Acids, Bases And Salts Very Short Answer Type:
Question 1. The aqueous solution of HCI gives the acid character. Which ion is responsible for it?
Answer: Hydronium ion (H3O+).
Question 2. Give the name and formula of the anion present in aqueous solution of bases.
Answer: Hydroxyl ion (OH–).
Question 3. What is the nature of aqueous solution of carbon dioxide?
Answer: Acidic
Question 4. What type of salt is this — NaHCO3?
Answer: Acidic.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Wbbse Madhyamik Physical Science And Environment Class 9 Question 5.
What is the use of methyl orange?
Answer: It is used as an indicator.
Question 6. Give an example of 2 neutral oxides.
Answer: Carbon monoxide (CO)
Question 7. What is the nature of ZnO?
Answer: Amphoteric oxide.
Question 8. Give an example of an acid which has oxidising properties.
Answer: Nitric acid (HNO3).
Question 9. What will be the colour of the solution if a few drops of phenolphthalein are added to the aqueous solution of sodium hydrogen carbonate?
Answer: Colourless.
Question 10. Which one is used in vanishing colour NH4OH or NaOH?
Answer: NH4OH.

WBBSE Class 9 acids bases and salts solutions
Question 11. What is the basicity of CH3COOH?
Answer: The basicity of CH3COOH is 1.
Question 12. Give an example of an oxide of metal which is not basic.
Answer: Mn3O4
Question 13. Give an example of an organic base.
Answer: Methyl amine (CH3NH2).
Question 14. Give an example of an inorganic gaseous compound which has basic properties.
Answer: Ammonia (NH3).
Question 15. Name a normal salt and an acid salt of the same acid.
Answer: Normal salt-sodium sulphate (Na2SO4) and acid salt-sodium bisulphate (NaHSO4) of acid H2SO4.
Question 16. Name a normal salt whose aqueous solution is basic.
Answer: Aqueous solution of sodium carbonate (Na2CO3) is basic.
Question 17. What is an oxide?
Answer: An oxide is a compound of oxygen formed with another element.
Question 18. Name 2 different classes of oxides.
Answer: Basic oxide and acidic oxide are two different classes of oxides.
Question 19. Name a binary compound of oxygen which is not an oxide.
Answer: Oxygen fluoride (OF2).
Question 20. What is an indicator?
Answer: An indicator is some weak organic acid or base which indicates distinctive colours in acid, alkali and neutral solutions.
Question 21. Which element is present in all acids?
Answer: Hydrogen is present in all acids.
Question 22. Which ion does an acid generate in an aqueous solution?
Answer: An acid generates hydrogen ion (H+) in aqueous solution.
Question 23. Why are CaH2 and CH4, not acids?
Answer: CaH2 and CH4 do not produce H+ ions in an aqueous solution, so they are not acids, for every acid produces H+ ions in an aqueous solution.
Acids bases and salts WBBSE Class 9 solutions with answers
Question 24. Which ions disappear in neutralization?
Answer: H+ ions generated by acid and OH ions generated by alkali disappear in a neutralization process.
Question 25. Name an acid salt and write its formula.
Answer: Sodium bisulphate is an acid salt. Its formula is NaHSO4.
Question 26. What is meant by dissociation?
Answer: The process of breaking a substance (electrolyte) into its constituent ions in solution is called dissociation. or ionisation.
Question 27. Which element must an acid contain?
Answer: Hydrogen.
Question 28. Give an example of normal salt.
Answer: Sodium chloride (NaCl).
Question 29. Which indicator is used in the titration of strong acid and weak base?
Answer: Methyl Orange.
Question 30. Name an acid salt.
Answer: Sodium bisulphate (NaHSO4).
Question 31. Which ions disappear in neutralization?
Answer: H+ ions generated by acid OH– ions generated by alkali disappear in a neutralization process.
Question 32. What is an oxide?
Answer: An oxide is a compound of oxygen formed with another element.
Question 33. Name a tribasic acid.
Answer: Phosphoric acid (H2PO4).
Question 34. Give an example of an organic acid.
Answer: Formic acid (HCOOH).
Question 35. What is the neutralisation reaction?
Answer: The reaction in which an equivalent amount of an acid reacts with a base is called a neutralisation reaction.
Question 36. Give an example of a neutral oxide.
Answer: Carbon monoxide (CO)
Question 37. What is a vanishing colour?
Answer: The aqueous solution of ammonium hydroxide mixed with phenolphthalein is called vanishing colour.
Question 38. What will be the colour of the solution if a few drops of phenolphthalein are added to in aqueous solution of sodium carbonate?
Answer: Pink colour.
Question 39. Which indicator is used in the titration of a weak acid and a strong base?
Answer: Phenolphthalein.
Question 40. What is the nature of ZnO?
Answer: Amphoteric oxide.
Question 41. How many replaceable hydrogen atoms are present in sulphuric acid?
Answer: Two replaceable hydrogen atoms are present in sulphuric acid.
Question 42. Give an example of a double salt.
Answer: K2SO4.Al2(SO4)3 , 24H2O.
Question 43. Give an example of an oxide of metal which is not basic.
Answer: Mn3O4.
Question 44. What is the acidity of NaOH?
Answer: The acidity of NaOH is 1.
Question 45. What is the basicity of CH3COOH?
Answer: The basicity of CH3COOH is 1.
Acids, Bases And Salts 2 Marks Questions And Answers:
Question 1. What are mineral acids?
Answer:
Mineral acids
Acids HCl, H2 SO4, HNO3 etc. are called mineral acids because all are derived from minerals. Mineral acids are strong acids also called inorganic acids.
Wbbse Class 9 Physical Science
Question 2. What is a universal indicator?
Answer:
Universal indicator
A universal indicator is a mixture of indicators, often sold ready-made as a solution, which can indicate pH values, usually in a range of 3 – 11. When this universal indicator is added in a solution, its colour changes and is then matched with the given indicator colour.
Question 3. Can a vanishing colour be prepared with a dilute NaOH solution?
Answer:
Vanishing colour cannot be prepared with dilute NaOH solution because sodium hydroxide (NaOH) does not evaporate.
Question 4. Why is an acid called a proton donor?
Answer:
Acid is called a proton donor: Acids produce cation (H+) in aqueous solution. H+ is nothing but a proton. So acid is called a proton donor.
Question 5. Why is alkali called a proton acceptor?
Answer:
Alkali is called a proton acceptor: Alkali produces hydroxyl ion (OH–) in an aqueous solution, and hydroxyl accepts a proton (H+) coming from an aqueous solution of acid to produce neutral water. So, alkali is called a proton acceptor.
WBBSE Class 9 Physical Science acids bases and salts notes
Question 6. Aqueous solution of HCl turns blue litmus red but HCl vapour does not, why?
Answer:
Aqueous solution of HCl turns blue litmus red but HCl vapour does not,
Reason: HCl vapour is a covalent compound, so it does not ionise in the vapour state. In an aqueous solution, HCI produces H3O+ (hydronium ion) and acts as an electrovalent compound which turns blue litmus red.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 7. Sodium carbonate is a neutral salt, but its aqueous solution is alkaline, why?
Answer:
Sodium carbonate is a neutral salt, but its aqueous solution is alkaline
Reason: Sodium carbonate reacts with water and produces caustic soda, a strong alkali, and a weak acid, carbonic acid. Due to strong alkali, after neutralisation of H*, there is excess OH (hydroxyl ion) in the solution. So the solution will be alkaline.
Equation : Na2CO3 + H2O \(\rightleftharpoons\)(Na+ + OH) +H2CO3
Question 8. What do you mean by the acidity of a base?
Answer:
The acidity of a base
The acidity of a base is expressed by the number of hydroxyl groups present in each molecule of the base.
For example :
| Base | Acidity |
| NaOH, KOH | 1. (Monoacidic base) |
| Ca(OH)l2, Zn(OH)l2, Mg(OH)l2 | 2. (Diacidic base) |
| AI(OH)3 | 3. (Triacidic base) |
The acidity of a base (metallic oxide) which does not contain a (OH) group is measured by the number of molecules of a monobasic acid required to react completely with one molecule of it to produce salt and water.
CaO + 2HCI = CaCl2 + H2O (Acidity of CaO is 2)
MgO + 2HNO3 = Mg(NO3)2 + H2O (Acidity of Mg is 2)
Question 9. Point out the differences between bases and alkalis.
Answer:
Differences between bases and alkalis: A base may or may not be soluble in water but all alkalis are soluble in water. So all alkalis are bases but all bases are not alkalis. NaOH is an alkali because it is soluble in water. Al(OH)3 is water insoluble. So it is a base but not an alkali.
Question 10. Which ions disappear in a neutralization reaction? Explain with an example.
Answer:
H+ ions generated by acid and OH– ions generated by alkali disappear in a neutralization reaction.
Example : 2HCI + Ca(OH)2 = CaCl2 + 2H2O. In this reaction, ions form as Ca(OH)2 \(\rightleftharpoons\)Ca2+ + 2OH–
2HCI\(\rightleftharpoons\) 2H+ + 2Cl.
Here, H+ ions generated from HCI combine with OH– ions generated from Ca(OH) producing water molecules.
Question 11. What colours are produced when a drop of methyl orange is added separately to the aqueous solution of sodium carbonate and ammonium sulphate?
Answer:
Yellow in the first case and pink-red in the latter, for, the aqueous solution of the former is alkaline and that of the latter is acidic. (Na2CO3 + 2H2O = 2NaOH + H2CO3) NaOH is a strong alkali while H2CO3 is a weak acid.
Again, (NH4)2SO4+ 2H2O = 2NH,OH + H2SO4 here H2SO4 is a strong acid but NH4OH is a weak alkali.
Wbbse Class 9 Physical Science
Question 12. Why does the reddish violet colour of a phenolphthalein-mixed KOH solution gradually decolourise when air is blown into it with the help of a pipe?
Answer:
When air is blown, CO2 gas mixes with the water of the alkaline KOH-solution producing carbonic acid (H2CO3), which in turn, neutralizes the given solution for which phenolphthalein turns colourless.
Question 13. What are oxides? What are the types of oxides?
Answer:
Oxides: Binary compounds of oxygen with any element (metallic and non-metallic) are called oxides.
Types of oxides :
(1) Acidic oxiae
(2) Basic oxide
(3) Neutral oxide
(4) Amphoteric oxide
(5) Peroxide
(6) Mixed oxide
(7) Polyoxide
(8) Sub-oxide
(9) Superoxide.
Question 14. Define acidic oxide.
Answer:
Acidic oxide: An acidic oxide is an oxide of a non-metal usually. It reacts with an alkali or a base to form salt and water.
Examples : CO2, SO2, P2O5 , etc.
Question 15. Define basic oxide.
Answer:
Important questions on acids bases and salts WBBSE Class 9
Basic oxide: A basic oxide is an oxide of a metal usually. It reacts with an acid to produce salt and water.
Examples: CaO, MgO, CuO, etc.
Question 16. Define neutral oxide.
Answer:
Neutral oxide: The oxides of certain non-metals which neither react with acid nor with base are called neutral oxides.
Examples: CO, H2O, NO, etc.
Question 17. Define amphoteric oxide.
Answer:
Amphoteric oxide: The oxides of certain weak electropositive metals which react with both acids and bases to form salt and water are called’ amphoteric oxides.
Examples: Al2O3, ZnO, PbO, etc.
Wbbse Class 9 Physical Science
Question 18. Define normal salts.
Answer:
Normal salts: The salts produced by the complete displacement of all the replaceable hydrogen atom or atoms present in the molecule of an acid by a metal or. a group equivalent to a metal are called normal salts.
Question 19. What are alkalis?
Answer:
Alkalis: Water-soluble hydroxides of metals are called alkalis.
Examples: NaOH, KOH, etc.
Question 20. What are salts? What are the types of salts?
Answer:
Salts: The replaceable hydrogen atoms in acid, when replaced by meta! or basic radical partially or fully then the compound so produced is called salt.
Types of salts :
(1) Normal salt
(2) Acid salt
(3) Basic salt
(4) Double salt
(5) Complex salt.
Question 21. What are bases?
Answer:
Bases: A base in general is an oxide or hydroxide of a metal and when reacts with an acid produces salt and water.
Examples: Al2O3, Al(OH)3, CaO, etc.
Wbbse Class 9 Physical Science Solutions
Question 22. What are acids?
Answer:
Acids: Ordinarily, an acid is a compound, the molecule of which contains one or more hydrogen atoms replaceable partially or completely, directly or indirectly by a metal or a group of elements behaving like a metal to form salt.
Examples: HCl, H2SO4, HNO3, etc.
Question 23. Define acid salts ‘or bi-salts.
Answer:
Acid-salts or bi-salts: The salts produced by the partial displacement of the replaceable hydrogen atoms present in the molecule of an acid by a metal or a radical acting like a metal, are known as acid-salts or bi-salts.
Examples : NaHSO4, NaHCO3, Na2HPO4 etc.
Question 24. How does vanishing colour act
Answer:
Action of vanishing colour: When the pink colour solution of vanishing colour is spread on a white linen, the linen turns pink. On allowing the pink-coloured wet linen to dryin air, the ammonia of the solution evaporates and the linen assumes its original white colour.
Wbbse Class 9 Physical Science Solutions
Question 25. Define basic salt.
Answer:
Basic salt: Salts formed by the partial displacement of oxide or hydroxide of alkalis by acid are known as basic salt.
Examples : Pb(OH)CI, Pb(OH)NO3, etc.
Question 26. What is a vanishing colour? How is it prepared?
Answer:
Vanishing colour: It is a coloured solution which becomes colourless on exposure to air for a length of time.Preparation of vanishing colour: It is a dilute solution of ammonia in water with a few drops of phenolphthalein. Its colour is deep pink.
WBBSE Class 9 solved exercises acids bases and salts
Question 27. Define double salt.
Answer:
Double salt: It is formed by the association of two or more normal salts.
Examples: K2SO4.Al,(SO4)3, 24H2O; (NH4)2 SO4.FeSO4, 6H2O, etc.
Question 28. Which ions disappear in neutralisation?
Answer:
lons disappear in neutralisation: H+ ions generated by acid and OH- ions generated by alkali disappear in a neutralisation process.
Question 29. Define complex salt.
Answer:
Complex salt: Complex salts contain complex ions which seem to be a complete salt.
Example : K4[Fe(CN)6], [Cu(NH3)4]SI4, [Ag(NH3)2Cl], etc.
Question 30. What is an indicator?
Answer:
Indicator: It is some weak organic acid or base which indicates distinctive colours in acid, alkali and neutral solutions,
Question 31. What is neutralisation ?
Answer:
Neutralisation: It is the process in which acids and alkalis in equivalent quantities in their aqueous solutions react to produce salt and the neutral substance water.
Question 32. What do you mean by titration?
Answer:
Titration: The process by which bases are neutralised with the help of acids or vice-versa.
Question 33. Why are CaH2 and CH4, not acids?
Answer:
CaH2 and CH4 are not acids: Calcium hydride (CaH2) and methane (CH4) do not produce H+ ions in aqueous solution, so these are not acids, for every acid produces H+ ions in aqueous solution.
Acids, Bases And Salts 3 Marks Questions And Answers:
Question 1. Classify acids with definitions of the types of acids and their respective examples.
Answer:
Acids are classified in many ways :
(1) based on composition :
Based on composition, acids can be classified into major two types :
(1) Hydracids and
(2) Oxy acids.
(1) Hydracids: The acids which contain hydrogen and a non-metallic element (other than oxygen) or a radical are known as hydracids.
For example:
HCI (Hydrochloric acid)
HI (Hydroiodic acid),
HCN (Hydrocyanic acid), etc.
(2) Oxyacids: The acids which contain oxygen besides hydrogen and a non-metallic element or a radical are called oxy-acids.
Examples of oxy-acids are :
HNO3 (Nitric acid),
H2SO4 (Sulphuric acid),
H3PO4 (Phosphoric acid), etc.
Wbbse Class 9 Physical Science Solutions
(2) According to sources :
According to sources, acids can be classified into:
(1) Inorganic acids and
(2) Organic acids.
(1) Inorganic acids: Those acids which are derived from inorganic sources (e.g. – minerals) are known as inorganic acids. For example – Hydrochloric acid (obtained from common salt NaCl), nitric acid (obtained from sodium or potassium nitrate), sulphuric acid (from sulphur), phosphoric acid (obtained from calcium phosphate), etc.
(2) Organic acids: The acids which contain carbon as an essential element and can be obtained from either plants or animals are designated as organic acids. Formic acid(HCOOH), acetic acid (CH3COOH), etc. are some of the organic acids.
(3) According to oxidising power, acids are also classified as:
(1) oxidising acid and
(2) non-oxidising acid.
(1) Oxidising acid: Acids which easily dissociate in normal conditions and produce nascent oxygen are known as oxidising acids:
Examples:
(1) Dissociation of concentrated sulphuric acid: H2SO4 H2O + SO2 + [O].
(2) Dissociation of concentrated nitric acid: 2HNO3 H2O + 2NO2 + [O].
The nascent oxygen atoms produced from these acids oxidise other substances, for this reason, they are called oxidising acids.
(2) Non-oxidising acids: Non-oxidising acids are those which do not give off nascent oxygen on dissociation.
Examples: Dilute sulphuric acid (H2SO4), hydrochloric acid (HCI), hydrocyanic. acid (HCN), organic acids, like formic acid (HCOOH), acetic acid (CH2COOR), etc.
(4) based on concentration: According to concentration, acids are of two types:
(1) Concentrated acid and
(2) Dilute acid.
(1) Concentrated acid: The acids which have less quantity of water in them are called concentrated acids.
Examples: conc. H2SO4, conc. HCl, etc.
(2) Dilute acids: Those acids which have more quantity of water mixed with them are called ditute acids.
Examples: dil. H2SO4, dil. HCl, etc.
(5) based on Basicity :
Definition of Basicity of acid: Basicity of an acid is its power of neutralising a base and it is measured by the number of replaceable hydrogen atoms present in one molecule of it.
Wbbse Class 9 Physical Science Solutions
According to basicity, acids are classified into three types:
(1) Monobasic acids
(2) Dibasic acids and
(3) Tribasic acids.
(1) Monobasic acids: Those acids which have only one replaceable hydrogen atom in their one molecule are called monobasic acids.
Examples: Hydrochloric acid (HCI), nitric acid (HNO), hydrocyanic acid (HCN), perchloric acid (HCIO4), formic acid (HCOOH), acetic acid (CH3COOH), etc.
(2) Dibasic acids: Those acids which have only two replaceable hydrogen atoms in their one molecule are called dibasic acids.
Examples: Sulphuric acid (H2SO4), Carbonic acid (H2CO3), hypophosphorous acid (H3PO2), etc.
(3) Tribasic acids: Those acids which have only three replaceable hydrogen atoms in their one molecule are known as tribasic acids. Phosphoric acid (H3PO4), citric acid (C6H8O7), etc.
Question 2. What is meant by ionisation? Explain with an example.
Answer:
Ionisation: The process of breaking up any compound into positively charged and negatively charged atoms or groups of atoms. by the mere process of dissolution is called ionisation. The positively charged particles are called cations and negatively charged particles are called anions.
Example: When common salt, i.e., sodium chloride (NaCl) is dissolved in water, it breaks up into positively charged sodium ions (Na+, cation) and negatively charged chloride ions (Cl–, anions) by the solvating action of water. This process of ionisation may be expressed by the equation.
NaCl \(\rightleftharpoons\) Na++ Cl–.
The process of ionisation is a reversible one (indicated by = sign), and consequently, an equilibrium is established between the ions and undissociated molecules.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 3. Calcium, sodium, sulphur, phosphorus, and carbon are burnt completely in separate oxygen jars. Each product is treated with water and then every solution is tested with blue and red litmus papers. Now complete the table.
Answer:
| Elements | Change of colour (1) Equation of reaction with oxygen. (2) Equation of reaction of product with water. |
Blue litmus | Red litmus |
| 1. Calcium | (1) 2Ca+O2= 2CaO (2) CaO + H2O= Ca(OH)2 |
blue | |
| 2. Sodium | (1) 2Na+O2= Na2O2 (2) 2Na2O2+2H2O=4NaOH +O2 |
blue | |
| 3. Sulphur | (1) S+O2 = SO2 (2) SO2 + H2 O = H2SO3 |
red | |
| 4. Phosphorus | (1) 4P + 5O2 = 2P2 O5 (2) P2O5+3H2O= 2H3PO4 |
red | |
| 5. Carbon | (1) C +O2 = CO2 (2) CO2 +H2 O = H2 CO3 |
red |
Question 4. What is Arrhenius’s concept of acid?
Answer:
Arrhenius’s concept of acid: According to Arrhenius, a Swedish scientist, an acid is a chemical compound that dissociates in water, producing H+ ions (cations) as the only Positive ions. H* ion is called proton. So, an acid is known as a proton donor. But H+ (proton) does not remain in a free state in solution; it attains stability being attached to a molecule of the solvent. In water solution proton combines with a water molecule to produce hydronium ion H3O+.
Examples : HCl + H2O \(\rightleftharpoons\)H3O+ + CI–
HNO3 + H2O \(\rightleftharpoons\) H3O+ + NO3–
Question 5. All acids are hydrogen compounds but all hydrogen compounds are not acids. Explain.
Answer:
Explanation: It is to be noted that any acid contains hydrogen but any compound containing hydrogen is not an acid. A compound containing one or more than one hydrogen atom in its molecule will be termed as acid only when its hydrogen atom or atoms are replaceable by metal and the product thus obtained must be a salt.
Example: None of the four hydrogen atoms of methane (CH4) can be replaced by a metal. Metals like sodium, and potassium displace hydrogen from water (H2O) but the product obtained in each case is not a salt. So, methane and water, though they contain hydrogen atoms in their molecules, are not acids.
Question 6. What are the important properties of acids?
Answer:
Important properties of acids :
(1) Taste: Generally, the aqueous solutions of acids have a sour taste.
(2) Colour changes of certain substances called indicators: Acids turn blue litmus solutions red. :
(3) In aqueous solution, the acids conduct electricity.
Wbbse Madhyamik Physical Science And Environment Class 9
(4) Reactions with metals: The solutions of acids in water react with many metals like zinc, magnesium, iron, etc. which are more electropositive than hydrogen with the liberation of hydrogen gas and formation of corresponding salts.
(5) Reactions with oxides and hydroxides of metals: Acids react with metallic oxides or hydroxides producing salts and water.
(6) Reactions with carbonates and bicarbonates: Acids are also characterised by their tendency to react with metal carbonates and bicarbonates evolving carbon dioxide.
(7) According to Arrhenius’s theory of electrolytic dissociation, the acids in aqueous solutions produce hydrogen ions as the positive ions or cations.
WBBSE Class 9 Physical Science chapter acids bases and salts solutions
Question 7. What is Arrhenius’s concept of base?
Answer:
Arrhenius’s concept of base: According to Arrhenius, a base is a chemical compound which produces OH- ions (hydroxyl ions) as the only anions when these are dissolved in water.
Examples : CaO + H2O \(\rightleftharpoons\) Ca(OH)2 ; Ca(OH)2 \(\rightleftharpoons\) Ca2+ + 2OH–
NaOH = Na+ + OH–
Wbbse Madhyamik Physical Science And Environment Class 9 Question 8.
Discuss the role of indicators in the case of titration.
Answer:
Uses of indicators in neutralisation reaction: Indicators which can determine the end-point of the neutralisation reaction are given below
| Indicator | Colour changes in | ||
| Neutral Solution | Acidic Solution | Alkaline Solution | |
| 1. Litmus | Violet | Red | Blue |
| 2. Methyl-orange | Orange | Red | Yellow |
| 3. Phenolphthalein | Colourless | Colourless | Pink |
The selection of a suitable indicator to determine the correct end-point of a reaction :
(1) Strong acid and weak base: Methyl orange
(2) Weak acid and strong base: Phenolphthalein
(3) Strong acid and strong base: Any indicator
(4) Weak acid and weak base: No suitable indicator.
Question 9. State the properties of acids and bases about indicators.
Answer:
Properties of acids about indicators: The aqueous solution of acids turn :
(1) Blue litmus to red
(2) Orange-coloured methyl orange to pinkish red
(3) Phenolphthalein remains colourless in aqueous solutions of an acid.
Properties of bases about indicators: The aqueous solution of bases turn:
(1) Red litmus to blue
(2) Orange-coloured methyl! orange to yellow
(3) Colourless. phenolphthalein solution to pink.
Question 10. What is the basicity of an acid and what is the acidity of a base?
Answer:
Basicity of an acid: Basicity of an acid is expressed by the number of replaceable hydrogen atoms present in each molecule of the acid.
Example :
Monobasic acid : (i.e., basicity is 1): HCl, HNO3
Dibasic acid (i.e., basicity is 2): H2 SO4, H2 CO3.
Tribasic acid : (i.e., basicity is 3): In H3PO4
Acidity of a base: Acidity of a base is expressed by the number of hydroxyl groups present in each molecule of the base.
Example :
Monoacidic base : NaOH, KOH.
Diacidic base : Ca(OH)2 , Mg(OH)2
Triacidic base : Al(OH)3.
Question 11. All alkalis are bases but all bases are not alkalis —Explain.
Answer:
All alkalis are bases but all bases are not alkalis
Explanation: Bases are the oxides or hydroxides of metals and react with acids to Produce salts and water. The bases which are soluble in water are known as alkalis. Ferric hydroxide Fe(OH)3, Zinc hydroxide Zn(OH)2, Aluminium hydroxide Al(OH)3, Sodium hydroxide NaOH, and Potassium hydroxide KOH all are bases but not alkalis. Among them only water-soluble metallic hydroxides NaOH, and KOH are alkalis. From the above examples of bases and alkalis, it is clear that all alkalis are bases but all bases are not alkalis.
Question 12. What are the properties of acids?
Answer:
Properties of acids :
(1) An acid produces H+ ion in aqueous solution.
(2) Generally acids are water-soluble and the solution has an acidic or sour taste.
(3) The aqueous solution of acids turns blue litmus to red, orange-coloured methyl orange to pinkish red and phenolphthalein remains colourless in the aqueous solution of an acid.
(4) Electropositive metals like Zn, Mg, and Fe (which belong above hydrogen in the electrochemical series) react with dilute acid to produce hydrogen.
Example : Zn + H2SO4 = ZnSO4 + H2 ↑
2Al + 6HCl = 2AICl3 + 3H2↑
(5) Acids form salt and water reacting with metallic oxides and hydroxides.
Example : 2NaOH + H2SO4 = H2SO4 + 2H2O
CaO + 2HCI = CaCl + H2O
(6) Acids form carbon dioxide by reacting with metallic carbonate and bicarbonate.
Example : Na2CO3 + 2HCI = 2NaCl + CO2 + H2O
NaHCO + HCI = NaCl + CO2 + H2O
Question 13. Classify and define the different types of oxides.
Answer:
Types of Oxides :
(1) Acidic oxide: An acidic oxide is an oxide which reacts with a base to form salt and water.
Non-metallic oxide : CO2 , SO2 , P2O5 , SiO2, etc.
Metallic oxide : CrO3 (Chromium trioxide), Mn2O7 (Manganese heptoxide), etc.
(2) Basic oxide: A basic oxide is an oxide which reacts with an acid to form salt and water. These are generally the oxides of metals.
Examples: CaO (Calcium oxide), MgO (magnesium oxide), FeO, CuO, etc.
(3) Neutral oxide: The oxides which neither react with acid nor with base are called neutral oxide.
Examples: CO (Carbon monoxide), H2O (Water), N2O (Nitrous oxide), NO (Nitric oxide), etc.
(4) Amphoteric oxide: The oxides which react with both acids and bases to produce salt and water are called amphoteric oxide.
Examples: Al2O3(Aluminium oxide), ZnO (Zinc oxide), SnO (Tanous oxide), PbO (Lead monoxide), etc.
(5) Peroxide: The oxides which react with a cold and dilute mineral acid to produce hydrogen peroxide and have peroxy linkage (— O — O -) are called peroxide.
Examples: Na2O2 (Sodium peroxide), BaO, (Barium peroxide), etc.
(6) Mixed oxide: An oxide which is formed by oxides of more than one oxide of a metal having variable valency is called mixed oxide.
Examples : Fe3 O4 [FeO + Fe2O3 ]; Mn3 O4 [2MnO + MnO2] ; Pb3 O4 [PbO2 + 2PbO], etc.
(7) Poly-oxide: The oxides which have oxygen atoms more than ordinary oxide and also do not react with an acid to produce hydrogen peroxide are called poly-oxides.
Examples: Mn2O7 (Manganese heptoxide), PbO2 (Lead dioxide), etc.
(8) Sub-oxide: An oxide which has fewer oxygen atoms rather than its oxygen atoms permitted for oxidation state: of the element is called sub-oxide.
Example: Carbon sub-oxide (C3O2).
(9) Super-oxide: Metals like Na, Li, and Ca form super-oxide and have negative ions (O — O) in them.
Examples : LiO2, KO2, etc.
Question 14. What are the properties of bases?
Answer:
Properties of bases :
(1) The aqueous solution of bases changes the colour of litmus from red to blue, and the orange methyl orange to yellow.
(2) Reacting with acid, bases produce salt and water.
(3) Concentrated solutions of some strong bases are slippery to the touch.
(4) The aqueous solution of bases conducts electricity.
(5) Strong bases when heated with electropositive elements (like Al, Zn, etc.) produce hydrogen.
Example : 2NaOH + Al + 2H2O = 2NaAlO2 + 3H2↑
Strong bases absorb carbon dioxide from the atmosphere to form carbonate salt and water.
Example : 2NaOH + CO2 = Na2 CO3 + H2O.
Question 15. Classify and define the different types of bases.
Answer:
Types of bases according to the acidity of bases :
(1) Monoacidic bases: These bases which produce one OH- ion in aqueous solution from one molecule are Called monoacidic bases.
Example : NaOH \(\rightleftharpoons\) Na+ + OH– ; KOH \(\rightleftharpoons\) K+ + OH–
(2) Diacidic bases: The bases which produce two OH- ions in aqueous solution from one molecule are called diacidic bases.
Example : Ca(OH)2\(\rightleftharpoons\) Ca2++ 2OH–
Types of bases according to their ionisation :
(1) Strong bases: The bases which ionise mostly in an aqueous solution and a small number of molecules exist in a molecular state are called strong bases.
Examples: NaOH, KOH, Ca(OH)2, etc.
Types of bases according to their source :
(1) Mineral bases: The bases which are produced from minerals are called mineral bases.
Examples: NaOH, KOH, etc.
(2) Organic bases: The bases containing nitrogen atoms and whose sources are animals and plants are called bases.
Example:
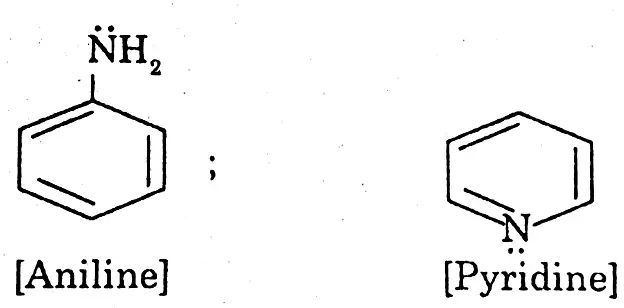
Properties of acids bases and salts Class 9 WBBSE notes
Question 16. Classify salt and define its different types.
Answer:
Types of salts :
(1) Normal salts: Normal salts are formed by the complete replacement of the hydrogen atoms in acid by metals or basic radicals.
Examples :
(1) NaCl [NaOH + HCI = NaCl + H2O].
(2) Na2SO4 [2NaOH + H2SO4 = Na2SO4 + H2O].
(2) Acid salt: An acid salt is formed only by partial replacement of hydrogen atoms in a molecule of an acid (di-basic, tri-basic) by metal or basic radical.
Examples :
(1) NaHSO4 (Sodium bisulphate).
(2) NaHCO3 (Sodium bicarbonate).
(3) Na2HPO4 (Di-sodium hydrogen phosphate).
(3) Basic salt: When the hydroxyl (OH–) or, oxide (O) radicals are partially neutralised by acid then basic salts are produced.
Examples :
(1) Pb(OH)CI (Basic lead chloride).
(2) Pb(OH)NO3 (Basic lead nitrate).
(4) Double salt: When two normal salts are mixed with their molecular weight ratio to form a solution and cooled the solution to make a combined crystal which is stable in solid state but ionises into different constituent ions in solution, the salts are called double salts.
Examples : K2SO4.Al2(SO4)3 .24H2O
(NH4)2 SO.FeSO4.6H2O
(5) Complex salts: When the solution of two mixed salts is concentrated and cooled then crystals of a new salt are produced. The constituent salts lose their identity in new salt. In an aqueous solution,, they produce new complex ions. These types of salts are called complex salts.
Examples : K4(Fe(CN6) , (Cu(NH3)4) SO4 .
Question 17. Write a short note on vanishing colours.
Answer:
Vanishing colours: Vanishing colour is a coloured solution which becomes colourless on exposure to air for a length of time.
Example: The aqueous solution of ammonium hydroxide (NH4OH) mixed with phenolphthalein turns pink in colour. Now if this solution is allowed to spray on cloth, all at once a pink colour is developed upon cloth. But since ammonia in NH4OH is highly volatile, after the lapse of time, NH3 volatilises and the solution becomes neutral. As a result, the pink colour of Phenolphthalein vanishes. Vanishing colour cannot be prepared with dilute NaOH solution, because NaOH does not evaporate.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 18. Write about the importance of pH.
Answer:
Importance of pH:
| Field | Utility |
| (1) Biotechnology | Biochemical and organic reactions at controlled pH value give best results. |
| (2) Agriculture | Alkaline soils are preferred for some crops such as citrus fruits grow better in alkaline soils. Sugarcane grows better in neutral soil, whereas rice. The pH of soil is tested before growing a particular crop. |
| (3) Medicine | Determination of pH value of blood and urine helps to diagnose certain diseases. |
| (4) Cosmetics | Special soaps, shampoos, face creams are prepared for consumers having different pH value of skin secretions. |
| (5) Milk plants | pH of milk is rigorously controlled at pH 6.8, otherwise, it turns sour. |
| (6) Breweries | Controlling pH value of wine to obtain a desired flavour. |
Question 19. Write a short note on the chemical properties of sulphuric acid.
Answer:
Chemical Properties of sulphuric acid :
(1) Acid Property: Sulphuric acid is a strong dibasic acid. In its aqueous solution, it is completely ionised. Its ionisation takes place in two steps
H2SO4 \(\rightleftharpoons\) H+ + SHO–4 ; H2SO4 \(\rightleftharpoons\) 2H + SO2-4
Its aqueous solution turns blue litmus red and reacts with bases and alkalis to produce salts and water.
(1) At ordinary temperature Sulphuric acid reacts with carbonates and bicarbonates with the formation of sulphates or bisulphates liberating carbon dioxide.
NaHCO3 + H2SO4→NaHSO4 + CO2 + H2O ; Na2CO3 + H2SO4→Na2SO4 + CO2 + H2O
(2) Since sulphuric acid has two replaceable hydrogen atoms, it forms two series of salts — normal salt (sulphates) and acid salts (bisulphates).
MgO + H2SO4→MgSO4 + H2O CaO + 2H2SO4→Ca(HSO4)2 + 2H2O
NaOH + H2SO4→NaHSO4+ H2O 2NaOH + H2SO4→ Na2SO4 + 2H2O
(3) Reaction with metals :
With zine :
(1) At ordinary temperature, dilute H2SO4 reacts with zinc to produce zinc sulphate and hydrogen gas. Zn + H2SO4→ ZnSO4 + H2
(2) Hot, concentrated H2SO4 reacts with zinc to produce zinc sulphate, water and sulphur dioxide gas. Zn + 2H2SO4→ZnSO4 + SO4 + 2H2O
With magnesium :
(1) At ordinary temperature, dilute H2SO4 reacts with magnesium to produce magnesium sulphate and hydrogen gas. Mg + H2SO→ MgSO4+ H2↑
(2) Hot, concentrated H2SO4 reacts with magnesium to produce magnesium sulphate, water and sulphur dioxide gas. Mg + 2H2SO4 →MgSO4 + SO2 + 2H2O
With copper :
(1) Cold, dilute H2SO4 does not react with copper.
(2) Hot, concentrated H2SO4 reacts with copper to produce copper sulphate, water and sulphur dioxide gas. Cu + 2H2SO4 → CuSO4 + SO2+ 2H2O
(2) Test of sulphuring acid: When barium chloride solution is added to dilute or concentrated H2SO4, a white precipitate of barium sulphate is formed which is insoluble in
HCl3. H2SO4 + BaCl2→ BaSO4 ↓+ 2HCI.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 20. Write a short note on the chemical properties of hydrochloric acid.
Answer:
Chemical Properties of hydrochloric acid :
(1) Acid Property: Hydrogen chloride in the gaseous state is not acidic, but the aqueous solution of the gas is highly acidic and one of the strongest acids. Hydrochloric acid is < monobasic acid and turns blue litmus red. It ionises almost completely in its aqueous solution.
HCl\(\rightleftharpoons\) H++Cl–
(1) Action on bases: Hydrochloric acid reacts with metallic oxides and hydroxide forming the corresponding chlorides and water.
CaO + 2HCI → CaCl2+H2O NaOH + HCI→ NaCl + H2O
MgO + 2HCIl → MgCl2 + H2O Fe(OH)3 + 3HCl →FeCl3 + 3H2O.
(2) Action on metallic carbonates and bicarbonates: Hydrochloric acid reacts with metallic carbonates and bicarbonates with the evolution of CO2.
Na2CO3+ 2HCI 2NaCl + CO2 + H2O ; NaHCO3 + HCl→ NaCl + CO2+ H2O
(3) Action on metals: The metals which are above hydrogen in the electrochemical series (Mg, Zn, Al, Fe, Sn, etc.) react with hydrochloric acid at ordinary temperature wit! the evolution of hydrogen and the formation of corresponding chloride.
Zn + 2HCI ZnCl2 + H2 ; Fe + 2HCl→ FeCl2 + H2 ; 2Al + 6HCl →2AICI3 + 3H2
Copper dissolves slowly on boiling with concentrated HCI in the presence of air or oxygen producing cupric chloride and water. 2Cu + 4HCl + O2 → 2CuCl2 + 2H2O
Question 21. Write a short note on the chemical properties of nitric acid.
Answer:
Chemical properties of nitric acid
Nitric acid is a strong monobasic acid. lt ionises almost completely from hydrogen ions and nitrate ions. HNO3 \(\rightleftharpoons\) H++ NO–
As nitric acid is a monobasic acid, it forms normal salts only. It turns blue litmus red.
(1) Action on alkalis: It reacts with oxides and hydroxides producing nitrate salts and water.
CaO + 2HNO3 →Ca(NO3)2+ H2O ; MgO + 2HNO3→Mg(NO3)2+ H2O
NaOH + HNO3 → NaNO3 + CO2 + H2O
(2) Action on metallic carbonates and bicarbonates: Nitric acid reacts with metallic carbonates and bicarbonates producing nitrates and liberating CO2.
Na2CO3 + 2HCl→ 2NaCl + CO2 + H2O ; NaHCO3+ HC] → NaCl + CO2 + H2O
(3) Action on metals: The metals which are above hydrogen in the electrochemical series (Mg, Zn, Al, Fe, Sn, etc.) react with hydrochloric acid at ordinary temperature with the evolution of hydrogen and the formation of corresponding chloride.
Zn + 2HCl —> ZnCl2 + H2 ; Fe + 2HCI → FeCl2 + H2 ; 2Al + 6HCl → 2AlCl3 + 2H2O
(4) Test of hydrochloric acid: An aqueous solution of HCl gives a curdy white precipitate of silver chloride with silver nitrate solution. The precipitate is insoluble in nitric acid but is
readily soluble more than the ammonium hydroxide solution. AgNO3 + HCl → AgCl + HNO3 .
Wbbse Madhyamik Physical Science And Environment Class 9 Question 22. Write a short note on the chemical properties of sodium hydroxide.
Answer:
Sodium hydroxide is popularly known as Caustic Soda.
Physical properties :
(1) NaOH has a sharp, bitter taste.
(2) It changes the colour of indicators turns red litmus to blue and turns colourless phenolphthalein to pink.
(3) It is a soapy substance, i.e., it is slippery to touch.
(4) NaQH is a strong electrolyte.
To note that: NaOH has a mild corrosive action which causes slight alkali burn on skin.
Chemical properties
(1) NaOH absorbs carbon dioxide from the air to form carbonates.
CO2+ 2NaOH→ Na2CO3 + H2O
(2) It produces ammonia gas when warmed with ammonium salt.
NH4Cl + NaOH → NaCl+ H2O + NH3 (g)
(3) NaOH, being an alkali or base, neutralizes acids to form salt and water.
NaOH + HCI → NaCl + H2O ; NaOH = HNO3→NaNO3 + H2O
2NaOH + H2SO4→ Na2SO4 + 2H2O
(4) Action on metals :
(1) With Aluminium: When powdered aluminium is heated with concentrated solution 4of NaOH, sodium aluminate and hydrogen gas are produced.
2Al + 2NaOH + 2H2O → 2NaAlO2+ 2H2
(2) With Zinc: When powdered zinc is heated with a solution of NaOH, sodium zincates 2nd hydrogen gas are produced.
Zn =2NaOH → Na2ZnO2 +H2
Question 23. How does acidity affect human life?
Answer:
(1) Decay of teeth-enamel: It has been inferred by researchers that in areas where drinking water has fluoride levels of 1 ppm (part per million) or above, children have 60% fewer cavities than do children living in areas with lesser fluoride concentrations. Dentists often suggest fluoride treatments and prescribe toothpaste containing fluoride. The enamel on teeth (and the bones of the body) are made of a compound called “hydroxyapatite [Ca10(PO4 )6(OH)2]. This is basic and it reacts with acids and thus decays.
Fluorides present in the body produce fluorapatite [Ca10(PO4 )6F2], which is more resistant to decay. Tooth cavities are caused when acids dissolve tooth enamel.
Ca10(PO4 )6(OH)2 + 8H+ (aq) → 10Ca2+ (aq) + 6HPO4 2-, (aq) + 2H2O (l)
(2) antacids: Drugs for stomach upset: Digestion is accompanied by the production of hydrochloric acid (HCI) in the stomach. Sometimes the stomach produces too much HCl. This may be due to emotional stress or overeating. Stomach upset and heartburn are the result. Antacids are the solution or care for such a problem.
Functioning of an antacid: The various antacid medications work by neutralizing the excess acid. The reaction is simply an acid-base reaction :
OH– +H+ H2O [for antacids that contain OH– ions]
CO32- + 2H+ → H2O+ CO2 [for antacides containing carbonate ions]
Mangesium hydroxide or magnesium carbonate are such compounds used in antacid.
Mg(OH)2 + 2HCI MgCl2+ 2H2O ; MgCO3 + 2HCI MgCl2 + H2O + CO2
These two compounds, in small doses, act as antacids and in large doses, they act as laxatives.
(3)In fishery agriculture:
(1) Acidification of inland waters induced by acid rain or other acidic pollutants causes various harmful physiological and behavioural effects on fish.
(2) At pH ≤ 5 it induces failure of immune and reproductive functions.
(3) pH around 4 efflux of Na+ and Cl– ions leading to mortality.
(4) Many populations of fish have vanished particularly due to the damage to aquatic ecosystems by acid rain.
Question 24. Write a short note on pH and soil quantity.
Answer:
pH and soil quality :
(1) pH of soil is the masier variable as it controls many chemical processes that take place. It specifically affects plant nutrients. The optimum pH range for most plants is 5.5→ 7.0.
(2) Acidity in soils comes from H+ and Al 3+ ions, which at pH = 4 →6, reacts with water to form Al(OH)3 and releases 3H+ ions.
(3) Many other processes may contibute to the formation of acid soils including rainfall, fertilizer use, plant root activity, acid rains and mine spoilings.
(4) Alkaline soils are characterized by the presence of carbonates.
The effects :
(1) Below pH = 4, H+ ions themselves damage root cell membranes.
(2) Al3+ and Mn2+ are more soluble at lower pH and dissolved Al3+is toxic to plants.
(3) If pH > 7.5 (alkaline), phosphorus (P) starts forming insoluble compounds with calcium (Ca).
Note that: The common and easy way to increase soil pH is to add lime (CaCO3 or MgCO3 ), wood ash or burnt lime CaO: CO2-3 + 2H+→ CO2 +H2O
Question 25. Write a short note on acid rain.
Answer:
Acid rain
Under normal conditions, rain is slightly acidic, with a pH = 5.6, due to dissolved CO2 ie., H2CO3. Recently, the acidity of rainwater, in many industrialized and populated areas, has increased to more than 100 times, to a PH 3 to 3.5. The major pollutants in acid rain are strong acids that arise from human activities, particularly automotive activities.
(2) The Chemistry behind acid rain In ‘the automobile internal combustion engines and electrical power stations NO2 is formed and gets further oxidized in air to form which in turn, forms nitric acid (and nitric oxide)
N2(g) + O2 (g) → 2NO (g) [at high temperature]
2NO (g) + O2 (g) → 2NO2 (g)
3NO2 (g) + 3H2O (l) → 2H3 O+(aq) + 2NO–3 (aq) + NO (g)
Sulphur dioxide (SO2) is produced as a by-product of the burning of fossil fuels. It reacts with water directly forming sulphurous acid (a weak acid) but produces sulphur trioxide (SO3) in the presence of particulate matter and aerosols. This SO3 combines with water forming sulphuric acid (H2SO4) (a strong acid)
SO2 (g) + 2H2O (l)→H2O (l) + H2SO4
2SO2(g) + O2 (g) → 2SO3 (g)
SO3 (g) + 2H2O (l) → H2O + H2SO4 (or H3O+ + HSO–4).
The effect of acid rain: In many processes, Nature likes and requires a balance of pH. Any drastic shift in the pH of rain, thus, is bound to affect it in many ways.
(1) Lakes may become so acidic that all fish life may disappear.
(2) Massive trees die off as acid rain has lowered the pH of the soil and has leached nutrients from leaves.
(3) Valuable and heritage marble statues have been slowly dissolved away as the calcium carbonate of marble has been attacked by acid rain :
CaCO3(s) + 2H + (aq) Ca2+ (aq) + H2O (l) + CO2 (g)
The problem will grow more serious as sources of low-sulphur coal are nearly exhausted and power plants are forced to rely on more abundant sources of high-sulphur coal.
What to do :
(1) Much more work on methods to control acidic emissions is to be done.
(2) To reduce and optimize the consumption of fossil fuel, and the consumption of energy in general.
(3) To change our attitude from ‘mastering’ nature (and thus exploiting and destroying to the “sustainable coexistence” with mother nature.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 26. Write a short note on neutralisation.
Answer:
Wherever acids and bases combine in the ratio of their equivalent weights, Salts and water are formed quantitatively. The resulting solution is neutral and this type of reaction is called neutralisation. Both acids and bases lose their identities completely. According to logic theory, neutralisation amounts to the formation of undissociated weak electrolyte H2O by thé interaction of H* ions of the acid and OH– ions of the alkalis.
HCI \(\rightleftharpoons\) H+ + Cl–
NaOH \(\rightleftharpoons\) Na+ + OH–
HCI + NaOH \(\rightleftharpoons\) Na+ + Cl– + H2 O
It is to be remembered that all indicators cannot be used for all types of acid-base titration. The choice of an indicator depends on the nature of acid and base.
Selection of Indicators :
| Titration of | Indicator to be used | Examples of neutralisation | |
| Acid | Base | ||
| 1. Strong | Strong | Any indicator | HCl + NaOH |
| 2. Strong | Weak | Methyl orange Methyl red |
HCI + NH4OH HCl+Na2CO3 |
| 3. Weak | Strong | Phenolphthalein | CH3COOH + NaOH |
| 4. Weak | Weak | No suitable indicator | CH3COOH + NH4OH |
Question 27. Write a short note on antacid.
Answer:
Antacid: Our stomach normally produces acids to help to acid in disgesting food and to kill germs. The pH of our stomach juice is 2-3. Secretion of hydrochloric acid increases when we consume too much of spicy food. Then we suffer from acidity, gas, etc.
Antacids work by counteracting (neutralising) the acid in our stomach. They do this because the chemicals in antacids are bases (alkalis). This neutralization reduces the acidity of the stomach juice. Antacids are the alkaline substances which can reduce the excess acid of stomach. They can maintain the proper pH of the stomach juice.
Some common antacids which are available in the market are gelusil, Digene, polyol, milk of magnesia, etc. All these antacids contain aluminium hydroxide and magnesium hydroxide. When we consume antacids, these alkaline substances react with the hydrochloric acid secreted in the stomach. Thus the acidity is reduced.
Nowadays, zinetac, acidic, pan-40, etc. are sold in the market as antacids. These compounds are to be taken in empty stomach. Actually these compounds can control the acid secretion from stomach.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 28. How do acids and bases react?
Answer:
According to Arrhenius’s theory, in an aqueous solution acids and bases produce H+ and OH– ions respecitvely. When acid-base are mixed together in aqueous solution, they produce water molecules.
H+ (aq) + OH– (aq) H2 O (l)
The other parts of the acid and base combine to give salt.
Ca(OH)2 + H2 SO4 → CaSO4 + 2H2O
(Base) + ( Acid ) → (Salt ) + (water)
2H+ ions form H2SO4 and 2OH– ions from Ca(OH)2 form 2H2O molecules. The Ca2+ ion and SO42- ions from the salt CaSO4. So acids and bases react to give salt and wate (some bases react with acids, given only salt no water is formed). e.g. 2NH3 + H2SO4= (NH4)2 SO4.
Question 29. The idea of nascent hydrogen (or oxygen) is at present considered unnecessary. Justify the statement.
Answer:
The idea of nascent hydrogen (or oxygen) is at present considered unnecessary.
The nascent state of an element is that state just at the time of its production from a compound. So, hydrogen just at the time of its production from its compound is called the nascent hydrogen.
The idea of nascent hydrogen and nascent oxygen as reducing and oxidising agents respectively at present is unnecessary, because the actual reducing agents produce hydrogen in acidic condition. These hydrogen-producing substances are stronger
reducing agents than hydrogen. On the other hand, oxidising agents are not producing
nascent oxygen, they are only oxiding agents, e.g.
(reduced)
Zn + 2FeCl3 (yellow) FeCl2(colourless) + ZnCl2
(oxidised)
Zn is a reducing agent which itself is oxidised and FeCl3 is an oxidising agent which itself is reduced. No participation of nascent hydrogen is required in the reduction process.
(oxidised)
Zn + 4HNO3 → Zn(NO3)2+ 2NO2↑+ 2H2O
(reduced)
In this reaction the idea of nascent oxygen as the oxidising agent is baseless. Here HNO3 itself is an oxidising agent, which is itself reduced to nitrogen dioxide (NO2) and oxidises zinc. Here zinc is a reducing agent.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 30.
Write the limitations of Arrhenius’s concept.
Answer:
Arrhenius’s concept has the following limitations :
(1) It recognises the dissociation of acids and acid-base behaviour in aqueous medium only. It cannot explain the acid-base behaviour in the absence of water and in non-aqueous medium.
(2) Arrhenius concept restricts acids to merely hydrogen-containing compounds and bases to merely hydroxyl-containing compounds. The substances such as CO2, SO2, SO3, etc. are not regarded as acids and the substances such as NH3, Na2O, CaO, MgO, and Na2CO3 are not regarded as bases though they exhibit properties of acids and bases, respectively.
CaO (s) + SO3(g) →CaSO3(s)
(Base) + (Acid)
NH3(g) + HCI (aq)→ NH4C (s) .
(Base) + (Acid)
Question 31. Write a short noie on indicator.
Answer:
Indicator: Indicators are complex.organic compounds which undergo a change of colour with a change of acidic, neutral or basic medium.
Litmus (a dye from plants), methyl orance (organic pigment)and phenolphthalein (an organic acid)are three commonly used indicators to determine the acidic or basic nature of a solution by a characteristic in colour. An indicator is a substance which change as to a characteristic colour in the presence of a particular concentration of ions such as H+ and OH– ions.
Some useful indicators
| Indicator | Colour in acid solution | Colour in alkaline solution | Colour in neutral soloution |
| Litmus | red | blue | purple |
| Phenolphthalein | colourless | pinkish red | colourless |
| Methyl orange | Pink | yellow | orange |
| Methyl red | red | yellow | light |
When litmus is dissolved in distilled water which is neither acidic nor basic; the colour of litmus solution is purple. In acidic medium the colour of litmus solution is red and in basic medium the colour of litmus solution is blue. Litmus solution is called an acid-base indicator. Examples of other acid-base indicators are phenolphthalein, methyl orange, and methyl red. The natural acid-base indicator is turmeric powder, which is used in cooking vegetables in almost all Indian homes. Its colour in acidic solution is yellow and in basic solution is brown.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 32. Write the chemical reactions of H2SO4, HCI & HNO, with carbonate and bicarbonate compounds.
Answer:
Chemical reactions of H2SO4, HCl & HNO3, with carbonate and bicarbonate compounds :
All mineral acids like H2SO4, HCl and HNO3, in reaction with metallic carbonates and bicarbonates, give corresponding metallic sulphate. metallic chloride or metallic nitrate, along with carbon dioxide. The gas (CO2) is liberated with brisk effervescence.
(1) Metallic carbonate + Hydrochloric acid Metallic chloride + water + carbon dioxide
Metallic carbonate + Sulphuric acid → Metallic sulphate + water + carbon dioxide
Metallic carbonate + Nitric acid → Metallic nitrate + water + carbon dioxide
Examples :
CaCO3 + 2HCI = CaCl2 + H2O + CO2
Na2CO3 + H2SO4 = Na2SO4 + H2O + CO2
K2CO3 + 2HNO3 = 2KNO3 + H2O + CO2
(2) Metallic bicarbonate + Hydrochloric acid —> Metallic chloride + water + carbon dioxide Metallic bicarbonate + sulphuric acid —> Metallic sulphate + water + carbon dioxide
Metallic bicarbonate + nitric acid —> Metallic nitrate + water + carbon dioxide
Example :
Ca(HCO3)2 + 2HCI = CaCl2 + H2O + CO2
2NaHCO + H2SO4= Na2SO4 +H2O + CO2
KHCO3 + HNO3 = KNO3+ H2O+CO2
Best study material for acids bases and salts WBBSE Class 9
Question 33. Write the chemical reaction of H2 SO4, HCl and HNO3 with alkali or base.
Answer:
Chemical reaction of H2 SO4, HCl and HNO3 with alkali or base
The chemical reaction of H2SO4, HCI and HN3 with alkali or base ” Bases are the metallic.oxides and alkalis are water-soluble hydroxides of metals. When acids like sulphuric acid (H2SO4), hydrochloric acid (HCI) and nitric acid (HNO3) react with these, they form salt and water. This is the main basic reaction for the neutralisation of acid or base.
Base + Sulphuric acid → Sulphate salt + water
Alkali + Sulphuric acid → Sulphate salt + water
Base + Hydrochloric acid → Chloride salt + water
Alkali + Hydrochloric acid → Chloride salt + water
Base + nitric acid → nitrate salt + water
Alkali + nitric acid → nitrate salt + water
Examples :
Na2O + H2SO4 = Na2SO4 + H2O
2NaOH + H2SO4 = Na2SO4 + H2O
[Sometime bisulphate salt can be formed by partial neutralisation of H2SO4,
NaOH + H2SO4 = NaHSO4+ H2O Sodium bisulphate]
CaO + 2HCI = CaCl2+ H2O
Ca(OH)2 + 2HCI = CaCl2+ 2H2O
MgO + 2HNO3 = Mg(NO3)2 + H2O
Mg(OH)2+ 2HNO3 = Mg(NO3)2 + 2H2O
Wbbse Madhyamik Physical Science And Environment Class 9
Question 34. Write the chemical reaction of H2SO4, HCI & HNO3 on metals.
Answer:
The chemical reaction of H2SO4, HCI & HNO3 with metals (like Cu, Zn, Mg): All the mineral acids like H2SO4(sulphuric acid), HCI (hydrochloric acid) and HNO, (nitric acid) react differently with the metals like Cu, Zn, Mg. Generally with H2SO4, they form sulphate salts, with HCI they form chloride salts and with HNO3 they form nitrate salts, under different conditions.
(1) With sulphuric acid :
(1) When copper metal is strongly heated with concentrated sulphuric acid, it forms blue-coloured copper sulphate, along with a gas having the pungent odour of burnt sulphur and water.
Cu + 2H2SO4= CuSO4+ SO2+ 2H2O
However copper does not react with cold and dilute sulphuric acid properly.
(2) When zinc granules are added to dilute sulphuric acid, it form hydrogen gas at room temperature.
Zn + H2SO4 = ZnSO4 +H2
(dilute)
H2 + H2SO4 = SO4+ H2O
(string)
Magnesium reacts with both dilute sulphuric acid, forming magnesium sulphate and hydrogen gas.
Mg + H2SO4 = MgSO4 + H2
WBBSE Class 9 indicators and pH scale solutions
(2) With hydrochloric acid: Dilute hydrochloric acid reacts with metals like zinc (Zn) and magnesium (Mg) forming corresponding metallic chlorides and liberating hydrogen gas, whereas concentrated hydrochloric acid reacts with copper in the presence of oxygen presence in the air, it forms cupric chloride and water. Here hydrogen gas is not produced.
Mg + 2HCI = MgCl2 + H2
Zn + 2HCI = ZnCl2 + H2
2Cu + 4HCI + O2 = 2CuCl2 + 2H2O
Copper does not react with dilute hydrochloric acid.
(3) With nitric acid: Very dilute nitric acid (1%) reacts with magnesium at low temperatures.
Mg + 2HNO3 = Mg(NO3)2 + H2
Strong or concentrated nitric acid in reaction with magnesium produces magnesium nitrate and nitric oxide.
3Mg + 8HNO3 = 3Mg (NO3)2 + 2NO + 4H2O
Zinc in reaction with dilute nitric acid at ordinary temperature forms zinc nitrate, nitric oxide (NO) and water.
Zn + 8HNO3 = 3Zn(NO3)2+ 2NO + 4H2O
When zinc reacts with concentrated nitric acid at high temperatures, it forms zinc, nitrate, nitrogen dioxide and water.
Zn + 4HNO3 = Zn(NO3 )2 + 2NO2 + 2H2O
Copper in reaction with dilute nitric acid at ordinary Temperature forms copper nitrate, nitric oxide (NO) and water.
3Cu + 8HNO3 = 3Cu(NO3 )2 + 2NO2 + 4H2O
Copper in reaction with concentrated nitric acid at high Gitte yey Temperature forms copper nitrate, nitrogen dioxide and water. of AgCl
Cu + 4HNO3= Cu(NO3)2 + 2NO2 + 2H2O
When nitric acid vapour is passed over red hot copper, copper immediately oxidises into cupric oxide (CuO) along with nitrogen gas and water vapour. This reaction proves the presence of nitrogen in nitric acid.
5Cu + 2HNO3 = 5CuO + N2+ H2O.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 35. Write about the identification test for HNO3, H2SO4 and HCl.
Answer:
Identification test for HNO3, H2SO4 and HCI (Wet Test) :
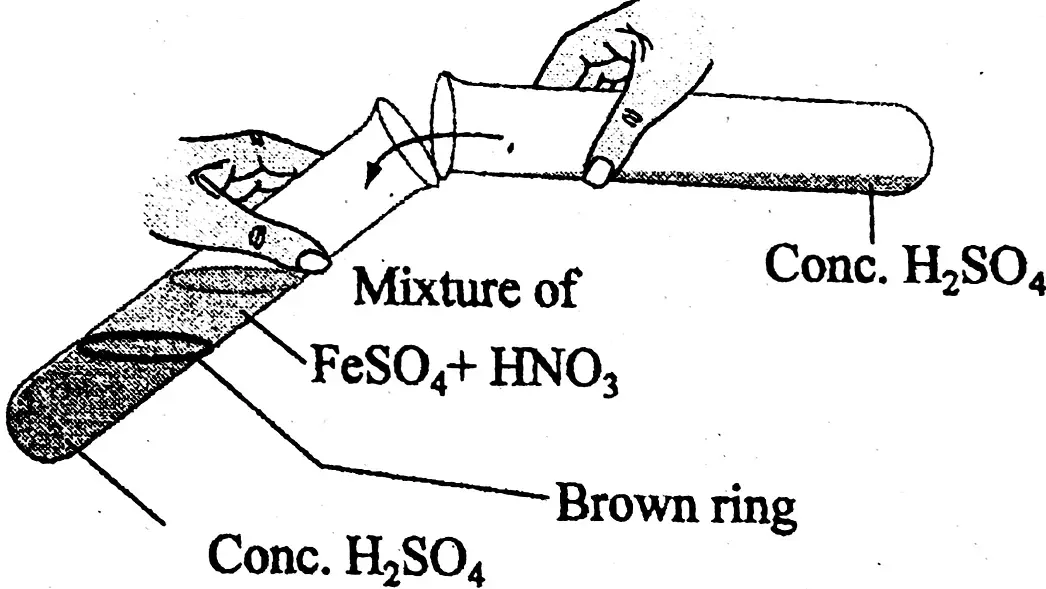
(1) Presence of nitric acid (HNO,) by ring test: A little amount of given dilute acid is taken in a test tube. Freshly prepared ferrous sulphate solution is added to it in same volume. Now concentrated sulphuric acid is taken in another test tube. Concentrated sulphuric acid taken in a tube is carefully poured into the liquid mixture of the first tube by the side of the test tube, so as to form a heavy layer at the bottom of the test tube. A brown ring is formed at the junction of the two liquids, which proves the presence of nitric acid in the test tube.
6FeSO4 + 2HNO3+ 3H2SO4= 3Fe2(SO4)3 + 4H2O + NO
FeSO4 + NO + 5H2O = Fe[(H2O)NO] SO4
(brown penta aqua nitroso ferrous sulphate)
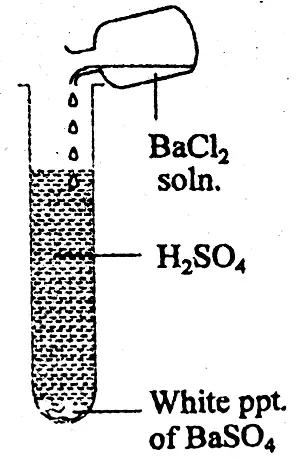
(2) Presence of sulphuric acid: In a test tube the given dilute solution of acid is taken. In that test tube, barium chloride solution is added. The test tube is well shaked. A white precipitate is formed at the bottom of the test tube which is insoluble in dilute HCI or dilute HNO3.
The precipitate is due to the formation of barium sulphate, which proves the presence of H2SO4.
BaCl2+ H2SO4 = BaSO4↓ + 2HCI
(white ppt )
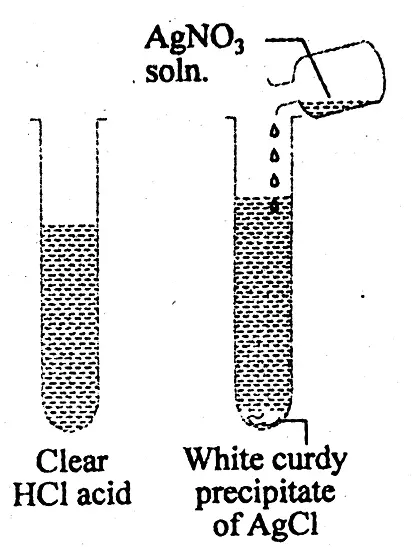
(3) Presence of Hydrochloric acid: In a test tube the given solution of acid is taken. In that test tube, silver nitrate solution is added. The test tube is well-shaken. A dense curdy white precipitate is formed at the bottom of the test tube which is insoluble in nitric acid but dissolves in ammonium hydroxide due to the formation of a complex salt, diamine silver chloride, which proves the presence of hydrochloric acid.
AgNO3 + HCI = AgCl + HNO3
( curdy white ppt)
AgCl + NH4OH = Ag(NH3)2 Cl + 2H2O
(diamine silver chloride)
Question 36. Write about the safe use of acids and alkalis.
Answer:
(1) A solution of hydrogen chloride is highly corrosive and blisters are caused on the skin by its touch.
(2) Pure and concentrated sulphuric acid is highly corrosive and burns the skin, turning it black due to the absorption of moisture from the lower layer of the skin.
(3) Dilute nitric acid reacts with the proteins of the skin and forms a yellow compound called xanthoproteic acid. Hence, the skin becomes yellow. Concentrated nitric acid causes blisters on the skin.
(4) Due to strong alkalinity, sodium hydroxide (NaOH) solution is soapy in touch and it is highly corrosive to the skin and makes blisters on the skin.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 37.
Write about the safety measures for acid & alkali burns.
Answer:
Safety measures for acid and alkali burns :
(1) In case of acid burns, wash the place with plenty of water, then wash with sodium bicarbonate and again wash with water.
(2) In case of alkali burns, wash the place with plenty of water; then with 1% per cent acetic acid and again with water. Finally, dry the skin and apply any medicated skin ointment.
(3) In case of eye accidents we proceed as follows :
(1) Acid in the eye: At once the eye must be washed with water a number of times and then with 1% sodium bicarbonate solution. Finally, add a dilute solution of any medicated eye drop.
(2) Alkali in the eye: At once the eye must be washed with water repeatedly and then it should be washed with 1% boric acid solution. Finally, add a dilute solution of any medicated eye drop or add a drop of liquid paraffin in the eye.
(3) If acid or alkali is swallowed: Immediately take plenty of drinking water to dilute it, followed by taking a dilute solution of milk of magnesia to destroy the effect of acid. Immediately take plenty of drinking water to dilute it, followed by lemon or orange juice to destroy the effect of alkali.
In all these cases a skilled medical attendant is necessary, which is very important in case of safety measures.
Question 38. Write about the importance of aqueous medium in the properties of acid and alkali.
Answer:
Importance of aqueous medium in the properties of acids and alkalis: According to the concept of Arrhenius, acids exhibit their acidic properties only in aqueous solution. In fact, in the absence of water or benzene and other non-aqueous solvents anhydrous HCl cannot turn blue litmus red. The anhydrous vapour of HNO3 cannot liberate CO2 from CaCO3. But such a reaction is a characteristic property of all acids. Aqueous solution of acids imparts hydronium (H3O+) ions.
Hydrogen chloride is a covalent compound. It does not ionise to form an H+ ion in a pure state. Since it does not give H+ ions, it is not acidic in a pure state. But in contact with water, HCI forms H3O+ and Cl– ions. The acid character of HCl is developed due to the formation of H3O+ ions in aqueous medium.
H2O+HCl → H3O+Cl–
Similarly, when a base like Na2O is dissolved in water it forms an alkali and in an aqueous medium, it generates OH– and shows alkalinity.
Na2O + H2O \(\rightleftharpoons\) 2NaOH \(\rightleftharpoons\) 2Na+ + 2OH–
So we observe that the aqueous medium plays an important role in case of acidity or alkalinity. Now, we carry out the test of dry baking soda (NaHCO3) and tartaric acid crystals. In a test tube dry NaHCO3 (or baking soda) and tartaric acid crystals are mixed and the mixture is kept in a test tube we observe that no; bubble of gas is evolved.
But when we add water in this mixture of test tube, i-e., in baking soda and tartaric acid, immediately bubbles of carbon dioxide gas come out. Here water is a liquid that behaves as a solvent, which separates the ions and molecules in the reaction.
At first, on dissolution in water, tartaric acid gives H+ ion which immediately reacts with NaHCO3, forming carbon dioxide gas and thus bubbles of CO2 comes out. But no bubble is evolved if a mixture of dry NaHCO3 and tartaric acid crystals is shaken with benzene or kerosene, which is no aqueous medium, solvents like kerosene, benzene can not dissolve ionic compounds. So, we observe, some reactions always occur in aqueous medium and water has an important role in case of acidity and alkalinity of those reactions.
Question 39. Write a short note on pH.
Answer:
pH
The acidity of a solution is expressed in terms of a parameter called pH, where pH stands for potenz of hydrogen (hydrogen power).
The quantity pH is defined as the logarithm of the reciprocal of hydrogen ion concentration (H+) and is equal to the logarithm of the hydrogen ion concentration with negative sign. Mathematically,
pH = − log10 [H+]
The scale indicating the hydrogen ion concentration of solutions in terms of pH values is called pH scale. The pH scale is expressed by a series of positive numbers between 0 and
14. The pH scale can be explained as a series of numbers which indicates the acidic or basic nature of a solution. Thus a neutral solution has the pH = 7, an acidic solution has a pH < 7 and an alkaline solution has a pH >7. The greater the hydrogen ion concentration, the smaller is the pH.
A mixture of several indicators which can show different colours at different concentrations on ‘hydrogen ions in a solution is called universal indicator. A paper impregnated with universal indicator which is used to measure the pH of a solution, is called pH paper.
The higher the concentration of H+ ions, the lower is the pH of a solution. For example, among two solutions A and B having pH 2 and_5 respectively, the solution A has higher hydrogen ion concentration.
Wbbse Madhyamik Physical Science And Environment Class 9
Question 40. Write about the acidity of water due to CO2, SO2 and NO2.
Answer:
Acidity of water due to CO2, SO2, NO2: When we final pH less than 5.1 in the rain water, that type of rain is called acid rain. The acidic gaseous oxides, such as SO2, NO2, and CO2 in the atmosphere cause acid rain. Such rainwater is acidic.
These oxides dissolve in rain water to form acids. Sometimes SO2 and NO2 undergo aerial oxidation catalysed by carbon particle or ozone gas to form SO3 & NO5 which form H2SO4 & HNO3. Carbon dioxide forms carbonic acid. These acids remain dissolved in rain water and so it becomes acidic. Due to the presence of CO2 in air, and other oxides like SO2 and NO2, these gases form acids with water by photochemical reaction in the atmosphere.
CO2 +H2O \(\rightleftharpoons\) H2CO3 \(\rightleftharpoons\)H+ +HCO–3
\(\mathrm{SO}_2+\frac{1}{2} \mathrm{O}_2 \frac{\text { carbon particles }}{\text { light }} \mathrm{SO}_3 \stackrel{\mathrm{H}_2 \mathrm{O}}{\longrightarrow} \mathrm{H}_2 \mathrm{SO}_4\)2NO2 + O3 → O2+ N2O5
N2O, + H2O→ 2HNO3
Acid rain affects plantation and agriculture by washing out the soil nutrients. It may even destory aquatic lives like fish. Exact pH value of acid rain is 5.1.
When acid rain reacts with limestone or slate, the following reaction shows the effect :
CaCO3 + H2SO4 = CaSO4 + CO2 + H2O
CaCO3 + 2HNO3 = Ca(NO3)2 + CO2 + H2O
Due to the presence of CaSO4 Ca(NO3)2, the marble stone of all historical monuments slowly becomes dull and the brightness of marble stone of historical monuments is slowly lost. The top layer of stone is corroded, small holes are created and bright layer of marble stone is totally lost. Such type of destruction of stone, due to air pollution, is called stone cancer.
Question 41. Write three points of the practical application of neutralisation.
Answer:
Practical Application of neutralisation :
(1) Farmers add slaked lime (calcium hydroxide) to reduce the acidity of soil.
(2) Persons suffering from acidity are given antacid tablets, containing magnesium hydroxide mainly, which neutralises excess HCI produced in stomach.
(3) Sting of ants and bees contains formic acid. This can be neutralised by rubbing soaps, which contains dissolved base.
Question 42. Explain the concept of antacid and classify it.
Answer:
Concept of antacid: The different foodstuffs which we eat are digested in the stomach where one of the important chemicals is hydrochloric acid having pH range 2 to 3. Sometimes due to the nature of food stuff and due to the different diseases, the production of this acid is uncontrolled and this excess secretion of acid produces disturbance in the
digestion and acidity is formed, which can produce blisters in the stomach in the long run.
So we can take antacid, which regulates. the acidity and pH level in the digestion. Generally antacids are taken after eating, as antacid does not decrease the production of acid in the stomach but controls the pH of the digestive system.
Antacids are of two types:
(1) systemic and
(2) non-systemic.
(1) Systemic antacids: They function in a regular manner. They are soluble in water and can be easily absorbed. But due to the absorption of this antacid the acid-base equilibirium of our body is disturbed.
Examples of: Such type is-sodium bicarbonate (NaHCO3), sodium citrate (Na3C6H5O7).
(2) Non-systemic antacids: They do not function in a regular manner. They are not soluble in water easily and they cannot be absorbed. Hence they do not disturb the acid base equilibrium of our body.
Examples of such type of antacids:
Magnesium compound—magnesium hydroxide
[Mg(OH)2], magnesium carbonate (MgCO3), aluminium compound—aluminium hydroxide
[Al(OH)3] and calcium compound-calcium carbonate (CaCO3).
The common composition of some antacids available in the market are:
(1) Magnesium trisilicate and aluminium hydroxide mixture.
(2) Aluminium hydroxide, magnesium hydroxide, and magnesium carbonate mixture.
(3) It is the gel of pure aluminium hydroxide, sodium carboxymethyl cellulose and magnesium hydroxide mixture.
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound
