WBBSE Chapter 3 Know About Some Common Gases
The study of chemistry involves many experiments carried out in the chemistry laboratory with the help of a number of laboratory apparatuses. Here we will describe about some simple chemical apparatus used in the chemistry laboratory which include :

Thermometer, electric cell, switch, wire, bulb, LED, chemical balance, clamp and stand, bunsen burner, spirit lamp, test tube, beaker, round bottom flask, conical flask, Woulfe bottle, gas jar; watch glass, measuring cylinder, tripod stand, wire gauze, glass rod, pipette and burette, funnel, thistle funnel, delivery tube, trough, filter paper, test tube holder, test tube rack.
Read and Learn more WBBSE Notes For Class 8 General Science And Environment
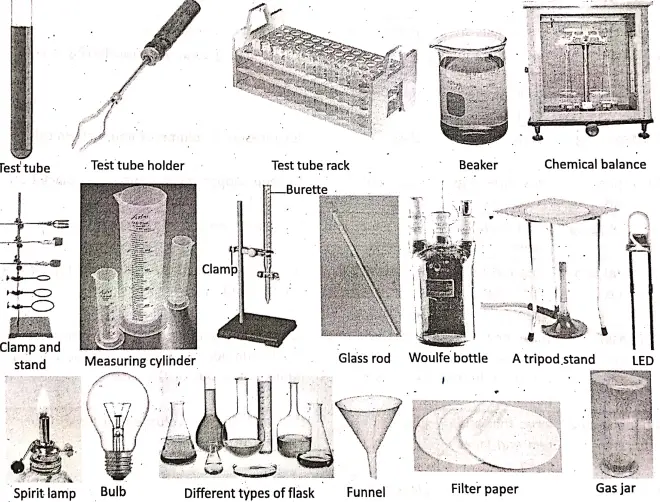
Different chemical apparatus and their uses :
Chapter 3 KnowAbout Some Common Gases Oxygen
Occurrence :
Oxygen is present in nature as a free element and in different compounds. Air contains about 20-6% by volume and 23% by mass, water contains 88-8% of oxygen by mass. It is the most abundant of all the elements present in the earth’s crust as oxides, nitrates, carbonates, sulphate compounds, etc.
“WBBSE Class 8 General Science Chapter 3 notes, Know About Some Common Gases”
| Class 8 General Science | Class 8 Maths |
| Class 8 History | Class 8 Science LAQs |
| Class 8 Geography | Class 8 Science SAQs |
| Class 8 Maths | Class 8 Geography |
| Class 8 History MCQs | Class 8 History |
Types Of Gas
Besides these, the plant and animal tissues contain 60-70% of oxygen by mass. During 1778, Swedish scientist Scheele, English chemist Joseph Priestly, and French scientist Lavoisier were able to prepare oxygen gas. Priestly named the gas ‘active air’ as it is very much reactive. Lavoisier gave the name ‘oxygen’, meaning ‘acid producer’.
Chapter 3 KnowAbout Some Common Gases Uses of oxygen :
Oxygen is used
- For artificial respiration to patients suffering from respiratory problems.
- For the production of oxy-hydrogen (2000°C) and oxy-acetylene (3200°C) flames are used for cutting and welding of metals.
- For the industrial manufacture of sulphuric acid, nitric acid, etc.
- As an oxidising agent in the laboratory.
- As a liquid fuel for rockets and missiles.
Physical properties of oxygen :
- Oxygen is a colourless, odourless, tasteless gas under normal conditions.
- It is slightly soluble in water (about 2ml in 100 ml of water) which is just sufficient to support the life of aquatic plants and animals.
- It is slightly heavier than air.
- The gas liquefies at -183°C, and on further cooling to -219°C, it solidifies.
Chemical properties of oxygen :
1. Oxygen is not combustible, but a great supporter of combustion. On introducing a glowing splint into a gas jar containing oxygen, the glowing splint rekindles inside the gas jar, but the gas does not burn itself.
2. Reaction with hydrogen :
Hydrogen burns in oxygen with a blue flame producing water vapour.
2H2 + 02 →2H20
3. Respiration :
In the living cells, food is oxidised in the presence of oxygen to produce energy. The process is known as respiration.
C6H12O6 + 602 6C02 + 6H2 + heat
- Oxygen is neutral—neither acidic nor basic.
- Oxide is a binary compound of two elements, one of which is oxygen,
- In general, non-metallic oxides are acidic and metallic oxides are basic.

4. Reactivity:
Oxygen gas reacts with most metals and non-metals to form their oxides.
“Class 8 General Science WBBSE notes, Common Gases chapter”
Reaction with non-metals :
1. Carbon (red hot charcoal) burns over oxygen to form carbon dioxide, which in reaction with water produces carbonic acid (H2CO3).
C + O2→ CO2
CO2 H2O→ H2CO3 (turns moist blue litmus red)

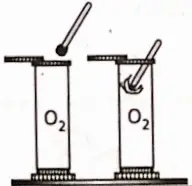
2. Sulphur burns in oxygen with a blue flame to form sulphur dioxide, which produces sulphurous acid (H2SO3) with water. It turns moist blue litmus red.
S + O2→ SO2 ; SO2 + H2O→ H2SO3
3. Phosphorus burns in oxygen forming dense white fumes of phosphorus pentoxide (P2O5), which on dissolution with water produces phosphoric acid (H3PO4).
4P + 5O2→ 2P2O5 ; P2O25+ BH2O →2H3PO4
Chapter 3 KnowAbout Some Common Gases Reaction With Metals:
Common Gases Reaction With Metals
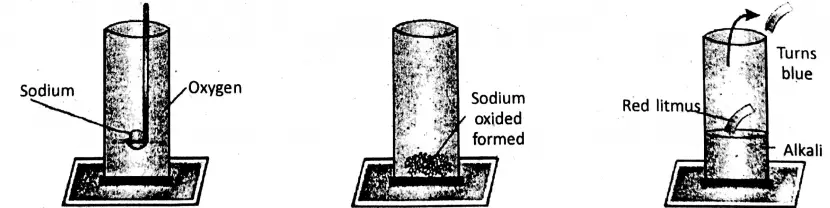
1. On heating a piece of sodium in a jar of oxygen, it bums with a golden yellow flame producing sodium oxide (Na20), which produces sodium hydroxide or caustic soda (NaOH) with water. The solution is alkaline and it turns moist red litmus blue.
4 Na + O2→ 2Na2O
Na2O + H2O → 2NaOH (alkali)
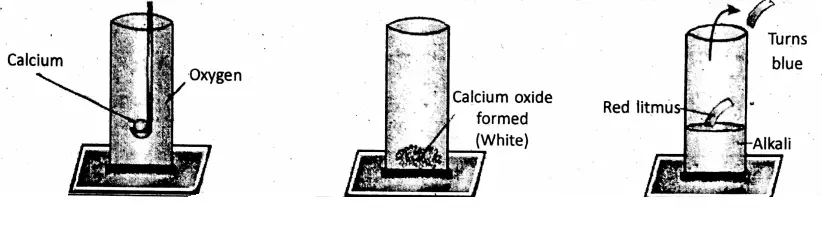
2. Burning calcium continues burning in a jar of 02 producing a white solid calcium oxide (CaO), which being treated with water produces calcium hydroxide [Ca(OH)2]. It is an alkali.
2Ca + O2→ 2CaO ; CaO + H2O→Ca(OH)2
3. Burning magnesium burns in 02 with a dazzling white flame and magnesium oxide (white powder) is produced, which on dissolution with water produces magnesium hydroxide [Mg (OH)2]; an alkali.
2Mg + O2 → 2MgO ; MgO + H2O→ Mg(OH)2
4. Zinc and aluminium react with oxygen to produce zinc oxide (ZnO) and aluminium oxide (Al203) respectively. These amphoteric oxides react with acids and bases separately to produce salt and water.

Absorber of Oxygen :
- Alkaline potassium pyrogallate solution absorbs oxygen and turns brown.
- Ammonical cuprous chloride solution absorbs oxygen and turns blue.
Preparation of oxygen gas :
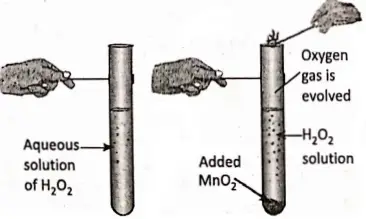
1. From a dilute solution of hydrogen peroxide using MnO2 as a catalyst :
An aqueous solution of hydrogen peroxide is taken in a test tube. A small amount of Mn02 is added to the solution. A reaction takes place with the evolution of a gas. The gas is oxygen as it rekindles a glowing splinter.
“WBBSE Class 8 Environment Chapter 3, Know About Some Common Gases study guide”
Reaction :
2H2O2 + [MnO2] 2H2O + O2↑ + [MnO2]
In the reaction, Mn02 enhances or accelerates (i.e. catalyses) the decomposition of H202 without undergoing any change in itself and thus/Mn02 acts as a positive catalyst. The reaction takes place at ordinary room temperature.
2. From solid sodium peroxide (Na2O2) :
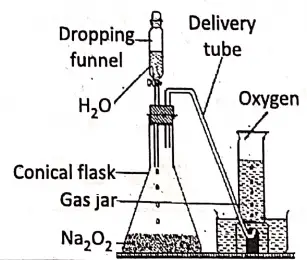
Chemicals required :
Sodium peroxide (Na2O2) and water.
Condition :
In the laboratory, oxygen is prepared by the action of water on Na202 at the ordinary room, temperature.
Reaction :
2Na2O2 + 2H2O → 2NaOH + O2↑
Procedure :
Solid sodium peroxide is taken in a conical flask filled with a dropping funnel and a bent delivery tube. The other end of the delivery tube is inserted into a vertical gas jar filled with water into a pneumatic trough.
“WBBSE Class 8 Know About Some Common Gases notes, General Science Chapter 3”
From the dropping funnel, water is slowly dropped over solid sodium peroxide. A vigorous reaction takes place with the evolution of oxygen gas which is collected in the gas jar via a delivery tube by downward displacement of water.
3. Laboratory preparation from potassium chlorate (KCIO3) :
Chemicals required :
A mixture of potassium chlorate (KClO3) and manganese dioxide (Mn02) in the ratio of 1: 4 by mass.
Condition :
On heating the mixture at a temperature of about 200-230°C, oxygen gas is prepared. In this reaction, Mn02 accelerates the decomposition of KClO3 rapidly and easily, but itself remains unchanged in mass and composition. Thus, MnO2 acts as a positive catalyst.
Reaction :
2KClO3 + [MnO2] = 2KCl + 3O2↑ + [MnO2] .
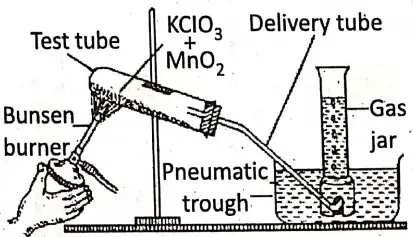
Procedure :
- A hard glass test tube is almost half filled with the said mixture and a bent delivery tube is filled at the mouth of the test tube through a rubber cork.
- The test tube is clamped on a stand at a slightly slanting position. The other end of the delivery tube is inserted into a pneumatic trough field with water.
- The test tube is heated gently by a bunsen burner. Then oxygen gas emerges through the delivery tube.
- Now an inverted gas jar filled with water is held over the delivery tube. O2 gas is collected In the gas jar by downward displacement of water. When the gas is filled completely, the mouth of the gas jar is covered with a glass lid and kept erect.
Precautions :
- KClO3 and MnO2 should be mixed well.
- Mn02 should be pure from charcoal dust, to avoid explosion.
- The test tube should be kept slightly slanting so that heating is done gently from front to backside.
- At the end of the experiment, the end of the delivery tube is removed from the trough before removing the bunsen burner otherwise water rushes into the tube causing a crack.
Chapter 3 KnowAbout Some Common Gases Hydrogen
Occurrence :
Hydrogen gas is rarely found in free elemental state in the air (only 1 part per million). It is found in minute traces in volcanic gases and the gases evolved from petroleum mines.
The main sources of hydrogen are water, acids, alkalis, cotton, wood, oil, fat, petroleum products and other organic substances. In plant and animal tissues, it occurs as carbohydrates, proteins and vitamins.
“WBBSE Class 8 Environment Chapter 3, Know About Some Common Gases study guide”
Hydrogen gas is the lightest of all the elements in the universe. In 1756, English scientist cavendish first prepared the gas. Lavoisier named it hydrogen (in Greek, hydro = water, gen = producer) as it produces water when burnt in oxygen.
Chapter 3 KnowAbout Some Common Gases Uses of Hydrogen:
Hydrogen gas is used
- As a reducing agent in the laboratory.
- In producing oxy-hydrogen flame (2000°C) used for cutting and welding of metals.
- For preparing hydrochloric acid, alcohols, etc.
- For the manufacture of fertilizers like ammonia, urea, etc.
- In converting vegetable oils into vanaspati ghee by hydrogenation.
- Liquid hydrogen as a liquid fuel.
- In hydrogenating coal for preparing fuels like petrol.
Physical properties of hydrogen :
- Hydrogen is a colourless, odourless and tasteless gas under normal conditions.
- It is the lightest of all the elements. Air is about 14-4 times as heavy as hydrogen. Balloons filled with hydrogen float in the air.
- The gas is almost insoluble in water. ‘
- Hydrogen is the best conductor of heat among the gases.
Chapter 3 KnowAbout Some Common Gases Chemical properties of hydrogen :
1. Combustibility :
Hydrogen gas is combustible, but not a supporter of combustion. When a lighted splinter is inserted into a gas jar filled with hydrogen, it is put off but the gas burns with a blue flame.
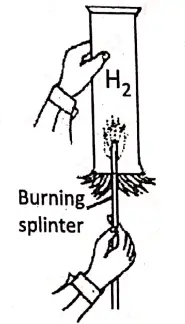
2. Reaction with non-metals :
Hydrogen forms non-metallic hydrides with non-metals.
Reaction with chlorine :
Equal volumes of hydrogen and chlorine react in diffused sunlight to form hydrogen chloride gas.
“WBBSE Class 8 Know About Some Common Gases notes, General Science Chapter 3”
The reaction is negligible in the dark and explosive in direct sunlight.
Reaction with sulphur:
Hydrogen gas reacts with boiling sulphur to form hydrogen sulphide gas.
H2 + S→H2 S
Reaction with nitrogen :
Hydrogen reacts with nitrogen at 500°C and 200-1000 atmospheric pressure in presence of finely divided iron (as a catalyst) to form gaseous ammonia.
 3. Reaction with metals :
3. Reaction with metals :
Strong electro-positive metals like Na, Ca, etc. react with hydrogen when they are heated and form metallic hydrides. The metallic hydrides react with water to produce hydrogen. For example,
4. Reducing Property :
Hydrogen is a very good reducing agent. If hydrogen is passed over heated black cupric oxide (Cuo), the latter is reduced into red metallic copper (Cu) and hydrogen is oxidised into water (H2 0).

5. Occlusion :
At ordinary temperatures, large quantities of hydrogen are adsorbed by certain finely divided metals like palladium, platinum, nickel, iron, and cobalt on their surfaces. Such adsorption is called occlusion. On heating, the adsorbed gas is set free.
Adsorption is the process of accumulation of gas on the surface of a metal.
Palladium can adsorb 900 times of its own volume at ordinary temperature.
Preparation of hydrogen gas :
Laboratory preparation
chemicals required :
Impure commercial granulated zinc (Zn) and dil. sulphuric acid (H2 S04 ).
Principle :
In the laboratory, hydrogen is prepared by the reaction of zinc turnings with dil. H2 S04 at room temperature.
Reaction :
Zn + H2 S04→ ZnS04 + H2 ↑
Procedure :
- Some granulated zinc is taken in a Woulfe bottle with air-tight corks containing water. A thistle funnel and a delivery tube are fitted through two corks of the woulf-bottle so that
- the lower end of the thistle funnel dips under water and the end of the delivery tube is kept under water within a bee-hive self in a pneumatic trough.
“WBBSE Class 8 Science Chapter 3 notes, Know About Some Common Gases PDF”
- The total set-up is made perfectly air-tight.
- Dil. H2 S04 is added through the thistle funnel. As dil. H2 SO4 comes in contact with Zn, the reaction takes place and hydrogen evolves and comes out through the delivery tube.
- After some time, a gas jar filled with water is inverted over the end of the delivery tube. Then, the gas is collected in the gas jar by the downward displacement of water.
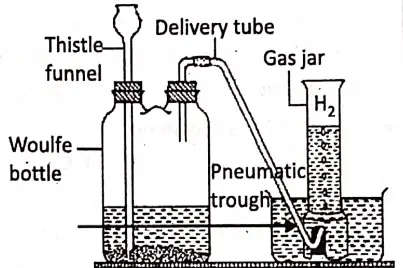
Precautions :
- The apparatus must be made air-tight. As H2 is a combustible gas, so no open flame should be brought near the apparatus.
- The lower end of the thistle funnel must be kept under water in the Woulfe bottle, otherwise hydrogen gas may leak through it.
- The zinc granules must be under the dilute acid.
- Hydrogen gas is collected after all the air in the Woulfe bottle escapes out.
Pure zinc reacts very slowly with dilute acids, and a thin film of H2 gas is formed on its outer surface. So, contact of Zn with acid is lost and the reaction I stop. On the other hand, in impure zinc, no such layer is formed.
“Class 8 WBBSE General Science Chapter 3, Common Gases summary”
The wholfe bottle and all the joints must be completely airtight since the mixture of hydrogen and air is explosive. Hydrogen gas is not collected by the downward displacement of air since the air-hydrogen mixture is highly explosive.
Conc. H2S04 is not used in the preparation of H2 gas from Zn granules. This is so because cone. H2S04 is a strong oxidising acid. It oxidises zinc into zinc sulphate (ZnSO4) and itself reduces to form sulphur dioxide gas (S02). Thus, the reaction does not produce hydrogen gas.
