium.
- Its aqueous or alcoholic solution is used for carrying out organic reactions.
Sodium chloride
Sodium chloride (NaCl), also called common salt, is found most abundantly in seawater. It is also found in salt wells and in deposits of rock salt. In countries like India, it is obtained mainly by the evaporation of seawater.
Very pure sodium chloride is prepared by crystallisation of a clear, saturated NaCl solution.
It generally contains sodium sulphate (Na2SO4), calcium sulphate (CaSO4), calcium chloride (CaCl2) and magnesium chloride (MgCl2) as impurities. Both CaCl2 and MgCl2, being deliquescent, absorb moisture from the atmosphere.
To obtain pure NaCl, the crude salt obtained is dissolved in water and then filtered. The insoluble impurities are removed and the filtrate is then saturated with HC1 gas when pure crystalline sodium chloride separates out (due to the common-ion effect).
Properties Of Sodium chloride
The melting point of sodium chloride is 801°C. It is soluble in water and glycerol, and slightly soluble in alcohol.
Uses Of Sodium chloride
- Sodium chloride is an essential constituent of our diet and is also used as a food preservative.
- On being mixed with water, NaCl gives a mixture that has a freezing point about 10°C lower than that of pure water.
- Sodium chloride mixed with ice (to achieve low temperatures) is used in the traditional method of making ice cream.
- It is used in the manufacture of caustic soda, sodium peroxide, sodium carbonate and other sodium compounds.
- NnCl is also employed in the manufacture of soap for salting out, and ion-exchange resins.
Sodium bicarbonate
It is commonly known as baking soda (NaHC03) and is obtained as the by-product of the ammonia-soda process (Solvay process) for the manufacture of sodium carbonate.
Ordinarily, sodium bicarbonate can be produced from sodium carbonate by passing carbon dioxide gas through its solution in water. Sodium bicarbonate, being sparingly soluble, precipitates out in the reaction.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaHCO}_3\)
Properties Of Sodium bicarbonate
Sodium bicarbonate is a white crystalline substance. Its aqueous solution is alkaline in nature (gives a yellow colour with methyl orange and no colour with phenolphthalein). On heating, it decomposes to form sodium carbonate and carbon dioxide is released.
⇒ \(2 \mathrm{NaHCO}_3 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2\)
“S-block elements, group 1 and group 2 properties, and periodic variations”
When used in baking powder the carbon dioxide gas released on heating leaves holes in cakes making them light and fluffy. NaHC03 reacts with adds with effervescence caused by to evolution of carbon dioxide.
⇒ \(\mathrm{NaHCO}_3+\mathrm{HCl} \rightarrow \mathrm{NaCl}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2\)
Uses Of Sodium bicarbonate
- It is used in medicine as an antacid. It is largely used in the treatment of acid spillage.
- Sodium bicarbonate is also used in fire extinguishers. NaHC03 reacts with add to produce C02, which extinguishes fires.
- It is also employed in the textiles, tanning, paper and ceramic industries.
- The hydrogen carbonate ion (HCO2–) has an important biological role as an intermediate between atmospheric CO2/H2CO3 and the carbonate ion (CO2–). For aquatic organisms, this is the most important and in some cases the only source of carbon.
Alkaline Earth Metals (Group 2 Elements)
The elements of Group 2 of the periodic table are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra).
All these elements are metallic in nature and are commonly known as alkaline earth metals.
The oxides of the metals magnesium, calcium, barium and strontium were known before the corresponding metals and were named alkaline earths since they were found in the earth’s crust and alkaline in nature.
Subsequently, all the corresponding metals were called alkaline earth metals. These metals are silvery-white and lustrous. They are soft but harder than alkali metals. were
Occurrence
Magnesium is the sixth most abundant element in the earth’s crust whereas calcium is the fifth most. Magnesium salts occur in seawater to the extent of 0.13%.
Calcium occurs as sedimentary deposits of CaCO3. Strontium and barium are much less abundant whereas beryllium is very rare. Radium, being radioactive, is extremely scarce.
Electronic Configuration
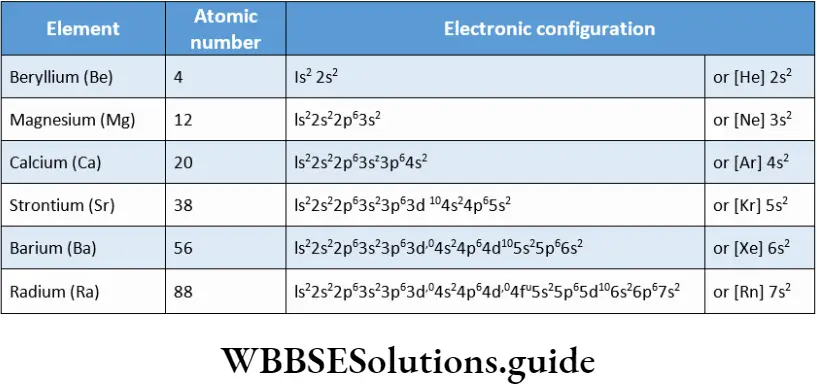
Alkaline earth metals have two electrons in the s orbital of their valence shell. Their general valence-shell electronic configuration is ns2, where n = 2 to 7.
The electronic configuration of the individual members.
Alkaline earth metals exhibit similar physical and chemical properties. Beryllium, the first member of the group, differs from the rest of the members.
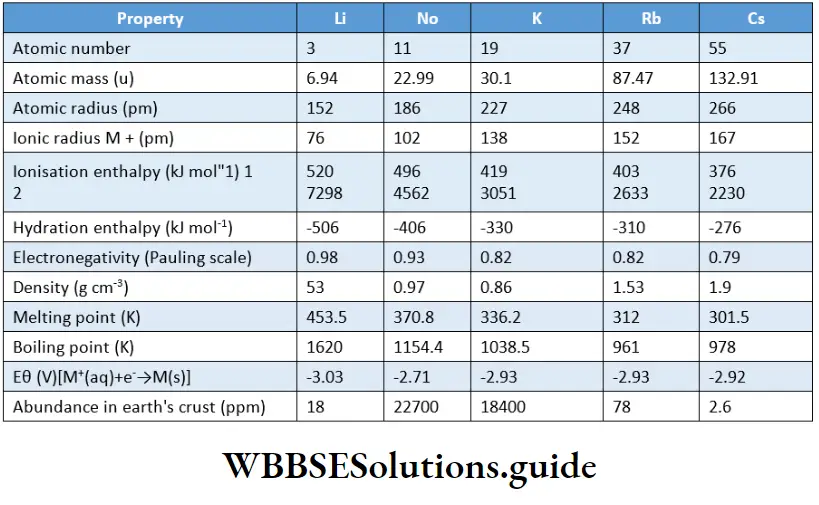
Let us now discuss the trend of variation in atomic and physical properties of the elements of Group 2. Group 2 elements show the same trend in properties as shown by the elements in Group 1.
Atomic And Ionic Radii
The atomic and ionic radii of elements of Group 2 increase from beryllium to radium because the addition of shells more than compensates for the increase in nuclear charge.
However, the atomic and ionic radii of alkaline earth metals are smaller than those of the corresponding alkali metals. This is because, in the same period, due to the extranuclear charge in the alkaline earth metal atom, the orbital electrons are drawn in.
Ionisation Enthalpy
The ionisation enthalpy of elements decreases within the group as the atomic number increases.
This is due to the increase in atomic size and in the magnitude of the screening effect caused by the electrons of the inner shells. Elements of Group 2 have higher values of first ionisation enthalpies than those of the corresponding elements of Group 1.
This is attributed to the smaller size and higher nuclear charge of alkaline earth metals due to which the electrons in their outermost shells are tightly held.
The values of the second ionisation enthalpies are higher than those of the first ionisation enthalpies in the case of alkaline earth metals since greater energy is required to pull out an electron from a positively charged ion than from a neutral atom.
However, the values of the second ionisation enthalpies of the alkaline earth metals are smaller than those of the corresponding alkali metals.
This is because the second electron is removed from a monovalent cation (in the case of an alkaline earth metal) when the atom has yet to acquire the stable noble-gas configuration.
Electropositive character and oxidation state
The electropositive character increases from beryllium to radium as the tendency of atoms to lose electrons increases.
However, alkaline earth metals are less electropositive than the corresponding alkali metals due to their high ionisation enthalpies.
The elements of Group 2 form bivalent cations and are in the bipositive (M2+) oxidation state, compared to the unipositive (M+) oxidation state of Group 1 elements.
Melting and boiling points
Alkaline earth metals have higher melting and boiling points than those of the corresponding alkali metals. This is because of the small atomic size and stronger metallic bonds due to the presence of two valence electrons in alkaline earth metals.
Density
The alkaline earth metals are denser than the corresponding alkali metals. The density decreases from beryllium to calcium and then increases from calcium to radium.
Flame colouration
Like alkali metals, alkaline earth metals also impart characteristic colours to a flame. Calcium, strontium and barium impart brick red, crimson and apple green colours respectively. The electrons absorb energy from the flame and get excited to higher energy levels.
When they drop back to the ground state, they emit this energy in the form of visible light.
Beryllium and magnesium do not give any flame colouration since their atoms are small in size and their electrons are strongly bound to the nucleus. They need a large amount of energy for excitation to higher energy levels and so much energy is not available in a Bunsen flame.
Photoelectric effect
Unlike alkali metals, alkaline earth metals do not exhibit a photoelectric effect. This is because their ionisation enthalpies are relatively high.
Hydration enthalpies
The hydration enthalpies of the cations of Group 2 elements are greater than those of the corresponding cations of Group 1 elements. This is due to the smaller size and increased charge on the cations formed by Group 2 elements.
The hydration enthalpies, decrease down the group as the size of the ions increases. Due to the high hydration enthalpies, the crystalline compounds of Group 2 elements contain more water of crystallisation than those of the corresponding Group 1 elements. For example, NaCl and KC1 are anhydrous but MgCl2–6H2O, CaG2–6H2O and BaCl2–2H2O have water of crystallisation.
Chemical properties
Alkaline earth elements are less reactive than alkali metals. Some of the important chemical properties of Group 2 elements are discussed below.
Nature of compounds
Like alkali metals, alkaline earth metals predominantly form ionic compounds. However, their salts are less ionic than those of alkali metals.
The tendency of the elements to form ionic compounds increases down the group due to the decrease in ionisation enthalpies.
The first member of the group, beryllium, forms covalent compounds because of its small size and high ionisation enthalpy. Magnesium sometimes exhibits covalency. All other elements form ionic compounds.
Reducing character
Alkaline earth metals have a lower reducing power than alkali metals. This is due to the higher ionisation enthalpies of alkaline earth metals than those of the corresponding alkali metals.
Reaction with oxygen
All the alkaline earth metals bum in oxygen to form oxides MO, except Sr and Ba, which form dioxides or peroxides (MOz) in the presence of an excess of oxygen.
Beryllium is relatively unreactive and bums brilliantly in the powdered form above 873 K.
Magnesium bums with dazzling brilliance in the air to give magnesium oxide and combine with the nitrogen of the air to give magnesium nitride (Mg3N2)
⇒ \(\begin{aligned}
2 \mathrm{Be}+\mathrm{O}_2 & \rightarrow 2 \mathrm{BeO} \\
2 \mathrm{Mg}+\mathrm{O}_2 & \rightarrow 2 \mathrm{MgO} \\
2 \mathrm{Ca}+\mathrm{O}_2 & \rightarrow 2 \mathrm{CaO} \\
\mathrm{Sr}+\mathrm{O}_2 & \rightarrow \mathrm{SrO}_2 \\
\mathrm{Ba}+\mathrm{O}_2 & \rightarrow \mathrm{BaO}_2
\end{aligned}\)
The oxides of alkaline earth metals (except that of beryllium, which is covalent) are basic in nature. The reactivity with oxygen increases from beryllium to barium. The oxide of beryllium (BeO) is amphoteric since it reacts with acids as well as alkalis.
⇒ \(\mathrm{BeO}+2 \mathrm{HCl} \rightarrow \mathrm{BeCl}_2+\mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{BeO}+2 \mathrm{NaOH} \rightarrow \underset{\text { sodium beryllate }}{\mathrm{Na}_2 \mathrm{BeO}_2}+\mathrm{H}_2 \mathrm{O}\)
Reaction With Water
The reactions of alkaline earth metals with water are less vigorous than those of the corresponding alkali metals.
Beryllium does not react with water, and magnesium reacts with steam or boiling water.
\(\mathrm{Mg}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{MgO}+\mathrm{H}_2\)The elements Ca, Sr and Ba react with cold water, liberating hydrogen and forming metal hydroxides.
\(\mathrm{Ca}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+\mathrm{H}_2\)Thus, the reactivity of water with alkaline earth metals increases as we move down the group.
The hydroxides of alkaline earth metals can also be obtained by dissolving their oxides (except BeO which is amphoteric) in water.
These hydroxides are strong bases. The hydroxides of alkaline earth metals are relatively less basic than those of the corresponding alkali metals.
This is because they have higher ionisation enthalpies and their ions are smaller in size and bipositive in character.
Consequently, the OH- ions are held more firmly by the M2+ ions and the M —OH bond does not break easily.
The basic character of the hydroxides increases from Be to Ba because of the decreasing ionisation enthalpy of the metal atoms.
⇒ \(\underset{\text { amphoteric }}{\mathrm{Be}(\mathrm{OH})_2}<\underset{\text { weak basc }}{\mathrm{Mg}(\mathrm{OH})_2}<\underset{\text { moderately strong hases }}{\mathrm{Ca}(\mathrm{OH})_2} \mathrm{Sr}(\mathrm{OH})_2<\underset{\text { strong base }}{\mathrm{Ba}(\mathrm{OH})_2}\)
These hydroxides are less soluble in water as compared to alkali metal hydroxides. However, they give strongly alkaline solutions because they readily form OH” ions in aqueous solutions. All these bases neutralise and are added to give salts and water.
⇒ \(\mathrm{M}(\mathrm{OH})_2+2 \mathrm{HCl} \rightarrow \mathrm{MCl}_2+2 \mathrm{H}_2 \mathrm{O}(\mathrm{M}=\mathrm{Mg}, \mathrm{Ca}, \mathrm{Sr}, \mathrm{Ba})\)
Ben-Ilium hydroxide, being amphoteric, reacts with alkalis as well to form the beryllate ion.
⇒ \(\mathrm{Be}(\mathrm{OH})_2+2 \mathrm{OH}^{-} \rightarrow\left[\mathrm{Be}(\mathrm{OH})_4\right]^{2-}beryllate ion\)
The solubility of the hydroxides in water increases with the increase in atomic number of the metal atom because of a decrease in lattice enthalpy with the increasing size of the metallic ion.
The hydroxides of beryllium and magnesium are almost insoluble, that of calcium is sparingly soluble while those of strontium and barium are increasingly more soluble.
The solubility of the hydroxides of alkaline earth metals depends on their lattice enthalpy and hydration enthalpy.
The lattice enthalpy of a crystal is the heat that would be released per mole if the ions (atoms/molecules) of the crystal were brought together from an infinite distance apart to form the lattice.
If the lattice enthalpy is higher, the ions are held more tightly in a crystal with the result that the solubility of the crystal in water is less.
The lattice enthalpy of the hydroxides of Group 2 elements decreases down the group due to the increase in the size of the cation.
Also, the greater the charge on the ion or the smaller the size of the ion, the greater the lattice enthalpy. If the hydration enthalpy of a salt is greater, the solubility of the salt in water is more.
The resultant of the lattice and the hydration enthalpies, Le., AÿH = A UlticeH – AhydH, becomes more as we move from Be(OH)2 to Ba(OH)2.
This accounts for the increase in solubility as we move down the group. Generally speaking, a hydroxide dissolves if its hydration enthalpy is more than its lattice enthalpy and vice versa.
The action of carbon dioxide on alkaline earth metal hydroxides On passing carbon dioxide gas through the solutions of alkaline earth metal hydroxides the corresponding carbonates are obtained.
⇒ \(\mathrm{M}(\mathrm{OH})_2+\mathrm{CO}_2 \rightarrow \mathrm{MCO}_3+\mathrm{H}_2 \mathrm{O}\)
The carbonates can also be obtained by the addition of sodium or ammonium carbonate to alkaline earth metal salts.
⇒ \(\mathrm{CaCl}_2+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow \mathrm{CaCO}_3+2 \mathrm{NaCl}\)
On being heated, the carbonates of alkaline earth metals decompose to give the corresponding oxides. The temperature of decomposition increases from beryllium to barium.
⇒ \(\mathrm{MCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{MO}+\mathrm{CO}_2\)
The solubility of the carbonates of alkaline earth metals decreases in the group. This is due to a decrease in hydration enthalpy as the lattice enthalpy remains almost unchanged.
Reaction with hydrogen
On heating, alkaline earth metals, except beryllium, combine with hydrogen to form metal hydrides.
⇒ \(\mathrm{M}+\mathrm{H}_2 \stackrel{\text { neat }}{\longrightarrow} \mathrm{MH}_2\)
Beryllium hydride can, however, be prepared by the reduction of beryllium chloride with lithium aluminium hydride.
⇒ \(2 \mathrm{BeCl}_2+\mathrm{LiAlH}_4 \rightarrow 2 \mathrm{BeH}_2+\mathrm{LiCl}+\mathrm{AlCl}_3\)
Among all the hydrides of Group 2 elements calcium hydride finds uses in the preparation of dry solvents.
Salts of oxoacids —sulphates, nitrates and carbonates
The sulphates of alkaline earth metals are thermally stable. The solubility of the sulphates in water decreases down the group. Thus, BeS04 and MgS04 are readily soluble but CaS04 is sparingly soluble.
The rest are virtually insoluble. The higher solubilities of BeS04 and MgS04 are due to the high enthalpy of the solution of Be2+ and Mg2+ ions. The sulphates of Be, Mg and Ca have water of crystallisation, viz BeS04-4H20, Mg$04-7H20 and CaS04-2H20.
The nitrates of alkaline earth metals are prepared by dissolving their carbonates in dilute nitric acid. On heating, the nitrates decompose, forming oxides.
⇒ \(2 \mathrm{M}\left(\mathrm{NO}_3\right)_2 \stackrel{\text { heat }}{\longrightarrow} 2 \mathrm{MO}+4 \mathrm{NO}_2+\mathrm{O}_2\)
The carbonates of alkaline earth metals are insoluble in water. The metal carbonates are more stable to heat as we move down the group.
The carbonates decompose on heating to give carbon dioxide and the oxide. Beryllium carbonate is unstable and can be kept only in an atmosphere of C02.
Reaction with halogens
On heating with halogens, alkaline earth metals form the corresponding halides.
⇒ \(\mathrm{M}+\mathrm{X}_2 \rightarrow \mathrm{MX}_2\)
The halides can also be obtained by the action of halogen acids on metals, their oxides, hydroxides and carbonates. Beryllium halides cannot be prepared this way due to the formation of the hydrated ion,[Be(H20)4 ]2
⇒ \(\begin{gathered}
\mathrm{M}+2 \mathrm{HX} \rightarrow \mathrm{MX}_2+\mathrm{H}_2 \\
\mathrm{MO}+2 \mathrm{HX} \rightarrow \mathrm{MX}_2+\mathrm{H}_2 \mathrm{O} \\
\mathrm{M}(\mathrm{OH})_2+2 \mathrm{HX} \rightarrow \mathrm{MX}_2+2 \mathrm{H}_2 \mathrm{O} \\
\mathrm{MCO}_3+2 \mathrm{HX} \rightarrow \mathrm{MX}_2+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}
\end{gathered}\)
Beryllium chloride is prepared from the oxide by heating with carbon and chlorine.
⇒ \(\mathrm{BeO}+\mathrm{C}+\mathrm{Cl}_2 \stackrel{870-1070 \mathrm{~K}}{=} \mathrm{BeCl}_2+\mathrm{CO}\)
General characteristics
The halides of alkaline earth metals (except those of beryllium) are ionic in nature; the ionic character increases as the size of the metal ion increases.
Beryllium chloride and fluoride, being covalent (due to the smaller size of Be2*), are soluble in organic solvents whereas the halides of other alkaline earth elements are insoluble. The fluorides of all alkaline earth metals except that of Be are almost insoluble in water. Beryllium fluoride is soluble because it has a high hydration energy. Also, the beryllium halides do not conduct electricity.
Beryllium chloride has low melting as compared to the halides of other alkaline earth metals.
The halides of alkaline earth metals (except those of beryllium) dissolve in water giving acidic solutions from which hydrates such as MgCl 2 -6H20, CaCl 2–6H20 and BaCl 2-2H20 crystallise out.
The tendency to form hydrated halides decreases with the increasing size of the metal ions structure of BeCl2 In the solid phase, BeCl 2 has the following polymeric chain structure.
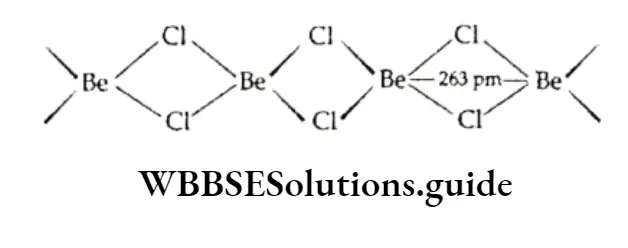
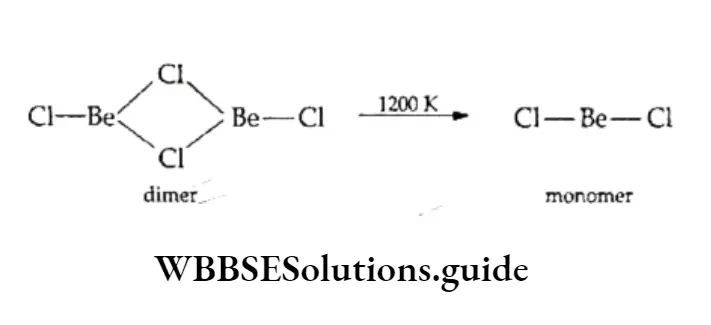
However, in the vapour phase, it forms a chloro-bridged dimer, which dissociates into the linear triatomic monomer at high temperatures (approximately 1200 K).
Anomalous behaviour of beryllium
Beryllium is anomalous in many of its properties. It differs much more from the rest of the members of Group 2 than lithium does from the other elements of Group 1.
The main reasons for the anomalous behaviour of beryllium are the small size of the atom, high ionisation enthalpy and the absence of d orbitals in its valence shell. Beryllium differs from the rest of the members of its group in many of its properties, as listed below.
- Beryllium is harder than the other elements of its group.
- It has higher melting and boiling points.
- It does not react with water even on heating, unlike the other members of the group.
- It does not react with ads to liberate hydrogen, unlike the other members of the group.
- Beryllium forms covalent compounds while the other members of Group 2 form ionic compounds.
- Beryllium oxide is amphoteric whereas the oxides of other metals of Group 2 are basic.
- Beryllium does not combine directly with hydrogen to form the hydride whereas other metals of this group form hydrides directly.
- Beryllium carbide reacts with water to give methane. In contrast, the other alkaline earth metals give acetylene.
⇒ \(\mathrm{MgC}_2+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Mg}(\mathrm{OH})_2+\mathrm{C}_2 \mathrm{H}_2\)
Diagonal Relationship Of Beryllium With Aluminium
Beryllium, the first element of Group 2, shows similarities in properties with aluminium, the second member of the next higher group, just as lithium shows a diagonal relationship with magnesium. Some of the properties in which beryllium resembles aluminium are as follows.
- Both beryllium and aluminium ions have comparable ionic radius and charge-to-radius ratio.
- Both form covalent compounds, which are soluble in organic solvents, e.g., BeCl2 and A1C13.
- The oxides of both beryllium and aluminium, viz. BeO and Al203, are high-melting, hard solids. Also, the two oxides are amphoteric and they dissolve both in acids and alkalies. With excess alkalies the hydroxides of Be and A1 form beryllate and aluminate ions respectively.
- Both beryllium and aluminium do not react readily with acids, due to the presence of an oxide on the surface of the metal.
- In reaction with water, the carbides of both beryllium and aluminium liberate methane.
⇒ \(\begin{gathered}
\mathrm{Be}_2 \mathrm{C}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{BeO}+\mathrm{CH}_4 \\
\mathrm{Al}_4 \mathrm{C}_3+6 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{Al}_2 \mathrm{O}_3+3 \mathrm{CH}_4
\end{gathered}\)
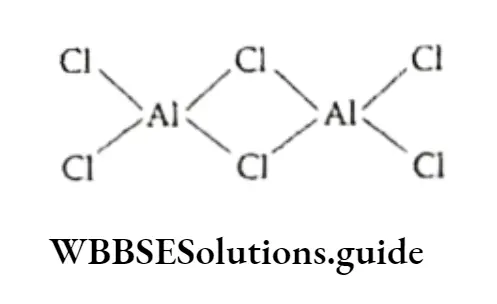
The chlorides of both metals have a bridged polymeric structure
Uses
Some of the important uses of alkaline earth metals are as follows.
- Beryllium finds use in making alloys like copper-beryllium, which is used in the preparation of high strength springs.
- Beryllium foil is also used in X-ray tubes to filter out visible light and allow only X-rays to pass through.
- Magnesium is also used in the preparation of alloys. Duralumin—a magnesium-aluminium-copper alloy—is very light and durable and, therefore, used in the manufacture of aeroplanes and automobile parts. Magnalium, an alloy of aluminium and magnesium, is used for making beam balances.
- Magnesium in the form of powder is used in incendiary bombs and signals and flash powders.
- Calcium is used in the extraction of metals from their oxides.
- Both calcium and barium are used to remove air from vacuum tubes because they can react with oxygen and nitrogen at elevated temperatures.
- Radium, being radioactive, is used in radiotherapy in the treatment of cancer.
Compounds Of Calcium
Some of the commercially important compounds of calcium are calcium oxide, calcium hydroxide, calcium carbonate, calcium sulphate and cement. The process of manufacture, properties and uses of these compounds are discussed here.
Calcium oxide
Calcium oxide (CaO) is a white, amorphous solid with a high melting point—2845 K. It is commonly known as quicklime and is obtained commercially by heating limestone at 1273 K in specially designed lime kilns.
⇒ \(\mathrm{CaCO}_3 \stackrel{1273 \mathrm{~K}}{\rightleftharpoons} \mathrm{CaO}+\mathrm{CO}_2\)
The reaction is reversible. Maximum yields of calcium oxide are obtained if the carbon dioxide is allowed to escape from the kiln. Carbon dioxide escapes above 1100 K.
Properties Of Calcium oxide
1. Quicklime readily absorbs moisture and carbon dioxide. Treatment of quicklime with water produces calcium hydroxide, Ca(OH)2. The reaction is highly exothermic and occurs with a hissing sound.
⇒ \(\mathrm{CaO}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{Ca}(\mathrm{OH})_2(\mathrm{aq}) \quad \Delta H=-645 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
“S-block elements, electronic configuration, oxidation states, and trends”
Calcium hydroxide is also called slaked lime. Calcium oxide is usually obtained in the form of hard lumps.
The lump gets disintegrated by the addition of a limited amount of water, and slaked lime is formed. The process is known as slaking of lime. Quicklime, slaked with soda (aqueous sodium hydroxide) gives a solid, soda lime.
Soda lime is a mixture of sodium hydroxide (NaOH) and calcium hydroxide, Ca(OH)2. It is much easier to handle soda lime than NaOH, which is corrosive.
Calcium oxide reacts with carbon dioxide to give calcium carbonate (CaO + COz ->CaC03). Though calcium carbonate occurs in nature as limestone, marble, etc., pure CaC03 is obtained by this process
Calcium oxide is a basic oxide and reacts with acids to form salts.
⇒ \(\mathrm{CaO}+2 \mathrm{HCl} \rightarrow \mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{O}\)
Calcium oxide combines with acidic oxides at a high temperature.
⇒ \(\begin{gathered}
\mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3 \\
6 \mathrm{CaO}+\mathrm{P}_4 \mathrm{O}_{10} \rightarrow 2 \mathrm{Ca}_3\left(\mathrm{PO}_4\right)_2
\end{gathered}\)
⇒ \(
\mathrm{CaO}+\mathrm{SO}_2 \rightarrow \mathrm{CaSO}_3
calcium sulphite\)
On heating with coke in an electric furnace at 2273-3273 K, calcium oxide forms calcium carbide.
⇒ \(\mathrm{CaO}+3 \mathrm{C} \stackrel{2273-3273 \mathrm{~K}}{\longrightarrow} \mathrm{CaC}_2+\mathrm{CO}\)
On heating with ammonium salts, calcium oxide liberates ammonia.
⇒ \(\mathrm{CaO}+2 \mathrm{NH}_4 \mathrm{Cl} \rightarrow \mathrm{CaCl}_2+2 \mathrm{NH}_3+\mathrm{H}_2 \mathrm{O}\)
Uses Of Calcium oxide
- Calcium oxide is used in the manufacture of bleaching powder, slaked lime, calcium carbide, cement, glass, mortar, etc.
- It is employed in making glass.
- It is used in steel-making to remove phosphates and silicates as slag.
- It is used as the basic lining in furnaces.
Calcium hydroxide
Also known as slaked lime, it is obtained by the action of water on calcium oxide.
⇒ \(\mathrm{CaO}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Ca}(\mathrm{OH})_2\)
It can also be obtained by the action of caustic alkalis on a soluble calcium salt.
⇒ \(\mathrm{CaCl}_2+2 \mathrm{NaOH} \rightarrow \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{NaCl}\)
Properties Of Calcium hydroxide
- Calcium hydroxide is a white, amorphous solid and is sparingly soluble in water. The aqueous solution is known as limewater.
- The suspension of calcium hydroxide in water is known as milk of lime. On heating above 700 K calcium hydroxide loses water to give calcium oxide (quicklime).
- Carbon dioxide reacts with limewater to give a white precipitate of calcium carbonate. This is the reason why carbon dioxide turns limewater milky. Thus, limewater is used to detect the presence of carbon dioxide gas.
⇒ \(\mathrm{Ca}(\mathrm{OH})_2+\mathrm{CO}_2 \rightarrow \mathrm{CaCO}_3 \downarrow+\mathrm{H}_2 \mathrm{O}\)
However, if carbon dioxide is passed for a longer time, the milkiness disappears because the insoluble CaC03 gets converted into soluble calcium hydrogen carbonate, Ca(HC03 )
⇒ \(
\mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \rightarrow \mathrm{Ca}\left(\mathrm{HCO}_3\right)_2
soluble\)
Like C02, sulphur dioxide also reacts with limewater to give a white precipitate—of calcium sulphite.
⇒ \(\mathrm{Ca}(\mathrm{OH})_2+\mathrm{SO}_2 \rightarrow \mathrm{CaSO}_3 \downarrow+\mathrm{H}_2 \mathrm{O}\)
Chlorine reacts with milk of lime below 308 K to give bleaching powder.
⇒ \(
3 \mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{Cl}_2 \stackrel{<308 \mathrm{~K}}{\longrightarrow} \mathrm{Ca}(\mathrm{OCl})_2 \cdot \mathrm{CaCl}_2 \cdot \mathrm{Ca}(\mathrm{OH})_2 \cdot 2 \mathrm{H}_2 \mathrm{O}
bleaching powder\)
Though the formula for bleaching powder is usually written as Ca (OCl2), it is really a mixture.
On heating with ammonium chloride, calcium hydroxide liberates ammonia and with acids, it forms salts.
⇒ \(\begin{gathered}
\mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{NH}_4 \mathrm{Cl} \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaCl}_2+2 \mathrm{NH}_3 \uparrow+2 \mathrm{H}_2 \mathrm{O} \\
\mathrm{Ca}(\mathrm{OH})_2+2 \mathrm{HCl} \longrightarrow \mathrm{CaCl}_2+2 \mathrm{H}_2 \mathrm{O}
\end{gathered}\)
Uses Of Calcium hydroxide
- Calcium hydroxide is used for whitewashing and construction purposes.
- It is used for softening water. As you have already studied in Chapter 9, the temporary hardness of can be removed by adding slaked lime (the carbonates precipitate). This is called lime softening.
- It is used in the manufacture of bleaching powder, caustic soda and glass.
- Soda lime (a mixture of calcium hydroxide and caustic soda) is used in the decarboxylation of sodium salts of fatty acids.
⇒ \(\mathrm{CH}_3 \mathrm{COONa} \underset{\text { hest }}{\stackrel{\text { soda lime }}{\longrightarrow}} \mathrm{CH}_4 \uparrow+\mathrm{Na}_2 \mathrm{CO}_3\)
Plaster of Paris
Plaster of Paris is calcium sulphate hemihydrate, i.e., it has one molecule of water for every two calcium and two sulphate ions.
It is obtained by the controlled heating of gypsum at 393 K. If the heating is not controlled, the anhydrous salt is produced instead of the hemihydrate.
⇒ \(\begin{aligned}
& 2\left[\mathrm{CaSO}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}\right] \stackrel{\text { heat }}{\longrightarrow}\left(2 \mathrm{CaSO}_4\right) \cdot \mathrm{H}_2 \mathrm{O}+3 \mathrm{H}_2 \mathrm{O} \\
& \text { gypsum } \\
& \text { plaster of Paris } \\
& \text { (calcium sulphate } \\
& \text { hemilhydrate) } \\
&
\end{aligned}\)
Gypsum is a naturally occurring mineral. Calcium sulphate can be obtained by the reaction of any soluble calcium salt either with dilute sulphuric acid or with sodium sulphate and then used to prepare gypsum.
⇒ \(\begin{gathered}
\mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CaSO}_4+2 \mathrm{HCl} \\
\mathrm{CaCl}_2+\mathrm{Na}_2 \mathrm{SO}_4 \rightarrow \mathrm{CaSO}_4+2 \mathrm{NaCl}
\end{gathered}\)
Properties Of Plaster of Paris
1. Plaster of Paris is a white, powdery substance. A paste of plaster of Paris with one-third of its weight of water sets to a hard mass when allowed to stand for about fifteen minutes.
The setting into a hard mass occurs due to the interlocking of crystals of gypsum. This property is made use of in the setting of broken bones and involves the following two stages.
⇒ \(\underset{\text { plaster of Paris }}{\left(2 \mathrm{CaSO}_4\right) \cdot \mathrm{H}_2 \mathrm{O}} \underset{\text { setting }}{\stackrel{\mathrm{H}_2 \mathrm{O}}{\longrightarrow}} \underset{\substack{\text { gypsum } \\ \text { (orthorhombic crystals) }}}{\mathrm{CaSO}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}} \stackrel{\text { hardening }}{\longrightarrow} \underset{\substack{\text { gypsum } \\ \text { (monoclinic crystals) }}}{\mathrm{CaSO}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}}\)
The rate of setting of plaster of Paris is made faster by sodium chloride and slower by alum or borax. The air addition of alum to the plaster of Paris makes it set into a hard mass. This mixture is known as Keene’s cement.
On being heated above 475 K, plaster of Paris gives anhydrous calcium sulphate, commonly known as dead burnt plaster. It takes up water very slowly and does not set at all.
Uses Of Plaster of Paris
- Plaster of Paris finds use in surgical bandages, dentistry and orthopaedic plaster for setting broken bones.
- It is also used in making casts for toys and statues.
- Plaster of Paris is also used in building materials.
Calcium carbonate
Calcium carbonate is a white solid that occurs naturally in two crystalline forms: calcite and aragonite.
These materials make up the bulk of such rocks as marble, limestone and chalk. Calcium carbonate can be prepared by passing a limited amount of carbon dioxide through slaked lime or by the addition of sodium carbonate to calcium chloride.
⇒ \(\begin{gathered}
\mathrm{Ca}(\mathrm{OH})_2+\mathrm{CO}_2 \rightarrow \mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{O} \\
\mathrm{CaCl}_2+\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow \mathrm{CaCO}_3+2 \mathrm{NaCl}
\end{gathered}\)
The use of excess carbon dioxide to prepare calcium carbonate results in the formation of calcium bicarbonate.
Properties Of Calcium carbonate
1. On heating, calcium carbonate decomposes and carbon dioxide is released
⇒ \(\mathrm{CaCO}_3 \stackrel{1200 \mathrm{~K}}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2\)
2. It reacts with acids to liberate CO2
⇒ \(\begin{gathered}
\mathrm{CaCO}_3+2 \mathrm{HCl} \rightarrow \mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 \\
\mathrm{CaCO}_3+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CaSO}_4+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2
\end{gathered}\)
Uses Of Calcium carbonate
- Calcium carbonate is used as a building material in the form of marble.
- It is employed in the manufacture of quicklime and also of sodium carbonate by the Solvay process.
- With magnesium carbonate, calcium carbonate is used as a flux in the extraction of metals like iron.
- Calcium carbonate is used in toothpaste, face powders, antacids, adhesives, paints and distempers.
Cement
Cement is one of the most important building materials. When mixed with water and allowed to stand, it sets to a very hard mass, which resembles Portland rock—a natural limestone in the Isle of Portland, England.
Hence, cement was given the name Portland cement in 1824 by Joseph Aspidin, a mason who lived in Leeds, England.
Composition of cement The composition of cement is given in terms of oxides. The average composition is:
- CaO 60-65%
- Fe203 2.5%
- Si02 22-25%
- MgO 2-3%
- A1203 6-8%
A lesser proportion of lime than that given above results in a decrease in the strength of the cement, while a higher proportion causes the cement to crack after setting.
Cement which sets slowly contains an excess of silica while quick-setting cement contains an excess of alumina.
At higher temperatures, the calcium oxide reacts with the aluminosilicates and silicates to form a mixture of various silicates and aluminates of calcium, chiefly tricalcium silicate (Ca3Si05), dicalcium silicate (Ca2Si04) and tricalcium aluminate (Ca3 A1206). Of these, tricalcium silicate is the most important constituent.
Manufacture Of Cement
The most important raw materials needed for the manufacture of cement are limestone (which provides lime) and clay (which provides silica, along with oxides of aluminium, iron and magnesium). When clay and lime are strongly heated together in a rotatory kiln, they fuse and react to form cement clinker.
The following reactions take place.
⇒ \(\begin{aligned}
2 \mathrm{CaO}+\mathrm{SiO}_2 & \rightarrow 2 \mathrm{CaO} \cdot \mathrm{SiO}_2 \\
3 \mathrm{CaO}+\mathrm{SiO}_2 & \rightarrow 3 \mathrm{CaO} \cdot \mathrm{SiO}_2 \\
3 \mathrm{CaO}+\mathrm{Al}_2 \mathrm{O}_3 & \rightarrow 3 \mathrm{CaO} \cdot \mathrm{Al}_2 \mathrm{O}_3 \\
2 \mathrm{CaO}+\mathrm{Al}_2 \mathrm{O}_3 & \rightarrow 2 \mathrm{CaO} \cdot \mathrm{Al}_2 \mathrm{O}_3 \\
4 \mathrm{CaO}+\mathrm{Al}_2 \mathrm{O}_3+\mathrm{Fe}_2 \mathrm{O}_3 & \rightarrow 4 \mathrm{CaO} \cdot \mathrm{Al}_2 \mathrm{O}_3 \cdot \mathrm{Fe}_2 \mathrm{O}_3
\end{aligned}\)
The mixture (cement clinker) obtained is composed of silicates and aluminates. It is powdered and mixed with gypsum (1.5-2%) (CoS04-2H20) to slow down the process of setting. The final mixture is ground to a fine powder which is grey in colour and filled in airtight bags.
Setting Of Cement
When cement is to be used, it is mixed with water. The cement reacts with water to form a gelatinous mass (the reaction is exothermic), which slowly sets into a hard mass which has —Si—O—Si— and —Si—O—Al— chains.
The setting time depends on the final composition of the cement. Tricalcium silicate sets quickly, within 2-3 days.
Dicalcium silicate sets slowly and develops strength in 3-4 weeks. However, tricalcium aluminate sets instantaneously in the presence of water.
The demand for cement has increased considerably due to the increase in construction. Attempts are being made to find a substitute for cement. When mixed with cement, fly ash, a waste product of the steel industry, reduces the cost of cement without affecting its setting quality.
Uses Of Cement
A mixture of cement and sand (in the ratio of 1:3) is mixed with water (the required amount) and is used as mortar for the construction and plastering of brick walls.
A mixture of cement, sand and gravel (in the ratio 1: 2: 4) is mixed with water (required amount) and is known as concrete, which is used as a building material.
Concrete used in conjunction with iron frameworks is known as reinforced cement concrete (RCC). It is used for the construction of roofs and pillars, and also for the construction of dams, bridges, etc.
High-alumina cement is obtained by fusing limestone and bauxite with small amounts of SiOz and Ti02 at 1750-1900 K in a rotary kiln.
“S-block elements, chemical and physical properties, and periodic table trends”
It is more expensive than the usual Portland cement. However, it has the advantage that it is much quicker and acquires high strength in a short time (24 hours). It is used for making beams for bridges and buildings.
It can withstand temperatures up to 1800 K and is, therefore, used with refractory bricks in furnaces. An additional advantage of high-alumina cement is its resistance to seawater and dilute mineral acids.
Biological Role of Sodium, Potassium, Magnesium and Calcium
A number of elements play a very important role in biological systems. Of the 27 essential elements, 15 are metals.
The metals required in major quantities are Na, K, Mg and Ca. Minor quantities of Mn, Fe, Co, Cu, Zn and Mo, and trace amounts of V, Cr, Sn, Ni and Al are also required in biological systems.
Sodium and potassium ions balance the electrical charges associated with the negatively charged organic macromolecules in the cell. They also help to maintain the osmotic pressure inside the cell in order to keep it turgid and prevent its collapse.
Though sodium and potassium are similar in their chemical properties, their biological functions are quite different. Sodium ions are actively expelled from cells into the extracellular fluid whereas potassium ions are not.
In red blood cells, the ratio of potassium to sodium is 7:1 in human beings, rabbits, rats and horses, and 1:15 in cats and dogs. The difference in the amounts of each ion (Na+ and K+) in biological components is due to the quantitative difference in the ability of Na+ and K+ to penetrate cell membranes.
However, the different ratios of Na+ and K+ inside and outside the cell produce an electrical potential across the cell membrane; this is essential for the functioning of nerve and muscle cells. The movement of glucose into the cells is associated with Na ions. They enter the cell together.
The K+ ions inside the cell are essential for the metabolism of glucose, the synthesis of proteins and the activation of enzymes.
Thus, a sodium-potassium pump operates across the cell membranes, which is fuelled by the hydrolysis of ATP (adenosine triphosphate—the energy-currency molecule of living systems) to ADP (adenosine diphosphate).
Magnesium ions are concentrated inside animal cells and calcium ions are concentrated in the body fluids outside the cell, in the same way as K+ concentrates inside the cell and Na+ outside it.
Both Mg2+ and Ca2+ ions play a vital role in biological systems for the storage of energy. They are also essential for the transmission of impulses along nerve fibres. Magnesium is an important constituent of chlorophyll in green plants.
Calcium is an important constituent of bones and teeth. The amount of calcium in bones is nearly 30 grams at birth and builds up to about 1200 grams in an adult. Hence the individual daily amounts should reach 400 milligrams during the adolescent growth spurt.
Calcium ions are also important in blood clotting and are required to trigger the contraction of muscles and to maintain the regular beating of the heart.
The S-Block Elements Multiple Choice Questions
Question 1. The reducing characteristics of alkali metals follow the order:
- Na < K < Rb < Cs < Li
- Li < Cs < Rb < K < Na
- K < Na < Eb < Li < Cs
- Rb < Li < Cs < K < N a
Answer: 1. Na < K < Rb < Cs < Li
Question 2. What happens when CO2 is passed into limewater?
- The limewater becomes turbid due to the formation of calcium bicarbonate.
- The limewater becomes turbid due to the formation of calcium carbonate.
- The turbidity formed disappears on passing C02 for a long time.
- There is no change.
Answer: 2. The limewater becomes turbid due to the formation of calcium carbonate
Question 3. Which of the following reacts slowly with water?
- Na
- Ca
- Li
- k
Answer: 3. Ca
Question 4. A salt imparts a brick-red colour to a flame. The salt can be one of
- Na
- Ca
- Mg
- Li
Answer: 2. Ca
Question 5. The chloride of a metal is soluble in an organic solvent. The chloride can be
- CaCl2
- NaCl
- MgCl2
- BeCl2
Answer: 4. BeCl2
Question 6. Alkali metals do not occur in nature because they
- Have A Small Size
- Are very reactive
- Are Monovalent
- Are Radioactive
Answer: 2. Are very reactive
Question 7. From among the following, name the metal, which and its salts will impart a characteristic blue colour to a Bunsen flame.
- Na
- K
- Rb
- Cs
Answer: 4. Cs
“S-block elements, reactivity, flame test colors, and periodic classification”
Question 8. Slaked lime reacts with chlorine to give
- CaCl2
- CaO
- CaOCl2
- None Of These
Answer: 3. CaOCl2
Question 9. Gypsum on heating to 393 K gives
- CaSO4-2H2O
- CaSO4
- CaSO4.1/2 H2O
- None Of These
Answer: 3. CaSO4.1/2 H2O
Question 10. The by-product of the Solvay process is
- CO2
- NH3
- CaCl2
- None Of These
Answer: 3. CaCl2
Question 11. The thermal stability of alkaline earth metal carbonates decreases in the order
- BaCO3 > SrCO3> MgCO3> CaCO3
- BaCO3> SrCO3> CaCO3> MgCO3
- MgCO3> CaCO3> SrCO3> BaCO3
- CaCO3> SrCO3> MgCO3> BaCO3
Answer: 2. BaCO3> SrCO3> CaCO3> MgCO3
Question 12. The raw materials used in the manufacture of Na2C03 by the Solvay process are
- Sodium Chloride, Limestone And Ammonia
- Sodium chloride, limestone and carbon dioxide
- Sodium chloride and carbon dioxide
- Limestone and calcium chloride
Answer: 1. Sodium Chloride, Limestone And Ammonia
Question 13. Among the following, the correct order of increasing ionic character is
- MgCl2 < BeCI2 < BaCl2 < CaCl2
- BeCl2 < MgCl2 < CaCl2 < BaCl2
- MgCl2 < BaCl2 < BeCl2 < CaCl2
- BaCl2 < CaCl2 < BeCl2 < MgCl2
Answer: 2. BeCl2 < MgCl2 < CaCl2 < BaCl2
Question 14. In the context of alkali metals, which of the following increases with an increase in atomic number?
- Solubility of sulphates
- Solubility of hydroxides
- Ionisation energy
- Electronegativity
Answer: 2. Solubility of hydroxides
Question 15. A solution of sodium in liquid ammonia is blue. This blue colour is due to
- Ammonia
- Sodium metal
- Solvated electrons
- Sodium ion
Answer: 3. Solvated electrons
Question 16. Which of the following gives only the monoxide on heating in an excess of air?
- Rb
- K
- Cs
- Li
Answer: 4. Li
Question 17. The increasing order of the basic characters of MgO, SrO, Kfi) and Cs20 is
- CS2O < K2O < SrO < MgO
- MgO < SrO < K2O < Cs20
- SrO < MgO < CS2O < K2O
- MgO < SrO < Cs2O < K2O
Answer: 2. MgO < SrO < K2O < Cs20
Question 18. Which of the following is less stable thermally?
- LiF
- CsF
- NaCl
- RbF
Answer: 2. CsF
Question 19. Which of the following is most stable to heat?
- MgCO3
- SrCO3
- CaCO3
- BaCO3
Answer: 4. BaCO3
Question 20. An electric potential is produced across the membrane of living cells by the different ratios of certain metal ions inside
and outside cells. The metal ions involved are
- Ca2+ and Na+
- K+ and Ba2+
- Na+ and k+
- Mg2+ and Ca2+
Answer: 3. Na+ and k+
Question 21. When beryllium hydroxide and aluminium hydroxide are dissolved in an excess of an alkali, the ions formed are
- \(\left[\mathrm{Al}(\mathrm{OH})_4\right]^{-} \text {and }\left[\mathrm{Be}(\mathrm{OH})_4\right]^{-}\)
- \(\left[\mathrm{Al}^{3+}, \mathrm{Be}^{2+}\right] \text { and } \mathrm{OH}^{-}\)
- \(\left[\mathrm{Al}(\mathrm{OH})_4\right\}^{2-} \text { and }\left[\mathrm{Be}(\mathrm{OH})_4\right]^{-}\)
- \(\left[\mathrm{Be}(\mathrm{OH})_4\right]^{2-} \text { and }\left[\mathrm{Al}(\mathrm{OH})_4\right]^{-}\)
Answer: 4. \(\left[\mathrm{Be}(\mathrm{OH})_4\right]^{2-} \text { and }\left[\mathrm{Al}(\mathrm{OH})_4\right]^{-}\)
