WBCHSE Class 11 Chemistry Notes On VSEPR Theory and Molecular Shapes
So far we have discussed Lewis structures and geometrical shapes of molecules. Perhaps, the Lewis theory was the first explanation of a covalent bond in terms of electrons. The theory, however, fails to explain the difference in energies of similar bonds.
Similarly, the VSEPR theory fails to explain the formation of chemical bonds. To overcome these limitations two modem theories were proposed—valence bond theory and molecular orbital theory—which are based on quantum mechanics.
“WBCHSE Class 11 Chemistry, Valence Bond Theory, notes, and key concepts”
This concept is based upon the fact that all systems in the universe tend to have the lowest possible potential energy because the lowering of energy leads to stability. According to the modern theory of bonding, atoms form bonds with each other in order to have the minimum possible energy.
Another way of putting this is that atoms combine with each other only if such a combination is accompanied by a decrease in energy, or that bond formation is accompanied by the release of energy.
Read and Learn More WBCHSE For Class11 Basic Chemistry Notes

Valence bond theory: This theory was proposed by Linus Pauling who was awarded the Nobel Prize for chemistry in 1954. The basis of this theory is the Lewis concept of electron-pair bond.
- To understand the theory qualitatively and know why the formation of a bond between two (or more) isolated atoms leads to the lowering of the energy of the ‘system’ of atoms of the molecule, let us consider the formation of a simple molecule—hydrogen.
- The hydrogen atom has only one electron, which is under the field of influence of the nucleus of the atom. Let us consider two such hydrogen atoms, HA and HB, which have electrons eA and eB in their valence shells respectively. When these two atoms are far apart there are no forces of attraction or repulsion between them. Now suppose the two atoms start approaching each other.
- At some point, the electron of one will start being attracted by the nucleus of the other. On the other hand, each nucleus will repel the other and the two electrons will also repel each other.
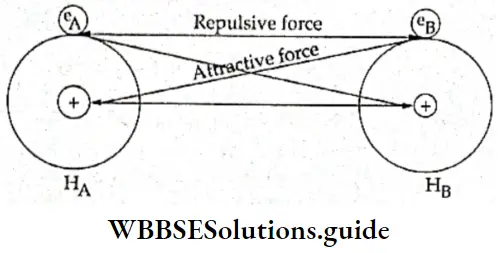
It has been found that in the beginning the magnitude of the forces of attraction is greater than that of the forces of repulsion. So, the atoms keep drawing closer and the potential energy of the system (i.e., the two atoms) keeps decreasing.
| Class 11 Biology | Class 11 Chemistry |
| Class 11 Chemistry | Class 11 Physics |
| Class 11 Biology MCQs | Class 11 Physics MCQs |
| Class 11 Biology | Class 11 Physics Notes |
“Valence Bond Theory, WBCHSE Class 11, chemistry notes, and explanation”
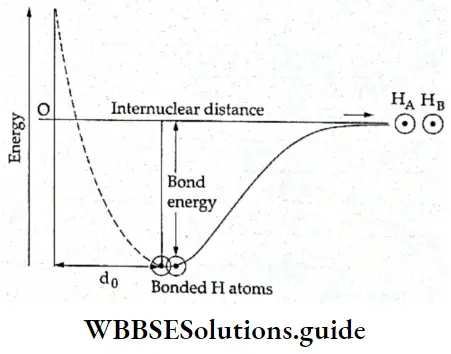
- However, as the atoms approach each other, the magnitude of the repulsion forces keeps increasing and a point is reached where the forces of repulsion just balance the forces of attraction. This is the point at which the system has the lowest potential energy, and consequently, the greatest stability.
- Let us call the distance between the two atoms at this point d0. If the atoms were to come closer than this distance, the magnitude of the repulsive forces would exceed that of the attractive forces and the energy of the system would increase, leading to the instability of the molecule. Figure shows the variation of the energy of the system with intemuclear distance (distance between the two atoms).
- As shown in Figure, the energy of the two-atom system (or the molecule) is the least when the distance between them is d0. It is far less than the energy of the two isolated atoms. This is why atoms form bonds—to gain stability. The greater the number of bonds formed, the greater is the lowering of energy and the stability of the system.
- In fact, some atoms even form more bonds than required to complete their octet, for example, phosphorus in PCI5, and sulphur in SF6. The quantum theory of bonding can, thus, explain why the octet rule is not always obeyed in bond formation.
Bond length: The distance d0 between the centres of the nuclei of two bonded atoms is called the bond length. At this distance the forces of attraction and repulsion between the bonded atoms just balance each other, also the orbitals of the two atoms overlap partially.
The greater the extent of overlapping, the shorter is the bond length, and the stronger the bond. The bond length depends upon
- The size of the atoms and
- The nature of the bonds.
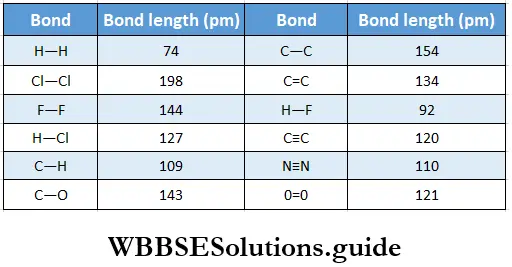
- It increases with the size of atoms because the extent of overlapping of the orbitals decreases as the size of the atoms increases. For example, the H—Cl bond length in the HCl molecule is 127 pm, whereas the C—Cl bond length is 177 pm.
- The bond length decreases with the multiplicity of bonds because the greater the number of electrons shared by two atoms, the greater is the force of attraction between them.
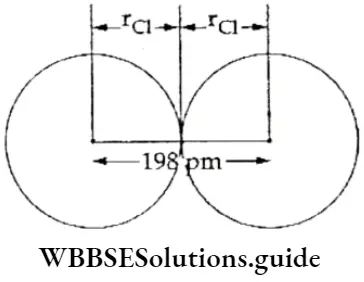
Each atom in a bond contributes to the bond length. The contribution of each atom, in case of a covalent bond, is the covalent radius of that atom. Consider a molecule of chlorine, where the two chlorine atoms are bonded together by a single covalent bond.
The bond length in the molecule is 198 pm. Here rCl is the covalent radius of the chlorine atom, which is 99 pm. Therefore, we may conclude that the bond length in a covalent molecule is equal to the sum of the covalent radii of the atoms which are bonded together.
Bond enthalpy: The energy corresponding to the lowest point (minima) in the potential energy curve is called the bond energy. It is the amount of energy released when one mole of covalent bonds is formed.
“WBCHSE Class 11, chemistry notes, on Valence Bond Theory, and its postulates”
The amount of energy released during the formation of a bond between two atoms is also the amount of energy required to break the bond between the two atoms or to separate them.
This is called the bond dissociation enthalpy (or bond enthalpy). Thus, the bond dissociation enthalpy is the amount of energy required to break one mole of bonds of the same kind so as to separate the bonded atoms in the gaseous state. For a homonuclear diatomic molecule, the bond enthalpy is equal to the bond dissociation enthalpy.
Consider an example of a homonuclear diatomic molecule like H2–
⇒ \(\mathrm{H}_2(\mathrm{~g}) \rightarrow \mathrm{H}(\mathrm{g})+\mathrm{H}(\mathrm{g}) ; \Delta_{\mathrm{g}} H^{\ominus}=435.8 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Similarly, for a heteronuclear diatomic molecule like HCl, we have
⇒ \(\mathrm{HCl}(\mathrm{g}) \rightarrow \mathrm{H}(\mathrm{g})+\mathrm{Cl}(\mathrm{g}) ; 4_{\mathrm{L}} H^{\ominus}=4310 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
“Valence Bond Theory, sigma and pi bonds, resonance, and bonding strength, WBCHSE syllabus”
Molecular Geometry And Bond Angles For WBCHSE Class 11 Chemistry
- The magnitude of the bond enthalpy depends upon the (1) size of the participating atoms and (2) the multiplicity of bonds. The larger the size of the atoms involved in bond formation, the lesser the extent of overlapping and the smaller the amount of energy released.
- For example, the bond enthalpy of the C—C bond is 348 kJ mol-1, whereas that of the H—H bond is 433 kJ mol-1. Tire magnitude of the bond enthalpy increases with the multiplicity of bonds.
- This is because the greater the number of shared electron pairs, the more is the attraction between two atoms, and the greater the energy released during bond formation. The bond enthalpies for the oxygen molecule (O=O) and nitrogen molecule (N≡N) are 498 kJ mol-1 and 946 kJ mol-1 respectively. A larger bond enthalpy implies a stronger bond.
Bond order: The bond order in a covalent molecule or polyatomic ion is the number of bonds present between the atoms of the molecule or ionic species.
- If we consider the examples, O2 and N2, the bond orders will be 1, 2, and 3 respectively. The bond orders correspond to the number of shared electron pairs, which is one in hydrogen (H—H), two in oxygen (O=O), and three in nitrogen (N≡N).
- Isoelectronic species have the same bond orders. Consider the example of the peroxide ion, \(\mathrm{O}_2^{2-}\) (Na2O2 contains a peroxide ion), and the fluorine molecule, F2. Both \(\mathrm{O}_2^{2-}\) and F2 have 18 electrons. The oxygen molecule contains a total of 16 electrons (2 + 6 = 8 in each oxygen atom).
- Therefore, the \(\mathrm{O}_2^{2-}\) ion has 18 electrons. The atomic number of fluorine is 9. Therefore, the F2 molecule has 18 electrons (2 + 7 = 9 electrons in each atom). Both \(\mathrm{O}_2^{2-}\) ion and F2 molecule are formed by sharing of one electron pair between the two atoms. Thus, both \(\mathrm{O}_2^{2-}\) and F2 have bond order 1.
- Generally speaking, with the increase in bond order, bond enthalpy increases and bond length decreases.
Bond angle: Covalent bonds are directional in nature. The atoms constituting a molecule have a three-dimensional arrangement which can be inferred from the spectral data of that compound. The positions of constituent atoms in a molecular structure are fixed.
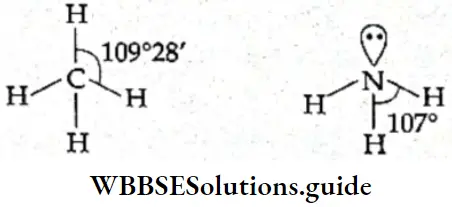
The position of each atom is determined by the nature of the chemical bond(s) that exist between that atom and the neighboring atoms. The angle that is formed between bonds sharing a common atom is called the bond angle.
The bond angle is expressed in degrees/minute/seconds. For example, the C—H bond angle in methane is 109°28′ and the N—H bond angle in ammonia is 107°.
“Valence Bond Theory, definition, importance, and applications, WBCHSE syllabus”
Concept of orbital overlap: When we say that two atoms are covalently bonded we mean that they share electrons. Take the case of the formation of the hydrogen molecule. The two isolated atoms form a molecule when their internuclear distance is d0 and the potential energy of the two atoms together is the least possible.
At this point, the electrons of the atoms are under the influence of both the nuclei. In other words, the two atoms share a pair of electrons.
- Now two atoms cannot share electrons if the electrons occupy two different regions of space. In other words, the two electrons are contained by the molecular orbital formed by the overlapping of the atomic orbitals. Tire filling of the molecular orbital must take place in accordance with the Pauli exclusion principle, i.e., since the two electrons occupy the same region of space, they must have opposite spins. The two atomic orbitals which participate in overlapping thus must be half-filled.
- This simple principle of orbital overlapping can explain not only the formation of the H2 molecule, but also why H3 and H4 are not formed, or why He2 is not formed. The hydrogen atom has a half-filled Is orbital. When two hydrogen atoms combine to form a molecule, the two Is orbitals (containing electrons with opposite spins) combine to form a molecular orbital.
“WBCHSE Class 11, chemistry notes, on Valence Bond Theory, and comparison with Molecular Orbital Theory”
- This molecular orbital gets completely filled by the two available electrons and no electron is left over. So, the H2 molecule does not have the capacity to form bonds with more hydrogen atoms. This is why H3 and H4 do not exist.
- The next question is why helium exists in the atomic state, or why He2 does not exist. The Is orbital of the helium atom is completely filled with the two electrons it contains. The Pauli exclusion principle forbids the overlapping of this orbital with the Is orbital of another helium atom. We can now summarise the orbital concept of the formation of covalent bonds.
- Covalent bonds are formed due to the overlapping of half-filled atomic orbitals.
- The atomic orbitals which overlap must contain electrons with opposite spins.
- Overlapping of atomic orbitals results in a decrease in energy.
The greater the extent of overlapping, the greater the energy released during bond formation and the stronger the bond.
Class 11 WBCHSE Chemistry Molecular Shapes And Geometry Explained
Types of covalent bonds: Covalent bonds are classified into two types based on the kind of overlapping that takes place between the atomic orbitals. These are
- The sigma (σ) bond and
- The pi (π) bond.
Sigma (σ) bond A σ bond is said to be formed between two atoms when their orbitals overlap along the internuclear axis. In other words, the axial or head-on overlapping of the orbitals of two atoms results in the formation of a σ bond between them.
“Valence Bond Theory, covalent bonding, hybridization, and limitations, WBCHSE notes”
The molecular orbital formed as a result of axial overlapping is symmetrical about the internuclear axis. This allows the rotation of the bonded atoms about the σ bond without breaking the bond.
The electrons constituting a σ bond are called sigma electrons. σ bonds can be formed by the overlapping of the following atomic orbitals.
s-s overlapping The s-s σ bond is formed when the half-filled (s) orbitals of two atoms overlap.
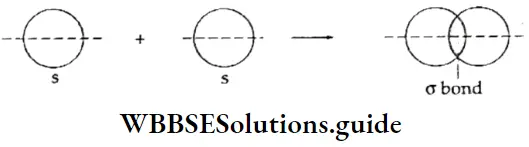
s-p overlapping The overlapping of the half-filled s orbital of one atom with the half-filled p orbital of another, leads to the formation of the s-p σ bond.

p-p overlap The p-p σ bond involves the overlapping of the half-filled p orbitals of two atoms. The first bond formed between two p orbitals is a sigma bond because these atomic orbitals can approach each other head on.
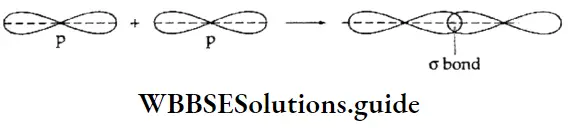
Pi (π) bond The lateral or sideways overlapping of atomic orbitals is called a π bond. The overlapping occurs in such a way that the orbitals are parallel to each other, but perpendicular to the intemuclear axis. Two electron clouds are formed in this kind of bond formation—one above and one below the plane of the atoms involved in the formation of the bond.
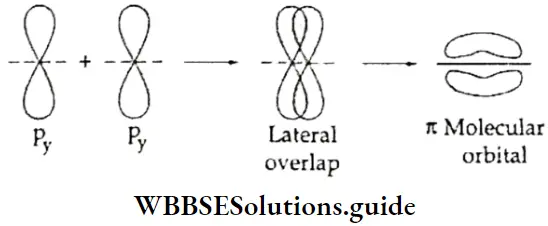
The electrons participating in the formation of a π bond are called π electrons. The formation of a π bond between two atoms restricts the rotation of the atoms about the π bond.
The first bond formed between two atoms is always a σ bond because the atomic orbitals are free to approach head on. The extent of overlapping is greater in a σ bond than in a π bond, so σ bonds are stronger.
Pi bonds are formed in addition to sigma bonds, if two atoms share more than one pair of electrons.
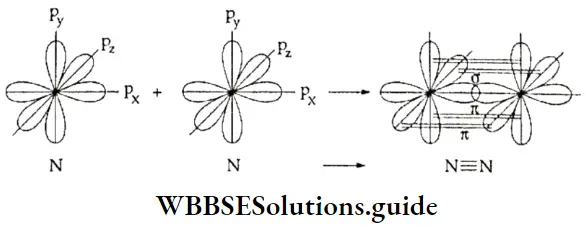
Formation of N2 and O2 molecules The electronic configuration of the oxygen atom is \(1 \mathrm{~s}^2, 2 \mathrm{~s}^2, 2 \mathrm{p}_x^2, 2 \mathrm{p}_y^1, 2 \mathrm{p}_z^1\). The py and pz orbitals, containing one electron each, are half-filled.
One of the p orbitals overlaps axially with a half-filled p orbital of another oxygen atom and forms a p-p σ sigma bond. After the formation of the sigma bond, the remaining half-filled orbitals of the two atoms are not free to approach head-on. So, these orbitals overlap sideways to form a π bond.
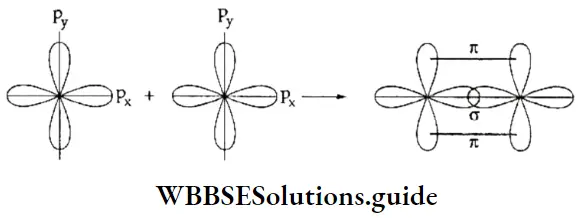
The electronic configuration of the nitrogen atom is \(I1 \mathrm{~s}^2 2 \mathrm{~s}^2 2 \mathrm{p}_x^1 2 \mathrm{p}_y^1 2 \mathrm{p}_z^1\). All three p orbitals are half-filled.
“WBCHSE Class 11 Chemistry, Valence Bond Theory, orbital overlap, and bonding”
The px orbital of one nitrogen atom overlaps with the px orbital of another nitrogen atom along the internuclear axis to form a σ bond. The py and pz orbitals of the two atoms overlap sideways to form two π bonds.