WBBSE Class 10 Physical Science Question Answer In English
Chapter 3 Chemical Calculations MCQs
Question 1. Which of the following contains the least number of molecules?
- 1.12 LSO2 at STP
- 1 gm-mole SO2
- 32g SO2
- 4 x 1023 molecules of SO2
Answer: 1. 1.12 L SO2 at STP.
Question 2. Who put forward the law of conservation of mass?
- Cannizzaro
- Lavoisier
- Dolton
- Ge Lusaka
Answer: 2. Lavoisier.
Read And Learn More: WBBSE Solutions For Class 10 Physical Science And Environment
Question 3. No. of atoms present in 3.7g. mole of nitrogen is :
- 4.45 x 1024
- 6.023 x 1024
- 1.204 x 1024
- 3.023 × 1023
Answer: 1. 4.45 × 1024.
“WBBSE Class 10 Physical Science and Environment Chapter 3 solutions, Chemical Calculations”
Question 4. The balanced equation for the chemical equation :
Ag2 SO4 + BaCl2 → BaSO4+ AgCl is:
- Ag2 SO4 + BaCl2 = BaSO4 + AgCl
- Ag2 SO2+ BaCl2 = BaSO4 + 2AgCl
- Ag2 SO4 + 2BaCl2= 2BaSO4 + AgCl
Answer: 2. Ag2 SO2+ BaCl2 = BaSO4 + 2AgCl
Question 5. The balanced equation for the chemical equation :
FeCl3 + SnCl2 → FeCl2 + Sn Cl4 is :
- Fecl3 + 2 SnCl2 = FeCl2 + 2SnCl4
- 2FeCl4 + SnCl2 = 2FeCl2+ SnCl4
- 3FeCl3 + SnCl2 = 3 FeCl2 + SnCl4
Answer: 2. 2FeCl4 + SnCl2 = 2FeCl2+ SnCl4

Question 6. NaOH + HCl = NaCl + H2O; the type of reaction is :
- Substitution reaction
- Addition reaction
- Neutralization reaction
Answer: 3. Neutralization.
Question 7. Ammonium cyanate changes to urea on heating is an example of :
- Decomposition reaction
- Direct combination reaction
- Rearrangement reaction
Answer: 3. Rearrangement reaction.
Question 8. The chemical equation provides :
- Some information
- All information
- No information
Answer: 1. Some information.
Question 9. The balanced equation for the chemical equation
MnO2 + HCl → MnCl2 + Cl2 + H2O is:
- MnO2 + 2HCl = 2MnCl2 + Cl2 + H2O
- 2MnO2 + HCI = 2MnCl2 + Cl2 +H2O
- MnO2+3HCI = MnCl2+ 3Cl2 + 2H2O
- MnO2 + 4HCI = MnCl2+ Cl2+2H2O
Answer: 4. MnO2 + 4HCI = MnCl2+ Cl2+2H2O
Question 10. N2 + O2 = 2NO – Q Cal; this reaction is :
- Endothermic
- Exothermic
- Rearrangement
- Decomposition
Answer: 1. Endothermic.
Question 11. Which of the following one takes part in a chemical reaction?
- Electron
- Proton
- Iron
- Positron
Answer: 1. Electron.
Question 12. NH3 + HCI = NH4Cl; it is a type of chemical reaction :
- Substitution
- Direct combination
- Addition reaction
- Rearrangement
Answer: 2. Direct combination.
Question 13. 27g of Al will react completely with how many grams of oxygen?
- 8g.
- 16g.
- 32g.
- 24g.
Answer: 4. 24g.
Question 14. The reaction in which two or more reactants combine directly forming molecules of new substances is called :
- Direct combination
- Neutralization reaction
- Addition reaction
- Decomposition
Answer: 1. Direct combination
Question 15. Calcium pyrophosphate is represented by the formula Ca2P2O7. The molecular formula of ferric pyrophosphate is :
- Fe2 (P2O7)4
- Fe2 (P2O7)
- Fe (P2O7)3
- Fe 4 (P2O7)3
Answer: 4. Fe 4 (P2O7)3
“Class 10 WBBSE Physical Science Chapter 3 solutions, Chemical Calculations study material”
Question 16. The chemical formula of a particular compound represents :
- The size of its molecule
- The shape of its molecule
- The total number of atoms in a molecule
- The number of different types of atoms in a molecule
Answer: 4. The number of different types of atoms in a molecule.
Question 17. The formula which represents the simple ratio of atoms in a compound is called :
- Empirical formula
- Molecular formula
- Rational formula
- Structural formula
Answer: 1. Empirical formula.
Question 18. If 0.5 mole of BaCl2 is mixed with 0-2 mol of Na3PO4, the maximum number of mol of Ba3 (PO4)2 that can be formed of:
- 0.7
- 0.5
- 0.30
- 0.10
Answer: 4. 0.10.
Question 19. 2.76 g. of silver carbonate on being strongly heated yields residue weighting:
- 2.4 8g
- 2.15g
- 2.32g
- 2.64g
Answer: 3. 2.32g.
Question 20. The reaction in which molecule or molecules of a compound break down into comparatively simpler molecules in the presence of heat or electricity is called :
- Decomposition
- Direct combination
- Double composition
- Neutralization reaction
Answer: 1. Decomposition.
Question 21. The balanced equation for the chemical equation :
2KCI03 → KCI +O2
- 2KCIO3 = 2KCI + 3O2
- KCIO2 = KCI + 2O2
- 4KCIO3 = 2KCI + 2O2
Answer: 1. 2KCIO2 = 2KCI + 3O2
Question 22. Fe+ S = FeS; it is a type of chemical reaction :
- Decomposition reaction
- Displacement reaction
- Combination reaction
- Dis placement reaction
Answer: 3. Combination reaction.
“WBBSE Class 10 Physical Science Chapter 3, Chemical Calculations solved examples”
Question 23. 1 amu = _________ g.
- 1.6605 10-24 g
- 1.660059 × 10-23 g
- 1.66 x 10-11 g
- 1.65 x 10-21 g
Answer: 1. 1.6605 10-24 g
Question 24. 2SO2 +O2…. 2SO3 ; What type of reaction is it?
- Reversible type of reaction
- Oxidation-reduction type of reaction
- Redox type of reaction
Answer: 1. Reversible type of reaction.
Question 25. A + B2 = A2 + B is an example of what type of reaction?
- Oxidation-reduction type of reaction
- Reversible type of reaction
- Redox type of reaction
Answer: 1. Oxidation – reduction type of reaction.
Question 26. CuO+H2 = Cu + H2O is an example of what type of reaction?
- Redox type of reaction
- Oxidation-reduction type of reaction
- Reversible type of reaction
Answer: 1. Redox type of reaction.
Question 27. Ag NO3 + NaCl = AgCl + NaN03 is an example of what type of reaction?
- Double decomposition type of reaction
- Direct combination type of reaction
- Substitution type of reaction
Answer: 1. Double decomposition type of reaction.
Question 28. What is the percentage of water in blue vitriol?
- 36.07 %
- 36.77%
- 49.07 %
- 55.77%
Answer: 1. 36.07%.
“WBBSE Class 10 Chemical Calculations solutions, Physical Science Chapter 3”
Question 29. 49g H2SO4 = ________ mole.
- 0.55 mol
- 0.5 mol
- 0.15 mol
- 0.1 mol
Answer: 2. 0.5 mol.
Question 30. The weight of oxygen obtained on heating 24.5g KCIO3 is :
- 19.6 g
- 29.6g
- 9.6 g
- 10.6g
Answer: 3. 9.6g.
Question 31. How much hydrogen is produced when steam is passed over 28kg of red hot iron?
- 1.33 kg
- 2.33 kg
- 3.33 kg
- 0.33 kg
Answer: 1. 1.33 kg.
Question 32. How much CaO is obtained from 10 kg? CaCO3 ?
- 5.6 kg.
- 6.6 kg
- 7.6 kg
- 3.6 kg
Answer: 1. 5.6 kg.
Question 33. NH4CNO (R) CO (NH2)2; What type of chemical reaction is it?
- Rearrangement reaction
- Substitution reaction
- Addition reaction
Answer: 1. Rearrangement reaction.
Question 34. In a balanced chemical equation, what sign needs to be placed instead of the arrow sign?
- Greater than sign
- Smaller than sign
- Equal to sign
Answer: 3. Equal to sign.
Question 35. Calcium carbonate contains what percentage of calcium?
- 40%
- 60%
- 50%
- 75%
Answer: 1. 40%.
Question 36. Zn + NaOH → H2 + ________.
- Na2 ZnO4
- Na2 ZnO2
- Na2 ZnO2
- NaZn 03
Answer: 2. Na2 ZnO2
Question 37. CO + Cl2 = COCl2What type of reaction is it?
- Rearrangement
- Substitution
- Addition
Answer: 3. addition.
Question 38. 2Mg + O2 = 2MgO; What type of chemical reaction is it?
- Neutralization reaction
- Substitution reaction
- Direct combination type of chemical reaction
Answer: 3. Direct combination type of chemical reaction.
Question 39. Molecular formula = _______ × empirical formula.
- n
- m
- t
- y
Answer: 1. n
Question 40. Atomic mass = Equivalent weight x _________.
- Volume
- Mass
- Valency
- Velocity
Answer: 3. Valency.
Chapter 3 Chemical Calculations Very Short Answer Type Questions
Question 1. State a limitation of a chemical equation.
Answer: A reaction whether exo-thermic or endo-thermic is not known from a chemical equation.
Question 2. Give an example of an addition reaction.
Answer: Carbon monoxide reacts with chlorine to form carbonyl chloride (COCL2) is an example of an addition reaction.
Question 3. What is the molecular weight of ammonium phosphate?
Answer: 149.
Question 4. How much CaCO3 will react with dil HCI to produce 22g CO2?
Answer: 50g.
Question 5. How much potassium chlorate is to be heated to produce as much oxygen as required to burn 6g, of carbon completely?
Answer: 40.83g
WB Class 10 Physical Science Question Answer
Question 6. Give an example of a thermal decomposition type of reaction.
Answer: Calcium carbonate on heating decomposes into calcium oxide and carbon dioxide is an example of a thermal decomposition type of reaction.
Question 7. What is the mole number of a substance?
Answer: \(\text { Mole number of a substance }=\frac{\text { Wt. of the substance in gram }}{\text { Gram-molecular weight of the substance }}\)
Question 8. How many grams of limestone are needed to get 48g? CO2?
Answer: 109.09g.
Question 9. How much quantity of silver chloride (AgCI) can be obtained from 1.0g silver nitrate?
Answer: 0.0844g.
Question 10. Balance the following reaction: Fe + H2O (R) Fe3O4 + H2
Answer: Balanced equation of \(\)
Question 11. Give an example of a substitution reaction.
Answer: Chlorine reacts with methane (CH4 ) to form successively CH3CI, CH2Cl2, CHCI3 and ultimately CCI4 in the presence of sunlight is an example of a substitution reaction.
\(\mathrm{CH}_4+\mathrm{Cl}_2 \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{CH}_3 \mathrm{Cl}+\mathrm{HCl}\) ⇒ \(\mathrm{CH}_3 \mathrm{Cl}+\mathrm{Cl}_2 \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{HCl}\)
\(\mathrm{CH}_2 \mathrm{Cl}_2+\mathrm{Cl}_2 \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{CHCl}_3+\mathrm{HCl}\) ⇒ \(\mathrm{CH}_2 \mathrm{Cl}_3+\mathrm{Cl}_2 \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{CCl}_4+\mathrm{HCl}\)
Question 12. Give an example of a polymerization type of chemical reaction.
Answer: \(\mathrm{n}\left(\mathrm{CH}_2=\mathrm{CH}_2\right) \rightarrow\left(-\mathrm{CH}_2-\mathrm{CH}_2-\right) \mathrm{n}\)
Question 13. Give an example of is isomerization type of chemical reaction.
Answer: \(\mathrm{NH}_4 \mathrm{CNO} \rightarrow \mathrm{NH}_2 \mathrm{CONH}_2 \text { (Urea) }\)
Question 14. Write the relation between molecular weight and vapor density.
Answer: Molecular = 2 x vapor density.
“Class 10 WBBSE Physical Science Chapter 3, Chemical Calculations easy explanation”
Question 15. What is the atomic mass unit?
Answer: It is the quantity of mass equal to [latexl\frac{1}{12}[/latex]th of the mass of a carbon atom (12C)
l amu 1.6605 × 10-24 g.
Question 16. What is solubility?
Answer: The amount of solute in grams that can be dissolved in 100 grams of a solvent to form a saturated solution at a definite temperature is called solubility.
Question 17. What is a gram’s atomic mass?
Answer: It is the atomic mass of an element expressed in grams.
Question 18. What is normality (N)?
Answer: It is the number of gram equivalents of solute dissolved per liter of a solution.
Question 19. Give an example of the catalytic type of chemical reaction.
Answer: \(\mathrm{N}_2+3 \mathrm{H}_2 \mathrm{Fe} \rightleftharpoons \mathrm{MO} 2 \mathrm{NH}_3\)
Question 20. What is atomic mass?
Answer: It is the average relative mass of an atom of an element as compared to the mass of a carbon atom (12C) taken as 12.
WB Class 10 Physical Science Question Answer Chapter 3 Chemical Calculations Fill In The Blanks
Question 1. KCIO3 → KCI + _________.
Answer: O2.
Question 2. The law of conservation of mass is shown to be ________ equation.
Answer: Valid.
Question 3. Fe + CuSO4= Cu + FeSO4; it is a _________reaction.
Answer: substitution.
Question 4. CuO + H2 = Cu + H2O; it is an ________ reaction.
Answer: oxidation-reduction.
Question 5. There are many
Answer: limitations.
Question 6. Zn + NaOH → H2 + __________.
Answer: Na2 ZnO2.
Question 7. mg + CO2→ _______ + C
Answer: MgO.
Question 8. 49g H2SO4 = ________ mole.
Answer: 0.5 mol.
Question 9. Calcium carbonate contains _______ % calcium.
Answer: 40.
“WBBSE Class 10 Physical Science Chapter 3 solutions, Chemical Calculations PDF”“
Question 10. Molecular formula = n x ________.
Answer: Empirical formula.
Question 11. A symbolic chemical reaction may be called a chemical equation only when it is balanced by putting the least numerals as formulae in it.
Answer: Coefficients.
Question 12. CO + Cl2 = COCl2 : This is a ________
Answer: Reaction.
Question 13. Chemical reaction means a permanent ________ between the atoms or radicals of the combining substances.
Answer: Rearrangement.
Question 14. In a balanced chemical equation _______ sign is placed instead of the arrow sign.
Answer: Equal.
Question 15. MnO2 + HCl → MnCl2 + _______ + H2O.
Answer: Cl2
Question 16. From a chemical equation, the time required for the completion of the reaction is not ________.
Answer: Known.
Question 17. From a chemical equation, the time required for the _______ of the reaction is not known.
Answer: Completion.
Question 18. If the reactants and products are gaseous then at the same temperature and pressure the ratio in ______ is known.
Answer: Volumes.
Question 19. 12g carbon combines with 32g oxygen producing ________ liter of carbon dioxide at NTP.
Answer: 22.4
Question 20. The empirical formula is the formula of a compound that gives the ______ whole number ratio of the atoms of various elements present in one molecule of the compound.
Answer: Simple.
Question 21. The molecular formula is the formula of a compound that gives the _______ number of the atoms of various elements present in one molecule of the compound.
Answer: Actual.
Question 22. The percentage of an element in a chemical compound is the number of parts by weight of its present _______ in parts by weight of the compound.
Answer: 100.
Question 23. The weight of oxygen obtained on heating 24.5g KCIO3 is
Answer: 9.6 g.
WBBSE Solutions Guide Class 10
Question 24. Decomposition or analysis is a process where a compound ________ into a simpler substance.
Answer: Splits.
Question 25. The empirical formula of a compound is HO and its molecular weight is 34. Therefore its molecular formula is __________.
Answer: H2O2.
Question 26. A chemical equation is one where the formulae of the ________ of a chemical reaction are connected with plus (+) signs in one set and those of the products are similarly connected in another set.
Answer: Reactants.
Question 27. In the double decomposition process, two different compounds, react chemically to produce two new compounds by mutual ________ radicals.
Answer: Interchange.
Question 28. NaOH + HCl = NaCl + H2O2; the type of reaction is
Answer: Neutralisation reaction.
Question 29. 27g of Al will react completely with _________ g of oxygen?
Answer: 24g.
Question 30. _______ takes part in a chemical reaction.
Answer: Electron.
Question 31. N2 + O2 2NO – Q cal; this reaction is ________.
Answer: Endothermic.
Question 32. Mag + H2SO4 = _______ + H2.
Answer: MgSO4.
WBBSE Solutions Guide Class 10 Chapter 3 Chemical Calculations Short Answer Type Questions
Question 1. What are the limitations of chemical formulas?
Answer:
Limitations of chemical formula
- If fails to convey whether the elements in a molecule are present in the form of atoms or ions.
- It does not tell anything about the binding force that holds atoms in a molecule together.
- It does not tell us about the arrangement of various atoms with respect to one another within the molecule.
Question 2. What is the limiting reactant?
Answer:
Limiting reactant: The reactant that is completely used and determines the amount of product formed is known as limiting reactant.
Question 3. Define reactants in a chemical reaction.
Answer:
Reactants:
The substances with which a chemical reaction is started are called the reactants.
Question 4. What is the percent yield?
Answer:
Percent yield: Percent yield which is the ratio of the actual yield to the theoretical yield multiplied by 100.
Question 5. What is meant by decomposition reaction?
Answer:
Decomposition: When a molecule of a substance is broken down or decomposed in a chemical reaction to form two (or more) new substances in the presence of heat or electricity. It is called decomposition.
Example:
Calcium carbonate on heating decomposes into calcium oxide and carbon dioxide. \(\mathrm{CaCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2\)
Electricity is passed through acidulated water hydrogen and oxygen are produced due to the decomposition of water. 2H2O = 2H2+O2
“WBBSE Class 10 Physical Science Chapter 3, Chemical Calculations important questions”
Question 6. What is meant by addition reaction?
Answer:
Addition reaction: The reaction in which one reactant molecule directly combines with other molecules of reactant forming new molecules of the product without leaving any part of the molecules of reactants is known as an addition reaction.
Example:
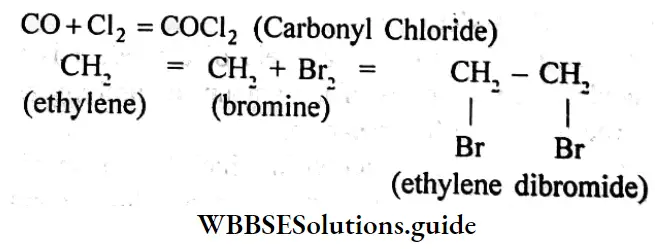
Question 7. What is the structural formula?
Answer:
Structural formula: A formula that gives the actual arrangement of the different atoms in the molecule or shows how the different atoms in the molecule are linked together is called a structural or a graphic formula of the compound.
Question 8. What do you mean by a chemical reaction?
Answer:
Chemical reaction: Any chemical change in matter that involves the transformation of matter into a new substance or new substance is termed a chemical reaction.
Question 9. What are mass volume relationship problems?
Answer:
Mass volume relationship problems: In this type of problem. mass or volume of one of the reactants or products is calculated from the volume or mass of other substances.
Question 10. Define products in a chemical reaction.
Answer:
Products: The substances formed as the result of the chemical reaction are called the products.
Question 11. What is the percentage of an element?
Answer:
Percentage of an element: The percentage of an element in a chemical compound is the number of parts by weight of it present in 100 parts by weight of the compound.
“Class 10 Physical Science and Environment Chemical Calculations solutions, WBBSE syllabus”
Question 12. Define double decomposition reaction.
Answer:
Double decomposition reaction
The reaction in which the constituent of the molecules of reactants change their position and form molecules of new substances is called. Double decomposition
Example: AgNO3 + NaCl = AgCl + NaNO3
Question 13. What is the molecular formula?
Answer:
Molecular formula: It is the formula of a compound that gives the actual number of atoms of various elements present in one molecule of the compound.
Question 14. Define oxidation-reduction type reaction.
Answer:
Oxidation-reduction reaction: The chemical reaction in which a chemical species loses electrons (s) is an oxidation reaction and the reaction in which a chemical species gains electrons (s) is called an oxidation-reduction reaction.
Question 15. What is the empirical formula?
Answer:
Empirical formula: It is the formula of a compound that gives the simple whole number ratio of the atoms of various elements in one molecule of the compound.
Question 16. What are mass-mass relationship problems?
Answer:
Moss-moss relationship problems: In this type of problem, the moss of one of the reactant products is to be calculated if that of the other reactant products is given.
Question 17. What are volume-volume relationship problems?
Answer:
Volume-volume relationship problems: In this type of problem, the volume of one of the reactants or products is to be calculated from the volume of some other reactant and product.
Question 18. What is a theoretical yield?
Answer:
Theoretical yield: The theoretical yield of a product is the maximum yield obtainable as calculated on the basis of the amount of limiting reactant used.
“WBBSE Class 10 Chapter 3 Physical Science, Chemical Calculations step-by-step solutions
Question 19. Define direct combination reaction.
Answer:
Direct combination: The reaction in which two or more reactions combine directly forming molecules of a new substance is called direct combination.
Example:
Burning magnesium wire reacts directly with oxygen forming new molecules of magnesium oxide. 2Mg +O2 = 2MgO
Question 20. What is meant by the acid-base reaction on neutralization reaction?
Answer:
Acid-base reaction or neutralization reaction: In this reaction, an acid reacts with a base forming salt and water. An equivalent amount of an acid neutralizes an equivalent amount of a base. This is known as a neutralization reaction.
Example:
\(\underset{\text { (acid) }}{\mathrm{HCl}}+\underset{\text { base }}{\mathrm{NaOH}}=\underset{\text { (salt) }}{\mathrm{NaCl}}+\underset{\text { (water) }}{\mathrm{H}_2 \mathrm{O}}\)Question 21. Define Chemical equation.
Answer:
Chemical equation: A brief representation of a chemical reaction by using symbols of atoms of the elements and formulae of molecules of reactants and products maintaining the balance in between is known as a chemical equation.
It reveals both the qualitative and quantitative aspects of a chemical change.
Question 22. Define substitution reaction.
Answer:
Substitution reaction: It is a chemical reaction in which one atom or molecule of a compound is replaced by another atom or molecule of another substance.
Example: Zn + CuSO4 = ZnSO4 + Cu.
Question 23. What is meant by rearrangement reaction?
Answer:
Rearrangement reaction: A reaction where a compound changes by internal arrangement of its atoms into another substance with different properties but having the same composition is known as a rearrangement reaction.
Example:
Ammonium cyanate changes to urea on heating.

Question 24. Why is a chemical equation called a balanced equation?
Answer:
A chemical equation must be balanced because, from the law of conservation of mass, the total reacting mass of the reactants must be equal to the total mass of the products.
Question 25. What is meant by ‘The vapor density of SO2 is 32′?
Answer:
‘The vapor density of SO2 is 32′
The vapor density of SO2 is 32 under the same conditions of temperature and pressure, a certain volume of SO2 is 32 times heavier than the same volume of hydrogen.
Question 26. Write the significance of a chemical equation.
Answer:
The chemical equation signifies
- The names of the reactants and the products our products formed as a result of chemical reactions.
- The relative number of atoms and molecules of the reactants taking part and also the product in a chemical reaction.
- Weight-weight, weight-volume, and volume-volume relations amongst the reactants and products are obtained from a chemical equation.
Question 27. What is the substitution reaction? Write an example.
Answer:
Substitution reaction: An atom or group in a molecule is replaced by another atom or group.
Example: CuSO4 Fe = FeSO4 + Cu.
Question 28. Write the relation between normal density and vapor density.
Answer:
At STP, normal density vapor density x 0.08.
Question 29. State the law of conservation of mass in chemical reactions.
Answer:
Law of conservation of mass in chemical reaction. In a chemical change, the total mass of the reaction is equal to the total mass of the products. Matter can neither be created nor destroyed.
Question 30. Short Note-Double decomposition.
Answer:
Double decomposition: Two compounds exchange their cations and anions to produce new compounds.
PbSO4 + Na2CO4 = PbCO3 + Na2SO4
Question 31. What are the classification of problems based upon the chemical reactions?
Answer:
The classifications of the problem based on the chemical reactions
- Mass-Mass relationship problems
- Mass-volume relationship problems
- Volume-volume relationship problems
Question 32. Short Note-Problems based on chemical equation.
Answer:
Problems based on chemical equation: The problems based upon chemical equation may be classified as :
- Mass-mass relationship problems – In this type of problem, the mass of one of the reactant products is to be calculated if that of the outer reactant products is given.
- Mass-volume relationship problems – In this type of problem, the mass or volume of one of the reactants or products is calculated from the volume or mass of other substances.
- Volume-volume relationship problems. In this type of problem, the volume of one of the reactants or products is to be calculated from the volume of some other reactant or product.
Question 33. Write the relation between molecular weight and vapor density.
Answer:
Molecular weight = 2 x Vapour density.
Question 34. Define-chemical equation.
Answer:
Chemical equation: Balancing the number of atoms of reactants and products, the representation of a chemical reaction in short by symbol and formula is called a chemical equation.
Question 35. Write the partial elimination of the limitations
Answer:
Partial elimination of the limitations
- (s), (i) or (g) may be written respectively for solid, liquid, and gaseous reactants and products.
- For exothermic reaction (4H) and for endothermic reaction (+ 4H) may be written.
- Temperature pressure and catalyst may be written on the arrow of the arrowheel equation.
& \mathrm{CaCO}_3(\mathrm{~S}) \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g}) \uparrow \\
& \begin{aligned}
\mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow & \mathrm{CO}_2(\mathrm{~g}) \uparrow \mathrm{CO}_2(\mathrm{~g}) \uparrow+(4 \mathrm{H}) \\
& +(4 \mathrm{H})
\end{aligned}
\end{aligned}\)
“WBBSE Class 10 Chemical Calculations, Physical Science Chapter 3 key concepts”
Question 36. Short Note-Addition reaction.
Answer:
Addition reaction: When two molecules combine chemically with the detachment of any part, the reaction is called an addition reaction, Generally unsaturated compounds are given this reaction.
CO + Cl2 = COCl2
Question 37. Write an example of a balanced equation.
Answer:
An example of a balance equation: CaCO3 + 2HCI = CO2 + CaCl2 + H2O
Class 10 Physical Science WBBSE
Question 38. Write the limitations of a chemical equation.
Answer:
The limitations of the chemical equation :
- The physical states of the reactants and products
- The reaction condition (i.e. temperature, pressure, and catalyst if required)
- Whether the reaction is exo thermic or endo thermic in nature.
- The concentration of the reactants
Question 39. \(\text { Prove, vapour density }=\frac{\text { Molecular weight of gas }}{\text { molecular weight of hydrogen }}\)
Answer:
\text { Vapour density of gas (D) } & =\frac{\mathrm{n} \text { molecules of gas }}{\mathrm{n} \text { molecules of hydrogen }} \\
& =\frac{\text { weight of } 1 \text { molecule of gas }}{\text { weight of } 1 \text { molecules of hydrogen }} \\
& =\frac{\text { molecular weight of mass }}{\text { molecular weight of hydrogen }}
\end{aligned}\)
“WBBSE Class 10 Physical Science Chapter 3, Chemical Calculations summary”
Question 40. Short Note Direct combination reaction.
Answer:
Direct combination reaction: The direct combination reaction takes place as soon as the reactants come into indirect contact. Sometimes heat, light, pressure, or catalyst is necessary to start the reaction.
Example: The reaction between Mg and O2 starts with the application of heat.
2Mg +O2= 2MgO
Question 41. Short Note-Decomposition reaction.
Answer:
Decomposition reaction: By this reaction, a compound is changed to different substances. This reaction is conducted by heat or electricity CaCO3 on heating decomposed calcium oxide (CaO) and carbon dioxide (CaO) and carbon dioxide (CaO) and carbon dioxide (CO2)
CaCO3 = CaO + CO2
Acidified water on electrolysis is decomposed to form H2 and O2.
2H2O=2H2 ↑ +O2↑
Question 42. State Avogadro’s law’
Answer:
Avogadro’s law: ‘Under the same conditions of temperature pressure, equal volumes of all gases contain the same number of molecules.
