Atomic Structure Very Short Answer Type Questions
Question 1. What is the term used to express the sum of the number of neutrons and protons present in an atom of an element?
Answer: As the nucleus is composed of protons and neutrons, they are collectively called nucleons.
Question 2. The number of which particle is different between 612C and 613C?
Answer: Neutron.
Question 3. State one difference between H and H+.
Answer: H is an unstable hydrogen atom carrying no net electric charge. H+ is a positive hydrogen ion carrying a unit positive charge.
Question 4. What are electron shells?
Answer: The electrons outside the nucleus of the atom rotate in definite numbers in certain specified circular paths around the nucleus. These concentric circular paths of the rotating electrons are called electron shells.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Wbbse Physical Science And Environment Class 9 Solutions
Question 5. What is an atom?
Answer: The smallest particle of an element which may not exist independently but contains all the properties of that element and takes part in chemical reactions, is called an atom.
Question 6. Which two particles of an atom are present in equal numbers?
Answer: The numbers of protons and electrons are equal in an atom.
Question 7. What are nucleons?
Answer: As the nucleus is composed of protons and neutrons, they are collectively called nucleons.
Question 8. Can the electrons stay inside the nucleus?
Answer: No, electrons can’t stay inside the nucleus.
Question 9. Name an atom that does not contain a neutron.
Ans: Neutron is absent in the nucleus of an ordinary hydrogen atom (11H).
Question 10. What is the force that holds together the nucleons in the nucleus of an atom?
Answer: Nuclear force firmly holds together the nucleons inside the nucleus.
Wbbse Physical Science And Environment Class 9 Solutions
Question 11. The atomic number of magnesium is 12; what is the number of electrons in the Mg2+ ion?
Answer: The number of electrons in the Mg2+ ion is 10.
Question 12. What is heavy water?
Answer: Heavy water is that kind of water where hydrogen has been substituted by deuterium. The formula of heavy water is D2O (molecular weight is 20).
Question 13. The atom of an element has 2 electrons in the K shell, 8 electrons in the L shell, and 7 electrons in the M shell. What is its atomic number?
Answer: The atomic number of the element is (2+8+7) = 17.
Question 14. What is the number of charged particles present in Cl– ion? (Atomic number of Class 17)
Answer:
(1) Number of negatively charged particles (Electrons) = 18.
(2) Number of positively charged particles (Protons) = 17.
Question 15. Sodium (atomic number 11) forms Na‘ after losing 1 electron. From which of the orbits is K, L, or M of the sodium atom the electron is lost?
Answer: The electron is lost from the ‘M’ orbit of the sodium atom.
Question 16. The number of electrons and that neutrons in an atom are 6 and 8 respectively. What is the mass number of the atom?
Answer: Mass number = Number of protons (= number of electrons) + number of neutrons =
6+8= 14.
Wbbse Physical Science And Environment Class 9 Solutions
Question 17. Which one is neutral and which one is positively charged among electron, proton, and neutron?
Answer: Neutrons are neutral and protons are positively charged.
Question 18. Name the fundamental constituent particles of an atomic nucleus.
Answer: The main and fundamental constituent particles of an atom are electrons, protons, and neutrons.
Question 19. What are the numbers of protons and neutrons in the atom, 92 X235?
Answer: Protons = 92, Neutrons = 143.
Question 20. Name the Indian sage who propounded the idea of the atom ‘para Manu’.
Answer: Maharshi Kanad.
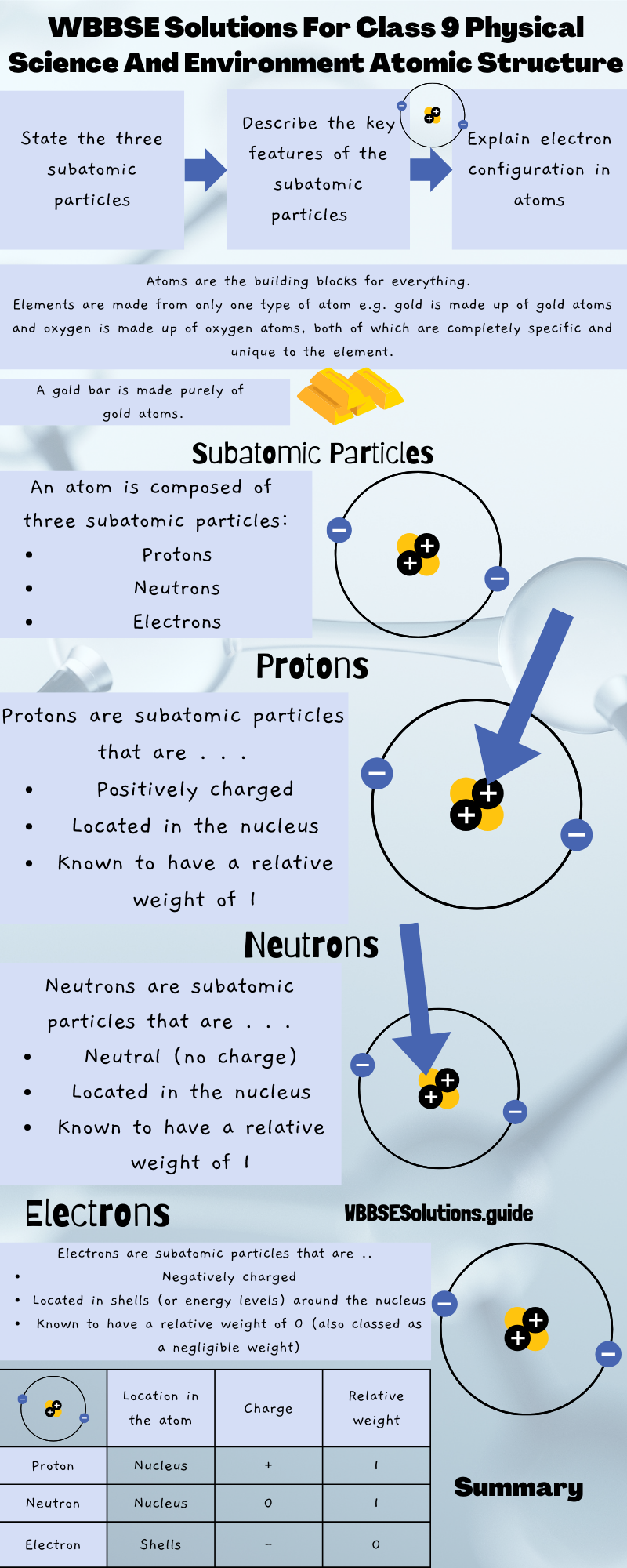
WBBSE Class 9 atomic structure solutions
Question 21. What is the meaning of “atom”?
Answer: In Greek, ‘atom’ means indivisible.
Question 22. Who proposed the name atom?
Answer: The Greek philosopher Democritus.
Question 23. Who did for the first time propound the modern theory of the atom? Or, By whom was the concept of the atom developed?
Answer: John Dalton in 1808.
Question 24. Who was the discoverer of the electron?
Answer: William Crookes.
Question 25. Name the scientist who showed that the proton is one of the constituents of an atom.
Answer: Goldstein.
Wbbse Physical Science And Environment Class 9 Solutions
Question 26. Who was the discoverer of the neutron?
Answer: J. Chadwick (1932).
Question 27. Who gave the name Neutron?
Answer: Rutherford.
Question 28. What is the symbol of an electron?
Answer: The symbol of the electron is e or _1 0e
Question 29. What is the symbol of the proton?
Answer: The symbol of the proton: is 11P Or P+
Question 30. What is the symbol! of a neutron?
Answer: The symbol of neutron: n or 01n
Question 31. Name the lightest atom.
Answer: The hydrogen atom is the lightest.
Question 32. What are protons?
Answer: Positively charged sub-atomic particles having unit mass and unit positive charge are called protons, i.e., a proton is a positively charged hydrogen ion (H1+).
WBBSE class 9 atomic structure question and answers
Question 33. Name the positively charged particle in the structure of an atom.
Answer: Proton present in the nucleus is positively charged.
Question 34. How is a proton formed?
Answer: A proton is formed by the removal of one electron from a hydrogen atom.
Question 35. State the relative mass and charge of a proton.
Answer: The relative mass of a proton is 1 amu and its relative charge is +1 (plus one).
Question 36. State the absolute charge and mass of a proton.
Answer: The absolute charge of a proton is 1.6 x10–27 coulomb of positive charge and its absolute mass is 1.6 x10_19 kg.
Atomic structure WBBSE Class 9 solutions with answers
Question 37. How many times is a proton heavier than an electron?
Answer: A proton is about 2000 times heavier than an electron.
Question 38. What is the mass of a neutron in a kilogram?
Answer: The mass of a neutron is 1.67 x 10–27kg.
Question 39. What is the absolute charge of an electron?
Answer: The absolute charge of an electron is 1.6 x10–19 coulomb.
Question 40. What is the mass of an electron?
Answer: The mass of an electron is 9.1 x 10–31 kg.
WBBSE class 9 atomic structure question and answers
Question 41. Why are electrons called planetary electrons?
Answer: Electrons revolve around the nucleus in different orbits as the planets revolve around the sun in various orbits.
Question 42. Why is the nucleus of an atom positively charged?
Answer: Because the nucleus contains protons which are positively charged particles.
Question 43. Name the isotope of an atom whose nucleus consists of one proton only.
Answer: The atom of protium (ordinary hydrogen) has a nucleus consisting of one proton only.
Question 44. State the main differences between proton and neutron.
Answer: The proton is a positively charged fundamental particle while the neutron is an electrically neutral particle.
Question 45. Write the name of the neutral particle present in the structure of an atom.
Answer: Neutron.
wbbse class 9 physical science solutions
Question 46. The number of which sub-atomic particle is fixed in the nuclei of all the atoms of an element?
Answer: Protons.
Question 47. Write the name of the fundamental particle of an atom that is not present in the nucleus of an atom.
Answer: Electron.
Question 48. Name two radioactive elements.
Answer: Radium and Uranium are two radioactive elements.
WBBSE class 9 atomic structure question and answers
Question 49. What is the maximum number of electrons that may be present in the M-shell of an atom when it is the outermost shell?
Answer: Eight (8).
Question 50. An atom has a mass number of 23 and an atomic number of 11. What is the number of electrons in it?
Answer: The number of electrons in this atom of atomic number 11 is also 11.
Question 51. An atom of an element has 11 protons, 11 electrons, and 12 neutrons. What is the atomic mass of the atom?
Answer: The atomic mass of the atom will be (11 + 12) or 23.
Question 52. If an element ‘X’ has mass number 24 and atomic number 12, how many neutrons does its atom contain?
Answer: The number of neutrons in the atom will be (24 − 12) or 12.
Question 53. What can be known about the nucleus of the atom from the symbol, 11Na23?
Answer: The symbol, 11Na23 reveals that the nucleus of sodium atom is made. Up of 11 protons and (23 − 11) or 12 neutrons.
Question 54. Find out the number of neutrons in 17Cl35.
Answer: (35 – 17) or 18 neutrons are present in 17Cl35
wbbse class 9 physical science solutions
Question 55. Name the fundamental property used to identify an element.
Answer: Atomic Number (Z).
Question 56. Write down the relation between mass number and atomic number.
Answer: Mass number of an atom = atomic number + number of neutrons in the atom.
Question 57. The atomic number of sodium is 11. What do you mean by the statement?
Answer: The statement means that the nucleus of a sodium atom has eleven units of positive charge, i.e., eleven protons.
Question 58. Give the atomic number of the atom in which the M-shell contains 4 electrons.
Answer: 14 (2, 8, 4)
WBBSE class 9 atomic structure question and answers
Question 59. What is the maximum number of electrons the M-shell of the atom can accommodate?
Answer: 18 electrons.
Question 60. State the number of electrons in H+, H, and H–
Answer: The number of electrons in H+, H, and H– are zero, 1, and 2 respectively.
Question 61. Give an example of a nuclide that contains 2 neutrons.
Answer: Helium (24He ).
Question 62. Define the term isotope.
Answer: Isotopes are atoms of the same element having the same atomic number but different mass numbers.
wbbse class 9 physical science solutions
Question 63. How do isotopes of an element differ from one another?
Answer: The isotopes of an element differ in the number of neutrons in their nuclei.
Question 64. What is the reason for the different atomic masses of the isotopes of an element?
Answer: The isotopes of an element have different mass numbers because they contain different numbers of neutrons.
65. What is the reason for identical chemical properties of all the isotopes of an element?
Answer: All the isotopes of an element have identical atomic numbers.
Question 66. Give one similarity between them. A pair of isotopes.
Answer: A pair of isotopes have the same number of protons in their nuclei.
WBBSE Class 9 Physical Science atomic structure notes
67. Give one difference between a pair of isotopes.
Answer: A pair of isotopes differ in the number of neutrons in their nuclei.
68. Write down the names of the three isotopes of hydrogen
Answer: Protium (1H1), Deuterium (1H2), Tritium (1H3).
WBBSE class 9 atomic structure question and answers
wbbse class 9 physical science solutions
Question 69. Of which element protium is an isotope?
Answer: Protium (ordinary Hydrogen) is an isotope of hydrogen.
Question 70. What is called the total number of protons and neutrons present in an atom of an element?
Answer: Mass number.
Question 71. Are the atomic masses of the elements always whole numbers?
Answer: No, atomic masses of many elements are fractions and not whole numbers.
Question72. Give an example of an element having fractional atomic mass.
Answer: Chlorine has a fractional atomic mass of 35.5.
Question 73. What is the reason for fractional atomic masses of elements?
Answer: The fractional atomic masses of elements are due to the existence of their isotopes having different masses.
Question74. The same element may have different nuclides. What are they called?
Answer: Isotopes.
Question 76. If three neutrons less are taken away from 228U92then how would you write the nuclide?
Answer: The formula of the new nuclide is 225U92
Question 77. The same element may have different nuclides. What are they called?
Answer: isotopes.
Question 78. Name the lightest particle of an atom.
Answer: Electron.
Question 79. Which particle of an atom is negatively charged?
Answer: Electron.
Question 80. Which particle of an atom is positively charged?
Answer: Proton.
Question 81. Which particle of an atom is neutral?
Answer: Neutron.
Important questions on atomic structure WBBSE Class 9
Question 82. What is a valence shell?
Answer: The outermost shell of an atom is called the valence shell.
Question 83. What do you mean by valence electrons?
Answer: Electrons present in the valence shell are called valence electrons.
Question 84. What are the isotopes of Helium?
Answer: 2He4 And 2He3.
Question 85. What is the maximum number of electrons that may exist in an orbit?
Answer: 2n2
86. In how many orbits the electrons may be distributed?
Answer: In 7 orbits numbered 1, 2, 3, 4, 5, 6, 7 or designated as K, L, M, N, O, P, Q.
Question 87. What do you mean by the statement that “The mass of a Hydrogen atom is almost equal to the mass of a proton”?
Answer: A hydrogen atom has only one proton in its nucleus and one electron in the outermost shell. The mass of an electron is negligible compared to that of the proton. Hence, it may be said that the mass of a Hydrogen atom is almost equal to the mass of a proton.
Question 88. Can an element have more than one atomic weight?
Answer: No.
Question 89. Which fundamental particle is responsible for producing isotopes?
Answer: Neutrons.
Question 90. Name the heaviest isotope of hydrogen.
Answer: Tritium is the heaviest isotope of hydrogen.
Question 91. Between ion and atom, which one is more stable and why?
Answer: Generally ion is more stable than an atom. Lons have stable electronic configurations. So, ions are more stable than atoms.
Question 92. In which parts of an atom do electrons, protons, and neutrons exist?
Answer: Neutrons and protons exist in the nucleus and electrons are present in different orbits around the nucleus.
Question 93. Name the heaviest particle and the lightest particle in an atom.
Answer: A neutron is the heaviest particle and an electron is the lightest particle in. an atom.
Question 94. Name the positively charged particle and the neutral particle in the structure of an atom.
Answer: The Proton present in the nucleus is positively charged and the neutron is neutral.
Question 95. What is a valence electron?
Answer: The electrons in the outermost. The orbit of an atom, which participates in chemical bonding, is called valence electrons.
Atomic Structure 2 Marks Questions And Answers
Question 1. What are isobaric elements? Give examples.
Answer:
Isobaric elements
If atoms of different elements have the same mass number but different atomic numbers, then these elements are known as isobaric elements.
For example, 26Fe57 and 2 7Co57 have the same mass number but their atomic weights are 26 and 27 respectively. Calcium – 46 and Titanium – 46 have the symbols, 20Ca46 and 22Ti45
and are isobaric elements. In the isobaric elements, the mass numbers are the same, but the numbers of protons and neutrons are different.
Question 2. Define Atom.
Answer:
Atom – The smallest particle of an element which may or may not exist independently but contains all the properties of that element and takes part in chemical reactions is called an atom.
Question 3. Why are the physical properties of isotopes different?
Answer: The mass numbers of the isotopes of an element are different. Hence, they contain different numbers of neutrons in their atoms. Due to differences in the number of neutrons, their physical properties like mass, density, N.P., B.P., etc. are different.
Question 4. What is Nuclide? Give example.
Answer:
Nuclide
A nuclide is a more or less unstable specific type of atom of definite mass no. and atomic number that exists for a measurable time. Eg.− 92U235
WBBSE Class 9 solved exercises on atomic structure
Question 5. What are fundamental particles? Why are they called ‘fundamental’?
Answer:
Fundamental particles: The sub-atomic particles, electrons, protons, and neutrons are known as fundamental particles.
Reason: Experimentally it is found that these particles are the primary components of all atoms of all elements, except ordinary hydrogen, the nucleus of which does not contain neutrons. That is why, they are known as fundamental particles.
Question 6. State some other sub-atomic particles other than electrons, protons, and neutrons.
Answer:
Other sub-atomic particles are :
(1) Positron
(2) Antiproton
(3) Messon
(4) Neutrino
(5) Antineutrino
(6) V-particle
(7) Deuteron, etc.
Question 7. Distinguish between atomic number and mass number.
Answer:
Difference between atomic number and mass number :
| Atomic Number | Mass Number |
| (1) It is equal to the number of protons present in the nucleus of an atom. | (1) It is equal to the sum of the number of protons and neutrons present in the nucleus of an atom. |
| (2) From the atomic number, several valence electrons can be determined, which in turn, gives the idea about the ability of the chemical combination of the atoms. | (2) Mass number gives an idea about the atomic mass of the element concerned but it does not give any idea about the chemical activity of the element unless the number of protons or the no. of neutrons is indicated. |
Question 8. Name the fundamental particles of an atom.
Answer:
An atom is mainly composed of three particles :
(1) Negatively charged particle: Electron
(2) Positively charged particle: Proton
(3) Electrically neutral particle: Neutron.
Question 9. Write the similarities and dissimilarities between,92U235 and 92U238 atoms.
Answer:
Similarities: Both of them contain the same number of protons and electrons (92).
Dissimilarities :
Number of neutrons in 92U235 = 235 – 92 = 143
Number of neutrons in 92U238 = 238 – 92 = 146
Question 10. Write the uses of isotopes.
Answer:
Uses of isotopes
(1) Radioactive isotopes are widely used for diagnostic purposes in medicine. Cobalt-60 is used in the treatment of cancer.
(2) The age of minerals, rocks, and earth can be determined with the help of a radioactive carbon-14 isotope.
Question 11. Define the atomic weight of any element in oxygen (O = 16) and carbon (C = 12) scales.
Answer:
(1) In oxygen (O = 16) scale: Atomic weight (relative atomic mass) of an element
From a specified source is the ratio of the average mass per atom of the element \(\frac{1}{16}\)to that of an oxygen atom.
∴ \(\text { Relative atomic mass of an element }\)=\(\frac{\text { average mass of an atom of the element }}{\frac{1}{16} \text { th jart of the mass of an oxygen atom }}\)
\(=\frac{\text { mass of an atom of the element }}{\text { mass of an oxygenatom }} \times 16\)
(2) In carbon (C =12) scale: An atomic weight or relative atomic mass of an element
From a given source is the ratio of the average mass per atom of the element to \(\frac{1}{12}\) the mass of an atom of 6C12.
∴ \(\text { Relative atomic mass of an element }\)=\(\frac{\text { average mass of an atom of the element }}{\frac{1}{12} \text { th jart of the mass of an carbon atom }}\)
\(=\frac{\text { averagemass of an atom of the element }}{\text { mass of a carbon atom }} \times 12\)WBBSE Class 9 Physical Science atomic models solutions
Question 12. Is there any difference between atomic weight and mass number? Explain with a suitable example. In most cases which is greater and why?
Answer:
Difference between atomic weight and mass number
| Atomic Weight | Mass Number |
| 1. Atomic weight represents the ratio of the average mass per atom of the element to 1/12th of an atom of 6c12 | 1. The mass number of an atom is the total number of protons and neutrons present in the nucleus. |
| 2. Atomic weight may be a fraction. | 2. A mass number is always a whole number and can’t be a fraction. |
In most of the cases mass number is greater than the atomic weight. Most of the elements have more than two isotopes. So, the mass number is greater than the atomic weight.
Example: Oxygen has three isotopes 8O16, 8O17, and 8O18. The atomic weight of oxygen is 16.
Mass numbers of 8O17 and 8O18atoms are greater than the atomic weight of the oxygen atom.
Question 13. Why is an atom electrically neutral?
Answer:
The number of protons in the nucleus and the total number of orbits of the atom are always equal. Again, the amount of positive charge is equal to the amount of negative charge of an electron. So the total negative charge is equal to the total positive charge and hence an atom is electrically neutral.
Question 14. Why do the electrons revolve around the nucleus?
Answer:
Electrons are revolving around the nucleus in different concentric circular paths or elliptical paths. There exists a strong electrostatic force of attraction between the positively charged nucleus and the negatively charged electrons. This force of attraction supplies the necessary centripetal force to the electrons to revolve around the nucleus.
Question 15. Why are the isotopes of an element placed at the same position in the periodic table? Or, Why are the chemical properties of the isotopes the same?
Answer:
Isotopes of an element with the same atomic number have the same number of electrons in their outermost shell Chemical property depends on the atomic number, i.e., the number of protons or, electronic configuration. As the electronic configuration of isotopes is identical, chemical properties are also identical. So, the isotopes are placed in the same position in the periodic table.
Question 16. What is the relation between 17A35 and 17A37 Are the chemical properties of these two above elements completely different or more or less the same? Justify with reasons.
Answer:
The atomic mass of 17A35= 35; the number of protons = 17. The atomic mass of 17A37= 37; the number of protons = 17; “both atoms contain 17 protons, but the number of neutrons is different. So, they are isotopes of each other. The chemical property of the element depends only upon the number of protons present in the nucleus of the constituent atom. In this case, as the number of protons is the same, so they have the same chemical properties.
Question 17. The atomic number is the fundamental property of an element but not atomic weight.
Answer:
The atomic number is the fundamental property of an element but not the atomic weight.
The atomic weight of an element may differ due to the presence of isotopes. However, the atomic number cannot be the same for two elements. Chemical properties change with a change in the atomic number. That is why, atomic number is considered as the fundamental property of an element rather than its atomic weight.
Question 18. The formula of an atom of an element is 92U238. Write the number of protons, and electrons.Neutronss are present in the atom.
Answer:
Given
The formula of an atom of an element is 92U238.
From 92U238 we know that atomic number = 92 and mass number = 238.
∴ The nucleus of 92U238nuclide contains 92 protons and (238-92) or 146 neutrons. Number of electrons = 92.
Subatomic particles Class 9 WBBSE notes
Question 19. What is the atomic mass unit? 1 amu = how many grams?
Answer:
Atomic mass unit
An atomic mass unit (amu) is a unit that is used to express the atomic or molecular masses. One atomic mass unit = 1/12 the part of the mass of one carbon atom (C-12) at rest and in its ground state.
1 amu or 1 u = 1°6603 X 10-24 g.
Question 20. Compare Proton and Neutron.
Answer:
Similarities :
(1) Both are heavier than electrons.
(2) Both exist in the nucleus.
Dissimilarities :
(1) Proton is positively charged. Neutron is neutral.
Question 21. Write the number of electrons in 40 K 19. Write the electronic arrangement of the atom having this number of electrons.
Answer:
Number of electrons – 19
Electronic configuration − K shell – 2, L shell – 8, M shell – 8, N shell – 1
Question 22. What is an ion? Between an atom and an ion of an element, which particle present in them differs in number?
Answer:
Ion
Lons are electrically charged atoms or groups of atoms. Lons are produced when an atom either gives up electron(s) or accepts electrons or a salt undergoes ionization by the effect of solvent or heat.
Anions are the acidic part of salts. As, Cl–, SO2-4, NO–3, and OH– are anions.
Electronic configuration ofMg+2= K shell = 2, L shell = 8.
Question 23. The atom of an element contains 2 electrons in the K shell, 8 electrons in the L shell, 8lectrons in the M shell, and 2 electrons in the N shell. What is the atomic number and valency of the element?
Answer:
Given
The atom of an element contains 2 electrons in the K shell, 8 electrons in the L shell, 8lectrons in the M shell, and 2 electrons in the N shell.
The atomic no. of the element = (2 + 8 + 8 + 2) = 20.
∴ Valency of the element = 2.
Question 24. There are 2 electrons in the K shell, 8 electrons in the L shell, and 6 electrons in the M shell in the atom of an element. What is the atomic number of the element? What is the maximum valency of the element? What is the number of neutrons in the atom of the element if the mass number of the element is 32?
Answer:
Given
There are 2 electrons in the K shell, 8 electrons in the L shell, and 6 electrons in the M shell in the atom of an element.
The atomic no. of the element = no. of protons
= total no. of electrons in K, L, and M shells.
=2+8+6=16.
Maximum valency of the element = 18 − 16 = 2.
No. of neutrons = Mass no − Atomic no.
= 32-16 = 16.
Question 25. What is meant by,8O16? Show its electronic configuration.
Answer:
8O16
(1) It is an isotope of oxygen.
(2) The symbol of the element is “O”.
(3) The atomic no. of the element is 8 and its mass no. is 16.
Electronic configuration of,8O16 is 2, 8, 6.
Question 26. How many protons and neutrons are there in the atom
143 57X
Answer: 143 57 X
Protons = 57
Neutrons = 143 − 57 = 86
Question 27. There are 2, 8, and 7 electrons in the K, L, and M shells respectively of an atom of an element. What is the atomic number of the element? What is its valency? What is the number of neutrons if the mass number of the element is 37? To which group of the periodic table does the element belong?
Answer:
Given
There are 2, 8, and 7 electrons in the K, L, and M shells respectively of an atom of an element.
The atomic no. of the element =2+8+7.
Valency of the element =18-17 =1
No. of neutrons = Mass no− Atomic no.
= 37-17 =20
It belongs to group VII.
Question 28. The atomic weight of chlorine is 35.46 concerning the atom “O”. What does it mean?
Answer:
One atom of chlorine is 35.46 times heavier than \(\frac{1}{16}\) th part.of an oxygen atom.
Question 29. What do you mean by nuclear force?
Answer:
Nuclear force
The protons and neutrons (nucleus) are bonded inside the nucleus by a strong attractive force which is called the nuclear force. The force exists between proton-proton, proton-neutron, and neutron-neutron. If, in a nucleus, the number of protons is much more than the number of neutrons or the number of neutrons is much more than the number of protons, the nuclear force becomes weak. The nuclei of such elements become unstable and tend to disintegrate.
Question 30. Find the relation between mass number and atomic number.
Answer:
Relation between mass number and atomic number
The mass number of an atom = several protons + several neutrons.
Let the number of protons = atomic number = Z
number of neutrons = N
and mass number of an atom = A
Then, A=Z+N.or Z=A-N
Atomic number = mass number – number of neutrons.
Question 31. Give the maximum number of electrons in the first four orbits.
Answer:
| ORBIT | Orbit Number (n) | Maximum number of electrons in the orbit |
| First orbit or K-Shell | 1 | 2 x 12= 2 |
| Second orbit or L-Shell | 2 | 2 x 22 = 8 |
| Third orbit or M-Shell | 3 | 2×32= 18 |
| Fourth orbit or N-Shell | 4 | 2 x 42= 32 |
Question 32. Explain how the mass number and atomic number of chlorine are represented symbolically.
Answer:
The symbol of Chlorine can be written as
17 Cl 35or 17 Cl 37
Here, A= 35, Z=17
N= A – Z
∴ N= 35 -17 =18
Question 33. Define isotopes. Give examples.
Answer:
Isotopes: The atoms of the same element having the same atomic number but different mass numbers are called isotopes.
For example, hydrogen has three isotopes: 1H1,1H2, and 1H3
Oxygen has three isotopes: 8 O16, 8 O17 and 8O18
Chlorine has two isotopes: 17 Cl 35 and 17 Cl 37
Question 34. State the properties of Isotopes.
Answer:
The main properties of isotopes are :
(1) The number of protons (atomic number) in all the isotopes of an element is the same.
(2) The electronic configurations of all the isotopes of the same element are similar.
(3) They have same number of valence electrons.
(4) The physical properties such as mass, density, melting points, boiling points, etc. of the isotopes of the same element are different.
Question 35. Why is the average atomic weight of chlorine fractional? Give a reason.
Answer:
The average atomic weight of chlorine is taken as 35.5. The fractional atomic weight of chlorine is because natural chlorine has three parts of 17 Cl 35 and one part of 7 Cl 37
The atomic mass of 3 atoms of17 Cl 35=3 x 35 = 105 a.m.u.
The atomic mass of 1 atom of 17 Cl 37 = 1 x 37 = 37 a.m.u.
Atomic mass of 4 atoms of natural chlorine = 105 + 37 = 142 a.m.u.
Average atomic mass of chlorine = 142 ÷ 4 = 35.5 am. u.
Hence, the average atomic weight is fractional.
Question 36. Define isobars. Give examples.
Answer:
Isobars: Isobars are the atoms of different elements having different atomic numbers but the same mass number. Isobars have different numbers of protons, electrons as well as neutrons. So they have different physical as well as chemical properties.
Example: 18Ar40, 19K40, 20Ca40 – each has the same mass number but different atomic numbers. That is why, they are known as isobars of each other.
Question 37. What are the differences between isotopes and isobars? Explain with suitable examples.
Answer:
The differences between isotopes and isobars
| Isotopes | Isobars |
| (1) Isotopes are the atoms of the element having the same atomic number but different mass numbers. | (1) Isobars are the atoms of different elements having different atomic numbers but the same mass number. |
| (2) Isotopes have identical chemical properties | (2) Isobars have different chemical properties. |
| Example: 8 O16 and 8 O17 are isotopes of each other as they have the same atomic number but different mass numbers. | Example: 18Ar40 and 19K40 are isobars of each other as they havethea same mass number but different atomic numbers. |
Question 38. Write the number of protons and neutrons present in the nuclide,6 A13. Write the electronic configuration of an atom of the above element. Write the difference between the structures of the nuclides 6A13 and,6 A12. Why are the chemical properties of these two nuclides the same? (A = symbol of an element).
Answer:
In, 6 A13 nuclide, the number of protons = 6 and the number of neutrons = (13 – 6) or 7.
(1) See of electrons = 6. Among these 6 electrons, 2 electrons are in K orbit and 4 electrons are in L orbit.
(2) The nucleus of 6 A13 nuclides contains 6 protons and 7 neutrons. On the other hand, the nucleus of 6 A12 contains 6 protons and 6 neutrons.
6 A13 contains one more neutron than, 6 A13.
(3) Due to the presence of the same number of protons, i.e., the same atomic number, the chemical properties of both are the same.
Question 39. Write the mass number and atomic number of the atom 92X235. If there is one more neutron, how will the element be expressed?
Answer:
(1)Mass number of 92X235, A = 235 and atomic number, Z = 92.
…Number of neutrons present, N = A – Z + 235 – 92 =143.
(2) If one more neutron is present in that atom, the mass number will also be increased by one unit.
In that case, the new element will be expressed as 92X235+1= 92X235.
Atomic Structure 3 Marks Questions And Answers:
Question 1. Describe J.J. Thomson’s atomic theory
Answer:
J.J. Thomson’s atomic theory
Thomson discovered the electron in the year 1897. His work put forward a new theory that the atom was made up of small particles. Thus, he discovered the electrons. He proved his theory using the cathode ray tube. J. Thomas used a highly evacuated discharge tube. He placed two anodes inside the tube.
He fixed two plates parallel to each other inside the tube. He passed a thin cathode ray through the pinhole of the tube. He could see the sides of the tube glowing in green color. The glow traveled in a straight line. The green glow was caused by the cathode rays. Now, Thomson applied an electric field. The ray deflected to the positively charged plate. Thus, Thomson discovered the electrons.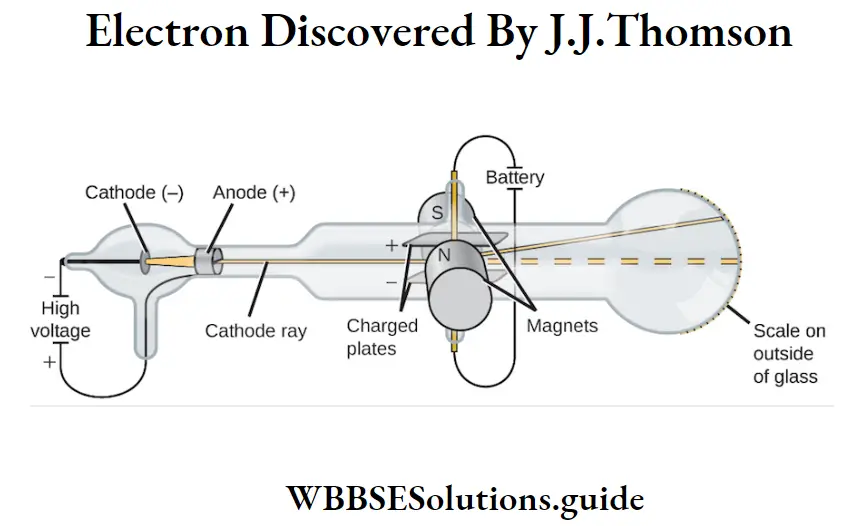
Question 2. State the importance of J.J. Thomas’s experiment.
Answer:
Importance of J.J. Thomas’s experiment
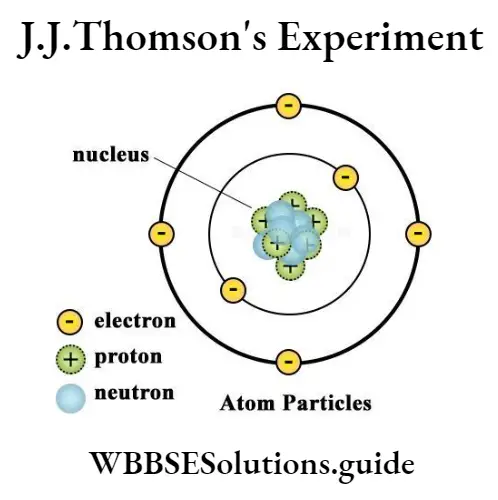
Thomson discovered the electrons and it was q proved that atoms were made up of protons, electrons, and neutrons. Thus, Thomson proved that the atom was divisible. Since the atom was neutral, Thomson ‘suggested that the negatively charged electron equaled the positively charged proton and neutrons had no charges.
Thomson suggested to consider the atom as a sphere. It has positively charged particles. The positively charged particles were surrounded by the negatively charged electrons. The electrons were placed there atom Particles due to electrostatic forces.
Question 3. Describe Rutherford’s alpha particle experiment.
Answer:
Rutherford’s alpha particle experiment
In 1910, a physicist from New Zealand, Ernest Rutherford performed an experiment known as Rutherford’s gold foil experiment. This experiment determined to find out the structure of an atom.
The alpha particles were confined to a narrow beam by passing them through a lead sheet through a slit. An extremely thin gold foil was bombarded with a narrow beam of fast-moving alpha particles.
On bombarding, the alpha particles were scattered in different directions with different angles and were detected by the fluorescent rotatable detector, which has a microscope and a screen coated with zinc sulfide. The whole experimental setup was placed in an evacuation.

Chamber to prevent scattering by the air molecules. These particles after striking the screen caused scintillations. Before performing this experiment it was assumed by Rutherford that most of the alpha particles would pass through the gold foil with less deflection.
He assumed this based on the theory proposed by J.J. Thomson. This was assumed because the alpha particles are heavy and the negative charge in the “plum pudding model” is widely spread.
Question 4. Describe Rutherford’s Atomic Model.
Answer:
Rutherford’s Atomic Model
Rutherford’s atomic model, also known as the planetary model is a model of the atom proposed by the physicist Ernest Rutherford.
The following are the main points of Rutherford’s theory :
(1) Most of the part of an atom is empty.
(2) Approximately all the mass of the atom is concentrated at the center of the atom which is now called the nucleus.
(3) In the central region of the atom the positively charged particles are present.
(4) The charge on the nucleus of an atom is positive and is equal to Z.e where Zis charge number, e is the charge of a proton.
(5) The negatively charged particles, i.e., electrons revolve around the central positive portion in different circular orbits.
(6) The central region (nucleus) is very small in size compared to the size of an atom.
Bohr Model Of Atom Class 9
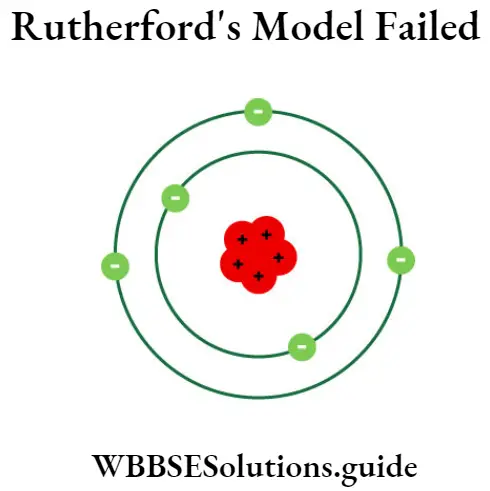
Question 5. State the limitations of Rutherford’s model.
Answer:
Limitations of Rutherford’s model
Rutherford’s model did not make any new headway in explaining the electronic structure of the atom. Rutherford’s concentration of most of the atom’s mass into a very small core made some type of planetary model, as such a core would contain most of the atom’s mass, similar to the sun containing most of the solar system’s mass. Rutherford’s model was later improved and quantified by one of his students, Niels Bohr, with the known Bohr’s model of the atom.
There were mainly two defects in Rutherford’s atomic theory which are shown as follows:
1. Being a charged particle, the electron must emit energy when it is accelerated, according to classical electromagnetic theory. We know that around the nucleus the motion of electrons is accelerated, hence it must radiate energy. But this does not happen in actual practice.
Assume that if it occurs then due to continuous loss of energy orbits of electrons must decrease continuously. As a_ result electrons will fall into the nucleus eventually after some time. But this is against the practical situation and hence this shows that the atom is unstable.
2. If the electrons emit energy continuously, a continuous spectrum should be formed. But practically, line spectrum is observed.
Bohr Model Of Atom Question 6. Describe Bohr’s model of the atom.
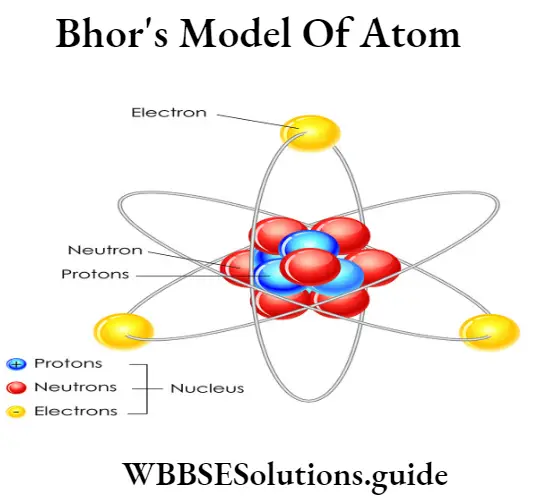
Answer:
According to Bohr’s theory :
(1) The atom consists of a small positively charged nucleus at its center.
(2) The whole mass of the atom is concentrated at the nucleus and the volume of the nucleus is smaller than the volume of the atom by a ratio of about 1:10°.
(3) The nucleus contains all the protons and neutrons of the atom.
(4) The electrons of the atom revolve around the nucleus in definite circular paths known as orbits which are designated as K, L, M, N or numbered as n=1, 2, 3, 4 outward from the nucleus.
(5) Each orbit is associated with a fixed amount of energy. Therefore, these orbits are also known as energy levels or energy shells.
Bohr Model Of Atom Class 9 WBBSE
Question 7. Compare all the proposed models of an atom by J. J. Thomson, Rutherford, and Neils Bohr.
Answer:
J. J. Thomson: Since discharge tube experiments suggested the presence of negatively charged particles in a neutral atom, J. J. Thomson suggested that electrons are embedded in a sphere of positive charge.
E. Rutherford: The good foil experiment of Rutherford suggested that all the positive charge is located in a very small space which is 10° times the radius of an atom. Therefore, Rutherford gave a model in which electrons are revolving around the nucleus.
Neils Bohr: To explain the stability of an atom and atomic spectra, Bohr suggested that electrons move around the nucleus in orbits that have fixed energy shells. There is a loss or gain in energy of an electron when it moves from one orbit to the other.
Question 8. How did Neils Bohr modify Rutherford’s model?
Answer:
Rutherford’s model failed to explain the electromagnetic principles, stability of an atom, line spectra of an atom, etc. In 1913 Neils Bohr modified Rutherford’s model by taking the help of quantum theory.
Structure Of Atom
Question 9. Describe the structure of the atom based on modern theory.
Answer:
Structure of the atom based on modern theory
Based on a modern concept, the atom is built up of tiny particles called sub-atomic particles—proton, and neutron. Atoms can be divided into two parts — the nucleus and the extranuclear part.
Bohr Model Of Atom Class 9 WBBSE
Nucleus: Protons and neutrons of an atom are packed in extremely small volumes of the Electron nucleus at the center of the atom. Shells All the protons are positively charged and hence nucleus has a positive charge. The proton and neutrons present in the nucleus together are called nuclei.
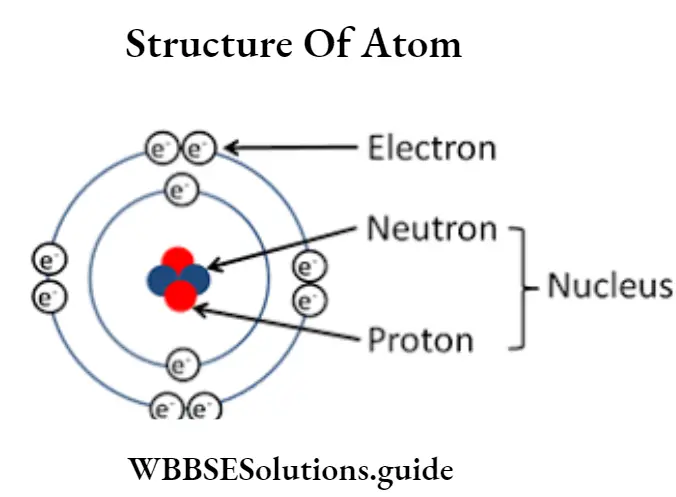
The whole of the mass of an atom is due to the nucleus Electron (e7) containing proton and neutron. Electrons revolving outside are of negligible mass. Hence, the sum of neutrons and protons in the nucleus is known as the mass number.
The nucleus is the central part of the atom which contains protons and neutrons. Protons are positively charged particles while neutrons have no charge. Electrons revolve around the nucleus in different orbits having negligible mass. Electrons are negatively charged particles. The number of electrons is equal to the number of protons in an atom. The number of neutrons may or may not be equal to that of a proton.
Structure Of Atom
Question 10. Describe the fundamental particles of an atom.
Answer:
Fundamental particles of an atom
The main and fundamental particles of an atom are electrons, protons, and neutrons.
Electron: Electrons are negatively charged particles revolving around the nucleus in the extranuclear part of the atom, in the definite orbit (marked C in the given figure). The number of electrons is the same as the number of protons in an atom.
The charge on an electron is 4.8033 x10-10 e.s.u and in S.l. unit is 1.6022 x 10-19
Coulomb. The mass of electron in C.G.S unit = 9.0196 x10-38 gm and in a.m.u. = 0.00054859.
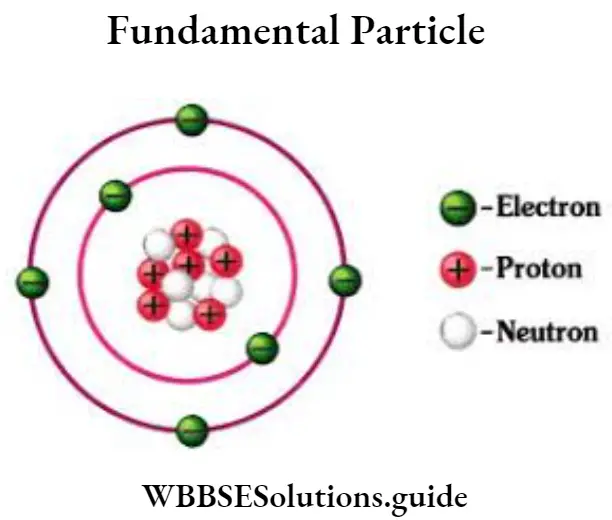
That electrons are negatively charged particles, is verified by the cathode rays tube.
Protons: Proton, the lightest positive charged particle, resides in the nucleus (marked A in the given figure). Its mass is about 1837 times the mass of an electron. The number of protons and electrons is equal in an atom.
The charge of a proton is 4.8033 x 10-10 e.s.u. and S.1. unit is 1.6022 x 10-19coulomb.
The mass of a proton is 1.6726 x 10-24 gm and in a.m.u. is 1.007277.
Neutron: Neutrons exist in the nucleus and contain no charge (marked. as B i). The number of neutrons is independent of the number of protons. Ordinary hydrogen contains no neutrons.
The mass of neutrons in grams is 1.6749 x10-24 and in amu, the unit is 1.008665.
Thus, the mass of one neutron is approximately equal to the mass of one proton.
Best study material for atomic structure WBBSE Class 9
Question 11. Define an atomic number of an element. State its main characteristics.
Answer:
Atomic number: The number of protons in the nucleus of an atom of an element is called its atomic number. Since an atom is electrically neutral, the number of protons is equal to the number of electrons in an atom.
The characteristics of atomic number are the following :
(1) The atomic number, of an element Represents its fundamental property. No two elements can have the same atomic number.
(2) An element is identified by its atomic number.
(3) The atomic number of an element is stable. It does not change during a chemical reaction.
(4) Atomic number of an element is usually denoted by the letter Z.
Question 12. What are the main points of similarities between the solar system and atomic structure?
Answer :
The similarities existing between the solar system and atomic structure are the following given below :
| Solar System | Atomic Structure |
| 1. In the solar system, planets revolve around the central sun in different fixed orbits. | 1. In atomic structure, the electrons revolve around the nucleus in different orbits. |
| 2. Most of the space between the sun and the planets is empty. | 2. Most of the space between the nucleus and electrons is empty. |
| 3. The mass of the sun is several times greater than that of any other planet. | 3. The mass of the nucleus is many times greater than the revolving electrons. |
| 4. The path of rotation of planets around the sun is not circular but slightly elliptical. | 4. The rotational paths of electrons are circular and elliptical. |
| 5. There exists a gravitational force between the sun and rotating planets, necessary for the rotation of planets. | 5. There exists an electrostatic attractive force between the positively charged nucleus and negatively charged extra nuclear electrons, necessary for the rotation of electrons. |
| 6. The planets rotate about their axis while revolving around the sun. | 6. Electrons rotate about their axis while revolving around the nucleus. |
Question 13. Explain the electronic configuration of an atom in its extranuclear part. Circular and elliptical both.
Answer:
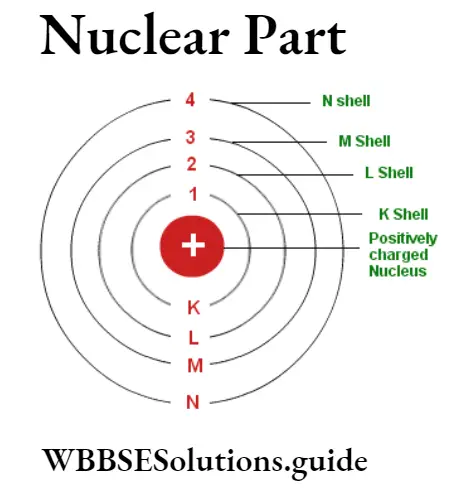
Electrons revolve around the nucleus part of the atom in definite orbits. These orbits are known as energy levels quantum numbers or shells. These orbits are known as first shell or K-orbit, second shell or L-orbit, third shell or M-orbit, etc. These orbits are K, L, M, N, O, P, and Q orbits.
The Bohr-Bury. scheme presented by Bohr and Bury in 1921 regarding filling the orbits of atoms with electrons is given as :
(1) An orbit can have a maximum number of2n2 electrons, where n represents the number of the orbit.
(2) The outermost orbit can not have more than 8 electrons and the inner orbit can not have more than 18 electrons.
(3) Before the completion of an orbit the next orbit starts filling, as soon as the outermost orbit gets 8 electrons, the next orbit starts filling.
(4)An atom becomes stable when it has 8 electrons in its outermost orbit.
Question 14. Mention the dissimilarities between the solar system and the structure of atoms.
Answer :
Following are the main points of dissimilarities between the solar system and the structure of atoms.
| Solar System | Atomic Structure |
| 1. In the solar system, there is one planet in one orbit. | 1. In atomic structure, the number of electrons in an orbit may be one or more than one. |
| 2. The force acting between the sun and the planets is gravitational. | 2. The force acting between the nucleus and electrons is electrostatic. |
| 3. The planets experience gravitational attraction between them. | 3. The force acting between electrons is repulsive. |
| 4. In the solar system the planets are of different masses and different volumes. | 4. In atomic structure all the electrons are of the same mass and volume. |
| 5. The planetary orbitals lie nearly in the same plane. | 5. In atomic structure, the electrons’ orbitals lie in different planes. |
| 6. The sun and planets are not electrically charged. | 6. The nucleus is charged positively and electrons are charged negatively. |
| 7. Some of the planets in the solar system have satellites as Earth has a moon. | 7. Electrons in an atom do not have satellite electrons. |
| 8. The planets do not escape from the solar system. | 8. Electrons can leave an atom or more electrons can be gained by an atom. |
Question 15. Define the mass number of an element. State its main characteristics.
Answer:
Mass number: The total number of protons and neutrons in one atom of an element is called its mass number.
The characteristics of mass number are the following :
(1) Mass number = number of protons + number of neutrons.
(2) It is usually denoted by the letter A.
(3) A mass number is always a whole number. Since the number of protons and the number of neutrons are in whole numbers, hencé mass number cannot be in fractions.
Question 16. How are the mass number and atomic number of an element represented symbolically? Give the example of oxygen.
Answer:
Generally mass number of an element is represented on the upper left or right side of the symbol and the atomic number in subscripts is on the lower left side of the symbol of the element.
For example, the symbol of oxygen can be written as 6O16 or 8O16
where, mass number = 16, atomic number = 8.
The number of neutrons = A- Z=16-8=8.
Question 17. Explain the electronic configurations of some elements from hydrogen to calcium element.
Answer:
The electronic configurations of some elements are given in the following table :
| Element | Symbol | Atomic number | Electronic arrangement In different orbit | |||||
| K | L | M | N | 0 | P | |||
| Hydrogen | H | 1 | 1 | |||||
| Helium | He | 2 | 2 | |||||
| Lithium | U | 3 | 2 | 1 | ||||
| Berylium | Be | 4 | 2 | 2 | ||||
| Boron | B | 5 | 2 | 3 | ||||
| Carbon. | C | 6 | 2 | 4 | ||||
| Nitrogen | N | 7 | 2 | 5 | ||||
| Oxygen | O | 8 | 2 | 6 | ||||
| Fluorine | F | 9 | 2 | 7 | ||||
| Neon | Ne | 10 | 2 | 8 | ||||
| Sodium | Na | 11 | 2 | 8 | 1 | |||
| Magnesium | Mg | 12 | 2 | 8 | 2 | |||
| Aluminium | Al | 13 | 2 | 8 | 3 | |||
| Silicon | Si | 14 | 2 | 8 | 4 | |||
| Phosphorus | P | 15 | 2 | 8 | 5 | |||
| Sulfur | s | 16 | 2 | 8 | 6 | |||
| Chlorine | s | 16 | 2 | 8 | 7 | |||
| Argon | Ar | 18 | 2 | 8 | 8 | |||
| Potassium | K | 19 | 2 | 8 | 8 | 1 | ||
| Calcium | Ca | 20 | 2 | 8 | 8 | 2 |
Question 18. What is orbital? What are the differences between orbit and orbital?
Answer:
Orbital: An orbital may be defined as a region in the space around the nucleus where the probability of finding the electron is maximum.
The main differences between orbits and orbitals are :
(1) Orbit is a well-defined circular path around the nucleus in| which electrons revolve. Orbital represents the region in space around the nucleus in which the probability of finding the electrons is maximum.
(2) Orbit represents the planar motion of an electron. Orbital represents the three-dimensional motion of an electron around the nucleus.
(3) All orbits are circular. Orbitals have different shapes. The orbitals are s, p, d, f, …, etc. The s – s-orbital is spherical, the p-orbital is dumbbell-shaped, etc.
(4) Orbits have a definite path of an electron, while orbitals do not specify any definite path.
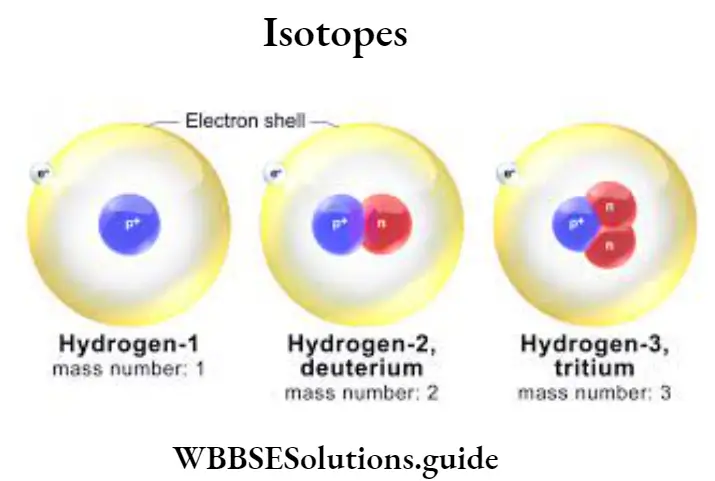
Question 19. Describe the different isotopes of hydrogen gas.
Answer :
Hydrogen gas has three isotopes :
(1) Ordinary hydrogen (Protium)
(2) Deuterium, and
(3) Tritium. Ordinary hydrogen (Protium) is represented by 1H1
Mass number = 1
Number of protons (Atomic number) = 1
Number of electrons = 1.
Number of neutrons = 0
Deuterium is represented by 1H2
Mass number = 2
Number of protons (Atomic number) = 1
Number of electrons = 1
Number of neutrons = 1
Tritium is represented by 1H3
Mass number = 3
Number of protons (Atomic number) = 1
Number of electrons = 1
Number of neutrons = 2
Diagrammatically they are expressed as below :
Question 20. What are the differences between atomic weight and the weight of an atom?
Answer:
The differences between atomic weight and the weight of an auto
| Atomic Weight | Weight of an atom |
| 1. Atomic weight does not represent the actual weight of an atom. | 1. The weight of an atom represents its actual weight. |
| 2. Atomic weight represents the ratio of the average mass per atom of the element to 1/12th of an atom of 6c12 | 2. The weight of an atom represents the summation of masses of all constituent particles present in that atom. |
| 3. Since atomic weight is a ratio of two weights, it is a dimensionless physical quantity. | 3. The weight of an atom has a definite unit |
| Example: The atomic weight of hydrogen is 1.0078, which is a pure number. | Example: The actual weight of a hydrogen atom is 1.6725 x 10-24g). |
WBBSE class 9 atomic structure question and answers
Question 21. Define an ion. State its different types.
Answer:
Ion
When an atom loses one or more electrons from an outermost orbit or gains one or more electrons in that orbit, then the resultant electronically Onaged particle is called an Ions are of two types: cations and anions.
(1) Cation: Cation is a positively charged atom or radical. When an atom loses one or more electrons from its outermost orbit, it becomes. A cation (positively charged particle).
For example : Na-e– → Na+
(2,8,1) (2,8)
(2) Anion: Anion is a negatively charged atom or radical. When an atom of an element accepts one or more electrons in its outermost orbit, the atom becomes an anion (negatively charged).
Cl +e– → Na+
(2,8,7) (2,8,8)
Question 22. Is there any difference between atomic weight and mass number? Explain with a suitable example. In most cases which is greater and why?
Answer:
| Atomic Weight | Mass Number |
| 1. Atomic weight represents the ratio of the average mass per atom of the element to 1/12th of an atom of 6c12. | 1. The mass number of an atom is the total number of protons and neutrons present in the nucleus. |
| 2. Atomic weight may be a fraction. | 2. A mass number is always a whole number and can’t be a fraction. |
In most of the cases mass number is greater than the atomic weight. Most of the elements have more than two isotopes. So mass number is greater than the atomic weight.
Example: Oxygen has three isotopes 8 O16, 8 O17, and 8 O18. The atomic weight of oxygen is 16. Mass numbers of 8 O17 and 8 O18 atoms are greater than the atomic weight of an oxygen atom.
Question 23. Why are atomic weight and mass number of all elements not the same? For which element are these same?
Answer:
Atomic weight and mass number have different values due to the presence of different numbers of isotopes of a particular element. This is because the atomic weight of those elements is the average of the mass numbers of isotopes present in different ratios.
(1)Example: isotopes of chlorine having mass numbers 35 and 37 are present in the chlorine gas in 75.4 % and 24.6 % respectively, so
atomic weight of chlorine\(=\frac{35 \times 75.4+37 \times 24.6}{100}\)\(=35.46 \approx 35.5\)
∴ The atomic weight and mass number of chlorine are different.
(2)Elements that have no isotopes have identical atomic weights and mass numbers.
(3)Example: Sodium has no isotope. The atomic weight and mass number of sodium are the same (23).
Question 24. Write the importance of Dalton’s atomic theory.
Answer:
Importance of Dalton’s atomic theory
(1) Dalton’s atomic theory for the first time stated that the atom is the smallest particle of an element which is a revolutionary idea in science.
(2) Atomic theory can explain how atoms of different elements combine to form compounds.
(3) Atomic theory can explain the law of conservation of mass and other laws of chemical combination (except Gay Lussac’s law).
(4) Avogadro’s hypothesis. The concept of molecules was derived from Dalton’s atomic theory.
WBBSE class 9 atomic structure question and answers
Question 25. Compare electron and proton.
Answer:
Similarities :
(1) Both are present in all atoms of all elements.
(2) Both are electrically charged particles.
Dissimilarities :
(1) The electron is negatively charged. The proton is positively charged.
(2) A proton is 1836 times heavier than an electron.
(3) Proton exists in the nucleus. Electrons rotate around the nucleus in definite orbits.
Question 26. State the difference between atoms and ions.
Answer:
Difference between atoms and ions
| Atoms | Ions |
| 1. An atom is electrically neutral. The number of protons in the nucleus is the same as the number of electrons in its shells. | 1. An ion is an electrically charged particle formed either by accepting an electron or donating an electron by an atom of an element. |
| 2. The electronic configuration of atoms in their outermost shell ranges from 1 to 7 electrons (except noble gases). | 2. All ions have an electronic configuration of either 2 or 8 in their outermost shells. |
| 3. An atom may or may not be able to exist independently. | 3. An ion cannot exist independently. |
| 4. The properties of an atom are independent of the properties of its ions. | 4. The properties of ions are different from that of atoms. |
WBBSE Class 9 Bohr’s and Rutherford’s model solutions
Question 27. How is the nucleus stable though it contains positively charged protons?
Answer:
In the nucleus, there should be repulsion between positively charged particles and protons and the nucleus should be unstable, but this is not happening. This contradiction was solved by Yukawa. According to him, protons emit π+ meson which is absorbed by neutron.
p −π+ → n
n+ π+ → p
Thus, the conversion of proton into neutron and neutron into proton is responsible for the origin of nuclear force between them which binds the nucleons. Thus, the nucleus becomes stable.
Question 28. Compare an electron, a proton, and a neutron concerning their masses, charges, and positions in the atom.
Answer:
| Name of the particle and symbol | Mass (g) | Amount and nature of charge | Position In atom |
| Electron (e or 1e0) | 9.11×10-28 | 4.8x 10-10esu ofcharge or 1.6×10-19 coulomb, negatively charged | In different orbits outside the nucleus of an atom |
| Proton (P or 1H1) | 1.6725×10-24 | 4.8x 10-10 esu o charge or 1.6×10-19 coulomb, positively charged | Inside the nucleus of an atom |
| Neutron(n or 0n1) | 1.675×10-24 | 0, neutral | Inside the nucleus of an atom |
Question 29. An ion is more stable than an atom. Explain.
Answer:
An ion is more stable than an atom.
An ion attains stability when its parent atom gains or loses electrons, as the case may be. The stability of the ion is total if its parent atom gains or loses electrons to its full capacity and the stability is partial if the process of gain or loss of electrons is partial at a certain stage. For example, a calcium atom of atomic number 20 (2, 8, 8, 2) can lose 2 electrons to establish the stable octet state, but if at a certain stage of its incomplete ionization process, it loses one electron producing the unstable Ca+ ion its
Stability is partial; ultimately when it loses both the electrons producing the Ca+ + ion, it is stable. An ion is stable if it does not at all tend to gain or lose electrons. Hence, an ion is more stable than an atom.
Question 30. The atomic number of an element is 2 and its mass number is A. What is the structure of the nucleus? Name the element.
Answer:
The atomic number of an element is 2 and its mass number is A.
The number of protons in the nucleus of the atom is 2 since the atomic number is 2. Again, its mass number is 4, number of neutrons is 4 – 2 of 2. Thus, the nucleus contains 2 protons and 2 neutrons.
Also, the number of protons equals the number of electrons. The number of electrons in the K shell is 2. The element is helium.
Question 31. Describe the structures of the nuclei of different isotopes of carbon.
Answer:
There are three isotopes of carbon: 6A12, 6A13 and 6A14
| Isotopes | Mass Number | Atomic Number | Number of protons =AtomNumberic | Number of neutrons =mass number -atomic number |
| 1.6c12 | 12 | 6 | 6 | 12 – 6 =6 |
| 2. 6c13 | 13 | 6 | 6 | 13-6=7 |
| 3. 6c14 | 14 | 6 | 6 | 14-6 = 8 |
Question 32. Describe the structures of the nuclei of the three isotopes of oxygen.
Answer:
| Isotopes | Mass Number | Atomic Number | Number of protons = Atomic Number | Number of neutrons = mass number -atomic number |
| 1. 8c16 | 16 | 8 | 8 | 16-8 = 8 |
| 2. 8c17 | 17 | 8 | 8 | 17-8 = 9 |
| 3. 8c18 | 18 | 8 | 8 | 18-8= 10 |
Question 33. Describe the structures of the nuclei of different isotopes of uranium.
Answer:
| Isotopes | Mass Number | Atomic Number | Number of protons = Atomic Number | Number of neutrons = mass number -atomic number |
| 1. 92U235 | 235 | 92 | 92 | 235 – 92 = 143 |
| 2. 92U238 | 238 | 92 | 92 | 238 – 92 = 146 |
| 3. 92U239 | 239 | 92 | 92 | 239 – 92 = 147 |
WBBSE class 9 atomic structure question and answers
Question 34. Write the limitations of Dalton’s atomic theory.
Answer:
Limitations of Dalton’s atomic theory
(1) The concept that atoms are indivisible is no longer valid. Now, it is known that atoms are divisible and composed of sub-atomic particles like protons, neutrons, electrons,s and other particles.
(2) This theory fails to explain Gay Lussac’s law of gaseous volumes.
(3) Atoms of the same element are always the same in all respects and atoms of different elements are different in all respects this statement is also not correct. After the discovery of isotopes, it is known that atoms of the same element may differ in mass and physical properties. Similarly, after the discovery of isobars, it is known that atoms of different elements may have the same mass.
(4) At present, the atoms of Uranium like heavy elements can be split to produce several atoms of different lighter elements by the process of nuclear fission. Again, an atom of a new heavy element can be formed from several hydrogen atom-like atoms at a very high temperature through the process of nuclear fusion. So, atoms can be created or destroyed.
(5) Dalton’s atomic theory fails to explain how atoms combine to form molecules.
(6) Dalton gave an idea regarding atoms. He was not able to give any idea regarding the molecules of an element or a compound. Thus, he even considered the smallest particle of a compound to be an atom. This is also a limitation of Dalton’s atomic theory.
Question 35. What are the numbers of protons and neutrons in a 4020 X atom? Write down the electronic configuration of the atom.
Answer:
4020X Number of protons = 20
Number of neutrons = 40 − 20 = 20
Electronic configuration
K (Shell) – 2
L (Shell) – 8
M (Shell) – 8
N (Shell)- 2 electrons and the number of protons are the same in these atoms.
Question 36. Define atomic mass. Can the atomic mass and molecular mass be the same for any element? Answer with reason.
Answer:
Atomic mass: The atomic mass of an element is defined as the average relative mass of an atom of an element as compared to the mass of an atom of Carbon (C12) taken as 12.
In other words, atomic mass is a number that expresses how. Many times an atom of the element is heavier than 1/12 th of the mass of a Carbon atom(C12). Therefore,
\(\text { Atomic mass }=\frac{\text { Mass of an atom }}{1 / 12 \text { Mass of a Carbon atom }\left(\mathrm{C}^{12}\right)}\)The atomic mass and molecular mass are the same for only monoatomic elements. Example Sodium. Because the symbol and formula for sodium is Na.
The atomic mass of sodium = 23.
The molecular mass of sodium = 23.
WBBSE class 9 atomic structure question and answers
Question 37. What is the relation between 35 17A and 37 17A? What are the number of neutrons in each of them? What is an ion?
Answer:
The relation between 35 17A and 37 17A
The nuclides 35 17A and 37 17A differ in mass number (35 & 37), and hence, in the number of neutrons. The former contains 2 more neutrons than the latter. The two species, despite having different mass numbers, have the same number of protons or atomic numbers (17), and hence, contain the same number of protons or electrons. So they are isotopes of each other and will have the same chemical properties.
Both have the same number of protons (17) and extra-nuclear electrons (17).
17 Cl 35 contains two more neutrons (20) than 17 Cl 35 (number of neutrons in it = 35 − 17 = 18).
38. What is the relationship between the two atoms 35 17A and 3717 B? Will the chemical properties of these be entirely different or approximately similar? Justify your answer.
Answer:
Relationship between the two atoms 35 17A and 3717 B
3517A and 3717B are isotopes of each other because they have the same atomic number but different mass numbers. They have the same chemical properties because their outermost electronic configurations are the same. The number of electrons in both atoms is 17, so they have approximately the same chemical properties.
Question 39. The electronic configuration of an element is K(2), L(8), and M(6).
(1) What will be its period and group in Mendeleev’s periodic table?
Answer:
(1) Period − 3rd
(2) Group − 6th
(2) What are the number of valence electrons?
Answer:
Number of valence electrons = 6.
(3) What will be its valency?
Answer:
Valency − 2
(4) Is the element a metal or non-metal?
Answer:
The element is a non-metal.
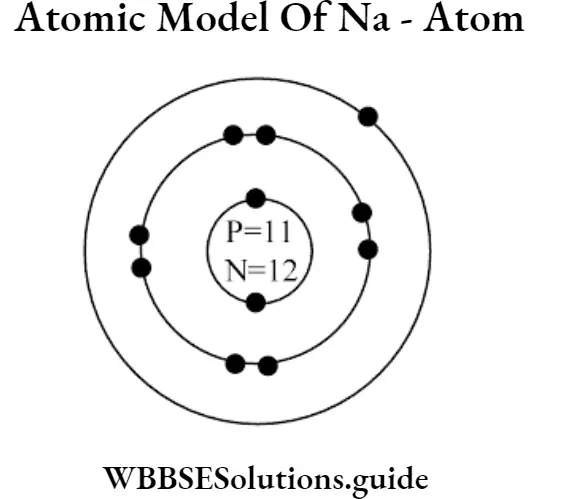
Question 40. Write the names of three particles present in an atom. What is the solar model of the atom? Show with diagram.
Answer:
(1) Electrons, protons, and neutrons are the particles present in an atom.
(2) Solar model of the atom :
(1) In the solar model of an atom the rotational paths of electrons are slightly elliptical.
(2) Nucleus lies at the focus.
(3) The nucleus is composed of neutrons and protons. They are called nucleons.
(4) The atom is not at all solid, most of the atomic space is empty.
(5) The atomic nucleus is positively charged because it.is composed of positively charged protons and electrically neutral neutrons.
(6) Nuclear density is very high because of its low volume.
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound
