WBCHSE Class 11 Chemistry Notes On Shapes Of Molecules
Molecules:
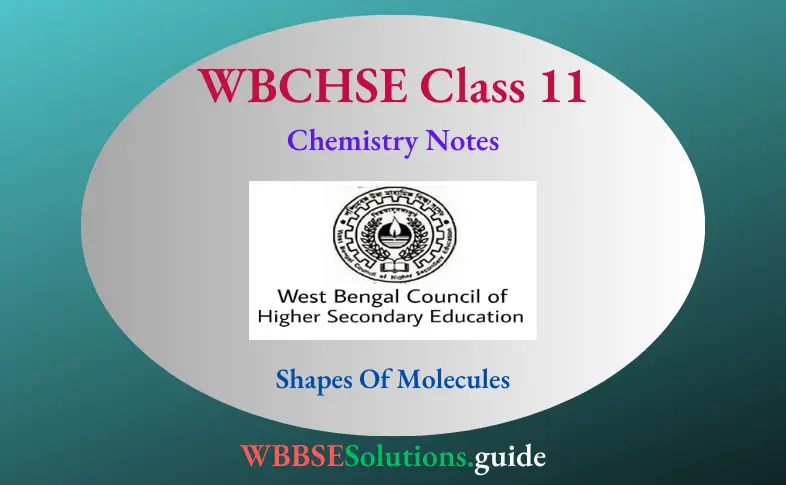
Molecules of different compounds exist in certain characteristic shapes. They exhibit linear, square planar, octahedral, trigonal planar, tetrahedral, or pentagonal bipyramidal geometric forms.
Many physical and chemical properties of a compound depend on the shape of its molecule.
- For example, the double spiral shape of DNA, a biomolecule, is responsible for many of its properties. Covalent bonds involve the overlapping of atomic orbitals. The bond is directional and tire shared electron pairs are localized in a region between the nuclei of the bonded atoms. So, the atoms constituting the molecule occupy definite positions concerning each other.
- This definite arrangement of atoms in a molecule is known as the geometry of the molecule and the three-dimensional model obtained by joining the points representing the bonded atoms represents the shape of the molecule.
- The study of molecular geometry is a very interesting branch of chemistry. One of the theories that explain the shapes of simple molecules is the valence shell electron pair repulsion (VSEPR) theory.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes
VSEPR theory: This theory was proposed by Gillespie and Nyholm in 1957. According to them, the orientation of bonds around the central atom of a molecule depends upon the total number of electron pairs (bonding as well as nonbonding) in its valence shell.
These electron pairs repel each other, so they would try to be as far away from each other as possible. In other words, the most favorable geometrical arrangement would be that in which the electron pairs are as far apart as possible to minimize repulsion so that the molecule can have maximum stability. This theory rests on the following four points.
“WBCHSE Class 11 Chemistry, shapes of molecules, notes and key concepts”
1. The electron pairs surrounding the central atom are as far apart as possible so that the repulsion between them may be the least and the molecule may have the maximum stability.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
2. The shape of the molecule depends upon the number of electron pairs (bonded and nonbonded) surrounding the central atom.
3. The repulsion between two lone pairs of electrons is the maximum and that between two bonded electron pairs is the minimum. The repulsion between electron pairs increases in the following order.
“Molecular geometry, bond angles, hybridization, and structural shapes, WBCHSE Class 11”
Bond pair-bond pair < bond pair-lone pair < lone pair-lone pair The difference in the force of repulsion exerted by electron pairs arises because a lone pair is under the field of influence of only one nucleus while a bond pair is under the influence of two nuclei.
4. Triple bonds cause maximum repulsion, followed by double and single bonds. Molecules have a regular geometry if the forces of repulsion between the various electron pairs around the central atom are equal. If all the electrons present in the valence shell of the central atom of a molecule participate in bonding then the central atom is surrounded only by bonded electron pairs which repel each other equally, so the molecule has a regular geometry.
 If, on the other hand, the central atom has both bonded and nonbonded electrons (lone pairs) in its valence shell, the molecule has an irregular geometry. This is because the different electron pairs do not repel each other equally.
If, on the other hand, the central atom has both bonded and nonbonded electrons (lone pairs) in its valence shell, the molecule has an irregular geometry. This is because the different electron pairs do not repel each other equally.
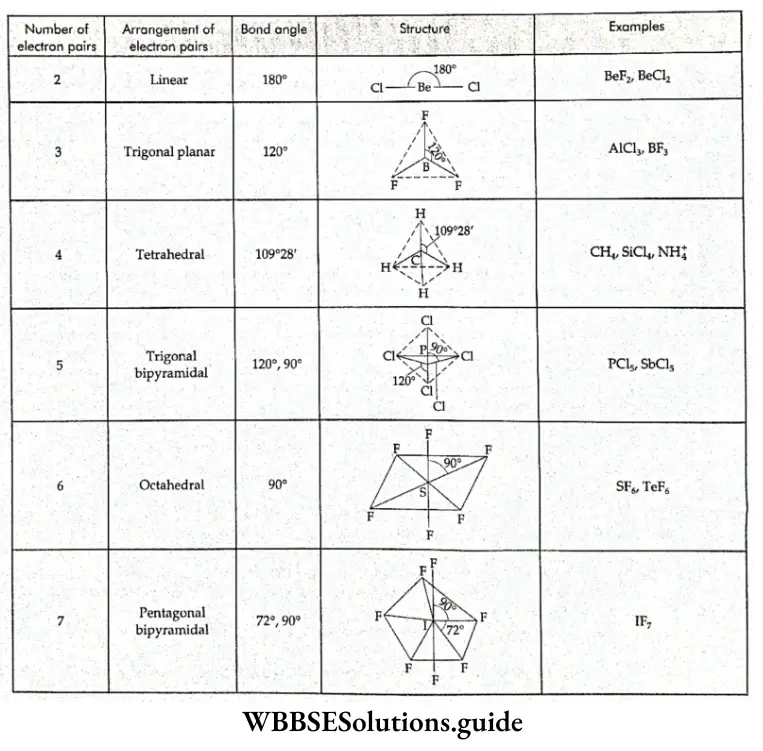
Shapes of BF3 and NF3 The electronic configuration of boron is 2, 3. In the BF3 molecule, the central atom (boron) has three electrons in its valence shell. It shares these with three fluorine atoms and forms three covalent bonds with these atoms.
Hence, all the electron pairs in the valence shell of the central atom of the BF3 molecule are bonded electron pairs, which repel each other equally. This is why the BF3 molecule has a regular trigonal planar shape and its bond angle is 120°.
“Shapes of molecules, WBCHSE Class 11, chemistry notes, and VSEPR theory”
Shapes Of Molecules: Theory And Examples For WBCHSE Class 11 Chemistry
Shapes of Molecules and Bond Angles in Chemistry for WBCHSE Class 11
- The electronic configuration of nitrogen is 2, 5. There are five electrons in the valence shell of the nitrogen atom but in the NF3 molecule, it uses only three of these to form covalent bonds with three fluorine atoms.
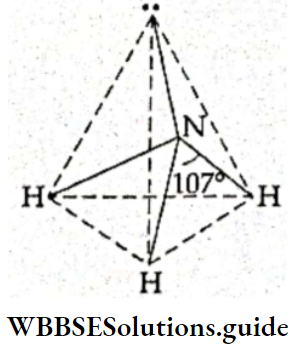
- Thus the valence shell of the central atom of the NF3 molecule contains 4 pairs of electrons—3 bonded pairs and a lone pair. The electron pairs surrounding the central atom repel each other unequally, so the electron pairs are arranged in the shape of a distorted tetrahedron. The bond angle (107°) is less than 109.5°, which is the bond angle of a regular tetrahedral molecule.
- Since one of the tetrahedral positions is occupied by a lone pair, the shape of the molecule is pyramidal.
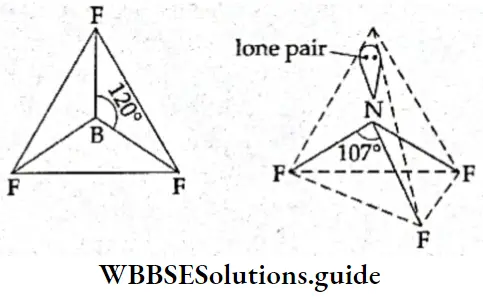
Shapes of CH4, NH3, and H2O In methane (CH4), the central carbon atom (Z = 6) has four electrons in its valence shell. It uses all of them to form bonds with four hydrogen atoms. Thus, the central atom in methane is surrounded by four bonded electron pairs, which repel each other equally, resulting in a regular tetrahedral geometry.
- In the ammonia molecule, the central nitrogen atom (Z = 7) has five electrons in its valence shell. It uses only three of these to form covalent bonds with three hydrogen atoms and is left with a lone pair of electrons. Thus, of the four pairs of electrons surrounding the central atom, three are bonded and one is the lone pair.
- Had all four electron pairs been bonded, the shape would have been tetrahedral. But due to unequal repulsion, the different pairs of electrons are arranged in the shape of a distorted tetrahedron and the bond angle reduces to 107°. One of the tetrahedral positions is occupied by a lone pair, so the shape of the molecule is pyramidal.
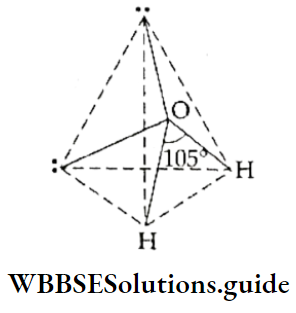
- In the water molecule, the central oxygen atom has six electrons in its valence shell (the electronic configuration is 2, 6). It uses two of these to form bonds with two hydrogen atoms and is left with two lone electron pairs. Thus, the central atom has four pairs of electrons in its valence shell and the shape of the molecule would have been tetrahedral, had all the pairs been bonded.
“WBCHSE Class 11 Chemistry, molecular shapes, electron pair repulsion, and bond angles”
But there are two bonded electron pairs and two nonbonded electron pairs which repel each other more strongly than the bonded pair. Tire electron pairs arrange themselves in the shape of a distorted tetrahedron and the angle reduces to 105°. Two of the tetrahedral positions are occupied by lone pairs, so the shape of the molecule is bent.
Shapes of PCl3 and PCl5 In PCl3 the central phosphorus atom has five electrons in its valence shell (electronic configuration 2, 8, 5). It uses only three of these to make three covalent bonds and is left with a lone pair of electrons.
Due to the unequal repulsion between the bonded electron pairs and the nonbonded electron pair, the arrangement of electron pairs is distorted tetrahedral with bond angle 107°. The shape of the molecule is pyramidal.
“WBCHSE Class 11, chemistry notes, on molecular geometry, and bonding theories”
Class 11 Chemistry Notes On Molecular Shapes And VSEPR Theory WBCHSE
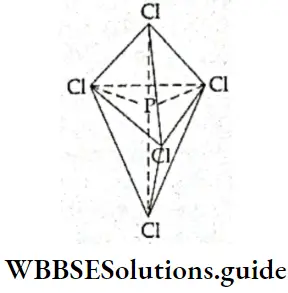
- In PCl3, phosphorus uses all the five valence electrons to form covalent bonds with five chlorine atoms. Thus, the valence shell of the central atom of PCl3 contains five bonded electron pairs which repel each other equally. Hence, the shape of the molecule is (regular) trigonal bipyramidal.
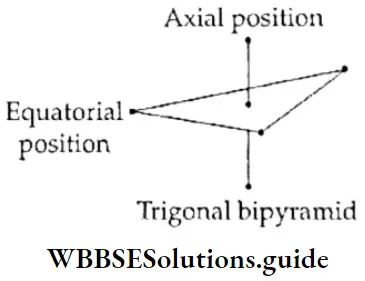
- Note that the bond angles in a trigonal bipyramidal arrangement arc not equal. This is one of the few cases (like a pentagonal bipyramid) where the bond angles arround an atom are not the same. Out of the five P—Cl sigma bonds, three lie in one plane and make an angle of 120° with each other.
- These are equatorial bonds. The other two P—Cl sigma bonds are oriented at right angles to the equatorial plane. One lies above and the other below the equatorial plane. In other words, the two bonds occupy axial positions and are known as axial bonds.
Shape of SF4, SF6, and ClF3 In SF4, the central atom (sulfur) has six electrons in the valence shell (electronic configuration, 2, 8, 6). It uses four of these to form covalent bonds with four fluorine atoms and is left with one unshared pair of electrons.
The total number of electron pairs surrounding the central atom is five, and the electron pairs arrange themselves in the shape of a trigonal bipyramid.
- In a trigonal bipyramidal structure, each axial bond experiences repulsion from three equatorial bonds at 90° and one axial bond at 180° to it, whereas each equatorial bond experiences repulsion from two equatorial bonds at 120° to it and two axial bonds at 90° to it.
- This means that the axial bond experiences greater repulsion from the other bonds. Therefore, if lone pairs are present in the molecule, they occupy equatorial positions to reduce repulsion. Also, the axial bonds are slightly longer and weaker than the equatorial bonds.
In SF4, the unshared pair of electrons occupies the equatorial position, and due to unequal repulsion, the arrangement of electron pairs is in the shape of a distorted trigonal bipyramid (see-saw shaped).
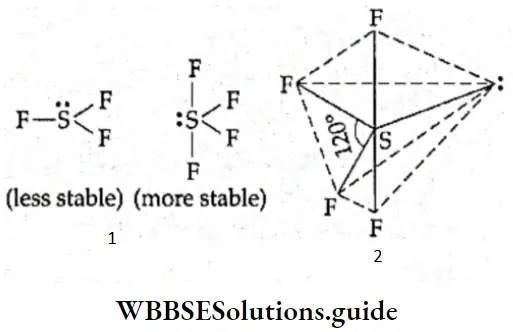
“Shapes of molecules, VSEPR theory, hybridization, and examples, WBCHSE Chemistry”
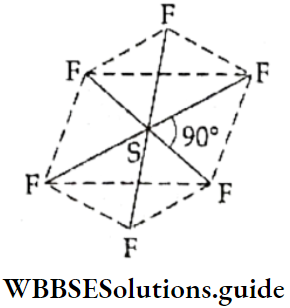
In sulphur hexafluoride (SF6), the central sulphur atom shares all six valence electrons to form covalent bonds with six fluorine atoms. Thus, there are six pairs of bonded electrons in the valence shell of the central atom of the molecule, which has an octahedral (regular) shape. The bond angle is 90°.
ClF3 or chlorine trifluoride is isoelectronic with SF4. Tire central atom of the molecule is chlorine with 7 valence electrons. It uses three electrons to form covalent bonds with three fluorine atoms and is left with four electrons or two lone pairs.
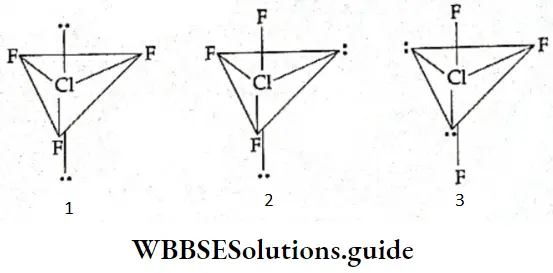
- Thus, Cl atom in the ClF3 molecule has five electron pairs and is therefore expected to have a basic trigonal bipyramidal shape. Since all the bond angles are not the same trigonal bipyramidal is not a regular shape. Lone pairs occupy two corners and fluorine atoms occupy the other three comers. Theoretically, three different arrangements are possible for this molecule.
- As a rule, if more than one lone pair exists in a trigonal bipyramid they will be located in the equatorial position rather than the axial positions to minimise the repulsive forces. On applying this to ClF3 molecule, we find the structure shown in Figure (T-shaped) to be the most stable, as lone pairs are in the equatorial position. Now let us summarise the shapes of few simple molecules with the central atom having one or more than one lone pair of electrons.
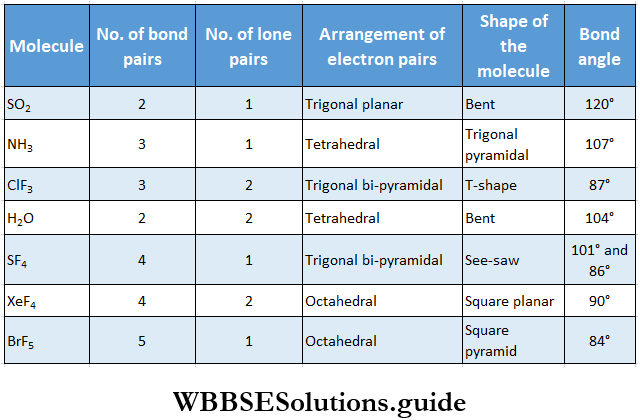
The VSEPR theory can also be extended to molecules with multiple bonds. For example, CO2 and SO2. Carbon is sp hybridized in CO3 and the shape of the molecule is linear. Sulfur is sp2 hybridized in SO2 and the shape of the molecule is bent. The bond angle reduces only a little from 120° to 119.5°, and although bond pair-lone pair repulsion exists, the double bond occupies more space.
