Class 9 Physical Science Water Very Short Answer Type :
Question 1. What is softening of water?
Answer:
The process of removing hardness from water, either temporary hardness or permanent hardness, is called softening of water.
Question 2. On which basis water is called hard and soft?
Answer:
Water is classified in hard and soft according to its ability to form lather with soap.
Question 3. What type of water is used in the boiler?
Answer:
Soft water.
Question 4. Which gas is produced when calcium reacts with water?
Answer:
Hydrogen.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Question 5. Which one has a greater affinity towards water Zn or Hg?
Answer:
Zn
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 6. State two causes of the water crisis.
Answer:
Causes of water crisis :
(1) Misuse of water
(2) Water pollution.
Question 7. What are the products obtained from the reaction of sodium and water?
Answer:
Sodium hydroxide and hydrogen.
Question 8. What is the cause of black foot disease?
Answer:
Black foot disease is due to arsenic poison.
Question 9. What is the cause of fluorosis disease?
Answer:
Excess fluoride in water is responsible for fluorosis disease.
Question 10. Which metal reacts with water at ordinary temperature?
Answer:
Potassium.
Wbbse Physical Science And Environment Class 9 Solutions
Question 11. Give one cause of the water crisis.
Answer:
Wastage of water.
Question 12. What amount of potable water is present in the total water of the earth?
Answer:
0:3%.
Wbbse Madhyamik Class 9 Physical Science And Environment Question 13.
Which metalloid is the cause of water pollution?
Answer:
Arsenic (As).
Question 14. Whose chemical activity is greater between potassium and magnesium?
Answer:
Potassium has greater chemical activity than magnesium.
Question 15. In the activity series of the metals which one has the upper position? Al or Au.
Answer:
Al.
Question 16. What is the temporary hardness of water?
Answer:
The temporary hardness of water is caused by the presence of soluble bicarbonates of calcium, magnesium and iron in it.
Question 17. What is the cause of the hardness of water?
Answer:
This is due to dissolved bicarbonate, chloride, and sulphate salts of Ca, Mg and Fe in water.
Wbbse Physical Science And Environment Class 9 Solutions
Question 18. What are the types of hardness of water?
Answer:
Two types of hardness of water :
(1) Temporary hardness
(2) Permanent hardness.
Question 19. Which one is more pure deionised water or distilled water?
Answer:
Distilled water.
Question 20. What is an electrochemical series?
Answer:
The electrochemical series is the arranged position of metals according to their standard oxidation potential values.
Question 21. What is the process by which both types of hardness are removed?
Answer:
Removal of both types of hardness is done by an ion exchange process.
Question 22. What is the mass ratio of hydrogen and oxygen in water?
Answer:
The mass ratio of hydrogen and oxygen in water is 1: 8.
Question 23. Who first recognised that water is a compound of two elements (hydrogen and oxygen) in 1783?
Answer:
Lavoisier first recognised that water is a compound of two elements (hydrogen and oxygen) in 1783.
Question 24. What is the fundamental property that makes water so important?
Answer:
Density makes water so important.
Question 25. In a pressure cooker, water boils at what temperature?
Answer:
In a pressure cooker, water boils at a temperature greater than 100°C.
Question 26. At 3.98°C (nearly 4°C), water has a special property. What is this
Answer:
At 4°C, the density of water is maximum.
Question 27. Mention one special property of water.
Answer:
Water can act as both an acid and base.
Question 28. Mention a unit of concentration equivalent to ppm.
Answer:
The required unit is m/l.
Question 29. Presence of what makes water temporarily hard?
Answer:
Temporary hardness: The hardness of water due to the presence of soluble bicarbonates of calcium, magnesium and iron is called temporary hardness.
Question 30. Presence of what makes water permanently hard?
Answer:
Permanent hardness: The hardness of water due to the presence of soluble chlorides, sulphates of calcium, magnesium and iron is called permanent hardness.
Class 9 Physical Science Water 2 Marks Questions And Answers:
Question 1. State the composition of water.
Answer:
Aris : Water is the principal constituent of earth’s surface. Of the total global water, the ocean and inland saline bodies hold 97.3 % and the freshwater amount 2.7 %. Water is made of two elements – hydrogen and oxygen.
The molecules of water consist of two hydrogen atoms bonded to one oxygen atom by covalent compound. The chemical formula of water is \(\mathrm{H}_2\). Water exists in three states solid, liquid and gaseous. At ordinary temperature waiter exists in liquid state.
Wbbse Physical Science And Environment Class 9 Solutions
Question 2. What do you mean by soft water and hard water?
Answer:
Natural water contains dissolved salts. Depending upon its behaviour towards soap solution, water may classified as soft water or hard water.
(1) Soft Water: Water which produces lather with soap solution readily is called soft water. For example, distilled water, rainwater and demineralized water.
(2) Hard water: Water which does not produce lather with soap solution readily is called hard water.
For example sea water, river water, tap water, etc.
Question 3. Describe the modern method of softening water (Calgon Process).
Answer:
In this modern method, the harmful \(\mathrm{Ca}^{+2}\) and \(\mathrm{Mg}^{+2}\)ions present in hard water are rendered ineffective in the form of soluble complexes by adding substance. For example, sodium poly metaphosphate \(\left(\mathrm{NaPO}_3\right)_{\mathrm{n}}\)(where is a very high number upto 1000), commonly known as calgon, is treated with hard water. Calgon combines with calcium ions and magnesium ions to form complex ions which are soluble in water.
Question 4. Water is regarded as a universal solvent. Explain it.
Answer:
Water can dissolve most of the inorganic substances. Most of the inorganic substances which are of ionic nature dissolve in water. A few organic substances such as urea, alcohol, sugar, etc. also dissolve in water. Many liquids are also miscible in water. Since water dissolves almost every common substance in it to some extent, therefore, water is considered to be a universal solvent.
Question 5. What is black foot disease?
Answer:
Black foot disease: Groundwater sometimes is contaminated with arsenic acid and arsenic acid as a deprotonated form. Consumption of this arsenic-contaminated water for a long period has some poisonous effect in the form of black spots on palms and feets, skin becomes rough. The disease caused by arsenic poison is called black foot disease.
Question 6. What is Fluorosis?
Answer:
Fluorosis: Sea-water, river water contain fluoride from natural source. Excess fluoride in water is responsible for fluorosis disease which is a cause of dental decay and bone decay.
Question 7. What is eutrophication? Explain its cause.
Answer:
Fertilizers, insecticides and pesticides pollute agricultural water. So, waters of rivers, ponds, lakes, canals are polluted as run-offs from agricultural lands flow to these water bodies. Nitrogen and phosphorus of fertilizers accumulate and help in the growth of algae in these water bodies which results in the depletion of oxygen in water, which again affects population of fish and other aquatic animals. This reduction of oxygen level in water is called eutrophication.
Question 8. What is water purification?
Answer:
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids and gases from contaminated water. The goal is to produce water fit for a specific purpose. Most water is disinfected for human consumption(drinking water), but water purification may also be designed for a variety of other purposes, including fulfilling the requirements of medical, pharmacological, chemical and industrial applications.
The methods used include physical processes such as filtration, sedimentation, and distillation, biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination and the use of electromagnetic radiation such as ultraviolet light.
Wbbse Physical Science And Environment Class 9 Solutions
Question 9. What are dental and skeletal fluorosis?
Answer:
Moderate amounts of fluoride ingestion can cause dental fluorosis, which is characterized by staining and pitting of the teeth. In more severe cases all the enamel may be damaged.Chronic high-level exposure to fluoride can lead to skeletal fluorosis.
In skeletal fluorosis, fluoride accumulates in the bone progressively over many years. The early symptoms of skeletal fluorosis include stiffness and pain in the joints. In severe cases, the bone structure may change and ligaments may calcify, resulting | in impairment of muscles and pain.
Question 10. What is arsenic poisoning?
Answer:
Arsenic poisoning caused by the widespread boring of wells for drinking water has become a major problem, maybe on a scale equivalent to the problem of unhygienic surface water causing diarrhoea disease and other diseases. The skin defects often shown are not the worst, but instead strong cancerogenous negatively affects the immunity defence.
Question 11. What are the sources of natural water?
Answer:
Sources of natural water: Natural water is available from various sources, such as seas, as seas, rivers, lakes or ponds, wells, springs and rain.
Question 12. What is water pollution?
Answer:
Water pollution: Some soluble and insoluble matters in water which produce some harmful effect to men and aquatic animals are called water pollution.
Question 13. What is the cause of hardness of water?
Answer:
Causes of hardness of water: The hardness of water is due to the presence of dissolved salts of metals (except Na, K) particularly calcium, magnesium and iron.
Question 14. Discuss the action of water on sodium and calcium metals.
Answer:
Action of sodium and calcium on water: Sodium and calcium react promptly with water at ordinary temperature with fire and sound. At the end of the reaction metallic hydroxides and hydrogen are found
Wbbse Physical Science And Environment Class 9 Solutions
Question 15. What is deionised water?
Answer:
Deionised water: The water that contains no cation other than H+ ion and anion there OH– ion, is called deionised water. Deionised water is prepared only when hard water is allowed to pass through both cation ion exchange and anion exchange resin.
Uses :
(1) In chemical laboratory for qualitative as well as quantitative analysis of presence of ions in a substance, other than OH– and H+ ions.
(2) In batteries having lead electrodes.
Question 16. What is permit?
Answer:
Permutit: Permutit is a trade name for artificially prepared zeolite which is sodium aluminosilicate (Na2O, Al2O3,3SiO2,2H2O). Permutit is used to remove the hardness (both types— temporary and permanent).
Question 17. What is meant by permanent hardness of water?
Answer:
Permanent hardness of water: Permanent hardness is caused by the presence of chloride and sulphate salts of calcium, magnesium and iron in water.
Question 18. What is meant by removal of hardness of water?
Answer:
Removal of hardness of water: Hardness is due to the dissolved calcium, magnesium and iron salts. Now if these soluble salts are converted to insoluble salts by any process, then hardness of water is removed.
Question 19. What is meant by temporary hardness of water?
Answer:
Temporary hardness of water: Temporary hardness is caused by the presence of soluble bicarbonates of calcium, magnesium and iron in water.
Question 20. Write down two processes of getting arsenic-free water.
Answer:
Two processes of getting arsenic-free water :
(1) Arsenic is removed from water if water is allowed to pass through a bag made of cloth containing alumina or ferric hydroxide replacing the candle used in the filter. Arsenic or arsenic ions are absorbed in the alumina bed or ferric hydroxide bed and thus are removed.
(2) Take 20 lit. of water, \(\frac{1}{4}\)amount of tea-spoonful of bleaching powder and the same amount of ferrous sulphate in a bucket. After stirring well, arsenic is removed.
Question 21. What is a water crisis?
Answer:
Water crisis: Within 2010 A.D. we are to face a critical situation for potable water. This is because the population is gradually increasing but the supply of sweet water is fixed. This crisis can be overcome by avoiding the wastage of water and by judicious use of available water.
Collection of rainwater and its uses for certain purposes like gardening, car-washing, floor-washing and even bathing can also mitigate the water crisis. Scientists in different countries are engaged in finding a solution to how saline water in sea is desalinated.
Wbbse Physical Science And Environment Class 9 Solutions
Question 22. How is the harvesting of water done for agriculture?
Answer:
Harvesting of water for agriculture: In India and also in some other parts of world excessive rain water is stored in underground reservoirs. Such subsurface stores of water are freed from huge loss of water by evaporation as it happens in surface reservoirs. This stored water may be used for agricultural works.
Question 23. What is the specific heat capacity of water?
Answer:
Specific heat capacity: High specific heat (1 cal\({g}^{-1}\)) controls environmental temperature. A fixed amount of heat (4.2 J or 1 calorie) absorbed by 1 g of water, when heated through 1°C is called its specific heat capacity. For this high specific heat capacity, the large amount of water on the earth’s surface is able to modify the climate of the nearby land areas, making warmer in winter and cooler in summer. Due to this moderating property land and sea breezes are set up.
Question 24. What is the latent heat of the vaporisation of water?
Answer:
Latent heat of vaporisation: High (540 Cal\({g}^{-1}\)). The heat energy required by water to change into its vapour at its boiling point without any change in temperature is called latent heat of vaporisation of water, its specific value is 540 Cal \({g}^{-1}\). The same amount of heat is released when 1g of steam condenses to form 1g of water at 100°C. Water is a good heat sink. This is the reason why steam causes more serious burns than water at 100°C.
West Bengal Board Class 9 Physical Science Solutions
Question 25. Comment on the density of water.
Answer:
Density: In 1gm/cc, maximum at 4°C, ice has low density. Water freezes on the surface, so aquatic life can survive in the water below even in winter. The latent heat of the fusion of ice is 80 cal/g. The same amount of heat is released when 1g of water solidifies to form 1g of ice at O°C. Due to the high specific latent heat of solidification, lakes and rivers do not freeze suddenly. Ice is a good insulator, the water under the ice can support life.
Question 26. Comment on the boiling point of water.
Answer:
Boiling point: At normal pressure boiling point of water is 100°C. The boiling point of water depends on pressure. If the pressure increases, the boiling point increases, and vice versa. The pressure cooker works on this principle. The high boiling point of water helps us to cook our food. The high boiling point of water is applied to sterilize surgical instruments in boiling water at higher pressure.
Question 27. What is the importance of dissolved matter in water?
Answer:
Importance of dissolved matters in water :
(1) The dissolved salts are essential for the growth and development of plants.
(2) The dissolved matters add taste to water.
(3) These dissolved salts supply the essential minerals needed in our body.
Question 28. What is the importance of dissolved air in water?
Answer:
Importance of dissolved air in water :
(1) Aquatic life-like fishes use the dissolved O, in water for their respiration. (1 dm3 of water contains 40 cm3,O2)
(2) Aquatic plants use dissolved CO2, for photosynthesis to prepare their foods.
(1) \(\mathrm{CO}_2+6 \mathrm{H}_2 \mathrm{O}\) \(\underset{\text { Sunlight }}{\stackrel{\text { Chlorophill }}{\rightleftharpoons}} \underset{\text { (glucose) }}{\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6}+6 \mathrm{O}_2\)
(2) CO2, reacts with limestone in water to form soluble calcium bicarbonate. Marine organisms form shells of calcium carbonate from calcium bicarbonate.
CaCO3+CO2+H2O → Ca(HCO3)2
(Calcium Carbonate) (Calcium Bicarbonate)
West Bengal Board Class 9 Physical Science Solutions
Question 29. Mention two quality parameters of drinking water.
Answer:
Quality parameters of drinking water. The drinking water must possess the following qualities :
(1) Must be clean, i.e., free from suspended particles
(2) Should be free from soluble salts like arsenic, fluoride, chloride, nitrate, etc.
Question 30. What are the limitations of water disinfection by UV radiation?
Answer:
Limitations of water disinfection by UV radiation :
(1) This process is applicable for small-scale water purifications and power supply dependent too.
(2) For better destruction of microorganisms the contact time of UV rays with water should be sufficient, i.e., Slow flow of water through the UV rays is necessary.
Question 32. Mention two uses of deionised water.
Answer:
Uses of deionised water :
(1) It is soft water like that of distilled water.
(2) There is difference between distilled water and deionised water. Distilled water contains no organic matter and can be used for medicinal preparations.
Question 33. What are the harmful effects of pesticides?
Answer:
Harmful effects of pesticides :
(1) Some of them are carcinogenic damage reproductive system, damage liver, kidney, etc.
(2) The organic chlorine and organophosphorus pesticides, damage the central nervous system (CNS).
(3) These insecticides cannot be removed by the purification of water so at present physiological and neurological problems are increasing.
(4) The inorganic pesticides, Hg, As, Pb, etc. damage liver, blood, brain, kidney Cd, Se damage bone and teeth. Lead poisoning causes mental retardation.
Question 34. What are the harmful effects of fertilizers?
Answer:
Harmful effects: Due to increase of plant nutrients in the form of nitrogen, carbon and phosphorus in water bodies increase algal growth (aquatic weeds), this is called eutrophication. The excessive growth of algae which deoxygenates water. As a result amount of dissolved oxygen will reduce to a great extent, the aquatic animals die, the water release foul odour and becomes unfit for use.
Class 9 Physical Science Water 3 Marks Questions And Answers:
Question 1. What are the causes of hardness of water?
Answer:
The hardness of water is due to the presence of the bicarbonates, chlorides and sulphate salts of calcium and magnesium metals. These salts dissolve in rainwater when it passes through the ground and rocks. Soaps are sodium salts of higher fatty acids like stearic acid (C17H35COOH), oleic acid (C17H35COOH), or palmitic acid (C17H31COOH)When soap is added to hard water, these anions combine with Ca2+ and Mg2+ ions to form calcium and magnesium salts which are insoluble in water.
MgCl2 + 2C17H35COONa= (C17H33COO)2 Mg+ 2Nacl
The magnesium chloride from hard water reacts with sodium stearate soap and precipitates insoluble magnesium stearate which is insoluble in water. Therefore, no lather is produced until all the calcium and magnesium ions are precipitated.
When soap is added to hard water, these anions combine with ions to form calcium and. magnesium salts which are insoluble in water.
MgCl2 + 2C17H35COONa= (C17H33COO)2 Mg+ 2Nacl
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 2. Describe the different types of hardness of water.
Answer :
The hardness of water is of two types :
(1) Temporary hardness and
(2) Permanent hardness.
(1) Temporary hardness: Temporary hardness in water is due to the presence of bicarbonates of calcium and magnesium dissolved in it. The hardness is called temporary because it can be very easily removed by simply boiling the hard water for some time.
(2) Permanent hardness: This type of hardness is due to the presence of sulphates and chlorides of calcium and magnesium dissolved in water. Since this type of hardness cannot be easily removed, therefore, it is called permanent hardness.
Question 3. State the methods of removing permanent hardness of water.
Answer:
The permanent hardness of water as well as the temporary hardiness (total hardness) of water can be removed by the following methods :
(1) By treating with washing soda
(2) By ion exchange method
(1) Inorganic cations exchanger (Permutit method)
(2) Organic ion exchanger
(3) By Calgon process (modern method).
West Bengal Board Class 9 Physical Science Solutions
Question 4. Describe the removal of permanent hardness of water by organic ion exchanger (lon exchange resin process).
Answer:
In this method various synthetic ion exchange resins are used to remove the permanent hardness of water. Resins are organic compounds containing the sulphonate group. In this method, hard water is first passed through a bed of cation exchange resin. The cations ofCa2+and Mg2+ will exchange with H* ions of the resin (represented by R) as
Ca2++2Cl–+2H+R → CaR2+2H++2Cl–
Mg2++SO42-+2H+R → MgR2+2H++SO42-
The water which comes out from the bottom of the first tank contains H+ ions. This water is passed into the second tank containing a bed of anion exchange resin. It exchanges its
OH– ions with Cr ions in water :
Cl–+HO–R → Cl–R+2OH–
Now,H++OH– = H2O
Thus, the water obtained by this method is free from all types of cations as well as anions and is called Deionized water.
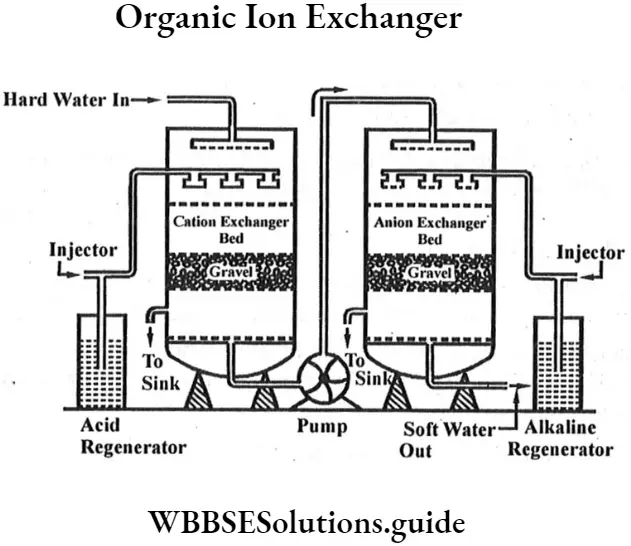 The exhausted resin in the first tank is regenerated by treatment with conc. hydrochloric acid or sulphuric acid.
The exhausted resin in the first tank is regenerated by treatment with conc. hydrochloric acid or sulphuric acid.
The exhausted resin in the second tank is regenerated by treatment with conc. solution of sodium hydroxide.
Question 5. State the physical properties of water.
Answer :
(1) Pure water is a colourless, odourless and tasteless liquid at ordinary temperature.
(2) The freezing point of water to ice is 0°C.
(3) The boiling point of pure water at normal pressure is 100°C.
(4) Its molecular mass is 18.015 (approx. 18).
(5) Its maximum density (gm/cubic cm) at 4°C is 1.
(6) It can dissolve most of inorganic substances.
West Bengal Board Class 9 Physical Science Solutions
Question 6. Describe the chemical properties of water.
Answer:
Water displays a versatile chemical behaviour. It behaves as an acid, a base, an oxidant, a reductant and acts on some metals.
Few of its main chemical properties are :
(1) Alkali metal as sodium readily decomposes water at ordinary temperature. It evolves heat and liberates hydrogen gas.
2Na+2H2O=2NaOH+H2↑
(2) Calcium reacts slowly with cold water and liberates hydrogen. With hot water, calcium reacts quickly to form hydrogen gas.
Ca+2H2O=Ca(OH)2+H2
(3) Water hydrolyses oxides of non-metals forming their respective acids.
SO2+H2O=H2SO3
(4) Water reacts with carbides, nitrides, etc. and gives. organic compounds. For example, calcium carbide (CaC2 )reacts with water giving acetylene gas.
CaC2+2H2O=Ca(OH)2+C2H2
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 7. Describe the methods of removal of temporary hardness of water.
Answer :
Temporary hardness of water can be removed generally by two methods :
(1) By boiling and
(2) By Clark’s process or addition of lime.
(1) By boiling: Temporary hardness of water can be removed by boiling the hard water in large boilers. During boiling the bicarbonates of calcium and magnesium decompose into insoluble carbonates and gives carbon dioxide. The insoluble carbonates can be removed by filtration.
Ca(HCO3)2=CaCO3+H2O+CO2
Mg(HCO3)2=MgCO3+H2O+CO2
(2) By Clark’s method or addition of lime: When temporary hard water is treated with calculated amount of lime, it reacts to form insoluble carbonates of calcium and magnesium, which can be filtered out and hence water becomes soft.
Ca(HCO3)2+Ca(OH)2=2CaCO3=2H2O
Mg(HCO3)2+Ca(OH)2=MgCO3+CaCO3+2H2O
Question 8. Describe the method of removing permanent hardness of water by washing soda.
Answer:
To remove the permanent hardness of water, it is treated with a calculated amount of washing soda (Na2CO3). Washing soda converts the chlorides and sulphates of calcium and magnesium dissolved in water into their respective carbonates which get precipitated.
CaCl2+Na2CO3=CaCO3+2NaCL
MgSO4+Na2CO3=MgCO3+Na2SO4
Hence, water becomes free from Ca2+ and Mg2+ ions, and it becomes soft.
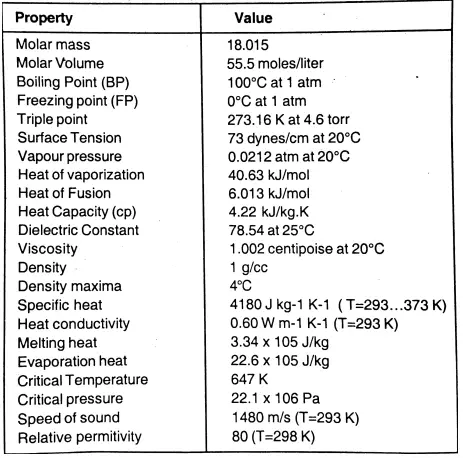
Question 9. Describe the physical properties of water.
Answer:
The Summary Table of Physical Properties :
Question 10. What are the water quality parameters for drinking water?
Answer:
Water quality parameters provide important information about the health of a water body. These parameters are used to find out if the quality of water is good enough for drinking water, recreation, irrigation, and aquatic life.
The water quality parameters for drinking water depend on:
(1) Alkalinity
(2) Ammonia,
(3) Dissolved Oxygen (DO)
(4) Faecal Coliform Bacteri
(5)Flow
(6)Hardness
(7)Nitrate
(8) Nitrite
(9)pH
(10)Phosphorus
(11)Specific Conductance
(12) Temperature
(13) Total Organic Carbon (TOC)
(14)Total Dissolved Solids (TDS)
(15)Total Suspended Solids (TSS)
(16) Turbidity.
West Bengal Board Class 9 Physical Science Solutions
Question 11. Describe thé inorganic cation exchanger (permutit method) for removing permanent hardness of water.
Answer :
Permutit method: Inorganic cations exchanger are naturally occurring minerals or artificially synthesised substances. ‘Its chemical name is hydrated sodium aluminium ortho silicate (Na2. Al2Si2O8.XH2O) It is commonly written as Na,Z where Z stands for (Al2Si2O8.XH2O), where x is ‘the number of molecules. Zeolite is also ‘known by the name permutit.
When Zeolite comes in contact with Ca2+ or Mg2+ions, it exchanges its sodium ions with them to form calcium Zeolite (CaZ) or magnesium zeolite.
Na2z+Ca2+ Cax+2Na+ Na2z+Mg2+ Mgz+2Na+
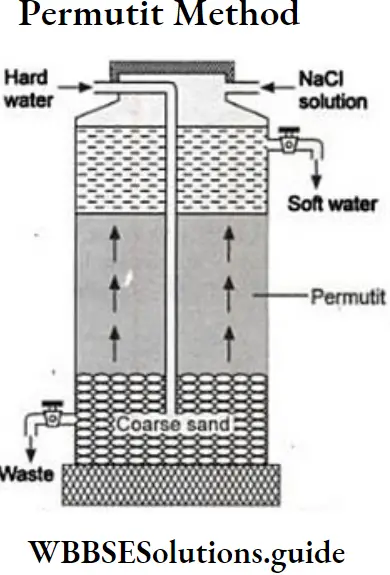
Procedure: The permutit softener consists of a long cylindrical tank. It contains one outlet at the bottom to remove soluble salts of calcium or Soft water magnesium. Another outlet is provided at the top for removing soft water. Two inlet pipes are ZF Permutitintroduced to bring hard water and saline solution of common salt The saline solution of common salt is introduced when zeolite gets exhausted.
Question 12. How is water treated for drinking?
Answer:
There are three acceptable ways to treat drinking water: boiling, chlorine bleach, or distilling. Distillation and treatment systems from the camping store have problems if you are trying to provide water for a household. In general, boiling and bleaching are the best.
Boiling: Water should be boiled for at least 5 minutes to sanitize it. Some agencies recommend boiling for 10 minutes just to be safe. Boiled water tends to taste flat because there is no air in it. You can add air by pouring the water back and forth between two clean containers. Doing this will also improve the taste of your stored or bottled water.
Bleach: Treating water with bleach is very effective at killing germs, and it doesn’t taste funny to most of us because ‘this is basically what most city water supplies do. You need to have a bottle of plain liquid chlorine bleach and a dropper. The bleach should be 5 to 6 percent sodium hypochlorite with no preservatives and no additional ingredients. Do not use scented
bleaches, colour safe bleaches, powdered bleaches, or bleaches with added cleaners.
Distillation: Distillation requires boiling the water and catching the vapour as it condenses back to water. Distillation has all the same problems as boiling and produces very small amounts of usable water. In addition, although distilled water is virtually free of germs, it is not necessarily free of chemicals.
Other Water Treatments: There are other ways to treat water for drinking. Camping, stores sell water treatment kits that use chemicals or filters to purify water. You can also buy filtering systems to purify water. These are not the filters you put on your faucet to make your water taste better.
They are special filters that are designed to make unsafe water pure enough to drink. Some use resins or charcoal to hold the germs and others have pores so small that bacteria can’t pass through them. Filters are not going to purify a lot of water and they quickly become used up or clogged up.
Question 13. What are the major causes of water pollution in India?
Answer:
There are several causes of water pollution in India.
The main causes are briefly described as under:
1. Urbanisation: Rapid urbanisation in India during the recent decades has given rise to a number of environmental problems such as water supply, wastewater generation and its collection, treatment and disposal.
2. Industries: Most Indian rivers and other sources of fresh water are polluted by industrial wastes or effluents. All these industrial wastes are toxic to life forms that consume this water. The total wastewater generated from all major industrial sources is 83,048 Mid which includes 66,700 Mid of cooling water generated from thermal power plants.
Use of chrome, tanning of leather, use of azo-dyes in fabrics, use of cadmium in ornaments and silver-ware, electroplating with cyanide baths, production of dye-intermediates and other refractory and toxic chemicals, etc.
3. Agricultural run-off and improper agricultural practices: Traces of fertilizers and pesticides are wasted into the nearest water-bodies at the onset of the monsoons or whenever there are heavy showers. As the point of entry of such agricultural inputs is diffused throughout the river basin, they are termed as non-point sources of pollution. Although irrigation has increased considerably in the country, little measures have been taken to tackle the problem of the high salinity return water.
4. Withdrawal of Water: Irrigation canals whisk away clean water soon after the rivers reach the plains, denying water to flow in the river downstream. The river-turned drain flow downstream with little or no fresh water unless a large river augments the depleted flows.
5. Religious and Social Practices: Religious faith and social practices also add to pollution of our river waters. Carcasses of cattle and other animals are disposed of in the rivers. Dead bodies are cremated on the river banks. Partially burnt bodies are also flung into the river. All this is done as a matter of religious faith and in keeping with ancient rituals.
These practices pollute the river water and adversely affect the water quality that offerings from a puja be immersed in a river. It is now common to see people immersing offerings in plastic bags. Plastic bags are very dangerous and further add to the pollution load of the river.
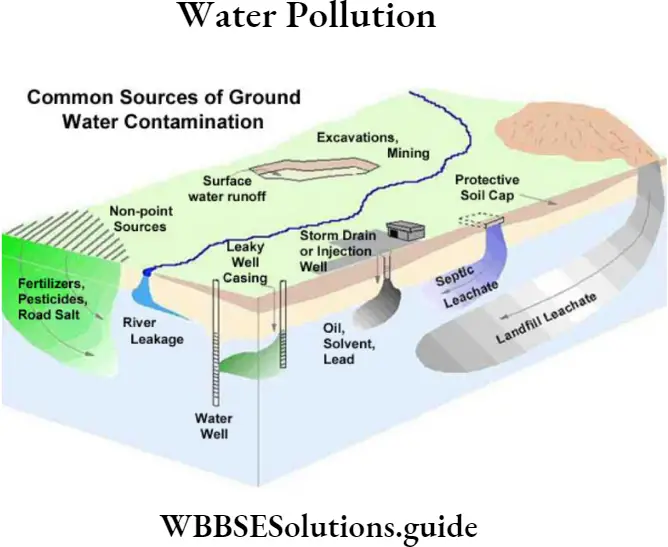
Question 14. What are the common sources of groundwater pollution?
Answer:
The following diagram explains the common sources of groundwater pollution.
West Bengal Board Class 9 Physical Science Solutions
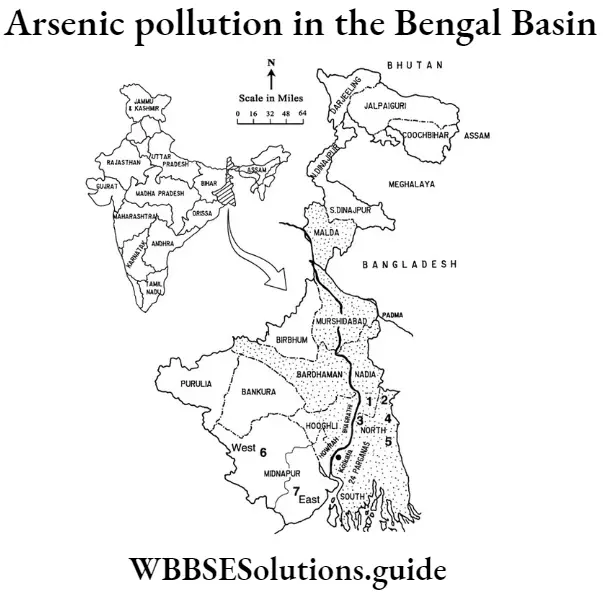
Question 15. Explain the groundwater arsenic pollution in the Bengal basin.
Answer:
The source of arsenic in India’s groundwater continues to elude scientists more than a decade after the toxin was discovered in the water supply of the Bengal delta in India.
The environmental crisis began after large traces of the element were detected in the groundwater in the Bengal Basin – an area inhabited by more than 60 million residents. This has caused a water shortage, illness and death in the region, leaving residents unable to use the water even for ordinary tasks like washing dishes or ablution. Arsenic pollution causes skin lesions, respiratory failure and cancer when present in high concentrations in drinking water.
Arsenic in groundwater constitutes a major human health issue in many countries globally. it is particularly acute in the Bengal Delta Plains in Bangladesh and india but has also prevalent in many other parts of the world, including Argentina, Chile, Mongolia, and the United States. The problem of arsenic poisoning in the Bengali countryside has received considerable media attention since it affects a maximum number of people, 30 million in Bangladesh and 5 million in West Bengal.
Question 16. Explain Fluoride contamination of groundwater.
Answer:
According to the Department of Drinking Water Supply, out of 593 districts from which data is available, water in 203 districts has shown high fluoride (Susheela, A. K, 2001). As seen from the map below, almost all states in India have districts where groundwater contains excess levels of fluoride.
Question 17. How is deionised water prepared?
Answer:
Preparation of deionised water: Water is treated with some resins which contain carboxylic acid radical __COOH or __SO3H (sulphonic acid radical). The cations, Ca2+ Na+ Fe2+, etc. present in water are removed on the exchange of these with H+ ions of —COOH or ___SO3H of resin. If the resin is represented as RH, where R is the resin polymer, H is hydrogen of acid radicals present in the resin, the chemical equations are given as follows
CaCl2+2RH=R2Ca +2HCl
NaCl+RH=RNa+HCl
After the removal of cations, water contains some acid radicals like HCl. To remove the acid radicals, water is treated with another type resin of basic nature. In this way, the water is freed from all types of ions, except Ht and OH ions and this is deionised water.
Question 18.
(1) What is fluoride?
(2) Name one harmful fluoride and state some of its bad effects on hum
(3) State some injurious effects of presence of the fluoride in water.
Answer:
(1) Fluoride is meant by any binary compound of fluorine.
(2) The most harmful compound of fluoride is hydrogen fluoride (HF). It violently attacks the skin, forming sores. Due to inhalation of vapour of this fluoride, loss of voice takes place and finally death results:
(3) The presence of traces of some fluorides in water is. very dangerous. Some of the injurious effects of fluoride are given below
(1) Loss of calcium from bones causing skeletal weakness.
(2) Discolouration of teeth.
(3) Bending of the kness of sides, known as, Genu Valgum.
West Bengal Board Class 9 Physical Science Solutions
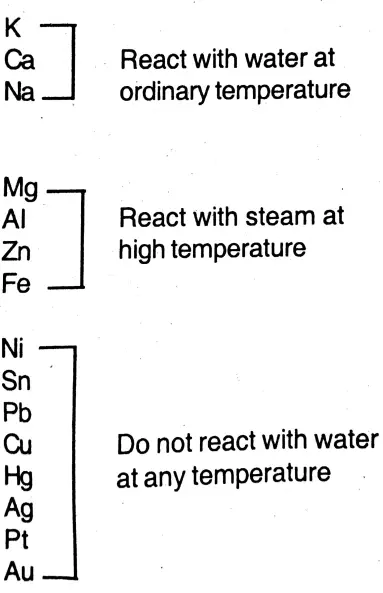
Question 19. Discuss the difference of activity of the metals with water on the basis of the position of the metals in electrochemical series.
Answer:
Difference of activity of the metals in reaction with water :
(1) The metal with highest electron-releasing ability is placed in the topmost position. Metals placed in the electrochemical series react with water according to their positions in the series.
(2) The chemical activity of metals entirely depends on their position in the electrochemical series. The metal which occupies a higher position than any other metal has greater value:
(1) Electropositive character,
(2) Reducing capacity,
(3) Chemical reactivity.
 Question 20.
Question 20.
(1) When may the water in a soil layer be considered arsenic-polluted?
(2) State two harmful effects of drinking water that contains arsenic beyond permissible limit.
(3) State one arrangement for supply or arsenic-free water.
Answer:
(1) According to the World Health Organisation (WHO): The permissible limit of arsenic in drinking.water is 0.05 mg/I. So, the water in a soil layer that contains more than 0:05 mg/I arsenic is polluted.
(2) Harmful effects of arsenic: On drinking water containing arsenic beyond the permissible limit, diseases like skin pigmentation, organ failure may occur.
(3) Supply of arsenic-free water :
(1) Arsenic is removed from water if water is allowed to pass through a bag made of cloth containing alumina or ferric hydroxide replacing the candle used in the filter. Arsenic or.arsenious ions are absorbed in the alumina bed or ferric hydroxide bed and thus are LES
(2) Take 20 litres of water, ¼ amount of tea-spoonful bleaching powder and the same amount of ferrous sulphate in a bucket. After stirring well, arsenic is removed.
West Bengal Board Class 9 Physical Science Solutions
Question 21. What is Permutit process?
Answer:
Permutit process: Both types of hardness can be removed cheaply in this process. Permutit is a commercial name for artificially prepared zeolite which is sodium aluminosilicate (Na2O, Al2O3, 3SiO2, 2H2O). It is a natural hydrated large sized crystalline substance.
A bed of granules of permit is kept in porous shelves staged with stone and sand are placed above and below the shelf containing bed of permutit. When hard water kept in a tank is allowed to pass through the bed of permutit, due to exchange of ions the hardness of water is removed and soft water is obtained. Let the ion-exchange material permit be formulated as Na2Ze, where Ze is the zeolite
Radical. The chemical reactions are given as follows :
Na2Ze+Ca(HCO3)2=CaZe+2NaHCO3
Na2Ze+CaCl2=CaZe+2NaCl
NaZe+MgSO4=MgZe+Na2SO4
After the use of permutit for some time when the permutit gets exhausted and loses its activity, it is regenerated by passing an aquecus solution of sodium chloride by which permutit gets back its exchange capacity.
CaZe+2NaCl=Na2Ze+CaCl2
MgZe+2NaCl=Na2Ze+MgCl2
Permutit water-softening plants are used in private houses and factories and also for softening public water supplies of towns.
Question 22. Water is a versatile solvent. Explain.
Answer:
Solvent properties: Water is a good solvent because :
(1) It is in liquid state in wide range of temperatures (0° − 100°C) at 1 atmospheric pressure.
(2) It has a high dielectric constant (80).
(3) Water is a polar solvent and it dissolves most of the ionic compounds, acids, bases, etc.
(4) It acts as an amphoteric solvent (acts as a base by accepting H+ to dissolve acid and acts as an acid to donate H+ to dissolve base), e.g. HCl+H2O → H3O+Cl– NH3+H2O → NH4OH
(5) Many covalent compounds like sugar, alcohol, and amines dissolve in water.
(6) Air and many gases dissolve in water in small amounts. Water can dissolve large class of compounds, so it is called the versatile. solvent.
West Bengal Board Class 9 Physical Science Solutions
Question 23. Discuss the process of disinfection of water by ultraviolet radiation.
Answer:
Disinfection of water by ultraviolet radiation: At present, domestic water purifiers are available, by this type of purifer water is purified in three stages.
(1) The tap water is first filtered through a al candle,
(2) the filtered water is then passed through a candle packed with activated charcoal and
(3) this filtered water is then passed through a chamber fitted with a U.V lamp. The UV-radiation destroys microorganisms like bacteria, etc. This process is an attractive option in many cases because the waier in this cases is chemical free.
 Limitations of this process :
Limitations of this process :
(1) This process is applicable for small scale water pu- Disinfection by UV-radiation ratifications and power supply dependent too.
(2) For better destruction of micro-organisms the contact time of UV rays with water should be sufficient, i.e., slow flow of water through the UV rays is necessary.
(3) The organic matters present in water are disintegrated to radicals, these free radicals are harmful for human tissues.
Question 24. Mention the limitations of chlorination to disinfect drinking water.
Answer:
Limitations of Chlorination to disinfect drinking water :
(1) Giardia and cryptosporidium are generally resistant to chlorine unless it is used in higher doses than those generally preferred for treatment.
(2) The residual chlorine in water imparts an unpleasant taste in treated water.
(3) Presence of too much residual chlorine may produce chemical byproducts with organic matters in water, some of which are carcinogenic.
Question 25. Mention the disadvantages of using drinking water.
Answer:
Disadvantages of using hard water :
(1) Water with high hardness is not good for health.
(2) Hardwater is unsuitable for cooking many hard foodstuffs like pulses.
(3) Hard water is unfit for laundries because it
(1) consumes too much soap and
(2) leaves dirty stains of Ca, Mg, and Fe salts of fatty acids on cloth (This problem does not cause by use of detergents).
(4) Hard water does not have a pleasant taste, so it is not suitable for drinking.
(5) Hard water is not suitable for use in boilers for the production of steam in industries. During boiling hard water produces white deposits of insoluble substances called scales. The scales consist mainly of CaCO3, MgCO3, MgCO3and CaSO4 The deposit on the walls of the boiler these scales are heat insulating. This causes greater consumption of fuels, they also block the pipes which may cause accidents.
(6) Hard water cannot be used in paper and dying industries.
Question 26. Mention the uses of deionised water.
Answer:
Use of deionised water :
(1) It is soft water like that of distilled water.
(2) There is difference between distilled water and deionised water. Distilled water contains no organic matter and can be used for medicinal preparations.
(3) Deionised water contains organic matter like pyrogen so it cannot be used for injection water.
(4) Deionised water is used in laboratories for analytical work, in wet batteries, boilers and laundries, etc.
Question 27. How did life originate in water ?
Answer:
Origin of life: The earth as a planet originated about 4600 million years ago. The atmosphere was composed of hydrogen, methane, ammonia and water vapour. There was hardly any oxygen and no life could develop because the surface was very hot. As the earth began to cool, the first steps were taken towards life about 3500 million years ago.
The water vapour condensed to form oceans and it is believed that the first living organisms appeared in the ocean. These unicellular organisms fed on the organic molecules surrounding them, bring them down to obtain their chemical energy without the help of oxygen. More than a billion years later, the green pigment chlorophyll developed and enabled certain organisms to create food using sulight and form oxygen gas.
Water evaporates from large surfaces like rivers, lakes and oceans The water vapour, on reaching the cooler upper regions of the atmosphere, condenses and falls as rain or snow. The water cycle is called this never-ending cycle of evaporation of water and condensation of water vapour.
Question 28. What is the role of water in the human body and in plants?
Answer:
Role of water in the human body :
(1) Water is a medium of transport of chemicals to and from cells.
(2) Water regulates the temperature of the body by the process of sweating and evaporation.
(3) Blood is a colloidal solution of many substances, such as salts, proteins, enzymes, glucose, etc. in water.
(4) Metabolic reactions occur in water. Role of water in plants :
(1) Water helps in the germination of seeds.
(2) Besides carbon dioxide, water is also used by plants for manufacturing food by photosynthesis.
(3) Water is the medium of transport of different minerals.
Question 29. What are the properties of water in the role of life?
Answer:
Properties of water in the role of life :
(1) Specific heat: The specific heat of water (4200 J/kg °C) is highest among the solid and liquid substances. So water is considered as a huge storehouse of heat energy. In hot water comparatively high heat is present for a long time, hence it is widely used in hot water bags or hot water bottles. In cold countries, the houses are kept hot by flowing hot water through pipes.
As it takes more health, it is also used as a coolant in different machines and engines of automobiles. Due to high specific heat, water becomes hot very slowly than the surface and again becomes cool by leaving heat. That is why both land breeze and sea breeze are formed at the sea shore and they control the rain and climate of a country.
(2) High boiling point: Pure water boils at 100°C at a pressure of 76 cm of mercury. Boiling point of water increases with increase in atmospheric pressure and decreases with decrease in atmospheric pressure. The boiling point of water increases due to the presence and concentration of dissolved impurities. Due to high boiling point and high specific heat itis used in hot water bag.
(3) Capillary action: Minerals present in the soil ‘dissolve in the water and form a solution. This solution is then absorbed by the roots and is conducted upwards through the plant tissues due to the capillary action of water.
(4) Solvent: Water is a covalent compound. It has a unique property to break the electrostatic forces holding the ionic compounds. Thus, ionic compounds rapidly dissolve in water. Covalent compounds like sugar, urea, and glucose are also dissolved in it because covalent compounds always dissolve in covalent solvents. For this reason, water is called as universal solvent.
Question 30. How does detergent pollute water?
Answer:
Pollution of water by detergent: Detergents are surfactants, i.e., surface active agents and builders. Surfactants have the following properties and hence cause water pollution.
(1) Bio-micro organisms (i.e., bacteria present in the water of lakes or rivers) are not able to bio-degrade the surfactants, i.e., surfactants are not decomposed by the bio-micro- organisms and hence large volumes of detergent foam make water polluted.
(2) Builders are generally sodium salts of phosphoric acid, sulphuric acid, etc. The builders form stable soluble complexes with Ca* and Mg?*. These complexes act as plant nutrients and cause a rapid growth of algae and weeds in the water. The growth of these plants appears as green sludge on the water surface. These plants do not generate oxygen in water and hence aquatic animals die.
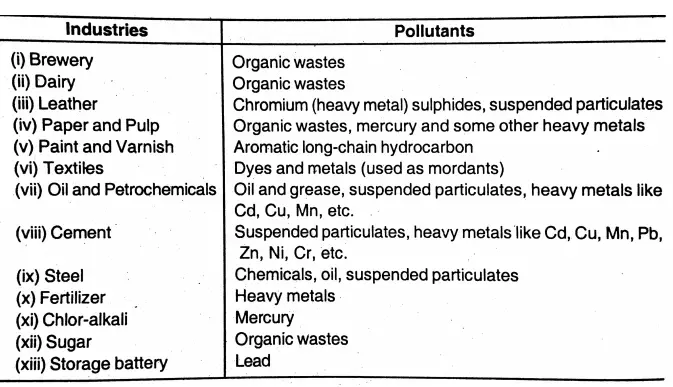
Question 31. Mention the pollutants generated by some common industries.
Answer:
Pollutants generated by some common industries: Industries Pollutants
Question 32. Mention the major water pollutants, their sources and effects.
Answer:
Major water pollutants, their sources and their effects :
(1) Pathogens:
Bacteria and other microorganisms: They are the agents responsible for so many of water-borne diseases.
Sources: Domestic sewage, animal excreta.
Example: Bacteria such as Escherichia coli, and streptococcus faecalis.
Effect: Diseases are caused like gastrointestinal disease, etc.
(2) Organic wastes: The wastes are biodegradable, which are decomposed by bacteria in water and thus consume dissolved oxygen in water (which is only 10 ppm or parts per million).
Sources: Domestic sewage, decaying and plant body.
Example: Leaves, grass, trash, etc.
Effect: Depletion of dissolved oxygen and thus causing a threat to the aquatic lives. It increases the BOD of water. BOD (Biochemical Oxygen Demand) measures organic material in water, in terms of how much oxygen will be required to break down that material. Clean water should have BOD less than 5 ppm, whereas polluted water may have BOD of 15 or more.
(3) Chemical pollutants : Inorganic: They include heavy metals, sulphates, nitrate, etc. They are dangerous mostly because our body can not excrete them.
Sources: Industries and chemical factories, mine drainage, etc.
Example: Heavy metals like lead (Pb), Cadmium (Cd), mercury (Hg). Salts like fluoride (in excess), sulphated, nitrates, detergent, etc.
Effect: Heavy metals damage kidneys, central nervous system, liver, etc. Acids, salts affect in many ways that include decay of tooth and bone (F°); laxative effect (sulphate), disease like ‘blue body syndrome’ (nitrate), etc.
(4) Chemical pollutants: Organic: It includes petroleum products, pesticides, fertilizers and many other industrial chemicals.
Sources and examples: Major oil spills in oceans, DDT, PVC (polyvinyl chloride), PCB (Polychlorinated biphenyls), phosphates in fertilizers, industrial sewage, etc.
Effects: Some of them are carcinogenic (PVC, PCB). Phosphates cause unlimited growth of algae that consume large amounts of oxygen and thus destroy the natural balance of water-body and aquatic life and subsequently cause loss of biodiversity. This process of algae bloom and what follows is known as “Eutrophication”.
(5) Other pollutants: They include sediments due to erosion of soil by agriculture and strip mining, radioactive substances (nuclear power plant, uranium mining) and heat (water from industrial cooling towers).
Question 33. Write a short note on water treatment.
Answer:
Purification of water: Water treatment: As we have already seen to purify water means to make water free from solid particles, suspended or colloidal particles, bacteria, ionic impurities, and other harmful impurities.
Depending on the methods used, the source water and the usage of treated water, the “water treatment” can be broadly classified into three categories as explained in the following chart.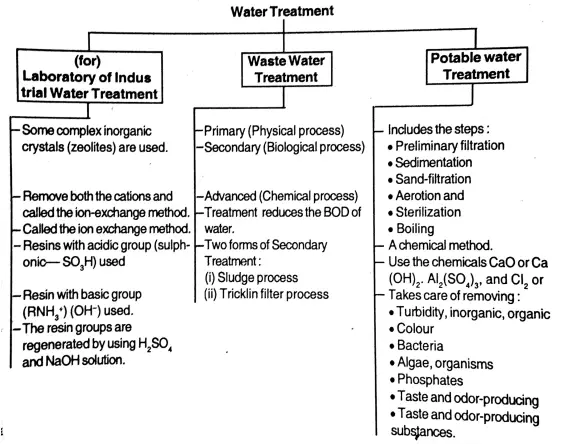
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound

