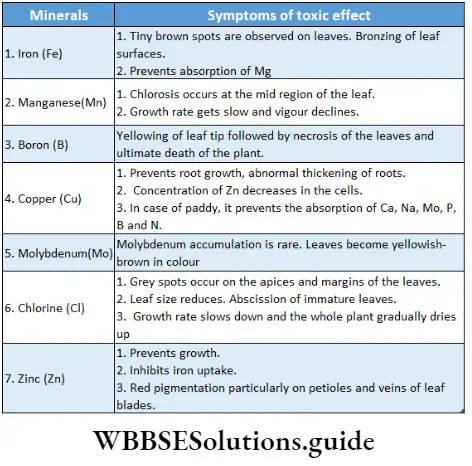
Toxicity Of Micronutrients
The overall growth and development of the plant are affected by higher concentrations (more than normal) of mineral nutrients especially micronutrients. If animals consume this plant with mineral toxicity, it will also affect the animal body.
Toxicity Of Micronutrients Definition: Mineral toxicity is the phenomenon in which a higher concentration of any mineral nutrient (especially micronutrients) causes a 10% reduction of the total dry weight of a plant along with different structural and functional abnormalities.
deficiency symptoms of micronutrients
The concentration of a micronutrient that reduces dry matter of tissue of a specific plant by 10% is called the critical toxicity level or toxic concentration of that micronutrient for that plant.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
“toxicity of micronutrients definition and examples”
Toxicity Of Micronutrient Characteristics:
- Toxicity level varies from plant to plant. For example, above 600//g/g concentration of manganese (Mn) is toxic for soybean plants, whereas in sunflower, the toxic concentration of manganese is 5300/rg/g.
- On the other hand, the uptake of other minerals may be reduced due to the toxic concentration of one mineral. For example, Mn toxicity leads to decreased absorption of Fe. Thus, Mn toxicity causes deficiency symptoms of Fe in plants.
- Heavy metals, in excess concentrations, are the most toxic elements for all types of plants.
Toxicity Of Micronutrients Class 11

Nutrient toxicity and interactions
- High levels of soil nitrogen can assist phosphorus, calcium, boron, iron, and zinc but an excess can dilute these elements.
- Low nitrogen levels in soil can reduce phosphorus, calcium, boron, iron, and zinc uptake.
- Ammonium nitrogen can make molybdenum deficiency appear less obvious.
- High levels of phosphorus reduce zinc and, to a lesser degree, calcium uptake. It is antagonistic to boron in low-pH soils.
- High levels of potassium reduce magnesium and to a lesser extent calcium, iron, copper, manganese, and zinc uptake.
- Boron levels can either be low or toxic under the influence of high potassium. Low levels can accentuate iron deficiency.
- High levels of copper, iron, or zinc can accentuate manganese deficiency, especially repeated applications of iron in soil.
- Uptake can be decreased by liming (adding Ca and Mg-rich materials like limestone) or increased by application of sulfur.
“types of micronutrient toxicity in plants and animals”
Toxicity Of Micronutrients Source:
- Chemical fertilizers used in agriculture.
- Industrial chemical wastes.
- Sail of mine regions contain higher amounts of a particular mineral, that may cause toxicity in plants of that area.
Mechanism Of Absorption And Translocation Of Mineral Nutrients
Mineral nutrients are absorbed by plants from the soil and then they are transported to the leaves. The mechanism of the whole process is discussed below.
Mechanism of absorption of mineral elements:
Uptake And Transport Of Mineral Nutrients
The soil solution contains mineral salts in dissociated condition. They are absorbed in forms of cations and anions, in different amounts by the roots. They are then translocated to different parts of the plant body through the xylem. The movement of ions into or out of the cells is called flux.
micronutrient deficiency in plants
The inward movement is called influx and the outward movement is called efflux. Previously it was believed that inorganic salts were absorbed passively along with the water. But, newer hypotheses show that mineral uptake takes place by two processes—
- First, there is a rapid uptake of ions into the apoplast i.e., intercellular spaces, without any expenditure of metabolic energy (ATP). It is called passive uptake
- Second, the ions move into the symplast i.e., plasma membrane, cytoplasm, vacuolar membrane, of cells. Such movement of ions requires the expense of energy (ATP). It is called active uptake. The mechanism of absorption of ions has already been described in Chapter 11 (Transport in Plants).
“functions of micronutrients and their toxicity effects”
Translocation of mineral elements:
The mineral elements are translocated or transported to different parts of the plant body through the tracheary elements of the xylem. The mineral salts move along the ascending stream of water through the xylem under the influence of transpiration pull. The detailed mechanism of translocation of mineral ions has already been discussed in Chapter 11.
Soil as a reservoir of essential elements
Minerals are present in soil and are absorbed by the plants. So, those can be used for metabolic purposes. Minerals are absorbed by root hairs, mycorrhizae, and the root epiblema (in very small amounts). These minerals occur in the soil due to —
- Weathering and breaking down of rocks, and
- Decomposition of organic matter of plant origin mainly leaves. Soil also contains colloid particles. The soil solution is found in small spaces among these particles. The easily available form of soil water is capillary water. It is found in the capillary spaces between soil particles.
Soil not only supplies minerals and water but also harbors nitrogen-fixing bacteria and other microbes which help the plant to gather necessary nutrients from the environment. Soil also supplies air to the roots and serves as a matrix for them.
“micronutrient toxicity symptoms and prevention in plants”
The nature of soil and the amount of minerals in it differs with geographical locations. Plants often suffer from different mineral deficiency diseases.
It especially affects the yield of crop plants. To avoid this, agricultural fields are often supplied with the necessary amount of fertilizers containing both micronutrients and macronutrients.
Nitrogen Metabolism
Nitrogen is the most abundant natural element found in the environment. It is the fourth most abundant element found in plant bodies. Nitrogen is found in many essential biomolecules such as nucleic acids, protein chlorophyll, some of the phytohormones, and many of the vitamins. So nitrogen as a component of these biomolecules is involved in most of the biochemical reactions contributing to various life processes.
Nitrogen is easily available in the atmosphere. The main source of nitrogen for the synthesis of nitrogenous organic compounds is the atmosphere. Nearly 80% of the earth’s atmosphere is composed of nitrogen, but most of the plants cannot utilize it in its elementary form.
Nitrogen Metabolism In Plants
So, nitrogen is known as the most critical element. Plants acquire nitrogen in the inorganic form either as ammonium (NH4+) or as nitrate compounds (NO33-) from the soil. Only certain microorganisms can use atmospheric nitrogen directly.
Plants synthesize different organic compounds such as protein, amino acids, etc., from the absorbed nitrogen. Animals get nitrogen by consuming other animals and plants. The nitrogen again returns to the atmosphere through plant and animal wastes.
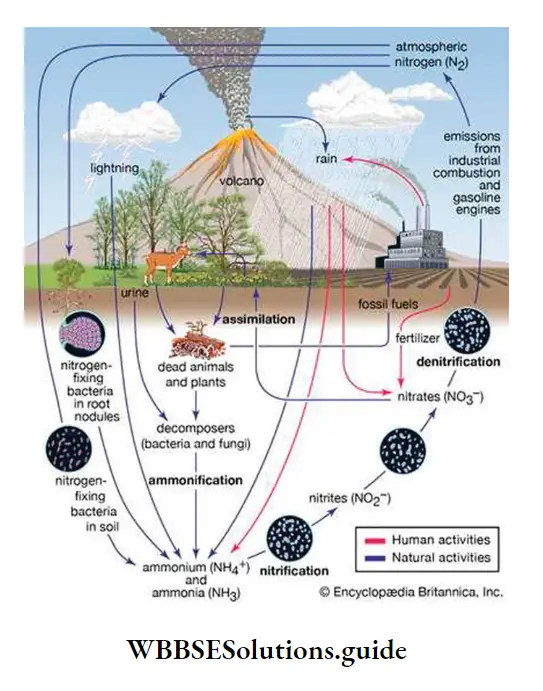
Nitrogen from these wastes is released into the air by the action of certain decomposers present in the soil. This whole process is known as the nitrogen cycle. This cycle maintains a continuous supply of nitrogen to organisms. Nitrogen metabolism helps to synthesize different organic compounds in the body.
“excess of iron, zinc, copper, and manganese toxicity effects”
Nitrogen Metabolism Definition: The process by which plants absorb nitrogen from the atmosphere and use the same for their different metabolic activities, is known as nitrogen metabolism.
Nitrogen metabolism plays an important role in various physiological processes of the plants.
Nitrogen cycle
Nitrogen cycle Definition: The continuous cyclic biogeochemical pathway through which circulation of nitrogen occurs among all living organisms, the cyclic pool of lithosphere and reservoir pool of atmosphere, to maintain the balance of nitrogen in the environment, is known as the nitrogen cycle.
Nitrogen Cycle Explanation or Nitrogen Cycle Importance:
- Nitrogen is present in different components of protoplasm such as protein, DNA, RNA, etc. Hence, nitrogen is essential for the growth and development of organisms.
- The nitrogen cycle represents one of the most important nutrient cycles and it also maintains the nitrogen balance in the atmosphere.
- It also maintains the continuous supply of nitrogen to the organisms.
” micronutrient deficiency plants”
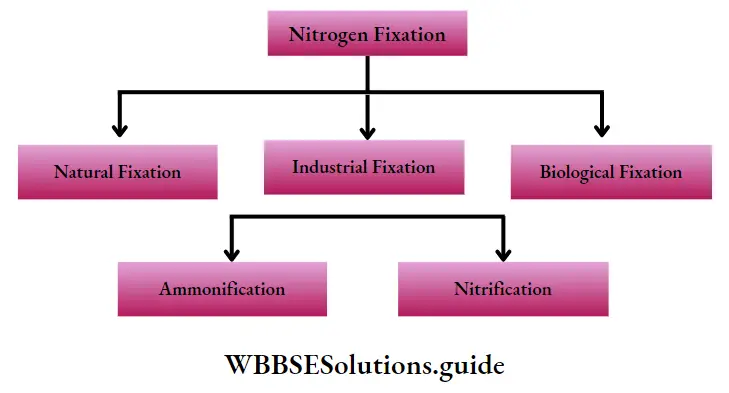
Phases of the nitrogen cycle: The nitrogen cycle comprises of mainly five phases—
- Nitrogen fixation,
- Nitrogen assimilation,
- Ammonification,
- Nitrification,
- Denitrification. These phases have been discussed under separate heads.
“role of micronutrients and their toxicity in plant growth”
Nitrogen Fixation
Nitrogen fixation Definition: The process of conversion of elementary, inactive atmospheric nitrogen (N2) into organic form, to make it available for plants, is known as nitrogen fixation.
Method of nitrogen fixation: There are three methods of nitrogen fixation
Nitrogen Fixation And Nitrogen Metabolism
Natural nitrogen fixation: 10% of the total nitrogen fixation occurs through this process. Due to lightning, water vapor and oxygen dissociate into hydroxyl ions, hydrogen ions, and then atomic oxygen respectively. The atomic oxygen and the hydroxyl ion being highly reactive combine readily with atmospheric nitrogen to form nitric acid.
⇒ \(\mathrm{H}_2 \mathrm{O} \stackrel{\text { Lightning }}{\longrightarrow} \mathrm{OH}^{-}+\mathrm{H}^{+}, \mathrm{O}_2 \stackrel{\text { Lightning }}{\longrightarrow} 2[\mathrm{O}]\)
⇒ \(\mathrm{N}_2+2 \mathrm{OH}^{-}+4[\mathrm{O}] \stackrel{\text { Lightning }}{\longrightarrow} 2 \mathrm{HNO}_3 \text { (Nitric acid) }\)
In another way, nitrogen directly reacts with oxygen j to form different oxides in the presence of ultraviolet rays or lightning.
⇒ \(\mathrm{N}_2+\mathrm{O}_2 \underset{\text { UV Rays }}{\stackrel{\text { Lightning }}{\longrightarrow}} 2 \mathrm{NO} \text { (Nitric oxide) }\)
⇒ \(2 \mathrm{NO}+\mathrm{O}_2 \underset{\text { UV Rays }}{\stackrel{\text { Lightning }}{\longrightarrow}} 2 \mathrm{NO}_2 \text { (Nitrogen dioxide) }\)
Some of the oxides of nitrogen are also generated by forest fires. These oxides get dissolved in rainwater or water vapor and get converted to nitric acid and nitrous acid.
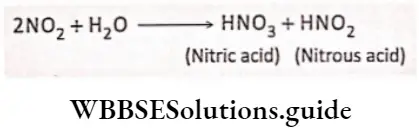
These two acids reach the soil through rainwater. In the soil, they react with other inorganic compounds such as salts of potassium, calcium, or magnesium, and form nitrite or nitrate salts of respective metals.
⇒ \(\mathrm{CaCl} 2+2 \mathrm{HNO}_3 \longrightarrow \mathrm{Ca}\left(\mathrm{NO}_3\right)_2+2 \mathrm{HCl}\)
Hence, the free atmospheric nitrogen is naturally fixed in the soil in the form of nitrite or nitrate.
industrial Rxathn: About 30% °f total nitrogen fixation is done industrially to produce fertilizers. Different nitrogen-containing fertilizers are prepared in industries.
For example, ammonia is prepared by combining hydrogen and nitrogen using Haber Bosch method at 300-400°C temperature and 35-100 MPa pressure. Fossil fuel is used as a source of both hydrogen and energy.
Nitrogen Fixation And Nitrogen Metabolism
⇒ \(\mathrm{N}_2+3 \mathrm{H}_2 \frac{300-400^{\circ} \mathrm{C}}{35-100 \mathrm{MPa}} 2 \mathrm{NH}_3\)
Nitrogen assimilation occurs as a result of using ammonia and other nitrogen-containing fertilizers in the Ammonification Nitrification soil.
“difference between deficiency and toxicity of micronutrients”
Biological nitrogen fixation: Some species of symbiotic, free-living bacteria and cyanobacteria can utilize free atmospheric nitrogen directly. These species are termed as ‘nitrogen-fixing’ organisms. These organisms convert this nitrogen into ammonia and other nitrogen-containing compounds with the help of enzymes, present in their cells.
These nitrogen-containing compounds are then assimilated by the microorganisms in their protoplasm. These microorganisms supply nitrogen to the plant body or plants get nitrogen from their dead remains. It is described later in this chapter.
⇒ \(\mathrm{N}_2+3 \mathrm{H}_2 \stackrel{\text { Nitrogenase }}{\longrightarrow} 2 \mathrm{NH}_3\)
Nitrogen Assimilation
Green plants absorb nitrogen in the form of nitrates from the soil. Some leguminous plants uptake ammonium compounds directly from root nodules. The nitrates absorbed by roots are then transported to leaves where nitrates are converted to amino acids through different biochemical reactions that take place inside the plant cells.
These amino acids then polymerize and produce proteins according to the plant’s needs. Animals consume these plants directly or indirectly to fulfill their nitrogen requirement. Thus nitrogen assimilation occurs in three steps
- Nitrate assimilation,
- Synthesis of amino acids and
- Synthesis of proteins. (These steps are discussed later in this chapter).
Ammonification
The organic nitrogen in the soil comes from nitrogen-containing organic compounds such as animal excreta as well as from decaying plant and animal remains. The process of production of ammonia from amino acids and other simpler nitrogenous substances and organic compounds with the help of different bacteria, is called ammonification.
These bacteria (Bacillus, Clostridium, Proteus, Pseudomonas, and Streptomyces) are called ammonifying bacteria. Ammonification of organic compounds is a very important step in the recycling of nitrogen in soils since most autotrophs are unable to absorb amino acids, nucleic acids, urea, and uric acid.

Urea is a major component of animal waste, it is hydrolyzed by urease enzyme into carbon dioxide and ammonia.
⇒ \(\text { Urea } \underset{\mathrm{H}_2 \mathrm{O}}{\stackrel{\text { Urease }}{\longrightarrow}} \mathrm{CO}_2 \mathrm{NH}_3\)
Ammonia immediately combines with hydrogen ions (H+) of water to form ammonium ions (NH4+).
⇒ \(\mathrm{NH}_3+\mathrm{H}^{+} \longrightarrow \mathrm{NH}_4^{+}\)
Toxicity Of Micronutrients Class 11
Nitrification
The process by which certain microorganisms convert ammonia (NH3) or ammonium ion (NH4+) present in the soil into nitrite (NO2–) and nitrate (N03~), is known as nitrification.
Nitrosomonas and Nitrococcus bacteria first convert NH4+to NO2–ions. Nitrobacter bacteria convert NO2-to NO3– These bacteria are known as nitrifying bacteria. In this reaction, some energy is also produced. This energy is used by the nitrifying bacteria for their carbon assimilation.
Nitrification requires oxygen as a reactant and occurs in aerobic soils. So, these bacteria are also known as chemoautotrophic bacteria.
⇒ \(\mathrm{NH}_4^{+}+2 \mathrm{O}_2 \stackrel{\text { Nitrosomonas }}{\longrightarrow} \mathrm{NO}_2^{-}+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(2 \mathrm{NO}_2^{-}+\mathrm{O}_2 \stackrel{\text { Nitrobacter }}{\longrightarrow} 2 \mathrm{NO}_3^{-}\)
Denitrification
The biological reduction of nitrogen-containing compounds such as nitrate (NO3–) to nitrogen gas (N2) by bacteria is called denitrification. The bacteria that take part in denitrification are known as denitrifying bacteria. Some examples of denitrifying bacteria are Thiobacillus denitrificans, Pseudomonas aeruginosa, etc. Denitrification occurs when oxygen levels are depleted and nitrate becomes the primary oxygen source for microorganisms. When nitrate (NO3–) is dissociated by the bacteria to get the oxygen (O2), the nitrate is reduced to nitrous oxide (N2O), and, in turn, nitrogen gas (N2). Since nitrogen gas has low water solubility, it escapes into the atmosphere as gas bubbles. Denitrification occurs only under anaerobic conditions.
⇒ \(\underset{\text { (Nitrate) }}{\mathrm{NO}_3^{-} \stackrel{\text { Reduction }}{\longrightarrow}} \mathrm{NO}_2^{-} \stackrel{\text { (Nitrite) }}{\underset{\text { (Nitrous oxide) }}{\longrightarrow}} \mathrm{N}_2 \mathrm{O} \text { (Nitrogen) }\)
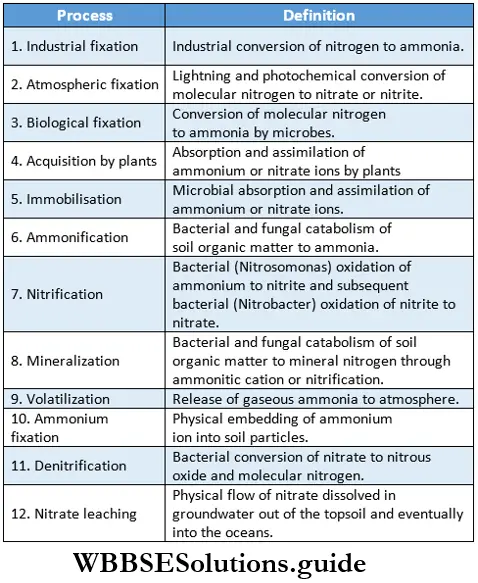
Biological Nitrogen Fixation
Definition: The physical process that involves the conversion of atmospheric nitrogen into ammonia through reduction by certain microorganisms in symbiotic or free-living conditions for the assimilation of nitrogen into protoplasm, is known as biological nitrogen fixation.
Nitrogen-fixing organisms: During the process of evolution, some bacterial species and cyanobacteria have acquired the capacity to fix atmospheric nitrogen in their body. These organisms are known as nitrogen-fixing organisms. They can be symbiotic or free-living.
Importance of biological nitrogen fixation:
- Molecular nitrogen becomes a part of protoplasm by the process of biological nitrogen fixation.
- Free-living nitrogen-fixing organisms can fix 5 kg of nitrogen per j hectare of land in one year. Hence, many nitrogen-fixing organisms such as Anabaena and Azotobacter are used as j biofertilisers.
- The rate of nitrogen fixation by symbiotic Rhizobium is higher than other bacteria. It is about 25-60 kg/year/hectare. Hence, the cultivation of legumes helps increase the nitrogen content of the soil naturally.
Types of biological nitrogen fixation:
Biological nitrogen fixation may be of two types—
- Symbiotic nitrogen fixation and
- Non-symbiotic nitrogen fixation. These are discussed under separate heads.
Special facts about nitrogen-fixing bacteria
The nitrogen-fixing bacteria remain more active under the soil in an alkaline or neutral medium. In the acidic soil, the nitrogen fixation process stops. The nitrogen-fixing bacteria, synthesize nitrogenase enzymes by the action of if genes present in them. A symbiotic bacteria, Rhizobium, is found in the roots of 90% of the leguminous plants. They live by forming nodules. The most common symbiotic bacteria is Rhizobium leguminosarum.
Symbiotic nitrogen fixation
Definition: The biological nitrogen fixation carried out by the microorganisms associated with other plants in symbiotic relationships is known as symbiotic nitrogen fixation.
Symbiotic nitrogen-fixing organisms:
Many species of bacteria and cyanobacteria fix atmospheric nitrogen in symbiotic association with other plants. These are known as symbiotic nitrogen-fixing organisms. Some common examples are given in Table 12.8 along with their host plants.
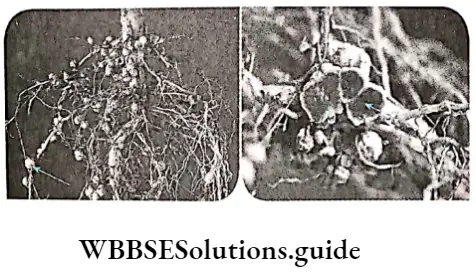
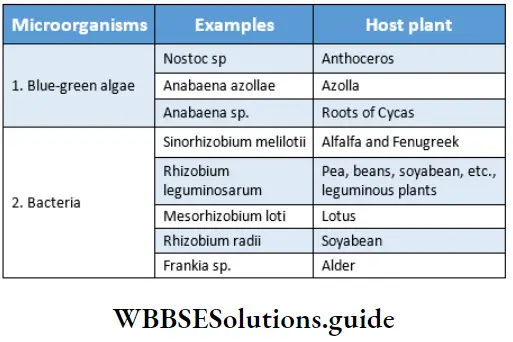
Process: Symbiotic N2 -fixation involving both legumes and Rhizobium is unique and ecologically very important for biological N2 -fixation. The physiological and biochemical process of nitrogen fixation by Rhizobium is discussed below—
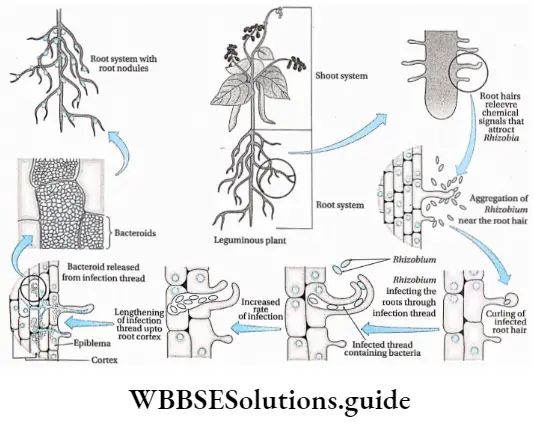
Physiological process of symbiotic nitrogen fixation
1. Pre-fixation stage: Rhizobium are gram-negative and rod-shaped bacteria. These bacteria cannot fix nitrogen when they are in free-living form. They can fix nitrogen only when they come in contact with their host plants.
The following series of events occur during this stage—
- Generation of chemical signals in the pre-infection phase: The bacteria are attracted by certain chemical components secreted from the roots of the host plants.
The secreted chemical compounds are—- Legume lectin, a carbohydrate-binding protein secreted by root hairs of the legumes creates a signal to attract bacteria toward the host plant.
- Homoserine, an amino acid secreted by root hair cells,
- Flavonoid, a secondary metabolite (chemoattractant) secreted from the root hairs.
- Bacterial association on plant root: Bacteria are attracted towards the root by the signal-producing chemicals secreted from the root. Then, certain reactions occur between the bacterial cell wall and the root hairs, that lead to the attachment of bacteria to the root hairs. The number of bacterial cells increases at this time due to the effect of chemoattractants.
- Nod gene activation: Flavonoid activates the nod gene present in bacteria. Activation of the nod gene results in the synthesis of the nod factor. This nod factor is a type of growth hormone (fi -indole acetic acid) that plays an important role during the infection stage.
2. Infection stage: Root hairs are curled due to the effect of RHCF (Root Hair Curling Factor) and nod factor. The nod factor causes the formation of pores at the tip of the root hairs, through which bacteria enter into the root hairs.
3. Formation of infection thread: After entering into the root hairs, the bacteria move deeper into the cortex of the root through an infection thread. This infection thread, made with the plasma membrane, grows inward from one host cell to another. The effect of the nod factor increases the number of bacteria in the infection thread.
4. Nodule and bacteroid formation: During the development of the infection thread, the individual cells near the vascular bundle form nodule primordia (singular: nodule primordium).
These nodule primordia are produced by the individual cells which divide rapidly by the effect of cytokinin hormone present in the root with the combined effect of nod factor of bacteria. The infection thread slowly combines with the nodule primordia.
The bacteria start dividing faster after entering into the nodule primordia. After some time, the bacteria stop dividing, become polyhedral in shape, and reside in the nodule primordia in immobile condition. This immobile state of bacteria is known as bacteroid and in this state, they are prepared for nitrogen fixation.
This bacteroid is morphologically different from the bacteria that entered the root hair. The membrane of individual cells that surrounds the bacteroid is known as the peribacteroid membrane.
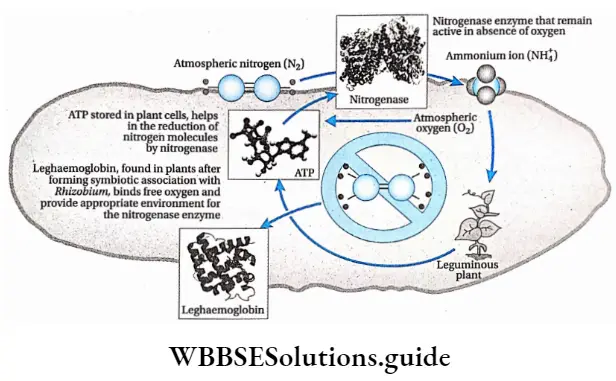
The cells of nodule primordia also start dividing and’ form root nodules. Inside the nodules, a pink color is seen. This coloration is developed by a pigment, named leghaemoglobin, produced by the joint activity of bacteria and the host cell cytoplasm.
Different nod factors are responsible for the formation of bacteroid and root nodules. The nodule thus formed, establishes a direct vascular connection with the host for exchange of nutrients.
Leghaemoglobin
Leghaemoglobin is a dark pink-colored pigment, present in the root nodules of leguminous plants, which is closely related to hemoglobin, the red pigment found in human red blood cells.
This pigment is an oxygen acceptor, which accepts free oxygen from the environment. During nitrogen fixation, leghaemoglobin absorbs the oxygen produced during the reaction and provides an anaerobic condition required for the process of nitrogen fixation.
Biochemical process of symbiotic nitrogen fixation:
Atmospheric nitrogen gas is reduced to ammonia by the enzyme nitrogenase present in the bacteroid. This enzyme remains active in the absence of oxygen. Leghaemoglobin traps the atmospheric oxygen and provides an anaerobic condition for the activity of nitrogenase. The steps of biochemical reactions are
1. Glucose and fructose, produced from the sucrose in the nodules, are converted to glucose-6-phosphate by the action of carbohydrate dissociating enzyme present in the bacteroid.
⇒ \(\text { Glucose } \longrightarrow \text { Glucose-6-phosphate }\)
2. Glucose-6-phosphate is converted to 6-phosphogluconic acid due to the presence of NADP+ (nicotinamide adenine dinucleotide phosphate, a co-enzyme, that acts as an electron acceptor) in the bacteroid cells. NADP+ is reduced to form NADPH+ H+
⇒ \(Glucose-6-phosphate +\mathrm{NADP}^{+}+\mathrm{H}_2 \mathrm{O} \longrightarrow 6-phosphoglucose acid + \mathrm{NADPH}+\mathrm{H}^{+}\)
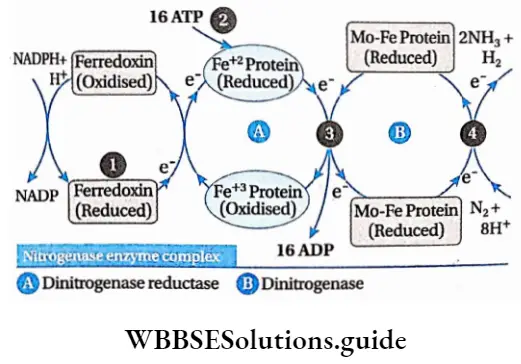
3. This reduced NADPH+ H+ gets oxidized by donating an electron to ferredoxin which acts as an electron carrier.

4. The reduced ferredoxin gets oxidized by reducing the Fe protein of the nitrogenase enzyme.
5. Now, reduced Fe protein gets oxidized by reducing the Mo-Fe protein of the nitrogenase enzyme.
6. Atmospheric nitrogen is reduced by this Mo-Fe protein. Hydrazine (N2H4) and ammonia (NH3) are produced in this process.
7. The energy required for the electron transport chain is supplied by ATP. 8 electrons are required for the fixation of 1 molecule of nitrogen.
⇒ \(\begin{array}{r} \mathrm{N}_2+8 \mathrm{e}^{-}+8 \mathrm{H}^{+}+16 \mathrm{ATP} \stackrel{\text { Nitrogenase }}{\longrightarrow} \\ 2 \mathrm{NH}_3+\mathrm{H}_2+16 \mathrm{ADP}+16 \mathrm{Pi} \end{array}\)
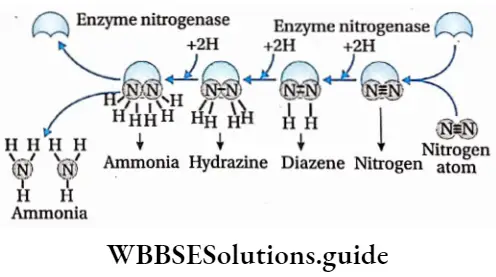
8. Ammonia is not released after completion of the reaction. A small amount of ammonia is very toxic for the microbes. Nitrogen-fixing bacteria protect themselves from this toxic effect of ammonia by synthesizing organic acids. The reaction between ammonia and organic acids produces amino acids. These amino acids are moved to all parts of the plant through the phloem.

Non-symbiotic Nitrogen Fixation
Definition: The process of biological nitrogen fixation, that is carried out by free-living bacteria in the soil, is known as non-symbiotic nitrogen fixation.
Non-symbiotic microorganisms: Some species of cyanobacteria and bacteria that fix nitrogen nonsymbiotically are known as non-symbiotic microbes or free-living microbes.
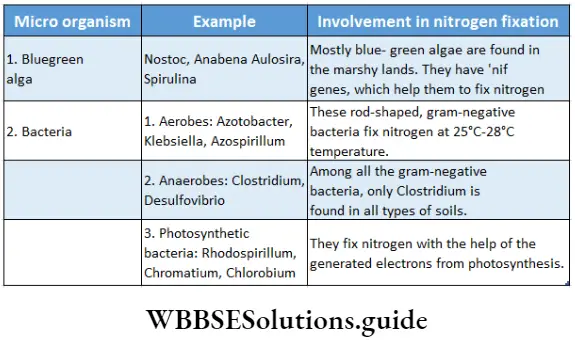

Process: Like symbiotic nitrogen fixation, nitrogenase enzyme is also involved in non-symbiotic nitrogen fixation. Besides nitrogenase enzyme hydrogenase, ferredoxin, Tpp (Thiamine pyrophosphate), pyruvate, Mg2+, inorganic phosphate, and Co-enzyme A (CoA) are also required for non-symbiotic nitrogen fixation.
Nitrogenase
Nitrogenase is a nitrogen-fixing enzyme, which plays a key role in the conversion of atmospheric nitrogen into ammonia under anaerobic conditions. Nitrogenase becomes inactive in the presence of oxygen. Nitrogen-fixing aerobe, Azotobacter, or symbiotic bacteria Rhizobium, helps the enzyme to remain active, by some special process. This enzyme is formed of two subunits—Fe protein and Mo-Fe protein. These two subunits transfer electrons separately.
According to Burris (1965), pyruvate provides energy and ferredoxin carries electrons during this process.
Hydrogen may also serve as the electron donor. Other sources of electrons are the photosynthetic water splitting, the assimilated carbon, and reduced sulfur compounds.
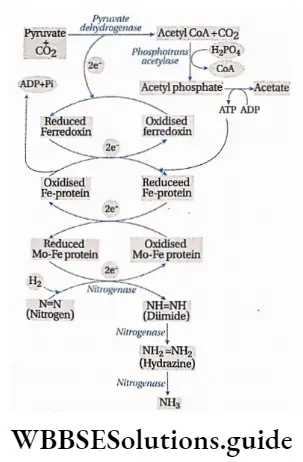
The end product, ammonia, reacts with organic acids to form amino acids (same as symbiotic nitrogen fixation).
Nitrate Assimilation
The nitrate absorbed from the soil is oxidized in two steps to form ammonia within the plants. The steps are—
Reduction of nitrate into nitrite: This occurs in the presence of the enzyme nitrate reductase. The nitrate reductase is a molybdenum-containing flavoprotein. FMN (flavin mononucleotide) and FAD (flavin adenine dinucleotide) are present as hydrogen acceptors. Molybdenum helps in electron transport.
⇒ \(\begin{aligned}
\mathrm{NO}_3^{-}+\mathrm{NAD}(\mathrm{P}) \mathrm{H}+\mathrm{H}^{+} & \frac{\text { Nitrate reductase }}{\mathrm{FMN} \text { or FAD }} \\
& \mathrm{NO}_2^{-}+\mathrm{NAD}(\mathrm{P})^{+}+\mathrm{H}_2 \mathrm{O}
\end{aligned}\)
Reduction of nitrite into ammonia: This reaction occurs in the presence of nitrite reductase. Two prosthetic groups—Fe-Cu complex and haem, are required for the activation of the enzyme. Ferredoxin is required for carrying electrons and co-enzyme NAD(P)H transports hydrogen.
The evolved NH3 converts to ammonium ion (NH4+) by accepting a proton. As minute amounts of these components are harmful to plants, they are converted to amino acids by reacting with organic acids, secreted by the nodules.
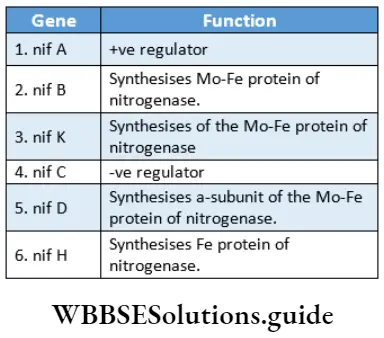
Nif gene
The gene that maintains the structure and controls the activity of the nitrogenase enzyme is known as the nif gene. This gene is present in the body of Rhizobium. The main genes are—
Synthesis Of Amino Acid
Amino acid is the structural unit of proteins. Amino acids are the primary compounds for nitrogen assimilation. The synthesis of amino acids involves two steps.
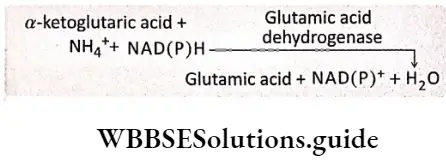
Reductive amination: Ammonia comes in contact with the organic acids by reductive amination. In this process, ammonia forms glutamic acid by reacting with ar-ketoglutarate. This process is controlled by the enzyme glutamate dehydrogenase.
Transamination: In this process, one amino group from one amino acid is transferred to a keto group of the keto acids. Glutamate can produce about 17 amino acids by the process of transamination. This reaction occurs in the presence of the enzyme transaminase.

Protein Synthesis
We know that amino acids are the structural units of proteins. Amino acids are the amino compounds of keto acids such as pyruvic acid, etc.
Amino acids contain three functional groups, they are—
- Amino group (— NH2),
- Carboxylic group (— COOH),
- Alkyl group (-R).
A single protein contains one or more polypeptide molecules. Polypeptides are combinations of some amino acids arranged in a long chain. The amino group of one amino acid remains attached to the carboxyl group of the adjacent amino acid by a peptide bond.
This peptide bond (— NH—CO—) is formed by the release of one molecule of water by the chemical bonding of the amino group and the carboxyl group. Chains of several amino acids form a polypeptide molecule. Living cells contain 20 different kinds of amino acids.

In the polypeptide molecule, amino acids are arranged according to the sequence of coded information present in mRNA. Protein synthesis takes place by the activity of both tRNA in cytosol and rRNA in the ribosome. The sequence of nitrogenous bases in mRNA is different in different species of animals.
Notes
Airstone: A piece of limestone or porous stone, used as aquarium furniture, which gradually diffuses air into the tank, eliminating noise and large bubbles of conventional air filtration system.
“micronutrient toxicity in humans – causes and symptoms”
Antioxidative: A property or a molecule that inhibits the oxidation of other molecules
Buffer: A solution that resists changes in pH when acid or alkali is added to it.
Calmodulin: A multifunctional intermediate Ca2+ binding messenger protein expressed in all eukaryotic cells.
Chelating agent: A substance whose molecules can form several bonds to a single metal ion. example ethylenediamine.
“toxicity of micronutrients short notes for NEET and exams”
Chemoattractant: A chemical substance that induces chemotactic movement of an organism towards a certain direction in which its concentration is increasing.
Ligand: A substance that forms a complex with the central metal atom of a biomolecule to serve a specific biological purpose.
Mottle: Mark with spots or smears of color. ‘
Necrosis: The word has originated from the Greek ‘nekros’ (dead body); death of tissue due to injury, radiation, infection, toxicity, or deficiency.
Rosette: A circular arrangement of leaves where all leaves are at a similar height.
