Chapter 2 Nature Of Matter Nature Of Matter
Physical and Chemical properties of matter
All substances have a set of characteristic properties utilizing which we can distinguish one substance from another. Properties of matter are of two types :
- Physical properties and
- Chemical properties.
Physical properties are those which are exhibited by a substance externally without any change in its composition. For example, water is a colourless, odourless, tasteless liquid. These are the physical properties of water.
Read and Learn more WBBSE Notes For Class 8 General Science And Environment
The important physical properties are :
colour, odour, taste, nature (touch), density, solubility, melting and boiling point and magnetic properties.

On the other hand, the properties that a substance exhibits when it undergoes a complete change of its initial composition and arrangement due to the action of some other substances or influenced by heat, electricity, sound, etc. are known as the chemical properties. For Example, when a magnesium ribbon burns in the air, it produces white fumes.
| Class 8 General Science | Class 8 Maths |
| Class 8 History | Class 8 Science LAQs |
| Class 8 Geography | Class 8 Science SAQs |
| Class 8 Maths | Class 8 Geography |
| Class 8 History MCQs | Class 8 History |
“WBBSE Class 8 General Science Chapter 2 notes, Nature of Matter”
These fumes get deposited over a cold surface as a white solid. The white solid is magnesium oxide, which is different from the original substance. It proves the fact that magnesium is combustible—it is a chemical property.
The important chemical properties are :
Chemical reactions with→Acids, Bases or other chemicals.

Physical Properties Of Some Substances
1. Colour :
It is such a physical property that helps us in recognising the substance.
Colour of solids :
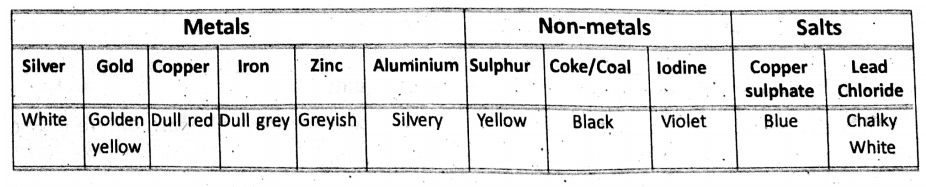
Colour of liquids :
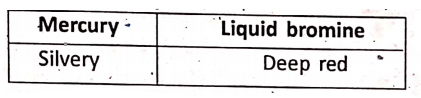
Colour of gases :

2. Odour:
Usually, we recognise naphthalene, bleaching powder, kerosene, petrol, etc. by their characteristic odour.
The odour of gases :

3. Taste: Some gases have taste and some are tasteless.

4. Touch :

5. Nature of some gases :

6. Solubility :
Common salt, sugar, copper sulphate, etc. are soluble in water while sulphur, chalk, etc. are insoluble in water.
Solubility of some gases:

7. Melting point of solids and boiling point of liquids :
Different solids melt at different temperatures. So, their melting points are different. For e.g., melting point of ice is 0°C, that of copper is 1080 C, etc. Every liquid has its own boiling point. For instance, under normal atmospheric pressure, the boiling point of water is 100°C, that of chloroform is 61°C, etc.
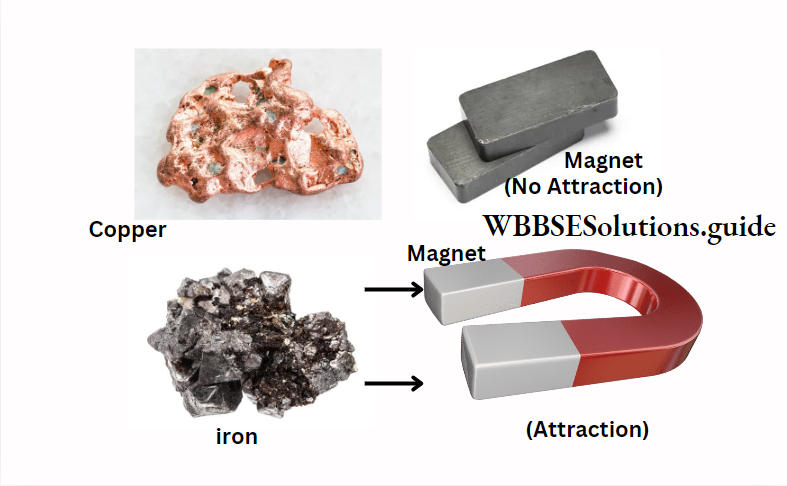
8. Magnetic properties :
Magnetic substances such as iron, nickel, cobalt and their alloys are attracted by a magnet whereas non-magnetic substances such as copper, aluminium, etc. are not attracted by a magnet.
Chapter 2 Nature Of Matter Nature Of Matter Chemical Properties Of Some Substances
1. Action of heat :
Different substances behave differently towards heat, using which their chemical properties are changed.

2. Treatment with water :

3. Action with acids :
Dilute sulphuric or’ dilute hydrochloric acid reacts with zinc granules or small pieces of magnesium wire or iron powder to produce hydrogen gas. The gas burns with a slight explosion (a ‘pop’ sound) when a glowing split is brought into the gas. But the acid makes no reaction in ordinary condition on copper.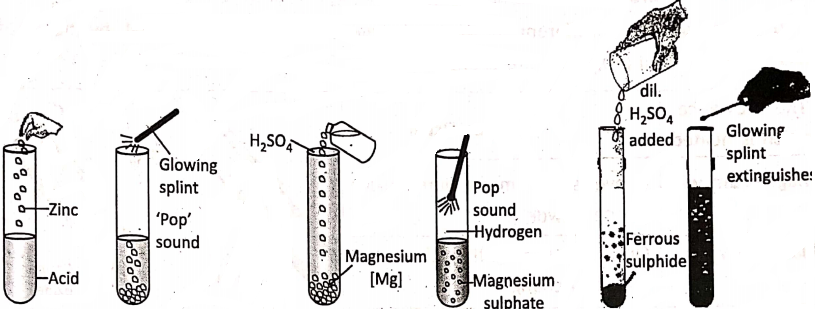
Also, when dilute sulphuric acid is added to ferrous sulphide, a gas with a rotten egg smell evolves, and the glowing splint gets extinguished. The evolved gas is hydrogen sulphide.
4. Action with alkalies :
Ammonium chloride is a colourless, odourless solid but soluble in water. If it is heated with a base like lime or caustic soda, ammonia gas is evolved.
“Class 8 General Science WBBSE notes, Nature of Matter chapter”
Again, sodium chloride (a colourless crystal) does not show any change on being heated with caustic soda. Thus, the action of caustic soda helps to distinguish ammonium chloride and sodium chloride from one another.
Chapter 2 Nature Of Matter Study Of Metals And Non-Metals
Based on their physical and chemical, properties, the elements are often divided into two main categories—metals and non-metals. Besides these, the elements which behave both as a metal and a non-metal constitute a third category—called metalloids or semi-metals.
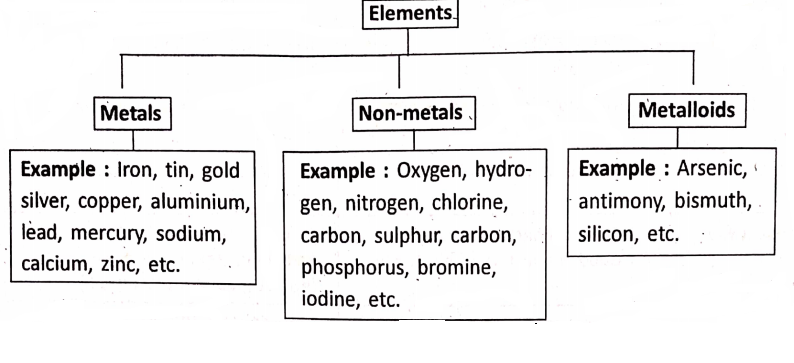
Metals are the elements which are, in general, lustrous (shiny), hard crystalline solids with high melting and boiling points, malleable and ductile, good conductors of heat and electricity and sonorous.
Non-metals are the elements which are, in general, not lustrous (non-shiny), exist as liquids or gases or soft solids with low melting and boiling points, are brittle if solids, are bad conductors of heat and electricity, and are not sonorous.
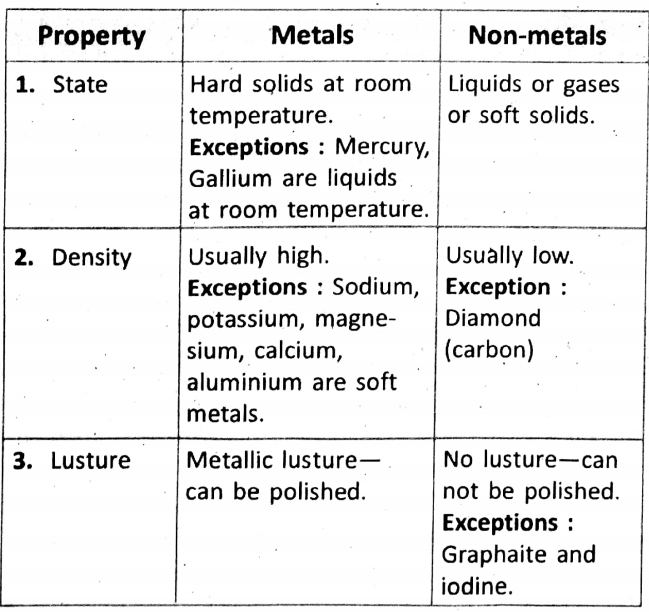
Chapter 2 Nature Of Matter General Physical Properties Of Metals And Non-Metals
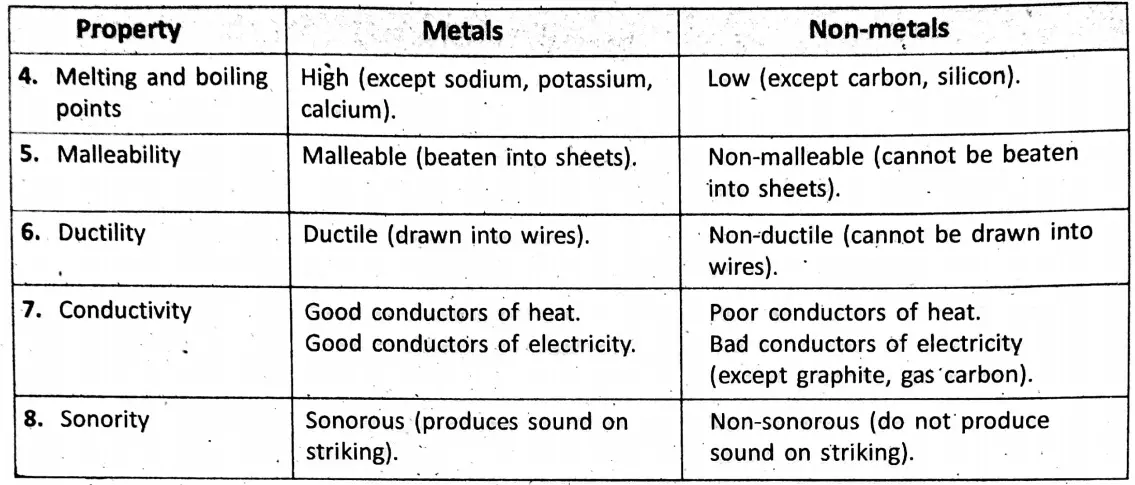
Chapter 2 Nature Of Matter General Chemical Properties Of Metals And Non-Metals
It is obvious that the physical properties of metals and non-metals explain only a partial description. So it is useful to mention their chemical properties also.
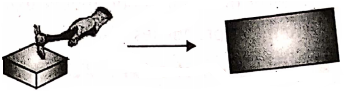
Malleability :
The ability of a substance that can be hammered into thin sheets.
Ductility :
The ability of a substance that can be drawn into wires.
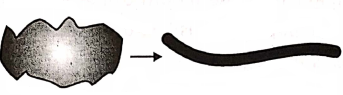
- Gold and silver are. highly malleable. Non-metals are not malleable, rather they are transformed, into powder when, hammered hard.
- Metals are ductile. Gold can be drawn into very thin wire.
- Metals conduct heat and electricity, but non-metals are unable to behave so, except diamond and graphite. Diamond is a very good conductor of heat, but a poor conductor of electricity.
The reaction of acids :
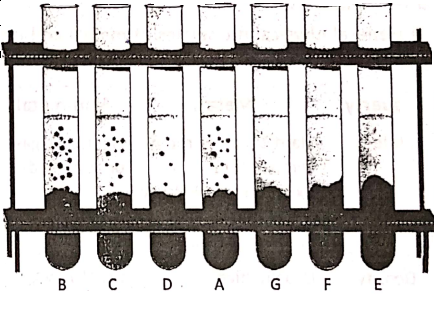
Acids react with active metals to liberate hydrogen gas. Let us take seven test tubes A, B, C, D, E, F, and G and fill them partially by dil. hydrochloric acid. Now add equal amounts of Fe, Mg, Al, Zn, Cu, S and C respectively in the test tubes.
“WBBSE Class 8 Environment Chapter 2, Nature of Matter study guide”
1. A colourless gas is evolved in the test tubes A, B, C and D. Hold a glowing splinter near the mouth of the test tubes and a ‘pop’ sound is heard. So the evolved gas is hydrogen.
Reactions : (In test tube A)
Fe + 2 HCl →FeCl2 + H2 ↑
(In test tube B)
Mg + 2HCl → MgCl2 + H2 ↑
(In test tube C)
2AI + 6 HCl →2ACl 3H2 ↑
(In test tube D) Zn + 2HCl→ZnCl2 + H2 ↑
(2) From the test tubes E, F and G, no gas is seen to evolve. This means that dil. HCI does not react with Cu, S and C.
Chapter 2 Nature Of Matter The Reaction Of Water With Metals
How do the metals react with water ?—
Metals react with water as follows
It can be explained with the help of the ‘Activity series of metals, which is a series of metals arranged in the decreasing order of their reactivity. The series helps us to explain many types of reactions (including displacement reactions) depending on the position of the metal in the activity series of metals. A metal which is placed higher in the activity series is more reactive than those placed below it.
1. Most active metals like K, Na and Ca which have a higher positions than hydrogen in the activity series, displace hydrogen from cold water.
For example,
2K + 2H2 O→2KOH + H2 ↑
2Na + 2H2 O → 2NaOH + H2 ↑
Ca + 2H2 O→ Ca(OH)2 + H2 ↑
2. Mg, Al, Zn, and Fe react with boiling water or steam producing their respective oxides.
Mg + H2O → MgO + H2 ↑
2AI + 3H2O→ Al2O3 + 3H2
Zn + H2O → ZnO + H2 ↑
3Fe + 4H2O Fe3O4 + 4H2 ↑
3. Metals placed below hydrogen in the activity series do not displace hydrogen from cold water or steam.
4. Iron, whose position is higher than copper in the activity series, reacts with copper sulphate solution. However, copper does not react with iron sulphate solution as copper is less reactive than iron.
“WBBSE Class 8 Nature of Matter notes, General Science Chapter 2”
5. Silver does not react with zinc sulphate.
Ag + ZnSo4 → No reaction
6. Silver does not react with copper sulphate solution.
Ag + CuS04 → No reaction
7. Zinc reacts with copper sulphate solution to form zinc sulphate and copper.
Zn + CuS04 → ZnS04 + Cu
Chapter 2 Nature Of Matter Uses Of Metals And Non-Metals
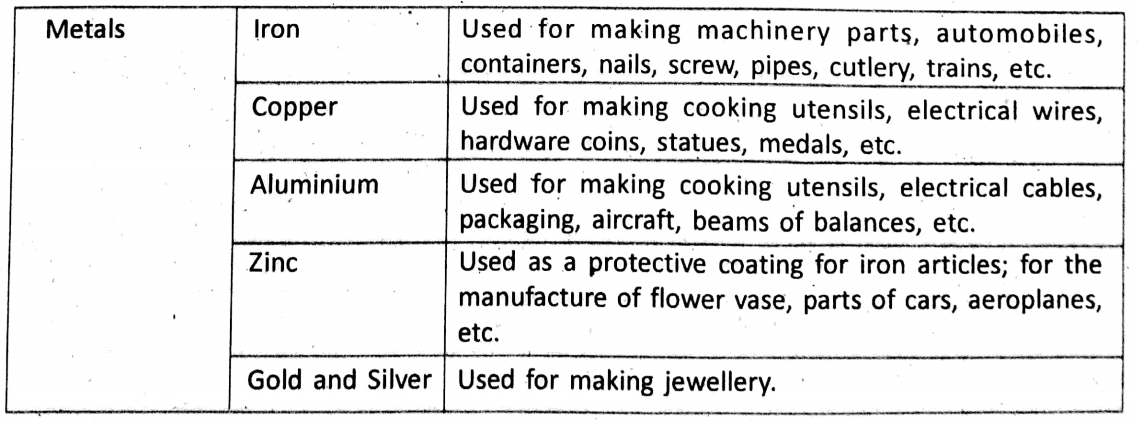
Uses Of Metals And Non-Metals
In our daily life, we use metals, non-metals and their alloys in various purposes. Here we mention the uses of some common metals and non-metals.

Importance Of Metals And Non-Metals In Human Life
Like all living organisms human body also needs food to obtain energy and different materials (mainly minerals) for the growth, development and repair of damaged cells, and tissues. Most of the foods that we take are of plant and animal origin containing C, 0, H, N, S, P, Na, K, Ca, Mg, and Fe.
In order to get all the essential nutrients (carbohydrates, fats, proteins, vitamins, minerals, etc.), we need to take a variety of food items. Especially, minerals which are the metallic and non-metallic elements are needed for the normal functioning of the body. They are the building materials of soft tissues, muscles, bones, teeth and blood.
The importance of some common minerals and their deficiency symptoms are listed “below :
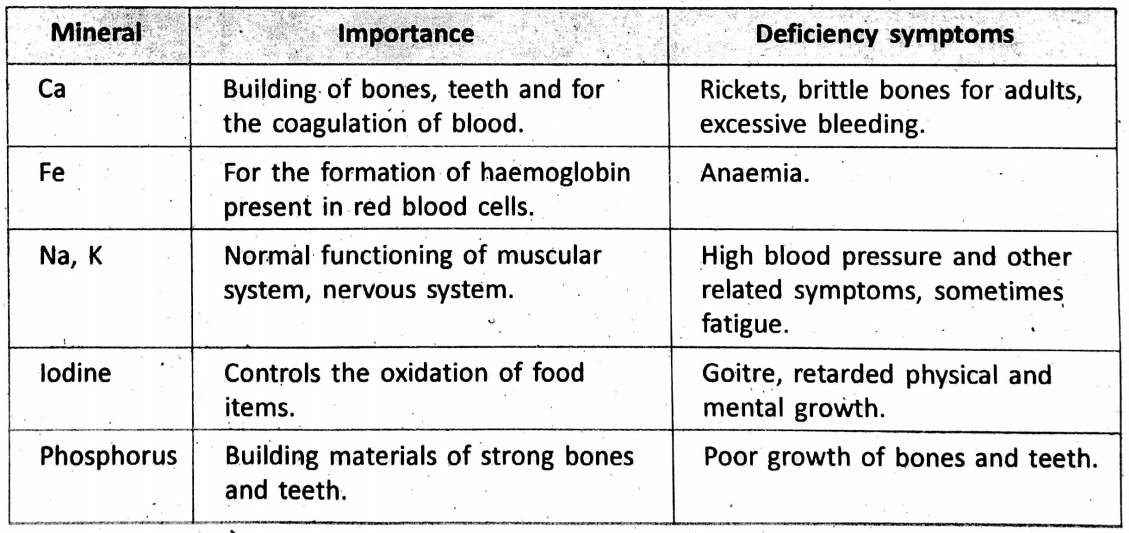
Some important life functions related to the usefulness of metals and non-metals are explained here :
1. Importance of water in human body :
About 90% of our body is made up of water. It is also the main component of most food items. In fact, water acts as a medium in most of the chemical reaction that takes part in our body. Water regulates our body temperature and helps in eliminating waste products from our bodies.
2. Formation of bones and teeth :
Ca, Mg and P4 are mainly useful for the building of strong bones and teeth.
3. Formation of blood in the body :
In adults, the RBCs are produced in the marrow of long bones. The RBCs contain a respiratory pigment called haemoglobin (Hb) which is formed of an iron-containing part (haemin) and a protein (globin).
4. Acid-base balancing :
Acidity inside cellular fluid increases with a lower intake of K, Na, Mo, etc. through different food items. An increase in acidity causes increases in alkalinity within extracellular fluids. The K, Na, etc. metals initiate to maintain acid-base balancing between the inner and outer fluid parts of a cell.
5. Well-functioning of heart :
The circulation of blood in the heart starts with the contraction of two auricles, and at the same time, the two ventricles relax. Therefore, blood passes from auricles to the ventricles. Next, the ventricles contract and the auricles relax. Such contraction and relaxation is controlled by metals like Ca, K, etc.
Now let us explain how several diseases are caused in human body due to excessive intake of some metals and non-metals like lead, aluminium, arsenic, fluorine, mercury, cadmium, etc. exceeding the limit of tolerance from the environment.

Chapter 2 Nature Of Matter Structure Of Atom
Consent of atoms
Kanad, the ancient Indian sage, for the first time introduced the idea that every matter is composed of very small particles. Greek philosopher Democritus also proposed the same idea and he named these particles as atoms.
“Class 8 WBBSE General Science Chapter 2, Nature of Matter summary”
The word ‘atom’ has been derived from the Greek word ‘atoms’ meaning ‘indivisible’. They approached no more and left the idea vague. In the 19th century a famous’ British chemist John Dalton first developed a scientific theory regarding the structure of matter.
He suggested that every substance consists of a large number of minute indivisible particles which are known as ‘atoms’. He proposed his John Dalton’s atomic theory’.
Chapter 2 Nature Of Matter The Postulates Of Dalton’s Atomic Theory Are
- All matter is made up of very minute, indivisible particles called ‘atoms’ which can neither be created nor destroyed.
- Atoms of the same element are all identical in size, mass and other properties. But, atoms of different elements have different properties.
- When different elements combine to form compounds, the combination takes place among the atoms in definite ratios of whole numbers.
Till the end of the 19th century, scientists believed that atoms are the smallest individual parts of an element. In the late 19th and early 20th century, experiments of J. J. Thomson, Goldstein, Rutherford, Chadwick, Sommerfield, Niels Bohr and many others brought a revolutionary change of this idea.

Modern research have proved that atoms are divisible. An atom is made up of three fundamental sub-atomic particles, namely, electrons, protons and neutrons.
Chapter 2 Nature Of Matter Discovery of Electrons
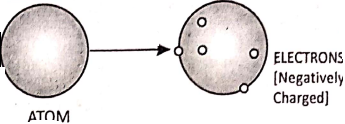
J. J. Thomson studied the structure of the atom and he found out by experiment that atoms contain negatively charged particles called ‘electrons’.
Chapter 2 Nature Of Matter Discovery of Protons
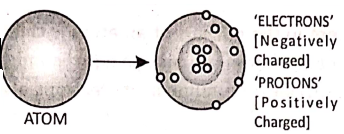
Goldstein further studied the structure of the atom. He assumed that the atoms are electrically neutral. To withstand with this idea, he considered the existence of positively charged particles in the atoms. These particles are called ‘protons’.
Chapter 2 Nature Of Matter Discovery of The Nucleus
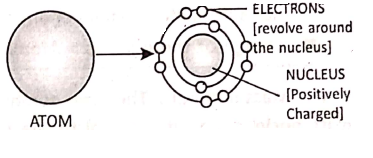
Ernest Rutherford, an eminent scientist, studied the ‘atomic model’ and concluded that each atom consists of a small, dense and positively charged space at the centre. It is known as the nucleus. It contains positively charged protons. The electrons revolve around the nucleus.
Chapter 2 Nature Of Matter Discovery of Neutrons
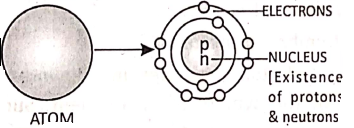
James Chadwick studied the sub-atomic particles and concluded that in the nucleus there are ‘neutral particles’ called ‘neutrons’.
Modern atomic model :
Niels Bohr proposed the modern atomic model. According to him, an atom contains a central nucleus having positively charged protons and neutral neutrons.
“WBBSE Class 8 Science Chapter 2 notes, Nature of Matter PDF”
The electrons revolve around the nucleus in different orbits or shells, where the number of electrons in the outer shells are exactly equal to the number of protons inside the nucleus. So, the net positive and negative charges are the same. That is why an atom is electrically neutral.
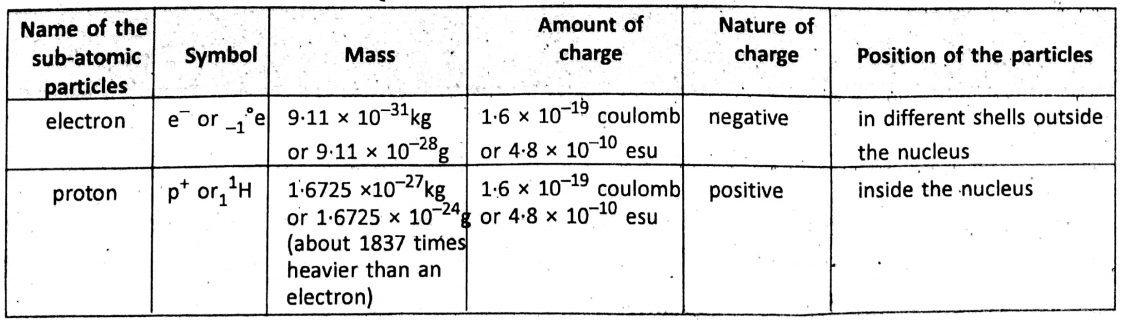
Features of sub-atomic particles :

Atomic number:
The number of protons present in the nucleus of an atom is called the atomic number. It is usually denoted by ‘Z’. Since a neutral atom contains an equal number of protons and electrons, so the atomic number also specifies the number of electrons also for a neutral atom.
The atomic number of each element is a fundamental property. Because, each element has a fixed, atomic number and no two elements can have the same atomic number.
Charge of the nucleus = Atomic number x Charge of an electron.
Mass number:
The mass number is the sum of the numbers of protons and neutrons in its nucleus, i.e., it is equal to the number of nucleons inside the nucleus. It is usually denoted by ‘A’. Thus,
Mass number (A) = Number of protons (Z) + Number of neutrons (n)
or, Number of neutrons (n) = Mass number (A) – Number of protons (Z)
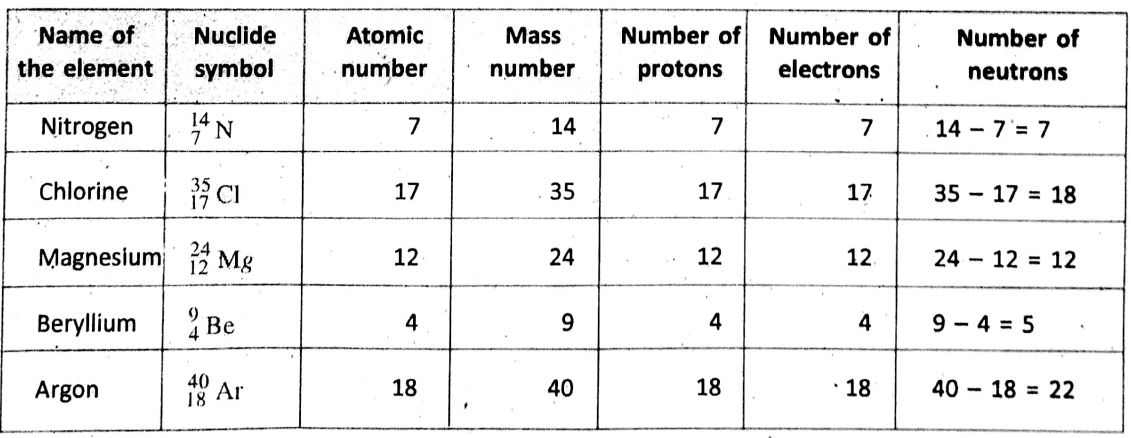
Nuclide symbol :
In general, the mass number and atomic number are shown as a superscript and a subscript respectively on the left of the symbol of an element. Such representation is known as the nuclide symbol. For example, an atom of element X is written as Axz. [A and Z are atomic numbers and mass number respectively].
Isotopes :
Atoms of the same element having the same atomic number but different mass numbers due to different numbers of neutrons in their nuclei are known as isotopes of one another.
Few examples of isotopes
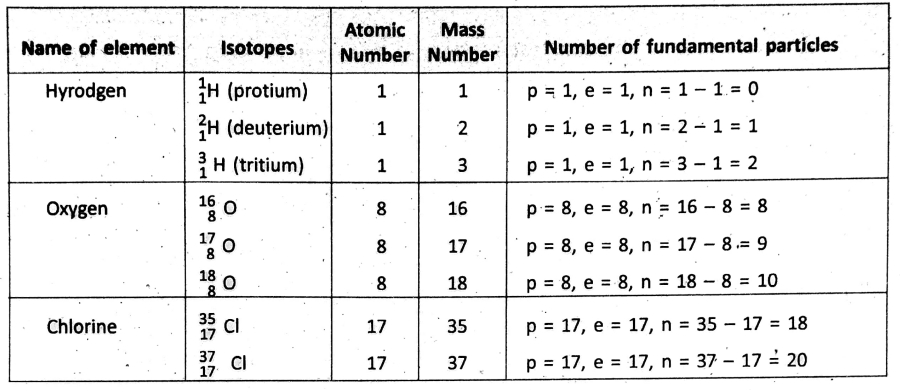
Physical properties like mass, density, etc. of the isotopes of an element are different. This is so because the isotopes contain a different number of neutrons in their nuclei. But, the chemical properties of all isotopes of an element are similar.
Because, the chemical properties of an element are mainly determined by the number of electrons in different shells and their arrangement—which is same for all the isotopes of an element. After the invention of isotopes, the postulate of Dalton’s atomic theory that all atoms of the same element have equal masses has been proved to be wrong.
Isobars and Isotones :
Atoms of different elements which have an equal mass numbers but different, atomic numbers are called isobars of one another.
Examples are : 14C6 and 14N7: 40Ar and 49Ca20 etc.
But, atoms of different elements which have different atomic numbers and different mass numbers but equal numbers of neutrons in their nuclei are called isotones of one another. For Example, ^60 and J4c are isotones of one another, as both the nuclei have 8 neutrons.
Chapter 2 Nature Of Matter Concept Of Molecules
John Dalton regarded an atom as the tiniest indivisible particle of an element taking part in a chemical reaction. He called atoms of elements as ‘simple atoms’ and atoms of compounds as ‘complex atoms. So, the distinction between element and compound became difficult.
This difficulty was first removed by Amedeo Avogadro. He told that the atoms of the gaseous elements like hydrogen, nitrogen, etc. cannot exist in nature freely, but they are present as particles joining two atoms together.

He called these P as molecules. That is, atoms of the same or different elements combine to form a molecule. Later Cannizzaro, Berzelius and others supported the existence of molecules. Thus, a molecule is the smallest particle of a substance than can normally exist independently in nature. For example—
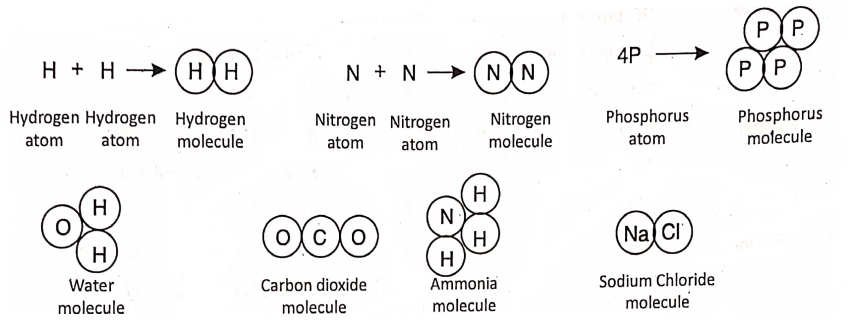
Chapter 2 Nature Of Matter Valency And Chemical Bond
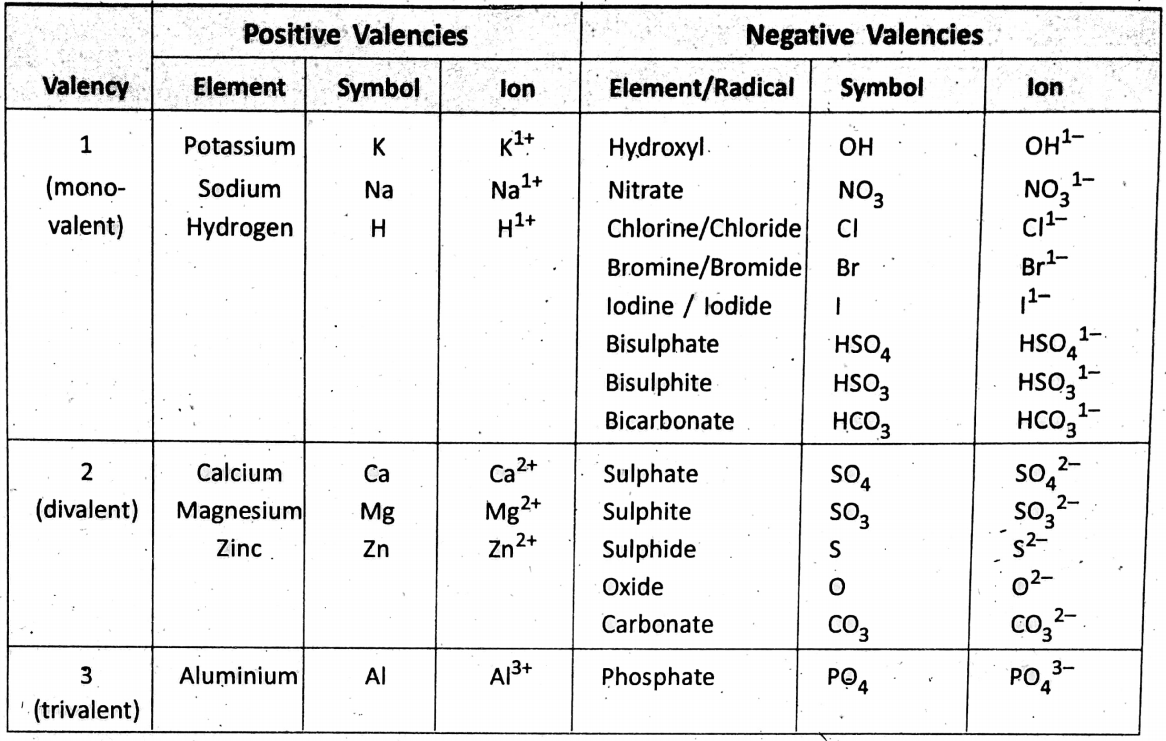
How a compound is formed ?—Atoms or molecules of different elements combine in a fixed proportion to form a compound. One atom of an element will combine with how many atoms of other elements depending on their combining capacities. The valency of an element is described as the combining capacity of an atom of the element.
It is found on analysis of compounds that the valency or combining capacity of hydrogen is the least and it is assumed to be 1 (one). Thus, in measuring valency, hydrogen has been chosen as the standard.
The valency of an element is expressed by the number of hydrogen atoms which can combine (or displace) one atom of the element (or radical) to form a compound.
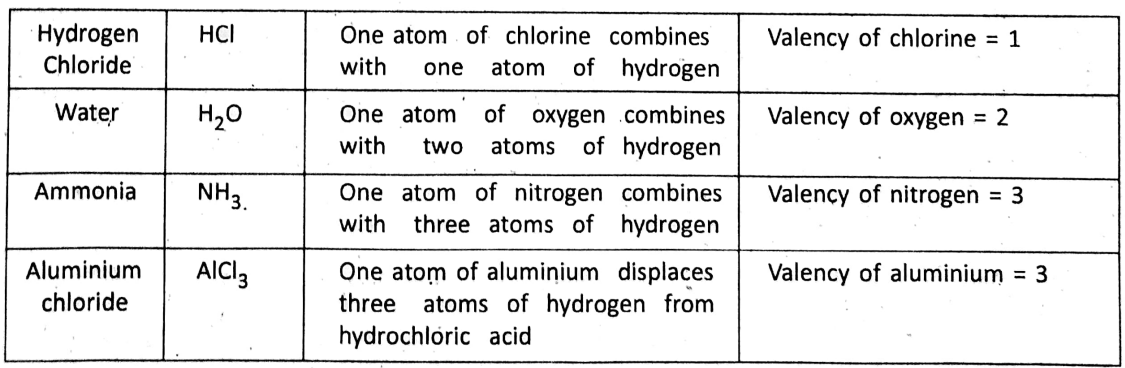
However, the valency of some elements which do not combine with hydrogen atoms is determined by the combining capacity with another element of known valency. For example, gold does not combine with hydrogen, and neither can displace hydrogen from its combination.
However, one atom of gold combines with three atoms of chlorine forming auric chloride -(AUCI3). Thus, the valency of gold = 3 (as the valency of chlorine = 1). Most of the elements can combine with oxygen and hence, it becomes convenient to find out valency with reference to oxygen. Remember that the valency of oxygen with reference to hydrogen is 2, and a valency is always a whole number.
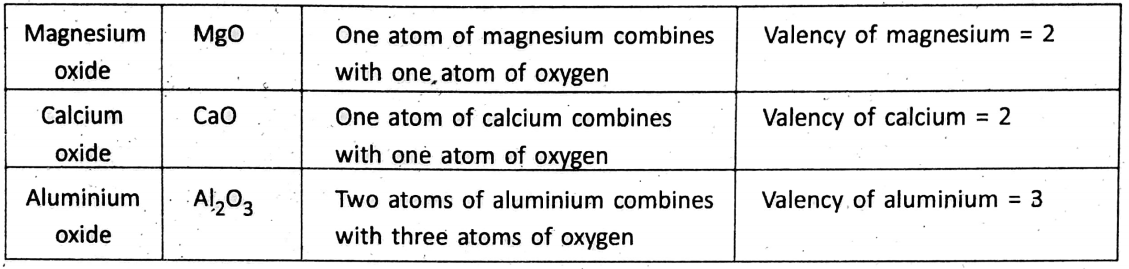
Some elements, however, have different valencies in different compounds.

If sodium sulphate (Na2S04 ) solution is added with barium chloride (BaCl2) solution, there appears a white precipitate of barium sulphate (BaS04 ).
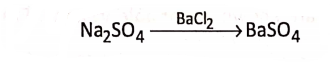
Here, S04, a group of atoms of different elements, passes from Na2S04 and enters unchanged into the formation of BaS04, such a group of atoms of different elements which acts collectively as a single atom, is called a radical.
Ions:
Under certain conditions, an atom can give up one or more electrons from its outermost shell or can accept one or more electrons. Due to this fact, a neutral atom is changed into an ion. Thus, ions are charged particles and they have an unequal number of protons and electrons, A cation is a positively charged ion. An anion is a negatively charged ion.
“Class 8 General Science Nature of Matter notes, WBBSE syllabus”
Chapter 2 Nature Of Matter Positive And Negative Valency
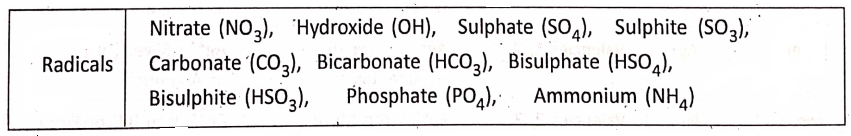
The valency of all metals (and hydrogen and ammonium radical) is considered ‘positive’; whereas the valency of all nonmetals (and non-metallic radicals) is considered ‘negative’.
Symbolic representation:
The ions M1+, M2+, and M3+ correspond to metallic ions and the ions A1-, A2-, and A3- correspond to non-metallic ions.
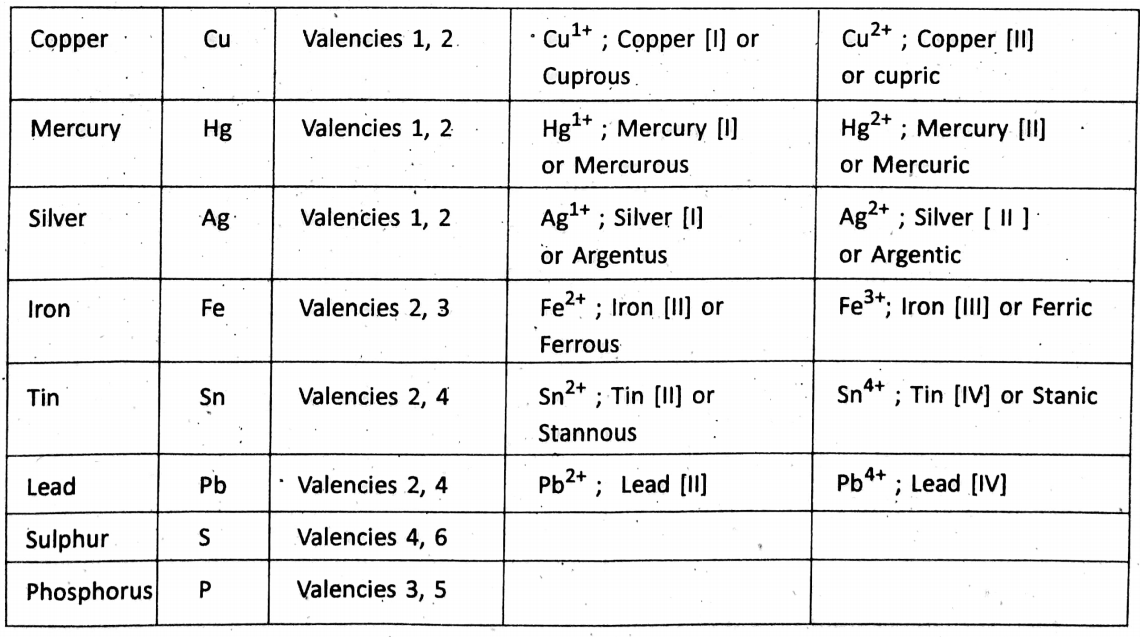
Variable valency :
Certain elements show more than one valency while the formation of different compounds. These.are known as variable valency. Examples are :
Chapter 2 Nature Of Matter Formation of Ionic Compounds :
Ionic Compounds
The ionic compounds are made up of oppositely charged ions held together by the electrostatic force of attraction. this gives rise to the formation of an ionic bonds such that the total positive charge on the cations must be equal to the total negative charge on the anions.
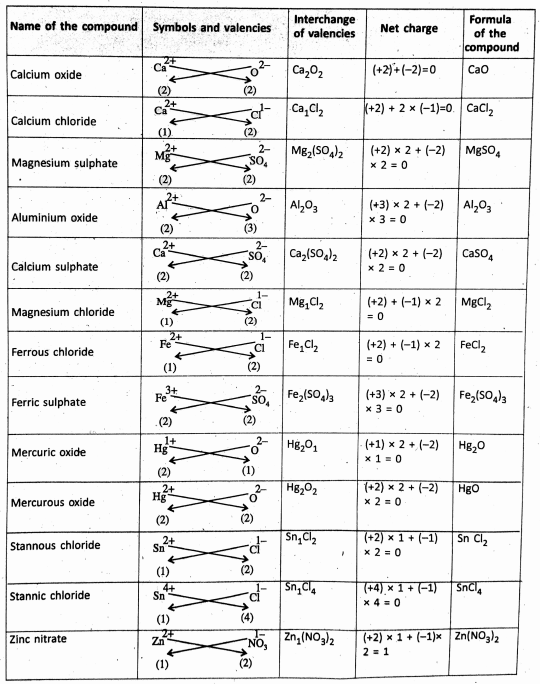
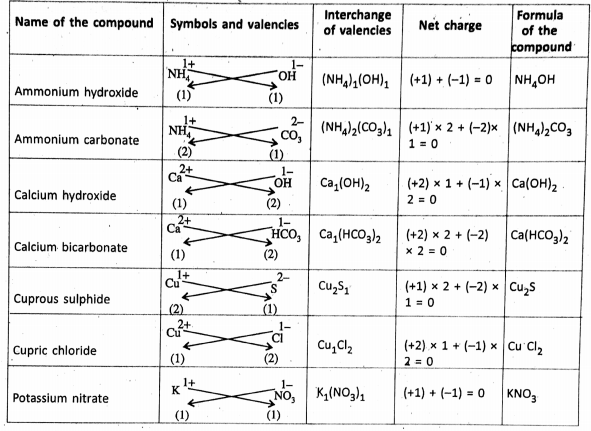
Chapter 2 Nature Of Matter Formation Of Covalent Compounds
Covalent Compounds
In covalent compounds (like 02, H2, H20, Cl2, CH4, CCl4; S02, P205, etc.), two or more atoms are bounded to each other by sharing of electron; pairs between them. Thus, a covalent bonding forms a true molecule by sharing electrons. In general, covalent ‘bonds are formed by non-metals. Let us now take some examples :
Covalent bonding :
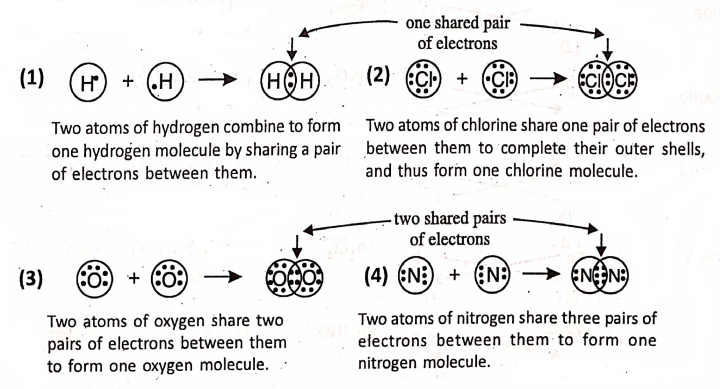
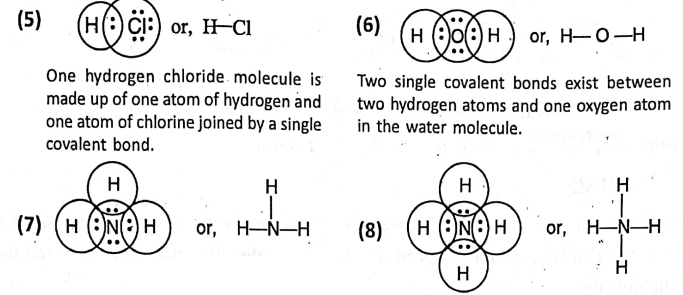
What is meant by the covalency of an atom ?—
The covalency of an atom refers to the number of electrons shared by it for the formation of shared electron pairs to form covalent bond. As hydrogen or chlorine shares one pair of electrons, so they have a covalence of 1.
Oxygen shares two pairs of electrons, so it has a covalence of 2. Nitrogen shares three pairs of electrons and has a covalence of 3. Carbon shares four pairs of electrons and has a covalence of 4.
“WBBSE Class 8 Science Chapter 2, Nature of Matter important questions”
Chapter 2 Nature Of Matter Chemical Reactions
Fetors affecting chemical reactions
Let us first know what is a chemical reaction.
A chemical reaction is a chemical change by means of which matter changes into a new substance or substance. A chemical equation represents the result of such a chemical change. For e.g. we may consider the simple reaction between zinc and dil. sulphuric acid.
Zinc + Sulphuric acid →Zinc sulphate’ + Hydrogen.
Zn + H2 SO4 (dil.) → ZnSO4 + H2 (g)
Here Zn and H2S04 (dil.) are the reactants; ZnS04 and H2(g) are the products; Also, the arrow sign indicates the direction of the reaction. Thus, reactants are the substances which take part in a chemical reaction and products are the substances which are produced as a result of a chemical reaction.
A chemical reaction is influenced by various conditions (or factors) such as the supply of heat, the presence of light, pressure, electricity, catalyst, solvent, etc.
Supply of heat:
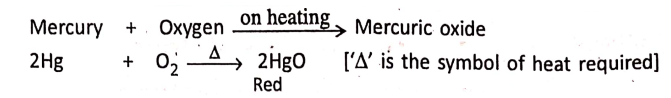
1. When mercury is heated in sufficient air or oxygen, it produces red mercuric oxide.
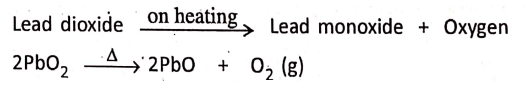
2. Lead dioxide on heating gives lead monoxide and oxygen.
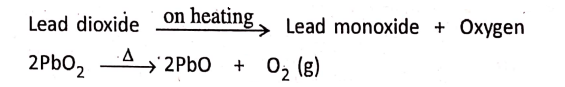
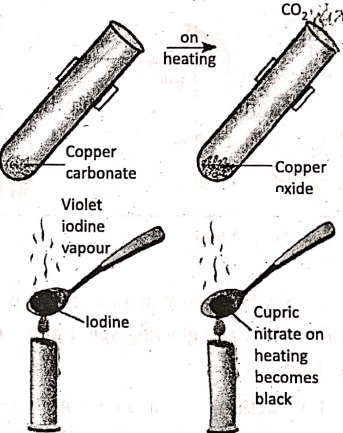
3. Blue, crystals of hydrated copper nitrate kept at room temperature, does not show any change; but when copper nitrate is heated, produces black powder of cupric oxide and reddish brown nitrogen dioxide gas.
Presence of light :
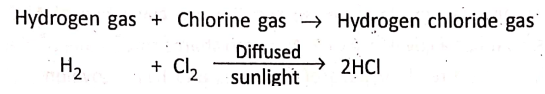
1. Hydrogen gas combines with chlorine in presence of diffused sunlight. The reaction is explosive in direct sunlight and negligible in dark.
Hydrogen gas + Chlorine gas→ Hydrogen chloride gas
2. Green plants use carbon dioxide and water to produce carbohydrates (sugar, starch, etc.) in the presence of light energy and chlorophyll. The reaction is
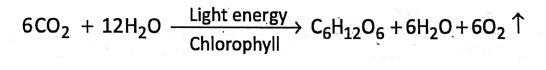
Presence of pressure :
Like heat, light, electricity, etc. pressure may sometimes bring ‘about chemical changes. Ordinary crackers (bhuipatka) made of potassium chlorate and sulphur or arsenic sulphide explode on percussion. There are some chemical reactions which take place only when the reactants are subjected to high pressure.
In the Haber process, nitrogen and hydrogen react only if the pressure is above 200 atmospheres. In this reaction, a finely divided iron catalyst (whose temperature is about 500°C) is used.
Nitrogen + Hydrogen→ Ammonia
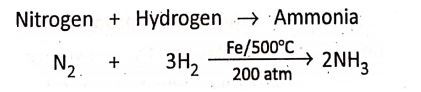
Presence of electricity :
On passing d.c. current through water acidulated with little amount of sulphuric acid, water is decomposed into hydrogen and oxygen. No gas is evolved unless d.c. current is passed.
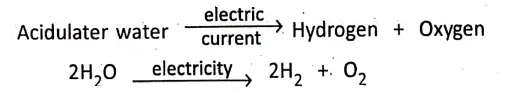
Chapter 2 Nature Of Matter Presence of Catalyst and Catalysis
Catalyst
Catalyst is a substance which being present in small amounts, increases or decreases the rate of a chemical reaction, without itself undergoing any permanent change in mass and composition.
The rate of a chemical reaction is accelerated by a positive catalyst and decelerated by negative catalyst. The action of a catalyst is known as catalysis.
For example:
1. potassium chlorate (KCI3) requires to be heated to a very high temperature of about 630°C, to yield oxygen gas, but it produces the same gas at a comparatively low temperature of 200-240°C on being mixed with a little amount of manganese dioxide (MnO2).
“Nature of Matter WBBSE Class 8 notes, General Science Chapter 2”
Here, manganese dioxide accelerates the decomposition of potassium chlorate, but itself remains unchanged chemically and in mass and composition. Hence, manganese dioxide acts as a positive catalyst.
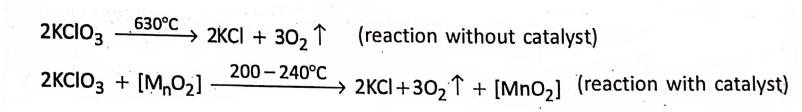
2. Hydrogen peroxide is a colourless liquid. At room temperature, it is not very stable and itself decomposes very slowly into water and oxygen gas.
2H2O → 2H2O + O2 ↑
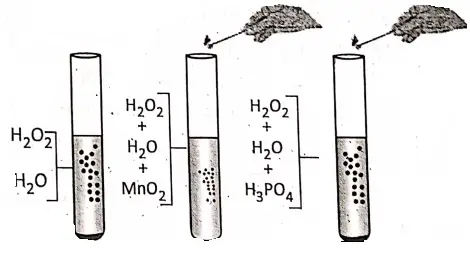
If a small quantity of manganese dioxide is added to the aqueous H202 solution, then the rate of evolution of oxygen increases rapidly. That is, Mn02 causes smooth decomposition of H202. Now, if phosphoric acid (H3P04) is added to the aqueous H202 solution, the rate of evolution of oxygen decreases. Hence, Mn02 acts a positive catalyst and H3P04 acts as a negative catalyst.
Chapter 2 Nature Of Matter Importance of Catalyst
Importance of Catalyst
Catalysts in different forms are very important for carrying out different chemical reactions like the industrial manufacture of ammonia, sulphuric acid, nitric acid, etc.
1. In the Haber process, for the manufacture of ammonia on a large scale, nitrogen and hydrogen in volume ratio 1 : 3 are treated in presence of a finely divided Fe catalyst at 500°C temperature and 200—1000 atmospheric pressure.

2. In Contact process, for the manufacture of sulphuric acid, the oxidation of sulphur dioxide to sulphur trioxide takes place in presence of a solid catalyst vanadium pentoxide V205 or platinized asbestos at 400-500c temperature.
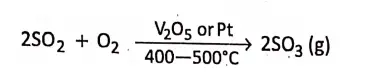
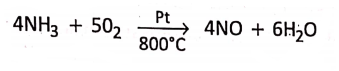
3. In Ostwald’s process, for the industrial manufacture of nitric acid, ammonia (NH3) gets oxidised by sufficient volume of air to nitric oxide (NO) while passing through platinum catalyst heated to 800°C, at about 8-8 atm pressure.
Organic catalysts or Enzymes are organic compounds. They control different biochemical reactions in the body.
Enzymes and hormones play a catalytic role in the chemical processes of life. There are some life processes such as digestion, respiration etc. Which are fulfilled by different enzymes which undergo different chemical changes.
Without Enzymes the digestion of carbohydrates, proteins, lipids of cell membranes etc. present in food cannot occur. Enzymes also specify different activities of body cells.
“WBBSE Class 8 Chapter 2 General Science, Nature of Matter solutions”
The inner wall of the stomach is lined by millions of gastric glands which secret gastric juice rich in enzymes such as pepsin and renin. Pepsin changes larger protein molecules into smaller protein molecules called peptones and proteoses. The gastric juice is also rich in hydrochloric acid and it kills bacteria and also helps in the digestion of proteins.
Chapter 2 Nature Of Matter Endothermic And Exothermic Reactions
Endothermic Changes
Physical or chemical changes which occur with the evolution of heat are called exothermic changes, while those which occur with the absorption of heat from the surroundings are called endothermic changes.
Exothermic Changes
In an exothermic change, the evolution of heat is due to the release of some other form of energy contained in the materials undergoing such a change. On the other hand, in an endothermic change, cooling occurs due to the absorption of heat from its surroundings by the materials undergoing such a change.
Let us now take some examples of these two types of changes :
Chapter 2 Nature Of Matter Exothermic physical change
When cone, sulphuric acid. (H2S04) mixes in water, or when caustic soda (NaOH) or caustic potash (KOH) dissolves in water, plenty of heat is evolved in each of the physical changes.
Chapter 2 Nature Of Matter Exothermic chemical change
1. When coal burns in the air, the evolution of heat takes place.
C + O2 → CO2 + heat
2. Slaking of lime :
When water is sprayed on small lumps of quicklime (CaO), then it changes into slaked lime [Ca(OH)2] with the evolution of a huge amount of heat. It is an example of exothermic chemical change.
CaO + H2O →Ca(OH)2 + heat
Quicklime Slakedlime
3. Combustion of methane (CH4) in air also takes place with the evolution of heat.
CH4 + 2O2→ CO2
4. Burning of magnesium wire in air or oxygen produces white powder of magnesium oxide (MgO), along with the evolution of heat.
2Mg + O2 → 2MgO + heat
Sometimes, in exothermic reactions both heat and light are produced. While burning wood, gas, candle, kerosene oil, etc. this occurs.
“WBBSE General Science Class 8 Nature of Matter, Chapter 2 explanations”
Oxy-acetylene flame (whose temperature is about 3200°C) used for welding, is produced by the burning of the mixture of oxygen and acetylene gas kept at high pressure. Here also heat and light are produced. 2C2H2+ 5O2→ 4CO2 + 2H2O + heat
“WBBSE Class 8 General Science Nature of Matter notes, easy explanation”
Chapter 2 Nature Of Matter Endothermic Physical Change
- When solid ammonium chloride (NH4CI) dissolves in water, a lowering of temperature occurs.
- A decrease of temperature also takes place when an aqueous solution of ammonium nitrate (NH4N03) is prepared.
Chapter 2 Nature Of Matter Endothermic Chemical Change
1. When the sulphur vapour is passed over red-hot charcoal, a colourless compound carbon disulphide (CS2) is formed with the absorption of heat.
C + 2S → CS2 – heat
2. When a mixture of hydrogen gas and iodine vapour is passed over hot platinum wire gauze, the hydroiodic acid (HI) is formed with a decrease of temperature.
H2 + l2 → 2Hl – heat
3. When methane and chlorine react with each other to form methyl chloride (CH3CI)and HCI, then a lowering of temperature takes place.
CH2 + Cl2→CH3Cl + HCl – heat

Reaction of solid hydrated barium hydroxide [Ba(0H)2.8H20] and solid NH4Cl is a very good example of an endothermic reaction. In this reaction, the temperature is decreased so much that the water droplets accumulated over the outside wall of the glass container soon freeze into ice. Ba(0H)2.8H20 + NH4CI→BaCl2.2H20 + 2NH3+ 8H20—heat
Chapter 2 Nature Of Matter Oxidation And Reduction
Concept of Oxidation :
Definition :
Oxidation is a chemical reaction which is associated with
- Addition or increase in the proportion of oxygen or any electronegative element or radical (Cl, F, N, OH etc.) to a substance or
- Removal or decrease in the proportion of hydrogen or any electropositive element or radical (K, Na, Ca, NH4+, etc.) from a compound.
Examples of oxidation :
Addition of oxygen :
1. Carbon, sulphur, phosphorus, sodium, etc. burn in oxygen to be oxidised respectively into carbon dioxide, sulphur dioxide, phosphorus pentoxide, sodium peroxide, etc.
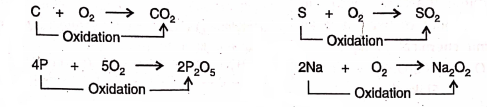
2. When copper is heated in sufficient air or oxygen, red-coloured copper is oxidised to black copper oxide. ‘ – . .
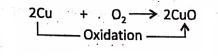
3. Burning of magnesium ribbon in oxygen :
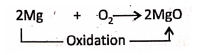
Increase in the proportion of organs:
Nitric oxide (NO) oxidised to nitrogen dioxide by the addition of oxygen.
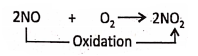
Chapter 2 Nature Of Matter Addition of Chlorine
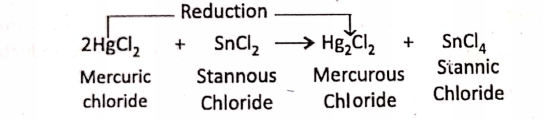
1. When stannous chloride (SnCl2) or
2. Ferrous chloride (FeCI2) is treated with an excess amount of chlorine gas, they are oxidised respectively to stannic chloride (SnCI4) or ferric chloride (FeCI3).
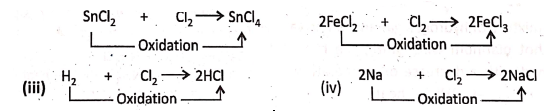
Chapter 2 Nature Of Matter Removal of Hydrogen
1. On passing hydrogen sulphide (H2S) gas into bromine water (red in colour), H2S is oxidised to sulphur (yellow in colour).
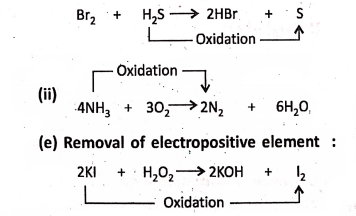
Chapter 2 Nature Of Matter Concept of Reduction
Definition :
Reduction is a chemical reaction which is associated with
- Addition or increase In the proportion of hydrogen or any electropositive element or radical (K, Na, Ca, NH4+ etc.) to a substance or
- Removal or decrease In the proportion of oxygen or any electronegative clement or radical (Cl, F, N, OH–, etc.) from a compound.
Examples of reduction :
Addition of hydrogen :
1. On passing chlorine gas through hydrogen sulphide (H2S), yellow deposits of sulphur along with the formation of hydrogen chloride (HCl) takes place. In this reaction, chlorine is reduced to hydrogen chloride.
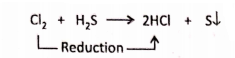
2. Nitrogen is reduced to ammonia.
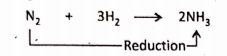
Removal of oxygen :
1. On passing H2 over red-hot black copper oxide (CuO), the later is reduced to red-coloured metallic copper.
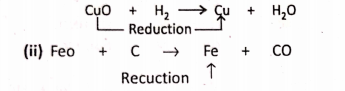
Addition Of Electropositive element :
Removal Of electronegative element:

Chapter 2 Nature Of Matter Modern Electronic Concept of Oxidation And Reduction
According to this concept, oxidation is such a chemical reaction in which an atom or ion loses electrons, while reduction is such a chemical reaction in which an atom or ion gains electrons. That is, on losing or gaining electrons, the atom or ion is oxidised or reduced respectively.
Examples of oxidation :
1. Atoms like Na, K, Ca, Al, etc. lose electrons to be oxidised into their corresponding ions.
Na – e →Na+; K – e -> K+ ; Ca – 2e → Ca2+ ; Al – 3e → Al3+
2. Cations like Fe2+ are oxidised to Fe3+ by the loss of electrons.
Fe2+-e → Fe3+ .
3. Anions like Cl–, S= etc. lose electrons to be oxidised into their corresponding atoms or molecules.
2Cl– —2e → ; S= — 2e → S
“Class 8 WBBSE Nature of Matter Chapter 2, General Science revision”
Examples of reduction :
1. Atoms like Cl, 0, S’ etc. gain electrons to be reduced into their corresponding anions.
Cl + e → Cl– ; 0 + 2e → 0= : S + 2e → S=
2. Cations like Cu2+, and Fe3+ gain electrons to be reduced to metals or lower valency ions.
Cu2+ + 2e → Cu ; Fe3+ + e → Fe
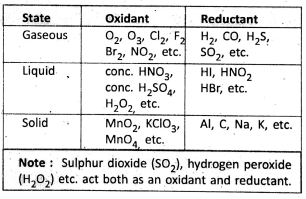
Oxidant (or oxidising agent) and Reductant (or reducing agent) :
Definition: A substance that oxidises another substance and itself gets reduced is called an oxidant.
A substance that reduces another substance and itself gets oxidised is called a reductant. On the basis of electronic theory, an oxidant is an electron acceptor while a reductant is an electron donator.
For example :
O +2e → O(0 is oxidant)
Cl + e → Cl– (Cl is oxidant)
H – e → H+ (H is reductant)
Al – 3e → Al3+ (Al is reductant)
Chapter 2 Nature Of Matter Oxidation And Reduction Take Place Simultaneously
When one substance is oxidised, another substance must be reduced. That is, oxidation and reduction go hand-in-hand. Such type of reactions are also known as Red-ox reactions.
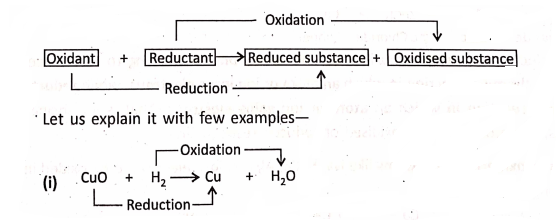
In this reaction, oxygen has been removed from CuO, so it is reduced; and oxygen has been added to H2, so it is oxidation. Hence, CuO has been reduced and H2 has been oxidised simultaneously. Here, CuO is the oxidant and H2 the reductant.
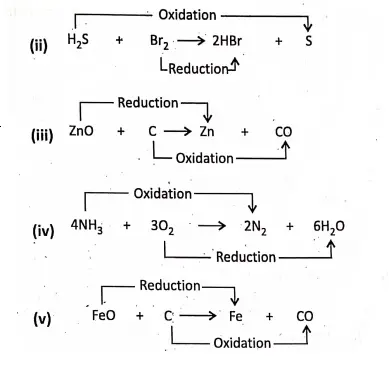
Chapter 2 Nature Of Matter Electronic Explanation of Red-ox Reactions
In such a reaction, the total number of electrons or total charge remains the same before and after the reaction.
For example :
1. If a shining naif is immersed in aqueous solution of copper sulphate, a reddish-brown coating of metallic copper deposits over the immersed portion of the nail.
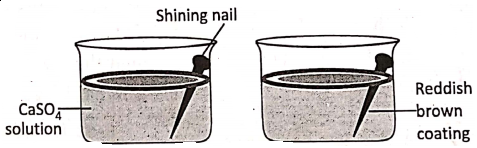
The reaction is :
Fe + CuS04 → FeS04 + Cu ↓
Before the reaction, there were cupricions (Cu2+) in the solution and Electronically,
Fe° – 2e→ Fe2+ (Oxidation)
Cu2+ + 2e → Cu (Reduction)
Hence, oxidation and reduction take place simultaneously.
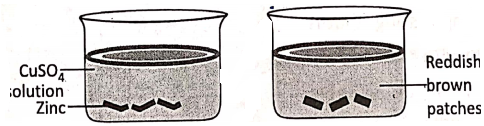
2. lf some small pieces of zinc (grey in colour) are put in an aqueous solution of copper sulphate, reddish-brown patches of copper are seen to appear over the surface of the zinc.
The reaction is :
Zn + CuS04 → ZnS04 + Cu↓. Before the reaction, there were Cu2+ ions in the solution and Zn atoms.
Electronically, Zn° – 2e→Zn2+ (Oxidation)
Cu2+ + 2e→Cu (Reduction).
3. When Cl2gas is passed into FeCl2 solution (light green in colour), the colour of the solution changes to yellow. The reaction is
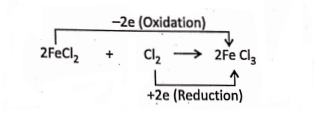
Electronically, 2Fe2+ – 2e →2Fe3+ (Oxidation)
Cl°2 + 2e → 2Cl– (Reduction)
4. H2S + Cl2→2HCl + S
Electronically, S2--2e→ S (Oxidation)
Cl°2 + 2e →2Cl– (Reduction)
5. Cu + 2AgN03 → CU(N03)2 + 2Ag
Electronically, Cu° – 2e → 4 Cu2+ (Oxidation)
2Ag+ + 2e → 2Ag° (Reduction)
Rusting of iron :
It is our common experience that when a piece of shining iron nail is kept in moist air, a reddish-brown, flaky coating is formed around it. We call the reddish-brown deposit rust and the phenomenon is known as rusting of iron. It is a chemical reaction.

Rust is hydrated ferric oxide. Its formula is Fe203. nH20, where n being the number of water molecules which is not fixed. Both water vapour and oxygen are essential for rusting.
During rusting, the iron atoms (Fe) first oxidised in presence of 02 to ferrous ions (Fe2+) and by further oxidation ferric ions (Fe3+) are produced. A small amount of heat is evolved also during rusting, that is why rusting is called a slow combustion process.
Prevention :
The rusting of iron is an undesirable reaction. Rusting once starts goes on rapidly. As a result, iron articles become weaker with time. Rusting can be prevented if iron is protected against the attack of water vapour and oxygen. This can be done in many ways, some of which are :

- By applying a coating of coal tar, oil paint, red oxide, etc. over iron.
- By galvanizing or tin-plating, nickel-plating, chromium-plating, etc. Galvanization is a process by which a coating of zinc is made over iron.
- By producing an adherent film of ferrosoferric oxide (Fe304) on the surface of iron on a dipping iron article in a cone. HN03.
Fact File :
Corrosion is a process of slow eating away of a metal due to its reaction with oxygen and moisture present in the atmosphere. Not only iron, but other metals also experience such a phenomenon. For example, copper articles get coated with a green substance (called copper carbonate) with time.

Chapter 2 Nature Of Matter Chemical Effects Of Electricity
Electrolytes and Non-electrolytes
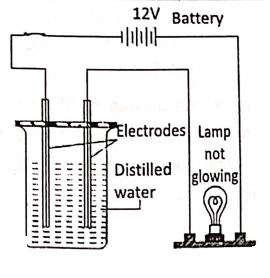
Compounds, which conduct electricity in a molten or aqueous solution state,-are called electrolytes. These compounds consist of ions in a molten or aqueous solution state e.g. sodium chloride, sodium bromide, etc. Compounds, which do not conduct electricity in a molten or aqueous solution state, are called non-electrolytes.
They consist of molecules, not the ions e.g. pure water, glycerol, molten sulphur, wax, kerosene, benzene, sugar solution, ether, etc. An electrolyte in the molten or aqueous solution dissociates into electrically charged ions [cations (+), anions (-)]. The electric current is carried by these ions.
But non-electrolytes (like cane sugar, pure water, wax, glycerol, kerosene etc.) do not conduct electricity because of the absence of such ions.
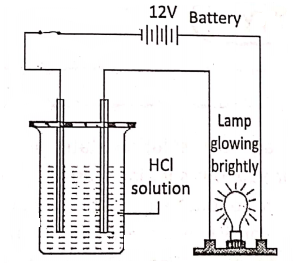
For example, acidulated
Strong electrolytes
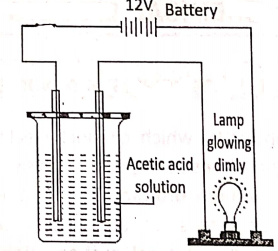
In the molten or aqueous solution, dissociate to a large extent e.g. H2S04′ NCI, HN03, NaOH, NaCl, KCI, etc: On the other hand, weak electrolytes in the molten or aqueous solution, dissociate only a slight and most of the electrolytes exist as unionized molecules.
Example : CH3COOH, HCOOH, NH4OH, etc.
Copper is a good conductor of electricity, but not an electrolyte :
Copper conducts electricity due to the presence of free electrons in it, but as it cannot be dissociated into cations or anions, it is not an electrolyte. This is the reason.
- Metals allow an electric current to pass through them by the flow of free electrons; but in electrolytes, the current is carried by the flow of ions.
- On the passage of electricity, metals undergo no chemical change; but electrolytes decomposed to form new products.
Chapter 2 Nature Of Matter Electrolysis
The chemical decomposition of a compound in a molten or aqueous solution state (i.e. electrolytes) into ions by the passage of electric current through it is called electrolysis. The called a Voltameter. Note that, a voltameter is used to carry out electrolysis, while a voltmeter is used to measure the potential difference between two points in an electric circuit.
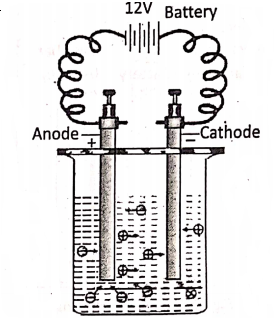
The electrode connected to the positive terminal of a battery is the anode and the electrode connected to the negative terminal of the battery is the cathode. During electrolysis, the cations (positive ions) move towards the cathode and are discharged at the cathode by gaining electrons.
“WBBSE Class 8 Science Chapter 2 Nature of Matter, key concepts”
Hence reduction occurs at the cathode. On the other hand, the anions (negative ions) move towards the anode and are discharged at the anode by losing electrons. Hence, oxidation occurs at the anode. From this point of view, electrolysis may be considered as a redox reaction.
Let us now consider two examples of electrolysis :
1. Dissociation of acidulated water using platinum electrode :
![]()
Reaction at the cathode : H+ + le– → H ; H + H →H2↑
Reaction at the anode : OH– – le– → OH ; 40H → 2H20 + 02↑
2. The electrolytic dissociation of molten sodium chloride can be represented as :
![]()
Na+ ions move towards the cathode and Cl– ions move towards the anode.
Reaction at the cathode : Na+ + le– → Na (reduction)
Reaction at the anode : CP– – le– → Cl (oxidation) ; Cl + Cl → Cl2
Chapter 2 Nature Of Matter Uses Of Electrolysis
Electrolysis is used in many ways in industry, such that,
- Purification of metals
- Extraction of metals from their ores and
- Electroplating.
Metals like copper and silver can be refined by electrolysis. In the electrorefining of Cu, a slab of impure Cu is made the anode and a thin plate of pure Cu is made the cathode and CuS04 solution is used as electrolyte. Highly electropositive metals like Na, Mg, Ca, Al are extracted from their ores by electrolysis.
What is electroplating?
Electroplating is an electrolytic process in which a thin layer of a superior metal is deposited on another metallic article. The article to be electroplated is made the cathode and a bar of the pure metal by which electroplating to.be made is taken as the anode and a solution of $ salt of the metal is used as electrolyte.
For example,
1. In silver-plating, the article to be electroplated is cleaned and made the cathode, a pure silver bar is made the anode and silver nitrate (AgN03) or potassium argento cyanide K[Ag(CN)2] is used as electrolyte.
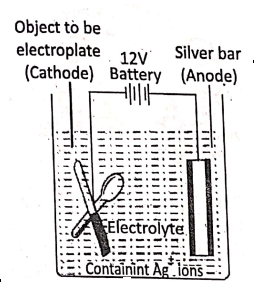
2. Electroplating of an article with nickel:
Cathode: The article to be electroplated. Anode: Pure block of nickel metal. Electrolyte: Nickel sulphate (NiSO4)
“WBBSE Class 8 Nature of Matter notes, Chapter 2 question answers”
3. For gold plating over a base metal :
Cathode: The article to be electroplated. Anode: Pure block of gold metal. Electrolyte: Potassium auro cyanide K[Au (CN)2].
4. Electro-refining of copper:
Cathode: Thin plate of pure copper. Anode: Slab of impure copper. Electrolyte: Acidified copper sulphate solution (CuS04).
Main purposes of electroplating :
- Electroplating helps to protect iron articles against rusting.
- It is also done for making artificial jewellery attractive.
