Separation Of Components Of Mixture Very Short Answer Type:
Question 1. What is a mixture?
Answer: A mixture refers to the physical combination of two or more elements or compounds at any proportion where the identities of all components are retained. Some mixtures can be separated into their components by physical means.
Question 2. What is a homogeneous mixture?
Answer: Most of the materials are neither pure elements nor pure compounds; they are mixtures. A solution of sugar in water is an example.
Question 3. Mention one reason to separate the components of a mixture.
Answer:
To get a pure sample of a substance: Pure substances are required in our daily life for various purposes like, in the laboratory for conducting various experiments, in industry, in medicines and for carrying out research works.
West Bengal Board Class 9 Physical Science Solutions
Question 4. Mention the principle of sedimentation and decantation.
Answer: Heavy solid insoluble in a liquid settles down at the bottom forming a sediment. The upper clear liquid is the supernatant liquid.
Read and Learn all WBBSE Solutions for Class 9 Physical Science And Environment
Example: Muddy water (mud forms the sediment).
Question 5. Mention the principle of filtration.
Answer: Light-weighed solid insoluble in a liquid can be separated by filtration.
Example: Sawdust + water (sawdust floats on water).
Question 6. Mention the principle of distillation.
Answer: One of the components is a solute solid. Both components are recovered.
Example: Salt + water.
Question 7. What is distillation?
Answer: The process of converting a liquid into its vapour by heating and the subsequent condensation of the vapours back into the original liquid is called distillation.
Wbbse Madhyamik Physical Science And Environment Class 9 Question 8.
What is fractional distillation?
Answer: The process of separation of two or more miscible liquids by distillation by making use of the difference in their boiling points is called fractional distillation.
Question 9. What is sublimation?
Answer:
Sublimation: A process of phase transition when a solid goes directly into the vapour phase without passing through the liquid phase.
Example: Lodine or \(\mathrm{NH}_4 \mathrm{Cl}\)
West Bengal Board Class 9 Physical Science Solutions
Question 10. What is an alloy?
Answer:
Alloy: Solid-solid mixture of metals or metals with non-metals.
Question 11. What is distillate?
Answer:
Distillate: In the distillation process, the condensed vapour is called the distillate.
Question 12. What is filtrate?
Answer:
Filtrate: The liquid that passes through the filter.
Question 13. What is residue?
Answer:
Residue: The insoluble solid left on the filter.
Question 14. What is sedimentation?
Answer:
Sedimentation: The process of settling down of the insoluble solid when the mixture of insoluble solid and liquid is allowed to stand.
Question 15. What is sediment?
Answer:
Sediment: The solid substance that settles down.
Question 16. What is supernatent liquid?
Answer:
Supernatant Liquid: The clear liquid left in sedimentation.
Question 17. What is decantation?
Answer:
Decantation: The process of pouring out of

Separation Of Components Of Mixture 2 Marks Questions And Answers:
Question 1. What is a mixture? State the types of mixtures.
Answer:
Mixture
If two or more substances (elements, compounds or both), mixed togther in any proportion, do not undergo any chemical change but retain their characteristics, the resulting mass is called mixture. A mixture may be of two types:
(1) Heterogeneous mixture: A mixture in which various constituents are not mixed uniformly is called a heterogeneous mixture. For example, a mixture of sand, salt, sulphur, etc.
(2)Homogeneous mixture: A mixture in which different constituents are mixed uniformly is called a homogeneous mixture. For example, all solutions are homogeneous mixtures.
Question 2. What are miscible and immiscible liquids? Give examples.
Answer:
Miscible and immiscible liquids
When two liquids are dissolved in each other forming a homogeneous solution, it is called a miscible liquids mixture. For example, a solution of-ethy| alcohol and water, a solution of methyl alcohol and acetone. When two liquids do not dissolve in one another, they are called immiscible liquids: For example, mixture of kerosene oil and water, a mixture of carbon disulphide and water.
Question 3. How are the miscible liquid mixture and immiscible liquid mixture separated?
Answer:
The liquid-liquid mixture are separated by use of diffrent physical methods depending on the state and nature of mixture. The miscible liquid mixture is separated by the method of fractional distillation while the mixture of immiscible liquids by separating funnel.
West Bengal Board Class 9 Physical Science Solutions
Question 4. What is the function of a fractionating column?
Answer:
Function of a fractionating column
The improved fractionating column is designed in such a way that the liquids of higher boiling point are mostly condensed in it and return in the form of liquid in the distillation flask.Only the liquid of lower boiling point is allowed to pass through it.
Question 5. Mention the principle of mechanical picking with example.
Answer:
Mechanical Picking: The components are to be of different sizes and colours.
Example: Tiny stones from grains and pulses.
Question 6. Mention the principle of magnetic separation with example.
Answer:
Magnetic Separation: One of the components being magnetic, magnetic separation can separate the mixture of of iron and powdered sulphur.
Wbbse Class 9 Physical Science Solutions
Question 7. Mention the principle of gravitational separation with example.
Answer:
Gravitational Separation: One of the component is much heavier than water and the other one is much lighter than water.
Example: A mixture of sand and saw dust.
Question 8. Mention the principle of sublimation with example.
Answer:
Sublimation: One of the components sublimes.
Example: a mixture of sand and iodine, a mixture of common salt (NaCl) and ammonium chloride (\(\mathrm{NH}_4 \mathrm{Cl}\)).
Wbbse Class 9 Physical Science Question Answers
Question 9. Mention the principle of solvent extraction with example.
Answer:
Solvent Extraction : One of the components is soluble in a solvent (say, water) and the other one is not.
Example: Mixture of sodium chloride (soluble in water) and chalk (not soluble in water).
Question 10. Mention the principle of fractional crystallization with example.
Answer:
Fractional Crystallization: The solubility of components in a solvent have to differ widely.
Example: Mixture of potassium nitrate (less soluble in water) and common salt.
Question 11. Mention the principle of sedimentation with example.
Answer:
Sedimentation or Decantation: A heterogeneous mixture containing an insoluble solid in a liquid.
Example: Mixture of sand or mud and water.
Wb Class 9 Physical Science Question Answers
Question 12. Mention the principle of evaporation with example.
Answer:
Evaporation: One component being non-volatile, may be soluble in water or not.
Example: Salt. solution [non-volatile salt recovered].
Wbbse Madhyamik Physical Science And Environment Class 9 Question 13.
Mention the principle of filtration with example.
Answer:
Filtration: A heterogeneous mixture containing an insoluble solid in liquid.
Example: Mixture of sand in water.
Question 14. Mention the principle of distillation with example.
Answer:
Distillation: A homogeneous mixture having a dissolved solute in a solvent. (Both components are recovered).
Example: A solution of common salt.
Wb Class 9 Physical Science Question Answers
Question 15. Mention the principle of separation by’ separating funnel with example.
Answer:
By Separating Funnel : A mixture of ifnmiscible liquids, differing widely in density.
Example : A mixture of oil (say, kerosene) and-water. Kerosene being much lighter than water.
Question 16. Mention the principle of fracti6nal -distillation with. example.
Answer:
Fractional Distillation: A homogéneus mixture of two miscible liquids, differing widely in their boiling points.
Example: A solution of alcohol and water. Alcohol boils at much lower temperature than water.
Question 17. What chromatography?
Answer:
Chromatography : It is a method by which in a mixture of different substances are separated. In this method due to difference in adsorption. of different substances in solid phase (adsorbent) and difference in migration of the substances in the mobile phase (liquid or gas) various substances are separated.
Wb Class 9 Physical Science Question Answers
Question 18. What is paper chromatography?
Answer:
Paper Chromatography : It is a very easy technique to separate the various organic dyes present in the writing ink or printing ink.
Question 19. Mention two advantages of chromatography.
Answer: Advantages of chromatography :
(1) A small amount of the compound present in the mixture can be separated.
(2) The properties of the individuals present in the mixture do not alter.
Separation Of Components Of Mixture 3 Marks Questions And Answers:
Question 1. Describe the method of separation of a mixture of two immiscible liquids using separating funnel.
Answer:
The method of separation of a mixture of two immiscible liquids using separating funnel
The components of a mixture of two immiscible liquids (such as oil and water) are separated by a separating funnel.Separating funnel is a long glass tube provided with a stop clock. The immiscible liquid is poured in the funnel and allowed to stand for some time. The immiscible liquids separate into two distinct layers. The liquid with lower density (oil) remains in the upper layer and the liquid with higher density (water) will be deposited in the lower layer.
A conical flask is placed under the nozzle of the separating funnel. The stop clock is gently opened so that the heavier liquid trickles in the flask drop by drop. Once the denser liquid is drained out, the stop clock is closed. Another conical flask is placed under the nozzle of the separating funnel. Stop clock is opened to drain the lighter liquid. In this way both the liquids are separated in two separate flasks.
Wbbse Class 9 Physical Science Solutions
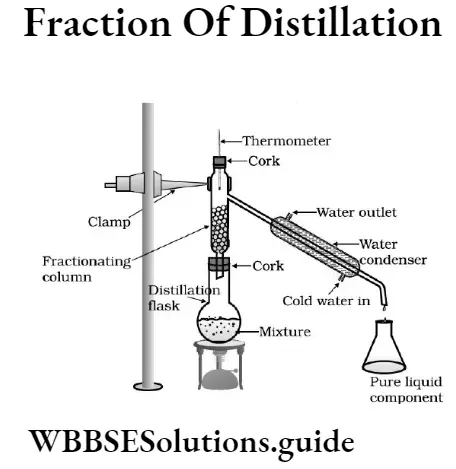
Question 2. What is fractional distillation? How is the mixture of two miscible liquids separated by the method of fractional distillation?
Answer:
Fractional distillatio
The process of separation of two miscible liquids by the process distillation, making use of their difference in boiling points, is called fractional distillation. The mixture of two miscible liquids is separated by a fractional distillation apparatus.
The fractional distillation apparatus contains a distillation flask fitted with a fractional columm. One end of a-Leibig’s condenser is connected with fractionating ‘column and other end with a receiving flask to collect the distilate as shown in the given figure. The fractionating column is designed in such a way so that the vapours of the higher boiling liquid are mostly
condensed in it and return into the distilating flask in the form of liquid.
The vapours of the lower boiling liquid passes through the Leibug’s condenser and are collected in a receiver. The thermometer shows a constant reading as long as the vapours of the first liquid are passing to Leibig’s condenser. As soon as the temperature starts rising, the receiver is replaced by another receiver to collect the second liquid.
Question 3. Why do we separate the components of a mixture?
Answer:
Reasons for separating the components of mixture: The components of a mixture are separated because of the following reasons :
(1) To get a pure sample of a substance: Pure substances are required in our daily life for various purposes like, in laboratory for conducting various experiments, in industry, in medicines and for carrying out research works.
(2) To remove any undesirable or harmful components: The undersirable components present in a mixture can sometimes be very harmful, hence they need to be removed in order to get the desirable component. For example, small stones are removed from tice before the rice is cooked, otherwise, they can harm us.
Wbbse Class 9 Physical Science Solutions
Wbbse Class 9 Physical Science Solutions
(3) To obtain the useful components of a mixture: Sometimes a mixture may comprise more than one useful components. These useful components need to be separated for their individual use. The best example of such a mixture is crude petroleum.
Question 4. Explain the method of separation of colouring matters of ink (made of three different coloured substances) by simple paper Chromatography.
Answer:
Take a strip of filter paper about 10 cm long and 5 cm broad and stick its smaller end to a glass plate. On the lower end about 1 cm from the bottom, mark a small point and put one or two drops of ink. Now suspend this filter paper in a tall cylinder and pour some water in the cylinder till the lower end of the filter paper slightly dips in the water. The cylinder is covered with a glass lid to prevent evaporation.
After some time the water rises up the filter paper upto the ink mark and slowly dissalves the various constitutes of ink in it. The various constitutes of ink get absorbed by the filter paper in different amounts. The components of the ink solution rise to different heights on the paper strip depending on their solubility in water. The constitutes that are more absorbed they move upward lesser. Thus different column bands are formed on the paper and colouring matters of the link are separated. The diagram given here shows the method of separation of colouring matters of ink by paper chromatography.
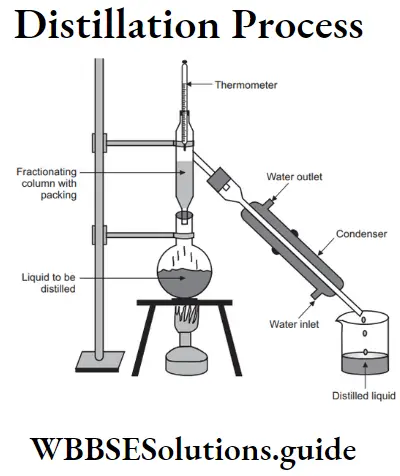
Question 5. Write a short note on distillation.
Answer:
Distillation: This process allows separation and recovery of both components of a solid-liquid mixture by the use of a distillation apparatus. The process of converting a liquid into its vapour by heating and the subsequent condensation of the vapours back into the original liquid is called distillation.
Procedure :
(1) The mixture is taken in a distillating flask fitted with a Leibig’s condenser. At the other end of the condenser a receiver is placed to collect the distance.
(2) When the flask is heated, the vapours of the solvent pass through the condenser. They are cooled, condensed into a liquid and collected as a distillate in the receiver.
(3) The solid component forms the residue in the flask.
Examples: Distillation of solid-liquid mixtures.
| Components of the Solid-Liquid Mixture | Soluble solid component | Distillate |
| 1. Iodine + Chloroform | Iodine | Chloroform |
| 2. Salt + Seawater | Common salt and other salts | Pure water |
| 3. Iodine + Alcohol | Iodine | Alcohol |
Wbbse Madhyamik Physical Science And Environment Class 9 Question 6.
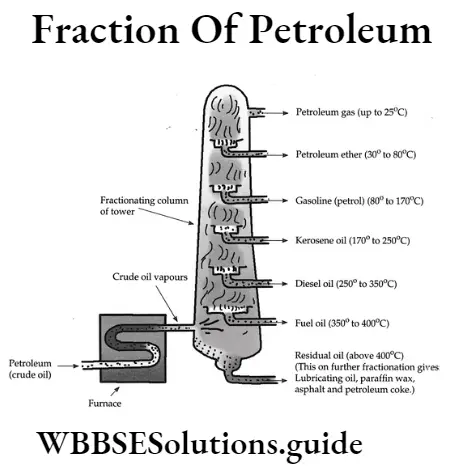
State the importance of fractionation of petroleum.
Answer:
Importance of fractionation of petroleum: The chief source of liquid fuels is petroleum. However, petroleum cannot be used directly because it burns with a highly sooty flame, producing harmful gases.
The petroleum is subjected to fractional distillation. The crude oil (petroleum) is separately boiled in a ‘retort’so as to form its vapour. The vapours of petroleum are passed into the fractionating column. The vapours of heavier molecules condense in the lower parts of the fractionating column. The vapours of lighter molecules condense in the upper part of fractionating column.
The crude petroleum obtained from mines is a mixture of hydrocarbons containing [laaatex]C_1 \text { to } C_{40}[/latex] carbon atoms. Its different fractions are collected by fractional distillation. This process of purification of petroleum into different fractions is called refining of petroleum.
Wbbse Class 9 Physical Science Solutions
Question 7. State the procedure of separation by separating funnel.
Answer:
Separation by separating funnel :
(1) Separating Funnel: It is an apparatus used for separating immiscible liquids. The liquids do not mix with each other and due to difference in densities the liquids remain in separate layers in the separatory funnel.
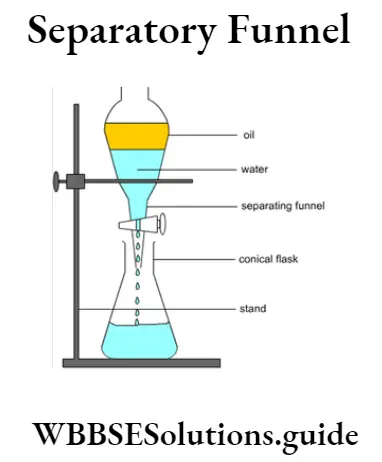
(2) Procedure :
(1) A mixture of the immiscible liquids is taken in a separatory funnel (with its stopcock closed) and allowed to remain on the stand
(2) The immiscible liquids get separated into two x Water distinct layers, the lighter component forming the upper layer and the denser component the lower g Separatory funnel layer.
(3) A conical flask is placed below the stem of the separatory funnel and the stopcock is carefully opened to run out the denser immiscible layer.
(4) The stopcock is then closed and a new conical flask is placed as before and the femaiing lighter upper iayer is carefully taken out.
Example : Separation of Liquid-Liquid pactres using a separatory funnel.
Wbbse Class 9 Physical Science
Question 8. State the different types of mixture giving one example each of homogeneous and heterogeneous mixtures.
Answer:
The different types of mixture giving one example each of homogeneous and heterogeneous mixtures
| Types of mixture | Homogeneous | Heterogeneous |
| (1) Solid in Solid (S in S) | Bronze (Mixture of Cu. Zn, Sn) | Gunpowder (Charcoal. Sulphur, Nitre) |
| (2) Solid in Liquid (S in L) | Sugar in water. Iodine in alcohol | Sand in water; Sugar in oil |
| (3) Liquid in Solid (L is S) | Hg-Au Amalgam | Water m sponge |
| (4) Liquid in Liquid (L in L) | Ethanol or methanol in water acetone in water | Oil in water |
| (5) Gas in Liquid (G in L) | Ammonia gas or hydrogen chloride gas in water. | Helium in water. |
| (6) Liquid in Gas (L in G) | Moisture in Air | ________ |
| (7) Gas in Gas (G in G) | Air | Industrial release of smoke |
Question 9. How does boiling point depend on superincumbent pressure ?
Answer:
Dependence of boiling point on superincumbent pressure : The boiling point of a liquid is directly proportional to the pressure exerted on it. It is known that when the vapour pressure of a liquid becomes equal to the atmospheric pressure, the liquid starts to boil. The vapour pressure of a liquid is also proportional to the pressure exerted on it. At low pressure, the liquid boils and evaporates at a lower temperature.
When any components of a solution decompose at normal boiling point (at normal pressure), then separation of the components is made by distillation at reduced pressure, i.e., the components boil below boiling points.
Wbbse Class 9 Physical Science Question 10. State the common separation techniques and their underlying working principles.
Answer:
Common separation techniques and their underlying working principles :
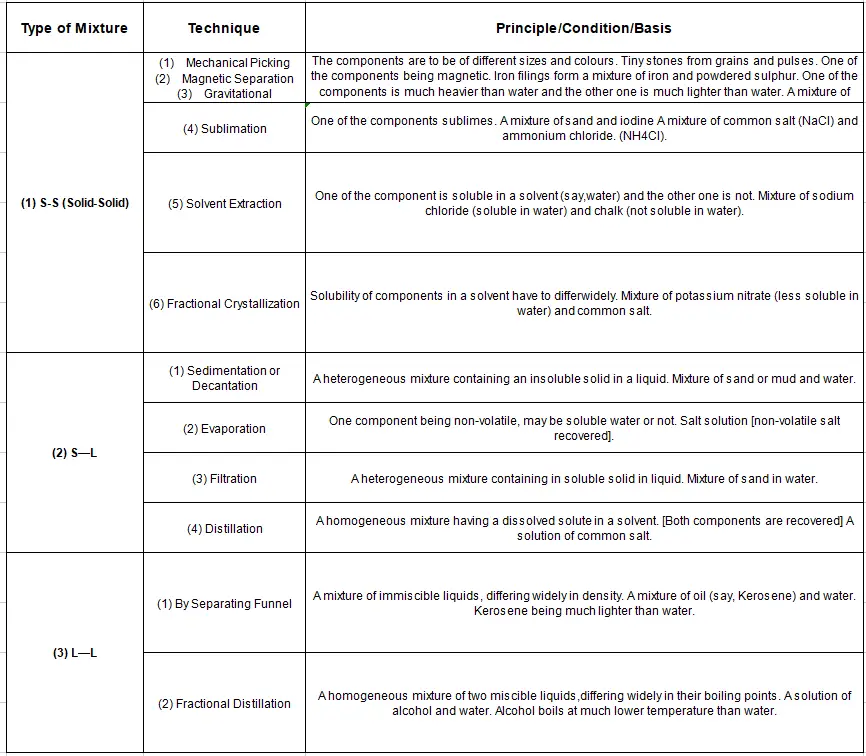
Question 11. Explain the method of separation of the compounds of crude petroleum.
Answer:
Seperation of Components of CrudePetroleum : Crude petroleum contains various hydrocarbon ingredients of close boiling points, the boiling points of these ingredients ranging from <293K to > 573K. These ingredients are collected in 313-443K a range of boiling points using high efficiency
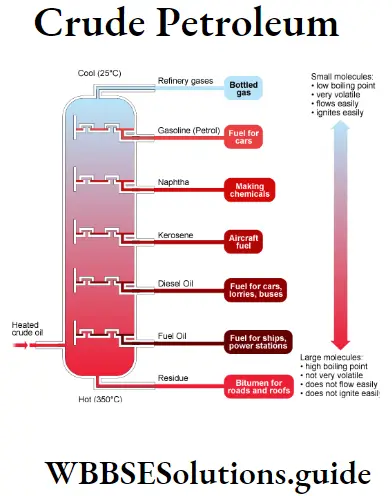
Fractionating column, Example:
(1) Natural gas (293K)
(2)Gasolene (293K-333k)
(3) Naptha (313K-453k)
(4)Kerosene (453K-533k)
(5) Diesel (533K-613K)
(6) Lubricating oil (613K-773K).
WBBSE Solutions for Class 9 Physical Science And Environment
- Chapter 1 Measurement
- Chapter 2 Force And Motion
- Chapter 3 Matter: Structure And Properties
- Chapter 4 Matter: Atomic Structure; Physical & Chemical Properties of Matter
- Chapter 5. Energy In Action: Work, Power & Energy
- Chapter 6 Heat
- Chapter 7 Sound
