WBBSE Class 10 Physical Science Chapter 2 Behaviour Of Gases Short Answer Type Questions
WBBSE Class 10 Gas Behaviour Question 1. State one point of difference between the expansion of gas and the expansion of solid.
Answer:
For any range of temperature rise all gases expand equally, but different solids expand by different amounts for the same range of temperature rise.
Question 2. Why does not the volume of a real gas become zero at absolute zero?
Answer:
Explanation: Any gas or vapour liquefies much before reaching this low temperature -273°C. Again, Charles’s law is valid for gases, not for liquids. Hence this situation of zero volume of gas is never attained practically.
“WBBSE Class 10 Physical Science Chapter 2 short answer questions, Behaviour of Gases”
WBBSE Class 10 Gas Behaviour
Question 3. Why type of automobile is inflated to a lesser pressure in summer than in winter?
Answer:
Explanation: In summer, the room temperature is much higher as compared to winter consequently, the same volume of air will exert greater pressure in summer. Therefore, it is always advisable to inflate the tyre to a lesser extent in summer as compared to winter to avoid bursting the tyre.

Question 4. Is it possible to cool a gas below absolute zero?
Answer:
Explanation: No, it is not possible to cool a gas below absolute zero. At absolute gas, the kinetic energy of the gas molecules becomes zero. The molecules come to rest at the bottom of the container. Therefore, the pressure of the gas is also zero. It is, therefore, impossible to cool a gas below absolute zero, since there is no heat left to be removed from the gas.
Question 5. What is an effusion of a gas?
Answer:
Effusion Of A Gas: It is the passage of a gas under pressure through a tiny hole in a container process of effusion is used to separate the isotopes of an element. During effusion, the lighter isotopes escape first from the pores of the container leaving behind the heavier isotopes.
Question 6. How does an ideal gas differ from a real gas?
Answer:
Explanation: Ideal gas obeys gas laws or the gas equation PV = nRT at all temperatures and pressures where as real gas obeys the gas laws or the gas equation only at high temperatures and low pressures.
There is no cooling or heating effect observed when an ideal gas expands in a vacuum whereas a real gas shows either a cooling or heating effect.
WBBSE Class 10 Gas Behaviour
Question 7. Why aerated bottles are kept underwater during summer?
Answer:
Explanation: Areated water bottles contain carbon dioxide gas dissolved in aqueous solution under pressure. In summer, the solubility of the gas in solution decreases with a rise in temperature.
Consequently, the amount of free gas in the bottle increases. This leads to an increase in pressure. Thus, the bottle may explode. To avoid explosion, bottles are kept under cold water in summer.
Question 8. Is it possible to keep liquid nitrogen in a sealed cylinder at ordinary temperatures like ammonia?
Answer:
Reason: No, it is not possible to keep the liquid nitrogen in a sealed container at an ordinary temperature like ammonia because the critical temperature of nitrogen is very low whereas the critical temperature of ammonia is lighter than that of ordinary temperature.
Question 9. What is normal temperature and pressure (NTP)?
Answer:
NTP or STP (Standard Temperature and Pressure): It is defined as the pressure exerted by a column of mercury 76 cm. in height at 0°C, at 45° latitude and at mean sea level.
Question 10. Calculate the value of normal pressure in the CGS and SI system.
Answer:
- Normal pressure in CGS system: 76 x 13.6 x 980 1.013 × 106 dyne/cm2
- Normal pressure in SI system: 1.013 x 105 N/m2 = 1.013 x 105 pascals.
Question 11. State Boyle’s law.
Answer:
Boyle’s law (1662): At constant temperature, the volume of a definite mass of any gas varies inversely to the pressure of the gas.
WBBSE Class 10 Gas Behaviour
Question 12. State Charle’s law.
Answer:
Charle’s law (1787): At constant pressure, the volume of a given mass of any gas increases or decreases by \(\frac{1}{273}\) of its volume at 0°C for each one-degree rise or fall in temperature.
“Class 10 WBBSE Physical Science Chapter 2 short answer questions, Behaviour of Gases study material”
Question 13. What is real gas? What is an ideal gas?
Answer:
- Real gas: It is a gas which does not obey the general gas equation and all other gas laws strictly but tends towards ideality at low pressure and high temperature.
- Ideal gas: It is a gas that obeys the general gas equation and other gas laws under all conditions of temperature and pressure.
Question 14. State the values of universal gas constant R in different units.
Answer:
Values of universal gas constant R in different units:
- 0.0821 lit atm mol-1 K-1
- 8.314 x 10′ erg mol-1 K-1
- 8.314 J mol-1 K-1
- 1.987 cal mol-1 K-1
WBBSE Class 10 Gas Behaviour
Question 15. What is the relation between the density and pressure of an ideal gas?
Answer:
The relation between the density and pressure of an ideal gas
According to the ideal gas equation
\(\mathrm{PV}=\mathrm{nRT}=\frac{\mathrm{m}}{\mathrm{M}} \mathrm{RT}\)Where ‘m’ is the mass of gas and m is its molecular mass.
\(\mathrm{P}=\frac{\mathrm{m}}{\mathrm{V}} \frac{\mathrm{RT}}{\mathrm{M}}\) \(\text { or, } P=d \frac{R T}{M} \text { where } d=\frac{m}{V}=\text { density of the gas }\)∴ d α p constant temperature.
Question 16. What is the equation of state? What is the universal gas constant?
Answer:
Equation of state: From the combination of. Boyle’s and Charle’s laws we get,
\(\frac{P V}{T}=K\)The numerical value of the constant of proportionality (K) depends upon the quantity of gas and the units in which volume and pressure are expressed but is totally independent of the nature of the gas.
For 1 mole of gas, the constant is termed a universal gas. Constant.
∴ \(\frac{P V}{T}=R\)
or, PV = RT.
This is known as the general gas equation for ‘n’ moles of the gas, therefore, the gas equation may be written as :
PV = nRT.
WBBSE Class 10 Gas Behaviour
“WBBSE Class 10 Physical Science Chapter 2, Behaviour of Gases short answer solutions”
Question 17. What is absolute zero? Define the absolute scale of temperature.
Answer:
Absolute zero: The lowest possible temperature at which a given mass of gas does not occupy any volume or does not exert any pressure is known as absolute zero.
Absolute scale of temperature. The temperature scale has been devised by taking -273°C as zero and using the magnitude of each degree as absolute or Kelvin scale of temperature.
Question 18. State two characteristics of gases.
Answer:
Characteristics of gases :
- A gas has no definite shape or volume. It takes the shape and attains the volume in which it is kept this tendency of scattering of the gas molecules is called the expansion of gas.
- If a few gases which do not react with each other, are kept in a container, these gases mix with each other and a homogeneous mixture is prepared thus the property is known as diffusion.
Question 19. State pressure-Temperature relationship por Gay Lussac’s law.
Answer:
Pressure-Temperature relationship or Gay-Lussac’s law:
At constant volume, the pressure of a given mass of a gas increases by \(\frac{1}{273}\) of its original value at 0°C for every 1°C rise in temperature.
WBBSE Class 10 Gas Behaviour
Question 20. Reduce the mathematical expression of Boyle’s law.
Answer:
The mathematical expression of Boyle’s law:
If P be the pressure and V the volume of a given mass of a gas, then according to Boyle’s law,
\(V \propto \frac{1}{P}\) (the mass and the temperature remaining constant)
or, \(V=\frac{K}{P}\) (K is a proportionality constant)
or, PV = K (constant)
This is the mathematical expression of Boyle’s law.
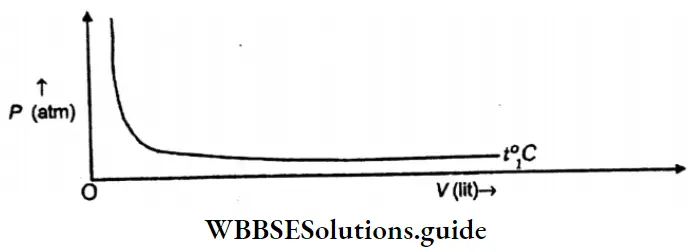
Question 21. What will be the nature of the graph is P is plotted against V for a given mass of gas at constant temperature?
Answer:
Explanation of graph: On plotting P against volume V fat at a given temperature, the graph will be a rectangular hyperbola as shown below.
Question 22. When a balloon inflates, it seems to violate Boyle’s law. Explain.
Answer:
When a balloon inflates, it seems to violate Boyle’s law.
Explanation: When a rubber balloon is inflated with the end of a pump, the pressure inside increases but the volume instead of suffering, decreases in accordance with Boyle’s law increases.
This happens because, with the introduction of air, the mass of the gas increases resulting in an increase in volume Thus, inflation of a balloon by mouth or pump appears to violate Boyle’s law but it is not so in the true sense.
Question 23. Deduce the mathematical expression of Charle’s law.
Answer:
The mathematical expression of Charle’s law:
Let Vo be the volume of a definite mass of a gas at 0°C, then the volume of 1°C rise in temperature will be = \(\left(\mathrm{Vo}+\mathrm{Vo} \times \frac{1}{273}\right)\)
The volume of t°C rise in temperature will be = \(\mathrm{Vo}+\mathrm{Vo} \times \frac{1}{273}\)
If Vt represents this volume at t°c
It \(V t+V_0+V_o+\frac{t}{273}=V_0\left(1+\frac{t}{273}\right)\)
similarly, when the temperature at the same mass of gas is gradually made to decrease its volume at t°C is given by
\(V t+V o+V o \times \frac{l}{273}=V_0\left(1-\frac{l}{273}\right)\)“WBBSE Class 10 Behaviour of Gases short answer questions, Physical Science Chapter 2”
Question 24. Establish the combined law of Boyle’s and Charle’s laws.
Answer:
Combination of Boyle’s law and Charle’s law:
Let V be the volume of a given mass of gas at pressure P and temperature T (absolute)
From Boyle’s law, \(\mathrm{V} \propto \frac{1}{\mathrm{P}}\) [When temperature and mass of the gas are constant]
From Charle’s law, V α T [When pressure and mass of the gas are constant]
∴ \(V \propto \frac{T}{P}\) when both T and P vary.
or, \(V=\frac{K T}{P} \text { or, } \frac{P V}{T}=K[K \text { constant }]\)
Now, if V1 is the volume of a gas at pressure P1 and temperature T1 and V2 is the volume of the same amount of gas at pressure P2 and temperature T2 then,
\(\frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2}\)“WBBSE Class 10 Physical Science Chapter 2, Behaviour of Gases important short answer questions”
Question 25. Define-Partial law.
Answer:
Partial law: The partial pressure of any constituent gas or vapour in a mixture is defined as the pressure which the said gas would exert if it alone had occupied the entire volume occupied by the mixture, with temperature in both cases remaining the same.
Question 26. Draw a graph between pressure (P) vs volume (1/V) at constant pressure.
Answer:
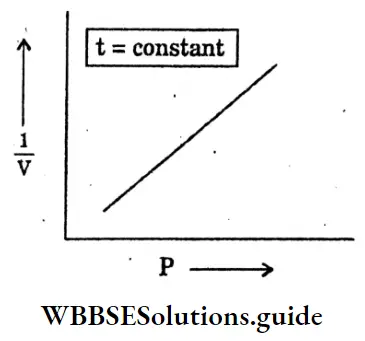
Question 27. Draw V vs t at constant pressure (P) for Charles law.
Answer:
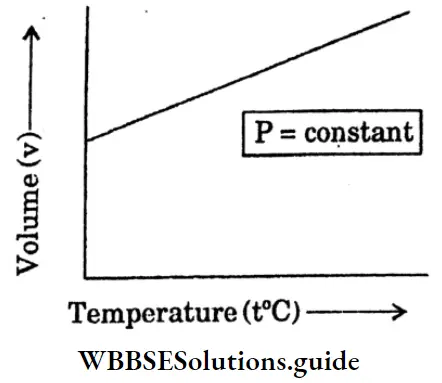
“Class 10 WBBSE Physical Science Chapter 2, Behaviour of Gases short answer key”
Question 28. What is the relation between pressure and density at a constant temperature?
Answer:
The relation between pressure and density at a constant temperature
The density of a gas at a constant temperature is proportional to pressure.
\(\frac{P}{P}=\text { Constant. }\)WBBSE Class 10 Physical Science Solutions
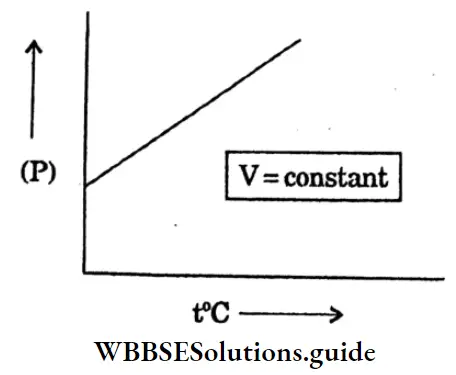
Question 29. Draw a graph of pressure (P) VS temperature (t) at constant volume.
Answer: