Chapter 6 Heat Very Short Answer Type:
Question 1. Which instrument is used to measure the temperature of a body?
Answer: Thermometer.
Question 2. Which instrument is used to measure heat gained or lost by a body?
Answer: Calorimeter.
Wbbse class 9 physical science chapter 6
Question 3. How many FahrenheiWBBSE Solutions For Class 9 Physical Science And Environment Chapter 6 Heatt degrees is equal to (1) Celsius degree?
Answer: \(\frac{9^{\circ}}{5} \mathrm{~F}\)
Question 4. What is the value of 100°C in the Fahrenheit scale?
Answer: In Fahrenheit scale the temperature is 212°F.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Question 5. Is heat a vector quantity?
Answer: No, heat is a scalar quantity.
Question 6. Is there any change of temperature during the change of state?
Answer: There is no change in temperature during the change of state.
Question 7. What is the value of the specific heat capacity of pure water?
Answer: The specific heat capacity of pure water is 4200 J/kg K.
Question 8. Is the specific heat of solids greater than that of liquids?
Answer: Generally specific heat of solids has a lower value than that of liquids.
Question 9. Express 45°C in Fahrenheit scale.
Answer: 45°C=111°F.
Question 10. What is a thermometer?
Answer: The instrument with the help of which the temperature of a body can be measured accurately is called a thermometer.

WBBSE Class 9 heat chapter solutions
Question 11. What will be the direction of heat flow when two bodies of different temperatures are kept in contact?
Answer: In thermal contact, heat will flow from the body with a higher temperature to the body at a lower temperature.
Question 12. What are the upper and lower fixed points in the Fahrenheit scale?
Answer: In the Fahrenheit scale the upper fixed point is 212°F and the lower fixed point is 32°F.
Wbbse class 9 physical science chapter
Question 13. How many divisions are there in the fundamental interval of a Celsius scale?
Answer: The fundamental interval of a Celsius scale is divided into 100 equal divisions.
Question 14. How many divisions are there in the fundamental interval of a Fahrenheit scale?
Answer: The fundamental interval of a Fahrenheit scale is divided into 180 equal parts.
Question 15. Of 0°C and 0°F – which one is less?
Answer: Of 0°C and 0°F, 0°F is less.
Question 16. In which scale is a clinical thermometer graduated?
Answer: A clinical thermometer is usually graduated in Fahrenheit scale. But, nowadays, a clinical thermometer is also being graduated in a Celsius scale.
Question 17. At what temperature are the readings in Celsius and Fahrenheit scales equal?
Answer: At -40 temperature the readings in Celsius and Fahrenheit scales are equal.
Question 18. What is the normal body temperature of a human being in a Celsius scale?
Answer: The normal body temperature of a human being in a Celsius scale = 36°9 °C.
Question 19. What is the normal body temperature of a human being?
Answer: The normal body temperature of a human being is 98°4°F.
Question 20. What is the relation between Celsius and Kelvin scales of temperature?
Answer: lf any temperature in the Kelvin scale be T K and in the Celsius scale it is t°C, then T K = (273 + t)°C.
Wbbse class 9 physical science chapter 6
Question 21. What is the relation between calories and joules?
Answer: (1)cal = 42 J.
Question 22. Which liquid has the highest specific heat?
Answer: The specific heat of water is the highest.
Question 23. What is the specific heat of pure water?
Answer: Specific heat of pure water in CGS system is 1cal \(g^{-1}\)°\( C^{-1}\) and in SI system it is 4200 J.K \(g^{-1}\)\(K^{-1}\)
Question 24. The water equivalent of a body is 20 g. What will be its thermal capacity?
Answer: As the water equivalent of the body is 20. g, its thermal capacity will be 20 cal °\( C^{-1}\).
Question 25. What is the SI unit of heat?
Answer: Joule (j) is the SI unit of heat.
Question 26. What is the SI unit of thermal capacity?
Answer: The SI unit of thermal capacity is Joule/Kelvin or Joule/°C.
Question 27. What is a clinical thermometer?
Answer: The thermometer used for measuring the temperature of the human body is called a clinical thermometer.
Question 28. What is the thermometric liquid in a clinical thermometer?
Answer: Mercury.
Heat WBBSE Class 9 solutions with answer
Question 29. Name the liquid which has the lowest specific heat. State one use of this property
Answer: Mercury. That is why, it is used as a thermometric liquid.
Question 30. What are the two special features of a clinical thermometer?
Answer:
(1) A very short temperature range from 35°C to 42°C.
(2) A constriction in the glass tube just above the mercury bulb.
Wbbse class 9 physical science chapter 6
Question 31. What is the value of 273K temperature in celsius scale?
Answer: Zero degrees centigrade (0°C).
Question 32. What is the unit of specific heat in the C.G.S. system?
Answer: Calorie per gram per degree Celsius (cal \(g^{-1}\)°\( C^{-1}\)).
Question 33. State the S.I. unit of specific heat (capacity).
Answer: The S.1. unit of specific heat capacity is J K \(g^{-1}\)\(K^{-1}\).
Question 34. What is the value of specific heat of the water in S.I. system?
Answer: The sp. heat of water in S.1. system is 4200 JK \(g^{-1}\)\(K^{-1}\).
Question 35. What is the value of specific heat of water in M.K.S. system?
Answer: In M.K.S. system the value of specific heat of water is 4200 J/kg°\(C^{-1}\).
Question 36. Equal masses of mercury and water are given equal amounts of heat. In which case will the temperature rise more and why?
Answer: Mercury, as it has lower specific heat than water.
Question 37. Mention two uses of high specific heat of water.
Answer: For heating purpose (hot water bag) and cooling purpose (car radiator).
Question 38. Mention the conditions under which the principle of Calorie a is valid.
Answer: No loss or gain of heat by the calorimeter.
Question 39. What is the unit of heat in CGS system?
Answer: Calorie is the unit of heat in the CGS system.
Question 40. What is the main principle of calorimetry?
Answer: Heat lost by the hot body = Heat gained by cold body.
Question 41. What will be the temperatures of the bodies at the state of thermal equilibrium?
Answer: The temperatures of the bodies at thermal equilibrium will be the same.
Question 42. What are the different kinds of heat?
Answer: Sensible heat, Latent heat and Radiant heat.
Wbbse class 9 physical science chapter 6
Question 43. What is steam point?
Answer: Steam point is the boiling point of water under normal pressure.
Question 44. What is (1)calorie?
Answer: 1 calorie is defined as the heat required to raise the temperature of 1g of water through one degree celsius.
Question 45. What is fundamental interval?
Answer: It is the range of temperature between the upper and lower fixed points in the scale of temperature.
Question 46. What are the factors for determining the quantities of heat in a body?
Answer:
The quantity of heat depends upon the following factors :
(1) Temperature
(2) Mass
(3) Material of the body.
Question 47. What are the lower fixed point and upper fixed point of the thermometer in Celsius scale?
Answer: Lower fixed point is 0°C and upper fixed point is 100°C.
Question 48. What is the fundamental interval of Celsius scale?
Answer: The fundamental interval is divided into 100 equal parts in celsius scale.
Question 49. What is the melting point of ice in Kelvin scale?
Answer: Melting point of ice in Kelvin scale is 273 K.
Question 50. Is specific heat of solid greater than that of liquid?
Answer: Generally specific heat of solid has. lower value than that of liquid.
Question 51. Give an advantage of use of mercury in thermometer.
Answer: Equal volume of mercury increases or decreases for each degree increase or decrease of temperature in a regular manner.
Wbbse class 9 physical science chapter 6
Question 52. What is radiant heat?
Answer: Heat which reaches us from a source by the process of radiation is called radiant heat.
Question 53. What are the different scales of thermometer?
Answer: Gelsius, Fahrenheit and Kelvin or Absolute scale of temperature.
Question 54. What is specific heat capacity?
Answer: Specific heat capacity is defined as the quantity of heat required to raise the temperature of unit mass of any substance through one degree.
Question 55. What do you mean by the statement that “S.H.C. of aluminium is 880 J k\(g^{-1}\)\(k^{-1}\)
Answer: The statement means 1kg of aluminum requires 880 J of heat for 1° K rise of temperature.
Question 56.The specific heat of copper is 0.09.’ — What does the statement mean?
Answer: ‘Specific heat of copper is 0.09’ means that 0.09 calorie of heat is required to raise the temperature of 1g of copper through 1°C.
Question 57. What is the temperature of 30°C in Kelvin scale?
Answer: The temperature of 30°C is 303 K in Kelvin scale.
Question 58. What is the boiling point of water in Kelvin scale?
Answer: The boiling point of water in Kelvin scale is 373 K.
WBBSE Class 9 Physical Science heat notes
Question 59. By which process heat reaches the earth from the sun?
Answer: Radiation.
Question 60. What is relation between work done and heat produced?
Answer: W =J x H.
W = work done
H = Heat produced
J = Mechanical equivalent of heat.
Question 61. Why do we feel warmer with two thin blankets than one blanket twice as thick?
Answer:
Air is a bad conductor of heat. When two blankets are used a layer of air is trapped in between. It does not allow the body temperature to go out. Hence, we feel warmer with two thin blankets than one blanket twice as thick.
Question 62. Why is it more convenient to cook when the cooking pot has a bottom made of copper?
Answer: Copper is an excellent conductor of heat. When the bottom of the cooking pot is made of copper, the heat is distributed very fast and equally. Hence, it is convenient to cook.
Question 63. Two coins, one of copper and the other of silver, are heated from room temperature to 100°C. Do they absorb same amount of heat? Explain your answer.
Answer: No. Since the specific heat of copper is more than that of silver, the copper coin will absorb more amount of heat.
Question 64. What is the melting point of ice in Kelvin scale?
Answer: Melting point of ice in Kelvin scale is 273K.
Question 65. The partial pressure of water vapor in the atmosphere is 10 mm of Hg at 20°C. Find the relative humidity. (Vapour pressure of water at 20°C = 17.5 mm of Hg)
Answer:
The vapour pressure of water at 20°C is 17.5 mm of Hg.
Hence, relative humidity \(=\frac{10}{17.5} \times 100\)= 57%.
Question 66. The rise in temperature of a body is 60° in celsius scale. What is the corresponding rise in temperature in Fahrenheit scale?
Answer:
We know, 1°C = \(=\frac{9}{5}^{\circ} \mathrm{F}\)
Question 67. How much heat would be produced by converting 42 Joule or work completely into heat?
Answer:
We know, W = JH
or, H\(=\frac{W}{J}\)=\(\frac{42 \text { Joule }}{4.2 \text { Joule } / \mathrm{Cal}}\)= 72 Joule/Cal = 10 Cal.
Question 68. Mass of a body is 150 g and its specific heat is 0.09. How much heat is required to raise its temperature by 10°C?
Answer:
Required heat = mass of the body x specific heat x rise in temperature = 150 x 0.09 x 10 = 135 cal.
Question 69. How much heat is required to raise the temperature of 50 g of lead from 20°C to 100°C? Specific heat of lead is 0.03.
Answer:
Heat gained by lead \(=m s\left(t_2-t_1\right)\)
= 50 x 0.03 x (100 −20) = 120 cal.
Question 70. What quantity of heat will be taken by 50 g of water at 20°C to reach the boiling point at normal pressure?
Answer:
Boiling point of water at normal pressure = 100°C.
So, heat taken by water = 50 x 1 x (100 −20) = 4000 cal.
Question 71. How many calories of heat are needed to melt 60 g of ice at 0°C?
Answer:
Given, the mass of ice, m = 60 g; temperature of ice = 0°C.
H = mL = 60 x 80 = 4800 cal.
Chapter 6 Heat 2 Marks Questions And Answers:
Wbbse class 9 physical science chapter 6
Question 1. Two bodies have the same heat capacity. If they are combined to form a single composite body, show that the equivalent specific heat of this composite body is independent of the masses of the individual bodies.
Answer:
Given
Two bodies have the same heat capacity. If they are combined to form a single composite body,
Let the two bodies have masses\(\mathrm{m}_1\) ,\(\mathrm{s}_1\) and specific heats\(\mathrm{s}_2\) and,,
then \(\mathrm{m}_1\)\(\mathrm{s}_1\)=\(\mathrm{m}_2\)\(\mathrm{s}_2\)
Let s = specific heat of the composite body.
Then,(\(\mathrm{m}_1\)+\(\mathrm{m}_2\)) S =\(\mathrm{m}_1\)\(\mathrm{s}_1\)+\(\mathrm{m}_2\)\(\mathrm{s}_2\)
Question 2. Define thermal capacity or heat capacity.
Answer:
Thermal capacity
Thermal capacity, also referred to as heat capacity, is the amount of heat required to change the temperature of an object by a certain degree.
Question 3. Define specific heat capacity.
Answer:
Specific heat capacity
The specific heat capacity, also referred to as the specific heat of a material, is the amount of heat needed to raise the temperature of an object per unit mass of that object.
Question 4. Define Latent Heat.
Answer
Latent Heat : Latent heat is the energy absorbed by or released from a substance during a phase change from a gas to a liquid or a solid or vice versa.
If a substance is changing from a solid to a liquid, for example, the substance needs to absorb energy from the surrounding environment in order to spread out the molecules into a larger, more fluid volume.
If the substance is changing from something with lower density, like a gas, to a phase with higher density like a liquid, the substance gives off energy as the molecules come closer together and lose energy from motion and vibration.
Question 5. Define Sensible Heat.
Answer:
Sensible Heat
Sensible heat is the energy required to change the temperature of a substance with no phase change. The temperature change can come from the absorption of sunlight by the soil or the air itself.
Or it can come from contact with the warmer air caused by the release of latent heat (by direct conduction). Energy moves through the atmosphere using both latent and sensible heats acting on the atmosphere to drive the movement of air molecules which create wind and vertical motions.
Important questions on heat WBBSE Class 9
Question 6. Define Latent Heat of Fusion and Vaporisation.
Answer:
Latent Heat of Fusion and Vaporisation
The energy required to change the phase of a substance is known as latent heat. The word latent means hidden. When the phase change is from solid to liquid we must use the term the latent heat of fusion, and when the phase change is from liquid to gas, we must use the term the latent heat of vaporisation.
Question 7. What is the mechanical equivalent of heat energy?
Answer:
Mechanical equivalent of heat energy
The amount of heat energy or thermal energy created is proportional to the work done. This relationship is called the mechanical equivalent of heat and can be expressed by the equation
W=JH
where,
W is the work done in joules (J),
J is the relationship constant 4.18 joules/calorie (J/c),
H is the thermal energy created from the work in calories (c).
Question 8. What is ‘Anomalous Expansion of Water’?
Answer:
‘Anomalous Expansion of Water’
Water shows unusual expansion when it is cooled from four degree centigrade to zero degree centigrade, this expansion is known as “anomalous expansion of water.” The unusual behaviour of water, when it expands below 4° celsius to 0°, is called anomalous expansion of water.
Question 9. How does the anomalous expansion of water help to preserve aquatic life?
Answer:
The anomalous expansion of water helps to preserve aquatic life during very cold weather. When temperature falls, the top layer of water in a pond contracts, becomes denser and sinks to the bottom.
A circulation is thus set up until the entire water in the pond reaches its maximum density at 4°C. If the temperature falls further, the top layer expands and remains on the top till it freezes. Thus, even though the upper layer is frozen, the water near the bottom is at 4°C and the fishes, etc. can survive in it easily.
Question 10. Give an example of the anomalous expansion of water.
Answer:
If we take a cube of ice at -5°C and heat it, it expands till ice starts melting. During melting its temperature remains 0°C but its volume decreases. If heat is continuously supplied to water at 0°C, it further contracts upto 4°C and then it starts expanding. Thus, water has its minimum volume and maximum density at 4°C.
Question 11. What is absolute scale or Kelvin scale of temperature?
Answer:
Absolute or Kelvin scale of temperature : In this scale the lower fixed point is 273K and the upper fixed point is 373K, the fundamental interval is divided into 100 equal divisions like that in the celsius scale. Each division represents 1K.
Question 12. What is specific heat capacity of a substance?
Answer:
Specific heat capacity of a substance: The specific heat in SI system is known as specific heat capacity.
Definition: Specific heat capacity of a substance is the quantity of heat required to raise the temperature of 1 kilogram of the substance through 1 K as 1°C.
Question 13. What does the first law of thermodynamics state?
Answer:
First law of thermodynamics: When. work is completely converted into heat or heat is completely converted into work, one is equivalent to the other.
Mathematical expression of the first law of thermodynamics: If by converting work W completely heat H is obtained then Wα H
Or, W=JH (J = mechanical equivalent of heat)
Question 14. What is mechanical equivalent of heat? What are the units of mechanical equivalent of heat in CGS and SI systems?
Answer:
Mechanical equivalent of heat: Work done to produce unit heat is called mechanical equivalent of heat.
Units of mechanical equivalent of heat :
CGS system : 4.18 x \(10^7\)erg/cal
SI system: 4.18 J/Cal
Question 15. Which indicates higher temperature, one division of Celsius scale or one division of Fahrenheit scale?
Answer:
One division of Celsius scale indicates higher temperature than one division of
Fahrenheit scale, for, 1°C \(=\frac{9}{5} \ °F\)
Question 16. Explain why a clinical thermometer should not be dipped into boiling water.
Answer: A clinical thermometer should not be dipped into boiling water. This is because the Temperature of boiling water is 212°F, much higher than the maximum limit (110°F) of the Clinical thermometer. So, if the thermometer is dipped in boiling water, the expanding mercury column will exert a great force which may break the thermometer tube.
Question 17. Why is the bulb of the thermometer made Cylindrical and not spherical?
Answer:
A thermometer becomes quick-acting if its bulb is made cylindrical in shape instead of spherical. Heat absorbed by a body due to conduction is directly proportional to its surface area in contact.
Volume being the same, the elongated cylindrical bulb has a larger contact area than a spherical bulb. The cylindrical bulb will, therefore, absorb more heat in the same time than the spherical bulb, and hence, will respond quickly.
Wbbse class 9 physical science chapter 6
Question 18. Two coins, one made of copper and another made of silver, are heated to 100°C at room temperature. Do they absorb same amount of heat? Explain.
Answer: The two coins will not absorb same quantity of heat.
Explanation : Heat absorbed = mass of the body x its specific heat x rise in temperature. Though the rise in temperature of both the copper and silver coins is the same, their masses and specific heats are different. So, the two coins will absorb different quantities of heat.
Question 19. A kettle containing water is cooled from 80°C to 40°C. Do they (the kettle and the water in it) release same amount of heat? Explain.
Answer: The kettle and the water inside it will not lose same amount of heat.
Explanation : Heat given up = mass of the body x its specific heat x fall in temperature.
Though the fall in temperature of both the kettle and the water in it is the same (80 – 40 = 40°C), their masses and specific heats are different and hence, the kettle and water in it will not release an equal amount of heat.
Question 20. Does milk get hot more quickly than water?
Answer:
The specific heat of milk is less than that of water. Hence, if the same amount of water and milk are heated separately at the same rate, the rate of increase in temperature is more for milk than for water. So, milk gets hot more. quickly than water.
Question 21. What is the specific heat of pure water?
Answer: S
pecific heat of pure water in CGS system is 1 cal\(g^{-1}\)\(C^{-1}\)and in SI system it is 4200 J. k\(g^{-1}\)
Question 22. What is the upper fixed point of a thermometer?
Answer: The upper fixed point or the steam point corresponds to the temperature of steam from water under normal atmospheric pressure (76 cm of mercury).
Wbbse class 9 physical science chapter 6
Question 23. Express 45°C of temperature in Fahrenheit scale.
Answer:
We know that, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
Or, \(\frac{45^0}{5}\)=\(\frac{F-32}{9}\)
Or, \(9\)=\(\frac{F-32}{9}\)
Or, F − 32 = 81
Or, F.= 81+ 32 = 113.
∴ In Fahrenheit scale 45°C is 113°F.
Question 24. A piece of copper of mass 100 gram is cooled from 60°C to 40°C. How much heat will be lost? (Specific heat of copper = 0.09 cal\(g^{-1}\)\(C^{-1}\)).
Answer:
Given
A piece of copper of mass 100 gram is cooled from 60°C to 40°C.
Heat lost = mass x specific heat x fall of temperature
= 100.g x 0.09 cal\(g^{-1}\)\(C^{-1}\) x (60 – 40)° C = 180 calories.
WBBSE Class 9 solved exercises on heat
Question 25. The mass of a piece of copper is 3 kilograms. The specific heat of copper is 0.09 cal \(g^{-1}\)°\(C^{-1}\). How much heat will be required to raise its temperature by 10°C?
Answer:
Given
The mass of a piece of copper is 3 kilograms. The specific heat of copper is 0.09 cal \(g^{-1}\)°\(C^{-1}\).
Here, mass (m) = 3 kg = 3000 gm.
Heat required to raise the temperature by 10°C
= mass x specific heat x rise in temperature
= 3000 x 0.09 x 10 calories = 2700 calories.
Wbbse class 9 physical science chapter 6
Question 26. Explain the term Heat.
Answer:
Heat
Heat is a form of energy. It can be experienced by our senses. If we touch a body, we can say whether it is hot or cold. A body appears hot when our hand receives heat from it, while it appears cold when heat passes from our hand to that body.
Heat: The external cause due to which a cold body becomes hot directly or indirectly, or a warm body becomes cold, is known as heat.
Since heat is a form of energy, so it cannot be-created or destroyed, but can be transformed into another form or a number of forms of energy. When heat is applied to a body, it gradually gets heated and when heat is taken out of it, the body becomes cold.
Question 27. What do you mean by the temperature of a body?
Answer:
Temperature : Temperature is a quantity which defines the thermal state of a body. It determines the direction of flow of heat when two bodies of coi temperatures are kept in contact.
Thus, temperature is the condition of a body which determines the direction along which heat will flow. Heat is the cause and temperature is the effect. Temperature indicates the
degree of hotness or coldness of the body. It does not tell us the quantity of heat energy contained in the body. Hence, temperature is the thermal state of a body.
Question 28. State and define the units of heat.
Answer:
Units of heat
The S.I. units of heat is 1 Joule (J).
The C.G.S. unit of heat is 1 Calorie (Cal).
Calorie:nOne calorie heat is the quantity of heat required to raise the temperature of (1)gm of water through 1°C (from 14. 5° C to 15.5°. C).
1 calorie (1 cal) = 4°2 Joules (J) nearly.
1 Kilo calorie = 1000 calorie
= 4200 Joules.
Question 29. How is temperature measured? Name the instrument to measure it.
Answer:
Temperature of a body is measured by an instrument called thermometer. The common type of thermometers used in the laboratory or to measure the temperature of a human body are mercury thermometers.
The most commonly used thermometers using the scale are:
(1) Celsius or centigrade scale and
(2) Fahrenheit scale.
WBBSE class 9 question and answers
Question 30. What are the different scales of temperature used in thermometers?
Answer:
To measure the temperature of a body accurately a standard temperature scale is essential.
A temperature scale is prepared by using fixed points on thermometers:
(1) Celsius scale of temperature, and
(2) Fahrenheit scale of temperature.
Question 31. What do you mean by calibration of thermometers?
Answer:
Calibration of thermometers
The calibration of thermometers is a process of fixing two points on it and then dividing the interval between them into convenient number of equal parts. The number of equal parts is called the scale of temperature. The fixed points are chosen in such a way that they can be easily obtained. These two fixed points are called lower fixed point and upper fixed point.
Question 32. Establish the relation between the two scales of temperature — Fahrenheit and Celsius.
Answer:
The relation between the two scales of temperature
The given figures shows the two scales of temperature, the Celsius scale and the Fahrenheit scale of temperature.
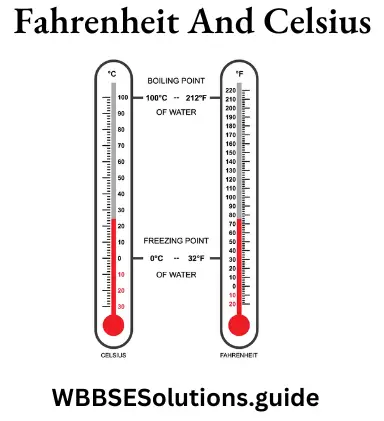
The interval between the upper and lower fixed points is the same in both the scales. Thus, 100 centigrade (div.) degree = 180 Fahrenheit (div.). degree.
∴\(\frac{C-0}{100}\)=\(\frac{F-32}{180}\)
Or, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
This the relation between the two scales of temperature.
Question 33. Explain Celsius scale (or centigrade scale) and Fahrenheit scale of temperature.
Answer:
Celsius scale (or centigrade scale): On this scale, the lower fixed point or ice point is marked as 0° C. The upper fixed point or steam point is marked as 100° C. The interval between the ice point and the steam point is divided into 100 equal parts. Each of these divisions is called one degree Celsius and is written as °C.
WBBSE class 9 question and answers
Fahrenheit scale of temperature: On the Fahrenheit scale of temperature, the ice point is marked as 32°F and the steam point is marked as 212°F. The interval between the two fixed points is divided into 180 equal parts.-Each division is called one degree Fahrenheit and is written as °F.
Question 34. Show by an experiment that two balls of different materials of same mass and temperature absorb different quantities of heat.
Answer:
Two balls of same mass but of different materials, say, lead and aluminum, are taken. They are immersed in water and heated for some time. After some time both balls are taken out of water and placed on a block of ice. It will be seen that the aluminum ball will melt more ice. This shows that the quantity of heat taken depends upon the material of the body if other conditions are constant. This property is known as the heat capacity of the body.
Question 35. Define thermal or heat capacity of a body.
Answer:
Thermal capacity: The thermal capacity (or heat capacity) is the amount of heat required to raise its temperature by 1° C.
Hence, thermal capacity =\(=\frac{\text { Amount of heat }}{\text { Rise in temperature }}\)
The unit of thermal capacity in S.I. system is Joule per degree.
The unit of thermal capacity in C.G.S. system is calorie per degree.
Question 36. The specific heat of lead is 0°03” – What is its meaning?
Answer:
The specific heat of lead is 0°03”
The specific heat of lead is 0°03” means that one gram of lead will require 0°03 calories of heat to raise the temperature of lead through 1° C.
Question 37. Do one gram of iron and one gram of copper contain equal quantities of heat at same temperature? Explain.
Answer:
The amount of heat required to raise the temperature of a body The specific heat of copper is less than that of iron. Hence the amount of heat required to raise the temperature of both metals of same mass will be different.
WBBSE class 9 question and answers
Question 38. Define water equivalent of a body.
Answer:
Water equivalent of a body
The amount of water whose temperature is raised through 1° C by the same quantity of heat which is required to raise the temperature of a body through 1° C, is known as the water equivalent of a body.
If the mass of a body is m gm and its specific heat be s, then the water equivalent (W) is W = ms gm.
The unit of water equivalent measured in gm or kg.
Question 39. State the difference between water equivalent and aennal capacity.
Answer:
The difference between water equivalent and aennal capacity
Water equivalent and thermal capacity of a body are numerically equal, but their units are different. Water equivalent is an amount of water and it is measured in gram, but thermal capacity is an amount of heat and is expressed in calories.
Question 40. State the principle of caiorimetry.
Answer:
The principle of caiorimetry
The principle of calorimetry is based on the law of conservation of energy. When a hot body is mixed (or kept in contact) with a cold body, heat passes from hot body to the cold body till both bodies attain the same temperature. If no heat is lost with the surrounding,then
Heat lost by the hot body = Heat gained by the cold body.
Question 41. Why is water used in hot water bottles?
Answer: Water has a high specific heat in comparison to other liquids. Hence for the same mass of water and other liquids, our body can get greater amount of heat from water.
Question 42. Why do farmers fill their fields with water to protect their crops from frost?
Answer:
Waiter having high specific heat does not allow the temperature in the surrounding area of plants to fall below 0° C. In the absence of water, if on a cold night, temperature falls below 0° C, the water in the fine capillaries of plants will freeze and veins will burst due to increase in volume of water on freezing. As a result the crops willbe destroyed.
Question 43. Why should the bore of a thermometer be uniform and also narrow?
Answer:
The bore should be uniform, otherwise, unequal expansion of mercury thread will take place at different parts of the bore for equal changes in temperature. The bore of thermometer tube should be narrow so that the expansion of mercury thread is long enough for a small change of temperature.
WBBSE class 9 question and answers
Question 44. What do you mean by a quick-acting thermometer? What are the requisites for making a thermometer quick-active?
Answer:
The thermometer with which the temperature of a body can be measured very quickly is called a quick-acting thermometer
To make a quick-acting thermometer:
(1) Volume of its bulb should be small
(2) Glass wall of the bulb should be thin
(3) The bulb should be made cylindrical and
(4) The thermo metric liquid should be of high thermal conductivity.
Question 45. On what factors does the total quantity of heat in a body depend?
Answer:
The quantity of heat in a body depends upon its
(1) Mass
(2) Specific heat and
(3) Temperature.
Question 46. The body temperature of an ill person is 104° F. What would be its temperature in Celsius scale and Kelvin scale?
Answer:
By relation,\(\frac{C}{5}\)=\(\frac{F-32}{9}\) given F = 104° F.
Then,\(\frac{C}{5}\)=\(\frac{104-32}{9}\) Or, C\(=\frac{5 \times 72}{9}\)=40°.
The required temperature in Celsius scale is 40° C.
∴ Reading in Kelvin Scale = 273 + 40 = 313K.
Question 47. The mass of a piece of copper is 3 kilogram. The specific heat of copper is 0°09 cal/g °\(C^{-1}\). How much heat will required to raise its temperature by 10° C?
Answer:
Given
The mass of a piece of copper is 3 kilogram. The specific heat of copper is 0°09 cal/g °\(C^{-1}\).
The mass of copper (m) = 3 Kg = 3000 Kg. Specific heat (s) = 0°09 cal/g\(C^{-1}\) and rise in temperature (1) = 10° C. Heat required = mass x specific heat x rise in temperature
= 3000 x 0°09 x 10 cal = 2700 caI.
Question 48. Calculate the amount of heat required to raise the temperature of 150 gm of water from 0°C to its boiling point.
Answer:
The rise in the température of water from 30° C to 100° C (boiling point)
= (100 – 30)°C = 70°C. 4
Heat taken by water = mass of water x specific heat x rise in temperature
= 150 x1 x 70 calories = 10500 calories.
Question 49. What quantity of heat will be taken by 50 gm of water at 20°C to reach its boiling point at normal pressure ?
Answer:
Mass m = 50 gm.
Temperature difference t = (100 − 20)°C = 80°C. ~ Specific heat of water s = 1.
We know that, H = mst
= 50 x (1)x 80
= 4000 cal
∴ Heat taken = 4000 cal.
Question 50. A thermometer having the corresponding upper and lower fixed points 95° and 15°, reads a temperature 65°, what will this temperature be in Celsius scale?
Answer:
Given
A thermometer having the corresponding upper and lower fixed points 95° and 15°, reads a temperature 65°
The relation between temperature of Celsius and the given scale can be written from the general relation between any two scales as, \(\frac{65-15}{95-15}\)=\(\frac{C-0}{100-0}\)
⇒ \(\text { or, } \frac{50}{80}\)=\(\frac{C}{100}\)
80C= 50×100
∴C\(=\frac{50 \times 100}{80}\)=6.25.
The required temperature is 62.5°C.
WBBSE class 9 question and answers
Question 51. In Fahrenheit scale the temperature of a body increases by 36°. What is the corresponding risé of temperature in Celsius scale?
Answer:
1°F\(=\left(\frac{5}{9}\right)^{\circ} \mathrm{C}\)
∴ 36° F\(=\left(36 \times \frac{5}{9}\right)^{\circ} \mathrm{C}\)
=20° C.
Question 52. What are the types of heat?
Answer:
Types of heat: There are three types of heat.These are :
(1) Sensible heat
(2) Latent heat
(3) Radiant heat.
Question 53. What is radiant heat?
Answer:
Radiant heat: It is that thermal condition of the body which determines whether it will absorb heat from other body or release heat to that body.
Question 54. What is lower fixed point of a thermometer?
Answer:
Lower fixed point of a thermometer: At normal atmospheric pressure, the temperature at which pure ice melts into water or pure water freezes into ice is called the lower fixed point of a thermometer.
Question 55. What is upper fixed point of a thermometer?
Answer:
Upper fixed point of a thermometer: It is the temperature at which pure water boils and transforms into steam under normal atmospheric pressure.
Question 56. What do you mean by fundamental interval?
Answer:
Fundamental interval: The range of temperature between the upper and the lower fixed points is called Fundamental interval.
Question 57. What is absolute scale or Kelvin scale of temperature?
Answer:
Absolute or Kelvin scale of temperature: In this scale the lower fixed point is 273 K and the upper fixed point is 273 K, the fundamental interval is divided into 100 equal divisions like that in the celsius scale. Each division represents 1K.
Question 58. What are the units of heat in CGS and SI systems?
Answer:
Unit of heat in CGS system is calorie: It is the amount of heat required to raise the temperature of one gram of pure water through 1°C. Unit of heat in SI system is joule : As heat is a form of energy, so now-a-days, heat is measured in the unit of energy. That is heat is expressed in joule. It is the SI unit of heat.
1 calorie = 4%8 joule.
Question 59. What do you mean specific heat of a substance?
Answer:
Specific Jeeat of a substance: It is defined as the quantity of heat required to raise the temperature of unit mass of it by 1 degree.
Question 60. What are the units of specific heat in CGS and SI systems?
Answer:
Unit of specific heat in CGS system is cal/g/°C.
Unit of specific heat in SI system is J/kg K.
Question 61. What are the units of heat capacity in CGS system and SI systems?
Answer:
Unit of heat capacity in CGS system is Calorie/°C.
Unit of heat capacity in SI system is Joule/kelvin.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 62. State the relation between thermal capacity and specific heat of a body.
Answer:
Relation between thermal capacity and specific heat of a body: Quantity of heat required to raise the temperature of a body of mass ‘m’ and specific heat is by 1° = m.s.1= m.s.
i.e., Thermal capacity = mass x specific heat.
Question 63. Show that specific heat is the heat capacity of unit mass.
Answer:
Thermal capacity of a body
= mass x specific heat of material of the body.
Hence, thermal capacity = ms.
Putting, m = 1, in the above relation,
Thermal capacity = 1 x specific heat.
So, it can be concluded, specific heat is the thermal capacity per unit mass.
Question 64. What are the units of water equivalent in CGS system and SI system?
Answer:
Unit of water equivalent in CGS system is gram (g).
Unit of water equivalent in SI system is Kilogram (kg).
Question 65. What are the differences between thermal capacity and water equivalent?
Answer:
Differences between thermal capacity and water equivalent :
| Thermal capacity | Water equivalent |
| (1) Thermal capacity represents some quantity of heat. | (1) Water equivalent represents some quantity of water. |
| (2) Units of thermal capacity in CGS and SI systems are calorie/°C and J/K respectively. | (2) Units of water equivalent in CGS and SI systems are gram and kg respectively. |
Question 66. What is the fundamental principle of calorimetry?
Answer:
Fundamental principle of calorimetry: If no heat flows to outside from the system of bodies and if no chemical reaction takes place between these, >, then from the principle of conservation of energy, it can be said, heat lost by the hotter bodies = heat gained by the colder bodies. This is the principle of calorimetry.
Question 67. What is the relation between heat and molecular velocity?
Answer:
Relation between heat and molecular velocity: The molecules in a hotter body have slower motions. Also, if heat is supplied to a body, its molecules move more rapidly. If heat is withdrawn from a body, the motion of its molecules becomes slower. Thus, increase or decrease of molecular velocity corresponds to higher or lower quantity of it.
Question 68. Why should the bore of a thermometer be uniform?
Answer:
Reason: The bore should be uniform, otherwise, unequal expansion of mercury thread will take place at different parts of the bore for equal change of temperature.
Question 69. Why is meant by sensitivity of a thermometer?
Answer:
Sensitivity of a thermometer: A thermometer is considered sensitive if it :
(1) Quickly picks up the temperature of the body with which it is kept in contact
(2) Can detect small changes of temperature.
Question 70. Why is water preferred to other liquids in a hot water bag?
Answer:
Reason: Since water has the highest specific heat, a certain mass of water heated to a certain temperature contains more heat than the same mass of any other liquid heated to the same temperature. So, hot water will take longer time to cool than any other liquid heated to the same temperature. So, for hot compression, it can be used for a sufficiently long time.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 71. When a mass m of hot water was added to a mass 3 m of cold water (initially at 10°C), the mixture attained a final temperature of 20°C. Find the initial temperature of hot water.
Answer:
Solution: Let s be the specific heat capacity of water and t be the initial temperature of hot water. Then we have,
Heat lost by hot water = ms(t −20)
and heat gained by cold water = 3ms (20 −10) = 30 ms.
Since heat lost = heat gained. :
We have, ms (t- 20) = 30ms_ or, t-20=30 or, t= 50°C
The initial temperature of the hot water was, therefore, 50°C.
WBBSE Class 9 Physical Science heat and temperature solutions
Question 72. One joule of work expended in winding a 30 g steel watch and the watch is wrapped in a heat insulator. How much warmer would the watch be by the time it has run down? Specific heat of steel = 0.(1)cal/g°c.
Answer:
W = JH or, H=W/J \(=\frac{1.0 \mathrm{~J}}{4.18 \mathrm{~J} / \mathrm{cal}}\) = 0.24 cal.
Now, H ( or Q ) = ms ΔT
∴ ΔT=\(\frac{\mathrm{H}}{\mathrm{ms}}\)=\(\frac{0.24 \mathrm{cal}}{30 \mathrm{~g} \times 0.1 \mathrm{cal} / \mathrm{g}^{\circ} \mathrm{C}}\)=0.08 C°.
Question 73. The temperature on a day was 94°F. What is it in Celsius sca
Answer:
We know that, \(\frac{C}{5}\)=\(\frac{F-32}{9}\) F=94°
or,\(\frac{C}{5}\)=\(\frac{94-32}{9}\) C=?
or, C\(=\frac{62 \times 5}{9}\) =34.4°.
Question 74. The lowest attainable temperature so far is — 270° Celsius. What is it in Fahrenheit scale?
Answer:
We know, C =− 270°
⇒ \(\frac{C}{5}\)=\(\frac{F-32}{9}[/latex, F=?
or, [latex]\frac{-270}{5}\)=\(\frac{F-32}{9}\)
or,−54 \(=\frac{F-32}{9}\)
or, F − 31=−54×9=−486
or, F = −486+32=− 454
∴ f=−454°5.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 75. Convert 50°C to Fahrenheit scale.
Answer:
We know, C=50°
⇒ \(\frac{C}{5}\)=\(\frac{F-32}{9}\) F=?
or, \(\frac{-50}{5}\)=\(\frac{F-32}{9}\)
or, 90=F−32
or, F = 90+32=122
Hence, 50°C =122°F
Question 76. The difference in the readings of a Fahrenheit and a Celsius Thermometer is 48°. What are the actual readings shown in the thermometers?
Answer:
Let the actual readings in the Rahsenhelt and the Celsius thermometers a to F and C.
∴ F−C = or,48°C F=C+48°
Again ,from the relation, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
or, \(\frac{C}{5}\)\(=\frac{F+48-32}{9}\)=\(\frac{C+16}{9}\)
or, 9C=5C+90
or, AC=80
or, C=20°
Now,F= C + 48° = 20° + 48° = 68°.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 77. A certain temperature is 104°F, what is its value in Kelvin scale?
Answer:
We know the relation between Fahrenheit and Kelvin scales is
⇒ \(\frac{F-32}{9}\)=\(\frac{K-273}{5}\), F=104°
or, \(\frac{104-32}{9}\)=\(\frac{K-273}{5}\), K=?
or, \(\frac{72}{9}\)=\(\frac{K-273}{5}\)
or, 8×5=K−273
or, K=313
∴ 104°F=313K
Question 78. The normal temperature of human body is 98.4°. What is the value of this temperature in centigrade scale?
Answer:
We know, F = 98.4°
⇒ \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
or, \(\frac{C}{5}\)=\(\frac{98.4-32}{9}\)
or,\(\frac{C}{5}\)=\(\frac{66.4}{9}\)
or, 9C=6.64×5
or, \(C\)=\(\frac{332.0}{9}\)=36.89
So, the required temperature = 36.89°C,
Question 79. Which temperature is same in both centigrade scale and Fahrenheit scale?
Answer:
Let the temperature be X°.
According to question, C = F = X.
We know, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
or, \(\frac{x}{5}\)=\(\frac{x-32}{9}\)
or, 9x=5x-160
or, 9x-5x= -160
or, 4x=-160
or,x\(=\frac{-160}{4}\)=−40°
So, (−)40° temperature will read same both in Fahrenheit and centigrade scales.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 80. Mass and specific heat of a substance 100 g and 0.09 respectively. Find the Thermal capacity and water equivalent of the body.
Answer:
We know, m= 100g; s = 0.09
Thermal capacity = ms
= 100 x 0.09 = 9 Cal/°C
Water equivalent = ms
= 100 x 0.09 =9g.
Question 81. Calculate the amount of heat required to raise the temperature of 60 g of water from 20°C to its boiling point.
Answer:
We know, m = 50g
Absorbed heat = ms\(\left(t_2-t_1\right)\) \(t_1\)= 20°C
=50 x 1x(100−20) ⇒ \(t_2\) = 200°C
=50×80, s=1
= 4000 cal.
Wb Class 9 Physical Science Question Answers
Question 82. A body of iron having mass 60 g is cooled from 200°C to 100°C. Calculate the amount of heat given out. Specific heat of iron = 0.12.
Answer:
We know, m = 60g
The amount of heat given out by iron \(t_1\)= 100°C
= ms\(\left(t_2-t_1\right)\) \(t_2\) = 200°C
= 60 x 0.12(200 − 100) s= 0.12
= 60 x 0.12 x 100 = 720 cal.
Question 83. Find the quantity of heat required to raise the temperature of 1 kg of water from 10°C to 90°C.
Answer:
Mass of water = 1 kg = 1000 g
Rise in temperature of water = 90 − 10 = 80°C
Specific heat of water = 1cal \(g^{-1}\) \( C^{-1}\)
Thus, the required heat = mst = 1000 x 1 x 80 = 80,000 cal.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 84. A kettle and the water in it are cooled down from 80°C to 40°C. Do the kettle and water give up the same amounts of heat? Explain
your answer.
Answer:
Heat lost by a body = mass of the body x specific heat of the body x fall in temperature. Although fall in temperature of water or kettle = 80 −40 = 40°C is same, their masses and specific heats being different, they would not give up same amounts of heat.
Question 85. If the specific heat of copper be 0.09, calculate the thermal capacity and water equivalent of a block of copper of mass 50 g.
Answer:
Thermal capacity of the piece of copper
= mass x specific heat = 50 x 0.09 = 4.5 cal/°C.
Water-equivalent of the piece of copper
= mass x specific heat = 50 x 0.09 = 4.5 g.
Question 86. What quantity of heat will be taken by 50 g of water at 20°C to reach its boiling point at normal pressure?
Answer:
Boiling point of water at normal pressure = 100°C.
So, heat taken by water = 50 x 1x (100 −20) = 4000 cal.
Question 87. The quantity of heat required to raise the temperature of one keane ofa metal is 2300 cal. Find the specific heat and water equivalent of the metal! piece.
Answer:
Given,
mass of the metal piece m = 1 kg = 1000 g;
rise in temperature = 20°C; total amount of heat Q = 2300 cal.
If specific heat of the metal s, then
Q=mst_ or, 2300 = 1000 x s x 20
∴ s\(=\frac{2300}{1000 \times 20}\)=0.115
Again, water-equivalent of the metal piece = ms = 1000 x 0.115 = 115 g.
Wbbse Madhyamik Class 9 Physical Science And Environment
Question 88. 300g of water at 15°C is mixed with 500 g of water at 30°C. What is the resultine temperature?
Answer:
Given, \(\mathrm{m}_1\)= 300g; \(\mathrm{m}_2\) = 500g; \(\theta_1\) = 15°C; \(\theta_1\) = 30°C.
Let the resulting temperature be θ°C.
Heat gained by cold water =\(\mathrm{m}_1\)xs (θ −\(\theta_1\)) = 300 x1x (θ−15) calorie
Heat lost by hot water = \(\mathrm{m}_2\)x s x (\(\theta_2\)− θ) = 500 x (1)x (30 − θ) calorie.
From the relation, Heat gained = Heat lost.
300(θ − 15) = 500(300 − θ )
or, (300 + 500)θ = 500 x 30 + 300 x 15
∴θ \(=\frac{15000+4500}{800}\)
=24.38°C.
Question 89. Calculate the amount of work to be done to convert 100g of water at 0°C to water at 100°C. Mechanical equivalent of heat = 4.2 Joule.
Answer:
Heat required, H = mst = 100 x 1x (100 −0) = 10,000 cal.
Work required, W = JH = 4.2 x 10,000 = 42,000 J.
Best study material for heat WBBSE Class 9
Question 90. A steel ball of specific heat 0.12 has its temperature increased by 0.5°C on falling through a certain height. If J = 4.2 x\(10^7\)erg\(\mathrm{cal}^{-1}\) , calculate the height.
Answer:
Given, specific heat, s = 0.12
Increase in temperature, θ = 0.5°C; J = 4.2x \(10^7\)erg\(\mathrm{cal}^{-1}\)
Let mass of the ball be m gram and the height be h cm. Then
PE of the ball = mgh.
Heat produced = msθ = m x 0.12 xθ
mgh=Jxmx0.12xθ
h\(=\frac{\mathrm{J} \times 0.12 \times 0.5}{g}\)=\(\frac{4.2 \times 107 \times 0.12 \times 0.5}{980}\)
=2.571×\(10^3\)cm=25.71m.
Question 91. A kilogram of water at 20°C is placed in a refrigerator. How much heat must be extracted before all the water turns to ice? Latent heat of ice = 80 Cal \(g^{-1}\).
Answer:
Mass of water m = (1)kg = 1,000 g; L = 80\(\text { calg }^{-1}\); \(t_1\) = 20°C; \(t_2\) = 0°C
Heat extracted from water, \(\mathrm{H}_1\) = ms(\(t_1\) −\(t_2\) ) = 1000 x (20 −0) = 20,000 cal.
Heat extracted from water to freeze into ice, \(\mathrm{H}_2\)= mL = 1000 x 80 = 80,000 cal.
Total heat extracted = \(\mathrm{H}_1\)+\(\mathrm{H}_2\)= 20,000 + 80,000 = 1,00,000 cal.
Question 92. After a few pieces of dry ice had been added to 120 g of water at 46°C, the final temperature of the mixture was found to be 0°C. What is the mass of ice added? Latent heat of ice = 80 cal\(g^{-1}\).
Answer:
Given, \(\mathrm{m}_1\)= mass of water = 120g \(t_1\) = temperture of water = 46°C.
Final temperature of the mixture,,\(t_2\)= 0°C; L = 80 cal\(g^{-1}\)
Let mass of the ice added be \(\mathrm{m}_2\)g.
Heat lost by water at 46°C to be water at 0°C = \(\mathrm{m}_1\)s(\(t_1\)−\(t_2\))
= 120 x (1)x (46 – 0)
= 120 x 46 cal.
Heat required by m,g ice at 0°C to water at 0°C.= \(\mathrm{m}_2\)L =\(\mathrm{m}_2\)x 80 cal.
Heat Lost = Heat gained; 120 x 46 = \(\mathrm{m}_2\) x 80
∴ \(\mathrm{m}_2\)
Wb Class 9 Physical Science Question Answers
Question 93. How much heat is released in converting 5g of steam at 100°C to water at 60°C ?Latent heat of steam = 540 calg”.
Answer:
Heat released in converting 5g steam at 100°C to 5g of water at 100°C = 5 x 540 cal.
Heat released in converting 5g of water at 100°C to 5g of water at 60° =5x 1(100-60) =5 x 40 cal.
∴ Total heat released = 5 x 540 + 5 x 40 = 5 x 580 = 2900 cal.
Question 94. A piece of copper heated to 35°C is dropped into 140 g of water at 15°C. The mass of copper is 150 g and the specific heat of copper is 400 J/kgK. Find the final temperature. (sp. heat of water = 4200 J/kgK)
Answer:
Here, the hot piece of copper will give up heat and cold water will absorb it. Let the final temperature be t°C.
Now, the heat lost by copper = its mass x sp. heat fall of temperature
= 0.15 x 400 x (35 – t) = 60 x (85-1) J.
And heat gained by water = its mass x sp. heat x rise of temperature
= 0.14 x 4200 x (t − 15) = 588(t − 15) J. Now, heat lost = heat gained
or, 60 x (35 − t) = 588 (t− 15) or, 175 – St = 49t – 735
∴ t= 16.85°C.
Question 95. A lump of copper of mass 100 g is cooled from 60°C to 40°C. How much heat will be rejected by the lump ? Specific heat of copper = 0.09 cal \(g^{-1}\) \( C^{-1}\)
Answer:
Amount of heat rejected = mass x sp. heat x fall in temperature
= 100 x 0.09 x (60 − 40) Cal = 180 Cal.
Question 96. A brass sphere of mass 0.4 kg at 100°C is dropped into 1 kg of water at 20°C. The final temperature of the mixture is 23°C. Find the $.H.C. of brass. Given, S.H.C. of water = 4200 J/kgK.
Answer:
Heat lost by brass sphere = m x s x (\(t_1\)−\(t_2\))
= 0.4 x s x (100 — 23) = 30.8 x sJ.
Heat gained by water
=mxsx (\(t_1\)−\(t_2\)) =1 x 4200 x (23 − 20) = 12600 J.
Now, Heat lost = Heat gained
30.8 x s = 12600
∴ s = 409.1 J/kgK.
Question 97. How many calories of heat will be evolved on the expenditure of 84 joules of energy?
Answer:
4.2 joule will produce 1 calorie heat.
∴ 84 joules will produce\(\frac{1 \times 84}{4.2}\) calorie = 20 calorie.
Wb Class 9 Physical Science Question Answers
Question 98. How much work is to be completely converted to heat to melt 50 g ice at orc?
Answer:
Heat required to melt 1 g ice at 0°C to water at 0°C = 80 calories.
∴ Heat required to melt 50 g ice at 0°C to water at 0°C = 80 x 50 calories = 4000 calories.
Now, for 1 calorie, 4.2-joule work is to be converted to heat.
∴ For 4000 calories, 4.2 x 4000 joule work is to be converted to heat = 16800 Joule.
Wb Class 9 Physical Science Question Answers
Question 99. A mass 10 kg falls a height 1 kilometer to the ground. If all the energy is converted into heat, find the amount of heat developed (given 4.2 joule = 1 calorie).
Answer:
The potential energy mgh stored in the body when it was 1 km above the ground converts to heat when it falls to the ground.
∴ Potential energy = mgh = 10 kg x 9.8 m/\( s^2\) x 1000 m = 98000 J.
∴ The required heat developed = 98000 J.
Question 100. How much heat is necessary to convert 10 g ice at 0°C to 10 g water at the same temperature?
Answer:
Latent heat of fusion of ice is 80 calories per gram or 336 J per gram.
∴ Heat required to convert (1)g ice at 0°C to water at 0°C = 80 calories or 336 J.
∴ Heat is required to convert 10 g ice at 0°C to water at 0°C.
= (80 x 10) calorie or (336 J x 10) = 800 calorie or 3360 J.
Question 101. Find the quantity of heat required to convert 15 g water at 100°C to 15 g steam at 100°C. (Specific latent heat of vapourization of water = 22.5 x 105 J/kg)
Answer:
15 g = 0.015 kg.
∴ Heat required to convert 1 kg water at 100°C to steam at 100°C = 22.5 x 10.
∴ Heat required to convert 0.015 kg water at 100°C to steam at 100°C
= 22.5 x \(10^5\) x 0.015 J = 33750 J.
Chapter 6 Heat 3 Marks Questions And Answers:
Question 1. State and explain the principle of calorimetry.
Answer:
The principle of calorimetry
When two bodies (one being solid and the other liquid or both being liquid) at different temperatures are mixed, heat will be transferred from the body at a higher temperature to the body at a lower temperature till both acquire the same temperature. The body at higher temperatures releases heat while the body at lower temperatures absorbs it, so that
Heat lost = Heat gained,
i.e., the principle of calorimetry represents the law of conservation of heat energy While using this principle always keep in mind that
(1) Temperature of the mixture (T) is always > lower temperature (\(T_1\)) and < higher temperature(\(T_2\)),i.e.,\(T_1\)<T<\(T_2\),
ie., the temperature of the mixture can never be lesser than lower temperature (as a body cannot be cooled below the temperature of the cooling body) and greater than higher temperature (as a body cannot be heated above the temperature of the heating body when there is no chemical reaction).
(2) When the temperature of a body changes, the body releases heat if its temperature falls and absorbs heat when its temperature rises. The heat released or absorbed by a body of mass m is given by:
Q=mc ΔT
Where c is the specific heat of the body and AT change in its temperature in °C or K.
(3) When the state of a body changes, change of state takes place at constant temperature [m.pt. or b.pt.] and heat released or absorbed is given by
Q=mL
Where L is latent heat. Heat is absorbed if solid converts into liquid (at m.pt.) or liquid converts into vapor (at b.pt.) and is released if liquid converts into solid or vapor converts into liquid.
Wb Class 9 Physical Science Question Answers
Question 2.20 gm steam at 100°C is let into a closed calorimeter of water equivalent to 10 gm containing 100 gm ice at — 10°C. Find the final temperature of the calorimeter and its contents. The latent heat of steam is 540 cal/gm, the latent heat of fusion of ice = 80 cal/gm, and the specific heat of ice = 0.5 cal/°C gm.
Answer:
Given
20 gm steam at 100°C is let into a closed calorimeter of water equivalent to 10 gm containing 100 gm ice at — 10°C.
Heat lost by steam = mL + ms (100 − t)
where, t is the equilibrium temperature
Heat lost by steam = 20 x 540 + 20 x (1)(100 − t) =10800 + 2000 – 20t.
Heat gained by (ice + calorimeter) = 100 x 80 + 100 x 0.5 x 10+ 100 x t.
Now, Heat lost = Heat gained :
or, t 10800 + 2000 − 20 t = 8000 + 500 + 100%
or, 120 = 4300 or t = 4300/120 = 35.83°C.
WBBSE Class 9 Physical Science latent heat and specific heat solutions
Question 3. A tube leads from a flask in which water is. boiling under atmospheric pressure to a calorimeter. The mass of the calorimeter is 150 gm, its specific heat capacity is 0.1 cal/g/°C, and it contains originally 340 gm of water at 15 °C. Steam is allowed to condense in the calorimeter until its temperature increases to 71°C, after which the total mass of the calorimeter and contents are found to be 525 gm. Compute the heat of condensation of steam.
Answer:
A tube leads from a flask in which water is. boiling under atmospheric pressure to a calorimeter. The mass of the calorimeter is 150 gm, its specific heat capacity is 0.1 cal/g/°C, and it contains originally 340 gm of water at 15 °C. Steam is allowed to condense in the calorimeter until its temperature increases to 71°C, after which the total mass of the calorimeter and contents are found to be 525 gm.
Mass of calorimeter and contents before passing steam = (150 + 340) = 490 gm.
Mass after passing steam = 525 gm.
=> mass of steam which condenses = (525 – 490) gm = 35 gm.
= 286
Let L = latent heat of steam.
Heat lost by steam = heat gained by water + heat gained by calorimeter.
35L +35 x 1 (100-71) =340 x 1 x (71-15) + 150 x 0.1x (71- 15), Thus, L=539 cal/gm.
Question 4. What is a Calorimeter?
Answer:
Calorimeter
A calorimeter is a device used to measure the heat of a reaction. It can be sophisticated and expensive or simple and cheap A styrofoam cup is used as a calorimeter because it is a container with good insulated walls to prevent heat exchange with the environment.
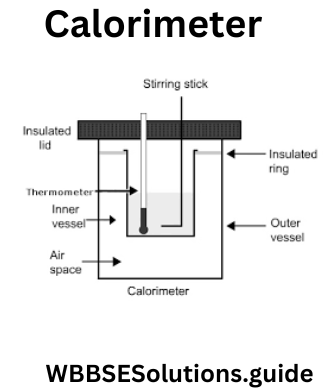
In order to measure heat of reactions, we often enclose reactants in a calorimeter, initiate the reaction, and measure the temperature difference before and after the reaction. The temperature difference enables us to evaluate the heat released in the reaction. A calorimeter may be operated under constant (atmosphere) pressure or constant volume.
Wb Class 9 Physical Science Question Answers
Question 5. State the laws of thermodynamics.
Answer:
There are two major laws concerning thermodynamics:
First Law of Thermodynamics: The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Instead, it is converted from one form to another, such as from mechanical work to heat, from heat to light, from chemical to heat or such. One example of that is how the kinetic energy of a moving car is converted into heat energy at the brakes and tire surfaces. Another example is when chemical energy is released in burning and is converted into light and heat energy.
Second Law of Thermodynamics: The Second Law of Thermodynamics has several variations which will be explained below. Some heat is wasted in conversion One version of the Second Law of Thermodynamics states that some heat is wasted when converting heat into mechanical energy. In other words, in a car engine, not all of the heat created from the exploding gasoline is used in turning the engine or moving the car. Some of the heat simply heats the engine. The percentage of heat turned to work is called the ‘thermal efficiency’ of the engine.
Question 6. Explain the terms saturation vapor pressure (s.v.p.) and unsaturated vapor.
Answer:
Saturation vapor pressure (s.v.p.) and unsaturated vapor
Let us suppose that some liquid is poured into a bottle which is then corked up. Owing to evaporation, the space above the liquid begins to fill with vapor. The vapor molecules move about in all directions and exert pressure when they bounce off the walls of the bottle.
They also strike the surface of the liquid and many re-enter it. Eventually, a state of dynamic equilibrium is reached in which the rate at which molecules leave the liquid is equal to the rate at which others return to it.
Under these conditions, the space above the liquid is said to be saturated with vapor, and the pressure exerted is called the saturation vapor pressure ( s.v.p.).
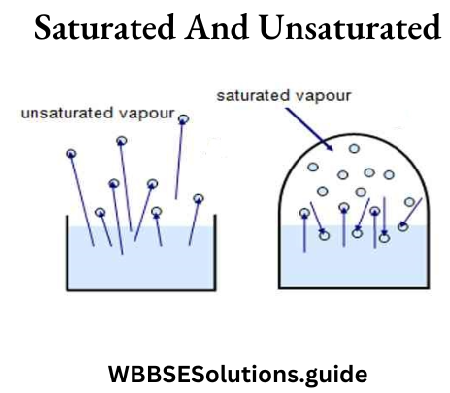
For a given temperature the saturation vapor pressure of a liquid is always the same whether there is air in the space above it or not. Before equilibrium has been reached in the manner described the vapour is said to be unsaturated.
Question 7. What are the kinds of heat?
Answer:
Heat is mainly of three types:
(1) Sensible heat: The kind of heat perceptible by senses or can be detected by a thermometer is known as Sensible heat.
(2) Latent heat: It is the quantity of heat absorbed or released when a substance of unit mass undergoes a physical change of state at constant temperature and pressure. This heat can not be measured by a thermometer.
(3) Radiant heat: The heat which radiates from its source to all directions is called Radiant heat. E.g. Sunlight.
Question 8. What are the conditions required to fulfill the principle of calorimetry?
Answer:
The conditions are:
(1) There should not be a transfer of heat from the bodies to the surroundings.
(2) There should not occur a change of state of any body.
(3) Chemical reaction must not take place between the components.
(4) The bodies should not be soluble in each other.
Question 9. Equal masses of milk and water are separately taken in two identical vessels. The vessels are heated simultaneously in the same oven. Which one will have the quicker rise of temperature, milk or water, and why?
Answer:
The specific heat of milk is less than that of water, so milk will require a smaller quantity of heat for each degree rise in temperature than that needed for the same mass of water. Hence, the supply of heat being the same to the equal masses of milk and water, the rise in the temperature of milk will be quicker than that of water.
(Mathematically, let H quantity heat be supplied to a body of mass m and specific, heat
S. Then if the rise of temperature is t, H = mst. Or, t\(=\frac{\mathrm{H}}{\mathrm{m}} \cdot \frac{1}{\mathrm{~S}}\)
Now if the same quantity in heat H be supplied to equal mass of different substance, then t ∝\(\frac{1}{s}\)
(∴ H, m are constants).
This means a substance of lower specific heat will have a greater rise in temperature. So, in this case, milk will have a quicker rise in temperature.)
Question 10. What do you mean by the sensitivity of a thermometer? Write down the characteristics of a sensitive thermometer.
Answer:
The efficiency of a thermometer to measure even a very little difference of temperature is called: its sensitivity. A thermometer becomes more sensitive if it can measure the slight differences in temperature more accurately.
Conditions for sensitivity :
Bulb:
(1) Should be of large volume (cylindrical)
Glass capillary tube:
(1) The bore of the capillary tube should be fine
(2) The area of the cross-section should be the same everywhere
(3) Lengthy
Thermometric liquid:
(1) Should be of high coefficient of volume expansion
(2) Must be of low thermal capacity
Wbbse Physical Science And Environment Class 9 Solutions
Question 11. Will the temperature of a body containing more heat will always be higher than that of another body containing less heat? Answer with an explanation.
Answer: No.
The quantity of heat in a body depends on three factors:
(1) Mass of the body,
(2) Specific heat of the body and
(3) Change in the temperature of the body. The flow of heat depends on the temperature of the bodies. Heat always flows from the body of high temperature to the body of low temperature.
Question 12. What are the effects of heat on a body?
Answer:
When heat is supplied to a body, the following changes are found to occur:
(1) Change in temperature
(2) Cange in volume
(3) Change of state
(4) Change in physical properties
(5) Change in chemical properties
(6) Creation of light energy, electric energy, burning, etc.
Question 13. Why is mercury used in thermometers?
Answer:
Mercury is an ideal substance for commonly used thermometers. It has the following advantages:
(1) Mercury is a liquid metal and a good conductor of heat, hence it easily acquires the temperature of the body with which it is kept in contact.
(2) The volume of mercury increases sufficiently for a small increase in temperature.
(3) Mercury is opaque and bright like silver, so it can be easily seen from outside the glass tube.
(4) While rising or falling, mercury does not stick to the walls of the glass tube.
(5) The freezing point of mercury is – 30°C and its boiling point is 357° C. Any temperature between this range is measurable by it:
(6)Pure mercury is available easily.
Wbbse Physical Science And Environment Class 9 Solutions
Question 14. Write three differences between heat and temperature.
Answer:
The main differences between heat and temperature are the following :
| Heat | Temperature |
| 1. Heal is a form of energy. | 1. Temperature is a thermal condition of the body, which shows how hot or cold the body is. |
| 2. Heat is the cause. If a body is heated without a change of states, its temperature increases. | 2. Temperature is the effect. |
| 3. The total quantity of heat contained in two bodies may not be equal but their temperatures may be same. | 3. The temperatures of two bodies may be the same, whether their quantity of heat contained be not equal |
| 4. The quantity of heat contained in two bodies may be same but temperatures may be different. | 4. The temperature of two bodies may be different but may have the same quantity of heat. |
| 5. The direction of flow of heat from one body to another does not depend upon the quantity of heat | 5. The direction of flow of heat from one body to another body depends upon |
| possessed by them. | their temperature difference. |
| 6. Heat is measured in Joules or in Calories | 6. Temperature is measured in degrees. |
| 7. During the change of state, the amount of heat in a body changes | 7. During the change of state, the temperature of the body remains constant. |
Modes of heat transfer Class 9 WBBSE notes
Question 15. Explain the factors on which the quantity of heat of a substance depends.
Answer:
If the change of state of a substance does not occur due to the application of heat, then the quantity of heat of the substance depends on its mass, material, and temperature.
(1) On Mass: The heat absorbed by a body is proportional to its mass. Let two balls of the same material, having a mass of one ball double the other, be immersed in boiling water at the same time. The heat gained by the first ball (whose mass is double) will be more than that of the second ball.
(2) On material: The quantity of heat taken by the body depends on the material of the body.
(3) On temperature: Heat taken by a body is directly proportional to the rise in temperature.
Question 16. Define specific heat (or, specific heat capacity). State its mathematical expression. State its units.
Answer:
Specific Heat: The heat required to raise the temperature of the unit mass of a substance through t°C is known as specific heat or specific heat capacity of the substance.
If Q is the quantity of heat supplied to a body of mass m so that its temperature rises through t? C, then specific heat (S) is given by
⇒ \(S\)=\(\frac{Q}{m t}\), Quantity of heat Q=mst.
Units of specific heat: The S.I. unit of specific heat is Joule per kilogram per degree C ( Joule/kg/ \(C^{-1}\)).
The C.G.S. unit of specific heat is calorie per gram per degree C cal/g °\(C^{-1}\).
Wbbse Physical Science And Environment Class 9 Solutions
Question 17. Explain mathematically that heat lost by the hot body is equal to the heat gained by the cold body if there is no loss of heat due to exchange with the surroundings.
Answer:
Let a substance A of mass \(m_1\), specific heat \(s_1\), and a higher temperature \(t_1\) is mixed with another substance B of lower temperature. Let the mass of the substance B is \(m_2\), specific heat be \(s_2\) and final temperature be \(t_2\)
Let the final temperature of the mixture be t.
Then, fall of temperature of A =\(t_1\)– \(t_2\) and gain of temperature of B =t−\(t_2\),
Heat lost by A = \(m_1\).\(s_1\). (\(t_1\) − t).
Heat gained by B =\(m_2\) \(s_1\)(t – \(t_2\)). If no heat is lost in surroundings then, heat lost by A = Heat gained by B
or,\(m_1\)\(s_1\)(\(t_1\)−t)=\(m_2\) \(s_1\)(t−\(t_2\))
Question 18. What will be the reading in Fahrenheit scale of temperature scale corresponding to 100 C? Sites Careak 392
Answer:
From the relation, \(\frac{C}{5}\)=\(\frac{F-32}{9}\), C=100° C
⇒ \(\frac{100}{5}\)=\(\frac{F-32}{9}\) or, F−32=20×9
or, F = 180 + 32 = 212° F.
Question 19. At what temperature is the reading on the Fahrenheit scale five times the reading on the centigrade scale?
Answer:
Let the reading on the centigrade scale be x°, then the reading on the Fahrenheit scale = 5x°.
From the relation,\(\frac{C}{5}\)=\(\frac{F-32}{9}\) or, \(\frac{x}{5}\)=\(\frac{5 x-32}{9}\)
or, 25 x − 160 = 9x or, 16 x = 160, or, x =10.
Therefore, reading on a centigrade scale is 10° C and Fahrenheit scale is = 10 x 5 = 50° F.
Question 20. A body of mass 500 gm requires 3000 joules of heat in order to raise its temperature from 35° C to 45° C. Calculate the specific heat of the body.
Answer:
Given, the mass of water (m) = 500 gm = 0.5 Kg
Heat supplied (Q) = 3000 J, and rise in temperature (t) = 45-35 = 10°C.
Let the specific heat of the body =s
Heat supplied = m.s.t
3000 =0°5x s x 10,
or, 5S = 3000, or s = 600.
Hence, specific heat of body = 600 J/Kg/° C
Question 21. An iron ball of mass of 50 gm is warmed in a furnace and then dropped in a vessel containing 240 gm of water at 30° C. The water equivalent of the vessel is 10 g. If the temperature of water rises to 50° C, find the temperature of the furnace. The specific heat of iron = 0.1.
Answer:
Let the required temperature of the furnace be t° C.
Heat gained by water = mass x specific heat x rise in temperature
= 240 x (1)x (50 – 30) = 4800 cal.
Heat gained by vessel = 10 x (50 – 30) = 200 cal.
Total heat gained by water and vessel = (4800 + 200 ) cal = 5000 cal.
Heat lost by iron ball = mass x specific heat x fall in temperature
= 50 x 0.1 x ( t −50) cal.
By the principle of calorimetry,
Heat lost by iron ball = Heat gained by water and vessel
50 x 0.1 x ( t− 50) = 5000
or, t= 1000 + 50 = 1050° C
Hence the temperature of the furnace = 1050° C.
Wbbse Physical Science And Environment Class 9 Solutions
Question 22. If a clinical thermometer reads 98°4°F as the temperature of a healthy person then what would be the reading of the temperature of this person, if measured in Celsius scale?
Answer:
From the relation, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
C=\(=\frac{5}{9}(F-32)\)
⇒ \(=\frac{5}{9}(98 \cdot 4-32)\)
⇒ \(=\frac{5}{9} \times 66.4\)
= 36.8°C.
Question 23. Calculate the amount of heat given out when the temperature of a piece of iron of mass 60 grams is lowered from 200°C to 100°C. (Specific heat of iron = 0°12)
Answer:
We know that H = mst
Mass of iron = 60 gm. Where t = rise in temperature
Fall in temperature = 200°C − 100°C and S = Specific heat = 100°C.
Specific heat of iron = 0.12
∴ H = 60 x 0.12 x 100:
= 720 cal.
∴ The amount of heat given out
= 720 cal.
Question 24. The difference in the readings of a Fahrenheit and a Celsius thermometer is 48°, What are the actual readings shown in the thermometers?
Answer:
Let the actual readings in the Fahrenheit and the Celsius thermometers correspond to F and C.
∴F−C = 48° or, F=C + 48°
Again, from the relation, \(\frac{C}{5}\)=\(\frac{F-32}{9}\)
⇒ \(\frac{C}{5}\)=\(\frac{C+48-32}{9}\)=\(\frac{C+16}{9}\)
∴ S =20°
Now, F = C + 48° = 20° + 48° = 68°.
∴ The reading in the Fahrenheit thermometer is 68° and that in the Celsius thermometer is 20°.
Question 25. What are the advantages of mercury as a thermometric substance?
Answer:
Advantages of mercury as a thermometric substance :
(1) Mercury being a metal, it absorbs very little heat from the source and thus there is almost no change in the temperature of the source.
(2) Mercury is a shining liquid, so it can be easily seen through glass.
(3) Mercury remains in the liquid state for a long range of temperatures (— 39°C to 357°C). So temperature can be measured over a long range.
(4) Mercury does not wet the glass wall of the tube, so it can move up and down easily through the glass tube.
Question 26. What steps should be taken to make a thermometer sensitive?
Answer:
Requisites of a sensitive thermometer :
(1) The thermometer bulb should have thin walls, so that heat from a body in its contact may easily reach the mercury inside it.
(2) The bore of the thermometer tube should be narrow so that the expansion of the mercury thread is long enough for a small change in temperature.
(3) The bore should be uniform, otherwise, unequal expansion of mercury thread will take place at different parts of the bore for equal changes in temperature.
(4) The thermometric liquid should be good conducting so that it quickly assumes the temperature of the body in contact of the thermometer.
Wbbse Physical Science And Environment Class 9 Solutions
Question 27. Obtain an expression for the heat absorbed or given out by a body.
Answer:
Calculation of the amount of heat absorbed or given out by a body: If S be the specific heat of a substance, then we can write from the definition of specific heat that,
1 unit mass of a substance, for 1° rise or fall of temperature, will absorb or give out ‘S’ units of heat.
m unit mass of a substance, for t° the rise or fall of temperature, will absorb or give out ‘mst’ units of heat.
That is,
Quantity of heat (absorbed or given out)
= mass of the body x specific heat x rise or fall of temperature.
Question 28. What is the relation between Celsius, Fahrenheit, and Kelvin scales?
Answer:
Relation between Celsius, Fahrenheit, and Kelvin scales: Since the range of temperature from lower fixed point to upper fixed point is equal in all three scales, 100 centigrade degrees = (212 — 32) or 180 Fahrenheit degrees = (373 − 273) or 100 absolute degrees.
We consider that the three thermometers in the above three scales are dipped simultaneously in a liquid of a certain temperature. Let the temperatures recorded in the Celsius, Fahrenheit, and Kelvin thermometers respectively be C, F, and K. Now, it can be proved that
⇒ \(\frac{C-0}{(100-0)}\)=\(\frac{F-32}{(212-32)}\)=\(\frac{K-273}{(373-273)}\)
or,\(\frac{C}{100}\)=\(\frac{F-32}{180}\)=\(\frac{K-273}{100}\)
or,\(\frac{C}{5}\)=\(\frac{F-32}{9}\)=\(\frac{K-273}{5}\)
Question 29. State and explain the equivalence of work and heat.
Answer:
The equivalence of work and heat
From various phenomena, it is possible to consider that there exists a relation between heat and work. In some phenomena, heat transforms to mechanical work and in some, the reverse happens.
In 1798 Rumford noticed the intense heat generated in a steel body that was being hollowed for making barrels of cannon. He concluded that the mechanical work done in the following process was converted into heat energy.
When a football bladder or a bicycle is pumped, the body of the pump is heated. Here, due to the push of the piston, the motion of the air particles inside the pump increases, i.e., their kinetic energy increases.
The kinetic energy of the air particles converts to heat energy. When heat is supplied to a gas confined in a vessel at constant pressure, the volume of the gas increases, which means, the gas particles do work against the pressure. Here, heat is converted to work.
Here, to cause expansion, i.e., to increase the space between the gas particles some heat energy from the gas particles is expanded, the loss of heat causes cooling. Thus, heat is used to do the mechanical work in increasing the separation between the gas particles.
From the above example, it is clear that heat and mechanical work are related to each other. So, if W amount of work produces H quantity of heat or H quantity of heat produces W mechanical work, according to Joule, W ∝ H or W = JH, where J is a constant, called Joule’s constant. J is also called the Mechanical equivalent of heat.
The above said relation, W=JH, J\(=\frac{W}{H}\)
lf H = 1, ie., when unit quantity of heat is produced, W = J. This means J, the mechanical equivalent of heat is equal to the mechanical work that produces a unit quantity of heat. So, the definition of the mechanical equivalent of heat is it is the quantity of mechanical work that can produce a unit quantity of heat.
If the unit of work chosen is erg, and that of heat, calorie, then in the C.G.S. system, the value of J is 4.2 Joule/calorie. Thus, the mechanical equivalent of heat is 4.2 Joule; it means that, if 4.2 Joule work is fully converted to heat, (1)calorie heat is produced.
This value of J has also been confirmed by many experiments.
Question 30. What is latent heat? Classify it and define its different types.
Answer:
Latent heat
The quantity of heat given out or absorbed per unit mass of a substance during the change from one state to another keeping the temperature constant at the temperature at which the change of state occurs is called the latent heat of the substance.
There are different types of change of states and the following are the corresponding latent heats:
Latent heat of fusion: The quantity of heat required to convert the unit mass of a solid substance at its melting point to its liquid state keeping the temperature constant is known as its latent heat of fusion.
The values of latent heat of fusion of ice in C.G.S. and SI systems are 80 calorie/g and 3.36 x \(10^5[l/latex] J/kg respectively.
Latent heat of vaporization: The quantity of heat given out to convert the unit mass of a liquid at its freezing point to its solid state keeping the temperature constant is known as its latent heat of solidification.
Latent heat of condensation: The quantity of heat given out to convert the unit mass of a vapor at its normal boiling point to its liquid state keeping the temperature constant is known as its latent heat of condensation.
Question 31. Distinguish between. saturated and unsaturated vapor.
Answer:
Difference between saturated and unsaturated vapor:
(1) A vapor in a closed enclosure at a given temperature is said to -be saturated if the pressure exerted by it is maximum at that temperature and the pressure exerted by it is called saturation vapor pressure at the temperature. A vapor in a closed enclosure at a given temperature is said to be unsaturated if the pressure exerted by it is less than the maximum value at that temperature.
(2) Saturated vapor does not obey Boyle’s law, while unsaturated vapor obeys Boyle’s law.
(3) Saturated vapor does not obey the pressure law, while unsaturated vapor obeys the pressure law.
(4) A certain quantity of unsaturated vapor can become saturated if the pressure is increased or the temperature is decreased at constant volume.
Question 32. Write a short note on the anomalous expansion of water on marine life.
Answer:
The anomalous expansion of water on marine life
Generally, liquids are found to expand on heating, i.e., their volumes increase with the rise in temperature. But in the case of water, this phenomenon does not hold true within a certain range of temperature.
When water is cooled from room temperature, it contracts uniformly till the temperature reaches 4°C. Below this temperature instead of contraction, water expands with fall in temperature till 0°C, when water freezes to form ice. This phenomenon is called the anomalous expansion of water.
This peculiar behavior of expansion of water has great importance on marine life in cold countries. In these countries when air above the surface of the water of a pond falls below 0°C, the water on the surface, cooling, becomes denser than that below and gradually sinks downwards. It keeps ‘on till the water temperature falls to 4°C. As the surface temperature falls further, it
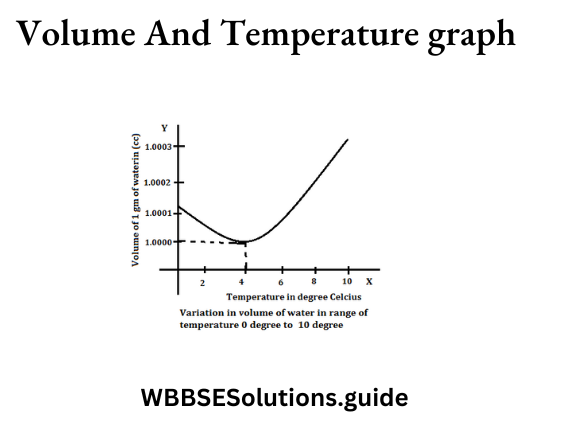
Becomes less dense than the water below, which is at 4°C, and is the densest. It thus remains at the top, cooling more and more and ultimately a layer of ice is formed there and remains there, as ice is lighter than water.
The layer of ice forms as a poor conducting layer on the top, which prevents the heat from the water below from passing into the colder atmosphere
above.
A layer of ice develops in thickness extremely slowly. The temperature of the deeper layers of the water in the pond remains nearly at 4°C and falls gradually to 0°C upwards till the layer of ice is reached. The marine life in the winter is thus saved.
Wbbse Physical Science And Environment Class 9 Solutions
Question 33. When 0.15 kg of ice at 0°C mixed with 0.30 kg of water at 50°C in a container, the resulting temperature is 6.7°C. Calculate the heat of the fusion of ice. ([latex]S_{\text {water }}\)=4186J\(\mathrm{kg}^{-1} \mathrm{~K}^{-1}\))
Answer:
Let mw = mass of water = 0.30 kg
⇒ \(m_i\)= mass of ice = 0.15 kg
⇒ \(\mathrm{T}_1\)= initial temperature of water.= 50°C
⇒ \(\mathrm{T}_2\)= final temperature of water = 6.7°C
⇒ \(\mathrm{L}_f\)= Heat of fusion of ice
⇒ \(\mathrm{S}_w\) = Specific heat of water = 4186 J\(
⇒ [latex]\mathrm{kg}^{-1} \mathrm{~K}^{-1}\)
Heat lost by hot water \(=m_w s_w\) \(\left(T_1-T_2\right)\) = (0.30kg) (4186 Jk\(g^{-1}\) (50 – 6.7)°C = 5437614 J
Heat needed to melt ice =\(m_i\)\(\mathrm{L}_f\)= (0.15 kg) (\(\mathrm{L}_f\))
Heat is needed to raise the temperature of ice water
=\(m_i\)\(\mathrm{S}_w\) (\(\mathrm{T}_2\)−0°C) = (0.15 kg) (4186 J\(\mathrm{kg}^{-1} \mathrm{~K}^{-1}\)) (6.7 −0)°C = 4206.93 J
Heat lost = Heat gained
∴ 54376.14 J = (0.15 kg)\(\mathrm{L}_f\) + 4206.93 J
or, \(\mathrm{L}_f\) = 3.34\(10^5\) J k\(g^{-1}\)
Question 34. 300g lead at 99°C is dropped in 100 g waster at 18°C. The final temperature of the mixture is 27°C. Find the specific heat capacity of lead if specific heat capacity of water is 4200 J k\(g^{-1}\)\(K^{-1}\)
Answer:
Let the specific heat capacity of lead be ‘s’ J/kg K.
Heat lost by 300g lead to cool to 27°C
⇒ \(=\frac{300}{1000} \times 5 \times(99-27) \text { Joules }\)
= 0.3 x s x 72 joules = 21.6s Joules
Heat gained by 100 g water when the temperature rises from 18°C to 27°C
⇒ \(=\frac{100}{1000} \times 42,000 \times(27-18) \text { joules }\)
= 3780 joules.
So, from the principle of calorimetry, heat lost = heat gained
∴21.68 = 3780
or, s\(=\frac{3780}{21.6}\)=\(175 \mathrm{JKg}^{-1} \mathrm{~K}^{-1}\).
Question 35. A piece of copper heated to 35°C is dropped into 140 g of water at 15°C. The mass of copper is 150 g and the specific heat of copper is 400 J/kg K. Find the final temperature. (Specific heat of water = 4200 J/kg.K)
Answer:
Let the final temperature be t°C.
Now, the heat lost by copper.
= 0.15 x 400 x (35 − t) = 60(35 – t) joule.
Heat gained by water
= 0.14 x 4200 x (t − 15) = 588 (t−15) joule.
So, from the principle of calorimetry,
heat lost = heat gained
60(36 − t) = 588 (t −15)
or, 60 x 35 −60t = 588t 588 x 15
or, 60 x 35 + 588 x 15 = 188t + 60t
∴ 648t = 60 35 +588 15
or, t\(=\frac{60 \times 35+588 \times 15}{648}\)
=16.85° C
Question 36. How much heat is required to convert fully 1 kg of ice at 0°C to steam at 100°C? (S.L.H. of fusion of ice = 3.36 x \(10^5\)J/kg, S.L.H. of vapourisation of water = 22.6 x \(10^5\) J/kg and specific heat capacity of water = 4.2 x \(10^3\) J/kg)
Answer:
Heat required to convert 1kg ice at 0°C to 1 kg water at 0°C = 3.36 × \(10^5\) J……….(1)
∴The heat required to raise the temperature of 1 kg water at 0°C to 100°C =1x 4.2 x\(10^3\) x 100 J = 4.2 x\(10^5\) J……….(2)
Heat required to convert (1)kg water at 100°C to 1 kg steam at 100°C = 22.6 x x\(10^5\) J………… (3)
∴ The total quantity of heat required to convert 1 kg ice at 0°C to (1)kg steam at 100°C
= (1) + (2) + (3) = (8.36 + 4.2 + 22.6) x \(10^5\) J
= 3016 x \(10^5\) J.
Question 37. 300 g lead at 99°C is dropped in 100 g water at 18°C. The final temperature of the mixture is 27°C. Find the specific heat capacity of lead if the specific heat capacity of water is 4200 J K\(g^{-1}\)\(K^{-1}\)
Answer:
Let the specific heat capacity of lead be ‘s’ J/kgK.
Heat lost by 300 g leads to cool from 99°C to 27°C
= \(\frac{300}{1000}\) xs x (99-27) J=0.3 xS x 72 J= 21.6 SJ.
Heat gained by 100 g water when its temperature rises from 18°C to 27°C
=\(\frac{100}{1000}\) x 42000 x (27 −18) J = 3780 J.
From the principle of calorimetry, heat lost = heat gained
or, 21.6 s= 3780
∴ S= \(\frac{3780}{21.6}\) Jkg-1K-(1)= 175 Jk\(g^{-1}\)\(K^{-1}\).
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound
