Class 10 Physical Science Solutions WBBSE
Chapter 7 Atomic Nucleus MCQs
Question 1. Which of the following are α-emitter?
- Helium-5
- Polonium-212
- Tritium
Answer: 2. Polonium-212
Question 2. Control rods of nuclear reactors are made up of:
- Cd
- Diamond
- Graphite
- Copper
Answer: 1. Cd
Read And Learn More: WBBSE Solutions For Class 10 Physical Science And Environment
Question 3. The moderator used in atomic pile is :
- Heavy water
- Uranium
- Iron
Answer: 1. Heavy water
“WBBSE Class 10 Physical Science and Environment Chapter 7 solutions, Atomic Nucleus”
Question 4. Which one of the following is not a synthetic element?
- Np
- Cm
- PU
- U
Answer: 4. U

Question 5. Which emits b-particle?
- 1H3
- 6 C14
- 19 K40
- All of these.
Answer: 4. All of these.
Question 6. Choose the element which is not radioactive :
- Cm
- NO
- Mo
- Md
Answer: 3. Mo
Question 7. 1H1 + 1H3 + 1H4 represents:
- β-decay
- Fusion
- Fussion
- D-decay
Answer: 2. Fusion.
Question 8. What is the order of reaction of the decay of 92 U235 ?
- Zero
- First
- Second
- Third
Answer: 2. First.
Question 9. Which of the following has the maximum ratio?
- 16Ne
- 16F
- 16O
- 16N
Answer: 4.16N
“Class 10 WBBSE Physical Science Chapter 7 solutions, Atomic Nucleus study material”
Question 10. Which of the following radioactive elements is soluble in water?
- Radium
- Tritium
- Radon
Answer: 3. Radon.
Question 11. The relative penetrating power of a,ß,y and neutron (n) follows the order:
- α > β > γ> n
- n > γ > β >α
- β >α > n > γ
- None of these
Answer: 2. n > γ > B > α.
Question 12. Which of the following nuclear reactions will generate an isotope?
- Newtron particle emission
- Positron emission
- β – particle emission
- α – particle emission
Answer: 1. Newtron particle emission.
Question 13. Nuclear energy is :
- Renewable
- Not renewable
- Sometimes renewable
- It is a matter of debate
Answer: 2. Not renewable.
Question 14. Nuclear fission is initiated by:
- Slow neutron
- By proton
- Fast newtron
- By α Particle
Answer: 1. Slow neutron.
Question 15. The source of energy of the sun is :
- Fission reaction.
- Fusion reaction
- Spalation reaction
- None of these
Answer: 2. Fusion reaction.
Question 16. Near the nuclear reactor, there is:
- Large neutron flux
- Large proton flux
- Large Particles flux
- Large β – ray flux
Answer: 1. Large neutron flux
Question 17. A decrease in atomic number is not observed during :
- α-emission
- β-emission
- Positron
- Electron capture
Answer: ????
Question 18. Identify the nuclear reaction that differs from the rest :
- Positron emission
- K-capture
- γ-decay
- β-decay
Answer: 3. γ- decay.
Question 19. Which of the following are β-emitters?
- Carbon-14
- Cobalt-60
- Tritium-3
- All of these
Answer: 4. All of these.
Question 20. Which of the following has the highest value for its radioactivity?
- 1g of Ra
- Ig of Ras 04
- 1g of RaBr2
- None of these
Answer: 1. Ig of Ra.
Question 21. Of the following which is not used as a moderator in a nuclear reactor?
- Heavy water
- Sodium
- Graphite
Answer: 2. Sodium.
Question 22. Control rods used in the nuclear reactor are made of:
- Nickel
- Iron
- Graphite
- Cadmium
Answer: 4. Cadmium.
Question 23. Which of the following processes causes the emission of an X-ray?
- Alpha emission
- Gamma Emission
- Electron emission
- Positron Emission
Answer: 3. Electron emission.
Question 24. Choose the incorrect one:
- 1 curie = 3.7 × 1010 ds-1
- 1 rutherford = 106 ds-1
- 1 fermi = 103 ds-1
- I becquerel = 1 ds-1
Answer: 3. fermi = 103 ds-1
Question 25. Mark the correct relation :
- No = Nett
- t= 1.44+0.5
- N= No(½)x
- To.5 = λln2
Answer: 4. To.5 = λln2
Question 26. Choose the natural element among the following:
- Uranium
- Astatine
- Neptunium
Answer: 1. Uranium.
Question 27. α-ray consists of a stream of
- H–
- He-2
- Only electrons
- Only Neutrons
Answer: 2. He-2
Question 28. Which of the following combinations will give the most stable nuclear?
- Odd Z and odd N
- Odd Z and even N
- Even Z and odd N
- Even Z and even N
Answer: 4. Even Z and even N.
Question 29. Emission of a ẞ particle by an atom of element results in the formation of its
- Iso tope
- Isomer
- Isobar
- Isomorph
Answer: 3. Isobar.
Question 30. Stable nuclides cannot be obtained for
- Z = 43, N = 35
- Z = 61, Z = 89
- A > 209
- All of these
Answer: 4. All of these.
Question 31. Which of the following nuclei are stable?
- 28 Ni60
- 6 C11
- 92 U233
- 4 Be8
Answer: 4. 4 Be8
Question 32. Which of the following nuclei is unstable?
- 5B10
- 7N14
- 8O16
- 4Be10
Answer: 4. 4 Be10
Question 33. A device used for the measurement of radioactivity is
- Mass spectrometer
- Cyclotron
- G-M-Counter
- Nuclear reactor
Answer: 3. G-M-Counter.
Question 34. Which of the following radiations, the one most easily stopped by air is
- X-rays
- α-rays
- β-rays
- γ -rays
Answer: 1. X-rays.
Question 35. y -Ray:
- Consist of particles that have mass
- Are energy waves.
- Have mass
- Are deflected by an electric field
Answer: 2. Are energy waves.
Question 36. Loss of B-particle is equivalent to
- Increase of one proton
- Decrease of one neutron
- Combination of (1) and 2)
- None of the above
Answer: 3. Combination of (1)and (2)
“WBBSE Class 10 Physical Science Chapter 7, Atomic Nucleus solved examples”
Question 37. Which of the following is the man-made radio-active disintegration series?
- Thorium series
- Actinium series
- Uranium series
- Neptunium series
Answer: 4. Neptunium series.
38. Question 13A27 is a stable isotope. It is expected to disintegrate by
- α-emission
- β – emission
- β–– emission
- Proton emission
Answer: 3. β–-emission.
Question 39. Positron has a mass equal to
- Electron
- α – particle
- Proton
- Deuteron
Answer: 1. Electron.
Question 40. Which of the following has a magic number of protons and neutrons?
- 8O17
- 13Al27
- 9Fl17
- 20Ca40
Answer: 4. 20Ca40
WBBSE Class 10 Physical Science Solutions Chapter 7 Atomic Nucleus Very Short Answer Type Questions
Question 1. Out of αβ and y rays which one is called hand rays?
Answer: γ rays.
Question 2. What are the magic numbers?
Answer: Magic numbers are 2, 8, 20, 50, 82 and 126.
Question 3. What is the value of the packing fraction for unstable nuclides?
Answer: Positive.
Question 4. What is the value of the packing fraction for stable nuclides?
Answer: Zero or negative.
Question 5. Do nuclear forces obey inverse square law?
Answer: Nuclear forces are not governed by inverse square law
Question 6. Out of a, ẞ and y rays which one causes maximum damage to the body tissues?
Answer: α-rays.
“WBBSE Class 10 Atomic Nucleus solutions, Physical Science and Environment Chapter 7”
Question 7. What is the density of the nucleus?
Answer: 1014/ Cm3
Question 8. What is the binding energy for a mass defect of 1 amu?
Answer: 931 5 Mev.
Question 9. What is the n/p ratio of lighter nuclides (Zup to 20)?
Answer: 1.
Question 10. What is the n/p ratio of 1ft1 ?
Answer: 0.
Question 11. What is the lifespan of a radioactive element?
Answer: The life span of a radioactive element is infinite.
Question 12. How many a and b particles are emitted from uranium series?
Answer: The uranium series emits 8α and 6β particles.
Question 13. What is artificial series?
Answer: (4n+1) is artificial series.
Question 14. What is the end product of the 4n series?
Answer: The end product of the 4n series is lead.
Question 15. Is the half-life of a radioactive element dependent on its physical and chemical state?
Answer: The half-life of a radioactive element is independent of its physical and chemical state.
Question 16. How mass defects can be converted into energy?
Answer: The mass defect can be converted into energy by using Einstein’s equation ΔE = mc2
“Class 10 WBBSE Physical Science Chapter 7, Atomic Nucleus easy explanation”
Question 17. Write an example of nuclear fission.
Answer:
92 U 235+ 0 n1 → 35 Br 87 +57 La146 + 3 0 n1
Question 18. Write an example of an isotope of carbon.
Answer: An example of an isotope of carbon C- 14.
Question 19. What is the value of the velocity of ẞ Particle?
Answer: The velocity of β particle is maybe up to \(\frac{9}{10}\) the velocity of light.
Question 20. Define.-Helions.
Answer: α particles are He– ions or bare helium nuclei sometimes called helions.
WB Class 10 Physical Science Question Answer Chapter 7 Atomic Nucleus Fill In The Blanks
Question 1. Radioactivity was proposed by_________
Answer: Madam Curie.
Question 2. Measurement of radioactivity is done by _________
Answer: Geiger-Muller counter.
Question 3. The process of transforming one element into another is known as _________
Answer: Alchemy
Question 4. NO2 and CO2, are the examples of _________
Answer: Isosteres
Question 5. The density of the nucleus is of the order of_________
Answer: 1014
Question 6. Magic numbers are 2, 8, 20, 50, 82 and _________
Answer: 126
“WBBSE Class 10 Physical Science Chapter 7 solutions, Atomic Nucleus PDF”
Question 7. The difference between the total mass of the particles present in the nucleus of a nuclide and its real mass is called _________
Answer: Mass defect
Question 8. Due to the large mass, α particles possess large _________
Answer: Kinetic energy
Question 9. β-particles are _________ charged
Answer: Negatively
Question 10. γ rays are _________
Answer: Neutral
Question 11. γ-rays have the least effect on _________
Answer: Photographic plates.
Question 12. Isotopes of the same element possess the same number of _________
Answer: Protons.
Question 13. 1H2+ 1H3 → 2He4 is a _________ reaction.
Answer: Fusion.
Question 14. The speed of y-rays is _________ speed of light reaction.
Answer: Equal to
Question 15. The atom bomb is based on nuclear _________ reaction.
Answer: Fission.
Question 16. A hydrogen bomb is based on nuclear _________ reaction.
Answer: Fusion.
Question 17. The source of the energy of the Sun is _________
Answer: Fusion.
Question 18. The number of neutrons in a radioactive isotope of hydrogen is _________
Answer: 2.
Question 19. The rate of disintegration of the active nucleus is known as _________
Answer: Activity.
Question 20. 7N14+0n→ a ____________ + 7H14
Answer: 6C14
Question 21. The energy equivalent to 1 amu is _________ me V.
Answer: 931.5.
Question 22. The time taken for the decay of half the initial amount of a radioactive nuclide is called _________.
Answer: Half-life period.
Question 23. The phenomenon of spontaneous emission of invisible radiation is called _________.
Answer: Radioactivity.
Question 24. B-particles are nothing but _________ moving at high speeds.
Answer: Electrons.
“WBBSE Class 10 Physical Science Chapter 7, Atomic Nucleus important questions”
Question 25. The phenomenon in which a stable nuclide is converted artificially into a radioactive substance is called _________.
Answer: Artificial radioactivity.
Question 26. Isotones are the nuclides having the same number of_________.
Answer: Neutrons.
Question 27. Atoms of the different elements possessing the same mass number are called _________.
Answer: Isobars.
Question 28. According to Einstein’s equation, the relation between mass and energy can be written as _________.
Answer: E = mc2
Question 29. _________ is α- a-emitter.
Answer: Polonium-212.
Question 30. Positron has a mass equal to _________.
Answer: Electron.
WBBSE Class 10 Physical Science Solutions Chapter 7 Atomic Nucleus Short Answer Type Questions
Question 1. What do you mean by nuclear forces?
Answer:
Nuclear forces: The forces which held the nuclear together within the small nucleus are called nuclear forces.
These forces exist among P-P, P-n and n-n
Question 2. What are nuclear reactions?
Answer:
Nuclear reactions: Nuclear reactions are the reaction in which the nuclear of an atom undergoes a change.
Question 3. What is packing fraction?
Answer: ‘
Packing fraction: Packing fraction was proposed by Aston and defined as the difference between actual isotopic mass and the mass number.
Question 4. What are nuclear isomers?
Answer:
Nuclear isomers: Nuclear species having the same atomic number and same mass number but different radioactive properties are called nuclear isomers.
Examples: 69Zn (7½ 13.8 hours) and 69Zn (7½, = 57 minute)
Question 5. What is Natural transmutation?
Answer:
Natural transmutation: It is a process in which elements such as radium undergo transmutation on their own.
Question 6. What do you mean by ‘Group Displacement Law?
Answer:
Group Displacement Law:
We know that an a-emission decreases the atomic number of the parent by 2 and P-emission increases the atomic number by 1.
Thus ‘In an a-emission, the parent element will be displaced to a group two places to the left and in ẞ-emission, it will be displaced to a group one place to the right.’
“Class 10 Physical Science and Environment Atomic Nucleus solutions, WBBSE syllabus”
Question 7. What is a spallation reaction?
Answer:
Spallation reaction: High-speed projectiles with energies of approximately 40M V may chip fragments from a heavy nucleus, leaving a small nucleus. This type of reaction is called spallation.
Examples: 92 U 235+ 0 n1 → 74W187 + 20 1H1+ 350 n1
Question 8. What is meant by ‘projectile capture reactions’?
Answer:
Projectile capture reaction: The bombarding particle is absorbed with or without the emission of γ radiations.
Examples: 92 U 238+2H4 → 92 U 239+ γ
Question 9. What is the disintegration series?
Answer:
Disintegration series: The whole series of elements starting with the parent radioactive element to the stable end product is called a radioactive disintegration series.
These series are- 4n (4n+1), (4n+2) and (4n+3)
Question 10. What do you mean by ‘Alchemy’?
Answer:
Alchemy: The process of transforming one element into another is known as Alchemy and the person involved in such experiments is called an alchemist.
Question 11. What is a disaster?
Answer:
Isoster: Molecules or ions with the same number of atoms and also the same number of electrons are said to form an isosteric group or more simple isosteres.
Examples: NO2 and CO2
Question 12. What is Radioactivity?
Answer:
Radioactivity: It is a process in which the nucleus of certain elements undergo spontaneous disintegration without excitation by any external me
“WBBSE Class 10 Chapter 7 Physical Science, Atomic Nucleus step-by-step solutions”
Question 13. What do you mean ny ‘isotopes’?
Answer:
Isotopes: The atoms of an element having the same atomic number but different mass numbers are called isotopes.
Examples: 1H1,1H2,1H3
Question 14. What is Nuclear fission?
Answer:
Nuclear fission: The process of artificial transmutation in which a heavy nucleus is broken down into two lighter nuclei of nearly comparable masses with the release of a large amount of energy is termed nuclear fission.
Examples: 92 U 235+ 0 n1 →56Ba140+ 36Kr93+ 3 0 n1
Question 15. What is a half-life period?
Answer:
Half-life period: The half-life period of a radioactive isotope is the time required for one-half of the isotope to decay.
⇒ \(t \frac{1}{2}=\frac{0.693}{\lambda}\)
(λ = Disintegration constant)
Question 16. What is nuclear fusion?
Answer:
Nuclear fusion: A nuclear reaction in which two lighter nuclei are fused together to form a heavier nucleus is called nuclear fusion. 2. 3 4
Examples: 1H2 + 1H3 → 2He4 +17.6MeV
Question 17. What is Artificial radioactivity?
Answer:
Artificial radioactivity: It is the phenomenon in which the artificial transmutation of a stable nucleus leads to the formation of a radioactive nuclide.
Question 18. What do you mean by ‘Rutherford’?
Answer:
Rutherford: If a radioactive substance has 106 disintegrations per second, it is said to have an activity of one Rutherford.
Question 19. What are magic numbers?
Answer:
Magic numbers: Magic numbers are the numbers 2, 8, 20, 50, 82 and 126. Nuclides having a magic number of either protons or neutrons or both are more stable.
Question 20. What is Radioactive equilibrium?
Answer:
Radioactive equilibrium: Radioactive change being an irreversible process shows equilibrium when a daughter element disinter grates at the same rate at which it is formed from the parent element.
Question 21. What is the packing fraction of
Answer:
Packing fraction (isotopic mass-mass number) mass number
Packing fraction= \(\frac{(\text { isotopic mass }- \text { mass number })}{\text { mass number }} \times 10^4\)
= \(\frac{55.92066-56}{560} \times 10^4\)
= – 14.167
So, the packing fraction of 26Fe56 is -14.167
Class 10 Physical Science WBBSE Question 22. How many a and ẞ particles will be emitted by 84 Ra218 in changing to 82 pb206?
Answer:
Let x and y be the number of a and ẞ particles involved in bringing about the change
⇒ 84 Ra218 → 82 pb206 +x 2He4+y -1e0
Comparing the mass number = 218
= 206 + 4x + 0y
⇒ 4x = 12
⇒ x = \(\frac{12}{4}\)
⇒ x= 3
Comparing the atomic numbers
84 = 82 + 2x + y
2x – y = 2
y = 2(3)- 2
y = 6- 2
y = 4
The number of a and ẞ particles emitted in the given nuclear reaction is 3 and 4.
Question 23. Calculate the number of neutrons in the remaining atom after the emission of a particle from the 92 U 238 atom.
Answer:
On emission of an a-particles atomic mass
If daughter element 238 – 4 = 234 (Mass)
Atomic number of daughter element = 92-2 = 90
Number of Neutrons =Atomic mass – Atomic number 234 – 90= 144.
Question 24. Short Note-y rays.
Answer:
γ rays: γ rays are high-energy electromagnetic waves. This can be shown by diffraction experiments. The penetration power of the y-rays is 10,000. There is no rest mase of γ -rays.
Question 25. Write four characteristics of γ-rays.
Answer:
Four characteristics of γ-rays
- The mass of y ray is none
- The nature of y-ray is electromagnetic waves
- The relative ionisation capacity of γ-rays is 1.
- The penetration power of γ-ray is 10,000.
Question 26. Short Note-a Gaiger Miller counter.
Answer:
Geiger Miller counter:
Particles produce scintillations on Zns screens and can easily be detected. One can detect and count the number of β -Particles with the help of the Geiger Muller counter. It consists of a tube fitted with two electrodes at the two ends and contains an inert gas like neon or argon.
Question 27. Short Note β -particles.
Answer:
β -Particles: By measuring the e/m of the B-particles by deflecting them in the an-electric field and a magnetic field it has been conclusively proved that the β – particles are nothing but high-speed electrons. In artificial radioactivity, however, β – particles can be emitted as positrons also.
Question 28. Short Notes a-particle.
Answer:
α-particles = α-particles are in fact. The ions or bare helium nuclei are sometimes called helions. That the a-particles are He– can be easily proved in a laboratory, acquire squirt electrons from the surrounding and get neutralised to produce helium atoms. The spectrum of the gas obtained from the a-particles emitted from a radioactive source confirms this point.
As α-particles are neutralised to give helium atoms, uranium mines are always found to be a very good source of helium gas. One can early count the number of particles emitted by a radioactive source, in a given time from the scintillation in an Instrument called Spintharis cope.
It is from the ionisation in a Gelger-Muller counter. One can also measure the amount of positive charge imparted to a metal plate during the same period, one can now determine the number of units of charge each particle carries by dividing the total charge imparted to the metal by the total number of particles emitted during the period. It is found to be two units (taking the amount of electronic charge as a unit). ]
Question 29. Radioactivity is a nuclear phenomenon Explain it.
Answer:
Radioactivity is a nuclear phenomenon
If we take two compounds of a radioactivity element containing the same amount of the element, the measured amounts of radioactivity in the two cases appear to be the same in both cases.
This fact shows that radioactivity is a property of an element, not of a compound. While discussing the cause of radioactivity Rutherford and Soddy commented that radioactivity was an unclear phenomenon.
They presented the following arguments in flavour of Heir’s view. When an atom of radioactive element emits a particle the element loses its atomic mass by 4 units and is converted into an atom written the Uranum series of radioactive elements.
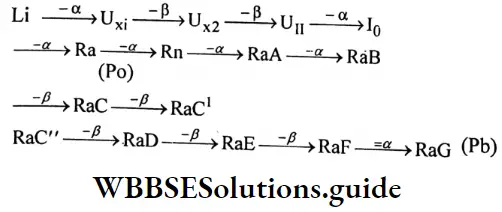
Question 30. Write four characteristics of -rays.
Answer:
Four characteristics of -rays
- The nature of β – rays is electrons
- The mass of β- the ray is \(\frac{1}{1856}\) AMU.
- The relative ionisation Capacity is 100
- The penetration power is 100.
Question 31. Short Note-Soddy Fajan’s rule.
Answer:
Soddy Fajan’s Rule:
A problem arose about the placement of the elements of the Uranium series in the periodic table soon after their discovery. It was observed that the element formed by the emission of a particle followed by the emission of two β – particles starting from U, with the atomic mass and atomic number producing an element which had the same chemical properties as that of U.
Question 32. Establish the equation of the rate of radioactive disintegration for a half-life period.
Answer:
We know \(\frac{\mathrm{dN}}{\mathrm{dt}}\) α N
\(\frac{\mathrm{dN}}{\mathrm{dt}}\) = λN ______ (1)
λ = Radioactive disintegration
Integrating the equation (1)
N= Noe – λt______ (2)
Taking Lagarithm and simplification
λ = \(\lambda\frac{1}{7} \ln \frac{\mathrm{No}}{\mathrm{N}}=\frac{2.303}{7} \log \frac{\mathrm{No}}{\mathrm{N}}\)
λ = \(\frac{1}{\frac{t_{01}}{2}} \ln _2=\frac{2.203}{\frac{t_1}{2}} \log 2=\frac{0.693}{t_{\frac{1}{2}}}\)
λ = \(\mathrm{t}_{\frac{1}{2}}=\frac{0.693}{\lambda}\)
“WBBSE Class 10 Atomic Nucleus, Physical Science Chapter 7 key concepts”
Question 33. What is the integration form of the rate of radioactive disintegration?
Answer:
We know, \(\frac{\mathrm{dN}}{\mathrm{dt}}\) α N
\(\frac{\mathrm{dN}}{\mathrm{dt}}\)α N
\(\frac{\mathrm{dN}}{\mathrm{dt}}\) = λn
λ = Radioactive disintegration
Integrating the equation, N Noe – λt
No initial number of atoms of the radioactive element.
Question 34. Define isotope. Write an example of the isotope.
Answer:
Isotope
Isotope Some elements have the same atomic numbers though they have different numbers. They are called isotopes as they occupy the same position in the periodic table.
Example: 92U238
“WBBSE Class 10 Physical Science Chapter 7, Atomic Nucleus summary”
Question 35. Write about the rate of radioactive disintegration.
Answer:
The rate of radioactive disintegration
If there are N number of atoms of a radioactive element in a sample at a
The particular instant of time, the rate of disintegration at that instant will be proportion to N.
\(\frac{\mathrm{dN}}{\mathrm{dt}}\)α N
\(\frac{\mathrm{dN}}{\mathrm{dt}}\)= – λN
γ = Constant (radioactive disintegration).
