Surface Chemistry
Surface chemistry deals with phenomena that occur at surfaces, or interfaces. A surface is an interface where one phase ends and another begins. It is a region in which the properties vary from one phase to another.
The interface is represented by putting a hyphen or a slash between the two bulk phases. For example, the interface between a solid and a gas may be represented as solid gas or solid/gas. An interface may be liquid/vapor, solid/liquid, or solid/gas, among others. No interface exists between gases as they are completely miscible.
The study of surface chemistry is important since many chemical reactions in industry and in biological systems take place at interfaces. Important phenomena that take place at interfaces are corrosion, heterogeneous catalysis, electrode processes, dissolution and crystallization, and so on.
For a proper study of this important branch of chemistry, an extremely clean surface is required. For example, if a metal is involved, a very clean sample stored in a vacuum (at a pressure of 10-8 -10-9 pascal) is required. Otherwise, the surface of the metal will be covered by atmospheric nitrogen and oxygen.
The topics we will cover in this chapter are the adsorption of gases and solutes on solid surfaces, and colloids and catalysis.
Adsorption
The molecules of a gas or a solute are attracted to and retained by the surface of the solid with which they come in contact. For example, if a piece of charcoal is introduced into a closed vessel containing ammonia gas, an appreciable quantity of ammonia is quickly taken up by the charcoal and the pressure of the gas in the enclosed vessel decreases. Not only ammonia but almost all gases (such as H2, O2, CO, Cl2, NH3, and SO2) are taken up by charcoal to a greater or lesser degree.
The gas that is thus taken up remains on the surface of the charcoal and does not pass into the interior. The simplest proof of this is that if the same sample of charcoal is more finely divided to produce a greater surface area per unit mass, it can take up more gas. The process of accumulation of any substance on the surface of another is called adsorption.
It is a fairly rapid process, as contrasted with absorption, which is a slow process as it involves diffusion into the interior of the material. The substance on the surface of which the concentration occurs is called the adsorbent and that taken up on the surface is called the adsorbate.
For example, in the example of charcoal adsorbing ammonia, ammonia is the adsorbate, and charcoal is the adsorbent.
How adsorption is different from absorption:
As you know, adsorption is purely a surface phenomenon. However, in absorption, the substance passes through the surface and is distributed throughout the bulk of the solid. For example, when a piece of chalk is dipped in ink, the surface of the chalk retains the color of the ink while the solvent of the ink goes into the bulk due to absorption.
On breaking the chalk, it is found to be white from the inside. Also, anhydrous CaCl2 absorbs water to form a hydrate while acetic acid is adsorbed from its solution by charcoal. Sometimes the word sorption is used to describe the phenomenon of adsorption and absorption taking place simultaneously.
Examples of adsorption:
- When a hot crucible is allowed to cool in the open, a film of moisture is formed on the die surface. This is an example of the adsorption of water vapor on the surface of the crucible.
- Acetic add is adsorbed from its solution by charcoal.
- When animal charcoal is stirred in a dilute solution of methylene blue, die color of the latter decreases in intensity because the charcoal adsorbs the coloured material and thus decolourises the solution.
- Water vapor gets adsorbed on the surface of silica gel. Hence, silica gel is used to keep the air in a confined space dry.
- When a raw sugar solution (yellowish brown) is stirred with animal charcoal, the solution becomes colorless as the coloring matter is adsorbed by the charcoal.
Mechanism Of Adsorption
All particles in the bulk of the adsorbent are surrounded by atoms or molecules of their own kind. Thus, the forces acting between the particles are balanced. However, on the surface, the particles are not surrounded by atoms or molecules of their own kind on all sides. Therefore, these particles have imbalanced attractive forces. As a result of these forces, the surface of the adsorbent has a tendency to attract and retain the adsorbate molecules. Therefore, the magnitude of adsorption increases with an increase in the surface area of the adsorbent.
When adsorption takes place, there is always a reduction in the attractive forces on the surface. As a result, there is a decrease in surface energy, which appears in the form of heat. This proves that adsorption is an exothermic process. In other words, adsorption is always accompanied by a decrease in the enthalpy of the system, i.e., is negative whenever a gas is adsorbed; the freedom of movement of its molecules is reduced. As a result, there is a decrease in the entropy of the gas, i.e., AS is negative. Thus, adsorption results in a decrease in both enthalpy and entropy of the system. The free energy change (AG) for the process of adsorption is given by
ΔG = ΔH -TΔS
The thermodynamic requirement for a spontaneous (or feasible) process is that, at constant temperature and pressure, there has to be a decrease in Gibbs energy, i.e., AG has to be negative. In the equation AG = AH- TAS, it is possible for AG to be negative only when AH has a sufficiently high negative value since -TAS is positive. As the process of adsorption continues, AH becomes less and less negative and finally equals TAS. At this point, AG becomes zero and equilibrium is attained. This is called adsorption equilibrium.
Types Of Adsorption
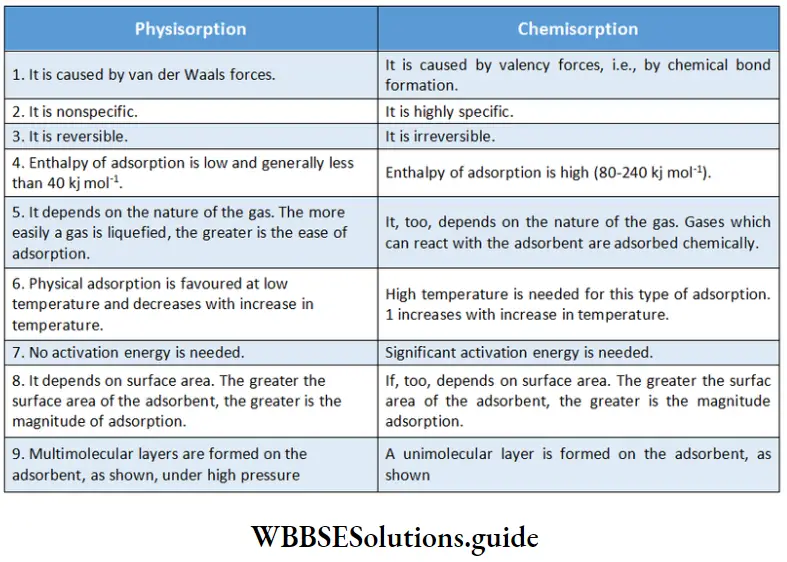
Adsorption is of two types—physisorption (physical adsorption) and chemisorption (chemical adsorption).
Physisorption:
If a gas is held on a solid surface by weak van der Waals forces, the adsorption is termed physisorption or physical adsorption. It is also known as van der Waals adsorption since the forces involved are of the van der Waals type. Absorption of gases by charcoal is an example of physisorption. Let us now discuss some characteristics of physisorption.
Lack of specificity:
Physical adsorption is caused by nonspecific van der Waals forces which act universally between any two molecules. The solid surface (adsorbent) does not show a stronger attraction for permanent gases like H2, O2, and N2.
Nature of adsorbate:
The nature of the gas determines the quantity adsorbed by a solid. It has been found that H2, O2, N2, etc., are adsorbed to a lesser extent and less readily than the more easily liquefiable gases such as NH3, HCl, and CO2. The more easily a gas is liquefied (i.e., the higher the critical temperature), the more is physical adsorption. This is because van der Waals forces are stronger near the critical temperature.
Volumes of various gases adsorbed by 1 g of activated charcoal:
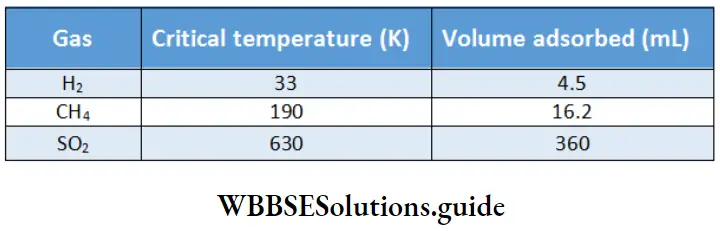
A look at Table 5.1 shows that 1 g of activated charcoal adsorbs more SO2 (critical temperature 630 K) than methane (critical temperature 190 K) and more methane than hydrogen (critical temperature 33 K).
Reversible nature The physical adsorption of a gas by a solid is reversible.
⇒ \(\text { Solid + gas } \rightleftharpoons \text { gas } / \text { solid + heat }\)
According to Le Chatelier’s principle, physical adsorption increases at low temperatures and high pressure and decreases with an increase in temperature and decrease in pressure.
Le Chatelier’s Principle:
Definition:
When a system at equilibrium is subjected to a change, the equilibrium shifts in such a direction as to annul the effect of the change.
Effect of temperature:
Let us consider the reaction
⇒ \(\text { gas }+ \text { solid } \rightleftharpoons \text { gas } / \text { solid }+ \text { heat }\)
Here, the gas is adsorbed on the solid with evolution of heat (exothermic reaction) and desorption takes place1 by absorption of heat (endothermic reaction).
If the temperature of the reaction is increased, the system will absorb heat, and therefore desorption will take! place. Hence in order to effect more adsorption, the temperature of the system will have to be reduced.
Therefore, if a reaction is exothermic then a low temperature will shift the equilibrium in the forward direction. A high temperature favors an endothermic reaction.
An increase in temperature favors endothermic reactions.
A decrease in temperature favors exothermic reactions.
Effect of pressure:
The adsorption of a gas leads to a decrease in pressure. Therefore, according to Le Chatelier’s principle, the magnitude of adsorption will increase with the increase in pressure and vice versa.
Surface area of adsorbent The magnitude of adsorption is directly proportional to the surface area of the adsorbent. So, finely divided metals and porous substances, which have a large surface area for a given mass, are good adsorbents.
Enthalpy of adsorption Since the attraction between gas molecules and a solid surface is only due to weak van der Waals forces, the enthalpy of adsorption is low (20-40 kJ mol-1 ).
Chemisorption:
When gas molecules or atoms are attracted to and retained by a solid surface through chemical bonds (ionic or covalent), the adsorption is known as chemical adsorption or chemisorption. Many chemisorption processes involve high activation energy.
Chemisorption is, therefore, often referred to as activated adsorption. Sometimes physisorption and chemisorption occur simultaneously. In such cases, it is very difficult to determine the exact kind of adsorption taking place. The physical adsorption of a gas at a low temperature may change to chemisorption at a high temperature.
We shall now discuss some characteristics of chemisorption. High specificity Just like chemical bond formation, chemisorption is highly specific and is caused by the same type of forces that help atoms combine chemically, i.e., by valency forces. Gases that can form compounds with the adsorbent are adsorbed chemically.
Examples are the adsorption of oxygen on a metal surface leading to the formation of an oxide, that of hydrogen on a transition element (such as Ni, Pt, or Pd) with unpaired d-orbitals, leading to the formation of a hydride, and the adsorption of oxygen on activated charcoal, resulting in the production of oxides of carbon.
Irreversibility Chemisorption is usually irreversible as it involves compound formation. Though exothermic, the process is very slow at low temperatures. The energy of activation being high, the rate of adsorption falls off rapidly, making chemisorption too slow to be observed at low temperatures. So chemical adsorption generally takes place at comparatively high temperatures. As is also true for a chemical combination, an increase in pressure increases chemisorption. This is one of the reasons why high pressure is often used in industrial catalysis.
Surface area Chemical adsorption, too, increases with the increase of the surface area of Jae adsorbent.
Enthalpy of adsorption The enthalpy of chemisorption ranges from 80-240 kJ mol-1. The high values can be attributed to the fact that chemisorption involves chemical bond formation.
Comparison of physisorption and chemisorption:

Adsorption Isotherm
The amount of gas adsorbed on the surface of the adsorbent depends on pressure and temperature. In other words, the amount of gas adsorbed is a function of temperature and pressure only. Mathematically, this can be represented as
⇒ \(\frac{x}{m}=f(p, T)\)
where x = amount of gas adsorbed on mass m of adsorbent at pressure p and temperature T.
If T is kept constant, then Equation 5.2 becomes
⇒ \(\frac{x}{m}=f(p)\)
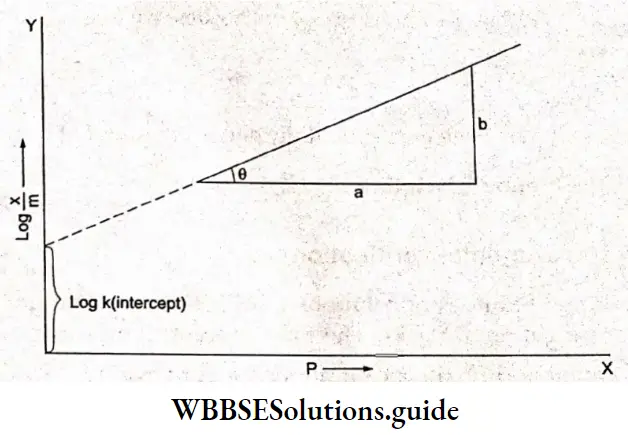
Equation (5.3) gives the variation of \(\frac{x}{m}\) with pressure at constant temperature. When \(\frac{x}{m}\) is plotted against p at a constant temperature, the curve obtained is called an adsorption isotherm. Thus, an adsorption isotherm is a mathematical expression or a graphical curve, which represents the variation of adsorption with pressure, at constant temperature.
An increased pressure of a gas causes increased adsorption. The increase of adsorption with increased pressure is experimentally found not to be proportional to pressure but somewhat less. So, Freundlich put the adsorption proportional to a fractional power of pressure. The resulting equation which gives the relationship between the amount adsorbed and the pressure is known as the Freundlich adsorption isotherm.
⇒ \(\frac{x}{m}=k p^{1 / n} \quad \text { or } \quad\left(\frac{x}{m}\right)^n=k p\)
where x is the amount adsorbed by m grams of adsorbent at a pressure p and k and n are constants that depend on the nature of the adsorbent and the gas at a particular temperature.
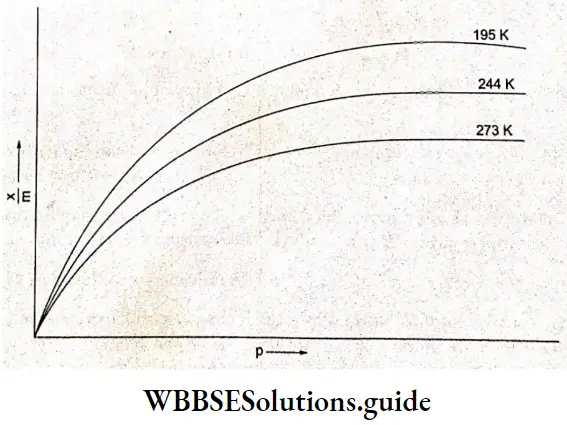
The relationship is generally represented in the form of a curve the mass of the gas adsorbed per gram of the adsorbent is plotted against pressure. A study of these curves reveals that, at a fixed pressure, there is a decrease in physical adsorption with an increase in temperature. These curves tend to approach a saturation or limiting value at high pressure. This shows that the Freundlich equation (or Freundlich adsorption isotherm) fails at high pressure.
On taking the logarithms of both sides of Equation 5.4, we have
⇒ \(\log \frac{x}{m}=\log k p^{1 / n}\)
or, \(\log \frac{x}{m}=\log k+\frac{1}{n} \log p\)
This is the equation of a straight line.
The validity of the Freundlich isotherm can be tested by plotting log \(\frac{x}{m}\) along the y-axis (ordinate) and log p along the x-axis (abscissa). If the plot is a straight line, then the Freundlich isotherm is valid. The slope of the straight line = \(\tan \theta=\frac{b}{a}=\frac{1}{n}\) The intercept on the y-axis \(\) gives the value of log k. E
The Freundlich isotherm only gives an approximation of the behavior of adsorption. The values of \(\frac{1}{n}\) lie between 0 and 1 (the probable range being 0.1-0.5).
Thus Equation 5.4 holds good over a limited range of pressure.
When \(\frac{1}{n}\) = 0
⇒ \(\frac{x}{m}=k=\text { constant }\)
Equation 5.6 shows that the magnitude of adsorption is independent of pressure when
⇒ \(\frac{1}{n}\) = 0
When, \(\frac{1}{n}=1\)
⇒ \(\frac{x}{m}=k p\)
or \(\frac{x}{m} \propto p\)
Equation 5.7 shows that the magnitude of adsorption is directly proportional to pressure when \(\frac{1}{n}\) = 1. Both the equations (Equations 5.6 and 5.7) are supported by experimental results.
Adsorption From The Solution Phase
Solid surfaces can also adsorb solutes from solutions. For example, when an aqueous solution of acetic acid is agitated with charcoal, a part of the acetic acid is adsorbed on the charcoal, resulting in a decrease in the concentration of acetic acid. Also, when a litmus solution is shaken with charcoal, it turns colorless because its coloring matter is adsorbed by the charcoal. To take another example, Mg(OH)2 becomes blue when it is precipitated in the presence of magnesium reagent (a blue dye). The color so obtained is due to the adsorption of the dye on the precipitate of Mg(OH)2.
However, adsorption from solutions is much less understood than that of gases by solids.
Adsorption of solutes has the following features:
- The magnitude of adsorption comes down with the increase in temperature.
- It rises with an increase in the surface area of the adsorbent.
- The magnitude of adsorption depends on the concentration of the solute.
- It also depends on the nature of the adsorbent and the solute.
Hie Freundlich equation approximately describes the behavior of adsorption from solutions. In such cases instead of pressure p we have to use concentration C. Thus the relevant equation becomes
⇒ \(\frac{x}{m}=k C^{1 / n}\)
Taking the logarithm of both sides, we get,
⇒ \(\log \frac{x}{m}=\log k+\frac{1}{n} \log C\)
It has been experimentally shown that the plot of log \(\frac{x}{m}\) against log C is a straight line, which shows the validity of the Freundlich isotherm.
Experimental verification:
Equal volumes of solutions of different concentrations of acetic acid are added to equal amounts of charcoal in four different flasks. The final concentration is determined in each flask after adsorption. The difference in the initial and final concentrations gives the values of x. log \(\frac{x}{m}\) values for the different bottles can be plotted against log C.
Applications of adsorption:
Adsorption finds innumerable applications both in the laboratory and in the industry. We shall now discuss some of the important applications of adsorption.
Creation of high vacuum A partially evacuated vessel is connected to another vessel containing activated charcoal cooled in liquid air. The charcoal adsorbs all the gas molecules from the first vessel, thus creating an almost total vacuum.
Gas masks are used for breathing in areas that contain toxic gases. Such areas include coal mines and places where a major fire has broken out. A gas mask consists of activated charcoal or a mixture of adsorbents which adsorbs all toxic gases and vapours and allows only pure air to pass through the pores.
Removal of colouring matter from solutions Animal charcoal can remove colouring matter from solutions. This property is used for decolorizing sugar solutions.
Heterogeneous catalysis A number of industrial processes involve heterogeneously catalyzed reactions. Most of these reactions take place through the adsorption of reactants on the surface of solid catalysts (adsorbents). Examples include the manufacture of ammonia using an iron catalyst, that of sulphuric acid by the contact process using V2O5 as a catalyst, and the hydrogenation of oils employing finely divided nickel.
Separation of inert gases Charcoal adsorbs different gases in varying degrees. This property is used to separate a mixture of inert gases on coconut charcoal at different temperatures.
Curing diseases Many drugs used to kill germs are adsorbed on the surface of such organisms.
Froth floatation In froth floatation, the ore is mixed with pine oil and agitated with water. Air is passed through this solution. The ore particles are adsorbed on the air-oil interface while the impurities remain in water. The adsorbed ore particles come to the surface in the form of foam.
Adsorption indicators Surfaces of silver halide precipitate have the property of adsorbing dyes like eosin and fluorescein. In the case of precipitation titrations (AgNO3 vs NaCl) the indicator is adsorbed at the endpoint producing a characteristic colour on the precipitate.
Chromatographic analysis based on adsorption is used for the separation, isolation, purification, and identification of the components of a mixture. This is achieved by passing the mixture (dissolved in petroleum ether, benzene, ethyl acetate, or some other solvent) through a column of a suitable adsorbent (alumina or silica gel).
Example 1. What is activated charcoal? How is the extent of absorption by charcoal increased?
Solution:
The extent of absorption by charcoal can be increased by subjecting charcoal to a process of activation. This involves the heating of wood charcoal between 625 K and 1275 K in a vacuum. During activation, hydrocarbons and other impurities are removed from charcoal and render it more porous, making available a very large surface area for adsorption. The resulting porous charcoal is called activated charcoal.
Example 2. What are the factors on which the amount of gas adsorbed by a solid depends?
Solution:
- The amount of a gas adsorbed by a solid depends upon the
- Nature of the gas and the adsorbent, the surface area of the adsorbent
- Temperature and pressure of the adsorbent-adsorbate system.
Example 3. What is desorption?
Solution:
Desorption is the process of release of the adsorbed molecules. Desorption may also be called the evaporation of the adsorbed molecules.
Example 4. Why are powdered substances more effective adsorbents than their crystalline forms?
Solution:
Powdered substances have greater surface area than their crystalline forms. The greater the surface area, the greater the adsorption.
Catalysis
For a given temperature and pressure, every chemical reaction occurs at a characteristic rate. If the rate is rapid, the products are formed in a short period of time. If the rate is slow, it takes a long time for the products to be formed. Scientists are interested in discovering ways to increase the rates of slow reactions, particularly when they are important to industrial processes.
In 1835, Berzelius observed that the speed of a number of reactions was enhanced by the mere presence of a foreign substance, which did not apparently take part in the chemical reaction. Let us consider the example of potassium chlorate decomposing to potassium chloride and oxygen when heated to 653-873 K.
2KClO3 → 2KCl+ 3O2
The decomposition happens at a lower temperature (473-633 K) if the potassium chlorate is mixed with manganese dioxide at a much-accelerated rate. The amount of manganese dioxide remains unchanged at the end of the reaction and the compound may be used again and again. A substance that can be used to alter the speed of a reaction (usually to speed it up) but itself remains unchanged chemically is called a catalyst and the phenomenon of the speeding up of a reaction using a catalyst is called catalysis.
The speed of a reaction is very important in the chemical industry. (The greater the speed, the more is the productivity.) Catalysts play a major role in this context.
In the manufacture of ammonia, iron is used as a catalyst in the presence of molybdenum to increase the speed of the reaction between nitrogen and hydrogen. Molybdenum acts as a promoter for the iron catalyst. Promoters increase the activity of a catalyst while poisons bring it down.
In the petroleum industry, methods have been found to break up the less useful larger molecules into the smaller, more useful ones. The process is known as cracking. Cracking was first done simply by heating the heavy fractions to a very high temperature (thermal cracking). More recently it has been found that by using a catalyst, a mixture of aluminum oxide and silicon oxide, cracking can be achieved at a temperature of only 773 K. This is known as catalytic cracking.
Catalysts are also very important in life processes. Photosynthesis is a chemical change that Is carried out in all green plants because of the catalyst chlorophyll which green plants contain. Countless other chemical changes are carried out in living cells of all kinds. They all depend on biological catalysts called enzymes. Enzymes in our saliva, stomach, and intestines control the digestion of our food.
Though most catalysts help speed up reactions, some slow reactions down. For example, in the decomposition of ammonia on a platinum surface, hydrogen (a product) is strongly adsorbed and inhibits the reaction. Such a condition is called inhibition of the catalyst.
One of the reactants or the products that gets strongly adsorbed on the surface of the catalyst and thereby decreases the reaction rate is called the inhibitor. It is also possible for a reaction to be inhibited by a foreign molecule that does not take part in the reaction. This type of inhibition is called catalytic poisoning. For example, in the manufacture of sulphuric acid by the contact process, arsenic compounds act as poison for the platinum catalyst in the conversion of SO2 into SO3.
Types Of Catalysis
Catalysis may be either homogeneous or heterogeneous.
Homogeneous catalysis:
In homogeneous catalysis, the catalyst and the reactants are present in one single phase (gaseous or liquid). The following are some classical examples of homogeneous catalysis.
1. The oxidation of sulphur dioxide to sulphur trioxide in the presence of nitric oxide as the catalyst in the lead-chamber process for the manufacture of sulphuric acid
⇒ \(2 \mathrm{SO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \stackrel{\mathrm{NO}(\mathrm{g})}{\longrightarrow} 2 \mathrm{SO}_3(\mathrm{~g})\)
All the reactants (SO2 and O2) and the catalyst (NO) are in the same phase (gaseous).
2. In the stratosphere, the decomposition of ozone occurs in the presence of Cl atoms as a catalyst
⇒ \(\mathrm{O}_3+\mathrm{O} \stackrel{\mathrm{Cl}}{\longrightarrow} 2 \mathrm{O}_2\)
Cl atoms are formed upon the decomposition of CFCl3 when the latter is exposed to UV radiation from sunlight.
3. The hydrolysis of ethyl acetate is catalyzed by hydrochloric acid.
⇒ \(\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5(\mathrm{l})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\mathrm{H}_3 \mathrm{O}(\mathrm{aq})}{\longrightarrow} \mathrm{CH}_3 \mathrm{COOH}(\mathrm{l})+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{l})\)
The reactants and the catalyst are in the same phase (liquid).
4. The process of esterification is catalyzed by concentrated
⇒ \(\mathrm{CH}_3 \mathrm{COOH}(\mathrm{l})+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{l}) \underset{\mathrm{H}_2 \mathrm{SO}_4(\mathrm{l})}{\stackrel{\text { conc. }}{\longrightarrow}} \mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5(\mathrm{l})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
The reactants and the catalyst are in the same phase (liquid).
5. The hydrolysis of cane sugar into glucose and fructose is catalyzed by a mineral acid (H2SO4)
⇒ \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\mathrm{H}_2 \mathrm{SO}_4(\mathrm{l})}{\longrightarrow} \underbrace{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})+\mathrm{Clucose}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})}_{\text {in solution }}\)
The reactants and the catalyst in this reaction are in the same phase (liquid).
Heterogeneous catalysis:
In heterogeneous catalysis, the catalyst and the reactants are present in different phases.
The following are some classical examples of heterogeneous catalysis:
1. The oxidation of sulfur dioxide into sulfur trioxide in the presence of platinum
⇒ \(2 \mathrm{SO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \stackrel{\mathrm{Pr}(\mathrm{s})}{\longrightarrow} 2 \mathrm{SO}_3(\mathrm{~g})\)
Both the reactants are in a gaseous state while the catalyst is in a solid state.
2. The combination of nitrogen and hydrogen to form ammonia in the presence of iron in the Haber process
⇒ \(\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \stackrel{\mathrm{Fe}(\mathrm{s})}{\longrightarrow} 2 \mathrm{NH}_3(\mathrm{~g})\)
The reactants are in the gaseous state while the catalyst is in the solid state.
3. The hydrogenation of vegetable oils (unsaturated fatty acids) in the presence of finely divided nickel as a catalyst
⇒ \(\text { vegetable oil }(\mathrm{l})+\mathrm{H}_2(\mathrm{~g}) \stackrel{\mathrm{Ni}(\mathrm{s})}{\longrightarrow} \text { vegetable ghee (dalda) }\)
The two reactants are in different states (liquid and gaseous). The catalyst is in the solid state.
4. The dehydration of formic acid in the presence of Al2O3 as a catalyst
⇒ \(\mathrm{HCOOH}(\mathrm{g}) \stackrel{\mathrm{Al}_2 \mathrm{O}_3(\mathrm{~s})}{\longrightarrow} \mathrm{H}_2 \mathrm{O}(\mathrm{g})+\mathrm{CO}(\mathrm{g})\)
The reactant is in the gaseous state while the catalyst is in the solid state.
5. The oxidation of ammonia into nitric oxide in the presence of platinum gauze (catalyst) in Ostwald’s process.
⇒ \(4 \mathrm{NH}_3(\mathrm{~g})+5 \mathrm{O}_2(\mathrm{~g}) \stackrel{\mathrm{Pt}(\mathrm{s})}{\longrightarrow} 4 \mathrm{NO}(\mathrm{g})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
Here, the reactants are in the gaseous state, and the catalyst is in the solid state.
Theories of heterogeneous catalysis:
The two old theories that explain the mechanism of heterogeneous catalysis are the intermediate compound theory and the adsorption theory.
Intermediate compound theory According to this theory, catalysis occurs due to the formation of a reaction intermediate between the reactant and the product. The catalyst is supposed to provide an alternate pathway b) reducing the activation energy between the reactants and products and hence lowering the potential energy barrier as shown in the figure given below.
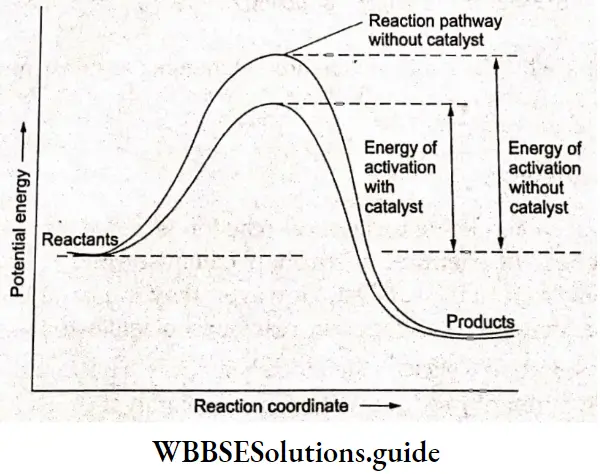
In view of Arrhenius’s equation
⇒ \(k=A e^{-E_a / R T}\)
it is clear that the lower the value of activation energy (Ea), the greater will be the rate of the reaction.
Adsorption theory The adsorption theory applies only to heterogeneous catalysis. According to this theory, the solid catalyst adsorbs the reactants, which are either in the gaseous state or in solution. The rise in concentration of the reacting molecules on the surface of the catalyst increases the rate of the reaction. As you know, adsorption is an exothermic process. The heat evolved due to adsorption is used in increasing the rate of the reaction.
The modem adsorption theory is a combination of the intermediate compound theory and the old adsorption theory. According to it, catalytic activity is localized on the surface of the solid catalyst, which has certain active spots at which there are free valencies. The mechanism of catalysis involves the following five steps.
- The reactants diffuse to the surface of the catalyst.
- They are adsorbed on the surface of the catalyst.
- A chemical reaction occurs on the surface of the catalyst through the formation of an intermediate.
- The products leave the surface of the catalyst to make room for fresh reactant molecules.
- The reaction products diffuse away from the surface of the catalyst.
When a gas comes in contact with the catalyst’s surface (adsorbent), its molecules form loose chemical bonds with the latter. If different molecules form loose chemical bonds with the catalyst’s surface side by side, they may react with each other, resulting in the formation of new molecules. These new molecules leave the catalyst’s surface to make the surface available for more reactant molecules.
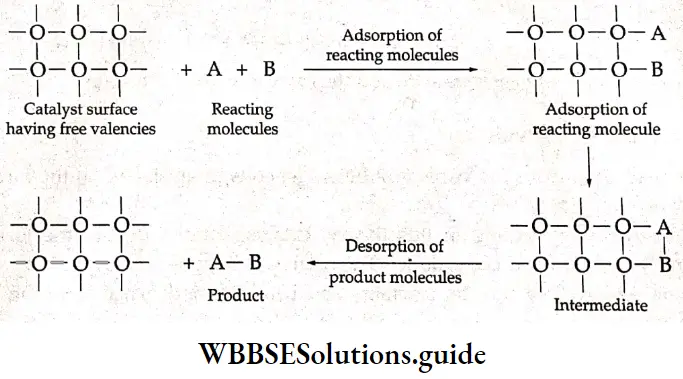
The modem theory explains why the catalyst remains unchanged in mass and chemical composition at the end of the reaction.
Important features of solid catalysts:
Activity The ability of a catalyst to accelerate a chemical reaction is called its activity. The activity of a catalyst largely depends upon the strength of chemical adsorption (chemisorption). The reactant molecules must get chemically absorbed fairly strongly onto the catalyst. However, they must not be adsorbed so strongly that they stick to surface of the catalyst, leaving no room for other reactant molecules to take their place.
Selectivity Catalysts are highly selective—each of them catalyzes only a particular reaction. Carbon monoxide, on treatment with hydrogen, yields different products with different catalysts.
⇒ \(\mathrm{CO}(\mathrm{g})+3 \mathrm{H}_2(\mathrm{~g}) \stackrel{\mathrm{Ni}}{\longrightarrow} \mathrm{CH}_4(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
⇒ \(\mathrm{CO}(\mathrm{g})+2 \mathrm{H}_2(\mathrm{~g}) \stackrel{\mathrm{Cu} / \mathrm{ZnO}-\mathrm{Cr}_2 \mathrm{O}_3}{\longrightarrow} \mathrm{CH}_3 \mathrm{OH}(\mathrm{g})\)
⇒ \(\mathrm{CO}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g}) \stackrel{\mathrm{Cu}}{\longrightarrow} \mathrm{HCHO}(\mathrm{g})\)
Shape-selective catalysis by zeolites:
A catalytic reaction that depends upon the size of the pores and the cavities of the catalyst and the size of the molecules of the reactants and products is called shape-selective catalysis.
On account of their honeycomb-like structures, zeolites are good shape-selective catalysts. They are microporous aluminosilicates which have a three-dimensional network of silicates in which aluminum atoms replace some silicon atoms, resulting In an Al-O-Si framework.
Zeolites (natural or synthetic) are healed in a vacuum so that the water trapped in the pores boils off. As a result, the zeolites become porous. The size of the pores ranges from 260 pm to 740 pm. Only those molecules which are small enough can be adsorbed in these pores.
In the petrochemical industry, zeolites are widely used as catalysts for the cracking of hydrocarbons and isomerization. An important zeolite catalyst used in the petroleum industry is ZSM-5 (zeolite sieve of molecular porosity 5). It converts alcohols directly into petrol (a mixture of hydrocarbons) by the process of dehydration.
Enzyme Catalysis
Living plants and animals produce complex nitrogenous organic compounds called enzymes. Enzymes are proteins with molecular weights ranging from 13000 to several million. They form colloidal solutions in water.
Enzymes are effective catalysts that are involved in a wide variety of biological processes. For example, enzymes in the saliva begin the process of breaking starch in food. Some enzymes catalyze the formation of blood clots when necessary; others dissolve the clots after a wound has healed.
Certain enzymes act to enable the body to fight infection or disease. Enzymes can disrupt the cell walls of certain bacteria. Fruits ripen because of the action of enzymes. Enzymes catalyze several reactions that occur in animals and plants. So they are termed biochemical catalysts.
Many enzymes are isolated in pure crystalline forms from living cells. The structures of some of them, e.g., insulin, have been determined and a few enzymes have recently been synthesized. The first enzyme was synthesized in the laboratory in 1969.
Let us now touch upon a few enzyme-catalyzed reactions:
1. Inversion of cane sugar The enzyme invertase converts sucrose (cane sugar) into glucose and fructose.
⇒ \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { invertase }}{\longrightarrow} \underset{\text { glucose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}+\underset{\text { fructose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6^{-}}\)
2. Conversion of glucose into ethyl alcohol The enzyme zymase converts glucose into ethyl alcohol and
⇒ \(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq}) \stackrel{\text { zymase }}{\longrightarrow} \underset{\text { ethyl alcohol }}{2 \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}(\mathrm{aq})}+2 \mathrm{CO}_2(\mathrm{~g})\)
This process is used in making wine (which contains alcohol) from grapes.
3. Conversion of starch into maltose The enzyme diastase catalyses starch into maltose by partial enzymatic hydrolysis.
⇒ \(\underset{\text { starch }}{2\left(\mathrm{C}_6 \mathrm{H}_{10} \mathrm{O}_5\right)_n(\mathrm{aq})+n \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { diastase }}{\longrightarrow}} \underset{\text { maltose }}{n \mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}(\mathrm{aq})}\)
4. Conversion of maltose into glucose The enzyme maltase catalyses the formation of glucose from maltose and water.
⇒ \(\underset{\text { maltose }}{\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}(\mathrm{aq})}+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { maltase }}{\longrightarrow} \underset{\text { glucose }}{2 \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6(\mathrm{aq})}\)
5. Decomposition of urea into ammonia and carbon dioxide The enzyme urease catalyzes the formation of ammonia and carbon dioxide from urea and water.
⇒ \(\mathrm{NH}_2 \mathrm{CONH}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { urease }}{\longrightarrow} 2 \mathrm{NH}_3(\mathrm{~g})+\mathrm{CO}_2(\mathrm{~g})\)
6. Digestive enzymes (such as pepsin and trypsin) hydrolyze large protein molecules into smaller groups of proteins. In the stomach, pepsin hydrolyses proteins into peptides while in the intestine, trypsin hydrolyses proteins into amino acids.
7. Conversion of milk into curd Lactobacilli enzymes present in curd convert milk into curd
8. Mycoderma acetic enzymes convert ethyl alcohol into acetic acid.
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}(\mathrm{l})+\mathrm{O}_2(\mathrm{~g}) \longrightarrow \mathrm{CH}_3 \mathrm{COOH}(\mathrm{l})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
9. At 310 K (body temperature), sucrose can be hydrolyzed using an enzyme, suocharase.
⇒ \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\text { saccharase }}{\longrightarrow} \underset{6}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}+\underset{\text { glucose }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6} \text { fractose }\)
10. An enzyme found in the mouth converts sucrose into dextran (a polysaccharide made of glucose units). About 10% of dental plaque is composed of dextran. That is why your dentist tells you not to eat candy.
Summary of some important enzymatic reactions:
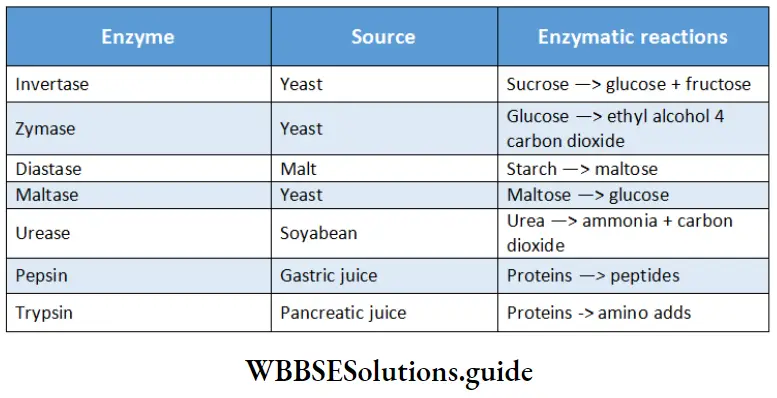
Characteristics of enzyme catalysis:
Efficiency Enzymes are extremely efficient. Some operate on as many as 25000 molecules in one second.
Highly specific An enzyme, like any other catalyst, is highly specific—it catalyzes only a certain reaction and not any other. The enzyme urease, for instance, catalyzes the hydrolysis of urea only. The hydrolysis of other amides is not catalyzed by urease.
The enzyme decomposing cane sugar does not decompose malt sugar. Lactic dehydrogenase oxidizes 1-lactic add but not d-lactic add.
Highly active at 298-310 K Enzymes are highly active at 298-310 K. At temperatures above 310 K, enzyme activity is reduced and extreme temperatures can even stop enzyme activity. Below 298 K too, enzyme activity decreases.
Enzymes in the body exhibit the greatest activity at normal body temperature (310 K). At much higher body temperatures, all physiological reactions cease due to loss of enzymatic activity. This is one reason why a high fever is dangerous.
Highly active under optimum pH Each enzyme exhibits maximum activity at its own optimum pH. The optimum pH for most enzymes is in the pH range 5-7.
On either side of that particular pH (or range), the activity of an enzyme is markedly decreased. Amylase in the saliva is the most active at a neutral pH and becomes inactive in the stomach where the pH is 1.6.
Activity increases in the presence of activators and coenzymes Some enzymes are initially in an inactive form. These are called proenzymes. They become active in the presence of another substance called an activatory Activators are generally inorganic ions (such as Ca2+, Zn2+, Mn2+, Mg2+, Na+ or K+, CO2+, Cu2+).
The catalytic activity of an enzyme is increased when these metal ions are weakly bonded to the enzyme molecule. For example, calcium activates prothrombin. Amylase in the presence of Na– ions catalyzes normal blood clotting.
A coenzyme is a nonprotein that increases the catalytic activity of the enzyme. Vitamins frequently form part of coenzyme molecules.
Inhibitors, poisons, and promotors Inhibitors reduce enzyme activity. They may be either organic or inorganic molecules. They react either directly with the enzyme or with the activator to prevent the activation of the enzyme. Many poisons are fatal because they inhibit vital enzyme activity
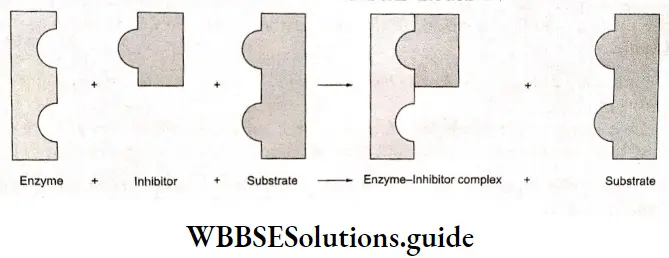
There are two types of inhibitors—competitive and noncompetitive. Since they resemble the substrate in their chemical nature, competitive inhibitors compete directly with the substrate. After linking with such an inhibitor, the enzyme is no longer able to perform its normal function.
Noncompetitive inhibitors react with certain nonspecific functional groups of the enzyme, altering the shape of the active site so that the substrate is not able to fit in.
An example is the cyanide ion. It combines with iron to form a very stable complex, interfering with cytochrome oxidase, which is essential for respiration. Respiration ceases and the person dies.
Mechanism of enzyme catalysis:
The catalytic activity of an enzyme is due to the presence of a specific site on its surface, called the active site. This site is characterized by the presence of functional groups (such as —NH2, —COOH, —SH, and —OH) which form weak bonds such as ionic bonds, hydrogen bonds, or van der Waals bonds with the molecules of the reactant (substrate).
A substrate that has a complementary shape fits into the active site like a key fits into a lock forming an enzyme-substrate complex. This complex intermediate then breaks apart into the products of the reaction, liberating the original enzyme.
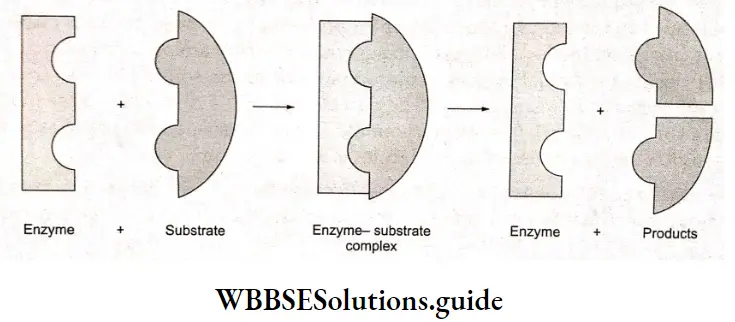
As the enzyme surface has no affinity for the product molecules, the latter leaves the enzyme surface quickly to make room for fresh molecules of substrates to be bound at the active site. Because of this recycling, only small amounts of enzymes are needed.
Example 1. What is the root of desorption in catalysis?
Solution:
Desorption makes room on the surface of the solid catalyst for fresh molecules of reactants to be adsorbed.
Example 2. Why is it necessary to remove carbon monoxide when ammonia is manufactured by the Haber process?
Solution:
Carbon monoxide acts as a poison for the iron catalyst used in the manufacture of ammonia by the Haber process. Carbon monoxide forms pentacarbonyl iron [Fe(CO)5] with iron at the reaction temperature.
Example 3. Why is a heterogeneous catalyst used in the form of a finely divided powder rather than as something with a smooth surface?
Solution:
For a heterogeneous catalyst to be effective, a large surface area is required. Since a finely divided powder has a large surface area per unit mass, a heterogeneous catalyst is employed in this form.
Example 4. Why is the hydrolysis of an ester slow in the beginning? Why does it become faster after some time?
Solution:
The acid hydrolysis of an ester may be expressed as
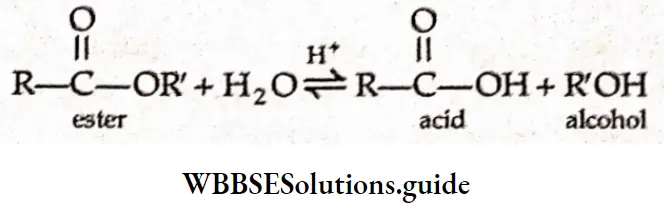
The add produced in the reaction acts as a catalyst (autocatalyst). The concentration of H+ ions increases due to the formation of the add.
Colloids
Fine particles of sand in water form what is known as a suspension. These particles settle down after a while. In a true solution, such as that of salt in water, the particles do not settle down.
In between the true solution and the suspension, there is a type of system that normally never separates; it is called a colloidal dispersion or sometimes just a colloid. A colloidal solution is also termed a sol in short.
A colloid can be defined as a stable two-phase (heterogeneous) system consisting of finely divided particles (usually a solid) dispersed in a continuous medium (often a liquid).
The finely divided particles form what is called the dispersed phase and the medium is called the dispersion medium. For example, in colloidal gold (also called gold sol), gold is the dispersed phase and water is the dispersion medium.
The basic difference between a true solution and a colloid involves particle size. In a true solution, the particles are ions or small molecules and in a colloid, the dispersed phase may comprise particles of very large individual molecules (e.g., a protein or a synthetic polymer) or an aggregate of many atoms, ions or small molecules.
The size of the dispersed particles in a colloidal system is more than that of the solute particles in a true solution and smaller than that of the particles in a suspension.
The particle size of the dispersed phase in a colloid lies in the range of 1 to 1000 nm (10-9 to 10-6 m). In a suspension, the size of the suspended particles is always larger than 1000 nm. In a true solution, the size of the solute molecules is less than 1 nm.
Colloids have a very large surface area per unit mass because they consist of many extremely small particles. Consider a cube of side 1 cm. The total surface area of the cube = 6a² = 6 cm². If the cube is divided into cubes of equal size, then
Volume of each small cube = \(\frac{1}{10^{12}} \mathrm{~cm}^3=10^{-12} \mathrm{~cm}^3\)
The side of each small cube = 10-4 cm [since volume is (side)³].
surface area of each small cube = \(6 a^2=6 \times\left(10^{-4}\right)^2=6 \times 10^{-8} \mathrm{~cm}^2\).
Total surface area of all the 1012 cubes = \(\left(6 \times 10^{-8}\right) \times 10^{12} \mathrm{~cm}^2=60,000 \mathrm{~cm}^2\)
This large surface area provides them with the property of adsorption, one of their most important properties
Classification Of Colloids
Colloids are classified based on the following criteria:
- The physical condition of the dispersed phase and the dispersion medium
- The affinity between the tine dispersed phase and dispersion medium
- The type of particles of the dispersed phase
One the basic physical condition of the dispersed phase and dispersion medium:
The following eight types of colloidal systems are possible, depending upon whether the dispersed phase and the dispersion medium are solids, liquids, or gases.
Types of colloidal systems:
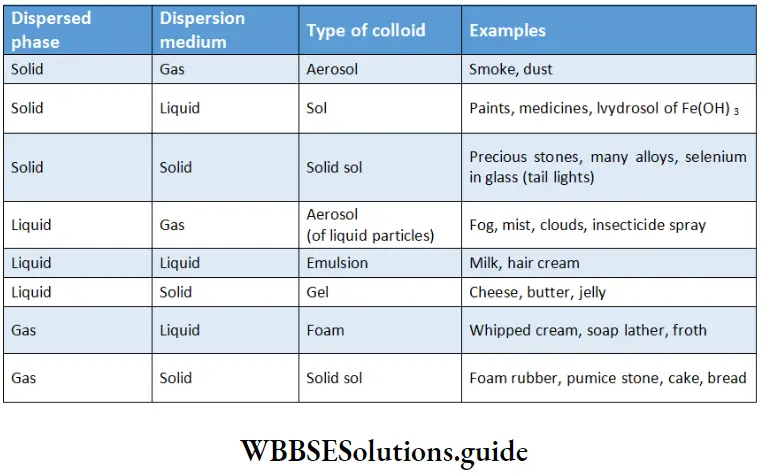
Many commercial products such as whipped cream and fire-fighting foam are colloids. Whipped cream is a foam (a gas dispersed in a liquid). Most biological fluids are aqueous solids (solids dispersed in water). In a cell, for example, proteins and nucleic acids are particles of colloidal size dispersed in an aqueous solution of ions and small molecules.
Of the eight colloids mentioned in the Table, the most common are sols (solids in liquids), emulsions (liquids in liquids), and gels (liquids in solids). In this chapter, we shall discuss only sols and emulsions.
If water is the dispersion medium, such systems are called hydrosols or simply sols, e.g., ferric hydroxide sol. If alcohol is the dispersion medium, the system is called an alcohol.
On the basis of affinity between the dispersed phase and dispersion medium:
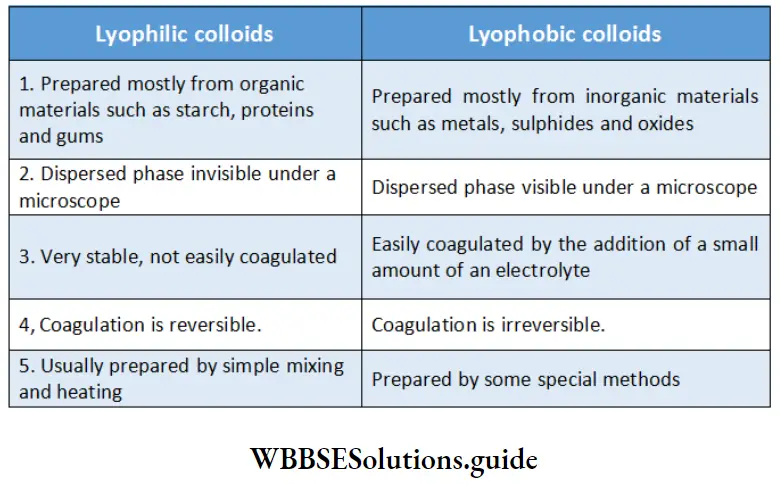
Based on the affinity between colloidal particles and the medium, solid-liquid sols can be classified as lyophilic and lyophobic.
Lyophilic colloids Lyophilic literally means ‘solvent-loving’. Lyophilic colloids include gelatin, gum-arabic, soaps, and starch, all of which have a marked affinity for water. These are simply prepared by mixing the solid (the dispersed phase) with the solvent (the dispersion medium) at a suitable temperature.
If the dispersion medium is separated from the dispersed phase by evaporation, the sol can be made again by simply remixing the dispersed phase with the dispersion medium. That is why these sols are sometimes called reversible sols. These are stable and cannot be easily coagulated.
Lyophobic colloids The word lyophobic means ‘solvent-hating’. When there Is little or no affinity between the dispersed phase and the dispersion medium, the colloid is called a lyophobic colloid.
Examples are gold sols, silver sols, and arsenic sulfide sols. Lyophobic sols cannot be prepared by simply mixing the solid (the dispersed phase) with the solvent (the dispersion medium) at a suitable temperature.
They are made by employing some special methods (as described later). These sols are relatively unstable and can be very easily coagulated by the addition of traces of electrolyte, on evaporation or by shaking.
Sometimes, they are called irreversible sols, because once the dispersed phase is precipitated out, it is not generally easy to get it back in the colloidal state on the addition of the dispersion medium. Lyophobic sols require stabilizing agents for their preservation.
Based on the types of particles of the dispersed phase:
Based on the nature of the particles of the dispersed phase, colloids may be classified as multimolecular, macromolecular, and associated.
Multimolecular colloids When a large number of atoms or molecules are dissolved in a solvent, they tend to form molecular aggregates of colloidal dimensions (diameter < 1 nm). The species formed in this fashion are called multimolecular colloids.
For example, a gold sol may have particles of various sizes possessing several atoms of gold. Similarly, a sulphur sol consists of particles which are aggregates of thousands of S8 molecules held by van der Waals forces. A silver sol (argyle) may contain particles of various sizes, each having several atoms of silver.
Macromolecular colloids True solutions of macromolecules in suitable solvents are called macromolecular colloids because the molecules themselves are large enough to be of colloidal dimensions.
Some naturally occurring macromolecules are starch, cellulose, proteins, and enzymes. Man-made macromolecules such as polyethylene, nylon, polystyrene, and synthetic rubber also belong to this class. These colloids are quite stable.
Associated colloids At low concentrations, some solutions behave as electrolytes. However, at higher concentrations, their solute particles aggregate, and colloids are formed.
Such species are called associated colloids. The molecules of associated colloids have both lyophobic and lyophilic groups. The molecular aggregates are called micelles and usually contain 20-100 molecules. The colloidal properties are due to these micelles.
Micelles are formed above a certain temperature called Kraft temperature (Tfc) and above a particular concentration called critical micelle concentration (CMC). Dyes, soaps, detergents, and many surface-active agents fall into this class. For soaps, the CMC is I -4 to 10-3 mol L-1.
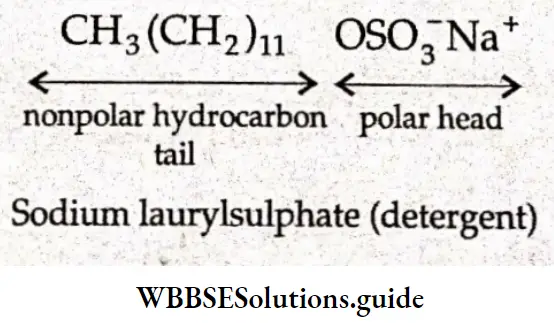
Mechanism of micelle formation Soaps are sodium or potassium salts of fatty acids and may be represented by a general formula, where R is a straight chain of 10-20 carbon atoms and M+ is Na– or K–. When a soap, say sodium stearate, is dissolved in water, it dissociates into Na+ and RCOCT (carboxylate ion).
The carboxylate ion is composed of a large hydrocarbon group (the hydrophobic (water-repelling) group) called, the tail’ and a polar group (tire hydrophilic (water-loving) group] called the ‘head’.
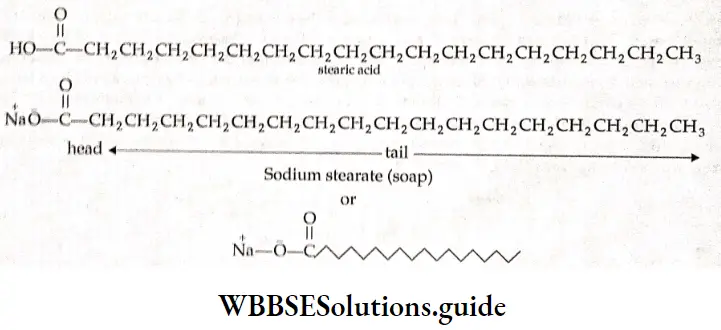
The \(\mathrm{C}_{17} \mathrm{H}_{35} \mathrm{COO}^{-}\)ions are, therefore, present on the surface with their —COO– groups in water and the hydrocarbon group away from water and remaining at the surface.
Long-chain carboxylate ions do not exist as individual ions in aqueous solutions, and at critical micelle concentration, the carboxylate ions are pulled into the bulk of the solution where they arrange themselves in a spherical cluster called a micelle.
Each micelle contains 50-100 long chain carboxylate ions. A micelle resembles a large ball. The polar carboxylate ions are on the outside of the ball because of their affinity for water, and the nonpolar tails are buried in the interior of the ball to minimize their contact with water.
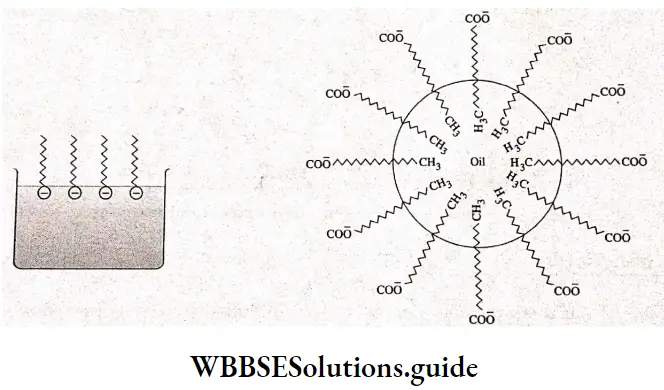
The mechanism of micelle formation in the case of detergents is the same as that in the case of soaps. Detergents, like soaps, have a polar group and a long-chain nonpolar hydrocarbon group that causes the molecules to form micelles in solution.
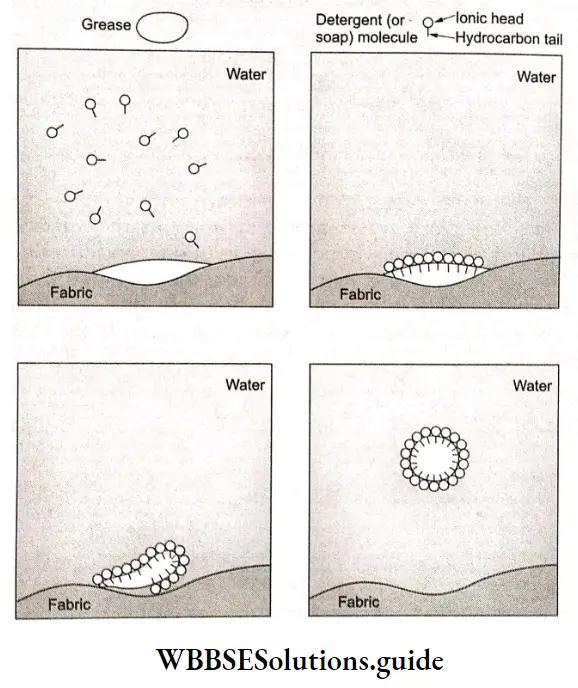
Cleansing action of soaps and detergents:
Soaps and detergents are composed of molecules containing large hydrocarbon groups [the hydrophobic (water-repelling) group] and one or more polar groups [hydrophilic (water-loving groups). The dirt particles that stick to textile fibers are generally covered by a layer of oil molecules called grease. The dies are and, so, repel water.
Therefore, water cannot wash away such dirt particles from doth by Or* the soap, grease molecules get attached to the nonpolar hydrocarbon tail of the stearate ion, and poor head of stearate ion is directed towards the water.
Since the polar heads of the stearate tons can interact with the wafer., surrounded by the stearate ions is gradually lifted off and pulled in water, The grease finally floats off completely surrounded by detergent (or soap) molecules.
The negatively charged sheath around them prevents them from coming together and forming aggregates. Because of this cleansing action of soap, grease and hence dirt is washed away from doth
Preparation Of Colloids
Preparing lyophilic colloids is fairly simple. Generally, vigorously shaking the dispersed phase in the dispersion medium results in the formation of lyophilic solids. For example, gelatine, starch, and soaps form colloidal solutions by simply dissolving in water.
Due to their low stability, lyophobic colloids present greater difficulty In their preparation. Such colloidal solutions are usually made by the following methods.
Chemical methods:
Many molecules produced in chemical reactions (such as oxidation, reduction, hydrolysis, and double decomposition) aggregate to give particles of colloidal dimensions. For example, sulfur solids can be prepared by the oxidation of hydrogen sulfide.
⇒ \(2 \mathrm{H}_2 \mathrm{~S}+\mathrm{SO}_2 \stackrel{\text { oxidation }}{\longrightarrow} 3 \mathrm{~S}(\mathrm{sol})+2 \mathrm{H}_2 \mathrm{O}\)
The gold sol can be prepared by the reduction of \(\mathrm{AuCl}_4\left(\mathrm{AuCl}_3+\mathrm{HCl}\right)\) using reducing agents like formaldehyde or hydrazine.
⇒ \(2 \mathrm{AuCl}_3+3 \mathrm{HCHO}+3 \mathrm{H}_2 \mathrm{O} \stackrel{\text { reduction }}{\longrightarrow} 2 \mathrm{Au}(\mathrm{sol})+3 \mathrm{HCOOH}+6 \mathrm{HCl}\)
Ferric hydroxide sol is prepared when freshly prepared ferric chloride solution is poured slowly into boiling water.
⇒ \(\mathrm{FeCl}_3+3 \mathrm{H}_2 \mathrm{O} \stackrel{\text { hydrolysis }}{\longrightarrow} \mathrm{Fe}(\mathrm{OH})_3(\mathrm{sol})+3 \mathrm{HCl}\)
Areunions sulfide sol may be prepared by passing H2S gas through an aqueous solution of arsenic oxide. double
⇒ \(\mathrm{As}_2 \mathrm{O}_3+3 \mathrm{H}_2 \mathrm{O} \underset{\text { decomposition }}{\stackrel{\text { double }}{\longrightarrow}} \mathrm{As}_2 \mathrm{~S}_3(\mathrm{sol})+3 \mathrm{H}_2 \mathrm{O}\)
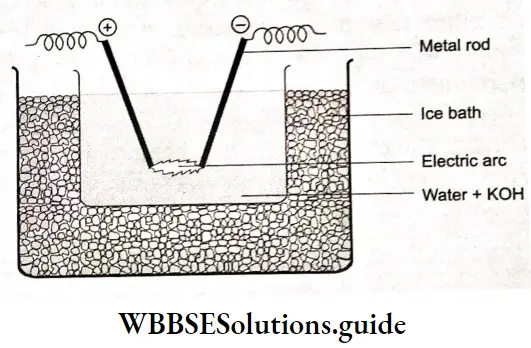
Electrical disintegration or Bredig’s Arc method:
This method is particularly suitable for tire preparation of colloidal sols of metals such as gold, silver, and platinum. Two wires of the same metal (say, gold or platinum) used as electrodes are placed in a suitable dispersion medium containing a small amount of alkali.
An electric arc is struck between them. The heat produced by the arc converts the tire’s solid metal into a light vapor state. These vapours condense into liquid form to give lire required colloidal sol.
Peptization:
The conversion of a freshly formed precipitate into a colloidal solution by the addition of a small amount of electrolyte to tire dispersion medium is called peptization.
The electrolyte used is called the peptizing agent. In the tire peptization process, the tire’s freshly formed precipitate adsorbs one of the ions of the electrolyte on its surface.
This causes the development of either positive or negative charged particles on the precipitate, which repel each other and ultimately break up into particles of colloidal size.
For example, a freshly prepared precipitate of Fe(OH)3 can be changed into a tire colloidal state when the precipitate is treated with water and a small amount of FeCl3 solution (peptizing agent).
The ferric ions (Fe3+) resulting from the ionization of ferric chloride are preferentially adsorbed on the surface of the Fe(OH)3 particles resulting in [Fe(OH)3]Fe3+.
These positively charged particles of ferric hydroxide repel each other and ultimately break up into particles of colloidal size and are dispersed throughout the medium as a colloidal sol of Fe(OH)3.
Purification Of Colloidal Solutions
Most lyophobic colloidal solutions prepared by the methods we have discussed contain massive amounts of electrolytes and other soluble impurities.
While the presence of a small amount of electrolytes is essential for the colloidal solution to be stable, larger quantities lead to coagulation. Therefore, the concentration of these soluble impurities must be minimized in order to make the colloidal solution stable. The following methods are generally used for the purification of colloidal solutions.
Ultrafiltration:
Colloidal particles cannot be filtered using ordinary filter papers because the pores are too large. However, the pore size in ordinary filter papers can be reduced by impregnating the filter paper with a solution of gelatin (an animal protein that can form gels) or collodion (a 4% solution of nitrocellulose in a mixture of alcohol and ether) to stop the flow of colloidal particles.
Colloidal particles can be filtered through specially prepared filter papers called ultrafilters. An ultrafilter can be made by dipping ordinary filter paper in a collodion solution, hardening it using formaldehyde, and then drying it.
The process of separating colloidal particles from the solvent and soluble solute present in the colloidal solution by ultrafilters is known as ultrafiltration.
This process is very slow. Sometimes, suction is applied to increase the rate of filtration. The colloidal particles left on the ultrafilter paper are then stirred in a fresh dispersion medium to get a pure colloidal solution.
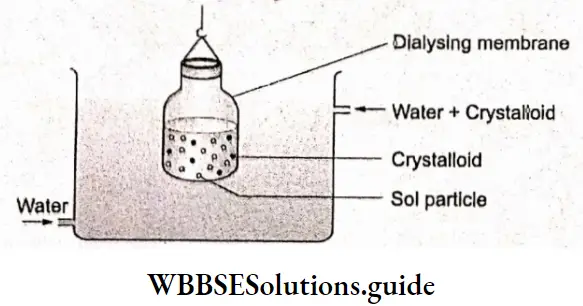
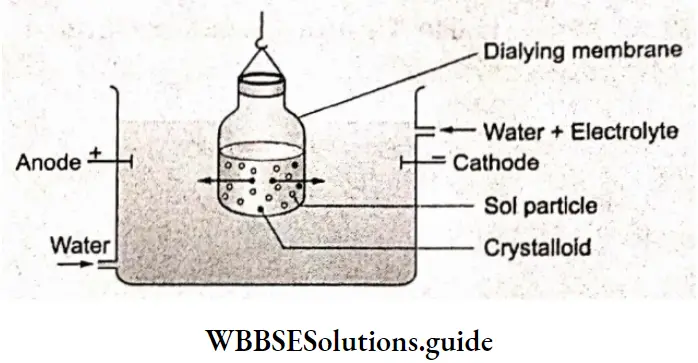
Dialysis:
When a dissolved substance is separated from a colloidal solution by diffusion through a suitable membrane, the process is called dialysis. Colloidal particles cannot pass through animal membranes (bladder), parchment paper or cellophane sheets while Ions or small molecules can. In dialysis, we use such a membrane to separate ions and small molecules from the colloidal solution.
The apparatus used in dialysis is called a dialyzer. It consists of a parchment or collodion bag containing the colloidal solution which is suspended in a vessel through which water is continuously flowing.
The ions and small molecules (crystalloids) inside the bag migrate out leaving the colloidal solution behind. The process of diffusion can be made faster by using hot water instead of cold water. Remember, excessive dialysis may lead to destabilization of the colloidal solution, and a precipitate may result.
Electrodialysis:
Electrodialysis is especially used when the impurities are ionic. A parchment bag containing the colloidal solution Is suspended in a vessel containing water. Two electrodes are fitted in water. When an electric field is applied across the electrodes, the positive ions move towards the anode through the membrane in water.
By the application of an electric field, the rate of diffusion is increased. This method is utilized in producing pure water from seawater on a large scale.
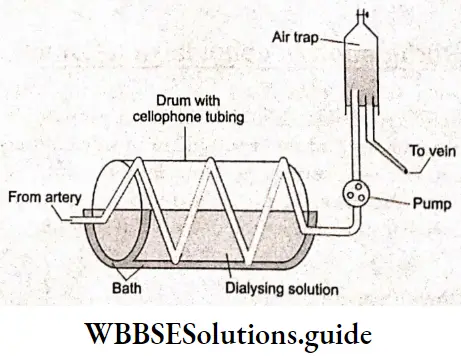
Artificial kidney (Haemodiaiyser):
The kidney is the filter of the body for waste products; its membrane allows the dissolved waste products to pass through but at the same time, prevents the passage of very large protein molecules. The artificial kidney machine uses tubular coiled cellophane as the dialyzing membrane to purify the blood of patients with renal excretion problems. This process is called hemodialysis.
When the patient’s blood is pumped through the coils, dialysis takes place and the soluble waste products and toxic products in the blood pass through the dialyzing membrane into the dialyzing solutions, the dialyzer thus functioning more or less as a real kidney
Properties Of Colloidal Solutions
Colloidal solutions show characteristic properties which are different from those of true solutions. We shall now discuss some important properties of colloidal solutions.
Colligative properties:
Like true solutions, colloidal solutions also show colligative properties. The four colligative properties, namely lowering of vapor pressure, depression in freezing point, the elevation of boiling point and osmotic pressure depend on the number of particles present in the solution.
In ordinary colloidal solutions, the number of colloidal particles is very small in comparison to the number of molecules or ions of a solute present in a true solution as colloids are aggregates of particles. Hence, the values of colligative properties of colloidal solutions are markedly smaller than those shown by true solutions at the same concentration.
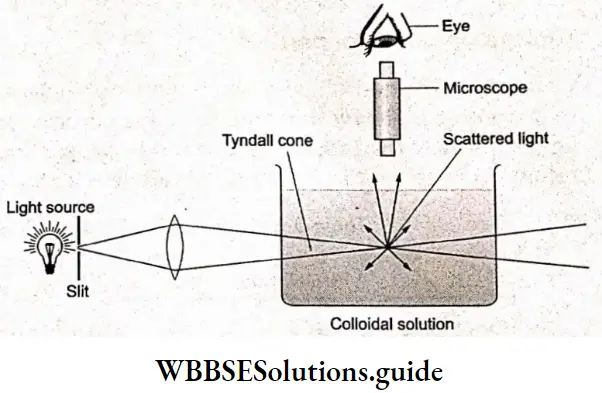
Tyndall effect:
When a beam of light is passed through a clear solution and viewed from a direction at right angles to the direction of the beam of light, it appears completely dark. However, in the case of a colloidal solution, we observe bluish light against this dark background.
This is due to the scattered light from the colloidal particles. The individual particles, which were otherwise invisible, can thus be observed. The above phenomenon was studied in detail by Tyndall and is known as the Tyndall effect.
The apparatus for viewing the colloidal particles is called an ultramicroscope, first constructed and used by Zsigmondy (1903). Using the ultramicroscope, we can distinguish between the different colloidal particles on the basis of light scattered by them.
A common example of the Tyndall effect can be observed in a cinema. You can observe the projection beam all the way from the projector to the screen due to the scattering of light by dust particles.
For the Tyndall effect to be observed, the following conditions must be satisfied.
- The refractive index of the dispersed phase and that of the dispersion medium must differ appreciably
in magnitude. - The size of the dispersed particles should be comparable to the wavelength of light used
Colour:
The color of a colloidal solution depends upon the wavelength of the light scattered by the dispersed particles, which in turn depends upon the size and nature of the particles. For example, the finest gold sol is red in color but as the size of the particles increases, it becomes purple, then blue, and ultimately golden.
The color also depends upon the manner in which the light is observed. For example, sulfur sols may be colorless, faint yellow, or deep yellow in reflected light and reddish in transmitted light. To cite another example, a mixture of milk and water appears blue when viewed through reflected light and red through transmitted light.
Brownian movement:
When viewed under a powerful ultramicroscope, colloidal particles appear to be in a state of continuous random motion. This ceaseless zigzag motion of the colloidal particles is called Brownian motion after the English botanist Robert Brown who for the first time observed such a movement in the case of the grains of pollen, suspended in water, with the help of a microscope.
Brownian motion is executed by all colloidal particles irrespective of their nature. Colloidal particles suspended in gaseous mediums also exhibit such motion. Brownian motion depends on the size of the colloidal particles and the viscosity of the dispersion medium. Smaller particles, and those in a less viscous medium, move faster.
Brownian motion occurs due to the unbalanced Impacts of the molecules of the dispersion medium colloidal particles. The colloidal units are constantly hit from all sides by the surrounding molecules and are tossed up and down just like a ship in a stormy sea.
Charge on colloidal particles:
Colloidal particles (both lyophobic and lyophilic) are electrically charged, in a given colloidal solution, the nature of the charge is always the same on all the particles of the dispersed phase.
The particles move in an electrical field either towards the cathode or the anode depending on whether they are positively or negatively charged. The following is a list of colloidal solutions along with the nature of charge on their particles.
Some examples of positively charged sols and negatively charged sols:
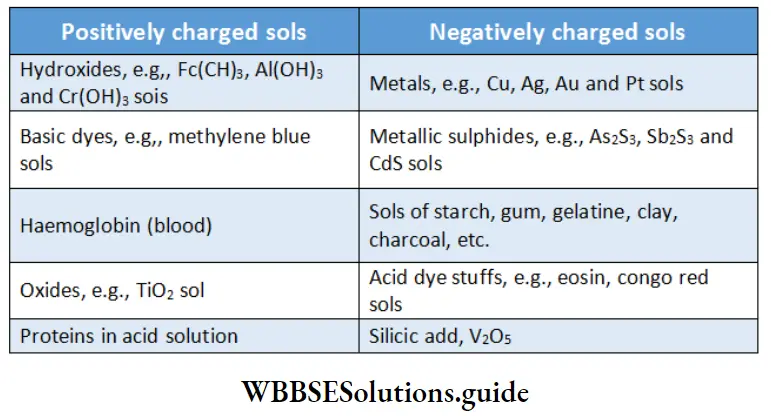
Sol particles require charge on account of one reason or more, One is electron capture by them during the electrodispersion of metals. Another is the formation of an electrical double layer.
A third, and most important, reason is the preferential adsorption of ions from the solution. Let us discuss the third process. On account of the large surface exposed by the colloidal particles, they preferentially adsorb either positively or negatively charged ions from their dispersion mediums.
When the dispersion medium has two ions or more, the ion is common to the dispersed phase, and the dispersion medium is preferentially adsorbed by the particles of the dispersed phase.
For example, when silver nitrate solution is added to a potassium iodide solution, the precipitated silver iodide sol particles adsorb the iodide ions from the solution and acquire a negative charge. However, when a KI solution is added to an AgNO3 solution, the precipitated silver iodideol particles adsorb the Ag+ ions from the solution and carry a positive charge.
⇒ \(\begin{array}{cc}
\mathrm{AgI} / \mathrm{I}^{-} & \mathrm{AgI} / \mathrm{Ag}^{+} \\
\text {Negatively charged } & \text { Positively charged }
\end{array}\)
Similarly, if FeCl3 is added to an excess of hot water, a positively charged sol of ferric hydroxide is formed due to the adsorption of Fe2+ ions. However, when ferric chloride is added to a NaOH solution, a negatively charged sol is obtained with the adsorption of OH– ions.
⇒ \(\begin{array}{ll}
\mathrm{Fe}(\mathrm{OH})_3 / \mathrm{Fe}^{3+} & \mathrm{Fe}(\mathrm{OH})_3 / \mathrm{OH}^{-} \\
\text {Positively charged } & \text { Negatively charged }
\end{array}\)
The charged colloidal particles repel one another and save themselves from agglomeration and subsequent precipitation. Electrical charge is thus the cause of their stability.
However, the charged colloidal particles affect the charge distribution In the dispersion medium. The particles attract ions of opposite charge (counter ions) and repel ions having similar charge (coions) present in the medium, forming a second layer ns shown below,
⇒ \(\mathrm{Agl} / \mathrm{I}^{-} / \mathrm{K}^{+} \quad \mathrm{AgI} / \mathrm{Ag}^{+} / \mathrm{I}^{-}\)
This electrical double layer formed at the interface between the dispersed phase and the dispersion medium is called the Helmholtz electrical double layer. According to the modem concept, the first layer of ions is firmly held and is termed the fixed layer while the second layer is mobile and is termed the diffused layer.
The charges of opposite signs on the fixed and diffused parts of the double layer result in a difference in potential between these layers. This potential difference is called the electrokinetic potential or zeta potential.
Electrophoresis or cataphoresis:
The existence of electrical charges on the colloidal particles is shown by the migration of the particles towards either the positive or the negative electrode when they are placed between two charged electrodes.
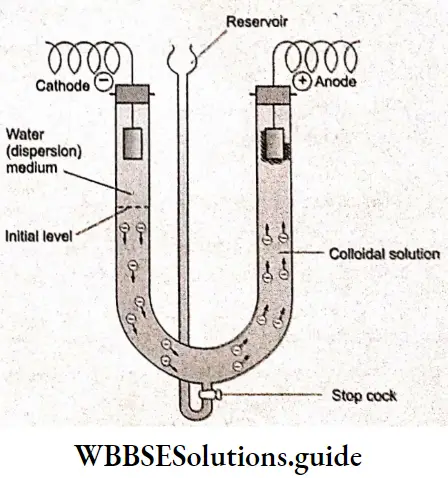
This migration of colloidal particles towards either the cathode or anode under the influence of an electrical field is known as electrophoresis or cataphoresis. The phenomenon of electrophoresis can be demonstrated by the following experiment.
Two platinum electrodes are fitted, one in each limb of a U-tube. The U-tube is partially filled with water (solvent). A requisite quantity of colloidal solution is placed in the reservoir. The stopcock is opened slightly and the tire reservoir is raised to introduce the colloidal solution into the tire U-tube.
The water is displaced upward. The platinum electrodes must be dipped in the water layer and a direct current voltage in the range of 50 V to 250 V is applied across the electrodes.
If the colloidal particles have a positive charge on their surface, they will move towards the cathode and the level in the cathodic limb will rise whereas the level in the anodic limb will correspondingly move downwards.
On the other hand, if the particles are negatively charged, movement will take place in the opposite direction in the two limbs. Electrophoresis has been used for tire separation of various types of colloidal particles from a mixture. Since different particles move at different velocities, their separation becomes easy.
If the colloidal particles are not allowed to move under the influence of an electric field by some suitable means, then the dispersion medium itself moves. This phenomenon is known as electro-osmosis.
Coagulation or precipitation:
Tire electrical charge on colloidal particles is the cause of the stability of lyophobic sols. The charged colloidal particles repel one another and save themselves from agglomeration and subsequent precipitation.
If, by any means, the charge is removed, the particles will come closer to each other to form aggregates (or coagulate) and settle down under the force of gravity. The settling of particles of the dispersed phase is called coagulation or precipitation of the sol.
Lyophobic sols can be coagulated in the following ways. By electrophoresis, The colloidal particles move towards oppositely charged electrodes where they lose their charge and get coagulated.
By mixing oppositely charged sols Two sols having colloidal particles carrying opposite charges on mixing together in equal proportions get neutralized and precipitated.
For example, the mixing of ferric hydroxide sol with arsenious sulfide sol results in the neutralization of the charge on both sols, and both of them are coagulated. Such coagulation is known as mutual coagulation.
By boiling On boiling a sol, the adsorbed layer is disturbed on account of increased collisions with the particles of the dispersion medium. This reduces the charge on the particles of the dispersed phase and ultimately they settle down as a precipitate.
By excessive dialysis On excessive dialysis, traces of the electrolyte present in the sol are almost totally removed, causing destabilization of the sol, which ultimately gets coagulated.
By the addition of electrolyte When an electrolyte is added to a sol, the colloidal particles are precipitated. In the process, the ions of the electrolyte are taken up by the colloidal particles. This results in the neutralisation of the charge on the particles leading to their coagulation.
The ion that causes the charge on the particles of the dispersed phase to be neutralized is called the coagulating ion or flocculating ion. A positive ion causes the precipitation of a negatively charged sol and a negative ion causes the precipitation of a positively charged sol.
The quantity of the electrolyte required to coagulate a definite amount of sol depends upon the valency of the coagulating ion. The higher the valency of the coagulating ion, the smaller the amount of the electrolyte required to bring about coagulation. The smaller the quantity needed, the higher will be the coagulating power of the ion. This is called the Hardy-Schulze rule.
Thus, for the coagulation of the negatively charged arsenious sulfide sol, trivalent cations (Al3+ ) are far more effective than the divalent Ba2+ ions, which in turn are more effective than the monovalent Na+ ions. In the coagulation of the positively charged ferric hydroxide sol, the trivalent PO3 ions are more effective than the divalent SO2 ions which in turn are more effective than the monovalent Cl– anions. The minimum concentration of an electrolyte in millimoles per liter required to cause coagulation in two hours is called the coagulation value.
Coagulation of lyophilic sols:
Lyophilic sols are more stable than lyophobic sols. Two factors that are responsible for the stability of lyophilic sols are the charges and solvation of the particles of the dispersed phase. Lyophilic sols can be coagulated by taking care of these two factors.
This is done by:
- Using a suitable second solvent or by
- Adding an electrolyte.
Introduction of a suitable solvent If a second solvent, which interacts strongly with the dispersion medium, is added to a lyophilic sol, coagulation takes place.
Thus, a hydrophilic sol (e.g., protein in water) can be coagulated by the introduction of alcohol. The water molecules interact strongly with the alcohol molecules, after which the protein molecules are desolvated. This brings about coagulation.
Introduction of an electrolyte When electrolytes are added to lyophilic solids, the ions of the electrolyte compete for the solvent molecules. If a sufficient amount of the electrolyte is added, the sol coagulates. This is called salting out of the sol.
Protection Of Colloids
Lyophobic sols, in contrast to lyophilic sols, are easily coagulated with the addition of a small amount of electrolyte. The relatively higher stability of lyophilic sols is due to the fact that lyophilic colloids are solvated, i.e., the particles of the dispersed phase are covered by a sheath of the liquid (dispersion medium).
The presence of a very small amount of a lyophilic colloid inhibits the precipitating action of an electrolyte on a lyophobic colloid. This is due to its formation of a very thin shell surrounding each particle of the lyophobic colloid through which the oppositely charged ions cannot easily penetrate to neutralize the charge on the particles of the lyophobic colloid.
Lyophilic colloids used for this purpose are called protective colloids. The photographic plate is a good example of protective action, where the lyophobic silver bromide is protected by the lyophilic gelatine.
Emulsions
An emulsion is simply a colloidal suspension of one liquid in another. It may be formed by shaking two immiscible liquids vigorously. Generally, one of the two liquids used is water.
Emulsions are of the following two types:
- Oil-in-water type (o/w type), e.g., milk and vanishing
- Water-in-oil type (w/o type), e.g., butter and cold cream
The oil-in-water emulsion has tiny droplets of an oily or waxy substance dispersed throughout a water solution (e.g., milk). In this system, water acts as the dispersion medium.
The water-in-oil emulsion has tiny droplets of water dispersed throughout an oil (e.g., natural petroleum, butter). In this system, oil acts as the dispersion medium. An oil-in-water emulsion can be washed off one’s hands with tap water, while a water-in-oil emulsion gives the hand a greasy water-repellent surface.
Emulsions are generally unstable and separate out into two distinct layers on standing. Thus, to get a stable emulsion, it is necessary to add a stabilizing agent.
This is called an emulsifier or emulsifying agent. Soap, gum, gelatine, and protein are some common emulsifying agents for o/w emulsions and heavy metal salts of fatty acids, long-chain alcohols, and lampblack are some common emulsifying agents for w/o emulsions.
Emulsions can be diluted by adding any amount of the liquid forming the dispersion medium. When an additional amount of the dispersed liquid is mixed with the emulsion, a separate layer is formed. Often, the liquid forming the dispersed phase has particles carrying a negative charge. These partides can be coagulated using a suitable electrolyte.
The stability of an emulsion can be reduced by adding a substance that negates the effect of the emulsifier. Sometimes centrifuging or freezing may also help in destabilizing an emulsion. The colloidal particles of an emulsion exhibit Brownian motion. The Tyndall effect is also observed.
Colloids Around Us
We come across many things which are colloidal in nature. Let us now briefly discuss a few of them.
Why the sky is blue:
The sky looks blue because dust particles, along with those of water suspended in air, scatter blue light, which reaches our eyes.
Fog, mist, and rain:
Air contains dust and moisture. When air is cooled below its dew point, the moisture condenses on the surface of dust particles forming tiny droplets that float in air, forming mist and fog.
Clouds are also a colloidal system in which tiny droplets of water are suspended in the air. As they reach the upper atmosphere (a cool region), the tiny colloidal droplets of water grow bigger and come down as rain. Sometimes, it rains when two oppositely charged clouds meet.
Artificial rain may be caused by spraying a sol carrying a charge opposite to the one on a cloud.
Food:
Much of the food we eat is colloidal in nature. Examples are milk, butter, ice cream, and fruit juices.
Ordinary milk is an emulsion of fat in water, the emulsifier being casein. Artificial beverages are either emulsions or colloidal solutions. Cocoa and coffee are emulsions but tea is a colloidal solution.
Blood:
Blood is a colloidal solution of negatively charged particles (albuminoid substance) in a liquid (blood plasma). The styptic action of alum and ferric chloride solution can be explained on the basis of coagulation. The Al3+ (from alum) or Fe3+ (from FeCl3) ions cause the coagulation of the negatively charged albuminoid particles forming a clot, and preventing bleeding.
Soil:
Fertile soil is colloidal. Humus acts as a protective colloid and imparts stability to the soil. Due to the presence of humus, the soil adsorbs moisture and nutrients.
Formation of a delta:
River water is a sol of clay. Sea water contains several electrolytes. When water from the river comes in contact with seawater, the electrolytes present in seawater coagulate the sol of clay. This causes the deposition of clay, forming a delta.
Applications Of Colloids
Colloids find many applications in the industry. We shall now briefly discuss some important ones.
Removal of smoke:
Smoke is a sol of solid particles (carbon, arsenic compounds, dust, etc.) in the air. Air pollution is considerably reduced by fitting what is known as a Cottrell apparatus in the chimneys of factories.
Before it emerges from the chimney, the smoke is made to pass through the Cottrel apparatus, which contains plates that have a charge opposite to that carried by the smoke particles.
On coming in contact with these plates, the smoke particles lose their charge, get precipitated, and settle down on the floor of the chamber, from where they are removed
Purification of drinking water:
Water obtained from natural sources (rivers, lakes, etc.) contains suspended impurities. Impure water is usually purified by the addition of alum, which coagulates the suspended impurities and makes water fit for drinking
Tanning:
Changing hide (the skin removed from a dead animal) into leather by chemical treatment is known as tanning. Hide contains protein in the colloidal state. These colloidal particles in hides are positively charged.
On soaking the hides in tannin, which is a negatively charged sol, mutual coagulation takes place. This results in the hardening of leather. In chrome-tanning, chromium compounds are used in place of tannin.
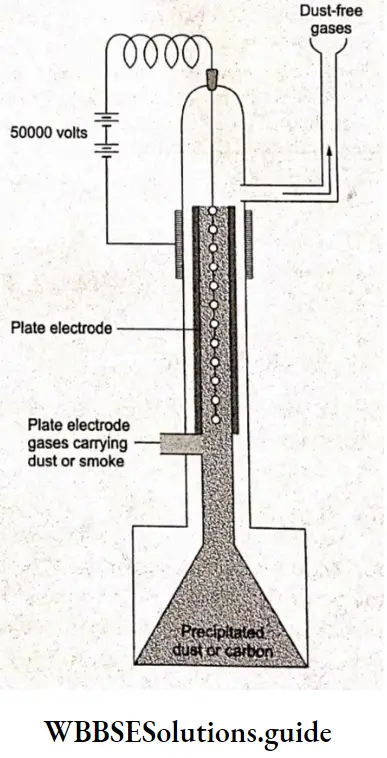
Medicines:
Many medicines in use are colloidal. For example, colloidal silver (argyrol) is used as an eye lotion. Colloidal antimony is effective in curing kala-azar. Colloidal gold is used for intramuscular injections. Milk of magnesia, an emulsion, is used for stomach disorders. Colloidal medicines are more effective because of their large surface area.
Photographic plates and films: An emulsion of the light-sensitive silver bromide in gelatin is coated over glass plates or celluloid films for preparing photographic plates or films.
Rubber industry: Latex is a colloidal suspension of negatively charged rubber particles in water. To obtain rubber, latex is coagulated.
Industrial products: Many industrial products like paints, varnishes, printing inks, gums, adhesives, and resins are colloidal in nature.
Example 1. Why do lyophobic sols containing gelatin not coagulate with the addition of an electrolyte?
Solution:
Gelatin forms a thin protective layer around particles of the dispersed phase and prevents the neutralization of charge by electrolytes.
Example 2. Why is it essential to wash a precipitate with water before estimating it quantitatively?
Solution:
Colloidal particles are precipitated by the addition of an electrolyte. However, some electrolytes remain adsorbed on the surface of the particles of the precipitate. Therefore, it is necessary to wash the precipitate with water repeatedly to remove any electrolytes from the precipitate before estimating it quantitatively.
Surface Chemistry Multiple-Choice Questions
Question 1. The rate of physisorption increases with
- Decrease in temperature
- Increase in temperature
- Decrease in pressure
- Decrease in surface area
Answer: 1. Decrease in temperature
Question 2. Which one of the following statements is incorrect?
- Chemical adsorption is reversible in nature.
- Physical adsorption is due to van der Waals forces.
- Physical adsorption is reversible in nature.
- Activation energy plays an important role in chemical adsorption.
Answer: 1. Chemical adsorption is reversible in nature.
Question 3. Which of the following equations represent (s) Freundlich’s adsorption isotherm?
- \(\frac{x}{m}=\left(\frac{1}{a}\right)+\left(\frac{b p}{a}\right)\)
- \(\frac{x}{m}=k C^{1 / n}\)
- \(\log \frac{x}{m}=\log k+n \log p\)
- \(\log \frac{x}{m}=\log k+\frac{1}{n} \log C\)
Answer:
2. \(\frac{x}{m}=k C^{1 / n}\)
4. \(\log \frac{x}{m}=\log k+\frac{1}{n} \log C\)
Question 4. Which of the following gases will be adsorbed most easily?
- N2
- H2
- O2
- CO2
Answer: 4. CO2
Question 5. According to the adsorption theory of catalysis, the speed of the reaction increases because
- The Concentration of the reactant molecules at the active centers of the catalyst becomes high due to adsorption
- In The Process of adsorption, the activation of like molecules becomes high
- Adsorption produces heat, which increases the speed of the reactions
- Adsorption lowers the activation energy of the reaction
Answer: 4. Adsorption lowers the activation energy of the reaction
Question 6. The function of an enzyme in an enzyme-catalyzed reaction is to
- Transport oxygen
- Conduct catalytic biochemical reactions
- Provide immunity
- Provide energy
Answer: 2. Conduct catalytic biochemical reactions
Question 7. Zeolites are
- Enzyme catalysts
- Shape-selective catalysts
- Liquid catalysts
- Nonspecific catalysts
Answer: 3. Liquid catalysts
Question 8. Which of the following is a true solution?
- Cement
- Muddy water
- CuSO4 solution
- Milk
Answer: 3. CuSO4 solution
Question 9. Which of the following is not a colloid?
- Chlorophyll
- Smoke
- Protein
- Blood
Answer: 1. Chlorophyll
Question 10. Substances that diffuse rapidly through water and certain membranes arc called
- Colloids
- Crystalloids
- Gels
- Emulsions
Answer: 2. Crystalloids
Question 11. The size of a particle in a colloidal solution is
- Greater Than 1000 Nm
- Within The Range of 1 Nm-1000 Nm
- Less Than 1 Nm
- None of these
Answer: 2. Within The Range of 1 Nm-1000 Nm
Question 12. A colloidal system consists of
- NaCl and water
- A Dispersion Medium Only
- A Dispersed Phase Only
- A Dispersed phase and a dispersion medium
Answer: 4. A Dispersed phase and a dispersion medium
Question 13. Sol is a general term usually applied to
- A Solid dispersed in a liquid
- A Solid dispersed in a solid
- A Solid dispersed in a gas
- All of these
Answer: 4. All of these
Question 14. Depending upon the types of the particles of the dispersed phase, colloids are classified as
- Multimolecular colloids
- Associated colloids
- Macromolecular colloids
- All of these
Answer: 4. All of these
Question 15. Fog is an example of a colloidal system of a
- Solid dispersed in a gas
- Gas dispersed in a gas
- Gas dispersed in a liquid
- Liquid dispersed in a gas
Answer: 4. Liquid dispersed in a gas
Question 16. Which of the following is/are lyophilic colloid(s)?
- Starch
- Gum arabic
- Gelatin
- Gold
Answer:
1. Starch
2. Gum arabic
3. Gelatin
Question 17. Lyophilic sols are more stable than lyophobic sols because
- The Colloidal particles have a positive charge
- The Colloidal particles have a negative charge
- The Colloidal particles are solvated
- There is strong electrostatic repulsion between the negatively charged colloidal particles
Answer: 3. The Colloidal particles are solvated
Question 18. The extra stability of lyophilic colloids is due to
- The charge on the particles
- A Protective film of the dispersion medium on the particles
- The smaller size of the particles
- The larger size of the particles
Answer: 2. A Protective film of the dispersion medium on the particles
Question 19. Lyophilic sols are
- Irreversible
- Coagulated by adding an electrolyte
- Prepared from inorganic compounds
- Self-stabilizing
Answer: 4. Self-stabilizing
Question 20. Which of the following statements is correct in the context of sodium stearate, \(\mathrm{CH}_3\left(\mathrm{CH}_{12}\right)_{16} \mathrm{COO}^{-} \mathrm{Na}^{+}\)?
- It is a major component of many bar soaps.
- The R-group is the nonpolar tail and is hydrophobic.
- The —COO group is the polar ionic head and is hydrophilic.
- All of these
Answer: 4. All of these
Question 21. When soap water interacts with grease, the grease
- Forms micelles, which are removed
- Forms macromolecular colloids, which are removed
- Forms colloids of low molecular mass, which are removed
- Forms multimolecular colloids, which are removed
Answer: 1. Forms micelles, which are removed
Question 22. Which of the following statements is correct?
- Micelles are formal above critical micelle concentration.
- Micelles are not associated with colloids.
- Micelles are formed below critical micelle concentration.
- Micelles are true solutions.
Answer: 1. Micelles are formal above critical micelle concentration.
Question 23. Micelles are also called
- Associated colloids
- Macromolecular colloids
- Macromolecular colloids
- Peptizing agents
Answer: 1. Associated colloids
Question 24. The cleansing action of soaps and detergents is due to
- Their dissociation into ions in water
- Their emulsifying nature in water
- Their decrease in viscosity in water
- The presence of bulky R-groups in them
Answer: 3. Their decrease in viscosity in water
Question 25. Which of the following method(s) is/are used in the preparation of colloidal solutions?
- Peptization
- Bredig’s arc method
- Chemical methods
- Electrophoresis
Answer:
1. Peptization
2. Bredig’s arc method
3. Chemical methods
Question 26. Peptization is a process
- Of precipitation of colloidal particles
- Of purification of colloids
- Of dispersing precipitates into colloidal solutions
- In which colloidal particles move in an electric field
Answer: 3. Of dispersing precipitates into colloidal solutions
Question 27. Adding a few drops of dilute HCl to freshly precipitated ferric hydroxide produces a red colloidal solution. This phenomenon is known as
- Protective action
- Peptization
- Dialysis
- Dissociation
Answer: 2. Peptization
Question 28. Colloidal solutions are purified by
- Dialysis
- Peptization
- Coagulation
- Flocculation
Answer: 1. Dialysis
Question 29. Tyndall effect can be observed in a
- True solution
- Solvent
- Colloidal solution
- Precipitate
Answer: 3. Colloidal solution
Question 30. Brownian motion is caused by
- Heat change in the liquid state
- Convection currents
- The collision of the molecules of the dispersion medium with the colloidal particles
- The attractive force between the colloidal particles and the molecules of the dispersion medium
Answer: 3. The collision of the molecules of the dispersion medium with the colloidal particles
Question 31. AS2S3 and Fe(OH)3 colloidal solutions are
- Respectively, positively and negatively charged
- Respectively, negatively and positively charged
- Both positively charged
- Both negatively charged
Answer: 2. Respectively, negatively and positively charged
Question 32. The movement of colloidal particles under an applied electric field is known as
- Dialysis
- Electrophoresis
- Electrodialysis
- None of these
Answer: 2. Electrophoresis
Question 33. The AS2S3 sol has a negative charge. Which of the following has the maximum power to precipitate it?
- H2SO4
- Na3PO4
- CaCl2
- AlCl3
Answer: 4. AlCl3
Question 34. Which of the following is arranged in order of decreasing coagulating power?
- NaCl > BaCl2 > AlCl3
- BaC22 > AlCl3 > NaCl
- AlCl3 > BaCl2 > NaCl
- BaCl2 > NaCl > AlCl3
Answer: 3. AlCl3 > BaCl2 > NaCl
Question 35. In the context of an AS2S3 sol, which of the following has minimum coagulating value?
- NaCl
- KCl
- BaCl2
- AlCl3
Answer: 1. NaCl
Question 36. The method usually employed for the precipitation of a colloidal solution is
- Dialysis
- Addition of an electrolyte
- Diffusion through an animal membrane
- Condensation
Answer: 2. Addition of an electrolyte
Question 37. In making ice cream, gelatine is used mainly to
- Prevent the formation of a colloidal sol
- Enrich fragrance
- Prevent crystallization and stabilize the mixture
- Modify the taste
Answer: 3. Prevent crystallization and stabilize the mixture
Question 38. Which of the following is/are correct?
- An Emulsion is a colloidal solution of a liquid in a liquid.
- A Gel is a colloidal solution of a solid in a liquid.
- An Aerosol is a colloidal solution of a liquid in a gas.
- Foam is a colloidal solution of a gas in a liquid.
Answer:
1. An Emulsion is a colloidal solution of a liquid in a liquid.
3. An Aerosol is a colloidal solution of a liquid in a gas.
4. Foam is a colloidal solution of a gas in a liquid.
Question 39. Which of the following is used to treat diseases of the eye?
- Colloidal gold
- Colloidal silver
- Colloidal antimony
- None of these
Answer: 3. Colloidal antimony